Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.111158
Revised: August 29, 2025
Accepted: September 22, 2025
Published online: October 28, 2025
Processing time: 123 Days and 14 Hours
Percutaneous liver biopsies, including coaxial needle biopsy (CNB), are the pre
We report a series of five image-guided liver biopsy cases undertaken due to a variety of clinical conditions followed by the use of a new electrocautery hemo
Hemostasis of CNB tracts using SinglePass Kronos was obtained in all cases. Ad
Core Tip: Percutaneous, image-guided medical liver biopsies are an invaluable tool in the diagnosis of a wide variety of hepatic diseases and conditions. Despite advances in tools and techniques, bleeding remains a frequent post-procedural complication, sometimes with significant sequelae. The SinglePass Kronos device was developed to provide hemostasis within the biopsy needle tract via electrocautery. This series of 5 case reports on Kronos use following percutaneous liver biopsy demonstrates Kronos integration into biopsy workflow with no post-procedural bleeding complications through 30 days. The Kronos device may be a valuable tool in preventing bleeding complications in percutaneous liver biopsies.
- Citation: Misono AS, Oftadeh B, Nguyen V, Dang Q, Young L, Patel T, Techasith T, Mesipam A, Baker C, Velling T. SinglePass Kronos electrocautery device for closure after percutaneous medical liver biopsy: Five case reports. World J Gastroenterol 2025; 31(40): 111158
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/111158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.111158
Liver biopsy is a common procedure integral to the diagnosis, treatment, and management of patients with hepatic conditions. While surgical and laparoscopic biopsies have historically been performed, particularly under certain clinical conditions, much more commonly in the modern era, percutaneous liver biopsy is the preferred method due to being less invasive and less expensive. Within the percutaneous category, there are several variants that employ different methods of visualization and different tools for harvesting tissue samples. Different needle types, including suction needles, cutting needles, and spring-loaded, triggered cutting needles, have been described, coupled with various visualization methods[1]. A brief comparison of various liver biopsy methodologies is presented in Table 1 below.
| Method | Indication | Advantages | Limitations | Risks | Relative bleeding risk |
| Palpation/percussion-guided | Diffuse disease | Simple, low-cost | Less accurate, poor sample yield | Collateral damage, need for repeat biopsy | +++ |
| Image-marked/blind | Focal lesions, abnormal anatomy | More accurate | Additional equipment required, inconsistent yield | Bleeding, collateral damage | ++ |
| Real-time image-guided | Focal lesions, abnormal anatomy | More accurate, more procedural flexibility | Additional equipment and training required | Bleeding, collateral damage | ++ |
| Plugged, real-time image-guided | Mild coagulopathy, higher bleeding risk | Lower bleeding risk, allows biopsy in higher-risk patients | Procedure complexity | Pain, extended observation | + |
| Trans-jugular | Coagulopathy, ascites, portal hypertension | Allows biopsy in high-risk patients | Smaller/inconsistent samples, vascular access required | Vascular damage | + |
| Laparoscopic/surgical | Direct visualization required or contraindication to percutaneous methods | Direct access, multiple sites can be sampled, complete samples | Significantly invasive, longer recovery time | Infection, pain, collateral damage | +++ |
As seen in Table 1, all biopsy procedures retain a risk of bleeding. This is pertinent to biopsies of all solid organs including liver, lungs, and kidney, as well as biopsies of various tumors[2]. The historical progression of techniques from open surgical to laparoscopic to percutaneous methods has been largely in an effort to limit risk of hemorrhage while retaining tissue quality. The addition of real-time image guidance has added precision and accuracy to the percutaneous procedure both in terms of tissue sample yield and in reducing collateral damage to blood vessels and target organs that can be a significant source of hemorrhagic complications. Finally, the addition of adjunctive post-biopsy techniques such as gel plugs, tract sealant, and ablation devices has further reduced the risk of post-procedure bleeding[3-5]. Some of these methods have been shown to be cost-effective when considering both direct and indirect costs[6].
Image-guided coaxial needle biopsy (CNB) is a common percutaneous technique used to obtain high-yield solid organ tissue samples in a percutaneous fashion. CNB has allowed for the minimally invasive diagnosis of various solid organ disorders without the need for surgery and is commonplace in the management and workup of liver disease[7,8]. However, percutaneous CNB procedures can also result in adverse events related to needle passes or capsular organ punctures during tissue collection. This may be further accentuated in high-risk patients who may have inherent coagulopathies, be on anticoagulation medication, or often a combination of the two[5,9,10].
The most common major adverse event related to these biopsies is solid organ hemorrhage, which can occur at rates as high as 5% in clinical practice[11]. In addition, it is difficult to predict and detect which patients may bleed or develop hematomas after CNB procedures[12] and the risk of bleeding necessitates temporarily halting antiplatelet and/or anticoagulant medications. Due to these factors, there has been increasing use of various techniques and agents amongst interventional radiologists to plug biopsy channels and promote hemostasis[5,13,14].
Despite these methods, patients still frequently require manual pressure, pressure bandages, bedrest, and/or close nursing observation for several hours and even overnight[15-17]. Serious hemorrhage requiring blood transfusions, endovascular intervention such as angiography and embolization, or surgical repair can be required; patient mortality is also known to occur[1,4,5,18]. The development of the SinglePass Kronos electrocautery device was undertaken to address this most common and potentially serious complication for percutaneous CNB in solid organs (e.g., liver, kidney, lung, etc.). In this case series, we introduce an initial experience of 5 medical liver biopsy cases using the SinglePass Kronos electrocautery device to perform active needle tract closure following its introduction onto the market in the United States.
Ultrasound-guided, percutaneous, medical liver CNB was performed in both inpatient and outpatient settings for various liver tissue assessments in five patients. Pre-biopsy preparation, screening, consenting, patient positioning, etc. were all performed in accordance with the Society of Interventional Radiology (SIR) consensus guidelines[15,19]. Briefly, pre-procedure preparation is performed by obtaining blood work to screen for bleeding diatheses, reviewing patient medical history, medications, and prior imaging to identify potential risks as well as the optimal access needle window.
Absolute contraindications: Inability of patient to cooperate with procedure; Significant coagulopathy or thrombocytopenia (unless corrected prior to procedure); International normalized ratio (INR) > 1.8; Platelets < 50000; And inability to identify an adequate biopsy site by imaging.
Relative contraindications: Ascites, which can often be modulated by performing a paracentesis prior to intervention; Blood thinner use (e.g., nonsteroidal anti-inflammatory drugs, anticoagulants), which can be managed by holding medications for a prescribed period of time per SIR consensus guidelines; Patient refusal to accept blood transfusion or inability to provide blood transfusion support; Procedure may proceed with adequate discussion of risks and benefits); Suspected vascular tumor, echinococcal cyst, or other atypical entity procedure may proceed with adequate discussion of risks and benefits); Dilated biliary tree (procedure may proceed with adequate discussion of risks and benefits as well as potentially administering antibiotics if infection is possible).
Patients are moderately sedated, and a local anesthetic is applied to the needle entry site. They are placed in a comfortable position that allows access to the needle entry site. Pre-procedure imaging should confirm the needle window. The skin is prepared to sterilize the entry site, and sterile technique is followed throughout the procedure. At our institution, medical liver biopsies are routinely performed with 17/18-gauge Bard Max-Core Disposable Core Biopsy Instruments (Becton Dickinson, Inc., Franklin Lakes, NJ, United States). The SinglePass Kronos electrocautery device (Single Pass, Inc., Lake Forest, CA, United States) was applied post-biopsy for hemostasis of the needle tract in each of these 5 cases. Informed consent to perform the procedure was obtained from each patient.
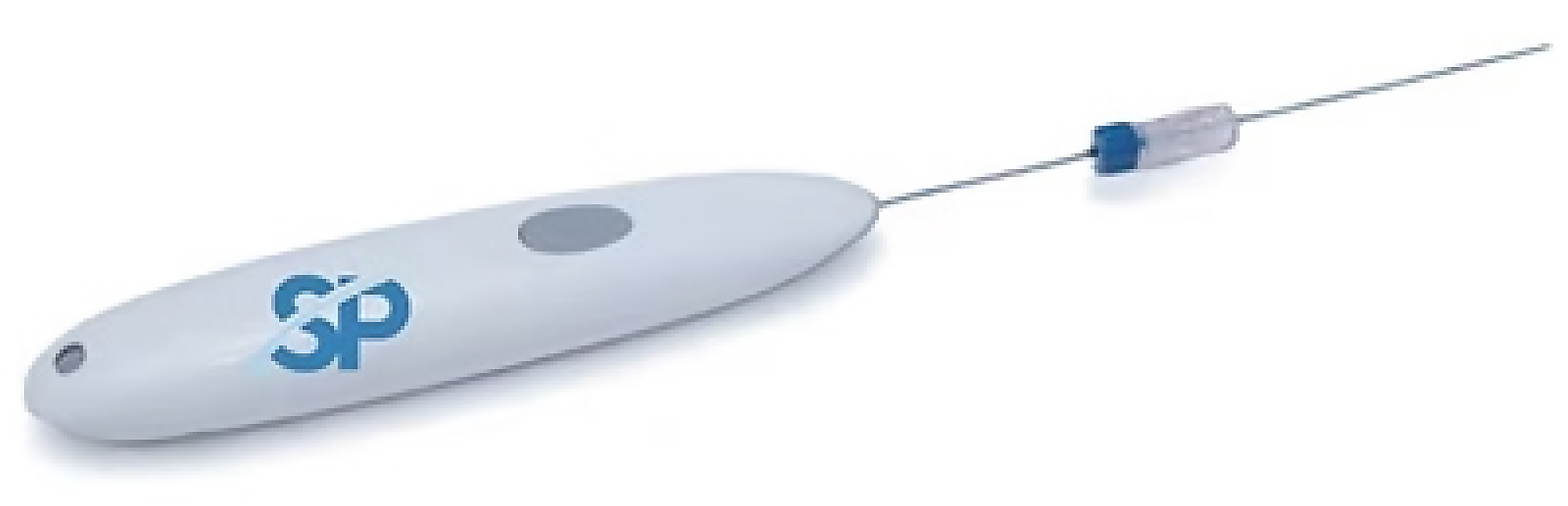
Kronos is a disposable, battery-operated electrocautery device utilized to control bleeding following CNB of solid organs (i.e., liver, kidney, lung, etc.). It consists of an ergonomic handle that activates a probe with an electrically activated tip that heats up to a maximum of 90 °C to cauterize tissue (see Figure 1 below).
Following biopsy procedures at our institution, all patients undergo immediate post-procedural ultrasound imaging to assess for hemorrhagic complications. They are then kept in a supine position and observed for 2 hours post-procedure for any additional procedure-related complications, which include pain, bleeding, injury to adjacent organs, cardiopulmonary compromise, and any other etiology for clinical decompensation. Absent any aberrations, patients are then either discharged (outpatient procedures) or released from bedrest (inpatient procedures). Following this, direct follow-up is performed with patients within 24-48 hours by a nurse navigator. Patients with any concerning signs and/or symptoms are sent for evaluation to the emergency department. Furthermore, all cases are performed within a community health system with a very tight referral system and coordinated follow-up is the norm. All patients are scheduled to be seen by their ordering/referring physician within one month. Furthermore, the nature of care in our community results in nearly all patients within this network being seen in-network for any complications. As such, a chart review was performed through 30 days post-procedure to identify complications in this case series.
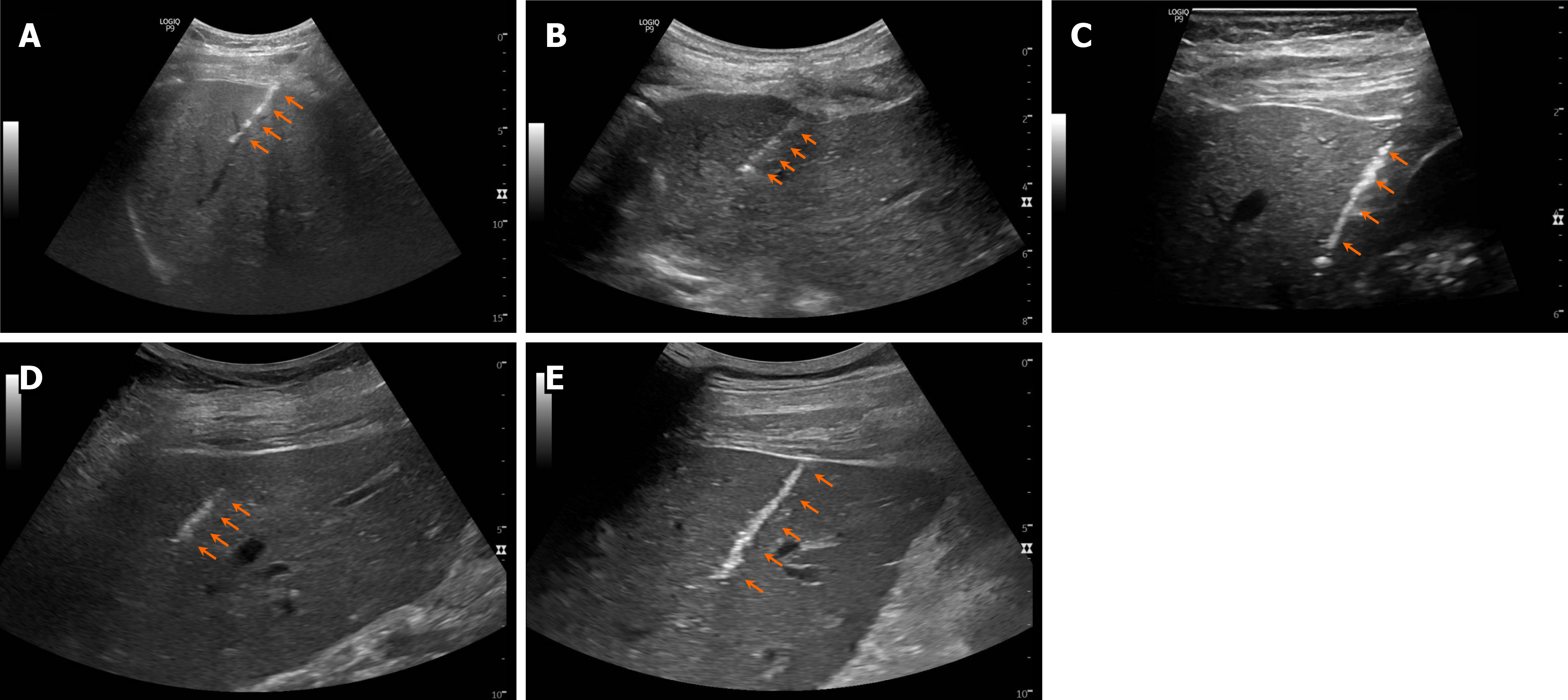
A summary of the cases presented in this series is provided in Table 2. Post-biopsy ultrasound images showing needle tract hemostasis associated with each case are shown in Figure 2.
| Case | Age (years)/gender (M/F) | Clinical presentation | Biopsy indication | Anti-platelet/anti-coagulant | Lab and imaging findings | Diagnosis/management | Post-biopsy bleeding? | Post-biopsy complications |
| 1 | 21/F | Obese, type 2 diabetes, NASH, fibrosis | Fibrosis staging | Neither | AST +, ALT + TBIL + | Weight loss program and semaglutide | No | None |
| 2 | 71/M | Hepatitis B e-antigen negative chronic hepatitis, HCC | Clinical trial inclusion | Neither | AST + | Immunotherapy via clinical trial, withdrew due to HCC progression | No | None |
| 3 | 64/F | Abnormal LFT, Hashimoto’s, | Rule out autoimmune hepatitis | Neither | AST +, ALT + | No intervention planned, counseled to avoid supplements, periodic lab surveillance | No | None |
| 4 | 67/F | Obese, metabolic syndrome, unexplained iron deficiency, mild steatosis | Fibrosis staging and rule out iron overload | Rivaroxaban | TBIL +, MRI showed mild steatosis and possible iron overload | Negative significant fibrosis and iron overload, follow-up MRI negative, periodic surveillance planned | No | None |
| 5 | 47/M | Substance and alcohol abuse, 3-year sobriety, jaundice post- cholecystectomy, increasing pruritus | Diagnostic assistance | Neither | INR +, AST +, ATL +, ALP +, TBIL +, imaging negative for stone, biliary or ductal obstruction | Medical management initially with ursodeoxycholic acid pending specific plan from hepatology | No | None |
Case 1: The patient presented to the hepatology clinic with obesity, type 2 diabetes. An outpatient biopsy was performed for fibrosis staging purposes.
Case 2: The patient was being screened for a clinical trial with an immunoconjugate, for which a biopsy was required by protocol.
Case 3: The patient presented to the hepatology clinic with incidentally abnormal liver function tests. There was a concern for autoimmune hepatitis, and a liver biopsy was therefore requested.
Case 4: The patient initially presented to the hepatology clinic with an unexplained iron deficiency. A liver biopsy was ordered to stage fibrosis and rule out iron overload.
Case 5: The patient presented with a chief complaint of jaundice to the hepatology clinic.
Case 1: The patient underwent a weight loss regimen with minimal change and was being considered for a metabolic dysfunction-associated steatohepatitis clinical trial.
Case 2: The patient developed progressive metastatic hepatocellular carcinoma (HCC) despite multiple lines of therapy including systemic therapy and locoregional therapy.
Case 3: The patient has a history of abnormal liver function tests.
Case 4: The patient has an unexplained iron deficiency.
Case 5: The patient’s jaundice initially was managed at an outside hospital, including undergoing a cholecystectomy. Subsequent to the surgery, however, he remained jaundiced and increasingly pruritic.
Case 1: The patient had a prior diagnosis of steatohepatitis following which a prior biopsy from over 2 years ago demonstrated stage III liver fibrosis.
Case 2: The patient has a history of hepatitis B e-antigen-negative chronic hepatitis.
Case 3: The patient has a history of Hashimoto’s thyroid disease alongside positive anti-nuclear antibody and anti-smooth muscle antibody results. She had no history of hepatotoxic medication exposure or other clear risk factors.
Case 4: The patient’s history was notable for obesity and metabolic syndrome.
Case 5: The patient’s history includes multi-substance abuse including alcohol although he reported a sobriety period of 3 years.
Case 1: The patient was a 21-year-old female, who was not taking antiplatelet or anticoagulant medications.
Case 2: The patient was a 70-year-old male who was not taking antiplatelet or anticoagulant medications.
Case 3: The patient was a 64-year-old female who was not taking antiplatelet or anticoagulant medication.
Case 4: The patient was a 67-year-old female who was taking rivaroxaban (15 mg/day) for atrial fibrillation, which was held for 48 hours prior to the procedure.
Case 5: The patient was a 47-year-old male who was not taking antiplatelet or anticoagulant medication.
Cases 1 and 4: Obesity.
Cases 2, 3, and 5: Not performed.
Case 1: The patient’s pre-procedure labs identified elevated levels of aspartate aminotransferase (AST) (325 IU/L), alanine aminotransferase (ALT) (429 IU/L), and total bilirubin (1.4 mg/dL).
Case 2: Pre-procedure lab work identified an elevated AST value (73 IU/L).
Case 3: The patient’s pre-procedure blood work indicated elevated AST (128 IU/L) and ALT (177 IU/L) values.
Case 4: Pre-procedure labs demonstrated an elevated total bilirubin (1.3 mg/dL).
Case 5: Pre-procedure lab work was significant with a slightly elevated INR (1.2) and elevated levels of AST (66 IU/L), ALT (73 IU/L), alkaline phosphate (214 IU/L), and total bilirubin (21.3 mg/dL).
Case 4: Magnetic resonance imaging revealed mild hepatic steatosis and possible mild hepatic iron overload.
Case 5: Further imaging did not reveal any etiologies of biliary obstruction, ductal injury, or retained stone.
Pathology results included a finding of steatohepatitis, severely active with foamy degeneration and moderate macrovesicular steatosis (33%), compatible with nonalcoholic steatohepatitis. Extensive pericellular fibrosis with areas of central-to-central bridging fibrosis were also identified.
Pathology results were not immediately available for this patient as they were sent to the clinical trial company directly, but the yield was deemed to be adequate.
A summary of the pathology results included nonspecific foci of spotty necrosis and minimal chronic inflammation of portal tracts with no significant fibrosis. There was no evidence for autoimmune hepatitis. However, a review of clinical history suggests possible drug-induced liver injury.
Pathology showed mild hepatic glycogenosis, but no significant steatosis. Mild Kupffer cell hemosiderosis was present. There was no significant steatosis or fibrosis seen. The diagnostic conclusion is that she does not have cirrhosis or other liver pathology at this time.
Pathology findings included mild canalicular cholestasis with minimal associated inflammatory infiltrates, minimal biliary ductular reaction, and mild sinusoidal congestion. A review of clinical history suggests possible drug-induced liver injury. The ultimate etiology for abnormal liver function tests remained indeterminate despite the results of the biopsy.
After the diagnosis, the patient was counseled on a weight loss regimen as the primary intervention, and she has now started taking Ozempic (semaglutide).
The patient was enrolled in the clinical trial and was placed on immunotherapy for HCC.
Initially, the clinical concern was for autoimmune hepatitis given a history of positive autoimmune markers. However, the pathological findings from the biopsy did not identify signs of autoimmune hepatitis and there was no evidence of fibrosis. Therefore, no further intervention was pursued.
Given a lack of significant steatosis, fibrosis, or signs of autoimmune hepatitis from this biopsy, the decision was made to perform follow-up imaging, which demonstrated no significant findings of liver stiffness or portal hypertension.
The patient was managed medically, initially with ursodiol (ursodeoxycholic acid).
No additional follow-up was performed.
Due to HCC progression, the patient ultimately withdrew from the clinical study.
The patient was advised to avoid any further supplements and is being surveilled with periodic blood work.
No further follow-up was planned.
A plan for further specific management from Hepatology is being developed.
Bleeding complications in percutaneous needle biopsies is an ongoing concern despite advances in equipment and techniques. In addition, some of these techniques can add complexity to the biopsy procedure by requiring additional passes into and out of the access needle and/or the implementation of additional equipment such as a radiofrequency generator and its associated cabling when performed in the context of ablation procedures. SinglePass Kronos is a novel electrocautery device that has demonstrated effectiveness in quickly and durably obtaining needle tract hemostasis during this early case series of liver CNB procedures. The advantages of the Kronos device include the fact that it is hand-held, requires no additional equipment, is operated with a single button push, and is disposable. In addition, its integration with the hub of the access needle allows Kronos to be automatically positioned just distal to the tip of the needle to perform electrocautery during needle withdrawal. In doing so, this adds minimal incremental effort to the standard workflow of the procedure.
There were no complications observed in this case series, most notably no evidence of immediate or delayed bleeding. Post-biopsy ultrasound imaging consistently showed clear evidence of thermal needle tract sealing and hemostasis. While one patient in this series was taking rivaroxaban, which is a relative contraindication, the medication was discontinued 48 hours prior to the procedure per standard protocol, thus allowing the procedure to proceed; this patient also had no bleeding complication.
As with other thermal devices, there is a risk of unintended collateral damage including potential vascular damage to blood vessels within the needle window or targets near the hepatic pedicle. While collateral damage is a risk with any device that introduces thermal energy into a patient, electrocautery with the Kronos device is obtained at temperatures at or below a maximum temperature of 90 °C. Furthermore, the device is only activated in conjunction with access needle withdrawal. The combination of this temperature limit and activation during withdrawal (i.e., movement of the heated probe tip) minimizes the risk of “steam pops” that can be caused by super heating local tissue.
Cost remains a concern with any new devices introduced into clinical practice. The human cost of a hemorrhagic complication would be difficult to fully quantify, particularly in the case of a mortality event. Financial costs linked with hemorrhagic complications can themselves be rather significant, whether requiring surgery, angiography, prolonged hospital stay, intensive care unit utilization, and/or other costs. Dedicated studies on the economics of this new technique, most likely due to cost avoidance should be pursued.
While the Kronos device performed perfectly in this limited series, its safety and performance in a larger experience has not yet been published, although a summary of the device’s clearance to the United States market by the United States Food and Drug Administration alludes to clinical experience in solid organ (liver, kidney, lung, etc.) biopsy procedures in 60 patients[20]. Larger-scale, comparative evaluations to determine the extent of Kronos’ ability to reduce bleeding events are warranted. This should include larger sample sizes, procedures in additional solid organs, comparisons to other hemostasis devices and/or techniques, product use in a higher-risk patient population (e.g., patients on anticoagulant or antiplatelet medication), and a cost-effectiveness analysis.
Routine Kronos use has the potential to overcome the unpredictable nature of bleeding complications thereby becoming a valuable tool in performing percutaneous solid organ biopsy procedures. Our findings in this limited case series suggest that durable hemostasis created by the SinglePass Kronos device has the potential to simplify patient post-operative care and improve patient safety.
| 1. | Chan M, Navarro VJ. Percutaneous Liver Biopsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 2. | Veltri A, Bargellini I, Giorgi L, Almeida PAMS, Akhan O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc Intervent Radiol. 2017;40:1501-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Wang H, Bao H, Yue L, Jiang T. A Novel Biopsy Method Based on Bipolar Radiofrequency Biopsy Needles. Front Oncol. 2022;12:838667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Rathod K, Deshmukh H, Sundaram S, Radhakrishnan H, Thakkar D, Ramani N, Bhatia S. Plugged percutaneous liver biopsy using Tru-cut needle and coils: A retrospective study. Indian J Gastroenterol. 2022;41:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Giunta D, Daddi N, Dolci G, Campisi A, Congiu S, Buia F, Bagni A, Dell'Amore A. A new image-guided technique for intraoperative localization of lung small solid nodules or ground-glass opacities with a self-expanding tract sealant device: a preliminary experience. Interact Cardiovasc Thorac Surg. 2019;28:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Suyama Y, Soga S, Mikoshi A, Hokari R, Shinmoto H, Tomita K. Initial experience of coaxial percutaneous liver biopsy with tract embolization using N-Butyl cyanoacrylate. Scand J Gastroenterol. 2023;58:1317-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Sheth RA, Baerlocher MO, Connolly BL, Dariushnia SR, Shyn PB, Vatsky S, Tam AL, Gupta S. Society of Interventional Radiology Quality Improvement Standards on Percutaneous Needle Biopsy in Adult and Pediatric Patients. J Vasc Interv Radiol. 2020;31:1840-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Hatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. AJR Am J Roentgenol. 2008;190:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Buckley A, Petrunia D. Practice guidelines for liver biopsy. Canadian Association of Gastroenterology. Can J Gastroenterol. 2000;14:481-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, Pointon K, Richardson C, Sawicka E; BTS. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 327] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Handke NA, Koch DC, Muschler E, Thomas D, Luetkens JA, Attenberger UI, Kuetting D, Pieper CC, Wilhelm K. Bleeding management in computed tomography-guided liver biopsies by biopsy tract plugging with gelatin sponge slurry. Sci Rep. 2021;11:24506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Potretzke TA, Gunderson TM, Aamodt D, Weisbrod AJ, Hesley GK, Welch TJ, Atwell TD. Incidence of bleeding complications after percutaneous core needle biopsy in hypertensive patients and comparison to normotensive patients. Abdom Radiol (NY). 2016;41:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | McDonald J, Amirabadi A, Farhat Z, Temple M, Parra D, Amaral J, Connolly B. Experience with Compressed Gelfoam Plugs in Children during Liver Biopsies and Other IR Procedures: A Retrospective Single-Center Case Series. J Vasc Interv Radiol. 2019;30:1855-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Singhal S, M D P, Inuganti S, Botcha S, Deepashree DT, Uthappa MC. Percutaneous ultrasound-guided plugged liver biopsy - a single-centre experience. Pol J Radiol. 2021;86:e239-e245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | DesRoche C, Callum J, Scholey A, Hajjaj OI, Flemming J, Mussari B, Tarulli E, Reza Nasirzadeh A, Menard A. Platelet and INR Thresholds and Bleeding Risk in Ultrasound Guided Percutaneous Liver Biopsy: A Before-After Implementation of the 2019 Society of Interventional Radiology Guidelines Observational Quality Improvement Study. Can Assoc Radiol J. 2024;75:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Davidson JC, Rahim S, Hanks SE, Patel IJ, Tam AL, Walker TG, Weinberg I, Wilkins LR, Sarode R. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part I: Review of Anticoagulation Agents and Clinical Considerations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30:1155-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, Wilkins LR, Sarode R, Weinberg I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30:1168-1184.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 18. | Yousem SA, Amin RM, Levy R, Baker N, Lee P. Pulmonary pathologic alterations associated with biopsy inserted hydrogel plugs. Hum Pathol. 2019;89:40-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Society of Interventional Radiology. ACR–SIR–SPR Practice Parameter for the Performance of Image-Guided Percutaneous Needle Biopsy (PNB). [cited September 17, 2025]. Available from: https://gravitas.acr.org/PPTS/DownloadPreviewDocument?ReleaseId=2&DocId=3. |
| 20. | United States Food and Drug Administration. Kronos Electrocautery Device. [cited September 17, 2025]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf23/K232805.pdf. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/