Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.111495
Revised: July 28, 2025
Accepted: September 9, 2025
Published online: October 21, 2025
Processing time: 112 Days and 17.1 Hours
Artificial intelligence (AI) has emerged as a transformative tool in the diagnosis and management of gastrointestinal (GI) and liver diseases. In clinical practice AI consists of overlapping technologies such as machine learning (ML), deep lear
Core Tip: Artificial intelligence (AI) has emerged as an invaluable transformative tool in diagnosis and management of gastrointestinal and liver diseases. In clinical practice AI technologies such as machine learning, deep learning, natural language processing, computer vision, and generative AI have been used in their applications. There is a need for continued development, validation, and real-world modeling of AI systems before its widespread adoption. Although it does not replace human clinical judgement, it can still be expected that AI application in gastroenterology and hepatology will further be enhanced in the future and become the standard of care in clinical practice.
- Citation: Shrestha UK. Emerging role of artificial intelligence in gastroenterology and hepatology. World J Gastroenterol 2025; 31(39): 111495
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/111495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.111495
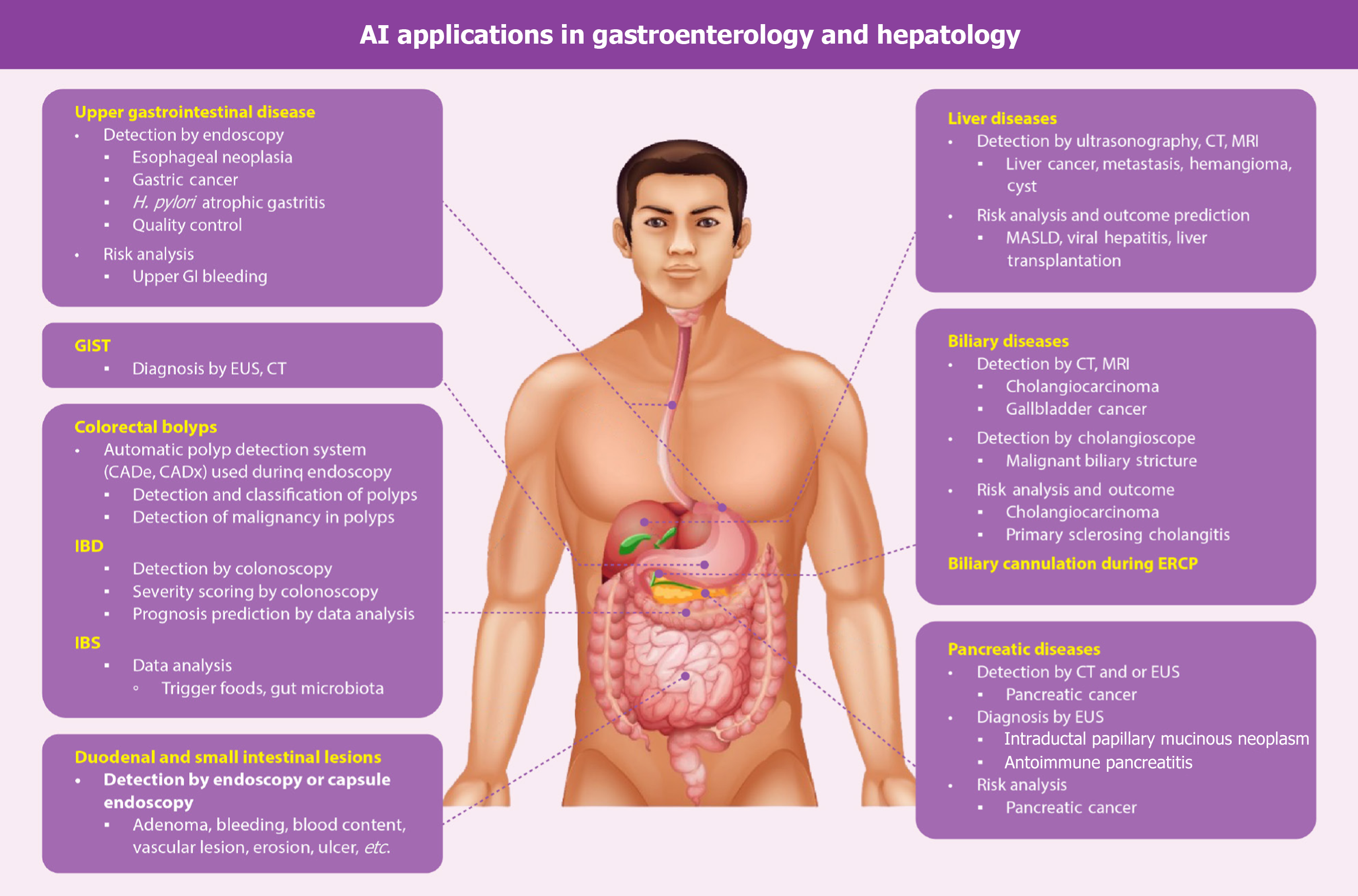
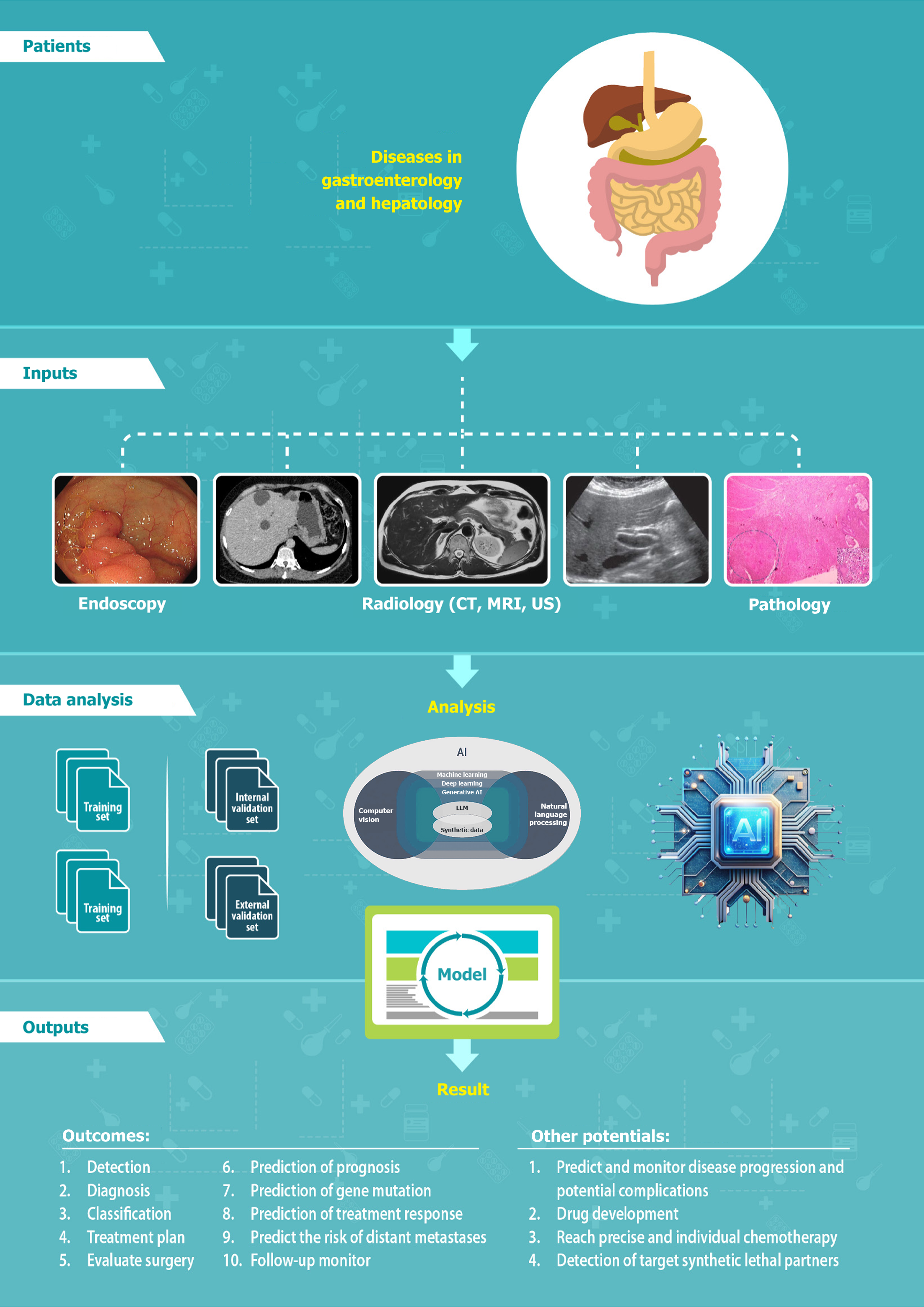
Artificial intelligence (AI) has drastically revolutionized the healthcare system, including the clinical diagnosis and treatment, resulting in the subsequent increase in the quality of life[1]. This has emerged as a transformative tool in the diagnosis and management of gastrointestinal (GI) and liver diseases. In one systematic review it was shown that among several published randomized controlled trials of AI-assisted tools in clinical practice, more studies were done in the field of gastroenterology (30 out of 39), and it was demonstrated that the performance of AI-assisted tools surpassed that of usual clinical care[2]. AI has been used in several GI diseases such as esophageal neoplasia, gastric cancer, Helicobacter pylori (H. pylori) infection, gastritis, GI stromal tumors (GISTs), colorectal polyps, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), GI bleeding, and pancreatobiliary diseases. The potential applications of AI in liver diseases encompass a variety of conditions such as liver masses, metabolic dysfunction-associated steatotic liver disease (MASLD), viral hepatitis, cirrhosis, and liver transplantation (LT). The applications of AI in gastroenterology and hepatology are shown in the Figure 1. This review discussed the common terminologies and the current status of AI in gastroenterology and hepatology, exploring its applications and ethical issues.
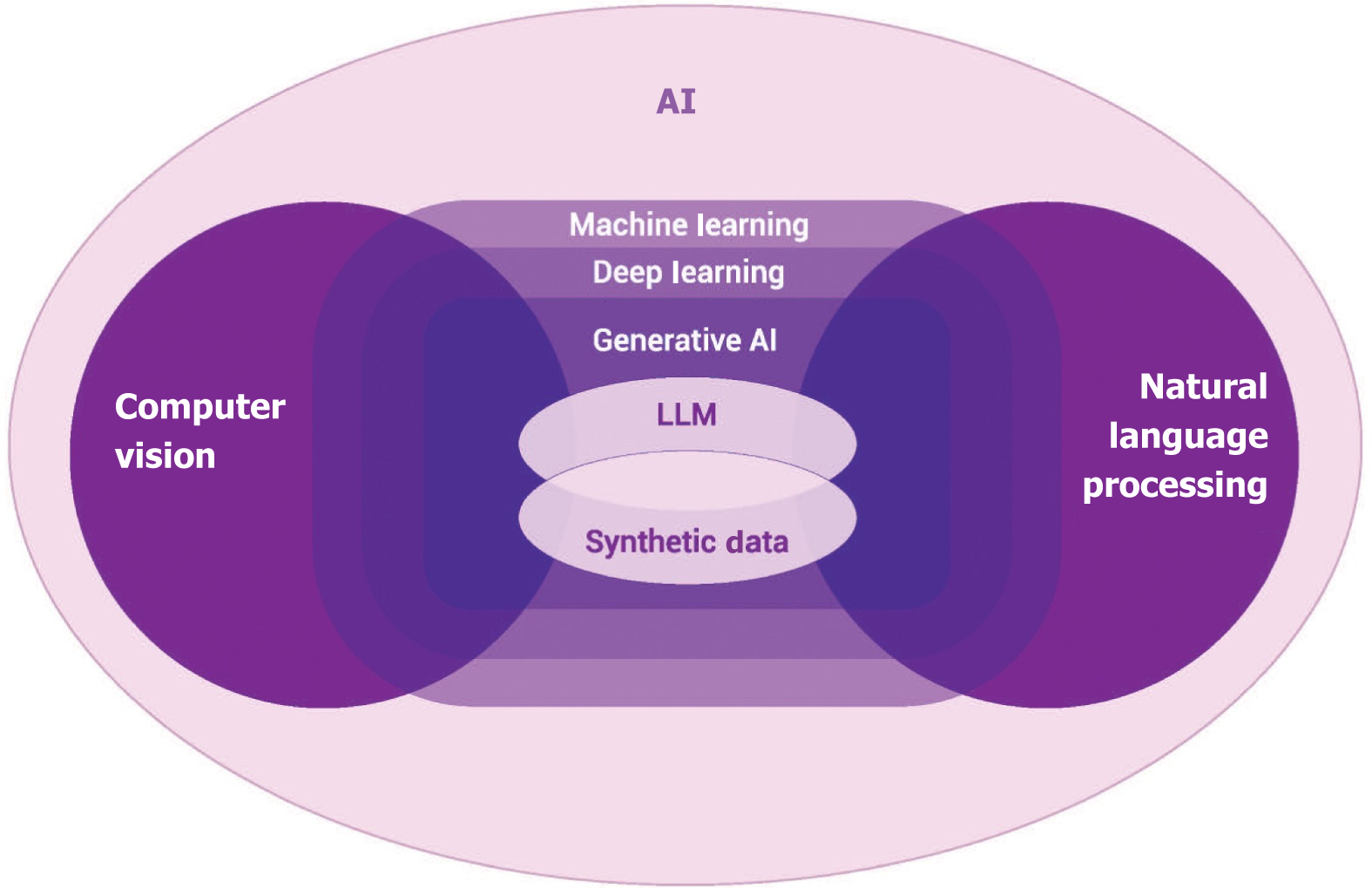
AI involves a computer program that uses a multitude of techniques to resolve different problems and mimics human intelligence[3]. In clinical practice several overlapping tools of AI are used, such as machine learning (ML), deep learning (DL), natural language processing (NLP), computer vision, and generative AI. The landscape of AI is depicted in Figure 2.
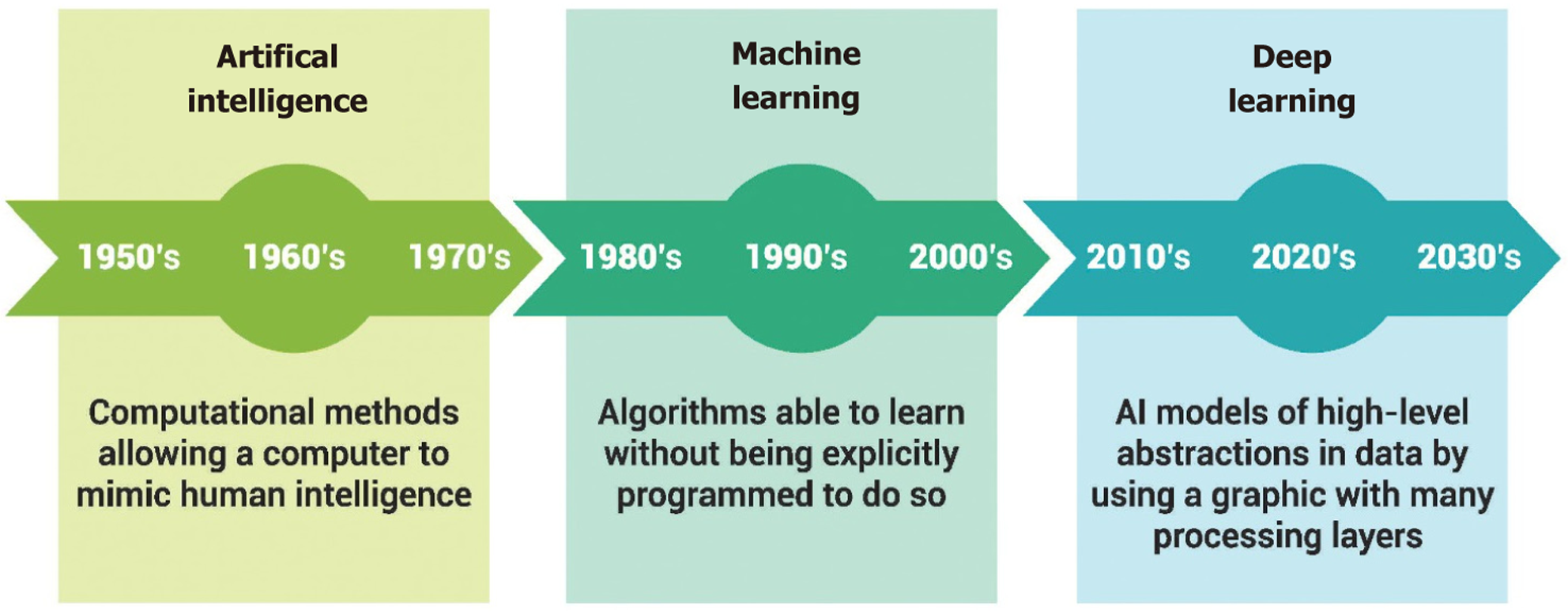
Going through the history timeline of AI, Turin A published his work “computing machinery and intelligence” in 1950 and introduced the Turing test, a measure of computer intelligence. After 6 years, McCarthy officially coined the term “AI” in 1956 at the Dartmouth Workshop[4]. The history timeline of AI is shown in Figure 3.
ML is a computer learning system that can provide insight on disease risk factors and phenotypes. The types of ML can be categorized into supervised, unsupervised, and reinforcement ML[5]. The supervised learning involves model training with labelled data and comprises classification and regression. Unsupervised learning includes unlabeled data and consists of clustering. In the reinforcement learning the model agent takes actions in the environment, and then the received state gives the update and feedback.
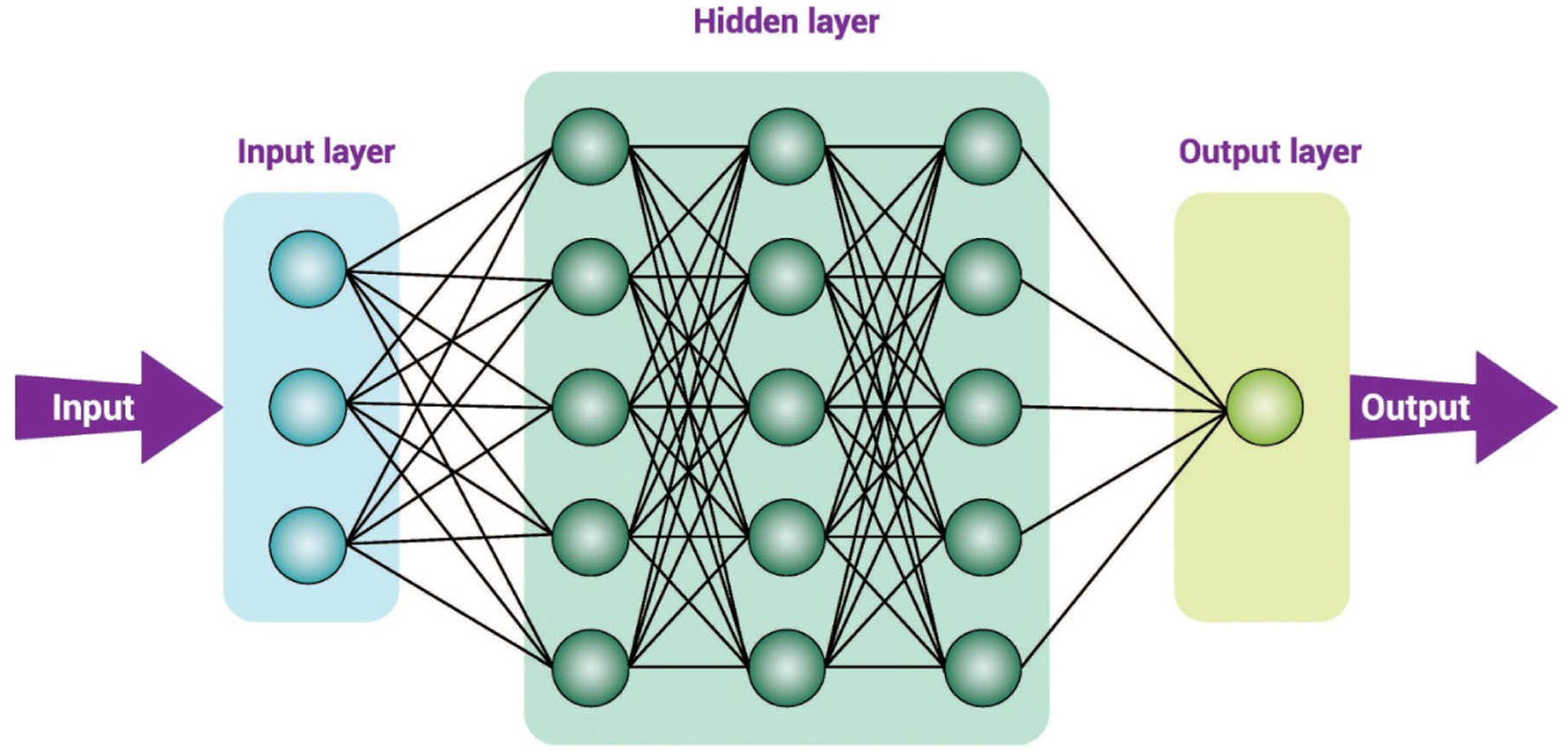
DL is an advanced and complex form of ML, consisting of an input layer, multiple hidden layers, and an output layer. A convolutional neural network (CNN) is a specific type of DL architecture powered with AI algorithms that can aut
NLP is a subfield of computational linguistics focused on AI models that interpret and generate human language[7]. It equips machines with the capability to comprehend, decode, and produce human language in a relevant and constructive manner and helps to analyze, understand, and derive actionable information from unstructured healthcare data, in
Computer vision is a branch of AI that helps computers to understand and interpret images and videos. Some examples of the application of this new system in endoscopy include: (1) Automated stratification and risk classification in esophageal varices; (2) Differentiation of ulcerative colitis and Crohn’s disease; (3) Differentiation between benign and malignant biliary strictures during endoscopic retrograde cholangiopancreatography (ERCP); (4) Assisting in selective biliary cannulation during ERCP; (5) Adequate assessment of mucosal examination during upper GI endoscopy or colo
Generative AI is a category of AI that employs ML, DL, NLP, and computer vision to create a new form of content by learning from innumerable data in the form of text, audio, images, or video following human input. It is often powered by large, pretrained models known as foundation models, which include large language models (LLMs), such as OpenAI’s ChatGPT and Google’s Gemini[9]. The chat interface is the simplest method of interacting with LLM in which prior programming experience is not required. In order to perform an intended task, the users input a prompt containing the necessary instructions and contextual information. The file uploads or adjustment of model characteristics like creativity are also allowed in some chat interfaces. Application programming interfaces are used in advanced LLM-based software for more complex or high-throughput use cases and provide simplified programming instruction sets for model interaction and customization[10].
The LLM brings a new era in gastroenterology and hepatology and is utilized in improving diagnostic accuracy during endoscopy, streamlines documentation, and enhances educational and patient engagement strategies, clinical decision support, research, and drug discovery[11]. Generative AI, particularly LLMs, plays a significant role in creating synthetic data. The concerns about the privacy of the patient, restriction of data sharing, and rarity of conditions can be the challenging problems in generating high-quality data required for the implementation of AI[12]; this can be solved by the use of synthetic data[13]. The flowchart of AI after data collection is shown in Figure 5.
Upper GI endoscopy fails to identify a number of neoplastic lesions because of the several factors obscuring the vision or improper navigation. This significant miss rate of neoplastic lesions can be properly rectified by the real-time application of AI that helps not only for the detection, characterization, treatment, and reporting of upper GI neoplasia but also improves the proper navigation of the scope. The computer-aided detection system helps to reduce the miss rate, whereas the computer-aided diagnosis system assists in histology prediction of precancerous lesions and depth of invasion. The AI also helps in lesion demarcation, which ensures the complete resection; moreover, AI-assisted anatomical landmarks help in adequate reporting[14].
Esophageal cancer: The two histological forms of esophageal cancer are squamous cell carcinoma and adenocarcinoma. Barrett’s esophagus, which correlates with the rise of gastroesophageal reflux disease, is a potential premalignant con
The early detection of dysplasia in Barrett’s esophagus and its timely treatment is believed to decrease the incidence of esophageal cancer. The histopathological examination has been the gold standard for the diagnosis of Barrett’s esophagus and dysplastic lesion, but it has the limitation of interobserver agreement[17]. In order to improve the histological diag
A study conducted in Japan utilized a CNN to detect early esophageal cancer and had a 98% sensitivity[19]. Another study utilized a computer-aided detection (CAD) system to identify Barrett’s esophagus as having nondysplastic or neoplastic lesions with an overall sensitivity, specificity, and accuracy of 90%, 88%, and 89%, respectively[20]. The analysis of morphological nuclear features performed by computerized morphometry as shown in one study helped to determine the degree of Barrett’s esophagus-associated dysplasia and predicted the time to progression to adenocarcinoma[21].
A study performed with volumetric laser endomicroscopy with an added feature of an algorithm to develop a prediction score had a sensitivity and specificity of 90% and 93%, respectively, for detecting early esophageal neoplasia[22]. In one study CAD was able to identify Barrett’s neoplasia in image-based validation with a sensitivity, specificity, and accuracy of 95.3%, 94.5%, and 94.7%, respectively, and in video-based external validation with sensitivity, specificity, and accuracy of 93.8%, 90.7%, and 92.0%, respectively[23]. These studies of AI in the identification of patients with esophageal neoplastic or preneoplastic lesions are shown in Table 1.
| Lesions | Diagnostic or predictive modality | AI classifier | AI validation methods | Number of images, slides or videos in training dataset | Number of images, slides or videos in test dataset | Best average results (%) | Ref. | |
| Accuracy | Sensitivity/specificity | |||||||
| NDBE, LGD, HGD | Histology | Deep learning | Training, validation, and test sets created (by a random 70/20/10 split) from 542 patients (164 NDBE, 226 LGD, and 152 HGD) | 8596 bounding boxes | 840 boxes | F1 score; NDBE: 0.91; LGD: 0.90; HGD: 1.0 | NDBE: > 90; LGD: 81.3/100; HGD: > 90 | Faghani et al[18] |
| ESCC and EAC | Upper GI endoscopy (WL or NBI) | CNN | 8428 from 384 patients (397 ESCC, 32 EAC) | 1118 (956/162) from 50 control, 41 ESCC, 8 EAC | 55.7 | 98.0/16.0 | Horie et al[19] | |
| EN-BE | Upper GI endoscopy | Deep learning CAD system | 494364 Labeled endoscopic images collected from all intestinal segments | 1704 unique esophageal high-resolution images of rigorously confirmed EN-BE and NDBE, derived from 669 patients | 89.0 | 88.0/90.0 | de Groof et al[20] | |
| EN-BE | Upper GI endoscopy | CNN-CAD | Phase 2 image-based validation; phase 3 video-based external validation | 75198 images and videos (96 patients) of neoplastic and 1014973 images and videos (65 patients) of nonneoplastic BE | Phase 2 107 images (20 patients) of neoplastic and 364 images (14 patients) of nonneoplastic BE; phase 3 32 videos (32 patients) of neoplastic and 43 videos (43 patients) of nonneoplastic BE | Phase 2: 94.7 | 95.3/94.5 | Abdelrahim et al[23] |
| Phase 3: 92.0 | 93.8/90.7 | |||||||
Gastric cancer: Gastric cancers are more common in the distal stomach (antrum and body), but proximal gastric cancers are increasing in frequency[24]. The two histological forms of gastric cancer are intestinal and diffuse-type cancers[25]. The potential premalignant gastric lesions, such as chronic atrophic gastritis, benign gastric ulcers, hypertrophic gastropathy, and gastric polyps, might increase the risk of gastric cancer[26]. Several algorithms have been devised to improve the detection of such premalignant gastric conditions. In one study CNN needed only 47s to analyze 2296 stomach images in identifying gastric cancer lesions with a sensitivity of 92.2%[27]. Another study showed that a CNN-CAD system was able to identify the invasion depth of gastric cancer with sensitivity, specificity, and accuracy of 76.47%, 95.56% and 89.16%, respectively[28]. A study conducted in China used a CNN system for the histopathological diagnosis of gastric cancer with a sensitivity and specificity of 100% and 80.6%, respectively[29]. These studies of AI in the identification of patients with early gastric cancer is shown in Table 2.
| Lesions | Diagnostic or predictive modality | AI classifier | Number of images in training dataset | Number of images in test dataset | Best average results (%) | Ref. | |
| Accuracy | Sensitivity/specificity | ||||||
| EGC | Upper GI endoscopy (WL, CE, NBI) | CNN | 13584 from 2639 lesions | 2296 from 77 lesions | NA | 92.2/NA | Hirasawa et al[27] |
| EGC | Upper GI endoscopy | CNN-CAD system | 790 images | 203 images | 89.16 | 76.47/95.56 | Zhu et al[28] |
| EGC | Histology | CNN | 2123 whole slide images | 3212 whole slide images | 100/80.6 | Song et al[29] | |
Chronic and recurrent H. pylori infection is a well-established risk factor for gastric adenocarcinoma. The diagnosis of H. pylori by assessing the endoscopic gastric mucosal biopsy has been an important part of gastric cancer screening, but the process of specific staining of a gastric biopsy is time consuming. Studies have shown that AI was able to predict H. pylori infection with a sensitivity and specificity of 87% and 86%, respectively[30]. Another study used AI in endo
Since the prevalence of chronic gastritis is high, it is mandatory to look not only for the H. pylori infection but also for atrophy, intestinal metaplasia, and the proper types of gastritis. One study showed that the use of CNN was able to identify gastritis subtypes, namely autoimmune (A), bacterial (B), and chemical (C) gastritis, with an overall accuracy of 84%. The sensitivity and specificity for gastritis B was 100% and 93%, respectively[32].
GISTs are the most common mesenchymal neoplasms of the GI tract, arising from the interstitial cells of Cajal within the myenteric plexus of the muscularis propria; they occur most commonly in the stomach followed by the small intestine[33-35]. Most of the GISTs are due to mutations in the proto-oncogene KIT[34]. Their three histological types are spindle, epithelioid, and mixed[33,36]. These are the tumors with malignant potential, and hence all GISTs > 2 cm and most GISTs < 2 cm with high risk factors should be resected[37]. A meta-analysis of seven studies showed that the endoscopic diagnosis of GISTs can be conducted with AI-assisted endoscopic ultrasound (EUS) with a pooled sensitivity and specificity of 92% and 82%, respectively[38]. These studies showed that AI could help in the early detection of GISTs, allowing for timely intervention.
Colorectal polyps are slow-growing overgrowths arising from the mucosal surface of the colon and rectum and may be neoplastic or non-neoplastic. Colorectal adenomas and serrated lesions are two main classes of pre-cancerous colorectal polyps. Adenomatous polyps will gradually show dysplastic changes, and those that develop high-grade dysplasia will become malignant with time[39-41]. Hence, early detection of adenomatous polyps is crucial for the timely treatment and better outcome.
Polyp detection: The colonoscopy for the detection of polyps has its own limitation resulting in a decreased adenoma detection rate (ADR) because of poor bowel preparation, inability to visualize the blind spots, difficulty navigating challenging situations, and fatigue. In one study it was found that ADR was inversely related to the risk of interval col
Polyp classification: The characterization of polyps is important to classify the polyps as malignant or non-malignant. AI helps in the detection and characterization of colorectal polyps[46]. Several studies have used computer-aided methods for the classification of colorectal polyps[47-50]. One study analyzed the use of narrow-band imaging (NBI) magnifying colonoscopy to predict the histology of colorectal tumors and found the accuracy of this system as 97.8%[51]. During endocytoscopy microscopic visualization is done using mini probes[52]. One study has shown that the management of diminutive and small colorectal polyps can be done effectively by the use of CAD in endocytoscopy[53].
Laser-induced autofluorescence has the capacity to detect colonic dysplasia in vivo[54]. Diagnostic algorithms based on fluorescence spectroscopy has been developed to diagnose the presence or absence of colonic adenoma by using the fluorescence excitation-emission matrices method[55]. One study has shown that chromoendoscopy, used to enhance lesion detection in colitis surveillance, identified more lesions than NBI, but most were not dysplastic[56]. The software designed for the automated classification of colonic polyps by probe-based confocal laser endomicroscopy had high performance, comparable to that of off-line diagnosis of probe-based confocal laser endomicroscopy videos established by expert endoscopists[57].
Detection of malignancy in polyps: It is very important to diagnose malignancy in polyps and determine the depth of submucosal invasion. Surgery is recommended for deep submucosal invasion because of the risk of local recurrence that can occur when treated with only endoscopic resection[58]. Endoscopic resectable malignant polyps are treated by different endoscopic treatments, such as endoscopic mucosal resection, submucosal dissection, and full-thickness resection[58-60]. Considering the different therapeutic modalities for malignant polyps, proper endoscopic diagnostic techniques should be available to use for the right therapeutic options. The depth of invasion can be determined by several endoscopic techniques, such as NBI, high-definition white light endoscopy, and EUS[61]. The CAD system used by one study utilized ultra-high magnification endocytoscopy to assess the depth of invasion and found a sensitivity and specificity of 98.1% and 100%, respectively[62].
During the treatment of IBD, it is important to achieve mucosal healing, which means endoscopic healing and histological healing. The persistence of histological mucosal inflammation increases the risk of disease exacerbation and dysplasia. However, it is difficult to assess the histological healing only by conventional colonoscopy. In this scenario the use of AI in the form of a CAD system might help to get the information about the mucosal healing, similar to that received by the histological examination. One study of a CAD system evaluated the images from the colonoscopies in patients with ulcerative colitis and showed the identification of persistent histological inflammation with a sensitivity and specificity of 74% and 97%, respectively[63]. In one study deep neural network evaluation of an ulcerative colitis algorithm was used to recognize patients with ulcerative colitis in endoscopic remission with a 90.1% accuracy and histological remission with a 92.9% accuracy[64]. A CNN study in patients with Crohn’s disease using the images of the small bowel mucosa during capsule endoscopy (CE) showed the mucosal condition, whether normal or mucosal ulcer, with an accuracy ranging from 95.4% to 96.7%[65].
IBS is characterized by its subtypes, namely IBS with diarrhea, IBS with constipation, mixed IBS, and IBS without a significant pattern of abnormal stool[66]. The pathophysiology of IBS is complex and involves the interplay of genetics, diet, gut microbiome, and the brain-gut axis, leading to altered motility, visceral hypersensitivity, and immune response[67]. The symptom flare-ups in most of the patients with IBS have been found to be triggered by certain foods, and unique AI-aided mobile applications have been developed to identify those potential trigger foods. One mobile application used photos of food to develop a personal informatics system, which allows patient-provider collaboration and supports precise individual management[68]. A novel food and symptom journal application was found to be a feasible and usable tool for patients with IBS and helped to determine the food triggers[69]. By using these AI-aided smartphone applications, frequent and continuous data can be received from the patients and can be utilized by the clinicians to provide precision feedback. Recently, the gut microbiota has been linked to the symptoms and pathogenesis of IBS[70]. One study of a unique AI prediction model analyzed gut microbiota to identify patients with IBS with a sensitivity and specificity of > 80% and > 90%, respectively[71].
The endoscopic examination identifies the cause of GI bleeding in most of the patients with upper or lower GI bleeding. In some patients with recurrent bleeding, the repeated endoscopic examination may be needed to identify the bleeding point and cause of the bleeding. ML models may be generally useful for the risk stratification of patients with GI bleeding and can predict the recurrent bleeding and outcome for these patients[72-76]. In a retrospective analysis of 22854 patients with peptic ulcers, an ML model was used and validated in 1265 patients. The ML model used the patient’s data such as age, level of hemoglobin, gastric ulcer, GI diseases, malignancies and infections to identify the patients with recurrent ulcer bleeding. In the study the ML model was able to identify the recurrent ulcer bleeding within 1 year with an acc
CE is used to evaluate the small bowel and can capture nearly 60000 images, making analysis cumbersome. The use of AI might help in analyzing those images more efficiently and accurately in a shorter period of time. The CAD system developed in one study used a CNN that identified six intestinal motility events with an accuracy of almost 96%[77]. The automatic bleeding system, which analyzed 10000 CE images based on a CNN, was used in another study to detect GI bleeding and found a 99.9% precision value[78]. A novel learning method has been devised for the detection of polyps and has an overall accuracy of 98% for polyps, bubbles, turbid, and clear images[79]. Moreover, a CAD system using a CNN was also able to detect angiectasia, the most common lesion of the small bowel, with a sensitivity and specificity of 100% and 96%, respectively[80]. Studies have shown that an AI system with DL is useful for detection of erosion, ulcer, and hookworm in CE images[81-83].
Pancreatic cancer: Pancreatic cancer is responsible for almost 5% of all cancer deaths worldwide[84]. The most significant prognostic factor in pancreatic cancer is the tumor size, which if less than 10 mm has a 5-year survival more than 80% and if more than 10 mm is less than 50%[85,86]. The early detection of pancreatic cancer by imaging faces a great challenge, and the sensitivity of detecting pancreatic cancer by CT scan is not high for small lesions[87,88]. One study showed that the AI algorithm used in CT images achieved a sensitivity and specificity of 80.2% and 90.2%, respectively, for the early detection of pancreatic cancer. They may be improved by a larger number of training images[89].
Another more powerful modality to detect small lesions in the pancreas is EUS[88,90]. The AI-based EUS used in one pilot study was able to diagnose pancreatic cancer successfully with an area under the curve (AUC) of 0.94[91]. Few studies have shown that the application of ML and DL to EUS imaging of the pancreas achieved equal or better results than endoscopists[92]. The detection of early pancreatic cancer is feasible only if the high-risk group can be identified properly. The study done in Taiwan showed that a logistic regression model could appropriately predict pancreatic cancer risk in patients with type 2 diabetes mellitus[93].
Intraductal papillary mucinous neoplasm: AI-based EUS models are under development to diagnose different types of pancreatic cystic lesions, including intraductal papillary mucinous neoplasm[94]. DL-based study for automatic identification of patients with pancreatic neoplasms at abdominal CT has shown good results with an AUC of 0.91 and a sensitivity for solid lesions of any size of 98%-100% and for cystic lesions measuring 1.0 cm or larger of 92%-93%[95]. A DL model was used in one AI-based EUS study to diagnose malignancies of intraductal papillary mucinous neoplasm and achieved a sensitivity, specificity and accuracy of 95.7%, 92.6% and 94.0%, respectively[96].
Autoimmune pancreatitis: The accurate diagnosis of autoimmune pancreatitis (AIP) poses a challenge as the mass for
Biliary tract cancers: AI technology such as DL has been used in medical imaging to improve diagnostic performance of biliary tract cancers, which include cholangiocarcinomas and gallbladder cancer[98]. The analysis of clinical data, serum biomarkers, imaging, and cell-free tumor DNA in whole genome sequencing can be analyzed by AI to diagnose early cholangiocarcinoma and predict patient outcomes[99].
Biliary stricture: AI-based digital single-operator cholangioscopy has been used during assessment of an indeterminate biliary stricture and helped experts and less-experienced endoscopists in obtaining better histopathological samples and cutting the interobserver disagreement among experts[100].
Primary sclerosing cholangitis: Primary sclerosing cholangitis (PSC) is a chronic cholestatic syndrome of unknown etiology and is characterized by fibrosing inflammatory destruction of intrahepatic or extrahepatic biliary ducts[101]. PSC is often progressive and leads to biliary cirrhosis and other complications. Because of the absence of proven medical therapy, orthotopic LT is an option with good outcomes in patients with end-stage PSC[102]. PSC is also associated with cholangiocarcinoma at an incidence of 10%-30%[102]. One study used ML in a PSC risk estimate tool to accurately predict outcomes of PSC. It outperformed the noninvasive prognostic scoring systems in accuracy to predict liver failure[103].
ERCP is an endoscopic procedure used with a therapeutic intent in biliary diseases. The procedure can be challenging in cannulating the papilla in cases of anatomical variation, and sometimes the post-ERCP pancreatitis may be the unwanted event that patients might encounter. An AI system based on CNN used during ERCP in one study assisted in identifying the ampulla in patients with anatomical variation with 76% precision and aided in biliary cannulation[104].
Hepatocellular carcinoma (HCC) is the fifth most common cancer globally[105] with a 5-year survival of 18%[106]. The study done with multivariate models has shown that hepatitis C virus infection, HBV surface antigen, HBV core an
Another study showed that DL was able to classify common hepatic lesions on typical MRI features with 92% accuracy, 92% sensitivity, and 98% specificity[109]. The study has shown that focal liver lesions could be identified and characterized (malignant vs benign) by DL using supervised-attention mechanism from liver ultrasound images[110]. In another study application of AI using EUS-based CNN model was able to identify and distinguish benign and malignant focal liver lesions accurately[111]. AI has also been used in histopathology to classify liver cancer histopathological images[112]. An AI algorithm was used in one study to evaluate the patients who were high risk for the development of HCC[113].
Another study showed that the AI-aided contrast-enhanced MRI was capable of preoperative prediction of micro
MASLD, previously known as nonalcoholic fatty liver disease, is the term for steatotic liver disease and is associated with cardiometabolic risk factors such as obesity, diabetes, hypertension, and dyslipidemia. The pathophysiology of MASLD begins with the buildup of fat in the liver, driven by metabolic dysfunction, followed by metabolic dysfunction-associated steatohepatitis, which progresses to inflammation and liver fibrosis, eventually resulting in cirrhosis, end-stage liver disease, and HCC[119]. Hence, the early detection of MASLD is vital to prevent the development of this category of HCC.
Liver steatosis can be defined histologically or radiologically. The liver biopsy is considered the gold standard for diagnosing metabolic dysfunction-associated steatohepatitis and monitoring fibrosis progression but is more invasive and costly. AI-enabled digital pathology has emerged as an important tool for achieving the more reliable and standar
The radiological method of detecting liver steatosis is more accessible to the patient due to its less invasive nature. Recently, the detection of liver steatosis by ultrasonography has been improved by the use of DL algorithms, such as CNNs[132,133]. The systematic review has shown that AI models integrated into noninvasive diagnostic tools such as ultrasonography, elastography, CT, MRI, and clinical parameters have promising diagnostic potential for liver fibrosis and nonalcoholic fatty liver disease[134]. Currently, elastography is regarded as the most commonly used modality for staging liver fibrosis[135]. One study used AI-assisted shear wave elastography based on a DL method to compare with liver biopsy and found the similar accuracy for diagnosing cirrhosis (AUC = 0.98) and fibrosis (AUC = 0.98)[136]. Another study used ultrasound shear wave elastography imaging with stiffness value-clustering and ML algorithm to classify chronic liver disease from healthy cases and found a sensitivity and specificity of 93.5% and 81.2%, respectively[137].
AI-assisted predictive models using the vast amounts of categorized patient data have been developed to identify individuals at high risk. One study used the application of ML methods to predict nonalcoholic steatohepatitis (NASH) in patients with nonalcoholic fatty livers[138]; the study used electronic health records from the Optum analytics to create supervised ML models trained on NASH and healthy patients and found AUCs of various models ranging from 83%-88%[138]. The NASH map model utilized a non-invasive tool using 14 variables to classify patients as probable NASH or non-NASH; the study showed good sensitivity in predicting NASH status[139].
Most HCC cases are linked to cirrhosis caused by chronic HBV or hepatitis C virus infections[140]. The predictive models for determining the risk of hepatitis-related cirrhosis have been developed by several AI-based tools[141-145]. An AI-based prediction model in one study showed that a universal gut-microbiome-derived metagenomic and metabolomic signature predicted cirrhosis with a diagnostic accuracy of 91%[146].
LT is a lifesaving procedure in acute and chronic end-stage liver disease in which other medical treatments are no longer effective. It restores normal health and lifestyle and extends lifespan by 15 years[147]. However, there are several challenges of LT including insufficient donors, high mortality on the waiting list, and graft failures. There is a lot of mismatch between the number of organs donated vs the number of organs needed and the availability of recipients. The model for end-stage liver disease (MELD), donor risk index, and D-MELD (the product of donor age and preoperative MELD) have been used as criteria for organ allocation during LT; however, these scoring systems may not ensure fair and equitable distribution of donated organs[148,149]. AI-generated synthetic liver transplant waitlist data from the United Network for Organ Sharing database showed that they replicate a complex hepatic dataset, mimic clinical correlations and survival patterns, and rigorously protect patient privacy[150]. AI has been used in LT with considerable outcomes for organ allocation, donor-recipient matching, post-LT survival, or graft failure[151]. One study created a neural network to predict 90-day LT waitlist mortality using the United Network for Organ Sharing database from 2002 to 2021 from which patients who were transplanted within 90 days of listing were excluded. The study showed that the neural network algorithm outperformed MELD and MELD-sodium scores with an AUC of 0.936 vs 0.860[152,153]. With the use of pre
During living donor LT (LDLT), the proper functioning of the liver graft in the recipient should be evaluated meticulously. At the same time it is essential to check whether the donor is left with enough liver parenchyma for future function and regeneration[155]. One study showed that ML can improve the accuracy of graft weight prediction in LDLT[156]. Another study showed that a DL algorithm-assisted CT volumetry method can accurately estimate the graft weight in LDLT[157]. Complications leading to graft failure or death after LT include graft dysfunction/rejection, infections (bacterial, fungal, viral), biliary complications (leaks, strictures), vascular complications (thrombosis, stenosis of arteries/veins), post-transplant malignancies, and organ system failures (renal, cardiovascular, neurological)[158]. AI applications can be used to facilitate the early identification and intervention of complications and possibly complication prediction.
ML algorithms can predict graft failure after LT by utilizing known donor, transplant, and recipient characteristics at the time of the transplant decision, thereby achieving high accuracy in matching donors and recipients[159]. AI has also been used to identify novel factors associated with death after transplantation[160,161]. AI plays a role in enhancing LT by analyzing complex data for preimplantation biopsy evaluation, developing robust recipient prognosis algorithms, improving imaging analysis, and providing decision-making support[162].
With the increasing development of AI in gastroenterology and hepatology, we face ethical dilemmas raising concerns about data privacy, algorithmic bias, and the potential for patient harm[163]. Large datasets are required for training and validation in DL-based AI models and might be an issue for the patient data privacy and security with the potential of data breaches[164,165]. The general data protection regulation is a comprehensive European Union regulation that harmonizes data privacy laws across Europe[165]. The trust and accountability of such AI-driven data are achieved only by implementation of regulatory frameworks[166].
Getting patient consent in the traditional methods may not be enough for the use of all patient data in AI applications. The right of the patient to understand the role of AI in diagnosis and treatment options should be addressed properly. The complexity of AI integration should be included in the informed consent to make patients aware of it[167]. Another important ethical concern is data ownership and sharing. AI algorithm bias can occur when they are trained on biased datasets and can perpetuate or amplify existing health inequities. Race, age, ethnicity, gender, and geographic region biases in algorithms can lead to discriminatory outcomes[168,169]. Because of the overdependence on AI-generated models, the role of clinician expertise in decision-making could be inadvertently affected[170]. Careful individualized approaches might be needed as poorly trained or validated algorithms can lead to misdiagnosis, biased care, and po
Apart from the ethical concerns, the financial constraint is the prime factor in implementation of AI in clinical practice as it requires substantial investment in technology infrastructure, ongoing maintenance, and specialized training. Moreover, the role of various stakeholders, including healthcare providers, patients, and regulatory organizations, is vital. In order to understand the potential and limitations of AI in clinical practice, the proper addressing of both the financial implications and stakeholder perspectives is essential[173].
In summary these ethical concerns need to be addressed properly before implementation of AI in gastroenterology and hepatology. We need to prioritize data privacy and security, use diverse and representative datasets to mitigate bias, and validate the studies thoroughly to assess the performance of AI tools across different populations. In order to achieve the trust and accountability, AI algorithms need to be transparent and explainable. It should be understood that AI is used to augment, not replace, human expertise. Hence, a balanced approach is needed by establishing clear guidelines and regulations for the development and deployment of AI in gastroenterology and hepatology.
AI has emerged as an invaluable transformative tool in diagnosis and management of GI and liver diseases. However, there is a need for continued development, validation, and real-world modeling of AI systems before its widespread adoption. Although it does not replace human clinical judgement, it can still be expected that AI applications in gastroenterology and hepatology will further be enhanced in the future and become the standard of care in clinical practice.
| 1. | Alagappan M, Brown JRG, Mori Y, Berzin TM. Artificial intelligence in gastrointestinal endoscopy: The future is almost here. World J Gastrointest Endosc. 2018;10:239-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (4)] |
| 2. | Lam TYT, Cheung MFK, Munro YL, Lim KM, Shung D, Sung JJY. Randomized Controlled Trials of Artificial Intelligence in Clinical Practice: Systematic Review. J Med Internet Res. 2022;24:e37188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 3. | Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues Clin Neurosci. 2010;12:489-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Haenlein M, Kaplan A. A Brief History of Artificial Intelligence: On the Past, Present, and Future of Artificial Intelligence. Calif Manage Rev. 2019;61:5-14. [DOI] [Full Text] |
| 5. | Darcy AM, Louie AK, Roberts LW. Machine Learning and the Profession of Medicine. JAMA. 2016;315:551-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 6. | Cao JS, Lu ZY, Chen MY, Zhang B, Juengpanich S, Hu JH, Li SJ, Topatana W, Zhou XY, Feng X, Shen JL, Liu Y, Cai XJ. Artificial intelligence in gastroenterology and hepatology: Status and challenges. World J Gastroenterol. 2021;27:1664-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 7. | Jerfy A, Selden O, Balkrishnan R. The Growing Impact of Natural Language Processing in Healthcare and Public Health. Inquiry. 2024;61:469580241290095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Penrice DD, Rattan P, Simonetto DA. Artificial Intelligence and the Future of Gastroenterology and Hepatology. Gastro Hep Adv. 2022;1:581-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Ahmed T, Rabinowitz LG, Rodman A, Berzin TM. Generative Artificial Intelligence Tools in Gastroenterology Training. Clin Gastroenterol Hepatol. 2024;22:1975-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Shahab O, El Kurdi B, Shaukat A, Nadkarni G, Soroush A. Large language models: a primer and gastroenterology applications. Therap Adv Gastroenterol. 2024;17:17562848241227031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Gong EJ, Bang CS. Revolutionizing gastrointestinal endoscopy: the emerging role of large language models. Clin Endosc. 2024;57:759-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Trachtenbarg DE, Asche C, Ramsahai S, Duling J, Ren J. The benefits, risks and costs of privacy: patient preferences and willingness to pay. Curr Med Res Opin. 2017;33:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Ahn JC, Noh YK, Hu M, Shen X, Shah VH, Kamath PS. Use of Artificial Intelligence-Generated Synthetic Data to Augment and Enhance the Performance of Clinical Prediction Models in Patients With Alcohol-Associated Hepatitis and Acute Cholangitis. Gastro Hep Adv. 2025;4:100643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Sharma P, Hassan C. Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia. Gastroenterology. 2022;162:1056-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 395] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Berbís MA, Aneiros-Fernández J, Mendoza Olivares FJ, Nava E, Luna A. Role of artificial intelligence in multidisciplinary imaging diagnosis of gastrointestinal diseases. World J Gastroenterol. 2021;27:4395-4412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (71)] |
| 18. | Faghani S, Codipilly DC, David Vogelsang, Moassefi M, Rouzrokh P, Khosravi B, Agarwal S, Dhaliwal L, Katzka DA, Hagen C, Lewis J, Leggett CL, Erickson BJ, Iyer PG. Development of a deep learning model for the histologic diagnosis of dysplasia in Barrett's esophagus. Gastrointest Endosc. 2022;96:918-925.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Horie Y, Yoshio T, Aoyama K, Yoshimizu S, Horiuchi Y, Ishiyama A, Hirasawa T, Tsuchida T, Ozawa T, Ishihara S, Kumagai Y, Fujishiro M, Maetani I, Fujisaki J, Tada T. Diagnostic outcomes of esophageal cancer by artificial intelligence using convolutional neural networks. Gastrointest Endosc. 2019;89:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (4)] |
| 20. | de Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology. 2020;158:915-929.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 21. | Sabo E, Beck AH, Montgomery EA, Bhattacharya B, Meitner P, Wang JY, Resnick MB. Computerized morphometry as an aid in determining the grade of dysplasia and progression to adenocarcinoma in Barrett's esophagus. Lab Invest. 2006;86:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Swager AF, van der Sommen F, Klomp SR, Zinger S, Meijer SL, Schoon EJ, Bergman JJGHM, de With PH, Curvers WL. Computer-aided detection of early Barrett's neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc. 2017;86:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 23. | Abdelrahim M, Saiko M, Maeda N, Hossain E, Alkandari A, Subramaniam S, Parra-Blanco A, Sanchez-Yague A, Coron E, Repici A, Bhandari P. Development and validation of artificial neural networks model for detection of Barrett's neoplasia: a multicenter pragmatic nonrandomized trial (with video). Gastrointest Endosc. 2023;97:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1163] [Article Influence: 290.8] [Reference Citation Analysis (0)] |
| 25. | Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, Pu J, Lv J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. 2023;29:2452-2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 343] [Cited by in RCA: 310] [Article Influence: 103.3] [Reference Citation Analysis (14)] |
| 26. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 970] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 27. | Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki J, Tada T. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (3)] |
| 28. | Zhu Y, Wang QC, Xu MD, Zhang Z, Cheng J, Zhong YS, Zhang YQ, Chen WF, Yao LQ, Zhou PH, Li QL. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest Endosc. 2019;89:806-815.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (3)] |
| 29. | Song Z, Zou S, Zhou W, Huang Y, Shao L, Yuan J, Gou X, Jin W, Wang Z, Chen X, Ding X, Liu J, Yu C, Ku C, Liu C, Sun Z, Xu G, Wang Y, Zhang X, Wang D, Wang S, Xu W, Davis RC, Shi H. Clinically applicable histopathological diagnosis system for gastric cancer detection using deep learning. Nat Commun. 2020;11:4294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 30. | Bang CS, Lee JJ, Baik GH. Artificial Intelligence for the Prediction of Helicobacter Pylori Infection in Endoscopic Images: Systematic Review and Meta-Analysis Of Diagnostic Test Accuracy. J Med Internet Res. 2020;22:e21983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Klein S, Gildenblat J, Ihle MA, Merkelbach-Bruse S, Noh KW, Peifer M, Quaas A, Büttner R. Deep learning for sensitive detection of Helicobacter Pylori in gastric biopsies. BMC Gastroenterol. 2020;20:417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Steinbuss G, Kriegsmann K, Kriegsmann M. Identification of Gastritis Subtypes by Convolutional Neuronal Networks on Histological Images of Antrum and Corpus Biopsies. Int J Mol Sci. 2020;21:6652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 344] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 34. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 832] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 35. | Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (9)] |
| 37. | Jariwala R, Bratton L, Romero R, Evans J, Shah J, El Chafic AH. Endoscopic resection of GI stromal tumor using full-thickness resection device: tips and tricks. VideoGIE. 2023;8:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Zhang B, Zhu F, Li P, Zhu J. Artificial intelligence-assisted endoscopic ultrasound in the diagnosis of gastrointestinal stromal tumors: a meta-analysis. Surg Endosc. 2023;37:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 39. | Chen EY, Vaccaro GM. Small Bowel Adenocarcinoma. Clin Colon Rectal Surg. 2018;31:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Hsieh YH, Leung FW. Increase your adenoma detection rate without using fancy adjunct tools. Tzu Chi Med J. 2018;30:127-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 41. | Turner JS, Henry D, Chase A, Kpodzo D, Flood MC, Clark CE. Adenoma Detection Rate in Colonoscopy: Does the Participation of a Resident Matter? Am Surg. 2018;84:1064-1068. [PubMed] |
| 42. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1666] [Article Influence: 138.8] [Reference Citation Analysis (1)] |
| 43. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, Iqbal N, Chandelier F, Rex DK. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 44. | Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang D, Li Y, Xu G, Tu M, Liu X. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 591] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 45. | Fernández-Esparrach G, Bernal J, López-Cerón M, Córdova H, Sánchez-Montes C, Rodríguez de Miguel C, Sánchez FJ. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy. 2016;48:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (2)] |
| 46. | Wang A, Mo J, Zhong C, Wu S, Wei S, Tu B, Liu C, Chen D, Xu Q, Cai M, Li Z, Xie W, Xie M, Kato M, Xi X, Zhang B. Artificial intelligence-assisted detection and classification of colorectal polyps under colonoscopy: a systematic review and meta-analysis. Ann Transl Med. 2021;9:1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 47. | Takemura Y, Yoshida S, Tanaka S, Onji K, Oka S, Tamaki T, Kaneda K, Yoshihara M, Chayama K. Quantitative analysis and development of a computer-aided system for identification of regular pit patterns of colorectal lesions. Gastrointest Endosc. 2010;72:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 48. | Tischendorf JJ, Gross S, Winograd R, Hecker H, Auer R, Behrens A, Trautwein C, Aach T, Stehle T. Computer-aided classification of colorectal polyps based on vascular patterns: a pilot study. Endoscopy. 2010;42:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (4)] |
| 50. | Mori Y, Kudo SE, Wakamura K, Misawa M, Ogawa Y, Kutsukawa M, Kudo T, Hayashi T, Miyachi H, Ishida F, Inoue H. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc. 2015;81:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 51. | Takemura Y, Yoshida S, Tanaka S, Kawase R, Onji K, Oka S, Tamaki T, Raytchev B, Kaneda K, Yoshihara M, Chayama K. Computer-aided system for predicting the histology of colorectal tumors by using narrow-band imaging magnifying colonoscopy (with video). Gastrointest Endosc. 2012;75:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Abad MRA, Shimamura Y, Fujiyoshi Y, Seewald S, Inoue H. Endocytoscopy: technology and clinical application in upper gastrointestinal tract. Transl Gastroenterol Hepatol. 2020;5:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Mori Y, Kudo SE, Chiu PW, Singh R, Misawa M, Wakamura K, Kudo T, Hayashi T, Katagiri A, Miyachi H, Ishida F, Maeda Y, Inoue H, Nimura Y, Oda M, Mori K. Impact of an automated system for endocytoscopic diagnosis of small colorectal lesions: an international web-based study. Endoscopy. 2016;48:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Cothren RM, Sivak MV Jr, Van Dam J, Petras RE, Fitzmaurice M, Crawford JM, Wu J, Brennan JF, Rava RP, Manoharan R, Feld MS. Detection of dysplasia at colonoscopy using laser-induced fluorescence: a blinded study. Gastrointest Endosc. 1996;44:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Richards-Kortum R, Rava RP, Petras RE, Fitzmaurice M, Sivak M, Feld MS. Spectroscopic diagnosis of colonic dysplasia. Photochem Photobiol. 1991;53:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 118] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Efthymiou M, Allen PB, Taylor AC, Desmond PV, Jayasakera C, De Cruz P, Kamm MA. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2132-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | André B, Vercauteren T, Buchner AM, Krishna M, Ayache N, Wallace MB. Software for automated classification of probe-based confocal laser endomicroscopy videos of colorectal polyps. World J Gastroenterol. 2012;18:5560-5569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Murakami Y, Fujimori T, Kaneko K, Saito Y. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551-9; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 59. | Yoda Y, Ikematsu H, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Fujimori T, Kaneko K, Saito Y. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy. 2013;45:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 797] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 61. | Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow Band Imaging, Magnifying Chromoendoscopy, and Gross Morphological Features for the Optical Diagnosis of T1 Colorectal Cancer and Deep Submucosal Invasion: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 62. | Takeda K, Kudo SE, Mori Y, Misawa M, Kudo T, Wakamura K, Katagiri A, Baba T, Hidaka E, Ishida F, Inoue H, Oda M, Mori K. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy. 2017;49:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Maeda Y, Kudo SE, Mori Y, Misawa M, Ogata N, Sasanuma S, Wakamura K, Oda M, Mori K, Ohtsuka K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019;89:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 64. | Takenaka K, Ohtsuka K, Fujii T, Negi M, Suzuki K, Shimizu H, Oshima S, Akiyama S, Motobayashi M, Nagahori M, Saito E, Matsuoka K, Watanabe M. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images From Patients With Ulcerative Colitis. Gastroenterology. 2020;158:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 65. | Klang E, Barash Y, Margalit RY, Soffer S, Shimon O, Albshesh A, Ben-Horin S, Amitai MM, Eliakim R, Kopylov U. Deep learning algorithms for automated detection of Crohn's disease ulcers by video capsule endoscopy. Gastrointest Endosc. 2020;91:606-613.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 66. | Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 540] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 67. | Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 68. | Chung CF, Wang Q, Schroeder J, Cole A, Zia J, Fogarty J, Munson SA. Identifying and Planning for Individualized Change: Patient-Provider Collaboration Using Lightweight Food Diaries in Healthy Eating and Irritable Bowel Syndrome. Proc ACM Interact Mob Wearable Ubiquitous Technol. 2019;3:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Zia J, Schroeder J, Munson S, Fogarty J, Nguyen L, Barney P, Heitkemper M, Ladabaum U. Feasibility and Usability Pilot Study of a Novel Irritable Bowel Syndrome Food and Gastrointestinal Symptom Journal Smartphone App. Clin Transl Gastroenterol. 2016;7:e147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Quigley EMM. Are diets the answers to colon ills? Food, irritable bowel syndrome and the microbiome. Curr Opin Gastroenterol. 2018;34:103-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Fukui H, Nishida A, Matsuda S, Kira F, Watanabe S, Kuriyama M, Kawakami K, Aikawa Y, Oda N, Arai K, Matsunaga A, Nonaka M, Nakai K, Shinmura W, Matsumoto M, Morishita S, Takeda AK, Miwa H. Usefulness of Machine Learning-Based Gut Microbiome Analysis for Identifying Patients with Irritable Bowels Syndrome. J Clin Med. 2020;9:2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Das A, Ben-Menachem T, Cooper GS, Chak A, Sivak MV Jr, Gonet JA, Wong RC. Prediction of outcome in acute lower-gastrointestinal haemorrhage based on an artificial neural network: internal and external validation of a predictive model. Lancet. 2003;362:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 73. | Das A, Ben-Menachem T, Farooq FT, Cooper GS, Chak A, Sivak MV Jr, Wong RC. Artificial neural network as a predictive instrument in patients with acute nonvariceal upper gastrointestinal hemorrhage. Gastroenterology. 2008;134:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Ayaru L, Ypsilantis PP, Nanapragasam A, Choi RC, Thillanathan A, Min-Ho L, Montana G. Prediction of Outcome in Acute Lower Gastrointestinal Bleeding Using Gradient Boosting. PLoS One. 2015;10:e0132485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Sengupta N, Tapper EB. Derivation and Internal Validation of a Clinical Prediction Tool for 30-Day Mortality in Lower Gastrointestinal Bleeding. Am J Med. 2017;130:601.e1-601.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 76. | Wong GL, Ma AJ, Deng H, Ching JY, Wong VW, Tse YK, Yip TC, Lau LH, Liu HH, Leung CM, Tsang SW, Chan CW, Lau JY, Yuen PC, Chan FK. Machine learning model to predict recurrent ulcer bleeding in patients with history of idiopathic gastroduodenal ulcer bleeding. Aliment Pharmacol Ther. 2019;49:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 77. | Seguí S, Drozdzal M, Pascual G, Radeva P, Malagelada C, Azpiroz F, Vitrià J. Generic feature learning for wireless capsule endoscopy analysis. Comput Biol Med. 2016;79:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Xiao Jia, Meng MQ. A deep convolutional neural network for bleeding detection in Wireless Capsule Endoscopy images. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 79. | Yuan Y, Meng MQ. Deep learning for polyp recognition in wireless capsule endoscopy images. Med Phys. 2017;44:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 80. | Leenhardt R, Li C, Le Mouel JP, Rahmi G, Saurin JC, Cholet F, Boureille A, Amiot X, Delvaux M, Duburque C, Leandri C, Gérard R, Lecleire S, Mesli F, Nion-Larmurier I, Romain O, Sacher-Huvelin S, Simon-Shane C, Vanbiervliet G, Marteau P, Histace A, Dray X. CAD-CAP: a 25,000-image database serving the development of artificial intelligence for capsule endoscopy. Endosc Int Open. 2020;8:E415-E420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Aoki T, Yamada A, Aoyama K, Saito H, Tsuboi A, Nakada A, Niikura R, Fujishiro M, Oka S, Ishihara S, Matsuda T, Tanaka S, Koike K, Tada T. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc. 2019;89:357-363.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 82. | Fan S, Xu L, Fan Y, Wei K, Li L. Computer-aided detection of small intestinal ulcer and erosion in wireless capsule endoscopy images. Phys Med Biol. 2018;63:165001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 83. | He JY, Wu X, Jiang YG, Peng Q, Jain R. Hookworm Detection in Wireless Capsule Endoscopy Images With Deep Learning. IEEE Trans Image Process. 2018;27:2379-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 84. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12644] [Article Influence: 6322.0] [Reference Citation Analysis (6)] |
| 85. | Hur C, Tramontano AC, Dowling EC, Brooks GA, Jeon A, Brugge WR, Gazelle GS, Kong CY, Pandharipande PV. Early Pancreatic Ductal Adenocarcinoma Survival Is Dependent on Size: Positive Implications for Future Targeted Screening. Pancreas. 2016;45:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 87. | Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 88. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 89. | Liu F, Xie L, Xia Y, Fishman E, Yuille A. Joint Shape Representation and Classification for Detecting PDAC. Available from: arXiv:1804.10684. [DOI] [Full Text] |
| 90. | Kanno A, Masamune A, Hanada K, Maguchi H, Shimizu Y, Ueki T, Hasebe O, Ohtsuka T, Nakamura M, Takenaka M, Kitano M, Kikuyama M, Gabata T, Yoshida K, Sasaki T, Serikawa M, Furukawa T, Yanagisawa A, Shimosegawa T; Japan Study Group on the Early Detection of Pancreatic Cancer (JEDPAC). Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 91. | Tonozuka R, Itoi T, Nagata N, Kojima H, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Nagakawa Y, Mukai S. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci. 2021;28:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 92. | Tonozuka R, Mukai S, Itoi T. The Role of Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Disorders. Diagnostics (Basel). 2020;11:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Hsieh MH, Sun LM, Lin CL, Hsieh MJ, Hsu CY, Kao CH. Development of a prediction model for pancreatic cancer in patients with type 2 diabetes using logistic regression and artificial neural network models. Cancer Manag Res. 2018;10:6317-6324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 94. | Dalal V, Carmicheal J, Dhaliwal A, Jain M, Kaur S, Batra SK. Radiomics in stratification of pancreatic cystic lesions: Machine learning in action. Cancer Lett. 2020;469:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 95. | Park HJ, Shin K, You MW, Kyung SG, Kim SY, Park SH, Byun JH, Kim N, Kim HJ. Deep Learning-based Detection of Solid and Cystic Pancreatic Neoplasms at Contrast-enhanced CT. Radiology. 2023;306:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 96. | Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Kurita Y, Koda H, Toriyama K, Onishi S, Ishihara M, Tanaka T, Tajika M, Niwa Y. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 97. | Marya NB, Powers PD, Chari ST, Gleeson FC, Leggett CL, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Majumder S, Pearson RK, Petersen BT, Rajan E, Sawas T, Storm AC, Vege SS, Chen S, Long Z, Hough DM, Mara K, Levy MJ. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut. 2021;70:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 98. | Gupta P, Basu S, Arora C. Applications of artificial intelligence in biliary tract cancers. Indian J Gastroenterol. 2024;43:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Huang J, Bai X, Qiu Y, He X. Application of AI on cholangiocarcinoma. Front Oncol. 2024;14:1324222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Ricaurte-Ciro J, Baquerizo-Burgos J, Carvajal-Gutierrez J, Mendez JC, Robles-Medranda C. Usefulness of artificial intelligence-assisted digital single-operator cholangioscopy as a second-opinion consultation tool during interhospital assessment of an indeterminate biliary stricture: a case report. VideoGIE. 2023;8:364-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 101. | Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology. 1999;30:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | Barnabas A, Chapman RW. Primary sclerosing cholangitis: is any treatment worthwhile? Curr Gastroenterol Rep. 2012;14:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Eaton JE, Vesterhus M, McCauley BM, Atkinson EJ, Schlicht EM, Juran BD, Gossard AA, LaRusso NF, Gores GJ, Karlsen TH, Lazaridis KN. Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) Predicts Outcomes of the Disease: A Derivation and Validation Study Using Machine Learning. Hepatology. 2020;71:214-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 104. | Kim T, Kim J, Choi HS, Kim ES, Keum B, Jeen YT, Lee HS, Chun HJ, Han SY, Kim DU, Kwon S, Choo J, Lee JM. Artificial intelligence-assisted analysis of endoscopic retrograde cholangiopancreatography image for identifying ampulla and difficulty of selective cannulation. Sci Rep. 2021;11:8381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20720] [Article Influence: 1883.6] [Reference Citation Analysis (23)] |
| 106. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109:djx030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1163] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 107. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 108. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 426] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 109. | Hamm CA, Wang CJ, Savic LJ, Ferrante M, Schobert I, Schlachter T, Lin M, Duncan JS, Weinreb JC, Chapiro J, Letzen B. Deep learning for liver tumor diagnosis part I: development of a convolutional neural network classifier for multi-phasic MRI. Eur Radiol. 2019;29:3338-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 110. | Schmauch B, Herent P, Jehanno P, Dehaene O, Saillard C, Aubé C, Luciani A, Lassau N, Jégou S. Diagnosis of focal liver lesions from ultrasound using deep learning. Diagn Interv Imaging. 2019;100:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 111. | Marya NB, Powers PD, Fujii-Lau L, Abu Dayyeh BK, Gleeson FC, Chen S, Long Z, Hough DM, Chandrasekhara V, Iyer PG, Rajan E, Sanchez W, Sawas T, Storm AC, Wang KK, Levy MJ. Application of artificial intelligence using a novel EUS-based convolutional neural network model to identify and distinguish benign and malignant hepatic masses. Gastrointest Endosc. 2021;93:1121-1130.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 112. | Sun C, Xu A, Liu D, Xiong Z, Zhao F, Ding W. Deep Learning-Based Classification of Liver Cancer Histopathology Images Using Only Global Labels. IEEE J Biomed Health Inform. 2020;24:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 114. | Feng ST, Jia Y, Liao B, Huang B, Zhou Q, Li X, Wei K, Chen L, Li B, Wang W, Chen S, He X, Wang H, Peng S, Chen ZB, Tang M, Chen Z, Hou Y, Peng Z, Kuang M. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 115. | Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Intra-arterial Therapies for Hepatocellular Carcinoma with the Use of Supervised Machine Learning-An Artificial Intelligence Concept. J Vasc Interv Radiol. 2018;29:850-857.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 116. | Saillard C, Schmauch B, Laifa O, Moarii M, Toldo S, Zaslavskiy M, Pronier E, Laurent A, Amaddeo G, Regnault H, Sommacale D, Ziol M, Pawlotsky JM, Mulé S, Luciani A, Wainrib G, Clozel T, Courtiol P, Calderaro J. Predicting Survival After Hepatocellular Carcinoma Resection Using Deep Learning on Histological Slides. Hepatology. 2020;72:2000-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 117. | Liu D, Liu F, Xie X, Su L, Liu M, Xie X, Kuang M, Huang G, Wang Y, Zhou H, Wang K, Lin M, Tian J. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol. 2020;30:2365-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 118. | Liu F, Liu D, Wang K, Xie X, Su L, Kuang M, Huang G, Peng B, Wang Y, Lin M, Tian J, Xie X. Deep Learning Radiomics Based on Contrast-Enhanced Ultrasound Might Optimize Curative Treatments for Very-Early or Early-Stage Hepatocellular Carcinoma Patients. Liver Cancer. 2020;9:397-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 119. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 955] [Article Influence: 477.5] [Reference Citation Analysis (1)] |
| 120. | Dinani AM, Kowdley KV, Noureddin M. Application of Artificial Intelligence for Diagnosis and Risk Stratification in NAFLD and NASH: The State of the Art. Hepatology. 2021;74:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Ratziu V, Hompesch M, Petitjean M, Serdjebi C, Iyer JS, Parwani AV, Tai D, Bugianesi E, Cusi K, Friedman SL, Lawitz E, Romero-Gómez M, Schuppan D, Loomba R, Paradis V, Behling C, Sanyal AJ. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: current status and future directions. J Hepatol. 2024;80:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 122. | Takahashi Y, Dungubat E, Kusano H, Fukusato T. Artificial intelligence and deep learning: New tools for histopathological diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Comput Struct Biotechnol J. 2023;21:2495-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 123. | Vanderbeck S, Bockhorst J, Komorowski R, Kleiner DE, Gawrieh S. Automatic classification of white regions in liver biopsies by supervised machine learning. Hum Pathol. 2014;45:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 124. | Munsterman ID, van Erp M, Weijers G, Bronkhorst C, de Korte CL, Drenth JPH, van der Laak JAWM, Tjwa ETTL. A Novel Automatic Digital Algorithm that Accurately Quantifies Steatosis in NAFLD on Histopathological Whole-Slide Images. Cytometry B Clin Cytom. 2019;96:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Gawrieh S, Sethunath D, Cummings OW, Kleiner DE, Vuppalanchi R, Chalasani N, Tuceryan M. Automated quantification and architectural pattern detection of hepatic fibrosis in NAFLD. Ann Diagn Pathol. 2020;47:151518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 126. | Ramot Y, Zandani G, Madar Z, Deshmukh S, Nyska A. Utilization of a Deep Learning Algorithm for Microscope-Based Fatty Vacuole Quantification in a Fatty Liver Model in Mice. Toxicol Pathol. 2020;48:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Soon G, Wee A. Updates in the quantitative assessment of liver fibrosis for nonalcoholic fatty liver disease: Histological perspective. Clin Mol Hepatol. 2021;27:44-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 128. | Brunt EM, Clouston AD, Goodman Z, Guy C, Kleiner DE, Lackner C, Tiniakos DG, Wee A, Yeh M, Leow WQ, Chng E, Ren Y, Boon Bee GG, Powell EE, Rinella M, Sanyal AJ, Neuschwander-Tetri B, Younossi Z, Charlton M, Ratziu V, Harrison SA, Tai D, Anstee QM. Complexity of ballooned hepatocyte feature recognition: Defining a training atlas for artificial intelligence-based imaging in NAFLD. J Hepatol. 2022;76:1030-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 129. | Naoumov NV, Brees D, Loeffler J, Chng E, Ren Y, Lopez P, Tai D, Lamle S, Sanyal AJ. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J Hepatol. 2022;77:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 130. | Wang Y, Wong GL, He FP, Sun J, Chan AW, Yang J, Shu SS, Liang X, Tse YK, Fan XT, Hou J, Chan HL, Wong VW. Quantifying and monitoring fibrosis in non-alcoholic fatty liver disease using dual-photon microscopy. Gut. 2020;69:1116-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 131. | Xu S, Wang Y, Tai DCS, Wang S, Cheng CL, Peng Q, Yan J, Chen Y, Sun J, Liang X, Zhu Y, Rajapakse JC, Welsch RE, So PTC, Wee A, Hou J, Yu H. qFibrosis: a fully-quantitative innovative method incorporating histological features to facilitate accurate fibrosis scoring in animal model and chronic hepatitis B patients. J Hepatol. 2014;61:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 132. | Biswas M, Kuppili V, Edla DR, Suri HS, Saba L, Marinhoe RT, Sanches JM, Suri JS. Symtosis: A liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Comput Methods Programs Biomed. 2018;155:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 133. | Byra M, Styczynski G, Szmigielski C, Kalinowski P, Michałowski Ł, Paluszkiewicz R, Ziarkiewicz-Wróblewska B, Zieniewicz K, Sobieraj P, Nowicki A. Transfer learning with deep convolutional neural network for liver steatosis assessment in ultrasound images. Int J Comput Assist Radiol Surg. 2018;13:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 134. | Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, Treeprasertsuk S. Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 135. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 540] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 136. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 385] [Article Influence: 55.0] [Reference Citation Analysis (1)] |
| 137. | Gatos I, Tsantis S, Spiliopoulos S, Karnabatidis D, Theotokas I, Zoumpoulis P, Loupas T, Hazle JD, Kagadis GC. A Machine-Learning Algorithm Toward Color Analysis for Chronic Liver Disease Classification, Employing Ultrasound Shear Wave Elastography. Ultrasound Med Biol. 2017;43:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 138. | Fialoke S, Malarstig A, Miller MR, Dumitriu A. Application of Machine Learning Methods to Predict Non-Alcoholic Steatohepatitis (NASH) in Non-Alcoholic Fatty Liver (NAFL) Patients. AMIA Annu Symp Proc. 2018;2018:430-439. [PubMed] |
| 139. | Schattenberg JM, Balp MM, Reinhart B, Tietz A, Regnier SA, Capkun G, Ye Q, Loeffler J, Pedrosa MC, Docherty M. NASHmap: clinical utility of a machine learning model to identify patients at risk of NASH in real-world settings. Sci Rep. 2023;13:5573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 140. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2562] [Article Influence: 183.0] [Reference Citation Analysis (3)] |
| 141. | Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, Layden TJ, Wright TL, White T, Cheung RC. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 142. | Lara J, López-Labrador F, González-Candelas F, Berenguer M, Khudyakov YE. Computational models of liver fibrosis progression for hepatitis C virus chronic infection. BMC Bioinformatics. 2014;15 Suppl 8:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Shousha HI, Awad AH, Omran DA, Elnegouly MM, Mabrouk M. Data Mining and Machine Learning Algorithms Using IL28B Genotype and Biochemical Markers Best Predicted Advanced Liver Fibrosis in Chronic Hepatitis C. Jpn J Infect Dis. 2018;71:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 144. | Wei R, Wang J, Wang X, Xie G, Wang Y, Zhang H, Peng CY, Rajani C, Kwee S, Liu P, Jia W. Clinical prediction of HBV and HCV related hepatic fibrosis using machine learning. EBioMedicine. 2018;35:124-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 145. | Konerman MA, Lu D, Zhang Y, Thomson M, Zhu J, Verma A, Liu B, Talaat N, Balis U, Higgins PDR, Lok ASF, Waljee AK. Assessing risk of fibrosis progression and liver-related clinical outcomes among patients with both early stage and advanced chronic hepatitis C. PLoS One. 2017;12:e0187344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 146. | Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, Singh S, Madamba EV, Bettencourt R, Richards L, Yu RT, Atkins AR, Huan T, Brenner DA, Sirlin CB, Downes M, Evans RM, Loomba R. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020;32:878-888.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 147. | Schnitzler MA, Whiting JF, Brennan DC, Lentine KL, Desai NM, Chapman W, Abbott KC, Kalo Z. The life-years saved by a deceased organ donor. Am J Transplant. 2005;5:2289-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 149. | Croome KP, Marotta P, Wall WJ, Dale C, Levstik MA, Chandok N, Hernandez-Alejandro R. Should a lower quality organ go to the least sick patient? Model for end-stage liver disease score and donor risk index as predictors of early allograft dysfunction. Transplant Proc. 2012;44:1303-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Ahn JC, Noh YK, Hu M, Shen X, Simonetto DA, Kamath PS, Loomba R, Shah VH. AI-driven synthetic data generation for accelerating hepatology research: A study of the United Network for Organ Sharing (UNOS) database. Hepatology. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 151. | Serban M, Balescu I, Petrea S, Gaspar B, Pop L, Varlas V, Stoian M, Diaconu C, Balalau C, Bacalbasa N. Artificial intelligence and liver transplantation; literature review. J Mind Med Sci. 2024;11:374-380. [DOI] [Full Text] |
| 152. | Nagai S, Nallabasannagari AR, Moonka D, Reddiboina M, Yeddula S, Kitajima T, Francis I, Abouljoud M. Use of neural network models to predict liver transplantation waitlist mortality. Liver Transpl. 2022;28:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 153. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 154. | Finlayson SG, Subbaswamy A, Singh K, Bowers J, Kupke A, Zittrain J, Kohane IS, Saria S. The Clinician and Dataset Shift in Artificial Intelligence. N Engl J Med. 2021;385:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 155. | Wang WC, Wu TH, Hung HC, Lee JC, Cheng CH, Wang YC, Lee CF, Wu TJ, Chou HS, Chan KM, Lee WC. Liver regeneration of living donor after liver donation for transplantation: Disparity in the left and right remnant liver. Medicine (Baltimore). 2024;103:e37632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 156. | Giglio MC, Zanfardino M, Franzese M, Zakaria H, Alobthani S, Zidan A, Ayoub II, Shoreem HA, Lee B, Han HS, Penna AD, Nadalin S, Troisi RI, Broering DC. Machine learning improves the accuracy of graft weight prediction in living donor liver transplantation. Liver Transpl. 2023;29:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 157. | Park R, Lee S, Sung Y, Yoon J, Suk HI, Kim H, Choi S. Accuracy and Efficiency of Right-Lobe Graft Weight Estimation Using Deep-Learning-Assisted CT Volumetry for Living-Donor Liver Transplantation. Diagnostics (Basel). 2022;12:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 158. | Craig EV, Heller MT. Complications of liver transplant. Abdom Radiol (NY). 2021;46:43-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 159. | Lau L, Kankanige Y, Rubinstein B, Jones R, Christophi C, Muralidharan V, Bailey J. Machine-Learning Algorithms Predict Graft Failure After Liver Transplantation. Transplantation. 2017;101:e125-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 160. | Bhat V, Tazari M, Watt KD, Bhat M. New-Onset Diabetes and Preexisting Diabetes Are Associated With Comparable Reduction in Long-Term Survival After Liver Transplant: A Machine Learning Approach. Mayo Clin Proc. 2018;93:1794-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 161. | Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernández D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC; International Expert Panel. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75:SS3-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 362] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 162. | Avramidou E, Todorov D, Katsanos G, Antoniadis N, Kofinas A, Vasileiadou S, Karakasi K, Tsoulfas G. AI Innovations in Liver Transplantation: From Big Data to Better Outcomes. Livers. 2025;5:14. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 163. | Aggarwal N, Singh A, Garcia P, Guha S. Ethical Implications of Artificial Intelligence in Gastroenterology. Clin Gastroenterol Hepatol. 2024;22:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 164. | Ueda D, Kakinuma T, Fujita S, Kamagata K, Fushimi Y, Ito R, Matsui Y, Nozaki T, Nakaura T, Fujima N, Tatsugami F, Yanagawa M, Hirata K, Yamada A, Tsuboyama T, Kawamura M, Fujioka T, Naganawa S. Fairness of artificial intelligence in healthcare: review and recommendations. Jpn J Radiol. 2024;42:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 198] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 165. | Vlahou A, Hallinan D, Apweiler R, Argiles A, Beige J, Benigni A, Bischoff R, Black PC, Boehm F, Céraline J, Chrousos GP, Delles C, Evenepoel P, Fridolin I, Glorieux G, van Gool AJ, Heidegger I, Ioannidis JPA, Jankowski J, Jankowski V, Jeronimo C, Kamat AM, Masereeuw R, Mayer G, Mischak H, Ortiz A, Remuzzi G, Rossing P, Schanstra JP, Schmitz-Dräger BJ, Spasovski G, Staessen JA, Stamatialis D, Stenvinkel P, Wanner C, Williams SB, Zannad F, Zoccali C, Vanholder R. Data Sharing Under the General Data Protection Regulation: Time to Harmonize Law and Research Ethics? Hypertension. 2021;77:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 166. | Youssef A, Nichol AA, Martinez-Martin N, Larson DB, Abramoff M, Wolf RM, Char D. Ethical Considerations in the Design and Conduct of Clinical Trials of Artificial Intelligence. JAMA Netw Open. 2024;7:e2432482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 167. | Ahmad OF, Stoyanov D, Lovat LB. Barriers and pitfalls for artificial intelligence in gastroenterology: Ethical and regulatory issues. Tech Innov Gastrointest Endosc. 2020;22:80-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 168. | Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1728] [Article Influence: 78.5] [Reference Citation Analysis (1)] |
| 169. | Khoury MJ, Bowen S, Dotson WD, Drzymalla E, Green RF, Goldstein R, Kolor K, Liburd LC, Sperling LS, Bunnell R. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet Med. 2022;24:1630-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 170. | Aggarwal N, Drew DA, Parikh RB, Guha S. Ethical Implications of Artificial Intelligence in Gastroenterology: The Co-pilot or the Captain? Dig Dis Sci. 2024;69:2727-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 171. | Fehr J, Jaramillo-Gutierrez G, Oala L, Gröschel MI, Bierwirth M, Balachandran P, Werneck-Leite A, Lippert C. Piloting a Survey-Based Assessment of Transparency and Trustworthiness with Three Medical AI Tools. Healthcare (Basel). 2022;10:1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | van Delden JJ, van der Graaf R. Revised CIOMS International Ethical Guidelines for Health-Related Research Involving Humans. JAMA. 2017;317:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 173. | Ramoni D, Scuricini A, Carbone F, Liberale L, Montecucco F. Artificial intelligence in gastroenterology: Ethical and diagnostic challenges in clinical practice. World J Gastroenterol. 2025;31:102725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/