Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.111380
Revised: August 2, 2025
Accepted: September 11, 2025
Published online: October 21, 2025
Processing time: 114 Days and 20.1 Hours
Antibiotic resistance significantly impacts the treatment failure rates of Helicobacter pylori (H. pylori) infections.
To investigate the trends in primary antibiotic resistance of H. pylori in Taiwan over the past six years.
We conducted a retrospective analysis of H. pylori isolates from Taiwanese who had not undergone previous treatments (n = 1408), collected between January 1, 2019 and December 31, 2024. Susceptibility of these strains to amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline was tested using the Epsilometer test. We analyzed the trends in single and dual resistance profiles over the study period, and compared antibiotic resistance across different regions (northern, southern and eastern areas) of Taiwan.
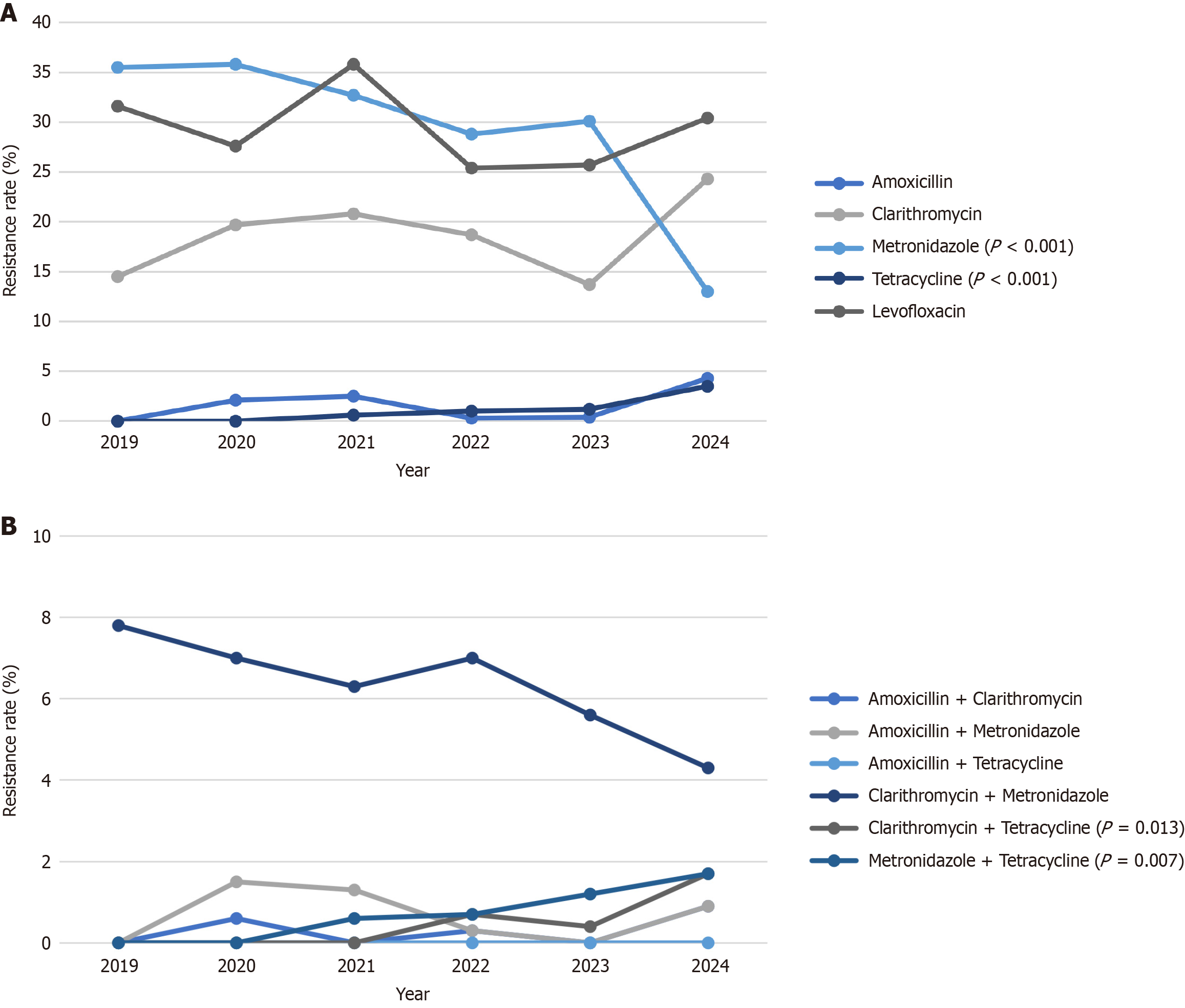
The overall resistance rates for H. pylori to amoxicillin, clarithromycin, metronidazole, tetracycline, and levofloxacin in Taiwan were 1.3%, 18.0%, 31.0%, 0.8%, and 28.7%, respectively. Tetracycline resistance increased significantly from 0% in 2019 to 3.5% in 2024 (P value in χ2 test for linear trend: < 0.001), while metronidazole resistance declined from 35.5% to 13.0% (P value in χ2 test for linear trend: < 0.001). No significant changes of amoxicillin, clarithromycin and levofloxacin resistances were observed. The dual resistances to clarithromycin plus tetracycline, and metronidazole plus tetracycline both increased significantly from 0% to 1.7% from 2019 to 2024 (P value in χ2 test for linear trend: < 0.05). Furthermore, no significant regional differences in resistance frequencies except for levofloxacin were detected.
Primary antibiotic resistance to tetracycline in H. pylori has increased in Taiwan from 2019 to 2024, while resistance to metronidazole has decreased during the same period. The dual resistance to clarithromycin plus tetracycline and metronidazole plus tetracycline both increased significantly.
Core Tip: Antibiotic resistance significantly impacts the treatment failure rates of Helicobacter pylori (H. pylori) infections. We analyzed the trends in single and dual resistance profiles of H. pylori treatment-naive Taiwanese between January 2019 and December 2024. Primary antibiotic resistance to tetracycline in H. pylori has increased in Taiwan from 2019 to 2024, while resistance to metronidazole have decreased during the same period. Additionally, the dual resistance to clarithromycin plus tetracycline and metronidazole plus tetracycline both increased significantly. These changes carry major clinical implications: It threatens the efficacy of bismuth-based quadruple therapy and limit the treatment options.
- Citation: Wu PJ, Tsay FW, Wu DC, Yang JC, Chuah SK, Chen KY, Chen CL, Lee CL, Shih CA, Liu YH, Shiu SI, Tai WC, Kuo CH, Lei WY, Kao SS, Tsai TJ, Feng IC, Koseki M, Hsu PI, Sheu MJ. Sequential changes of antibiotic resistances of Helicobacter pylori in Taiwan from 2019 to 2024. World J Gastroenterol 2025; 31(39): 111380
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/111380.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.111380
Helicobacter pylori (H. pylori) infection is associated with chronic gastritis, gastric ulcer, duodenal ulcer, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma[1,2]. The Real-world Practice and Expectation of Asia-Pacific Physicians and Patients in H. pylori Eradication Survey indicated that standard triple therapy remains the most commonly prescribed regimen in the Asia-Pacific region[3]. However, its efficacy has declined to below 80% in most countries[4].
Treatment failures, particularly with standard triple therapy, are attributed to factors such as antibiotic resistance, insufficient acid suppression, poor patient compliance[5] and rapid proton pump inhibitor (PPI) metabolism[6]. In response, alternative eradication regimens including bismuth quadruple therapy, non-bismuth quadruple therapies (e.g., sequential therapy, concomitant therapy and hybrid therapy), and high-dose dual therapy have been developed[4,7,8]. The Maastricht VI/Florence Consensus Report[9] recommends using bismuth quadruple therapy, concomitant therapy or hybrid therapy in regions where clarithromycin resistance exceeds 15%, and advocates for culture with antimicrobial susceptibility test for third-line therapies. For second line rescue therapy, levofloxacin-containing triple or quadruple therapy, preferably a 14-day course, is recommended in most consensus reports[9,10]. However, eradication rates for levofloxacin-containing therapies also decline markedly in the presence of levofloxacin-resistant strains.
Antibiotic resistance is one of the most essential factors that determines the eradication success. On the other hand, antibiotic resistance has increased in Taiwan and other countries over time. The clinical decision for optimal eradication regimens should be supported by referring to the relative efficacies stratified by antibiotic resistance and safety profiles. Therefore, continuously monitoring the regional resistance rates was essential. A previous Taiwanese study[11] demon
This multicenter retrospective study was conducted at Kaohsiung Chang Gung Memorial Hospital, Kaohsiung Medical University and Hospital, Kaohsiung Veterans General Hospital, An Nan Hospital, Buddhist Tzu Chi Hospital, National Taiwan University Hospital, Shin Kong Wu Ho-Su Memorial Hospital, Cathay General Hospital Renai Branch of Taipei City Hospital, Antai Tian-Sheng Memorial Hospital. The study protocol was approved by the Institutional Review Board and the Ethics Committee of An Nan Hospital. The requirement for informed consent was waived, and patient data were anonymized. No minors were included. None of our patients were minors.
We retrospectively analyzed H. pylori isolates from treatment-naïve patients (n = 1408) between January 1, 2019 and December 31, 2024. Demographic and clinical data, including age, sex, smoking status, and alcohol, coffee, and tea consumption, were obtained from each patient. Smoking was defined as ≥ 1 pack of cigarettes per week, and coffee or tea consumption as ≥ 1 cup per day. Exclusion criteria were prior H. pylori treatment, recent antibiotic, PPI, or bismuth use within 4 weeks, prior gastric surgery, severe comorbidities (e.g., decompensated cirrhosis, uremia), pregnancy, or age < 20 years.
One antral gastric biopsy specimen was obtained for cultured for H. pylori, and susceptibility to amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline was determined by Epsilometer test method (E-test) according to European Committee on Antimicrobial Susceptibility Testing guidelines[12]. All stock cultures were maintained at -80 °C in Brucella broth (Difco. Detroit, MI, United States) supplemented with 20% glycerol. Organisms were identified as H. pylori via Gram stain, colony morphology, and positive oxidase, catalase, and urease reactions. The antibiotic susceptibility is tested by E-test (AB Biodisck, Solna, Sweden). Minimum inhibitory concentration value of resistance breakpoints were > 0.5 μg/mL for amoxicillin, > 1 μg/mL for levofloxacin, > 4 μg/mL for tetracycline, > 8 μg/mL for metronidazole, and > 1 μg/mL for clarithromycin.
The primary outcome was antibiotic resistance rates for the five antibiotics by the years. Trends over time were assessed using the χ2 test for linear trends. Dual resistance for combinations excluding levofloxacin were also evaluated. Regional comparisons were made among northern, southern, and eastern Taiwan. Analyses were performed using SPSS v26 (Chicago, IL, United States). A P value < 0.05 was considered statistically significant.
The baseline characteristics of the study population are showed in Table 1. The mean age of the patients was 54.96 years. The proportions of male and female patients were 46.0% and 54.0%. With regard to endoscopic findings, showed 71.4% of patients presented with gastritis, 29.8% with gastric ulcer, and 17.3% with duodenal ulcer. The overall primary antibiotic resistance rates of H. pylori isolates in Taiwan were 1.3% for amoxicillin, 18.0% for clarithromycin, 31.0% for metroni
| Variables | Patient characteristics (n = 1408) |
| Age (years), IQR | 54.96 (46-65) |
| Gender | |
| Male | 648 (46.02) |
| Female | 760 (53.98) |
| Cigarette smoking | 233 (16.5) |
| Alcohol drinking | 198 (14.1) |
| Ingestion of coffee | 558 (39.6) |
| Ingestion of tea | 569 (40.4) |
| Endoscopic findings | |
| Gastritis | 1005 (71.4) |
| Gastric ulcer | 420 (29.8) |
| Duodenal ulcer | 244 (17.3) |
| Antibiotic resistance | 558 (39.6) |
| Amoxicillin resistance | 18 (1.3) |
| Clarithromycin resistance | 253 (18.0) |
| Metronidazole resistance | 437 (31.0) |
| Tetracycline resistance | 11 (0.8) |
| Levofloxacin resistance | 404 (28.7) |
Table 2 Lists the antibiotic resistances of H. pylori from 2019 to 2024 in Taiwan. Tetracycline resistance rates in 2019, 2020, 2021, 2022, 2023 and 2024 were 0%, 0%, 0.6%, 1.0%, 1.2%, 3.5%, respectively. A significant increasing trend was observed in tetracycline resistance from 2019 to 2024 among treatment naïve patients (Figure 1A shows P value in χ² test for linear trend: < 0.001).
| Year | Patient number | Proportion, % (95%CI) | P value (χ2 test for linear trend) | |
| Amoxicillin | 2019 | 0/256 | 0.0 (0.0-0.0) | 0.289 |
| 2020 | 7/330 | 2.1 (0.6-3.7) | ||
| 2021 | 4/159 | 2.5 (0.1-5.0) | ||
| 2022 | 1/299 | 0.3 (0.0-1.0) | ||
| 2023 | 1/249 | 0.4 (0.0-1.2) | ||
| 2024 | 5/115 | 4.3 (0.6-8.1) | ||
| Clarithromycin | 2019 | 37/256 | 14.5 (10.1-18.8) | 0.514 |
| 2020 | 65/330 | 19.7 (15.4-24.0) | ||
| 2021 | 33/159 | 20.8 (14.4-27.1) | ||
| 2022 | 56/299 | 18.7 (14.3-23.3) | ||
| 2023 | 34/249 | 13.7 (9.4-17.9) | ||
| 2024 | 28/115 | 24.3 (16.4-32.3) | ||
| Metronidazole | 2019 | 91/256 | 35.5 (29.6-41.4) | 0.000 |
| 2020 | 118/330 | 35.8 (30.5-41.0) | ||
| 2021 | 52/159 | 32.7 (25.3-40.1) | ||
| 2022 | 86/299 | 28.8 (23.6-33.9) | ||
| 2023 | 75/249 | 30.1 (24.4-35.9) | ||
| 2024 | 15/115 | 13.0 (6.8-19.3) | ||
| Tetracycline | 2019 | 0/256 | 0.0 (0.0-0.0) | 0.000 |
| 2020 | 0/330 | 0.0 (0.0-0.0) | ||
| 2021 | 1/159 | 0.6 (0.0-2.0) | ||
| 2022 | 3/299 | 1.0 (0.0-2.3) | ||
| 2023 | 3/249 | 1.2 (0.0-2.8) | ||
| 2024 | 4/115 | 3.5 (0.1-7.2) | ||
| Levofloxacin | 2019 | 81/256 | 31.6 (25.9-37.3) | 0.269 |
| 2020 | 91/330 | 27.6 (22.7-32.4) | ||
| 2021 | 57/159 | 35.8 (28.3-43.4) | ||
| 2022 | 76/299 | 25.4 (20.5-30.4) | ||
| 2023 | 64/249 | 25.7 (20.2-31.2) | ||
| 2024 | 35/115 | 30.4 (21.9-39.0) |
In contrast, metronidazole resistance rates in 2019, 2020, 2021, 2022, 2023 and 2024 were 35.5%, 35.8%, 32.7%, 28.8%, 30.1%, 13.0%, respectively. There was a statistically significant decreasing trend from 2019 to 2024 (Figure 1A shows P value in χ2 test for linear trend: < 0.001). No significant changes were observed in the resistance rates for amoxicillin, clarithromycin, or levofloxacin during the same period.
Table 3 displays the dual resistance of H. pylori from 2019 to 2024 in Taiwan. The dual resistance to clarithromycin plus tetracycline and metronidazole plus tetracycline both increased significantly from 0% to 1.7% during the study period (P value in χ2 test for linear trend: < 0.05). There were no significant changes in dual resistance for amoxicillin plus clarithromycin, amoxicillin plus metronidazole, or clarithromycin plus metronidazole (P value were 0.720, 0.543, 0.208 respectively). No cases of dual resistance to amoxicillin plus tetracycline were detected (Figure 1B shows P value in χ2 test for linear trend: < 0.001).
| Year | Patient number | Proportion, % (95%CI) | P value (χ2 test for linear trend) | |
| Amoxicillin + clarithromycin | 2019 | 0/256 | 0 (0.0-0.0) | 0.720 |
| 2020 | 2/330 | 0.6 (0.0-1.0) | ||
| 2021 | 0/159 | 0 (0.0-0.0) | ||
| 2022 | 1/299 | 0.3 (0.0-1.1) | ||
| 2023 | 0/249 | 0 (0.0-0.0) | ||
| 2024 | 1/115 | 0.9 (0.0-2.9) | ||
| Amoxicillin + metronidazole | 2019 | 0/256 | 0 (0.0-0.0) | 0.543 |
| 2020 | 5/330 | 1.5 (0.3-3.0) | ||
| 2021 | 2/159 | 1.3 (0.0-3.2) | ||
| 2022 | 1/299 | 0.3 (0.0-1.1) | ||
| 2023 | 0/249 | 0.0 (0.0-0.0) | ||
| 2024 | 1/115 | 0.9 (0.0-2.9) | ||
| Amoxicillin + tetracycline | 2019 | 0/256 | 0.0 (0.0-0.0) | 0 |
| 2020 | 0/330 | 0.0 (0.0-0.0) | ||
| 2021 | 0/159 | 0.0 (0.0-0.0) | ||
| 2022 | 0/299 | 0.0 (0.0-0.0) | ||
| 2023 | 0/249 | 0.0 (0.0-0.0) | ||
| 2024 | 0/115 | 0.0 (0.0-0.0) | ||
| Clarithromycin + metronidazole | 2019 | 20/256 | 7.8 (4.5-11.1) | 0.208 |
| 2020 | 23/330 | 7.0 (4.2-9.7) | ||
| 2021 | 10/159 | 6.3 (2.5-10.1) | ||
| 2022 | 21/299 | 7.0 (4.1-9.9) | ||
| 2023 | 14/249 | 5.6 (2.7-8.5) | ||
| 2024 | 5/115 | 4.3 (0.6-8.1) | ||
| Clarithromycin + tetracycline | 2019 | 0/256 | 0.0 (0.0-0.0) | 0.013 |
| 2020 | 0/330 | 0.0 (0.0-0.0) | ||
| 2021 | 0/159 | 0.0 (0.0-0.0) | ||
| 2022 | 2/299 | 0.7 (0.0-1.7) | ||
| 2023 | 1/249 | 0.4 (0.0-1.3) | ||
| 2024 | 2/115 | 1.7 (0.0-4.5) | ||
| Metronidazole + tetracycline | 2019 | 0/256 | 0.0 (0.0-0.0) | 0.007 |
| 2020 | 0/330 | 0.0 (0.0-0.0) | ||
| 2021 | 1/159 | 0.6 (0.0-2.1) | ||
| 2022 | 2/299 | 0.7 (0.0-1.7) | ||
| 2023 | 3/249 | 1.2 (0.0-2.8) | ||
| 2024 | 2/115 | 1.7 (0.0-4.5) |
Table 4 demonstrated antibiotic resistance rates of H. pylori according to different regions in Taiwan. The antibiotic resistance rates for levofloxacin in northern, southern and eastern Taiwan were 26.5%, 30.9%, 20.2%, respectively. The antibiotic resistance rate for levofloxacin in southern Taiwan was significantly higher than that in eastern and north Taiwan (P < 0.05). No significant regional differences in antibiotic resistance rates for amoxicillin, clarithromycin, metronidazole, tetracycline were observed among northern, southern, and eastern Taiwan.
| Region | Patient number | Proportion, % (95%CI) | P value (χ2 test for linear trend) | |
| Amoxicillin | South | 11/818 | 1.3 (0.6-2.2) | 0.606 |
| North | 5/501 | 1.0 (0.2-1.9) | ||
| East | 2/89 | 2.2 (0.0-5.8) | ||
| Clarithromycin | South | 144/818 | 17.6 (15.0-12.2) | 0.360 |
| North | 88/501 | 17.6 (14.2-20.9) | ||
| East | 21/89 | 23.6 (14.6-32.6) | ||
| Metronidazole | South | 248/818 | 30.3 (27.2-33.5) | 0.784 |
| North | 160/501 | 31.9 (27.8-36.0) | ||
| East | 29/89 | 32.6 (22.7-42.5) | ||
| Tetracycline | South | 8/818 | 1.0 (0.3-1.7) | 0.080 |
| North | 1/501 | 0.2 (0.0-0.6) | ||
| East | 2/89 | 2.2 (0.0-5.8) | ||
| Levofloxacin | South | 253/818 | 30.9 (27.8-34.1) | 0.044 |
| North | 133/501 | 26.5 (22.7-30.4) | ||
| East | 18/89 | 20.2 (11.7-28.7) |
Antibiotic resistance remains a major cause of H. pylori eradication failure and the observed resistance rates direct impact on treatment choices. In prior Taiwanese study (2013-2019) discussing the trend of changes in antibiotic resistance in H. pylori[11] reported rising primary resistance to clarithromycin, levofloxacin, and metronidazole. In contrast, our current surveillance of primary resistance rate in H. pylori from 2019 to 2024 found clarithromycin and levofloxacin resistance rates remained no significant changes, while metronidazole resistance decreased significantly.
In our large multicenter survey, we observed a notable emergence of tetracycline resistance (from 0% to 3.5%) in Taiwanese H. pylori isolates, in contrast to its historically negligible prevalence in Taiwan and globally (< 2%-5%)[11,13-15]. One possible explanation is that tetracycline is widely used in medicine, aquaculture, livestock industry in early Taiwan, and still residual tetracyclines been detected in aquaculture environments and animal food products[16]. On the other hand, the abrupt emergence in tetracycline resistance likely reflects genetic changes in H. pylori. Tetracycline binds the bacterial 30S ribosome at a 16S rRNA target[17]. In H. pylori, tetracycline resistance arises from mutations in the 16S rRNA gene that block tetracycline binding. Point mutations cause low-level tetracycline resistance, whereas high-level resistance requires multiple mutations in the 16S rRNA binding site[18,19]. For example, a triple-base substitution (AGA965-967TTC) in the 16S rRNA has been directly linked to high-level resistance[18]. In addition, expression of hefA gene attributing to upregulation of the efflux pump[18,20], which can lower intracellular tetracycline concentration and increased drug efflux. These suggest new tetracycline-resistant H. pylori in Taiwan have likely evolved through multiple ribosomal mutations (and/or accompanying efflux).
Tetracycline has been a cornerstone of bismuth-based quadruple therapy (PPI-bismuth-tetracycline-metronidazole) because of traditionally low resistance rates[13]. The sudden rise of tetracycline-resistant strains is therefore a significant clinical concern, as it could undermine the efficacy of the bismuth quadruple therapy, that is widely recommended as first-line therapy in regions with elevated clarithromycin resistance[21]. Notably, international guidelines[9] (e.g., Maastricht VI) now endorse optimized bismuth quadruple therapy as the preferred empiric regimen when clarithromycin resistance exceeds 15%. Our findings suggest that in Taiwan, the new incidence of tetracycline resistance may decrease the high success rates historically with quadruple regimens, potentially necessitating alternative or susceptibility-guided therapies. In particular, the emergence of dual-resistant strains containing tetracycline in combination with another drug (e.g., metronidazole or levofloxacin) is worrisome, as these dual resistances dramatically limit effective options. By contrast, some prior work showed that bismuth quadruple therapy performs well even in the face of dual clarithromycin/metronidazole resistance[13], but rising tetracycline resistance could challenge this advantage. Furthermore, the rising resistance to tetracycline in Taiwan could also compromise the effectiveness of vonoprazan-tetracycline dual therapy in patients with penicillin allergy. In summary, the new 3.5% resistance rate serves as a warning sign: It is far above Taiwan’s prior baseline (approximately 0%) and approaches the upper end of expected rates internationally, signaling that continued surveillance and alternative strategies may soon be needed.
In parallel, we observed a decline in metronidazole resistance over the study period. This is striking given that global and regional data document very high rates of metronidazole resistance (often 30%-60% or more)[13,14,22]. The downward trend in metronidazole resistance is encouraging, since metronidazole is a key component of bismuth quadruple therapy. Lower resistance to metronidazole may help preserve the efficacy of bismuth quadruple therapy, even as we face new challenges with tetracycline resistance. Overall, the rising resistance to tetracycline and the declining resistance to metronidazole observed in Taiwan may have implications for the effectiveness of bismuth quadruple therapy, although the clinical significance remains to be determined.
By contrast, clarithromycin resistance (13.7%-24.3%) remained relatively stable throughout 2019-2024 in our cohort, with just slightly risen from past Taiwanese baseline (approximately 15%). These findings differ from many global reports of steadily rising clarithromycin resistance; for example, recent Asian data report primary clarithromycin resistance around 30%[22], mainland China (approximately 50%), Japan (approximately 30%)[15] and Europe (approximately 23%)[13]. However, our results may reflect local practice patterns. Since clarithromycin-based triple therapy has been used sparingly in Taiwan in recent years, and this may have contained further increases in clarithromycin resistance. This result approaches the 15% threshold which clarithromycin-based triple therapy is no longer recommended as first-line therapy in Taiwan by Maastricht VI and other consensus guidelines[9]. Similarly, levofloxacin resistance, which is known to reduce the efficacy of quinolone-containing regimens[21], did not climb appreciably in our data. Importantly, both drugs remain moderately resistant at present, reinforcing that empiric use of clarithromycin or levofloxacin without prior sensitivity testing would not achieve reliable cure rates. Our findings support the replacement of conventional clarithromycin-based triple therapy with bismuth quadruple therapy or concomitant therapy for empiric first-line treatment in Taiwan.
The trend of dual resistance also warrants emphasis. We noted an increase in strains resistant to two antibiotics simultaneously, especially those combinations involving tetracycline (e.g., tetracycline + metronidazole or tetracycline + levofloxacin), that reflected the truth of increasing primary resistance of tetracycline. Dual resistance is clinically important because it corresponds to very low eradication rates with standard regimens. For example, dual clarithromycin/metronidazole resistance is known to reduce eradication success to around 50%[21], necessitating use of salvage therapies. To date, no first line eradication regimen including clarithromycin plus tetracycline. In contrast, although bismuth quadruple therapy can overcome single-agent resistance, the appearance of tetracycline-metronidazole dual resistance could compromise even this regimen. Although increasing metronidazole doses can overcome resistance, the rising dual resistance observed in our survey imply that if tetracycline-metronidazole dual resistance increased, even these quadruple regimens could encounter treatment failure. This also indicates that empiric therapy must be chosen carefully, and may increasingly require culture-based susceptibility guidance.
Furthermore, we found that no significant reginal differences in resistance rates except for levofloxacin, which only used in second line therapy of H. pylori eradication. This result likely reflects Taiwan’s centralized health system and antibiotic stewardship. Since the National Health Insurance provides universal coverage and follows national guidelines, prescribing behaviors are relatively consistent from north to south to east. These factors probably contribute to the lack of regional differences of resistance. In turn, this suggests that our data on antibiotic resistance are broadly representative of the country, without major local outliers. Levofloxacin resistance in H. pylori appears to be linked to community fluo
Our study has several limitations. Firstly, by virtue of its retrospective design, causality cannot be established. The isolates were obtained at medical centers and may not fully represent community clinics, causing potential selection bias. Second, we did not include molecular analyses, so we cannot specify the genetic basis of observed resistance or track clonal spread of resistant strains. Third, we are lacking data of clinical outcomes linking to antibiotic resistance, so we cannot measure how these resistance trends impacted eradication rate. Finally, although our consortium includes multiple hospitals, coverage may still be incomplete (for example, eastern regions were under-represented), potentially missing local pockets of resistance.
In summary, our 2019-2024 data reveal both stability and change in Taiwan’s H. pylori resistance pattern. Primary antibiotic resistance to tetracycline in H. pylori has increased in Taiwan from 2019 to 2024, while resistance to metroni
We would like to express our sincere gratitude to the statistician, Ho CH, who conducted the data analysis for this study. We thank him for his valuable insights and advice on the statistical methods used in this study.
| 1. | Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1935] [Article Influence: 80.6] [Reference Citation Analysis (3)] |
| 3. | Chuah YY, Wu DC, Chuah SK, Chen KY, Yang JC, Lee CL, Chen CL, Shiu SI, Shie CB, Shih CA, Tsay FW, Liu YH, Hsu PI; Taiwan Acid-related Disease & Microbiota Consortium (TARD-M). REAP-HP survey 2020: Comparing the real-world practice and expectation in Helicobacter pylori eradication of the Taiwanese gastroenterologists in 2015 and 2020. Helicobacter. 2022;27:e12931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S, Monno R, Stoppino V, Morini S, Panella C, Ierardi E. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Kita T, Sakaeda T, Aoyama N, Sakai T, Kawahara Y, Kasuga M, Okumura K. Optimal dose of omeprazole for CYP2C19 extensive metabolizers in anti-Helicobacter pylori therapy: pharmacokinetic considerations. Biol Pharm Bull. 2002;25:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F; Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Liou JM, Fang YJ, Chen CC, Bair MJ, Chang CY, Lee YC, Chen MJ, Chen CC, Tseng CH, Hsu YC, Lee JY, Yang TH, Luo JC, Chang CC, Chen CY, Chen PY, Shun CT, Hsu WF, Hu WH, Chen YN, Sheu BS, Lin JT, Wu JY, El-Omar EM, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2016;388:2355-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 848] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 10. | Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Liang CM, Tai WC, Hsu PI, Wu DC, Kuo CH, Tsay FW, Lee CL, Chen KY, Chuah SK. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019: a multicentre report from Taiwan. Therap Adv Gastroenterol. 2020;13:1756284820976990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | EUCAST. Archive of EUCAST tables and documents. [cited August 20, 2025]. Available from: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents. |
| 13. | Katelaris P, Hunt R, Bazzoli F, Cohen H, Fock KM, Gemilyan M, Malfertheiner P, Mégraud F, Piscoya A, Quach D, Vakil N, Vaz Coelho LG, LeMair A, Melberg J. Helicobacter pylori World Gastroenterology Organization Global Guideline. J Clin Gastroenterol. 2023;57:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 14. | Boyanova L, Hadzhiyski P, Gergova R, Markovska R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics (Basel). 2023;12:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 586] [Article Influence: 58.6] [Reference Citation Analysis (2)] |
| 16. | Lu TH, Chen CY, Wang WM, Liao CM. A Risk-Based Approach for Managing Aquaculture Used Oxytetracycline-Induced TetR in Surface Water Across Taiwan Regions. Front Pharmacol. 2021;12:803499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232-60 ; second page, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2824] [Cited by in RCA: 2731] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 18. | Dailidiene D, Bertoli MT, Miciuleviciene J, Mukhopadhyay AK, Dailide G, Pascasio MA, Kupcinskas L, Berg DE. Emergence of tetracycline resistance in Helicobacter pylori: multiple mutational changes in 16S ribosomal DNA and other genetic loci. Antimicrob Agents Chemother. 2002;46:3940-3946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Hasanuzzaman M, Bang CS, Gong EJ. Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications. J Korean Med Sci. 2024;39:e44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Liu ZQ, Zheng PY, Yang PC. Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J Gastroenterol. 2008;14:5217-5222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Chey WD, Howden CW, Moss SF, Morgan DR, Greer KB, Grover S, Shah SC. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2024;119:1730-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 22. | Hong TC, El-Omar EM, Kuo YT, Wu JY, Chen MJ, Chen CC, Fang YJ, Leow AHR, Lu H, Lin JT, Tu YK, Yamaoka Y, Wu MS, Liou JM; Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2024;9:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 23. | Dascălu RI, Bolocan A, Păduaru DN, Constantinescu A, Mitache MM, Stoica AD, Andronic O. Multidrug resistance in Helicobacter pylori infection. Front Microbiol. 2023;14:1128497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/