Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.111934
Revised: July 27, 2025
Accepted: August 19, 2025
Published online: September 21, 2025
Processing time: 67 Days and 1.4 Hours
Pediatric inflammatory bowel disease (IBD), encompassing Crohn’s disease, ulcerative colitis, and IBD-unclassified, has become increasingly prevalent worldwide, including in previously low-incidence regions. Children often present with more extensive and aggressive disease, creating unique diagnostic and management challenges that differ significantly from adult-onset IBD. This review aims to synthesize current knowledge on pediatric IBD, highlighting historical challenges while exploring emerging frontiers in diagnosis, treatment, and long-term care strategies. A narrative synthesis of global and regional epidemiological data, clinical classifications, diagnostic advancements, management approaches, and psychosocial considerations was conducted, with a particular emphasis on innovations in precision medicine, microbiome-targeted therapy, and multidisciplinary care models. Pediatric IBD continues to rise globally, driven by environmental and genetic interactions, especially in rapidly industrializing regions. Novel diagnostic tools, age-specific treatment protocols, biologics, nutritional strategies, and psychosocial support are reshaping care. Emphasis on very early-onset IBD, transition care, and regional policy adaptations underscores the evolving complexity of managing pediatric IBD. The landscape of pediatric IBD care is rapidly evolving. Addressing the distinct pathophysiology, developmental impact, and healthcare challenges of pediatric patients requires an integrated, child-centered approach. Ongoing research into genetics, immune pathways, and the microbiome will be essential in tailoring precision therapies and improving outcomes globally.
Core Tip: Pediatric inflammatory bowel disease (IBD) is increasing globally, with rising incidence in previously low-prevalence regions such as the Arabian Gulf. This review highlights the unique challenges in pediatric IBD diagnosis, including atypical presentations and very early-onset IBD, and emphasizes the need for age-specific treatment approaches. It also explores emerging frontiers such as personalized medicine, biologic therapies, and multidisciplinary care. By integrating recent data on epidemiology, pathogenesis, and psychosocial impact, this article presents a comprehensive framework for optimizing outcomes in children with IBD, guiding future research and policy priorities in this evolving field.
- Citation: Al-Beltagi M, Saeed NK, Mani PKC, Bediwy AS, Elbeltagi R. Inflammatory bowel disease in paediatrics: Navigating the old challenges and emerging frontiers. World J Gastroenterol 2025; 31(35): 111934
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/111934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.111934
Inflammatory bowel disease (IBD) refers to a group of chronic, relapsing, immune-mediated disorders primarily affecting the gastrointestinal tract, including Crohn’s disease (CD), ulcerative colitis (UC), and IBD-unclassified (IBD-U). While historically considered a condition of adulthood, IBD has gained increasing recognition in pediatric populations[1]. Currently, approximately 25% of IBD cases are diagnosed before the age of 20, with a noticeable rise in incidence among younger age groups. The rising global incidence, particularly in regions like the Arabian Gulf that were once considered low incidence, has brought pediatric IBD into sharper clinical focus. This shift has prompted heightened clinical and research attention toward the unique manifestations and challenges of pediatric IBD[2].
Historically, the understanding and management of IBD have been mainly based on adult populations. However, the appearance of IBD in children presents unique clinical and developmental challenges that differ significantly from adult-onset disease. Pediatric patients often show more widespread inflammation, faster disease progression, and complications such as growth delays, delayed puberty, and nutritional deficiencies[3]. Particularly, very early-onset IBD (VEO-IBD), diagnosed before age six, may be a distinct entity often linked to monogenic mutations, immune dysregulation, and treatment resistance. These pediatric-specific features require customized diagnostic algorithms, treatments, and long-term care plans[4]. Besides physiological issues, IBD during childhood and adolescence carries significant psychosocial burdens, impacting mental health, academic performance, social relationships, and family life. The transition from pediatric to adult care adds further complexity, requiring coordinated, multidisciplinary management[5].
This review aims to provide a comprehensive and current analysis of pediatric IBD, framed around two core di
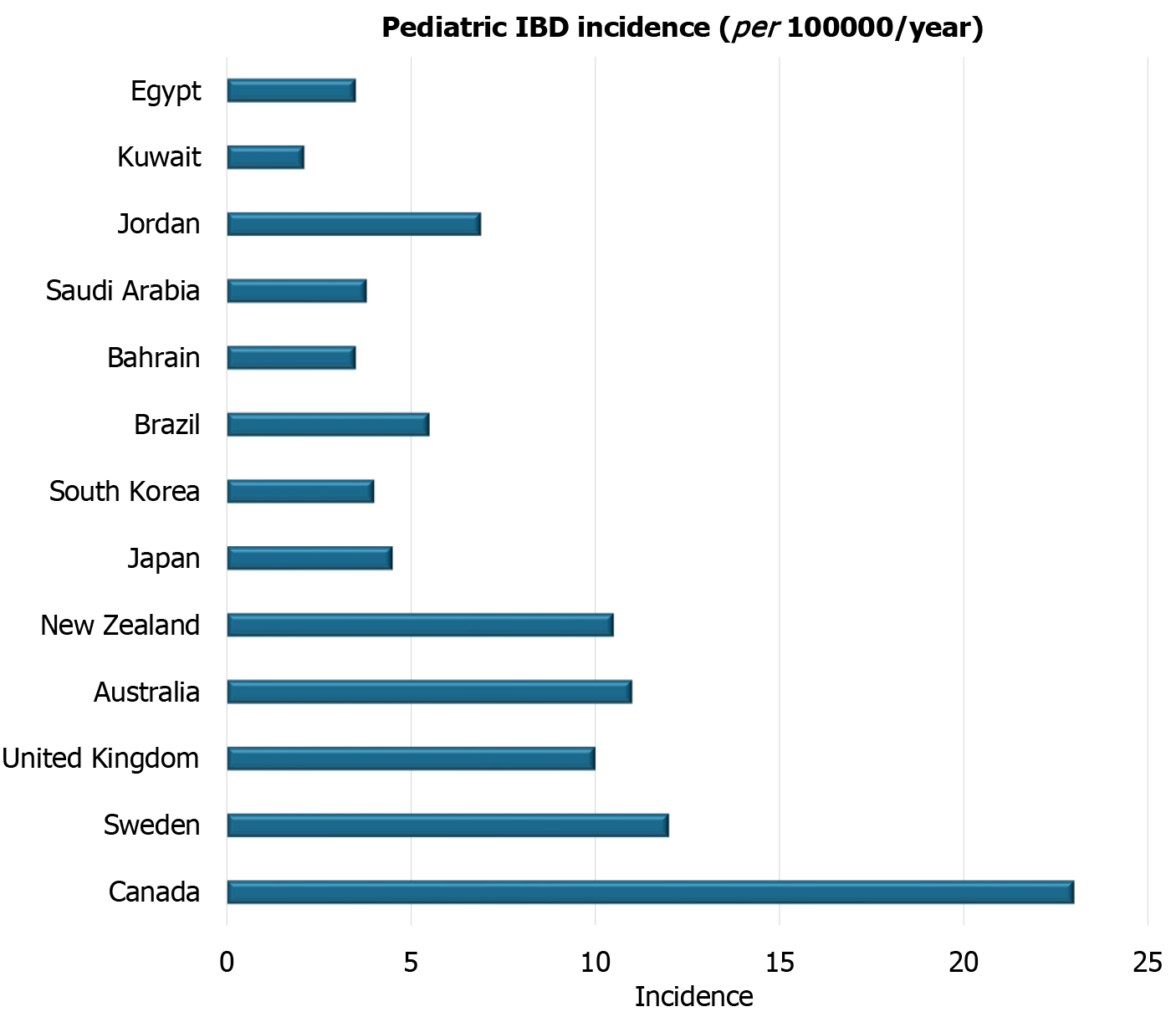
Over the past few decades, the incidence and prevalence of pediatric IBD have demonstrated a striking global increase, transforming IBD into a growing public health concern in both developed and developing nations[6]. Historically, the highest rates of pediatric IBD were reported in industrialized regions such as North America, Northern and Western Europe, and Oceania. In these regions, pediatric IBD has reached a plateau or modestly increased, yet the burden remains substantial. For instance, studies from Canada and Scandinavian countries report incidence rates exceeding 10 per 100000 children annually, with CD being more prevalent than UC[2].
On the other hand, the most significant epidemiological changes have occurred in developing and newly industrialized regions, including parts of Asia, the Middle East, Africa, and Latin America. These regions, once considered low-incidence areas, are now seeing a rapid increase in pediatric IBD cases[6]. This rise is often linked to urbanization, the westernization of diets, reduced microbial exposure during early life, and improvements in healthcare systems that lead to better diagnosis and reporting. Notably, countries like China, India, Saudi Arabia, and the United Arab Emirates have shown a steady upward trend in pediatric IBD diagnoses over the past twenty years. This trend spans various pediatric age groups, with some studies indicating that the highest rates occur among 10 to 16-year-old[7].
The disparity in disease emergence between developed and developing regions suggests a strong role for environmental modifiers, including hygiene, antibiotic use, dietary patterns, and lifestyle changes, in genetically susceptible individuals. Moreover, this rise in pediatric cases is often associated with more severe disease phenotypes and younger ages of onset, including VEO-IBD, placing additional strain on healthcare systems that may lack pediatric IBD-specific resources[8]. This global epidemiologic transition highlights the urgency for collaborative surveillance programs, region-specific research, and tailored public health strategies to address the rising burden of pediatric IBD in both high-income and resource-limited settings[9].
Over the past two decades, extensive research has unequivocally demonstrated a substantial global rise in pediatric IBD. This increase is particularly pronounced in high-income regions such as North America and Europe, which continue to report some of the highest incidence and prevalence rates worldwide.
In North America, Canada stands out with persistently high rates of pediatric IBD. For instance, the incidence of pediatric IBD in Canada often exceeds 15 per 100000 children per year, with specific provinces like Nova Scotia and Alberta reporting rates that can surpass 20 per 100000, positioning them among the highest globally. National registry data indicate a predominance of CD over UC among Canadian pediatric cases, and early-onset cases continue to increase annually by approximately 6%-7%[10]. Studies from Ontario, for example, show a significant increase in incidence, particularly in younger age groups (0-9 years old), highlighting an accelerated rise in this population[11]. In the United States, IBD continues to affect a substantial proportion of the adult and pediatric population, reaching 1 in 209 among adults and 1 in 1299 among children aged 2-17 in 2016. The prevalence of IBD has been increasing compared with previously published 2009 data[12].
In Europe, a comprehensive meta-analysis by Roberts et al[13] highlighted that the incidence rates of pediatric CD and UC are generally higher in Northern Europe compared to other regions of the continent. Over the past 50 years, significant increases in the incidence of both CD and UC have been observed across Europe, indicating a widespread rise. The most substantial increases for CD have been reported in Sweden, Wales, England, the Czech Republic, Denmark, and Hungary. At the same time, UC has seen its most significant increases in the Czech Republic, Ireland, Sweden, and Hungary. Incidence rates for pediatric CD have climbed to as high as 9 or 10 per 100000 population in parts of Europe, including Scandinavia, with pediatric UC rates typically remaining slightly lower than CD. Prevalence rates for CD have been reported to range from approximately 8.2 to 60 per 100000, and for UC, from approximately 8.3 to 30 per 100000[13].
Asia, traditionally considered a low-incidence region, is currently experiencing the most rapid relative increase in pediatric IBD, often mirroring the trends observed in Western countries decades ago. In Japan, recent studies indicate a pediatric IBD incidence ranging from 3.0 to 4.5 per 100000 per year, a substantial rise from less than 1.0 two decades ago. This surge is often linked to the adoption of Westernized diets[14]. Similarly, South Korea has observed a marked increase, with recent incidence estimates typically ranging from 2.5 to 4.0 per 100000, alongside a notable rise in pediatric CD prevalence. The incidence of pediatric IBD in Korea has steeply increased since 2000 and continues to rise[15]. In China, large urban centers report a growing number of pediatric IBD cases. While population-wide estimates remain somewhat limited due to uneven surveillance infrastructure, studies from urban regions have shown increasing prevalence and incidence, particularly in Eastern China[16,17].
In South America, the incidence and prevalence of IBD have been steadily increasing, although generally remaining lower than in North America and Europe. For instance, in Brazil, the incidence of CD has shown a significant rise, from 0.08 per 100000 person-years in 1988 to 5.5 per 100000 person-years in 2015[18]. Argentina has also reported increasing prevalence rates, with some studies indicating a pediatric IBD incidence below 0.4 per 100000 children under 18 years. Across the region, a shift towards more severe disease phenotypes and an increasing use of biological therapies are being observed, reflecting the evolving landscape of IBD[19]. UC often remains more prevalent than CD in many Latin American countries, although this ratio is changing in some areas[18].
Oceania, particularly Australia and New Zealand, reports some of the highest pediatric IBD rates globally, comparable to or even exceeding those in North America and Northern Europe. A meta-analysis of 19 studies has confirmed a dramatic rise in pediatric-onset IBD over the past two decades, with a steep increase in the incidence of CD[20]. New Zealand is also noted for having one of the highest rates of pediatric IBD, with estimates around 5.2 per 100000 children[21]. The region’s high rates are consistent with other highly industrialized Western nations, suggesting similar environmental influences at play.
In Africa, high-quality, population-based epidemiological data for pediatric IBD remain scarce. However, indirect evidence, such as increasing search interest for IBD-related terms on platforms like Google trends, suggests a growing awareness and potentially an emerging rise in IBD cases across the continent[22]. As many African nations undergo rapid socioeconomic development and urbanization, they may experience similar epidemiological shifts seen in newly industrialized Asian countries, highlighting an urgent need for robust surveillance and data collection efforts[23].
These regional differences underscore the complex interplay of various factors, including variations in genetic susceptibility, environmental exposures, and disparities in healthcare infrastructure, surveillance capabilities, and diagnostic practices. Nonetheless, the convergence of rising trends globally, particularly the acceleration in newly industrialized regions, strongly suggests a broader influence of changing environmental risk factors[24]. These factors include, but are not limited to, urbanization, adoption of Westernized diets, reduced microbial exposure, and increased antibiotic use, all of which transcend traditional geographic and socioeconomic boundaries. Continued multinational surveillance, harmonization of registry data, and focused research into the unique environmental and genetic factors within diverse populations remain essential for accurately tracking these evolving patterns and informing targeted public health interventions.
The Arabian Gulf and broader Middle East and North Africa (MENA) region have witnessed a remarkable surge in the incidence and recognition of pediatric IBD over the past two decades, posing a growing challenge to regional healthcare systems. Once considered rare, IBD particularly CD is now increasingly diagnosed among children and adolescents, a trend reflecting both a genuine rise in disease incidence and significant improvements in clinical awareness, diagnostic capabilities, and healthcare access[25].
Recent epidemiological reports from Gulf countries consistently demonstrate this growing trend, with several centers reporting an annual increase in new pediatric IBD diagnoses. This notable uptick directly parallels rapid socioeconomic development, widespread urbanization, and a discernible shift toward Westernized dietary habits and lifestyles environmental factors robustly linked to increased IBD risk globally[26]. Concurrently, enhanced training among pediatric gastroenterologists, wider availability of advanced endoscopy, and the nascent implementation of IBD registries have collectively contributed to improved detection rates, revealing a previously underrecognized burden[27].
Distinctive features have also been observed in the regional pediatric IBD phenotype. Studies from Saudi Arabia and Kuwait, for instance, consistently report a predominance of CD over UC, with many children presenting with moderate to severe disease activity, extensive ileocolonic involvement, and notable growth failure at diagnosis[28]. Moreover, VEO-IBD including genetically driven monogenic forms appears to be relatively more common in consanguineous populations within the region, further complicating diagnostic pathways and management prognoses due to their often severe and refractory nature[29].
Despite these advancements in recognition and diagnosis, the region continues to face significant challenges. These include ongoing delays in diagnosis, often caused by overlapping symptoms with more common infectious or nutritional disorders, a notable lack of dedicated multidisciplinary pediatric IBD centers, and frequently limited or unequal access to biologics and advanced therapies in some areas[30]. Additionally, the lack of comprehensive, population-based epidemiological data remains a significant limitation, as many current studies are hospital-based, which restricts their generalizability and overall understanding of the actual disease burden across the diverse populations of the MENA region[25].
While the lack of comprehensive national registries limits the ability to provide precise, generalizable population-based prevalence figures for every country in the MENA region, available studies from tertiary centers and systematic reviews offer valuable insights into the burden of pediatric IBD. These prevalence figures, typically expressed per 100000 pediatric individuals, provide a snapshot of the disease burden[31].
In Bahrain, the overall pediatric IBD prevalence is notably high at 25.64 per 100000, with CD making up 9.32 per 100000 and UC accounting for 16.3 per 100000[32,33]. Saudi Arabia reports a total pediatric IBD prevalence of approximately 6.86 per 100000, where CD is slightly more common, at 4.1 per 100000, compared to an estimated UC prevalence of 2.76 per 100000, with some variation across different regions in the kingdom[34]. In Kuwait, the total pediatric IBD prevalence is 2.16 per 100000, with CD estimated at 1.53 per 100000 and UC at 0.6 per 100000[35]. Oman has a comparatively lower total pediatric IBD prevalence of around 0.5 per 100000, with CD at 0.21 per 100000 and UC at 0.24 per 100000[28]. For Qatar, the estimated total pediatric IBD prevalence is approximately 5.5 per 100000, with CD around 3.0 and UC about 2.5 per 100000[26].
While specific pediatric prevalence rates for CD and UC in Jordan are not available, the overall incidence of IBD across all age groups in the country was notably high at 6.9 per 100000 in 2019, indicating a significant burden that affects the younger population[25,36]. In Egypt, the estimated total pediatric IBD prevalence is about 3.5 per 100000, with CD around 2.0 per 100000 and UC at 1.5 per 100000. This pattern differs from adults in Egypt, where UC is considerably more com
| Feature | Global | Arabian Gulf region |
| Overall trend | Rising incidence worldwide, particularly in newly industrialized and urbanizing regions | Significant and consistent increase in incidence and recognition, especially over the past two decades |
| Incidence change (1990-2019) | Increase 22.8% globally in children/adolescents (Global Burden of Disease data) | Increasing across Gulf countries (e.g., Saudi Arabia, Qatar, Bahrain) with documented annual case growth |
| Proportion diagnosed before age 20 | Approximately 25%-30% of total IBD cases | Similar or higher in some regional cohorts, with peak onset between ages 10-16 |
| VEO-IBD (age < 6) | Rising; accounts for approximately 15% of pediatric IBD in some registries; often severe or monogenic | Presumed to be rising; more common in consanguineous populations; monogenic IBD increasingly reported |
| High-incidence regions | Canada, Northern Europe (Sweden, United Kingdom), Oceania (NZ, Australia): ≥ 10-20/100000/year | Transitioning from low to moderate incidence; still lower than North America/Europe but rapidly increasing |
| Recent pediatric incidence (per 100000/year) | Canada: 15-20 +; Sweden: 10-12; Japan: 3-4.5; South Korea: 2.5-4.0; Brazil: Approximately 5.5 | Bahrain: CD 1.0, UC 2.5; Saudi Arabia: Regional estimates 3-4; Jordan (all ages): 6.9 |
| Prevalence (per 100000 pediatric population) | Canada: CD 50-60, UC 30; United States (2016): Approximately 77 total; Europe: CD 8.2-60, UC 8.3-30 | Bahrain: CD 9.3, UC 16.3; Saudi Arabia: CD 4.1, UC 2.76; Kuwait: CD 1.53, UC 0.6; Egypt: CD 2.0, UC 1.5 |
| Highest incidence | Canada: Up to 23 per 100000/year (Nova Scotia, Alberta); among the highest globally | Jordan: 6.9 per 100000/year (all ages); Bahrain: Pediatric incidence of approximately 3.5 per 100000/year |
| Genetic role | About 20% have a family history; monogenic IBD is common in VEO-IBD | Consanguinity increases familial clustering and monogenic IBD risk in early-onset cases |
| Environmental risk factors | Westernization, hygiene hypothesis, antibiotic exposure, sedentary lifestyle | Similar environmental changes linked to rising IBD (urbanization, western diets, reduced microbial exposure) |
| Common pediatric phenotype | CD more common than UC; aggressive disease in younger children (CD) | CD predominates; ileocolonic involvement, more extensive disease, frequent growth failure, severe onset in many cohorts |
| IBD-U | Approximately 10%-15% at initial diagnosis; requires further subclassification | Common at presentation due to overlapping features and limited access to advanced diagnostics |
| Data gaps | Better registry coverage in North America/Europe; underreported in parts of Asia, South America | Hospital-based data dominate; lack of national registries in many MENA states |
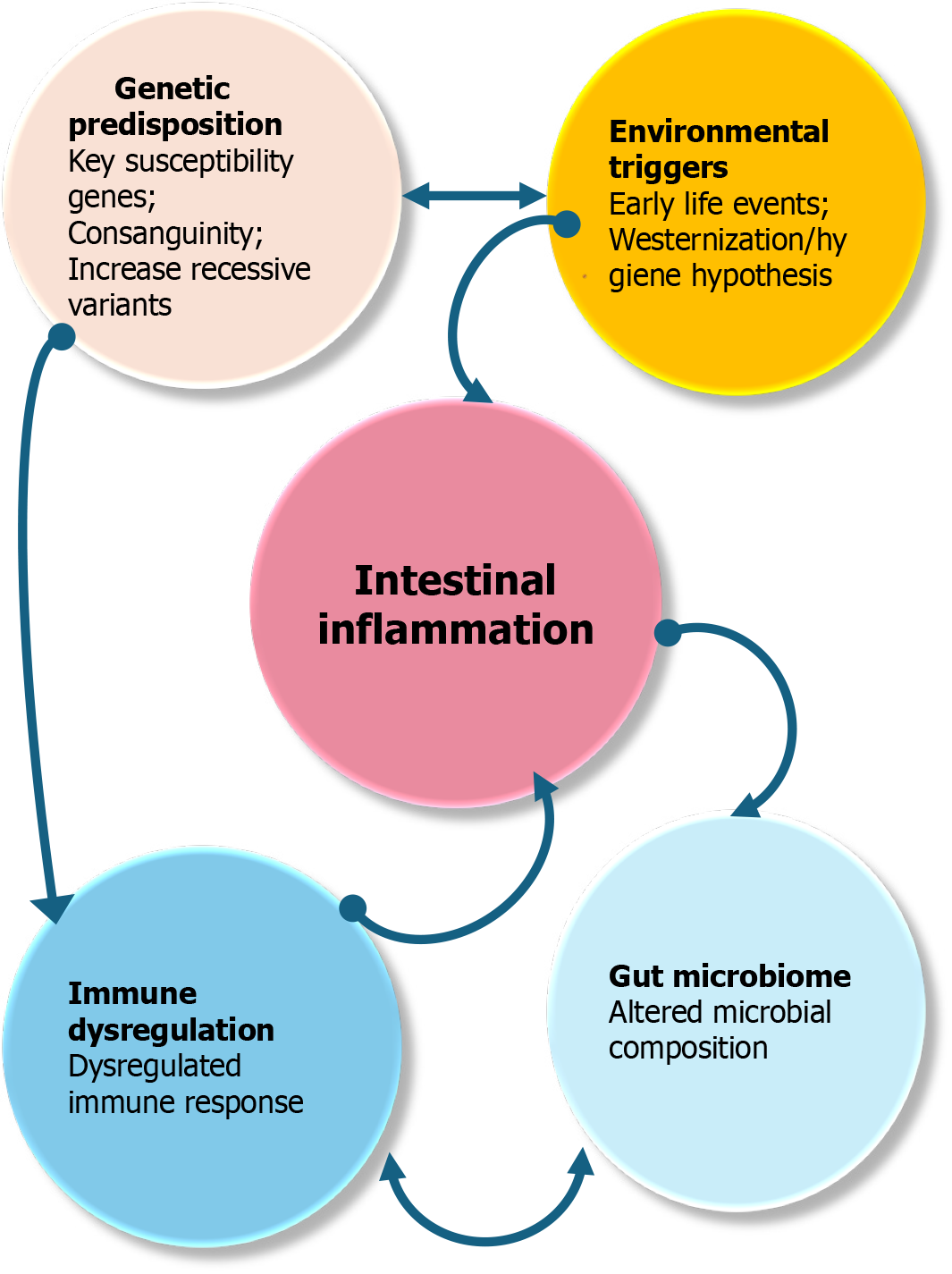
The rising incidence of pediatric IBD in regions such as the Arabian Gulf and MENA is increasingly attributed to the interplay of environmental and sociocultural factors, particularly westernization, urbanization, and consanguinity[39].
Westernization has dramatically reshaped dietary patterns, lifestyle habits, and exposure to environmental agents in many traditionally low-incidence countries. The transition from fiber-rich, traditional diets to processed, high-fat, and low-residue foods has been associated with alterations in gut microbiota, mucosal immunity, and intestinal barrier function factors implicated in the pathogenesis of IBD[40]. Moreover, reduced exposure to enteric infections, overuse of antibiotics, and increased cesarean deliveries have also contributed to the so-called “hygiene hypothesis”, whereby a lack of microbial diversity in early life may predispose genetically susceptible children to immune dysregulation and chronic intestinal inflammation[41]. Figure 2 shows the conceptual diagram of pediatric IBD pathogenesis.
Urbanization compounds these risks by introducing more sedentary behaviors, increasing psychosocial stress, reducing breastfeeding rates, and limiting exposure to natural environments all of which may negatively impact immune development and gut microbial composition. Rapid development in the Arabian Gulf has created urban ecosystems that mirror Western societies, which, in turn, has been paralleled by a marked uptick in autoimmune and allergic diseases, including pediatric IBD[6].
Consanguinity, a common cultural practice in many Middle Eastern populations, plays a unique role in shaping the pediatric IBD landscape, especially in early-onset cases. High rates of consanguineous marriages increase the chance of autosomal recessive inheritance patterns and monogenic forms of IBD, particularly in children diagnosed before age six VEO-IBD[42]. These monogenic variants often exhibit atypical features, a poor response to standard treatments, and a more severe disease course, necessitating genetic testing and targeted immunological interventions. Recognizing the impact of consanguinity in these populations is crucial for early diagnosis, personalized treatment, and genetic cou
Taken together, the convergence of Western lifestyle adoption, urban living conditions, and unique genetic backgrounds underscores the complex, multifactorial etiology of pediatric IBD in the Arabian Gulf and similar regions. Understanding these contextual factors is crucial for tailoring prevention strategies, public health policies, and clinical care to meet the evolving needs of affected children[44].
Accurate and comprehensive epidemiological data on pediatric IBD are crucial for understanding disease burden, identifying risk factors, and informing effective health policies. While significant strides have been made, data collection remains a complex endeavor with distinct challenges across different global settings.
In many developing countries, particularly within the MENA region, accurate and comprehensive epidemiological data on pediatric IBD remain limited. This scarcity poses significant obstacles to understanding the true disease burden, identifying local risk factors, and creating effective health policies tailored to specific populations[25].
One major challenge is the absence or infancy of national or regional IBD registries, which severely hampers efforts to track incidence, prevalence, and disease trends over time. Unlike developed countries, which often benefit from extensive databases and established surveillance systems that support long-term studies, many developing areas frequently rely on single-center or retrospective research. This limits the generalizability and scope of their findings[45].
Underdiagnosis and misdiagnosis are significant concerns. Limited access to specialized pediatric gastroenterologists, advanced endoscopic services, and modern diagnostic tools, such as fecal calprotectin, comprehensive genetic panels, and specialized histopathological expertise can delay or hinder accurate diagnosis. In some regions, children presenting with chronic diarrhea, weight loss, or anemia may initially be treated for common infectious diseases, malnutrition, or other endemic conditions, leading to substantial delays in recognizing and diagnosing IBD[46].
Variability and resource limitations in health infrastructure further exacerbate these challenges. Health systems in many developing countries are often overwhelmed and under-resourced, prioritizing acute infectious diseases over chronic, non-communicable illnesses like IBD. Inconsistent referral processes, fragmented care models, and poor integration of electronic medical records collectively diminish the quality and completeness of data collection[6].
Furthermore, sociopolitical instability and conflict in parts of the developing world, including areas of the MENA region, profoundly disrupt healthcare delivery and research efforts. War, mass displacement, and economic crises restrict access to medical care, drive rapid population movement, and interrupt long-term follow-up, thereby skewing epidemiological assessments and making consistent data collection exceedingly difficult[47]. Cultural stigma and awareness gaps can also delay diagnosis and reporting. In some communities, symptoms of gastrointestinal disease may be normalized or hidden due to embarrassment or a lack of knowledge, particularly among adolescents, leading to significant underreporting and distorted prevalence estimates[48].
While developed nations generally boast more robust healthcare infrastructures and established registry systems, they’re not immune to data collection challenges in pediatric IBD[49]. These challenges include inconsistencies in diagnostic criteria and medical coding practices, particularly for IBD-U, which can result in discrepancies in reported incidence and prevalence rates. Furthermore, despite the widespread adoption of electronic medical records, data fragmentation and interoperability issues often result in information being siloed across different healthcare providers, making it difficult to compile comprehensive, long-term patient data[50]. Patient mobility within and between these countries can also complicate long-term follow-up, potentially resulting in incomplete data on disease course and an underestimation of prevalence. Even well-established registries can suffer from selection bias if participation is voluntary or limited to specific tertiary centers, thus affecting the data’s representativeness[51]. Many studies rely on administrative health databases, which, while providing large datasets, often lack granular clinical details like disease activity or treatment response, potentially leading to an underestimation of disease severity or misclassification of IBD subtypes. Finally, the cost and sustainability of maintaining comprehensive, high-quality IBD registries pose significant challenges even for well-funded systems[52].
Addressing the complex challenges in pediatric IBD data collection worldwide requires collaborative efforts that recognize both common and region-specific obstacles. This involves establishing and strengthening pediatric IBD registries, especially promoting new ones in developing regions while improving the comprehensiveness and interoperability of existing registries globally[53]. It also includes enhancing diagnostic capacity by increasing access to pediatric gastroenterologists, advanced endoscopic services, and comprehensive diagnostic tools, particularly in underserved areas. At the same time, raising public and professional awareness is essential for educating both the public and heal
IBD in children is generally categorized into three main clinical types: CD, UC, and IBD-U. Each of these subtypes has distinct pathophysiological features, clinical signs, and disease courses, which can be further specified in the pediatric population due to age-related developmental differences. Table 2 shows the main differences between CD and UC. Additionally, specific pediatric subtypes may include IBD associated with primary sclerosing cholangitis and monogenic forms of IBD[54].
| Feature | CD | UC | IBD-U |
| Location | Any part of the GI tract (mouth to anus) | Limited to the colon and rectum | Colon only |
| Inflammation pattern | Discontinuous (skip lesions) | Continuous | Colonic, but with features unclear for CD/UC |
| Depth of involvement | Transmural (full thickness) | Mucosal and submucosal (superficial) | Overlapping or ambiguous features |
| Rectal involvement | Often spared (rectal sparing) | Always involved (proctitis) | Variable, can be involved |
| Microscopic features | Non-caseating granulomas (characteristic) | Crypt abscesses (common), no granulomas | Ambiguous; may have some transmural features but no granulomas |
| Fistulas/strictures | Common | Rare (unless long-standing, severe disease) | Rare, but can develop features over time |
| Perianal disease | Common | Rare | Rare |
| Cobble stoning | Characteristic endoscopic appearance (CD) | Absent | Absent (classic UC) |
| Surgical cure | Not curative (disease can recur) | Curative for GI manifestations | Variable, depends on evolving phenotype |
CD is a transmural, segmental, and often granulomatous inflammation that can affect any part of the gastrointestinal tract, from the mouth to the anus. In children, the terminal ileum and colon are the most frequently involved sites. CD is characterized by patchy areas of inflammation, often described as “skip lesions”, which may lead to strictures, fistulas, and abscesses over time. Pediatric-onset CD is notable for its more aggressive behavior compared to adult-onset forms, with a higher likelihood of extensive involvement, perianal disease, and extraintestinal manifestations such as growth retardation, pubertal delay, and nutritional deficiencies. Symptoms often include abdominal pain, chronic diarrhea, weight loss, fatigue, and perianal complications[55].
UC, by contrast, is a continuous, mucosal inflammation confined to the colon, usually starting at the rectum and extending proximally. In pediatric cases, extensive colitis or pancolitis occurs more frequently than in adults, while left-sided or proctitis-only involvement is relatively uncommon. Children with UC often present with bloody diarrhea, abdominal cramping, urgency, tenesmus, and systemic symptoms such as fever or weight loss in more severe cases. Although UC generally does not involve the full thickness of the bowel wall like CD does, severe cases can still cause significant complications, including toxic megacolon and a higher risk of colectomy[56].
IBD-U includes cases where clinical, endoscopic, histologic, and radiologic features do not definitively identify as either CD or UC, even after a thorough diagnostic evaluation. IBD-U is more frequently seen in children, partly because of incomplete disease progression or atypical presentations. This diagnosis may evolve into CD or UC over time as more clinical or histological features become apparent. Accurate and timely classification is vital because it greatly influences treatment choices and long-term management[57]. Overall, identifying the distinct phenotypic features of CD, UC, and IBD-U in children is crucial for accurate diagnosis, risk evaluation, and personalized treatment planning. The next section will explore the Montreal and Paris classification systems, which provide structured frameworks for categorizing IBD phenotypes in both adults and pediatric patients[58].
Pediatric-onset IBD often exhibits different clinical behavior, disease distribution, and progression compared to adult-onset disease, requiring a specialized approach to care (Table 3). These differences, due to variations in genetic predisposition, immune response, environmental exposures, and developmental physiology, significantly influence diagnostic methods, treatment options, and long-term outcomes[59].
| Feature | Pediatric-onset IBD | Adult-onset IBD |
| Disease extent | More extensive disease (e.g., pancolitis in UC, panenteric CD) | More localized (e.g., left-sided colitis in UC) |
| Severity at onset | Often more severe with rapid progression | Variable; may have a milder course at onset |
| Growth and development | Commonly affected (growth failure, delayed puberty, bone density loss) | Growth is not an issue |
| Perianal disease | More common in pediatric Crohn’s disease | Less frequent |
| Extraintestinal manifestations | More frequent and severe | Present, but generally less common |
| Disease behavior over time | More aggressive with higher risk of complications (stricturing, penetrating) | Slower progression in many cases |
| Response to therapy | Often good response, but long-term therapy and toxicity concerns | Shorter treatment duration; toxicity concerns more manageable |
| Psychosocial impact | High impact on quality of life, schooling, and emotional development | Significant, but generally with better coping mechanisms |
| Treatment adherence | More challenging, especially during adolescence | Usually, better self-management and adherence |
Children frequently present with more extensive and severe disease at diagnosis. For instance, pediatric CD commonly involves the entire gastrointestinal tract (panenteric disease, affecting both the small and large intestines). It has a higher incidence of perianal manifestations, while pediatric UC is more often characterized by pancolitis[60]. This aggressive disease phenotype necessitates a proactive diagnostic workup. It usually requires the earlier initiation of potent im
A critical distinguishing feature of pediatric IBD is its significant impact on growth and pubertal development, a complication rarely observed in adults. Chronic inflammation, malabsorption, and the use of corticosteroids can lead to linear growth failure, delayed puberty, and decreased bone mineral density[3]. The potential for growth impairment, even preceding overt gastrointestinal symptoms, serving as an early indicator of an underlying disease and underscores the need for vigilant growth monitoring in all children with suspected or diagnosed IBD and prioritizes steroid-sparing treatment strategies and intensive nutritional support [such as exclusive enteral nutrition (EEN)] to optimize linear growth[62].
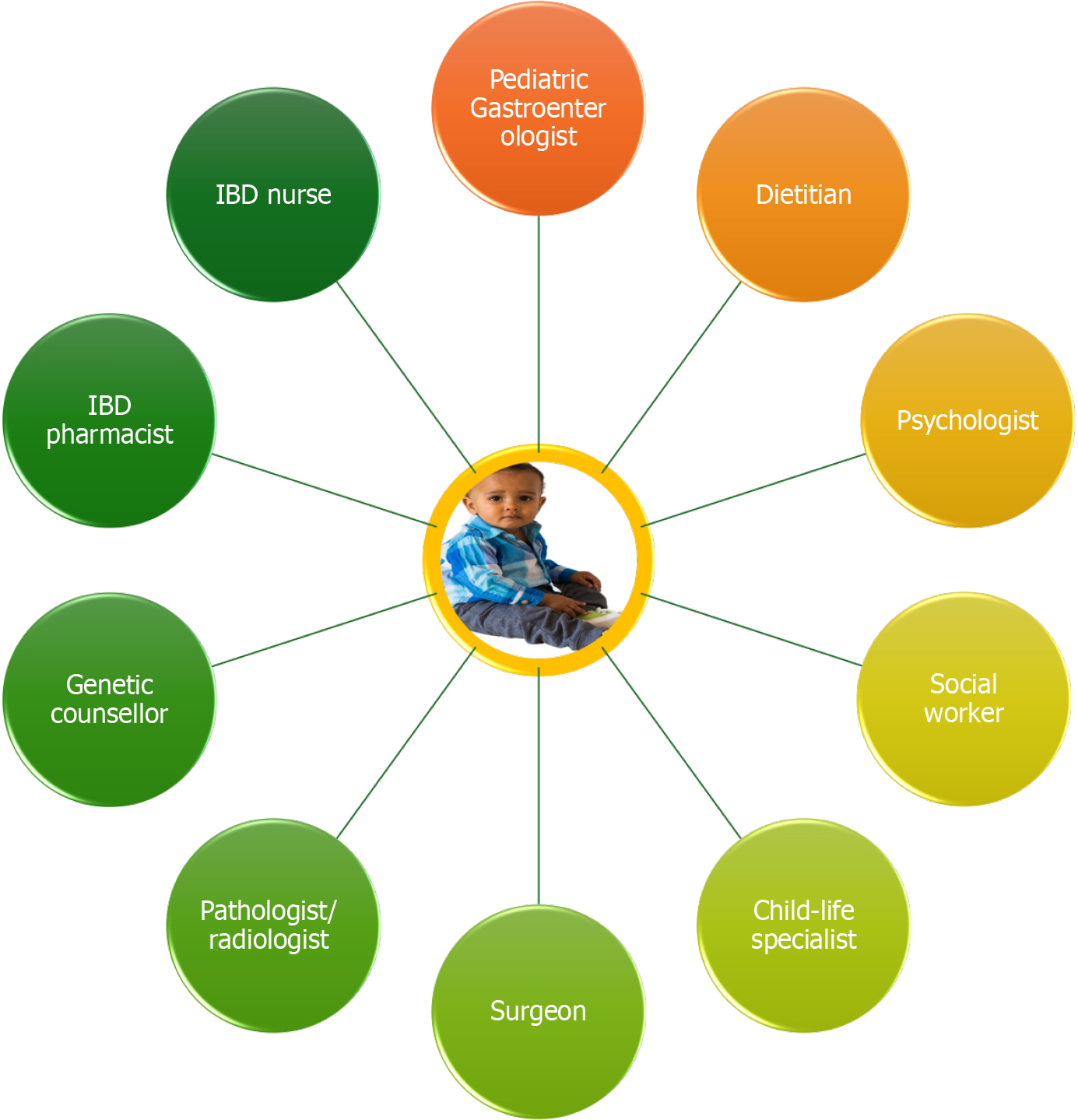
Furthermore, children with IBD are more susceptible to extraintestinal manifestations and tend to exhibit a more aggressive disease course over time, increasing the risk of developing structuring and penetrating complications, particularly in CD. The frequent occurrence of extraintestinal manifestations, such as arthritis, uveitis, erythema nodosum, and primary sclerosing cholangitis, necessitates a multidisciplinary care approach involving specialists beyond gastroenterology[63]. The heightened risk of complications underscores the importance of close disease monitoring and the strategic use of advanced therapies, such as immunomodulators or biologics, to alter the natural course of the disease[64].
Finally, IBD in children and adolescents occurs during crucial periods of physical, psychological, and social development. The burden of chronic illness, including frequent medical visits, invasive procedures, and social stigma, can significantly impact mental health, academic performance, and overall quality of life[65]. This unique developmental context necessitates tailored psychosocial support interventions and age-appropriate education to promote adherence to complex treatment regimens and facilitate a smoother transition into adult care. Recognizing these fundamental differences is paramount for optimizing both short-term disease control and long-term quality of life in pediatric IBD patients[66].
The Paris classification, introduced in 2011 as a pediatric adaptation of the Montreal system, is widely used to categorize IBD in children and adolescents. Recognizing the distinct clinical features and disease courses in pediatric patients compared to adults, this classification provides age-appropriate criteria for defining disease phenotype, which is crucial for accurate diagnosis, management, and prognosis[67]. One of its key modifications is the subdivision of age at diagnosis into two groups: Early childhood onset (0 to under 10 years) and adolescence (10 to under 17 years), reflecting the differing disease behavior and severity in these age groups[68].
For CD, the Paris classification refines the anatomical location into ileal, colonic, and ileocolonic, and further subdivides upper gastrointestinal involvement into proximal (above the ligament of Treitz) and distal segments, which can coexist with other locations. It also categorizes disease behavior as non-stricturing/non-penetrating, stricturing, penetrating, or a combination, and includes a perianal disease modifier to capture this crucial clinical feature[67]. For UC, the extent of disease is classified from proctitis to left-sided colitis, extensive colitis, and pancolitis, reflecting the spread of disease within the colon[67]. A unique addition to the Paris classification is the assessment of growth delay, a crucial consideration in pediatric IBD that is not included in the adult Montreal system. This growth parameter helps identify children at risk for poor physical development due to chronic inflammation or malnutrition[62].
The Paris classification is valuable in clinical practice because it standardizes the description of pediatric IBD phenotypes, guides treatment decisions by identifying more aggressive disease patterns, and aids in prognostication. It also facilitates research by providing a common framework to compare pediatric IBD cohorts[69]. Despite its strengths, the classification does not yet incorporate emerging molecular or genetic markers or patient-reported outcomes (PROs), which are increasingly recognized as necessary for personalized care. Nonetheless, the Paris classification remains a cornerstone in pediatric IBD management, highlighting the necessity for pediatric-specific tools in understanding and treating this complex disease[67].
Diagnosing IBD in children can be especially difficult due to atypical or subtle symptoms that differ significantly from adult-onset disease. Although classic symptoms like chronic diarrhea, abdominal pain, and rectal bleeding are seen in children, many pediatric patients particularly younger ones initially show extraintestinal or nonspecific signs that can delay diagnosis[70].
Growth failure is a key atypical feature in pediatric IBD and may appear months or even years before gastrointestinal symptoms. Chronic intestinal inflammation, combined with reduced caloric intake and increased metabolic demands, interferes with standard growth patterns. A decline in height velocity or failure to reach expected growth milestones should lead clinicians to consider IBD, especially after ruling out other common causes[71]. Delayed puberty is another important sign of pediatric IBD, often overlooked in early disease stages. Systemic inflammation affects the hypo
Additional atypical presentations include fatigue, iron deficiency anemia, arthralgia, and oral manifestations such as aphthous ulcers. In some children, these may be the only signs initially, leading to misdiagnosis or referral to multiple specialists before the underlying IBD is identified. The diagnostic challenge is even greater in very young children, especially those under 6 years old classified as VEO-IBD, as their symptoms can resemble infections, immunodeficiencies, or allergic colitis[4,72]. Recognizing these atypical signs is crucial for early diagnosis, reducing complications, and initiating prompt treatment to support growth, development, and overall quality of life in affected children.
Accurate diagnosis of pediatric IBD requires a comprehensive, multi-modal approach that combines clinical evaluation with advanced diagnostic techniques. Due to the variability of presentation in children and overlap with other gas
Endoscopy remains the essential tool for diagnosing IBD. Upper gastrointestinal endoscopy and ileocolonoscopy, with multiple biopsies from both inflamed and non-inflamed areas, enable direct visualization and histologic confirmation of mucosal inflammation. This is especially important for distinguishing between CD and UC, as well as identifying features of IBD-U[74]. Endoscopy also helps rule out infectious and other non-IBD causes, particularly in cases of very early onset. However, the requirement for general anesthesia and bowel preparation creates logistical and safety challenges in pediatric patients[75].
Imaging complements endoscopy by providing non-invasive evaluation of the small bowel and extraintestinal complications. Magnetic resonance enterography (MRE) is preferred due to its superior soft tissue contrast and lack of ionizing radiation, making it ideal for children who require repeated assessments. MRE is beneficial in detecting transmural inflammation, fistulas, strictures, and abscesses features more commonly associated with CD[76]. Ultrasound is increasingly utilized as a radiation-free, accessible tool for initial assessment or follow-up in the hands of experienced practitioners[77].
Biomarkers serve as valuable adjuncts in both the diagnostic and monitoring phases. C-reactive protein and ery
Histopathology provides definitive confirmation of chronic intestinal inflammation and is critical for distinguishing IBD from mimics such as infectious colitis, allergic colitis, or primary immunodeficiencies. Characteristic features, such as crypt architectural distortion, granulomas (indicative of CD), basal plasmacytosis, and goblet cell depletion, are key diagnostic clues. In very young children, histopathologic findings may be less specific, necessitating expert review by pediatric pathologists[79,80]. Together, these modalities form the diagnostic framework for pediatric IBD, with each contributing essential information to establish an accurate diagnosis, define the disease phenotype, and guide individualized treatment strategies.
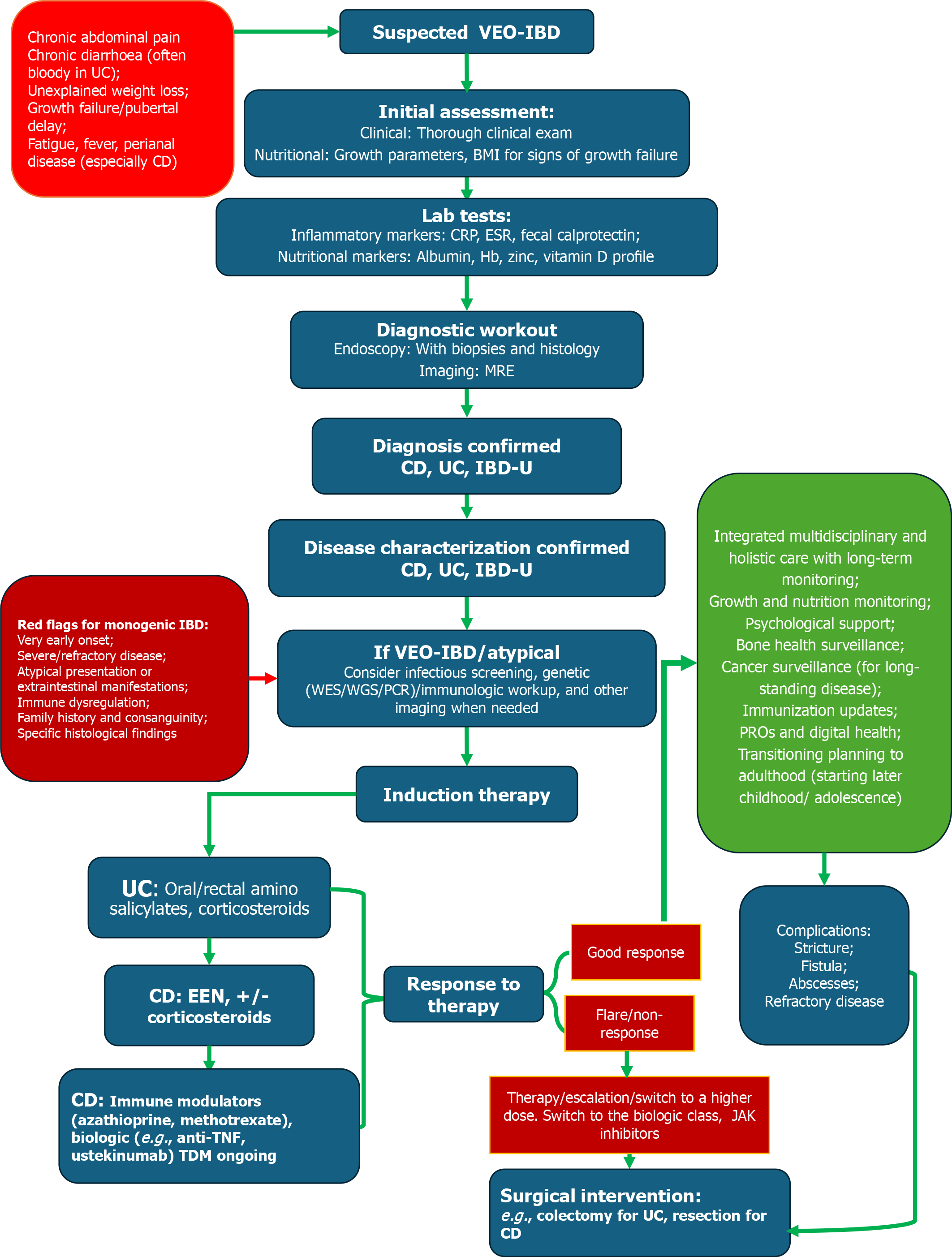
VEO-IBD, defined as IBD diagnosed before the age of 6 years, presents unique diagnostic and management challenges that differentiate it from later-onset pediatric IBD. A significant number of these cases are now recognized as monogenic in origin, caused by single-gene defects that impair immune regulation, epithelial barrier function, or microbial interactions in the gut. As a result, genetic testing and immunological evaluation have become crucial parts of the diagnostic process in this subgroup[4]. Unlike classic polygenic IBD, VEO-IBD often shows severe, treatment-resistant disease, perianal involvement, or unusual features such as recurrent infections, oral ulcers, or autoimmune phenomena. These red flags should lead clinicians to consider underlying inborn errors of immunity or primary immunodeficiencies that can mimic IBD[81]. More than 80 monogenic disorders, including mutations in IL10, IL10RA, XIAP, FOXP3, TTC7A, and CYBB, have been linked to VEO-IBD, each associated with specific immune dysregulation pathways[82].
Next-generation sequencing techniques, such as whole-exome sequencing or targeted gene panels, have significantly enhanced the ability to detect these mutations. Early genetic diagnosis has significant implications not only for prognosis and genetic counseling but also for treatment decisions[83]. Some monogenic forms, for example, may be curable with hematopoietic stem cell transplantation, a treatment not usually considered in conventional IBD[84]. Simultaneously, functional immunological assays (e.g., lymphocyte subsets, neutrophil oxidative burst, immunoglobulin levels, cytokine profiling) help evaluate immune competence and aid in diagnosing immune-related IBD mimics. In some cases, abnormal immune findings may support the use of targeted immunosuppressive or biologic therapies tailored to the specific immune defect[85]. Ultimately, adding genetic and immunologic screening to the diagnostic process for infants and very young children with suspected IBD not only facilitates accurate classification but also enables precision medicine approaches, thereby improving care for this especially vulnerable group[86].
Despite significant advancements in the understanding and diagnosis of pediatric IBD, several limitations persist in current diagnostic pathways that can delay timely and accurate diagnosis, especially in younger children. These limitations arise from both clinical complexities and systemic constraints[87]. One major challenge lies in the non-specific and often subtle early symptoms in children, such as intermittent abdominal pain, fatigue, poor weight gain, or anemia, which may be misattributed to more common pediatric conditions like functional gastrointestinal disorders, iron-deficiency anemia, or malnutrition. This diagnostic ambiguity often results in delayed referrals to pediatric gastroenterologists, postponing definitive evaluation[88].
Moreover, diagnostic tools validated in adults may not be fully applicable to children. For instance, disease activity indices like the CD activity index or Mayo score are limited in pediatric contexts. Although pediatric-specific tools, such as the pediatric CD activity index (PCDAI) and the pediatric UC activity index (PUCAI), exist, their use is not uniformly adopted across all clinical settings[89]. Access to specialized investigations including pediatric endoscopy, MRE, and genetic testing may be limited in resource-constrained regions. Sedation risks, equipment limitations, and a lack of pediatric-trained endoscopists can hinder timely endoscopic evaluation. Similarly, advanced genetic or immunological testing, which is necessary for diagnosing monogenic IBD (e.g., in VEO-IBD), is not routinely available in many centers, especially in developing countries[90].
Another gap is the lack of standardized diagnostic algorithms that integrate clinical, endoscopic, histologic, imaging, serologic, and genetic findings in a pediatric context. This leads to variation in diagnostic approaches between institutions and may contribute to inconsistencies in diagnosis, classification, and subsequent treatment[91].
Finally, insufficient awareness among general paediatricians and primary care providers regarding the early warning signs of pediatric IBD adds to diagnostic delays. Educational initiatives targeting frontline providers are needed to improve early recognition and prompt referral[92]. In sum, while diagnostic capabilities for paediatric IBD have advanced, they remain fragmented and unevenly applied, especially in cases of early-onset or atypical disease. Add
Pediatric IBD extends its burden beyond the gastrointestinal tract, exerting profound effects on a child’s physical growth, pubertal development, and psychological well-being. These impacts are often more severe than in adult-onset IBD due to the disease’s interference with key developmental milestones[93].
Growth failure is a hallmark feature of pediatric IBD, particularly in CD. Chronic intestinal inflammation leads to malabsorption, increased metabolic demands, anorexia, and cytokine-mediated growth suppression, all of which contribute to impaired linear growth[71]. Corticosteroid therapy, often used to manage disease flares, can further disrupt growth through suppression of the hypothalamic-pituitary-adrenal axis. Notably, growth impairment may be the first sign of disease, occurring even in the absence of overt gastrointestinal symptoms. Up to 85% of children with CD may experience growth deceleration, with some failing to reach their genetic potential if diagnosis and treatment are delayed[62].
Children with IBD frequently experience delayed puberty, which can result from both chronic inflammation and nutritional deficiencies, including low levels of zinc, vitamin D, and other essential micronutrients. Hormonal imbalances, especially in those with prolonged or poorly controlled disease, may contribute to hypogonadotropic hypogonadism[3]. Additionally, reduced bone mineral density is a recognized complication in pediatric IBD, stemming from chronic inflammation, malnutrition, limited physical activity, and prolonged corticosteroid use. This places children at increased risk of fractures and long-term skeletal complications[94].
Malnutrition is a common and complex issue in pediatric IBD, often resulting from decreased oral intake, increased nutrient loss, and increased energy requirements. Children may present with deficiencies in iron, vitamin B12, folate, vitamin D, calcium, and zinc. These deficiencies not only contribute to anemia and growth failure but also impair immune function and wound healing, further complicating disease management[95].
The chronic, unpredictable nature of IBD can significantly affect a child’s emotional and psychological development. Children with IBD report higher rates of anxiety, depression, social isolation, and body image concerns. Frequent hospital visits, school absences, dietary restrictions, and stigma associated with symptoms such as diarrhea or fecal incontinence can hinder academic success and social interactions[65]. Adolescents, in particular, may struggle with treatment adherence due to a desire for autonomy or denial of illness. Moreover, the transition from pediatric to adult care can be a source of emotional distress if not properly coordinated[96].
Overall health-related quality of life is often diminished in children with IBD. Even during periods of remission, fears of relapse, medication side effects, and long-term disease implications can create psychological strain. Instruments such as the impact questionnaire (explicitly developed for pediatric IBD patients) have shown that quality of life is closely tied to disease activity, nutritional status, and social support[66].
The management of pediatric IBD requires a personalized, step-up or top-down approach aimed at inducing remission, sustaining mucosal healing, preventing complications, and promoting normal growth and psychosocial development. The treatment modalities include aminosalicylates, corticosteroids, immunomodulators, biologic therapies, and small-molecule agents, each with distinct mechanisms, indications, and limitations[97].
Aminosalicylates: Aminosalicylates, including mesalamine and sulfasalazine, are locally acting anti-inflammatory agents used predominantly as first-line therapy for mild to moderate UC. They exert their effects on the intestinal mucosa and are generally ineffective in CD. Owing to their favorable safety profile, aminosalicylates are widely employed for long-term maintenance therapy in UC, achieving clinical remission rates of approximately 40%-60% in mild cases. Mesalamine is typically administered at 40-60 mg/kg/day in 2-3 divided doses (maximum 4.8 g/day). Despite their safety, the effectiveness of aminosalicylates diminishes in moderate to severe disease, and adherence issues, including frequent dosing, pill burden, and side effects such as headache, abdominal pain, and rare nephrotoxicity, may hinder their use in children[98,99].
Corticosteroids: Corticosteroids, such as prednisone, prednisolone, and budesonide, are potent medications primarily used to induce remission in moderate to severe UC or CD. For pediatric patients, prednisone is typically dosed at 1-2 mg/kg/day (with a maximum of 40-60 mg/day), and this is usually tapered over 8-10 weeks. Prednisone and prednisolone are highly effective for inducing remission during moderate to severe flares, with an efficacy rate of approximately 60%-80%. However, their use is strictly limited to induction therapy only. It is not recommended for long-term maintenance due to their significant systemic toxicity and lack of efficacy for mucosal healing. Their well-known adverse effects in children include growth retardation, mood disorders, osteoporosis, and adrenal suppression. To minimize these risks, strategies focus on the early introduction of steroid-sparing agents, like immunomodulators or biologics, to reduce the duration and dosage of corticosteroid use[100,101].
Budesonide is a glucocorticoid steroid that acts locally in the intestinal mucosa, offering a low risk of systemic side effects (approximately 10% systemic absorption) due to its high first-pass metabolism. It’s effective for inducing remission in mild to moderate CD in the ileum and/or ascending colon, typically starting at 9 mg/day and tapering down. While a Cochrane review found it superior to placebo and mesalamine for remission induction in CD after 8 weeks (with 42%-55% efficacy in pediatric trials), a 6 mg/day dose is ineffective for relapse prevention, only for short-term maintenance. Importantly, budesonide hasn’t shown superiority over prednisone, which may even be more effective in cases where budesonide fails. Therefore, budesonide is best reserved for specific CD cases where its localized action is beneficial[102].
Immunomodulators: Thiopurines, such as azathioprine (1.5-2.5 mg/kg/day), 6-mercaptopurine (1.0-1.5 mg/kg/day), and methotrexate (15 mg/m²/week, administered subcutaneously), are used to maintain remission, especially in patients who are steroid-dependent or steroid-refractory. They maintain remission after steroid induction in 50%-70% of cases and can be used for years; reassessed based on disease course and response. These agents work by modulating the immune response but require close monitoring due to potential adverse effects, including hepatotoxicity, myelosuppression, pancreatitis, and increased risk of infections and malignancy[103]. Pharmacogenetic testing (e.g., thiopurine methyltransferase activity) is recommended before initiating thiopurine therapy to guide dosing and minimize the risk of toxicity[104].
Biologics: Biologic therapies have revolutionized the treatment of moderate to severe pediatric IBD, particularly in CD and UC, as well as in fistulizing and steroid-refractory disease. These agents work by targeting specific immunological pathways and have been shown to improve rates of clinical remission, promote mucosal healing, and support normal growth and development in affected children[30]. The main classes of biologics used in pediatric IBD include anti-tumor necrosis factor (TNF) agents such as infliximab (IFX) and adalimumab, anti-integrin agents like vedolizumab (VDZ), and anti- interleukin-12/23 agents such as ustekinumab (UST). IFX is typically administered at a dose of 5-10 mg/kg in
Despite their effectiveness, biologics are associated with several limitations. These include the risk of infusion or injection-site reactions, severe infections such as tuberculosis or opportunistic pathogens, immunogenicity leading to the formation of anti-drug antibodies, and the psychological burden of regular injections or infusions[108]. Additionally, the high cost of biologic agents may restrict access in certain healthcare systems. Rare but severe complications, including lymphoma and hepatosplenic T-cell lymphoma particularly when biologics are combined with thiopurines have also been reported. Management of secondary loss of response often involves therapeutic drug monitoring to assess serum drug levels and detect anti-drug antibodies[109]. Based on therapeutic drug monitoring findings, clinicians may optimize therapy through dose escalation, shortening dosing intervals, or switching therapies either within the same class (e.g., IFX to adalimumab) or to a different class (e.g., to VDZ or UST). While biologics have transformed outcomes for many children with IBD, their use requires ongoing monitoring and individualized care to balance efficacy, safety, and long-term quality of life[110].
Small-molecule agents (JAK inhibitors): Small-molecule agents, particularly JAK inhibitors such as tofacitinib (JAK1/3 inhibitor) and upadacitinib (JAK1-selective), represent a novel class of oral immunomodulatory drugs that inhibit key pathways involved in cytokine signaling[111]. These agents are currently approved for the treatment of moderate to severe UC in adults, with growing interest in their potential use in pediatric populations. Although pediatric trials are ongoing, their use in children remains off-label and is largely limited to clinical research settings[112]. One of the key advantages of JAK inhibitors is their oral administration and rapid onset of action, which may improve adherence compared to injectable biologics. However, safety concerns have tempered their widespread adoption, especially in the pediatric context[113]. Reported risks include serious infections such as herpes zoster, elevated lipid levels, and a potential increased risk of thromboembolic events. As such, these agents are currently reserved for select adolescent patients with refractory UC or are used within the framework of controlled clinical trials. Ongoing research is needed to establish their long-term safety and efficacy in children with IBD[97].
Nutritional therapy and growth monitoring: Nutrition plays a pivotal role in the management of pediatric IBD, not only as a therapeutic intervention but also as a cornerstone for monitoring overall health, growth, and development. Unlike in adults, where pharmacologic management dominates, nutritional therapy in children serves dual purposes: Inducing remission and supporting the unique physiological demands of growth[114].
EEN is a well-established, first-line therapy for inducing remission in pediatric CD, particularly in Europe. It involves the exclusive use of a nutritionally complete liquid formula for 6-8 weeks, with the exclusion of normal food[115]. EEN has demonstrated remission rates comparable to corticosteroids but with added benefits such as mucosal healing, improved nutritional status, and fewer side effects. Despite its efficacy, adherence can be challenging due to palatability and psychosocial factors, and its role in UC remains limited[116]. While EEN is primarily used for induction, partial enteral nutrition (PEN), where the formula is combined with regular food, has been explored for maintenance of remission. Though evidence is still evolving, some studies suggest that PEN may help prolong remission and reduce relapse rates, especially when combined with other therapies[117].
Children with IBD are at risk of various micronutrient deficiencies, including iron, vitamin D, vitamin B12, folate, and zinc, due to poor intake, malabsorption, or chronic inflammation. Routine monitoring and timely supplementation are essential, particularly as deficiencies can contribute to fatigue, poor bone health, and anemia, exacerbating the disease burden[118]. Growth impairment and delayed puberty are significant concerns in pediatric IBD, often signaling subclinical disease activity or undertreated inflammation. Height velocity, weight gain, and pubertal staging should be regularly assessed using standardized growth charts. Growth failure can precede clinical relapse, highlighting the need for vigilant monitoring and early therapeutic escalation when growth falters[3]. Growth hormone resistance due to chronic inflammation is another mechanism that complicates linear growth recovery. Beyond EEN, several dietary strategies, such as the CD exclusion diet (CDED) and specific carbohydrate diet (SCD), are being studied for their potential in modulating inflammation and altering the gut microbiome[62]. While promising, most of these approaches lack robust evidence in children and should be applied under the guidance of a dietitian experienced in pediatric IBD.
Pediatric IBD can lead to a variety of complications that significantly affect a child’s long-term health, growth, and quality of life, often following a more aggressive course than adult-onset disease[119]. In CD, intestinal complications such as strictures (narrowing) causing obstructive symptoms, fistulas (abnormal connections), and abscesses, particularly those in the perianal area, are common outcomes of transmural inflammation. Management of strictures involves distinguishing between inflammatory (treated medically with anti-TNF agents or corticosteroids) and fibrotic (requiring endoscopic dilation or surgery) types. In contrast, fistulas and abscesses often require combined medical (e.g., biologics) and surgical interventions (e.g., seton placement)[120]. For UC, chronic colonic inflammation significantly increases the long-term risk of colorectal dysplasia and cancer, especially in cases of pancolitis or early-onset, long-standing disease. This necessitates regular surveillance colonoscopies with chromoendoscopy and targeted biopsies, typically starting 8-10 years post-diagnosis, with intervals adjusted based on individual risk factors like family history or the presence of primary sclerosing cholangitis, a hepatobiliary comorbidity more common with UC that itself elevates colorectal cancer risk and requires annual colonoscopic surveillance from diagnosis[46]. Beyond the gut, other systemic complications include growth failure and pubertal delay, more pronounced in CD due to nutrient malabsorption, making early, effective therapy (like EEN or biologics) critical for restoring growth[62]. Osteopenia/osteoporosis and delayed psychosocial development are also concerns, especially with poorly controlled disease. Proactive management involves a multidisciplinary approach, incorporating regular imaging and endoscopic evaluation, nutritional assessment, psychosocial support, timely escalation of therapy, and early surgical consultation for refractory complications[121]. This proactive and comprehensive care is vital for optimizing long-term outcomes for children with IBD.
Corticosteroids have long been a cornerstone in the induction of remission for moderate-to-severe pediatric IBD, particularly in both CD and UC. However, their use is fraught with well-recognized challenges that are especially concerning in the pediatric population[46]. A significant subset of children with IBD becomes steroid-dependent defined as the inability to taper steroids without disease relapse or steroid-refractory, where there is no significant clinical response to ap
The adverse effects of long-term steroid therapy are particularly pronounced in children. These include impaired linear growth, delayed puberty, reduced bone mineral density, hypertension, hyperglycemia, cushingoid appearance, mood disturbances, and increased infection risk. Even short-term use can significantly suppress the hypothalamic-pituitary-adrenal axis, with implications for stress response and recovery[123]. Steroid-related cosmetic changes such as weight gain, facial rounding, and acne can have a profound psychosocial impact, particularly during adolescence, a stage of heightened self-image and social sensitivity. This can contribute to poor adherence, emotional distress, and decreased quality of life. Furthermore, the unpredictable nature of steroid response and frequent relapses may cause frustration for both patients and caregivers, increasing the risk of nonadherence or medication fatigue[124].
Given these risks, clinical guidelines now emphasize minimizing steroid exposure. The use of EEN for induction in CD, early initiation of immunomodulators (e.g., azathioprine, methotrexate), and biologics (e.g., IFX, adalimumab) is preferred in children with moderate-to-severe disease or those who exhibit poor response to initial steroid therapy[125]. Addi
Surgical intervention remains a vital component in the multidisciplinary management of pediatric IBD, particularly for patients with medically refractory disease, growth failure, or complications such as strictures, fistulas, and toxic megacolon. While surgery does not cure IBD, especially CD, it can significantly improve quality of life and disease control when medical therapies fall short[127].
In pediatric CD, surgery is typically indicated for stricturing or penetrating complications (e.g., abscesses, fistulas), localized disease resistant to medical therapy, or growth failure due to persistent inflammation[128]. In UC, colectomy may be considered in cases of fulminant colitis, steroid-dependence, dysplasia or cancer risk, or failure to achieve remission despite optimal medical therapy, including biologics. In UC, total proctocolectomy with ileal pouch-anal anastomosis remains the surgical standard for refractory cases[129]. For CD, segmental resections, strictureplasties (widening of narrowed sections), and drainage of abscesses are commonly performed depending on the disease location and complications[130]. Minimally invasive techniques, such as laparoscopic or robotic-assisted surgery, are increasingly used in children due to their advantages in postoperative recovery and cosmesis[131].
The timing of surgery is essential and often involves shared decision-making among pediatric gastroenterologists, surgeons, and families. Delaying surgery in hopes of achieving further pharmacologic response can worsen nutritional status and post-surgical outcomes[132]. Conversely, performing surgery too early without fully exploring medical options may result in unnecessary complications. Post-surgical recurrence is a key concern, especially in CD, where inflammation frequently recurs at the anastomotic site[133]. Postoperative prophylaxis with immunosuppressive therapy or biologics might be recommended to lower recurrence risk. Long-term follow-up is vital to monitor nutritional health, growth, and disease recurrence[134]. Surgery can also have a significant psychological impact, particularly in adolescents who may be dealing with issues related to body image, independence, and peer relationships. The presence of stomas or surgical scars can affect self-esteem. Therefore, preoperative counseling and postoperative psychosocial support are essential parts of care[66].
The management of pediatric IBD requires age-specific treatment protocols that address the unique physiological, developmental, and psychosocial needs of children. Disease presentation often varies by age, with younger patients especially those with VEO-IBD more likely to display atypical or monogenic forms of the disease, necessitating distinct diagnostic and therapeutic strategies[66]. Moreover, pharmacokinetics and drug safety profiles differ across pediatric age groups. For example, corticosteroids may hinder growth and bone development in young children. At the same time, immunosuppressive therapies carry increased risks due to an immature immune system, underscoring the importance of tailored dosing and careful monitoring[120].
Nutritional status and growth are also critical concerns in pediatric IBD. Treatment plans must support linear growth and pubertal development, with therapies like EEN often playing a primary role in inducing remission, particularly in CD[135]. Unlike adults, children may benefit from therapies that combine nutritional rehabilitation with anti-inflammatory effects. Moreover, psychosocial factors such as treatment adherence, school participation, and coping with chronic illness require age-appropriate interventions and family-centered care[136]. Adolescents, in particular, face challenges related to autonomy, self-management, and transitioning to adult care services, necessitating structured transition protocols. Ultimately, the development and implementation of age-specific treatment guidelines are essential for optimizing long-term outcomes in pediatric IBD[137]. These protocols should integrate a multidisciplinary approach that includes pediatric gastroenterologists, nutritionists, psychologists, and social workers, ensuring that treatment not only addresses inflammation but also supports the child’s overall development and quality of life. Figure 3 illustrates a proposed VEO-IBD diagnostic and management pathway.
The evolution of pediatric IBD care is increasingly shaped by the principles of personalized medicine, which aims to tailor treatment strategies based on individual patient characteristics, including genetic, molecular, microbial, and clinical profiles. This approach is particularly relevant in children, where heterogeneity in disease behavior, therapeutic response, and long-term outcomes is more pronounced than in adults[138]. Biologic therapies have transformed the treatment landscape for moderate to severe IBD, with TNF-α inhibitors such as IFX and adalimumab becoming mainstays in pediatric care. More recently, newer biologics targeting alternative inflammatory pathways, such as VDZ (anti-integrin) and UST (anti-interleukin-12/23) have shown promise in refractory pediatric cases or those with intolerance to anti-TNF agents[139]. These agents offer improved efficacy and safety profiles and are helping to shift the paradigm toward earlier introduction of advanced therapies to prevent disease progression and complications[140].
Advances in pharmacogenomics and therapeutic drug monitoring are further refining the biologic use. By identifying genetic markers and pharmacokinetic parameters that predict treatment response or adverse events, clinicians can optimize drug selection and dosing on an individual basis. For example, understanding variations in the TNF receptor pathway or drug clearance rates can guide the decision between different biologic agents or the timing of dose escalation[141]. Moreover, molecular profiling and next-generation sequencing are becoming essential tools in identifying monogenic forms of IBD, particularly in VEO-IBD, allowing for highly specific interventions, including hematopoietic stem cell transplantation in selected cases[142]. As pediatric IBD care moves toward precision medicine, the integration of biologic therapies, genetic insights, and individualized monitoring holds the potential to significantly improve disease control, reduce long-term morbidity, and enhance quality of life for affected children. Ongoing research and clinical trials focused on pediatric populations are crucial to ensuring that these innovations translate into accessible and effective therapies across diverse clinical settings[143].
In recent years, the field of pediatric IBD treatment has grown to include innovative strategies beyond traditional immunosuppressants and biologics. Notable advancements include small molecule therapies, such as JAK inhibitors, and cellular therapies like hematopoietic stem cell transplantation, especially for monogenic IBD cases[144]. JAK inhibitors, which interfere with the JAK-signal transducer and activator of transcription signaling pathway that plays a role in inflammation and immune cell function, such as tofacitinib, upadacitinib, and filgotinib, offer an oral, targeted approach to modulating intracellular signaling pathways involved in cytokine-mediated inflammation[145]. These agents have already been approved for use in adult UC and are under investigation in pediatric trials. Their ease of administration, rapid onset of action, and potential to treat both luminal and extraintestinal manifestations make them attractive options for selected pediatric patients, especially those who are refractory to or intolerant of biologic therapy[146]. However, concerns regarding immunosuppression, infection risk, and long-term safety in the pediatric population warrant cautious and closely monitored use.
In parallel, hematopoietic stem cell transplantation has emerged as a curative option in children with monogenic IBD, particularly those with mutations in genes involved in immune regulation (e.g., IL10, FOXP3, XIAP). These rare but severe forms of VEO-IBD are often unresponsive to standard therapies, and genetic diagnosis is critical in guiding the decision toward hematopoietic stem cell transplantation. Successful transplantation can lead to complete resolution of gastrointestinal symptoms and immune dysregulation, although it carries significant risks, including graft-versus-host disease and treatment-related morbidity[147,148]. The introduction of these novel therapies highlights the shift toward precision medicine in pediatric IBD care. As our understanding of disease heterogeneity deepens particularly in the context of genetics, immunology, and molecular pathology so too does the potential for individualized and curative treatment approaches[149]. Ongoing clinical trials, long-term safety studies, and real-world data will be crucial in refining the indications and integrating these therapies into routine pediatric practice.
The gut microbiome has become a key factor in the development of IBD, especially in children, where early microbial exposures influence immune development[150]. In genetically prone children, changes in microbial diversity often characterized by a decrease in beneficial microbes (such as Faecalibacterium prausnitzii) and an increase in pro-inflammatory bacteria (like Enterobacteriaceae) are thought to contribute to immune imbalance and ongoing intestinal inflammation[151,152]. The interaction of genetics, diet, antibiotics, and environment causes microbial imbalance, which may trigger or worsen IBD. This is particularly important in VEO-IBD, where unusual microbial colonization in infancy could have lasting immune effects. Recent research suggests that addressing dysbiosis may offer treatment options, thereby increasing interest in microbiome-focused therapies[153,154].
Microbiome-targeted therapies include the use of prebiotics, probiotics, postbiotics, and synbiotics, all aimed at restoring a balanced microbial ecosystem. Although widely available, their effectiveness in IBD remains inconsistent, especially in moderate to severe cases. However, ongoing research aims to identify specific strains or microbial signatures that could provide targeted therapeutic benefits[155]. One of the most innovative and promising strategies is fecal microbiota transplantation (FMT), which involves transferring processed stool from a healthy donor into a patient’s gastrointestinal tract. FMT has shown remarkable success in treating recurrent Clostridioides difficile infection and is being studied for its potential to modulate intestinal inflammation in IBD[156]. Pediatric trials, though still limited, suggest that FMT may induce remission in certain cases of UC. Safety, donor selection, and regulatory issues remain significant challenges, but the potential of FMT to durably reshape the microbiome offers great promise[157]. As our understanding of microbial-host interactions advances, microbiome-based interventions could become a key part of personalized medicine in pediatric IBD. Future research will likely focus on engineered microbial communities, defined microbial therapeutics, and individualized microbiome profiling to guide treatment and predict responses.
Nutritional therapy has long been a key part of pediatric IBD management, especially in CD. As knowledge of the gut-immune-microbiome axis has expanded, so has the concept of precision nutrition. This emerging field focuses on tailoring dietary plans to individual patient profiles, disease type, microbial composition, and inflammation levels[158]. EEN remains the preferred induction therapy for pediatric CD in many regions. It provides similar effectiveness to corticosteroids in inducing remission, with additional benefits such as promoting mucosal healing, enhancing nutritional status, and supporting growth, all without the side effects of immunosuppression. However, sticking to EEN for the long term can be tough, and relapse rates when reintroducing food are still a concern[115]. This has led to increased interest in PEN and specific food-based diets, such as the CDED, the SCD, and the mediterranean diet. These strategies aim to re
Precision nutrition goes beyond generalized diet plans by incorporating insights from nutrigenomics, microbiome sequencing, and metabolomics to identify individualized dietary triggers or deficiencies. For example, identifying children with microbiota profiles predictive of diet responsiveness may allow clinicians to choose between enteral nutrition, food-based exclusion diets, or probiotic supplementation with greater accuracy and efficacy[114].
Moreover, diet-based interventions are increasingly recognized not only for their role in reducing disease activity but also in addressing extraintestinal complications such as growth failure, bone demineralization, and micronutrient deficiencies. Regular assessment of caloric intake, macronutrient balance, and levels of critical micronutrients, such as iron, vitamin D, and zinc, is essential, particularly in children with restricted diets[160]. In sum, diet is no longer considered ancillary but rather integral to both the treatment and personalization of care in pediatric IBD. As research advances, dietitians and clinicians will play an increasingly central role in designing evidence-based, individualized nutrition plans that address both inflammation and long-term developmental needs.
Monitoring pediatric IBD traditionally relies on clinical indices, endoscopy, imaging, and laboratory markers such as C-reactive protein and fecal calprotectin. However, repeated invasive procedures and blood draws pose practical and psychological challenges for children. In recent years, non-invasive biomarkers and digital health innovations have emerged as transformative tools in improving disease surveillance while minimizing patient burden[161,162]. Non-invasive biomarkers, particularly fecal calprotectin, are increasingly used to monitor mucosal inflammation and predict relapse. Advances in home-based fecal testing kits allow families to collect and submit samples remotely, facilitating real-time monitoring and reducing the need for frequent clinic visits[163]. Blood-based markers such as serum cytokine panels and novel proteins like leucine-rich alpha-2-glycoprotein are also being investigated for their potential to offer more specific and sensitive assessments of disease activity[164].
Wearable technologies represent a new frontier in pediatric IBD monitoring. Devices that track vital signs, physical activity, sleep patterns, and gastrointestinal symptoms offer continuous, real-world data that can enhance early detection of flares or complications. For instance, wearable biosensors capable of tracking heart rate variability or gut motility may serve as early indicators of disease exacerbation, potentially allowing for proactive intervention before clinical deterioration occurs[165].
Parallel to these technological advances is the rise of telemedicine and digital health platforms, especially catalyzed by the corona virus disease 2019 pandemic. Virtual consultations have improved access to specialized care, especially for patients in remote or underserved areas[166]. Moreover, digital apps tailored for IBD featuring symptom tracking, medication reminders, nutritional logs, and educational resources promote self-management and enhance commu
Importantly, PROs are becoming integral to care models, reflecting a shift towards more patient-centered approaches. PROs directly capture the child’s and family’s perspective on symptoms, functional status, quality of life, and treatment side effects, providing invaluable real-world data that complements objective clinical and laboratory findings[168]. Interactive platforms that utilize gamification, virtual reality, or personalized dashboards can enhance health literacy and empower pediatric patients to take an active role in managing their conditions. When children and caregivers are equipped with real-time PRO data and decision-making support, shared decision-making is enhanced, leading to improved adherence to therapy, clinic attendance, and overall outcomes[169].
Despite these promising developments, challenges remain. Data integration into electronic health records, standardization of wearable metrics, ensuring digital equity across diverse socioeconomic backgrounds, and addressing privacy concerns must be tackled to ensure equitable and secure implementation of these tools[170]. Collectively, the integration of non-invasive diagnostics, wearable technology, digital engagement tools, and particularly the systematic incorporation of PROs is reshaping the landscape of pediatric IBD care, shifting it toward more personalized, proactive, and participatory medicine.
Pediatric IBD is more than just a physical health issue, it’s a chronic, relapsing illness that significantly impacts a child’s emotional well-being, cognitive development, academic success, and family life. The unpredictable nature of flares, the necessity for invasive treatments, and the long-term consequences of living with a life-changing disease can create a substantial psychosocial burden[65]. Mental health challenges are especially common among children and adolescents with IBD. Research consistently shows higher rates of anxiety, depression, and emotional distress in this group compared to their healthy peers. Factors that contribute to psychological vulnerability include the chronic and uncertain course of the illness, frequent hospital stays, concerns about body image (particularly due to steroid use or surgical scars), and social isolation[171]. Adolescents, who are in a crucial stage of identity development, may struggle to accept a “different” body or lifestyle, leading to decreased self-esteem and a lower quality of life. Additionally, mental health symptoms can adversely impact disease outcomes by reducing treatment adherence, triggering flares, and worsening physical symptoms such as pain or fatigue[172].
Academic performance and school attendance are also commonly disrupted. Recurrent symptoms like abdominal pain, urgency, and fatigue, along with frequent medical appointments or hospital stays, can lead to absenteeism and academic underachievement[173]. Children may fall behind in school or face difficulties maintaining concentration due to discomfort or anxiety. Some may also experience stigma or bullying, particularly when bathroom access or dietary restrictions make them stand out. A lack of school-based support or understanding can further exacerbate stress, underscoring the importance of individualized education plans and strong collaboration between healthcare providers, families, and educators[174].
Family dynamics are deeply affected by pediatric IBD. Parents often experience significant emotional strain, including guilt, fear, and burnout from the demands of managing a chronic disease. Siblings may feel neglected or confused, especially when attention and resources are disproportionately focused on the affected child[175]. Financial pressures related to treatment costs, travel for specialty care, or job disruptions can further compound family stress. Over time, the entire household may adopt a “disease-centered” dynamic, which can affect interpersonal relationships and overall family functioning[176].
Importantly, resilience and adaptability can also emerge in families dealing with chronic illness. With appropriate psychosocial support, including counseling, peer support groups, and integrated care models involving psychologists or social workers children and families can develop strong coping mechanisms and maintain a good quality of life[177]. As pediatric IBD affects much more than the gastrointestinal tract it touches every aspect of a child’s and family’s life. A holistic, multidisciplinary approach that includes psychological and social care is essential to truly optimize outcomes and support the long-term well-being of children with IBD (Figure 4).
Transitioning from pediatric to adult care is a pivotal milestone in the management of IBD, and for many adolescents, it represents both a developmental and healthcare challenge. While the goal is a seamless handover that ensures continuity of care, the transition period is often fraught with emotional, psychological, and logistical complexities that can jeopardize disease control and overall well-being[178]. Emotionally, adolescents may experience anxiety, uncertainty, or resistance about leaving the familiarity of pediatric care providers, with whom they may have established strong, trust-based relationships. The pediatric model of care is typically family-centered, nurturing, and holistic, whereas adult care settings often expect a greater degree of independence and self-management[179]. This shift can be intimidating, particularly for patients who are still grappling with self-identity, treatment fatigue, or coexisting mental health concerns. Some individuals may feel unprepared to assume responsibility for tasks such as medication adherence, scheduling appointments, or reporting symptoms[180].
Practically, many healthcare systems still lack standardized, well-defined protocols for transition, often resulting in poorly coordinated or abrupt transfers to adult services. Crucial medical history may not be communicated effectively, and patients may face gaps in care, inconsistent treatment strategies, or even reluctance from adult providers to manage complex pediatric-onset IBD cases, especially those with complications like growth failure, prior surgeries, or significant psychosocial comorbidities[181]. Beyond the clinical handover, external factors such as insurance changes, relocation for higher education, and differences in healthcare access further compound the barriers during this vulnerable period[182]. Evidence shows that poorly managed transitions are associated with increased risk of non-adherence, loss to follow-up, disease flares, and hospitalization. Therefore, structured transition programs are essential. Best practices for these programs dictate that they should begin early ideally during early adolescence and involve gradual, planned preparation of both the patient and their family for the increased autonomy required in adult care[183]. Key components include comprehensive education about IBD, fostering independent self-management skills (e.g., medication management, symptom recognition, communication with providers), targeted psychological support to address anxieties, and active, continuous collaboration between pediatric and adult IBD teams[184]. Tools such as validated transition readiness assessments, joint pediatric-adult clinic visits, and individualized written care plans with clear transfer summaries can significantly improve outcomes and promote confidence among transitioning youth[185].
Despite the recognized benefits and outlined best practices, a key remaining challenge lies in the widespread and consistent implementation of these structured transition programs. Barriers include a lack of dedicated funding, insufficiently trained personnel (e.g., transition coordinators or patient navigators), and varied levels of engagement or understanding among healthcare providers in both pediatric and adult settings regarding the specific needs of this population[186]. Therefore, successful transition in pediatric IBD care is not merely a change of setting it is a carefully orchestrated process that recognizes the developmental needs and vulnerabilities of adolescents. Addressing emotional and practical challenges with comprehensive, structured support can ensure continuity of care, empower young patients, and lay the foundation for long-term health autonomy[187].
Managing pediatric IBD goes far beyond medical treatment, encompassing essential psychological and social support. Since IBD is chronic and relapsing, and its onset happens during critical developmental years, children and teenagers need a comprehensive care approach that addresses their mental, emotional, and social needs alongside their physical health[188]. Psychological support is essential because of the high rates of mood and anxiety disorders in this group. Persistent symptoms, fear of flare-ups, concerns about growth, appearance, or the future, and social isolation can lead to emotional distress, depression, and even post-traumatic stress, particularly in those with repeated hospital stays or invasive procedures[189]. Identifying and addressing these issues early can significantly enhance quality of life, treatment adherence, and disease outcomes. Pediatric IBD teams should include psychologists or mental health professionals trained to assist children and teens coping with chronic illness[190].
Social support is equally vital. Peer relationships are crucial for social development, but children with IBD may struggle to participate in typical social, school, or extracurricular activities due to fatigue, dietary restrictions, or fear of accidents and embarrassment. These issues can lead to withdrawal, low self-esteem, and feelings of isolation. Support groups either in person or online can provide a strong sense of community, reduce stigma, and help kids realize they are not alone[191,192].
Family support is also essential. Parents and caregivers frequently encounter emotional stress, financial difficulties, and caregiver fatigue. Siblings may feel overlooked or confused. Offering family counseling, educational resources, and respite services can enhance family connections and reduce caregiver stress. Equipping parents with tools to communicate effectively with their child and manage care-related concerns helps sustain a supportive and steady home[177].
Schools are a vital area where support can make a meaningful difference. Teachers and school staff should understand the child’s condition and recommend necessary accommodations, such as bathroom access, flexible schedules, or modified physical activities[193]. Creating individualized education plans or 504 plans can help ensure academic progress without worsening the child’s physical or emotional symptoms. Therefore, psychological and social support are not just extras in pediatric IBD care; they are essential. A comprehensive approach involving mental health professionals, social workers, peer networks, and school cooperation can significantly enhance resilience, treatment success, and long-term well-being for young patients living with IBD[194].
Advances in molecular medicine are quickly changing our understanding of pediatric IBD, especially with growing insights into genetic and immunologic foundations. Unlike adult-onset IBD, pediatric forms particularly VEO-IBD are increasingly seen as a diverse group of diseases with different underlying mechanisms, many of which are based on single-gene defects or complex immune system dysregulation[195].
Monogenic IBD is among the most significant recent discoveries. This form, primarily seen in children under six, involves rare mutations in genes that regulate immune pathways, epithelial barrier integrity, and microbial sensing, such as IL10, IL10RA/B, FOXP3, NEMO, and CYBB. Finding these mutations is not just academically interesting; it has direct treatment implications[196]. For instance, patients with IL10 pathway mutations may benefit from hematopoietic stem cell transplantation, a therapy not usually employed for conventional IBD. However, challenges persist in translating these discoveries into routine clinical practice[197]. Access to comprehensive genetic testing remains limited in many regions worldwide, especially in resource-constrained settings, and there is a critical need to establish standardized, evidence-based criteria for when such testing should be conducted to ensure appropriate resource utilization. Fur
Beyond monogenic forms, polygenic risk profiling and genome-wide association studies have identified many susceptibility loci shared between pediatric and adult IBD. However, emerging evidence indicates that some genetic variants may have stronger effects or show unique penetrance in pediatric patients, highlighting the importance of age-specific genomic studies[199]. A key research priority is to understand how these polygenic risk scores can be integrated into clinical algorithms to predict disease course, guide treatment selection, or identify high-risk individuals for early intervention, as their practical utility beyond risk stratification is still evolving[200].
On the immunological front, pediatric IBD has been associated with distinct immune signatures, including exaggerated Th17 responses, impaired regulatory T cell function, and altered cytokine profiles. Additionally, early-life immune development, shaped by exposure to microbiota, nutrition, and infections, may influence susceptibility or resistance to IBD[201]. Future research must focus on translating these complex immune signatures into actionable clinical biomarkers or novel therapeutic targets, as their precise role in guiding personalized medicine beyond general immune suppression remains largely undefined. Understanding these developmental immune pathways could open opportunities for primary prevention or early treatment[202].
Utilizing multi-omics methods, such as transcriptomics, proteomics, and epigenomics, can enhance disease subtyping and guide the field toward precision medicine. For example, immune profiling may help classify patients based on their likely response to specific biologics or better predict disease progression than relying solely on clinical features. The current limitation lies in validating these multi-omics findings in large, diverse pediatric cohorts and developing cost-effective, scalable platforms for their routine clinical application[203]. Therefore, expanding our genetic and immunologic understanding of pediatric IBD offers significant potential not only for understanding disease mechanisms but also for developing targeted, personalized treatments that can change the disease course early in life. Ongoing investment in translational research and international collaborative registries is crucial to fully realize this potential, overcoming the current hurdles of accessibility, standardization, and clinical implementation.
The development of pediatric-specific clinical trials is a vital yet often overlooked area in improving care for children with IBD. Historically, treatment protocols for pediatric IBD have mainly been adapted from adult data, despite apparent differences in disease characteristics, immune responses, pharmacokinetics, and psychosocial factors between the two groups. This reliance on extrapolation creates significant limitations, which can result in less effective treatment or unexpected side effects in younger patients[204].
Children, especially those with early-onset and VEO-IBD, often face more extensive and aggressive disease types. As a result, there is an urgent need for clinical trials specifically designed to assess therapeutic options, dosing methods, and treatment lengths suitable for children. These trials must also consider growth, puberty, neurodevelopment, and long-term safety factors that adult studies do not adequately address[205]. A critical gap remains in the availability of sufficient long-term safety and efficacy data for many established and emerging therapies when applied to a pediatric population that will likely be on treatment for decades[206].
Recent efforts by international consortia, including the pediatric IBD consortium and the International Organization for the Study of IBD, have started to address these gaps by promoting age-appropriate endpoints, standardized disease activity indices for children (such as PUCAI and PCDAI), and the inclusion of PROs relevant to pediatric populations[207]. Furthermore, the rise of biologics and small molecule therapies (like JAK inhibitors and integrin blockers) highlights the importance of evaluating their safety and effectiveness in children through well-designed trials, rather than relying solely on post-marketing surveillance[208].
However, significant regulatory, ethical, and logistical barriers continue to hinder pediatric trial enrollment. These include the complexities of informed consent/assent, limited patient populations for rare disease subtypes, and concerns about long-term follow-up for a lifelong condition[209]. Future efforts must focus on developing innovative trial designs, such as adaptive trials and master protocols, that can efficiently evaluate multiple therapies and overcome challenges posed by small patient numbers. Streamlined protocols, multicenter collaborations, and increased funding for pediatric-specific research are crucial to overcoming these hurdles and enhancing participation[210]. Investing in pediatric-specific clinical trials is crucial for optimizing therapy, minimizing harm, and ensuring evidence-based, child-centered care in IBD. Such efforts will not only improve outcomes for children currently affected but also inform early intervention strategies that may alter the disease trajectory altogether[30].
VEO-IBD is defined as IBD diagnosed before the age of 6, representing a distinct and heterogeneous clinical entity with a unique pathophysiology often rooted in monogenic defects, primary immunodeficiencies, or epithelial barrier dysfunctions[4]. Given its rarity, variable presentation, and complex underlying etiologies, standardized management pathways for VEO-IBD remain underdeveloped and inconsistently applied across healthcare settings globally. Currently, the diagnosis and treatment of VEO-IBD vary significantly by institution and region, leading to delays in appropriate interventions and missed opportunities for targeted therapy[211]. Establishing standardized diagnostic algorithms including mandatory early genetic screening, immunologic profiling, and functional immune assays is crucial for timely identification of monogenic forms, many of which may benefit from interventions beyond conventional IBD therapies, such as hematopoietic stem cell transplantation. However, challenges exist in achieving consensus on universally applicable genetic screening panels and ensuring equitable access to these specialized tests, especially in regions with limited resources[68].
Therapeutically, a unified framework is needed to guide escalation strategies based on clinical phenotype, genetic findings, and response to initial treatment. For instance, the traditional step-up approach may be inadequate for certain monogenic IBDs, where early introduction of biologics or immunomodulators or even curative therapies may be warranted[212]. Moreover, nutrition-based therapies and microbiota-targeted interventions must be integrated thoughtfully, especially in cases with coexisting malabsorption or immunodeficiency. A significant limitation is the lack of robust, prospective data from large cohorts to definitively establish optimal treatment sequences and guide the transition from conventional therapies to targeted interventions for specific VEO-IBD subtypes[213].
Equally important is the standardization of monitoring protocols. VEO-IBD patients often require more frequent assessments of growth, development, nutritional status, and immune function. Centralized registries and collaborative databases, such as those developed by the Global IBD Genetics Consortium and ESPGHAN’s pediatric IBD working group can facilitate the collection of longitudinal data to refine prognostic indicators and treatment algorithms. Ultimately, the development of internationally recognized, evidence-based guidelines tailored to VEO-IBD will ensure early and accurate diagnosis, streamline personalized treatment strategies, and reduce disease burden[214]. Achieving widespread adoption and consistent implementation of these guidelines, particularly in diverse healthcare systems with varying resources and expertise, will be a significant ongoing challenge. Standardization will also support better outcomes through multidisciplinary collaboration among gastroenterologists, immunologists, geneticists, dietitians, and psychologists, highlighting the need for a holistic and coordinated care model in this vulnerable population[215].
Effective management of pediatric IBD necessitates a shift from a purely disease-centered paradigm to a holistic and multidisciplinary model of care. This approach integrates medical, psychological, nutritional, and social support services to address the full spectrum of challenges faced by children and adolescents with IBD, aiming not only for disease control but also for optimal growth, development, and quality of life[216]. At the center of this model is a dedicated multidisciplinary team, typically including pediatric gastroenterologists, dietitians, psychologists or mental health professionals, IBD-specialized nurses, social workers, and, when needed, surgeons and genetic counselors. Each discipline brings a unique perspective and sets of skills that contribute to comprehensive, individualized care. Regular interdisciplinary case discussions and care coordination ensure that decisions are patient-centered and responsive to the evolving needs of both the child and their family[188].
Integration of psychosocial care is vital. As emotional well-being strongly influences disease perception and adherence, routine psychological screening and early mental health interventions should be embedded in IBD clinics. Similarly, dietitians should routinely assess nutritional status and develop personalized meal plans that support growth and alleviate gastrointestinal symptoms, while also considering cultural preferences and socioeconomic realities[65]. Family involvement is another essential element. Educating parents about the disease, treatment choices, and coping strategies empowers them to support their child effectively. Additionally, family counseling can address role changes, emotional stress, and improve communication within the household. School coordination also plays a vital role in the multidisciplinary approach[217]. Collaborating with educational institutions ensures that necessary accommodations are provided and that children can remain academically engaged despite variations in their illness. School re-entry programs, academic flexibility, and counseling services help bridge the gap between illness and educational achievement[218]. Importantly, transition planning from pediatric to adult care must start early and be handled with structure and sensitivity. Ado
Despite the clear benefits, the widespread implementation of a truly holistic and multidisciplinary model faces significant barriers. These include a severe shortage of specialized pediatric IBD health professionals in many regions, inadequate funding for comprehensive support services (e.g., psychologists, social workers), and the structural complexities of integrating diverse specialties within existing healthcare systems[220]. Future research should focus on developing scalable and cost-effective models of multidisciplinary care that are adaptable to various healthcare settings, as well as demonstrating the long-term cost-effectiveness and superior patient outcomes of this approach in diverse real-world contexts. Notably, the holistic and multidisciplinary approach is not just ideal it is essential for pediatric IBD care. By addressing the biological, psychological, and social facets of illness in a coordinated manner, this model promotes resilience, improves clinical outcomes, and helps children with IBD succeed into adulthood.
As the global occurrence of pediatric IBD continues to increase especially in regions previously considered low-burden, such as the MENA, and parts of Asia there is an urgent need to adapt healthcare systems and policies to meet the changing demands of this growing patient population. The rise in disease burden requires a shift from reactive, fragmented care to proactive, integrated, and sustainable healthcare planning[221]. One of the main priorities is developing and implementing national pediatric IBD registries to monitor incidence trends, clinical outcomes, and treatment responses. These registries are crucial for evidence-based decision-making, resource allocation, and establishing benchmarks for care standards across institutions and regions[222]. Without dependable epidemiological data, policymakers in areas experiencing increased incidence face significant challenges in predicting healthcare needs and identifying high-risk populations. A key limitation is the absence of robust, sustained funding and the technical expertise required to establish and maintain such registries, particularly in regions with nascent public health data infrastructure[223].
Infrastructure investment is another essential element. Many healthcare systems in regions with increasing incidence rates lack specialized pediatric gastroenterology services, endoscopic capabilities for children, or multidisciplinary care teams[224]. National health policies should prioritize training and retaining pediatric IBD specialists, as well as establishing referral centers equipped to handle complex cases, especially for VEO-IBD and cases requiring advanced therapies. Achieving this requires substantial and sustained government commitment and investment, which can be challenging amidst competing public health priorities[225]. Equally important is ensuring equitable access to advanced diagnostics, such as genetic testing and immunologic assays, along with innovative treatments like biologics and small molecules. In many developing countries, cost and regulatory barriers limit the availability of these treatments, leading to disparities in outcomes[226]. Strategic negotiations on drug pricing, including essential IBD medications in national formularies, and partnerships with international health agencies can help overcome these challenges. However, even with policy changes, the on-the-ground implementation and consistent affordability of these high-cost therapies remain significant hurdles for many patients and healthcare systems[227].
Public awareness campaigns and physician education programs should also be essential parts of policy efforts. Delayed diagnosis remains common in emerging regions, often due to primary care providers having low awareness or because symptoms are misdiagnosed as infections or malnutrition[228]. Training programs can enhance early recognition and prompt referrals, resulting in improved outcomes. Finally, healthcare planning must include psychosocial support services, nutrition counseling, and transition care models areas that are often underfunded or overlooked in emerging systems[229]. Since IBD is a lifelong condition, early investment in pediatric care will offer long-term benefits by reducing complications, hospitalizations, and overall healthcare costs. Future policy research needs to focus on implementation science to identify effective strategies for translating these recommendations into practical, sustainable, and equitable healthcare services across diverse regional contexts[119].
To effectively address the increasing burden and growing complexity of pediatric IBD, a set of strategic priorities must be collaboratively pursued by clinicians, researchers, and healthcare policymakers, all of which are crucial for optimizing patient outcomes and enhancing the quality of life for children worldwide. This necessitates the development and rigorous implementation of standardized, pediatric-specific guidelines to ensure consistent and evidence-based approaches to diagnosis, treatment, and long-term management across different age groups and regions. Integral to this is establishing and expanding multidisciplinary care teams encompassing pediatric gastroenterologists, IBD-specialized nurses, dietitians, psychologists/mental health professionals, and social workers as they are essential for addressing the medical, nutritional, psychosocial, and developmental needs unique to children, thereby improving clinical outcomes and quality of life. Furthermore, implementing formal and structured programs to facilitate the seamless transition of adolescents from pediatric to adult IBD care is vital for empowering young patients with self-management skills, ensuring continuity of care, and mitigating the risk of disease flares. In regions experiencing a rapid increase in IBD, such as the Arabian Gulf and MENA areas, prioritizing and funding national and regional epidemiological registries is fundamental for accurately monitoring disease trends, informing healthcare resource allocation, and supporting local research initiatives. Alongside this, integrating mental health and psychosocial support services into routine IBD care is critical, given the high prevalence of anxiety, depression, and school-related challenges among children with IBD, where proactive screening and early intervention can significantly improve emotional well-being. Looking forward, intensified research efforts into VEO-IBD, focusing on comprehensive genetic and immunologic profiling, are crucial for developing truly personalized and potentially curative treatment strategies. Concurrently, dedicated pediatric clinical trials are imperative to rigorously assess the safety, efficacy, and long-term effects of emerging therapies, as children remain underrepresented in research. The advancement of precision nutrition and dietary modulation as therapeutic strategies also holds promise, requiring further research to understand individual responses to specific diets and their impact on gut microbiota. Finally, health policy reforms are essential in high-growth regions to ensure equitable access to specialized pediatric IBD care, advanced diagnostics, and innovative treatments, overcoming current cost and regulatory barriers. Collectively, these comprehensive efforts aim to transform the landscape of pediatric IBD care, enabling children and adolescents to lead healthier, more fulfilling lives.
While this review provides a comprehensive overview of current knowledge and emerging directions in pediatric IBD, several limitations should be recognized. First, the narrative review design may inherently introduce selection bias because it depends on available literature rather than a systematic search strategy. As a result, some relevant studies might have been unintentionally left out. Additionally, the evolving nature of pediatric IBD research especially in genomics, microbiome science, and personalized medicine means that some findings could quickly become outdated as new evidence emerges. The review also heavily relies on data from high-income countries, which may limit its applicability to resource-limited settings where diagnostic and treatment infrastructure can differ greatly. Moreover, there is limited availability of strong pediatric-specific clinical trial data, particularly in newly industrializing regions, which restricts the depth of evidence-based recommendations for emerging therapies. Lastly, although the review includes regional insights from the Arabian Gulf and MENA regions, variability in epidemiologic data collection across countries may influence the accuracy of regional comparisons and trends.
Pediatric IBD is a rapidly evolving and increasingly recognized global health challenge, characterized by unique clinical features, developmental effects, and management needs compared to adult-onset disease. As incidence rises worldwide including in previously low-incidence regions like the Arabian Gulf there is an urgent need for age-specific, multidisciplinary, and proactive care approaches. Advances in biologics, nutritional strategies, precision medicine, and psychosocial support are transforming pediatric IBD management, but significant challenges remain, especially in accessing specialized care, ensuring diagnostic consistency, and incorporating emerging research into clinical practice. The future of pediatric IBD care depends on collaborative efforts that address regional disparities, increase pediatric-focused research, and turn scientific discoveries into equitable, personalized treatments. Ultimately, a holistic, child-centered approach that includes medical, psychological, nutritional, and social care is essential for improving long-term outcomes and quality of life for affected children and their families.
The authors would like to extend their sincere gratitude to the editors and the anonymous peer reviewers for their valuable time, constructive feedback, and insightful comments, which greatly contributed to the refinement and clarity of this manuscript. Their dedication to scientific rigor and editorial excellence is deeply appreciated.
| 1. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1319] [Article Influence: 188.4] [Reference Citation Analysis (2)] |
| 2. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (6)] |
| 3. | Amaro F, Chiarelli F. Growth and Puberty in Children with Inflammatory Bowel Diseases. Biomedicines. 2020;8:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, Field M, Grushkin-Lerner L, Pai SY, Bousvaros A, Cho J, Argmann C, Schadt E, Mcgovern DPB, Mokry M, Nieuwenhuis E, Clevers H, Powrie F, Uhlig H, Klein C, Muise A, Dubinsky M, Snapper SB. Very Early Onset Inflammatory Bowel Disease: A Clinical Approach With a Focus on the Role of Genetics and Underlying Immune Deficiencies. Inflamm Bowel Dis. 2020;26:820-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Sălcudean A, Nan AG, Bodo CR, Cosma MC, Strete EG, Lica MM. Association between Childhood Onset Inflammatory Bowel Disease and Psychiatric Comorbidities in Adulthood. Diagnostics (Basel). 2023;13:1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | M'Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 7. | Aniwan S, Santiago P, Loftus EV Jr, Park SH. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United European Gastroenterol J. 2022;10:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Church DL. Major factors affecting the emergence and re-emergence of infectious diseases. Clin Lab Med. 2004;24:559-586, v. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Fan Z, Zhou H, Tian L, Wu T, Zhang J, Lin J, Wang C, He J, Zhao L, Chen J, Liang J. The epidemiological trends of inflammatory bowel disease among women of reproductive age: a global analysis from 1990 to 2021. Immunol Res. 2025;73:101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, Vutcovici M, El-Matary W, Nguyen GC, Griffiths AM, Mack DR, Jacobson K, Mojaverian N, Tanyingoh D, Cui Y, Nugent ZJ, Coulombe J, Targownik LE, Jones JL, Leddin D, Murthy SK, Kaplan GG. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am J Gastroenterol. 2017;112:1120-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Benchimol EI, Guttmann A, Griffiths AM, Rabeneck L, Mack DR, Brill H, Howard J, Guan J, To T. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Ye Y, Manne S, Treem WR, Bennett D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the United States, 2007-2016. Inflamm Bowel Dis. 2020;26:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Roberts SE, Thorne K, Thapar N, Broekaert I, Benninga MA, Dolinsek J, Mas E, Miele E, Orel R, Pienar C, Ribes-Koninckx C, Thomson M, Tzivinikos C, Morrison-Rees S, John A, Williams JG. A Systematic Review and Meta-analysis of Paediatric Inflammatory Bowel Disease Incidence and Prevalence Across Europe. J Crohns Colitis. 2020;14:1119-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Huang JG, Wong YKY, Chew KS, Tanpowpong P, Calixto Mercado KS, Reodica A, Rajindrajith S, Chang KC, Ni YH, Treepongkaruna S, Lee WS, Aw MM. Epidemiological characteristics of Asian children with inflammatory bowel disease at diagnosis: Insights from an Asian-Pacific multi-centre registry network. World J Gastroenterol. 2022;28:1830-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, Park DI, Kim SH, Kim TJ. Emerging trends of inflammatory bowel disease in South Korea: A nationwide population-based study. J Gastroenterol Hepatol. 2019;34:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Shao B, Yang W, Cao Q. Landscape and predictions of inflammatory bowel disease in China: China will enter the Compounding Prevalence stage around 2030. Front Public Health. 2022;10:1032679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 17. | Yang H, Qian J. Epidemiological research, burden, and clinical advances of inflammatory bowel disease in China. Chin Med J (Engl). 2024;137:1009-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Kotze PG, Underwood FE, Damião AOMC, Ferraz JGP, Saad-Hossne R, Toro M, Iade B, Bosques-Padilla F, Teixeira FV, Juliao-Banos F, Simian D, Ghosh S, Panaccione R, Ng SC, Kaplan GG. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 19. | Arcucci MS, Contreras MB, Gallo J, Antoniska MA, Busoni V, Tennina C, D'Agostino D, Kakisu MH, Weyersberg C, Orsi M. Pediatric Inflammatory Bowel Disease: A Multicenter Study of Changing Trends in Argentina Over the Past 30 Years. Pediatr Gastroenterol Hepatol Nutr. 2022;25:218-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Forbes AJ, Frampton CMA, Day AS, Vernon-Roberts A, Gearry RB. Descriptive Epidemiology of Pediatric Inflammatory Bowel Disease in Oceania: A Systematic Review and Meta-Analysis. J Pediatr Gastroenterol Nutr. 2023;77:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Lopez RN, Evans HM, Appleton L, Bishop J, Chin S, Mouat S, Gearry RB, Day AS. Prospective Incidence of Paediatric Inflammatory Bowel Disease in New Zealand in 2015: Results From the Paediatric Inflammatory Bowel Disease in New Zealand (PINZ) Study. J Pediatr Gastroenterol Nutr. 2018;66:e122-e126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Omede M, Itam-Eyo A, Park A, Ikobah J, Ibrahim MK, Chukwudike E, Ali-Ibrahim A, Lydston M, Asombang AW, Ananthakrishnan AN. Epidemiology, Natural History, and Treatment of Inflammatory Bowel Disease in Africa: A Scoping Review. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Caron B, Honap S, Peyrin-Biroulet L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J Crohns Colitis. 2024;18:ii3-ii15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 24. | Amador C, Xia C, Nagy R, Campbell A, Porteous D, Smith BH, Hastie N, Vitart V, Hayward C, Navarro P, Haley CS. Regional variation in health is predominantly driven by lifestyle rather than genetics. Nat Commun. 2017;8:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Alsakarneh S, Ahmed M, Jaber F, Abuassi M, Mourad FH, Francis FF, Barada K, Tfayli R, Al-Bawardy B, Farraye FA, Hashash JG. Inflammatory bowel disease burden in the Middle East and North Africa Region: a comprehensive analysis of incidence, prevalence, and mortality from 1990-2019. Ann Gastroenterol. 2024;37:527-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Zhang ZM, Lin ZL, He BX, Yan WT, Zhang XY, Zhang ZH, Wang L, Wang JQ, Liu DM, Zhang W, Li ZH. Epidemiological analysis reveals a surge in inflammatory bowel disease among children and adolescents: A global, regional, and national perspective from 1990 to 2019 - insights from the China study. J Glob Health. 2023;13:04174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 27. | Khan HH, Whatley JS, Suppa C. Improving Procedural Documentation of Newly Diagnosed Pediatric Inflammatory Bowel Disease Patients: A Single-center Quality Improvement Study. Pediatr Qual Saf. 2025;10:e819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Al Lawati TT, Al Rawahi Y, Al Bahlani AQ, Al Jamei A, Ramatalla D, Saadah OI. Incidence and clinical characteristics of pediatric inflammatory bowel disease in Oman. Saudi J Gastroenterol. 2023;29:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Moran CJ. Very early onset inflammatory bowel disease. Semin Pediatr Surg. 2017;26:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Bhalla A, Shahi A, Maity M, Safa F, Srividya V, Clementina R, Anugu GR, Younas S. Inflammatory Bowel Disease in Children: Current Diagnosis and Treatment Strategies. Cureus. 2025;17:e78462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Hracs L, Windsor JW, Gorospe J, Cummings M, Coward S, Buie MJ, Quan J, Goddard Q, Caplan L, Markovinović A, Williamson T, Abbey Y, Abdullah M, Abreu MT, Ahuja V, Raja Ali RA, Altuwaijri M, Balderramo D, Banerjee R, Benchimol EI, Bernstein CN, Brunet-Mas E, Burisch J, Chong VH, Dotan I, Dutta U, El Ouali S, Forbes A, Forss A, Gearry R, Dao VH, Hartono JL, Hilmi I, Hodges P, Jones GR, Juliao-Baños F, Kaibullayeva J, Kelly P, Kobayashi T, Kotze PG, Lakatos PL, Lees CW, Limsrivilai J, Lo B, Loftus EV Jr, Ludvigsson JF, Mak JWY, Miao Y, Ng KK, Okabayashi S, Olén O, Panaccione R, Paudel MS, Quaresma AB, Rubin DT, Simadibrata M, Sun Y, Suzuki H, Toro M, Turner D, Iade B, Wei SC, Yamamoto-Furusho JK, Yang SK, Ng SC, Kaplan GG; Global IBD Visualization of Epidemiology Studies in the 21st Century (GIVES-21) Research Group. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature. 2025;642:458-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 115.0] [Reference Citation Analysis (4)] |
| 32. | Isa HM, Mohamed AM, Al-Jowder HE, Matrook KA, Althawadi HH. Pediatric Crohn's Disease in Bahrain. Oman Med J. 2018;33:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Isa HM. Pediatric Ulcerative Colitis from Prevalence to Outcome. J Clin Gastroenterol Treat. 2017;3. [DOI] [Full Text] |
| 34. | El Mouzan MI, AlEdreesi MH, Hasosah MY, Al-Hussaini AA, Al Sarkhy AA, Assiri AA. Regional variation of pediatric inflammatory bowel disease in Saudi Arabia: Results from a multicenter study. World J Gastroenterol. 2020;26:416-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Al-Qabandi WA, Buhamrah EK, Hamadi KA, Al-Osaimi SA, Al-Ruwayeh AA, Madda J. Inflammatory bowel disease in children, an evolving problem in Kuwait. Saudi J Gastroenterol. 2011;17:323-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Mosli M, Alawadhi S, Hasan F, Abou Rached A, Sanai F, Danese S. Incidence, Prevalence, and Clinical Epidemiology of Inflammatory Bowel Disease in the Arab World: A Systematic Review and Meta-Analysis. Inflamm Intest Dis. 2021;6:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 37. | Elbadry M, Nour MO, Hussien M, Ghoneem EA, Medhat MA, Shehab H, Galal S, Eltabbakh M, El-Raey F, Negm M, Afify S, Abdelhamed W, Sherief A, Abdelaziz A, Abo Elkasem M, Mahrous A, Kamal G, Maher M, Abdel-Hameed O, Elbasuny A, El-Zayyadi I, Bassiony A, Moussa A, Bedewy E, Elfert A, El Kassas M. Clinico-Epidemiological Characteristics of Patients With Inflammatory Bowel Disease in Egypt: A Nationwide Multicenter Study. Front Med (Lausanne). 2022;9:867293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Ahmaida A, Al-Shaikhi S. Childhood Inflammatory Bowel Disease in Libya: Epidemiological and Clinical features. Libyan J Med. 2009;4:70-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | El Mouzan MI, Saadah O, Al-Saleem K, Al Edreesi M, Hasosah M, Alanazi A, Al Mofarreh M, Asery A, Al Qourain A, Nouli K, Al Hussaini A, Telmesani A, AlReheili K, Alghamdi S, Alrobiaa N, Alzaben A, Mehmadi A, Al Hebbi H, Al Sarkhy A, Al Mehaidib A, Al Saleem B, Assiri A, Wali S. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis. 2014;20:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 41. | Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 2015;4:143-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | El Mouzan M, Al-Mofarreh M, Assiri A, Hamid Y, Saeed A. Consanguinity and inflammatory bowel diseases: is there a relation? J Pediatr Gastroenterol Nutr. 2013;56:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Uhlig HH, Charbit-Henrion F, Kotlarz D, Shouval DS, Schwerd T, Strisciuglio C, de Ridder L, van Limbergen J, Macchi M, Snapper SB, Ruemmele FM, Wilson DC, Travis SPL, Griffiths AM, Turner D, Klein C, Muise AM, Russell RK; Paediatric IBD Porto group of ESPGHAN. Clinical Genomics for the Diagnosis of Monogenic Forms of Inflammatory Bowel Disease: A Position Paper From the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2021;72:456-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 44. | Hammer T, Langholz E. The epidemiology of inflammatory bowel disease: balance between East and West? A narrative review. Dig Med Res. 2020;3:48-48. [DOI] [Full Text] |
| 45. | Chen L, Xu Y, Ai F, Shen S, Luo Y, Li X. Dissecting the rising tide of inflammatory bowel disease among youth in a changing world: insights from GBD 2021. Int J Colorectal Dis. 2025;40:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 47. | Al Ta'ani O, Al-Ajlouni Y, Lahoud C, Almasaid S, Alhalalmeh Y, Oweis Z, Danpanichkul P, Baidoun A, Alsakarneh S, Dahiya DS, Njei B. Socioeconomic and demographic disparities in the impact of digestive diseases in the middle East and North Africa (MENA) region. Int J Equity Health. 2025;24:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 48. | Pandit AU, Tomasino KN, Aswani Omprakash T, Epstein DE. Cultural considerations in gastroenterology: barriers to care and a call for humility and action. Transl Gastroenterol Hepatol. 2024;9:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 49. | Seyed Tabib NS, Madgwick M, Sudhakar P, Verstockt B, Korcsmaros T, Vermeire S. Big data in IBD: big progress for clinical practice. Gut. 2020;69:1520-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 50. | Fiske HW, Ward C, Shah SA, Holubar SD, Al-Bawardy B, Barnes EL, Binion D, Bohm M, Brand M, Clarke K, Cohen BL, Cross RK, Dueker J, Engels M, Farraye FA, Fine S, Forster E, Gaidos J, Ginsburg P, Goyal A, Hanson J, Herfath H, Hull T, Kelly CR, Lazarev M, Levy LC, Melia J, Philpott J, Qazi T, Siegel CA, Watson A, Wexner SD, Williams ED, Regueiro M. Clinical Decision Making in Inflammatory Bowel Disease Mimics: Practice Management from Inflammatory Bowel Disease LIVE. Crohns Colitis 360. 2024;6:otae022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Kumar A, Yassin N, Marley A, Bellato V, Foppa C, Pellino G, Myrelid P, Millan M, Gros B, Avellaneda N, Catalan-Serra I, El-Hussuna A, Cunha Neves JA, Roseira J, Cunha MF, Verstockt B, Bettenworth D, Mege D, Brookes MJ. Crossing barriers: the burden of inflammatory bowel disease across Western Europe. Therap Adv Gastroenterol. 2023;16:17562848231218615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Homsi M. The deficiencies in using large databases to assess the risk of future development of inflammatory bowel disease. United European Gastroenterol J. 2024;12:162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Bousvaros A, Sylvester F, Kugathasan S, Szigethy E, Fiocchi C, Colletti R, Otley A, Amre D, Ferry G, Czinn SJ, Splawski JB, Oliva-Hemker M, Hyams JS, Faubion WA, Kirschner BS, Dubinsky MC; Challenges in Pediatric IBD Study Groups. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Andrews AR, Putra J. Special Considerations in Pediatric Inflammatory Bowel Disease Pathology. Diagnostics (Basel). 2025;15:831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 56. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1652] [Article Influence: 118.0] [Reference Citation Analysis (6)] |
| 57. | Thurgate LE, Lemberg DA, Day AS, Leach ST. An Overview of Inflammatory Bowel Disease Unclassified in Children. Inflamm Intest Dis. 2019;4:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Saadah OI, AlAmeel T, Al Sarkhy A, Hasosah M, Al-Hussaini A, Almadi MA, Al-Bawardy B, Altuwaijri TA, AlEdreesi M, Bakkari SA, Alharbi OR, Azzam NA, Almutairdi A, Alenzi KA, Al-Omari BA, Almudaiheem HY, Al-Jedai AH, Mosli MH. Saudi consensus guidance for the diagnosis and management of inflammatory bowel disease in children and adolescents. Saudi J Gastroenterol. 2025;31:107-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Granot M, Kopylov U, Loberman-Nachum N, Krauthammer A, Abitbol CM, Ben-Horin S, Weiss B, Haberman Y. Differences in disease characteristics and treatment exposures between paediatric and adult-onset inflammatory bowel disease using a registry-based cohort. Aliment Pharmacol Ther. 2024;60:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Bay M C, Núñez F P, Quera R, Yarur AJ. Current perspectives on pediatric inflammatory bowel disease focusing on transitional care management. What should we consider? Gastroenterol Hepatol. 2023;46:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Yoon H. Another Piece of Evidence for Early Administration of Biologics in Children with Crohn's Disease Who Start as an Inflammatory Phenotype. Gut Liver. 2021;15:791-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Gasparetto M, Guariso G. Crohn's disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219-13233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Jose FA, Garnett EA, Vittinghoff E, Ferry GD, Winter HS, Baldassano RN, Kirschner BS, Cohen SA, Gold BD, Abramson O, Heyman MB. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Dulai PS, Singh S, Casteele NV, Boland BS, Sandborn WJ. How Will Evolving Future Therapies and Strategies Change How We Position the Use of Biologics in Moderate to Severely Active Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Mackner LM, Greenley RN, Szigethy E, Herzer M, Deer K, Hommel KA. Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;56:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 66. | Bishop J, Lemberg DA, Day A. Managing inflammatory bowel disease in adolescent patients. Adolesc Health Med Ther. 2014;5:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M, Dubinsky M, Hyams JS. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1200] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 68. | Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D, Klein C, Snapper SB, Muise AM; COLORS in IBD Study Group and NEOPICS. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990-1007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 69. | Eszter Müller K, Laszlo Lakatos P, Papp M, Veres G. Incidence and paris classification of pediatric inflammatory bowel disease. Gastroenterol Res Pract. 2014;2014:904307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Cheema AY, Munir M, Zainab K, Ogedegbe OJ. An Atypical Presentation of Crohn's Disease: A Case Report. Cureus. 2022;14:e29431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 71. | Ishige T. Growth failure in pediatric onset inflammatory bowel disease: mechanisms, epidemiology, and management. Transl Pediatr. 2019;8:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Lauritano D, Boccalari E, Di Stasio D, Della Vella F, Carinci F, Lucchese A, Petruzzi M. Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics (Basel). 2019;9:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 73. | Spyropoulou V, Russo G, Rossi ED, Ruggiero C, Volpe D, D'Arcangelo G, Papoff P, Civitelli F, Aloi M, Oliva S. Diagnostic accuracy of multimodal noninvasive follow-up for pediatric ulcerative colitis: A single-center prospective study. J Pediatr Gastroenterol Nutr. 2024;78:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Moran CP, Neary B, Doherty GA. Endoscopic evaluation in diagnosis and management of inflammatory bowel disease. World J Gastrointest Endosc. 2016;8:723-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 75. | Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J Gastroenterol. 2018;24:4014-4020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 76. | Krzesiek E, Nienartowicz E, Iwańczak B. Value of magnetic resonance enterography in diagnosis and treatment follow up in Crohn's disease in children. Adv Med Sci. 2020;65:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | El-Assaly H, Mohamed AAB, Mustafa HAAF. Could ultrasound alone substitute MR imaging in evaluation of Crohn’s disease complications? Egypt J Radiol Nucl Med. 2024;55:170. [DOI] [Full Text] |
| 78. | Clough J, Colwill M, Poullis A, Pollok R, Patel K, Honap S. Biomarkers in inflammatory bowel disease: a practical guide. Therap Adv Gastroenterol. 2024;17:17562848241251600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 79. | Villanacci V, Reggiani-Bonetti L, Salviato T, Leoncini G, Cadei M, Albarello L, Caputo A, Aquilano MC, Battista S, Parente P. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica. 2021;113:39-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 80. | Kellermann L, Riis LB. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig Med Res. 2021;4:3-3. [DOI] [Full Text] |
| 81. | Parente P, Pastore M, Grillo F, Fassan M, Francalanci P, Dirodi A, Rossi C, Arpa G, De Angelis P, Gullo I, Mastracci L, Alaggio R, Vanoli A. Very Early Onset-IBD: evidence for the need of a multidisciplinary approach. Pathologica. 2022;114:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Collen LV, Kim DY, Field M, Okoroafor I, Saccocia G, Whitcomb SD, Green J, Dong MD, Barends J, Carey B, Weatherly ME; Regeneron Genetics centre, Rockowitz S, Sliz P, Liu E, Eran A, Grushkin-Lerner L, Bousvaros A, Muise AM, Klein C, Mitsialis V, Ouahed J, Snapper SB. Clinical Phenotypes and Outcomes in Monogenic Versus Non-monogenic Very Early Onset Inflammatory Bowel Disease. J Crohns Colitis. 2022;16:1380-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 83. | Yska HAF, Elsink K, Kuijpers TW, Frederix GWJ, van Gijn ME, van Montfrans JM. Diagnostic Yield of Next Generation Sequencing in Genetically Undiagnosed Patients with Primary Immunodeficiencies: a Systematic Review. J Clin Immunol. 2019;39:577-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Arai K. Very Early-Onset Inflammatory Bowel Disease: A Challenging Field for Pediatric Gastroenterologists. Pediatr Gastroenterol Hepatol Nutr. 2020;23:411-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Abraham RS. Relevance of laboratory testing for the diagnosis of primary immunodeficiencies: a review of case-based examples of selected immunodeficiencies. Clin Mol Allergy. 2011;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Levine AE, Zheng HB, Suskind DL. Linking Genetic Diagnosis to Therapeutic Approach in Very Early Onset Inflammatory Bowel Disease: Pharmacologic Considerations. Paediatr Drugs. 2022;24:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 87. | Ajbar A, Cross E, Matoi S, Hay CA, Baines LM, Saunders B, Farmer AD, Prior JA. Diagnostic Delay in Pediatric Inflammatory Bowel Disease: A Systematic Review. Dig Dis Sci. 2022;67:5444-5454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Olczyk M, Frankowska A, Tkaczyk M, Socha-Banasiak A, Czkwianianc E. Early Symptoms in Children with Inflammatory Bowel Disease: Implications for Subsequent Bone Mineral Deficiency. Children (Basel). 2024;11:1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Shaoul R, Day AS. An Overview of Tools to Score Severity in Pediatric Inflammatory Bowel Disease. Front Pediatr. 2021;9:615216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | Hauer AC, Sultan M, Darma A, Altamimi E, Serban DE, Assa A, Franco CPS, de Ridder L, Wilson DC, Afzal NA, Bronsky J, Georgieva M, Aloi M, Urbonas V, Navas-López VM, Medina JR, Turner D, Amil-Dias J. Management of pediatric inflammatory bowel diseases in limited-resource settings: A position paper from the Paediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Jevon GP, Madhur R. Endoscopic and histologic findings in pediatric inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2010;6:174-180. [PubMed] |
| 92. | Vernon-Roberts A, Day AS. Promoting early testing and appropriate referral to reduce diagnostic delay for children with suspected inflammatory bowel disease, a narrative review. Transl Pediatr. 2023;12:1416-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Moeeni V, Day AS. Impact of Inflammatory Bowel Disease upon Growth in Children and Adolescents. ISRN Pediatr. 2011;2011:365712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Sen P, Uday S. Bone Health in Paediatric Inflammatory Bowel Disease. Diagnostics (Basel). 2025;15:580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Ghishan FK, Kiela PR. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 96. | Gray WN, Resmini AR, Baker KD, Holbrook E, Morgan PJ, Ryan J, Saeed SA, Denson LA, Hommel KA. Concerns, Barriers, and Recommendations to Improve Transition from Pediatric to Adult IBD Care: Perspectives of Patients, Parents, and Health Professionals. Inflamm Bowel Dis. 2015;21:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 97. | Conrad MA, Kelsen JR. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr Gastroenterol Rep. 2020;22:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 98. | Barberio B, Segal JP, Quraishi MN, Black CJ, Savarino EV, Ford AC. Efficacy of Oral, Topical, or Combined Oral and Topical 5-Aminosalicylates, in Ulcerative Colitis: Systematic Review and Network Meta-analysis. J Crohns Colitis. 2021;15:1184-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Mansuri I, Wang S, Fishman L, Rufo PA, Liu E, Chan C, Bousvaros A. Clinical outcomes of maintenance therapy with sulfasalazine compared to 5-aminosalicylates in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2025;80:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | Lahad A, Weiss B. Current therapy of pediatric Crohn's disease. World J Gastrointest Pathophysiol. 2015;6:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Kandavel P, Eder SJ, Adler J; and the ImproveCareNow Network Pediatric IBD Learning Health System. Reduced Systemic Corticosteroid Use among Pediatric Patients With Inflammatory Bowel Disease in a Large Learning Health System. J Pediatr Gastroenterol Nutr. 2021;73:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, Kaplan GG, Seow CH. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;2015:CD000296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Singh A, Mahajan R, Kedia S, Dutta AK, Anand A, Bernstein CN, Desai D, Pai CG, Makharia G, Tevethia HV, Mak JW, Kaur K, Peddi K, Ranjan MK, Arkkila P, Kochhar R, Banerjee R, Sinha SK, Ng SC, Hanauer S, Verma S, Dutta U, Midha V, Mehta V, Ahuja V, Sood A. Use of thiopurines in inflammatory bowel disease: an update. Intest Res. 2022;20:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 104. | Konidari A, Anagnostopoulos A, Bonnett LJ, Pirmohamed M, El-Matary W. Thiopurine monitoring in children with inflammatory bowel disease: a systematic review. Br J Clin Pharmacol. 2014;78:467-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Lofland JH, Mallow P, Rizzo J. Cost-per-remission analysis of infliximab compared to adalimumab among adults with moderate-to-severe ulcerative colitis. J Med Econ. 2013;16:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Ledder O, Turner D. Multi-item Measures for Paediatric Inflammatory Bowel Diseases: The ABCs of All Those Acronyms. J Crohns Colitis. 2023;17:1154-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 107. | Fanizzi F, Allocca M, Fiorino G, Zilli A, Furfaro F, Parigi TL, Peyrin-Biroulet L, Danese S, D'Amico F. Raising the bar in ulcerative colitis management. Therap Adv Gastroenterol. 2024;17:17562848241273066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 108. | Velikova T, Sekulovski M, Peshevska-Sekulovska M. Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development. Antibodies (Basel). 2024;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 109. | Shah ED, Coburn ES, Nayyar A, Lee KJ, Koliani-Pace JL, Siegel CA. Systematic review: hepatosplenic T-cell lymphoma on biologic therapy for inflammatory bowel disease, including data from the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther. 2020;51:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 110. | Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr, Osterman MT, Saroufim A, Siegel CA, Yarur AJ, Melmed GY, Papamichael K. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am J Gastroenterol. 2021;116:2014-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 111. | Honap S, Agorogianni A, Colwill MJ, Mehta SK, Donovan F, Pollok R, Poullis A, Patel K. JAK inhibitors for inflammatory bowel disease: recent advances. Frontline Gastroenterol. 2024;15:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 112. | Ashton J, Lee KY, Thangarajah A, Rodrigues A, Kammermeier J. Therapeutic options for children and young people with moderate-to-severe ulcerative colitis. Frontline Gastroenterol. 2024;15:387-394. [DOI] [Full Text] |
| 113. | Herrera-deGuise C, Serra-Ruiz X, Lastiri E, Borruel N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne). 2023;10:1089099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 114. | Cucinotta U, Romano C, Dipasquale V. Diet and Nutrition in Pediatric Inflammatory Bowel Diseases. Nutrients. 2021;13:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 115. | Day AS, Lopez RN. Exclusive enteral nutrition in children with Crohn's disease. World J Gastroenterol. 2015;21:6809-6816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 116. | Bargas A, Palmela C, Glória L. Enteral Nutrition in Crohn's Disease: A Comprehensive Review of Its Role in Induction and Maintenance of Remission and Perioperative Management in Adult Patients. Nutrients. 2025;17:1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 117. | Jatkowska A, White B, Gkikas K, Seenan JP, MacDonald J, Gerasimidis K. Partial Enteral Nutrition in the Management of Crohn's Disease: A Systematic Review and Meta-Analysis. J Crohns Colitis. 2025;19:jjae177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 118. | Ehrlich S, Mark AG, Rinawi F, Shamir R, Assa A. Micronutrient Deficiencies in Children With Inflammatory Bowel Diseases. Nutr Clin Pract. 2020;35:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Turunen P, Ashorn M, Auvinen A, Iltanen S, Huhtala H, Kolho KL. Long-term health outcomes in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2009;15:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 120. | Dubinsky M. Special issues in pediatric inflammatory bowel disease. World J Gastroenterol. 2008;14:413-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Olczyk M, Czkwianianc E, Socha-Banasiak A. Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases. Life (Basel). 2022;12:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 165] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 123. | Targownik LE, Nugent Z, Singh H, Bernstein CN. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 124. | Beese SE, Harris IM, Dretzke J, Moore D. Body image dissatisfaction in patients with inflammatory bowel disease: a systematic review. BMJ Open Gastroenterol. 2019;6:e000255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Jongsma MME, Vuijk SA, Cozijnsen MA, van Pieterson M, Norbruis OF, Groeneweg M, Wolters VM, van Wering HM, Hojsak I, Kolho KL, van Wijk MP, Teklenburg-Roord STA, de Meij TGJ, Escher JC, de Ridder L. Evaluation of exclusive enteral nutrition and corticosteroid induction treatment in new-onset moderate-to-severe luminal paediatric Crohn's disease. Eur J Pediatr. 2022;181:3055-3065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 126. | Barrett K, Saxena S, Pollok R. Using corticosteroids appropriately in inflammatory bowel disease: a guide for primary care. Br J Gen Pract. 2018;68:497-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Triantafillidis JK. Surgical treatment of inflammatory bowel disease: From the gastroenterologist's stand-point. World J Gastrointest Surg. 2024;16:1235-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 128. | Kim S. Surgery in Pediatric Crohn's Disease: Indications, Timing and Post-Operative Management. Pediatr Gastroenterol Hepatol Nutr. 2017;20:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Liu S, Eisenstein S. State-of-the-art surgery for ulcerative colitis. Langenbecks Arch Surg. 2021;406:1751-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Meima-van Praag EM, Buskens CJ, Hompes R, Bemelman WA. Surgical management of Crohn's disease: a state of the art review. Int J Colorectal Dis. 2021;36:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 131. | Abad CCU, Llumiquinga DBL, Imbaquingo HJR, Rueda RAL, Rueda JEL, Moreno JDN, Zambrano IIZ. The Use of Minimally Invasive Surgery in the Treatment of Crohn's Disease: A Systematic Review of Evidence. Int J Med Sci Clin Res Stud. 2024;04. [DOI] [Full Text] |
| 132. | Kelay A, Tullie L, Stanton M. Surgery and paediatric inflammatory bowel disease. Transl Pediatr. 2019;8:436-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 133. | Colombo F, Frontali A, Baldi C, Cigognini M, Lamperti G, Manzo CA, Maconi G, Ardizzone S, Foschi D, Sampietro GM. Repeated surgery for recurrent Crohn's disease: does the outcome keep worsening operation after operation? A comparative study of 1224 consecutive procedures. Updates Surg. 2022;74:73-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 134. | Aljabri R, Al-Saraie S, Alhouti A. Optimizing Biologic Therapy for the Prevention of Post-Operative Recurrence in Crohn's Disease: Current Evidence and Future Perspectives. Biomedicines. 2025;13:1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 135. | Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 136. | Yeh AM, Wren A, Golianu B. Mind-Body Interventions for Pediatric Inflammatory Bowel Disease. Children (Basel). 2017;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Green Z, Ashton JJ, Beattie RM. Adolescent IBD: recent data and practical management. Frontline Gastroenterol. 2025. [DOI] [Full Text] |
| 138. | Konidari A, Dickens D, Pirmohamed M. Inflammatory Bowel Disease: A Personalized Approach. Front Pediatr. 2020;8:620545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 139. | Juillerat P, Grueber MM, Ruetsch R, Santi G, Vuillèmoz M, Michetti P. Positioning biologics in the treatment of IBD: A practical guide - Which mechanism of action for whom? Curr Res Pharmacol Drug Discov. 2022;3:100104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 140. | Berg DR, Colombel JF, Ungaro R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 141. | Cheifetz A. Overview of Therapeutic Drug Monitoring of Biologic Agents in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2017;13:556-559. [PubMed] |
| 142. | Kelsen JR, Baldassano RN. The role of monogenic disease in children with very early onset inflammatory bowel disease. Curr Opin Pediatr. 2017;29:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Uhlig HH, Muise AM. Clinical Genomics in Inflammatory Bowel Disease. Trends Genet. 2017;33:629-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 144. | Caballero Mateos AM, Cañadas de la Fuente GA, Gros B. Paradigm Shift in Inflammatory Bowel Disease Management: Precision Medicine, Artificial Intelligence, and Emerging Therapies. J Clin Med. 2025;14:1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 145. | Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 146. | Fansiwala K, Sauk JS. Small Molecules, Big Results: How JAK Inhibitors Have Transformed the Treatment of Patients with IBD. Dig Dis Sci. 2025;70:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 147. | Baccarella A, Patel T, Conrad MA, Macchi M, Boyer B, Pickering O, Borodyanskaya Y, Gaddipati S, Cohen M, Cubero A, Dawany N, Heimall J, Bunin N, Sullivan KE, Kelsen JR. Outcomes of Allogeneic Hematopoietic Stem Cell Transplant in Monogenic Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Meena S, Varla H, Swaminathan VV, Chandar R, Jayakumar I, Ramakrishnan B, Uppuluri R, Raj R. Hematopoietic stem cell Transplantation in Children with very Early Onset Inflammatory Bowel Disease Secondary to Monogenic Disorders of immune-dysregulation. Indian J Hematol Blood Transfus. 2023;39:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 149. | Spencer EA, Dubinsky MC. Precision Medicine in Pediatric Inflammatory Bowel Disease. Pediatr Clin North Am. 2021;68:1171-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Mogoş GFR, Manciulea Profir M, Enache RM, Pavelescu LA, Popescu Roşu OA, Cretoiu SM, Marinescu I. Intestinal Microbiota in Early Life: Latest Findings Regarding the Role of Probiotics as a Treatment Approach for Dysbiosis. Nutrients. 2025;17:2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 151. | Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (7)] |
| 152. | Lee M, Chang EB. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology. 2021;160:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 451] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 153. | Miró-González ÁA, Maldonado-Chaar SM, Zambrana-Valenzuela R, Iglesias-Escabi IM, Arciniegas-Medina NJ. Development of Very-Early-Onset Inflammatory Bowel Disease After Multiple Early-Life Antibiotic Exposures: A Case Report and Literature Review. Cureus. 2023;15:e33813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 154. | Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, de Souza HSP. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int J Mol Sci. 2022;23:3464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 155. | Murgiano M, Bartocci B, Puca P, di Vincenzo F, Del Gaudio A, Papa A, Cammarota G, Gasbarrini A, Scaldaferri F, Lopetuso LR. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. Int J Mol Sci. 2025;26:3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 156. | Fanizzi F, D'Amico F, Zanotelli Bombassaro I, Zilli A, Furfaro F, Parigi TL, Cicerone C, Fiorino G, Peyrin-Biroulet L, Danese S, Allocca M. The Role of Fecal Microbiota Transplantation in IBD. Microorganisms. 2024;12:1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 157. | Borody TJ, Clancy A. Fecal microbiota transplantation for ulcerative colitis-where to from here? Transl Gastroenterol Hepatol. 2019;4:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 158. | Gerasimidis K, Russell RK, Giachero F, Gkikas K, Tel B, Assa A, Bronsky J, de Ridder L, Hojsak I, Jenke A, Norsa L, Sigall-Boneh R, Sila S, Wine E, Zilbauer M, Strisciuglio C, Gasparetto M; ESPGHAN Special Interest Group in Basic and Translational Research; the ESPGHAN IBD Porto Working Group; the ESPGHAN Allied Health Professionals. Precision nutrition in pediatric IBD: A position paper from the ESPGHAN special interest group for basic science and translational research, the IBD Porto group, and allied health professionals. J Pediatr Gastroenterol Nutr. 2024;78:428-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Xu D, Peng Z, Li Y, Hou Q, Peng Y, Liu X. Progress and Clinical Applications of Crohn's Disease Exclusion Diet in Crohn's Disease. Gut Liver. 2024;18:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 160. | Kim S, Koh H. Nutritional aspect of pediatric inflammatory bowel disease: its clinical importance. Korean J Pediatr. 2015;58:363-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Al-Beltagi M, Saeed NK, Bediwy AS, Elbeltagi R. Fecal calprotectin in pediatric gastrointestinal diseases: Pros and cons. World J Clin Pediatr. 2024;13:93341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (4)] |
| 162. | Hong SM, Baek DH. Diagnostic Procedures for Inflammatory Bowel Disease: Laboratory, Endoscopy, Pathology, Imaging, and Beyond. Diagnostics (Basel). 2024;14:1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 163. | Kapel N, Ouni H, Benahmed NA, Barbot-Trystram L. Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases. Clin Transl Gastroenterol. 2023;14:e00617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 164. | Shimoyama T, Yamamoto T, Yoshiyama S, Nishikawa R, Umegae S. Leucine-Rich Alpha-2 Glycoprotein Is a Reliable Serum Biomarker for Evaluating Clinical and Endoscopic Disease Activity in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2023;29:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 165. | Pathak P, Choudhury A, Marikanty A, Jena A, Sebastian S, Sharma V. Wearable Technologies in Inflammatory Bowel Disease: A Scoping Review. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Rowan C, Hirten R. The future of telemedicine and wearable technology in IBD. Curr Opin Gastroenterol. 2022;38:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 167. | Horrigan JM, Louis E, Spinelli A, Travis S, Moum B, Salwen-Deremer J, Halfvarson J, Panaccione R, Dubinsky MC, Munkholm P, Siegel CA. The Real-World Global Use of Patient-Reported Outcomes for the Care of Patients With Inflammatory Bowel Disease. Crohns Colitis 360. 2023;5:otad006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 168. | Yin AL, Hachuel D, Pollak JP, Scherl EJ, Estrin D. Digital Health Apps in the Clinical Care of Inflammatory Bowel Disease: Scoping Review. J Med Internet Res. 2019;21:e14630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 169. | Anand R, George AT, Rubin DT, Spiegel BMR, Bernstein CN. The role of virtual reality in managing inflammatory bowel disease: a novel approach to bridging mental and physical health. J Can Assoc Gastroenterol. 2025;8:S15-S20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 170. | Sedano R, Solitano V, Vuyyuru SK, Yuan Y, Hanžel J, Ma C, Nardone OM, Jairath V. Artificial intelligence to revolutionize IBD clinical trials: a comprehensive review. Therap Adv Gastroenterol. 2025;18:17562848251321915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 171. | Stapersma L, van den Brink G, Szigethy EM, Escher JC, Utens EMWJ. Systematic review with meta-analysis: anxiety and depression in children and adolescents with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 172. | Volpato E, Bosio C, Previtali E, Leone S, Armuzzi A, Pagnini F, Graffigna G. The evolution of IBD perceived engagement and care needs across the life-cycle: a scoping review. BMC Gastroenterol. 2021;21:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 173. | Mosli MH, Alamri AA, Saadah OI. Work and School Absenteeism in Inflammatory Bowel Disease Patients in Jeddah, Saudi Arabia: A Cross-Sectional Study. Saudi J Med Med Sci. 2021;9:159-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 174. | Barned C, Fabricius A, Stintzi A, Mack DR, O'Doherty KC. "The Rest of my Childhood was Lost": Canadian Children and Adolescents' Experiences Navigating Inflammatory Bowel Disease. Qual Health Res. 2022;32:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 175. | Cushman G, Shih S, Reed B. Parent and Family Functioning in Pediatric Inflammatory Bowel Disease. Children (Basel). 2020;7:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 176. | Borren NZ, Conway G, Tan W, Andrews E, Garber JJ, Yajnik V, Ananthakrishnan AN. Distance to Specialist Care and Disease Outcomes in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 177. | Thapwong P, Norton C, Rowland E, Czuber-Dochan W. Our Life Is a Rollercoaster! A Qualitative Phenomenological Study Exploring the Impact of IBD on Family Members. Inflamm Bowel Dis. 2024;30:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 178. | Kim J, Ye BD. Successful Transition from Pediatric to Adult Care in Inflammatory Bowel Disease: What is the Key? Pediatr Gastroenterol Hepatol Nutr. 2019;22:28-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | MacKay LJ, Chang U, Kreiter E, Nickel E, Kamke J, Bahia R, Shantz S, Meyerhoff H. Exploration of trust between pediatric nurses and children with a medical diagnosis and their caregivers on inpatient care units: A scoping review. J Pediatr Nurs. 2024;78:e1-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 180. | Taft TH, Ballou S, Bedell A, Lincenberg D. Psychological Considerations and Interventions in Inflammatory Bowel Disease Patient Care. Gastroenterol Clin North Am. 2017;46:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 181. | Tan B, Ong D. Pediatric to adult inflammatory bowel disease transition: the Asian experience. Intest Res. 2020;18:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 182. | Raeisi A, Rarani MA, Soltani F. Challenges of patient handover process in healthcare services: A systematic review. J Educ Health Promot. 2019;8:173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 183. | Tóbi L, Prehoda B, Balogh AM, Nagypál P, Kovács K, Miheller P, Iliás Á, Dezsőfi-Gottl A, Cseh Á. Transition is associated with lower disease activity, fewer relapses, better medication adherence, and lower lost-to-follow-up rate as opposed to self-transfer in pediatric-onset inflammatory bowel disease patients: results of a longitudinal, follow-up, controlled study. Therap Adv Gastroenterol. 2024;17:17562848241252947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 184. | Singh A, Bhardwaj A, Sharma R, Midha V, Sood A. Developing IBD counsellors in low- and middle-income countries: bridging gaps in patient care. EClinicalMedicine. 2025;83:103218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 185. | Jensen PT, Paul GV, LaCount S, Peng J, Spencer CH, Higgins GC, Boyle B, Kamboj M, Smallwood C, Ardoin SP. Assessment of transition readiness in adolescents and young adults with chronic health conditions. Pediatr Rheumatol Online J. 2017;15:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 186. | Rosen MA, DiazGranados D, Dietz AS, Benishek LE, Thompson D, Pronovost PJ, Weaver SJ. Teamwork in healthcare: Key discoveries enabling safer, high-quality care. Am Psychol. 2018;73:433-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 187. | Kaul K, Schumann S, Felder J, Däbritz J, de Laffolie J. Patient Empowerment Among Children and Adolescents with Inflammatory Bowel Disease (IBD) and Parents of IBD Patients-Use of Counseling Services and Lack of Knowledge About Transition. Children (Basel). 2025;12:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 188. | Wren AA, Maddux MH. Integrated Multidisciplinary Treatment for Pediatric Inflammatory Bowel Disease. Children (Basel). 2021;8:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 189. | Brandt L, Liu S, Heim C, Heinz A. The effects of social isolation stress and discrimination on mental health. Transl Psychiatry. 2022;12:398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 190. | Russo K. Assessment and Treatment of Adolescents With Chronic Medical Conditions. J Health Serv Psychol. 2022;48:69-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 191. | Nicholas DB, Otley A, Smith C, Avolio J, Munk M, Griffiths AM. Challenges and strategies of children and adolescents with inflammatory bowel disease: a qualitative examination. Health Qual Life Outcomes. 2007;5:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 192. | Taft TH, Ballou S, Keefer L. A preliminary evaluation of internalized stigma and stigma resistance in inflammatory bowel disease. J Health Psychol. 2013;18:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 193. | Pulimeno M, Piscitelli P, Colazzo S, Colao A, Miani A. School as ideal setting to promote health and wellbeing among young people. Health Promot Perspect. 2020;10:316-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 194. | Spiel CF, Evans SW, Langberg JM. Evaluating the content of Individualized Education Programs and 504 Plans of young adolescents with attention deficit/hyperactivity disorder. Sch Psychol Q. 2014;29:452-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 195. | Shim JO, Seo JK. Very early-onset inflammatory bowel disease (IBD) in infancy is a different disease entity from adult-onset IBD; one form of interleukin-10 receptor mutations. J Hum Genet. 2014;59:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 196. | Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1:462-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 197. | Wang Y, Liu D, Gao H, Liu W, Mao Y. Treatment of IL-10RA deficiency of pediatric patients with very early onset inflammatory bowel disease by allogeneic haematopoietic stem cell transplantation. Sci Rep. 2025;15:9606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 198. | Lee CL, Chuang CK, Chiu HC, Chang YH, Tu YR, Lo YT, Lin HY, Lin SP. Understanding Genetic Screening: Harnessing Health Information to Prevent Disease Risks. Int J Med Sci. 2025;22:903-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 199. | Kullo IJ, Lewis CM, Inouye M, Martin AR, Ripatti S, Chatterjee N. Polygenic scores in biomedical research. Nat Rev Genet. 2022;23:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 200. | Gettler K, Levantovsky R, Moscati A, Giri M, Wu Y, Hsu NY, Chuang LS, Sazonovs A, Venkateswaran S, Korie U, Chasteau C; UK IBD Genetics Consortium, National Institute of Diabetes, Digestive and Kidney Diseases Inflammatory Bowel Disease Genetics Consortium, Duerr RH, Silverberg MS, Snapper SB, Daly MJ, McGovern DP, Brant SR, Rioux JD, Kugathasan S, Anderson CA, Itan Y, Cho JH. Common and Rare Variant Prediction and Penetrance of IBD in a Large, Multi-ethnic, Health System-based Biobank Cohort. Gastroenterology. 2021;160:1546-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 201. | Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, Che T, Zhang C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019;8:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 552] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 202. | Denson LA, Curran M, McGovern DPB, Koltun WA, Duerr RH, Kim SC, Sartor RB, Sylvester FA, Abraham C, de Zoeten EF, Siegel CA, Burns RM, Dobes AM, Shtraizent N, Honig G, Heller CA, Hurtado-Lorenzo A, Cho JH. Challenges in IBD Research: Precision Medicine. Inflamm Bowel Dis. 2019;25:S31-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 203. | Cannarozzi AL, Latiano A, Massimino L, Bossa F, Giuliani F, Riva M, Ungaro F, Guerra M, Brina ALD, Biscaglia G, Tavano F, Carparelli S, Fiorino G, Danese S, Perri F, Palmieri O. Inflammatory bowel disease genomics, transcriptomics, proteomics and metagenomics meet artificial intelligence. United European Gastroenterol J. 2024;12:1461-1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 204. | Kumagai H, Shimizu T, Iwama I, Hagiwara SI, Kudo T, Takahashi M, Saito T, Kunisaki R, Uchino M, Hiraoka S, Naganuma M, Sugimoto K, Miyoshi J, Shibuya T, Hisamatsu T. A consensus statement on health-care transition for childhood-onset inflammatory bowel disease patients. Pediatr Int. 2022;64:e15241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 205. | Krauthammer A, Weintraub I, Shaoul R, Lev-Tzion R, Broide E, Wilschanski M, Lerner A, Yerushalmi B, Shouval DS, Shamaly H, Haberman-Ziv Y, Weiss B. Infantile-onset inflammatory bowel disease has variable long-term outcomes. Front Pediatr. 2023;11:1097779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 206. | Vuijk SA, Camman AE, de Ridder L. Considerations in Paediatric and Adolescent Inflammatory Bowel Disease. J Crohns Colitis. 2024;18:ii31-ii45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 207. | Picoraro JA, Lee D, Heller CA, Weaver A, Hyams JS, Conklin LS, Otley A, Ziring D, Kugathasan S, Rosh JR, Mulberg A, Denson LA, Kappelman MD, Grossman AB, Bousvaros A, Park KT. Pediatric Inflammatory Bowel Disease Clinical Innovations Meeting of the Crohn's & Colitis Foundation: Charting the Future of Pediatric IBD. Inflamm Bowel Dis. 2019;25:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 208. | Aljabri A, Soliman GM, Ramadan YN, Medhat MA, Hetta HF. Biosimilars versus biological therapy in inflammatory bowel disease: challenges and targeting strategies using drug delivery systems. Clin Exp Med. 2025;25:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 209. | Sahn B, Maltz RM, Rosh JR. Children With Inflammatory Bowel Diseases are Disadvantaged by Current Drug Approval Policies: A Call for Urgent Change. Crohns Colitis 360. 2025;7:otaf036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 210. | PRACTICAL, PANTHER, TRAITS, INCEPT; and REMAP-CAP investigators. The Rise of Adaptive Platform Trials in Critical Care. Am J Respir Crit Care Med. 2024;209:491-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 211. | Wilkins BJ, Kelsen JR, Conrad MA. A Pattern-based Pathology Approach to Very Early-onset Inflammatory Bowel Disease: Thinking Beyond Crohn Disease and Ulcerative Colitis. Adv Anat Pathol. 2022;29:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 212. | Cai Z, Wang S, Li J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front Med (Lausanne). 2021;8:765474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 213. | Bueno-Hernández N, Yamamoto-Furusho JK, Mendoza-Martínez VM. Nutrition in Inflammatory Bowel Disease: Strategies to Improve Prognosis and New Therapeutic Approaches. Diseases. 2025;13:139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 214. | Birimberg-Schwartz L, Zucker DM, Akriv A, Cucchiara S, Cameron FL, Wilson DC, Lazowska I, Yianni L, Paul SP, Romano C, Kolacek S, Buderus S, Pærregaard A, Russell RK, Escher JC, Turner D; Pediatric IBD Porto group of ESPGHAN. Development and Validation of Diagnostic Criteria for IBD Subtypes Including IBD-unclassified in Children: a Multicentre Study From the Pediatric IBD Porto Group of ESPGHAN. J Crohns Colitis. 2017;11:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 215. | Ye BD, Travis S. Improving the quality of care for inflammatory bowel disease. Intest Res. 2019;17:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 216. | Raffaele A, Ferlini CM, Fusi G, Lenti MV, Cereda E, Caimmi SME, Bertozzi M, Riccipetitoni G. Navigating the transition: a multidisciplinary approach to inflammatory bowel disease in children. Pediatr Surg Int. 2024;40:245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 217. | Prates PEG, Correa-Júnior AJS, Russo TMDS, Paraizo-Horvath CMS, Teles AADS, Sonobe HM. Effectiveness of Family Coping Interventions in Improving Problem-Solving Skills in the Care of Children and Adolescent Cancer Survivors during and after Treatment: A Scoping Review. Nurs Rep. 2024;14:2153-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 218. | Kaffenberger C. School Reentry for Students with a Chronic Illness: A Role for Professional School Counselors. Prof Sch Couns. 2006;9:223-230. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 219. | Campbell F, Biggs K, Aldiss SK, O'Neill PM, Clowes M, McDonagh J, While A, Gibson F. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev. 2016;4:CD009794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 220. | Dotson JL, Bricker J, Chisolm DJ, Mackner LM. Patient, Parent, and Provider Perceptions of Barriers to Pediatric Inflammatory Bowel Disease Care. JPGN Rep. 2023;4:e386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 221. | Naidoo CM, Leach ST, Day AS, Lemberg DA. Inflammatory bowel disease in children of middle eastern descent. Int J Pediatr. 2014;2014:906128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 222. | Croft NM, de Ridder L, Griffiths AM, Hyams JS, Ruemmele FM, Turner D, Cheng K, Lutsar I, Greco M, Gołębiewska Z, Laumond F, Cavaller-Bellaubi M, Elgreey A, Altepeter TA, Pallidis C, Norga K, Nelson R, Crandall W, Vassal G. Paediatric Inflammatory Bowel Disease: A Multi-Stakeholder Perspective to Improve Development of Drugs for Children and Adolescents. J Crohns Colitis. 2023;17:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 223. | Wiczynska-Ryl J, Krogulska A. Incidence of Inflammatory Bowel Disease in Children. Gastroenterology Res. 2025;18:71-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 224. | Rao MS, Gaur A, Bharadwaj HR, Imran S, Tan JK, Abbas S, Fuad M, Abuhashem S, Shah MH, Dalal P, Al Khatib AN, Abbasher Hussien Mohamed Ahmed K. The current state of pediatric gastroenterology in under-resourced nations. Ann Med Surg (Lond). 2025;87:2218-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 225. | Barfield E, Sockolow R, Hoffenberg E, Saeed S, Kim S, Siebold L, Picoraro J, Moses J, Dykes D, Grossman A, Wahbeh G, Park KT. Assuring Quality for Non-hospital-based Biologic Infusions in Pediatric Inflammatory Bowel Disease: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 226. | Adedara VO, Adedara CA, Ruth ND, Alozie GU, Nettagul N. Advancements in the Management of Pediatric and Adult Inflammatory Bowel Disease: A Systematic Review of Treatment Strategies and Long-Term Outcomes. Cureus. 2024;16:e72324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 227. | Constant BD, Adler J, Gold BD, Dotson J, Lightdale JR, Scott F, Saeed S, Kim S, Moses J, de Zoeten EF, Mirea L, Ritchey A, Pasternak B. National perspectives of barriers by insurance and pharmacy benefit managers in pediatric inflammatory bowel disease. JPGN Rep. 2025;6:80-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 228. | Lv H, Li HY, Zhang HN, Liu Y. Delayed diagnosis in inflammatory bowel disease: Time to consider solutions. World J Gastroenterol. 2024;30:3954-3958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 229. | van Gaalen MAC, van Pieterson M, Waaijenberg P, Kindermann A, Wolters VM, Dijkstra A, van Wering H, Wessels M, de Ridder L, Rizopoulos D, Derikx CLAAP, Escher JC; Kids with Crohn’s, Colitis (KiCC) Working Group for Collaborative Paediatric IBD Research in the Netherlands, the Dutch Initiative on Crohn and Colitis (ICC) and Dutch Nurses Network Inflammatory Bowel Disease (NIBD). Effectiveness of Transitional Care in Inflammatory Bowel Disease; Development, Validation, and Initial Outcomes of a Transition Success Score. J Crohns Colitis. 2025;19:jjae166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/