Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.110241
Revised: June 20, 2025
Accepted: August 13, 2025
Published online: September 21, 2025
Processing time: 108 Days and 13.8 Hours
Management of portal hypertension has been the focus of the Baveno guidelines since 1990. This article explores the evolution of these recommendations and their impact on clinical practice. Initially reliant on invasive diagnostics such as the hepatic venous pressure gradient, later editions have incorporated non-invasive methods such as elastography and serum biomarkers. Management strategies have evolved substantially. Endoscopic surveillance has shifted from routine annual endoscopy to an individualized approach based on liver stiffness and platelet count. The role of non-selective beta-blockers (NSBBs) in primary prophy
Core Tip: This is the first and only comprehensive review to date covering all editions of the Baveno guidelines on portal hypertension. It traces the evolution of diagnostic and management strategies from invasive approaches to modern non-invasive tools, and outlines key shifts in surveillance, prophylaxis, and rescue therapy. The review summarizes resolved controversies, identifies current gaps in knowledge, and highlights future research directions that could further enhance clinical outcomes in portal hypertension.
- Citation: Brzdęk M, Dobrowolska K, Janczura J, Wajdowicz M, Brzdęk K, Zarębska-Michaluk D, Gąsiorowska A, Mangia A. Advances in portal hypertension management: Evolution of the Baveno guidelines. World J Gastroenterol 2025; 31(35): 110241
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/110241.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.110241
Portal hypertension (PH) is a clinical syndrome caused by an increased blood flow through the portal vein combined with increased intrahepatic resistance. Liver cirrhosis is considered the most common cause of PH[1]. According to the latest global estimates published in 2025, it was estimated that in 2021 more than 58 million new cases of cirrhosis and other chronic liver diseases (CLDs) occurred globally, with more than 1.4 million associated deaths[2]. The onset of PH in patients with liver cirrhosis is considered a turning point in the course of the disease, which may be accompanied by the development of life-threatening complications, such as gastroesophageal varices (GOVs), ascites, or hepatocellular carcinoma[3-5]. It is estimated that at the time of diagnosis of cirrhosis, varices are present in 30% of patients with compensated and in 60% of patients with decompensated cirrhosis. The incidence of bleeding from esophageal varices (EVs) is estimated at 5% per year, but in patients with large EVs, the risk can be as high as 30%. The first acute variceal bleeding is fatal in up to 50% of patients[6]. Notably, recent data show that bleeding-related mortality has decreased over time but remains substantial[7].
Given the high incidence and severity of PH complications, the Baveno guidelines were created and first published in 1990. The guidelines are named after Baveno, a small town in northern Italy, where the second-ever meeting of experts on the subject was organized[8]. The first meeting, held in Groningen in 1986 and organized by Andrew Burroughs, resulted in the publication of a review paper, which is not considered part of the official Baveno guidelines[9]. Ensuing conferences were held not only in Baveno but also in Stresa (Italy) and Reston (VA, United States), and were organized by the New Italian Endoscopic Club.
The most recent Baveno VII guidelines were published following a conference originally scheduled for March 2020 but postponed to October 2021 due to the coronavirus disease 2019 pandemic; it was held online[1,10]. According to available information, the next meeting, Baveno VIII, is planned in Baveno in March 2026 and was preceded by the Baveno VIII online symposium in March 2025[11-13]. Since 2016, the guidelines have been supported by the European Association for the Study of Liver (EASL). At that time, the Baveno Cooperation was named an official EASL consortium, aiming to simplify and streamline the collaboration of experts in the field of PH and its complications[14].
The Baveno guidelines are useful tools for clinicians and researchers worldwide. They provide an overview of the rapidly changing diagnostic and therapeutic standards for PH and up-to-date, evidence-based definitions and management strategies.
The aim of this paper is to analyze the changes that have occurred in the Baveno guidelines over the years and their impact on the approach to the diagnosis and treatment of PH and its complications.
A comprehensive literature search was conducted using PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar. The search included publications from 2015 onwards, excluding original Baveno guidelines and other seminal publications necessary to provide context for the earliest Baveno guidelines. Keywords used were combinations of “portal hypertension”, “Baveno guidelines”, “hepatic venous pressure gradient”, “varices”, and related terms. Articles published in English and relevant clinical studies, guidelines, and reviews were included.
The Baveno guidelines have played a key role in shaping standards for the diagnosis of PH. Various approaches exist, ranging from invasive methods such as hepatic venous pressure gradient (HVPG) measurement, endoscopy to non-invasive imaging techniques.
Currently, HVPG is considered the gold standard for assessing the severity of PH, and values above 5 mmHg indicate the presence of PH[15]. HVPG is measured by inserting a catheter through the internal jugular vein or femoral vein into one of the hepatic veins, to determine the difference between wedged hepatic venous pressure and free hepatic venous pressure[1]. HVPG measurement was first introduced in the second official Baveno guidelines in 1996 as an optional parameter to be used in addition to endoscopic findings in assessing bleeding risk[16]. The third edition of the recommendations (2000) defined clinically significant PH (CSPH) as HVPG greater than 10 mmHg and listed both HVPG measurements and endoscopic evaluation as reliable tools for diagnosing CSPH[17]. In Baveno IV, although more emphasis was placed on predictive models, the use of HVPG as a diagnostic method remained strongly emphasized, particularly its role in identifying patients at risk for complications such as varices and ascites[18]. Baveno V continued to support this approach, but the guidelines recommended routine HVPG measurements in centers with adequate expertise and resources[19]. Baveno VI (2015) introduced the concept of compensated advanced CLD (cACLD). This is particularly relevant from a clinical perspective, as differentiating between advanced fibrosis and cirrhosis remains challenging, especially in obese patients. Both stages can present with similar clinical features, and in the absence of more advanced diagnostic techniques, such as liver biopsy or elastography, it is difficult to distinguish them based solely on clinical signs and symptoms. This edition fully established HVPG as the gold standard for the diagnosis of CSPH and HVPG value > 5 mmHg has also become a recognized method for confirming the diagnosis of cACLD[20].
The latest guidelines have focused primarily on the importance and indications for HVPG measurement[1]. In addition to technical advances, this edition highlights specific patient subgroups that present diagnostic challenges, such as patients with primary biliary cholangitis, in whom the additional presinusoidal component of PH may not be picked up by HVPG, potentially leading to an underestimation of its prevalence and severity. Similarly, in patients with cirrhosis associated with non-alcoholic steatohepatitis and CLD who show clinical signs of PH despite HVPG < 10 mmHg, the accuracy of HVPG as the sole diagnostic tool is limited. Additionally, when PH is suspected despite HVPG is < 10 mmHg, portosystemic vascular disorder should be considered and ruled out[1,21]. Over the years, HVPG has evolved from a novel measurement tool to the gold standard for diagnosing and monitoring PH. Although non-invasive alternatives continue to evolve, HVPG remains a valuable tool for prognostic assessment and treatment stratification, especially in research and specialized clinical settings.
Endoscopy remains an essential tool in the diagnosis, staging, and treatment of varices, one of the most life-threatening complications of PH. The first official Baveno guidelines laid the groundwork for endoscopic classification of esophageal variceal features in PH. The system was simple and standardized, offering a two-stage classification of EVs (large/small), although the cutoff point was not clearly defined. Other parameters, such as color and location, were listed, but only the presence or absence of red dots was considered clinically relevant. Although endoscopy was identified as the most valuable tool for assessing bleeding risk, no screening protocol was proposed[8]. In the 1996 recommendations, experts underlined the importance of evaluating all patients with cirrhosis for the presence of PH and identified endoscopy with ultrasound as the recommended method of evaluation in patients with cirrhosis without a previous bleeding episode[16]. Moreover, endoscopy became the recommended method for the routine diagnosis of gastric varices, and the guidelines suggested using the New Italian Endoscopic Club classification in their evaluation[16,22]. Additionally, the Baveno II recommendations mentioned gastric antral vascular ectasia (GAVE) for the first time as a condition contributing to gastrointestinal bleeding in patients with PH, while emphasizing the need for further research to better understand the bleeding patterns associated with GAVE[23]. An important change came in Baveno III, which proposed clear guidelines for the interval between endoscopies depending on the clinical condition of patients[17]. Baveno III also endorsed the Sarin classification for diagnosing gastric varices. According to this system, varices are categorized into GOVs and isolated gastric varices (IGVs). GOVs include GOV type 1, which extend 2-5 cm below the gastroesophageal junction along the lesser curvature as extensions of EVs; and GOV type 2, which extend to the bottom of the stomach as large, tortuous, nodular varices. IGVs, occurring without EVs, include IGV type 1s, which are localized to the fundus as tortuous and nodular varices often showing a red color; and IGV type 2s, which are ectopic varices that can appear anywhere in the stomach and can also include duodenal varices[17,24]. The Baveno III consensus distinguished GAVE as a separate clinical entity rather than a consequence of PH. It was described as clusters of red spots arranged in a linear or diffuse fashion within the gastric antrum, with confirmation requiring biopsy. The guidelines also emphasized that GAVE can be seen in conditions other than PH[17]. The 2005 and 2007 sessions confirmed that endoscopic screening remained the most effective method for detecting varices[18,19]. Moreover, for the first time, Baveno IV introduced an expanded classification of EVs, adding a category for medium-sized varices. This change was specifically addressed in the treatment guidelines, according to which non-selective beta-blockers (NSBBs) were recommended for the prevention of first bleeding only in patients with medium and large EVs[18]. With the sixth edition, the introduction of cACLD brought further refinements, designating upper gastrointestinal endoscopy as one of the methods to confirm the diagnosis of cACLD, while identifying a subgroup of patients with cACLD among whom the diagnosis of CSPH can be made without the use of endoscopy[20]. The latest consensus upholds these principles while advocating for further evaluation of the utility, safety and accuracy of endoscopic ultrasonography. In addition, guidelines for specific groups of patients who may require tailored endoscopic treatment have also been expanded[1].
Due to the limitations of invasive methods, such as a high risk of complications, significant cost, and the inability to implement them routinely in daily clinical practice, non-invasive diagnostic tests (NIDTs) have been developed and extensively studied[25]. These tests offer alternative tools for assessing the progression of CLD and PH, as well as for predicting the risk of their complications and mortality[26]. With the advancement of imaging techniques, expert re
In the initial guidelines, hepatic vessel angiography was recognized as the "gold standard" among imaging studies for the assessment of PH[8]. This technique was primarily recommended for patients being considered for surgical treatment and in cases of clinical uncertainty. For screening purposes, experts recommended ultrasonography.
The development of ultrasonography techniques using the Doppler method influenced the modification of recommendations in the subsequent edition[16]. This examination was recommended for the screening assessment of all patients with liver cirrhosis for signs of PH and for monitoring patients with already diagnosed PH who had not yet experienced a bleeding episode. The indication for the use of hepatic vessel angiography was maintained, particularly for patients qualified for surgical treatment and in cases where Doppler ultrasonography was insufficient.
The third edition of the Baveno consensus introduced the definition of CSPH, with its diagnosis relying exclusively on invasive techniques. However, none of the NIDT, including Doppler ultrasonography, were accepted for routine clinical use in diagnosing CSPH[17].
The fourth and fifth Baveno consensus editions did not introduce changes in the recommendations for diagnostics and endoscopic screening[18,19]. The previously used NIDT were considered merely helpful in identifying patients with CSPH. At the same time, it was emphasized that no satisfactory non-endoscopic indicators of EVs exist.
Baveno VI introduced significant changes regarding the use of NIDT for diagnosing and monitoring patients with PH[20]. The role of elastography was emphasized in identifying patients with CLD at risk of developing CSPH. For the first time, Baveno VI introduced the concept of cACLD, highlighting that it encompasses both advanced fibrosis and cirrhosis, which form a continuum of disease. It was noted that, in daily clinical practice, a clear distinction between these conditions is often impossible, which served as the basis for recognizing them as a single diagnostic category. The precise criteria for exclusion, suspicion, and diagnosis of cACLD, as well as indications for endoscopy based on non-invasive test results, are presented in Table 1.
| Baveno VI[20], 2015 | Baveno VII[1], 2022 | |
| Exclusion of cACLD | TE1 < 10 kPa and absence of clinical symptoms of disease | TE1 < 10 kPa and absence of clinical and imaging signs of disease |
| Suspicion of cACLD | TE1 10-15 kPa | TE1 10-15 kPa |
| High suspicion of cACLD | TE1 > 15 kPa | TE1 > 15 kPa |
| Diagnosis of cACLD | Advanced fibrosis or cirrhosis in histopathological examination; Esophageal varices; HVPG > 5 mmHg | Definition of cACLD based on liver stiffness measurement |
| Exclusion of CSPH in cACLD | No recommendation | TE1 ≤ 15 kPa and PLT ≥ 150000/μL |
| Confirmation of CSPH in cACLD | HVPG ≥ 10 mmHg; TE1 ≤ 20-25 kPa alone or in combination with PLT and spleen size assessment (in the etiology of viral liver disease); Imaging studies showing collateral circulation | TE1 ≥ 25 kPa (applies to patients with cACLD related to virus and/or alcohol and/or NASH without obesity) |
| Indications for endoscopy | TE1 ≥ 20 kPa or PLT ≤ 150000/μL | TE1 ≥ 20 kPa or PLT ≤ 150000/μL |
| SSM | No recommendation | It can be used in chronic viral hepatitis to exclude CSPH (< 21 kPa) and diagnose CSPH (> 50 kPa); In individuals with SSM ≤ 40 kPa, endoscopy can be omitted |
One of the key modifications in Baveno VII is the more precise definition of the role of elastography in identifying cACLD[1]. Additionally, for individuals suspected of having cACLD, it is recommended to repeat elastography and laboratory tests every 12 months to monitor disease progression. A novel aspect of Baveno VII is the introduction of spleen stiffness measurement (SSM) as an additional tool in the assessment of CSPH. In patients with viral hepatitis, SSM < 21 kPa excludes CSPH, while a value > 50 kPa confirms its presence. These modifications aim to reduce the number of unnecessary invasive procedures while improving the early detection of patients at risk of decompensation and complications related to PH. Furthermore, the use of fibrosis biomarkers was recommended as a complementary diagnostic tool when liver stiffness measurement (LSM) exceeds 10 kPa. These biomarkers include fibrosis-4 (≥ 2.67), enhanced liverfibrosis test (≥ 9.8), and FibroTest (≥ 0.58 for alcohol-related or viral liver disease, ≥ 0.48 for non-alcoholic fatty liver disease).
Beyond its diagnostic applications, HVPG has proven to be a powerful prognostic tool, providing valuable insights into the risk of disease progression, complications, and treatment outcomes. Already at the second Baveno conference in 1996, HVPG was recognized not only for its role in assessing the risk of variceal bleeding but also as an essential parameter for evaluating the efficacy of emerging pharmacological treatments. This early acknowledgment highlighted the growing importance of hemodynamic measurements in both risk stratification and therapeutic monitoring[16]. At Baveno III, HVPG’s was emphasized as the only parameter currently suitable for monitoring the response to pharmacological therapy. However, their clinical use was not recommended[17]. By the time of Baveno IV in 2005, the prognostic role of HVPG had expanded significantly. HVPG was identified as the most reliable predictor for the development of EVs and was recognized as the only established predictor for the onset of ascites. Additionally, an HVPG value exceeding 12 mmHg was considered a critical threshold for identifying patients at high risk of variceal formation and progression[18]. Since Baveno V, it was recommended that studies evaluating drug therapy for primary prevention should include HVPG measurements[19]. In addition, an HVPG value above 20 mmHg was identified as one of the most important factors for consistently predicting treatment failure within the first 5 days after the initiation of therapy for acute variceal bleeding[19]. At Baveno VI, the prognostic role of HVPG in guiding therapy became even more clearly defined. HVPG change was recognized as a relevant surrogate outcome, and measurement of the hemodynamic response to therapy was recommended as a clinically meaningful strategy. Specifically, in patients undergoing primary prophylaxis with NSBBs, a reduction of HVPG by at least 10% from baseline, or achieving an absolute HVPG value of 12 mmHg or lower, was associated with a significant reduction in the risk of first variceal bleeding and hepatic decompensation[20]. Finally, Baveno VII further expanded the prognostic use of HVPG by noting that values above 16 mmHg are associated with an increased risk of short-term mortality following abdominal surgery. Moreover, in cases of alcohol- or virus-related cirrhosis, a reduction in HVPG in response to NSBBs has been shown to significantly lower the risk of variceal bleeding and other decompensation events[1].
In Baveno I, annual endoscopy was recommended for all patients with liver cirrhosis[8]. In the second edition of the guidelines, endoscopy was also recommended for all patients with liver cirrhosis, but it was further specified that only untreated patients with low or moderate bleeding risk should undergo follow-up endoscopy every 12 months[16].
In the guidelines from the year 2000, endoscopy continued to be recommended for all patients with liver cirrhosis[17]. It was also advised that in patients without EVs, endoscopy should be repeated every 2-3 years to assess their development, while in individuals with small EVs, endoscopy should be performed every 1-2 years. In the case of large EVs, no further follow-up examinations were recommended, as additional endoscopic findings did not influence the treatment indications.
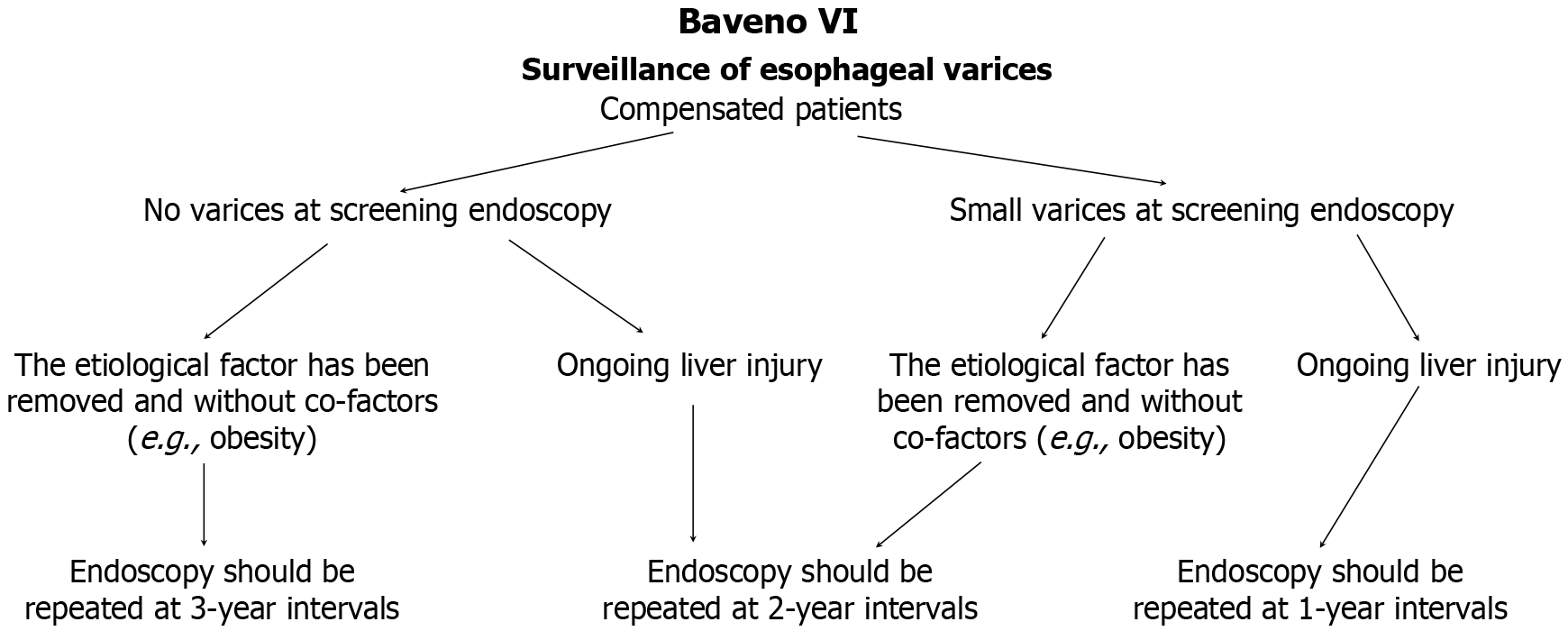
In Baveno IV and V consensus, no significant changes were introduced regarding the recommendations for endoscopic examinations[18,19]. However, Baveno VI marked a notable shift toward a less invasive and less expensive approach was made. Gastroscopy was recommended for patients with cACLD, with the proviso that it could be omitted in individuals with LSM < 20 kPa and PLT > 150 × 109/L[20]. However, annual monitoring of LSM and PLT was recommended. If LSM increased above 20 kPa or PLT dropped below 150 × 109/L, gastroscopy was necessary. The time between successive gastroscopies was dependent on the removal of the underlying cause of CLD. In patients with compensated cirrhosis and without varices, with persistent liver damage, follow-up gastroscopy was recommended every 2 years, and in the case of small varices, every 1 year. If the etiological factor was removed and no additional risk factors were present, gastroscopy in patients without varices should be repeated every 3 years, and in patients with small varices, every 2 years (Figure 1).
In the latest guidelines, the principles of endoscopic surveillance established in Baveno VI were maintained, with additional recommendations introduced[1,20]. First, in patients who cannot take NSBBs, SSM ≤ 40 kPa may indicate a low risk of EVs requiring treatment, allowing the omission of gastroscopy. Furthermore, patients with cACLD caused by hepatitis C virus infection who have achieved sustained virologic response and show sustained improvement after treatment, with LSM values below 12 kPa and PLT above 150 × 109/L, may be exempted from endoscopic surveillance, provided there are no additional risk factors.
The use of NSBBs as a form of pre-primary prevention was first discussed in the third edition of the Baveno guidelines, where it was acknowledged that further in-depth research and discussion were needed[17]. In the 2005 guidelines (Baveno IV), HVPG was recognized as a predictor of variceal development, while NSBBs as pre-primary prophylaxis were contraindicated[18]. In the fifth edition of the recommendations, as in previous years, no evidence was found to support the implementation of NSBBs as part of pre-primary prevention of EVs. HVPG measurement, as a predictor of variceal development, was reserved for clinical trials[19]. The need to remove the etiological factor in order to reduce PH and prevent complications is underlined many times in different editions of the recommendations[18-20]. Preclinical studies have shown that the removal of the damaging factor in rats, such as an inappropriate diet, resulted in regression of fibrosis and thus PH[27]. In a real-world setting, treatment of CHC with direct-acting antivirals is an ample example of the importance of etiological factor removal. In a multicenter prospective study, which included 226 patients with cirrhosis and CSPH, achieving a sustained virological response resulted in a clinically significant decrease in HVPG: Before treatment 15 [interquartile range (IQR): 12-18], after treatment 13 (IQR: 10-16)[23]. Similar improvements have been observed in patients with liver fibrosis of metabolic etiology. In a study that included overweight or obese patients with cirrhosis and PH, lifestyle changes and the introduction of moderate physical exercise resulted in a significant decrease in HVPG after 16 weeks[28].
The Baveno guidelines address key aspects of preventing first variceal bleeding, including risk assessment and stratification, pharmacological prophylaxis, and endoscopic therapies. Over the years, these areas have been refined and updated to reflect the growing body of evidence and clinical practice (Table 2).
| Baveno version (reference) - year of publication | NSBBs indications | Carvedilol | EBL |
| I[8], 1992 | Recommended for high-risk varices, but not for small varices | Not mentioned | Not recommended |
| II[16], 1996 | NSBBs confirmed as primary therapy for large varices | Not mentioned | Potentially valuable but role unclear |
| III[17], 2000 | First-line treatment for large varices, but not indicated for small varices | Not mentioned | Alternative to NSBBs for large varices, but role uncertain |
| IV[18], 2005 | Expanded to include small varices with high-risk features | First mentioned as a possible alternative to traditional NSBBs | Recommended for patients intolerant to NSBBs, but long-term benefits uncertain |
| V[19], 2010 | Further expanded to all small varices, even without high-risk signs | Suggested to have potential advantages over propranolol/nadolol | Recommended for medium/Large varices |
| VI[20], 2015 | Confirmed for all small varices to prevent progression and bleeding | More effective than traditional NSBBs in reducing HVPG | Recommended for medium/Large varices |
| VII[1], 20221 | Recommended for CSPH to prevent decompensation | Preferred for compensated cirrhosis | Recommended for high-risk varices in NSBB-intolerant patients |
Pharmacological prophylaxis: In the first edition of the Baveno guidelines, NSBBs were recommended exclusively for patients with large EVs and a high risk of bleeding, while their use for smaller varices was not yet recommended[8]. The guidelines also emphasized the need for further research into combined therapies and alternative pharmacological approaches.
In Baveno II, NSBBs were established as the primary pharmacological treatment for large EVs, while isosorbide mo
In subsequent editions of the Baveno guidelines, NSBBs remained the first-line treatment for patients with large EVs, while their use was not recommended for small varices[17]. Greater emphasis was placed on dosing strategies, suggesting that NSBB doses should be adjusted to achieve a 25% reduction in resting heart rate. However, it was acknowledged that reaching this target alone does not guarantee complete protection against bleeding. There was also no consensus on the treatment of patients with contraindications to NSBBs, although preliminary data suggested a potential role for isosorbide mononitrate as an alternative. Combination therapy with NSBBs and nitrovasodilators remained unsupported due to insufficient scientific evidence.
In Baveno IV and V, the eligibility criteria for NSBBs treatment were expanded to include patients with small EVs, even if they had no red signs or were not classified as Child-Pugh C[18,19]. NSBBs were reaffirmed as the primary prophylactic method for patients with medium and large EVs. At the same time, stronger evidence emerged against the use of isosorbide mononitrate as monotherapy for primary bleeding prevention. Combination therapy with NSBBs and drugs such as spironolactone or isosorbide-5-mononitrate was also not recommended due to insufficient evidence of its effectiveness. Baveno V introduced significant changes, recognizing carvedilol as a promising alternative to traditional NSBBs (propranolol, nadolol), suggesting its superiority in reducing portal pressure. For the first time, it was also acknowledged that despite the lack of specific preventive studies, NSBBs could be considered for patients with gastric varices.
In Baveno VI, it was confirmed that even patients with small EVs without signs of high risk should be considered for NSBB treatment to prevent their progression and bleeding[20]. However, further research was needed to evaluate the effectiveness of this strategy in this group of patients. NSBBs remained the first-line treatment for primary bleeding prevention in patients with medium and large EVs, with carvedilol shown to more effectively reduce HVPG compared to traditional NSBBs. Additionally, a single study suggested that cyanoacrylate injections might be more effective than NSBBs in preventing the first bleeding in patients with large GOV2 or IGV1. Nevertheless, a thorough evaluation of the benefit-risk ratio of this method was necessary before issuing official recommendations.
In the latest Baveno guidelines, NSBB treatment was recommended not only for primary bleeding prevention but also for preventing decompensation in patients with CSPH[1]. Carvedilol became the preferred NSBB, especially in patients with compensated liver cirrhosis. However, carvedilol is contraindicated in patients with clinically evident liver dysfunction, and caution should be exercised in those with moderate to severe ascites due to the risk of hypotension and the potential development of hepatorenal syndrome[29,30].
Endoscopic therapies: In the early guidelines, endoscopic interventions such as sclerotherapy and band ligation were not recommended for primary prevention of esophageal variceal bleeding[8].
In Baveno II, it was clearly stated that sclerotherapy should not be used for primary prevention of esophageal variceal bleeding[16]. At the same time, the potential value of endoscopic band ligation (EBL) as a therapeutic method for EVs began to be recognized, although its role in primary prevention had not yet been fully established.
In Baveno III, EBL of EVs was acknowledged a potential alternative to NSBBs for preventing the first bleeding in patients with large EVs, although its effectiveness in this regard had not been conclusively proven[17]. Additionally, combination therapy involving both pharmacological and endoscopic approaches was not recommended at that time due to a lack of data confirming its effectiveness.
In Baveno IV, EBL of EVs was recommended for patients with contraindications or intolerance to NSBBs, although the long-term benefits of this strategy remained uncertain[18].
In Baveno V and VI, EBL of EVs was officially recommended as a strategy for primary bleeding prevention in patients with medium and large EVs[19,20]. The choice between pharmacological and endoscopic therapy depended on the individual characteristics of the patient, their preferences, and the availability of local specialists. At the same time, combination therapy (pharmacological + endoscopic treatment) was not recommended in Baveno IV and V due to insufficient scientific evidence supporting its efficacy.
In Baveno VII, there were no clear indications for EBL of EVs, with the guideline stating only that it should be used in patients with compensated liver cirrhosis and high-risk varices who had contraindications or intolerance to NSBBs, as primary bleeding prevention[1]. NSBBs remained the treatment of choice for patients with gastric varices to prevent decompensation, although one study[31] suggested the potential effectiveness of cyanoacrylate injections in this patient group. It is important to note that balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt (TIPS) techniques were not included in the recommendations for primary prevention of gastric variceal bleeding.
Management of acute bleeding from esophageal and gastric varices has evolved considerably across successive Baveno guidelines (Table 3). The first consensus established the basic definitions and diagnostic criteria for variceal bleeding[8]. It emphasized the need for rapid endoscopy and introduced the concept of "zero time", defined as the moment the patient is admitted to the hospital. At this stage, there was no established consensus on the management of acute esophageal variceal bleeding, although endoscopic sclerotherapy was proposed as the primary treatment. In the case of acute gastric variceal bleeding, the use of vasoactive drugs or balloon tamponade was recommended. The lack of clear guidelines during this early period reflected the limited availability of scientific evidence, underscoring the need for further research and standardization of therapeutic strategies.
| Baveno version (reference), year of publication | Endoscopic treatment | Pharmacological treatment | Balloon tamponade | TIPS | Antibiotic prophylaxis | Failure to control bleeding criteria |
| I[8], 1992 | ASAP; AEVB: No consensus, sclerotherapy proposed as the primary treatment | Vasoactive drugs for gastric variceal bleeding | If continued bleeding (or rebleeding within 24-36 hours) despite treatment | No recommendations | No recommendations | No consensus |
| II[16], 1996 | Endoscopic techniques were the treatment of choice for AEVB; Tissue adhesives and thrombin suggested for AGVB | Terlipressin and somatostatin shown to be effective; Insufficient data on octreotide | Reserved for emergency cases | Rescue option if endoscopic and pharmacological treatments fail | No recommendations | Two failure timeframes: < 6 hours and > 6 hours; rebleeding defined > 48 hours |
| III[17], 2000 | ASAP (within 12 hours); EBL established as superior to sclerotherapy for AEVB; Insufficient data on tissue adhesives and EBL for AGVB | Vasoactive drugs recommended in suspected AEVB; ASAP (before endoscopy); Use to 5 days; In combination with endoscopic techniques; Vasoactive drugs suggested for bleeding from PHG | Reserved for massive bleeding as a bridge to definitive treatment | TIPS or shunt surgery for PHG if pharmacological therapy fails | No recommendations | As above |
| IV[18], 2005 | ASAP (within 12 hours); EBL preferred for AEVB (sclerotherapy as an alternative); Tissue adhesive is recommended for AGVB | Vasoactive drugs (terlipressin, somatostatin, vapreotide, octreotide) recommended in suspected AEVB; ASAP (before endoscopy); Use to 5 days; In combination with endoscopic techniques | As above | TIPS with PTFE-covered stents recommended in case of treatment failure | No recommendations | Fresh hematemesis 2 hours after treatment, 3 g drop in Hb without transfusion, increased blood transfusion requirement1, death |
| V[19], 2010 | ASAP (within 12 hours); EBL preferred, sclerotherapy as an alternative; Tissue adhesive is recommended for AGVB from IGV and GOV2; EBL or tissue adhesive can be used in AGVB from GOV1 | As above | As above | Early TIPS within 72 hours for high-risk patients; TIPS with PTFE-covered stents recommended in case of treatment failure | Antibiotic prophylaxis became an integral part of therapy in with cirrhosis | As above |
| VI[20], 2015 | Requirement for 24/7 availability of an endoscopist proficient in hemostasis; ASAP (within 12 hours); EBL recommended for AEVB; Tissue adhesive is recommended for AGVB from IGV and GOV2; EBL or tissue adhesive can be used in AGVB from GOV1 | As above, but vapreotide was not mentioned | Only in refractory esophageal bleeding, as a temporary ‘‘bridge’’ (for a maximum of 24 hours) | Early TIPS within 72 hours for high-risk patients (further specification of indications); TIPS with PTFE-covered stents recommended in case of treatment failure | As above | No changes |
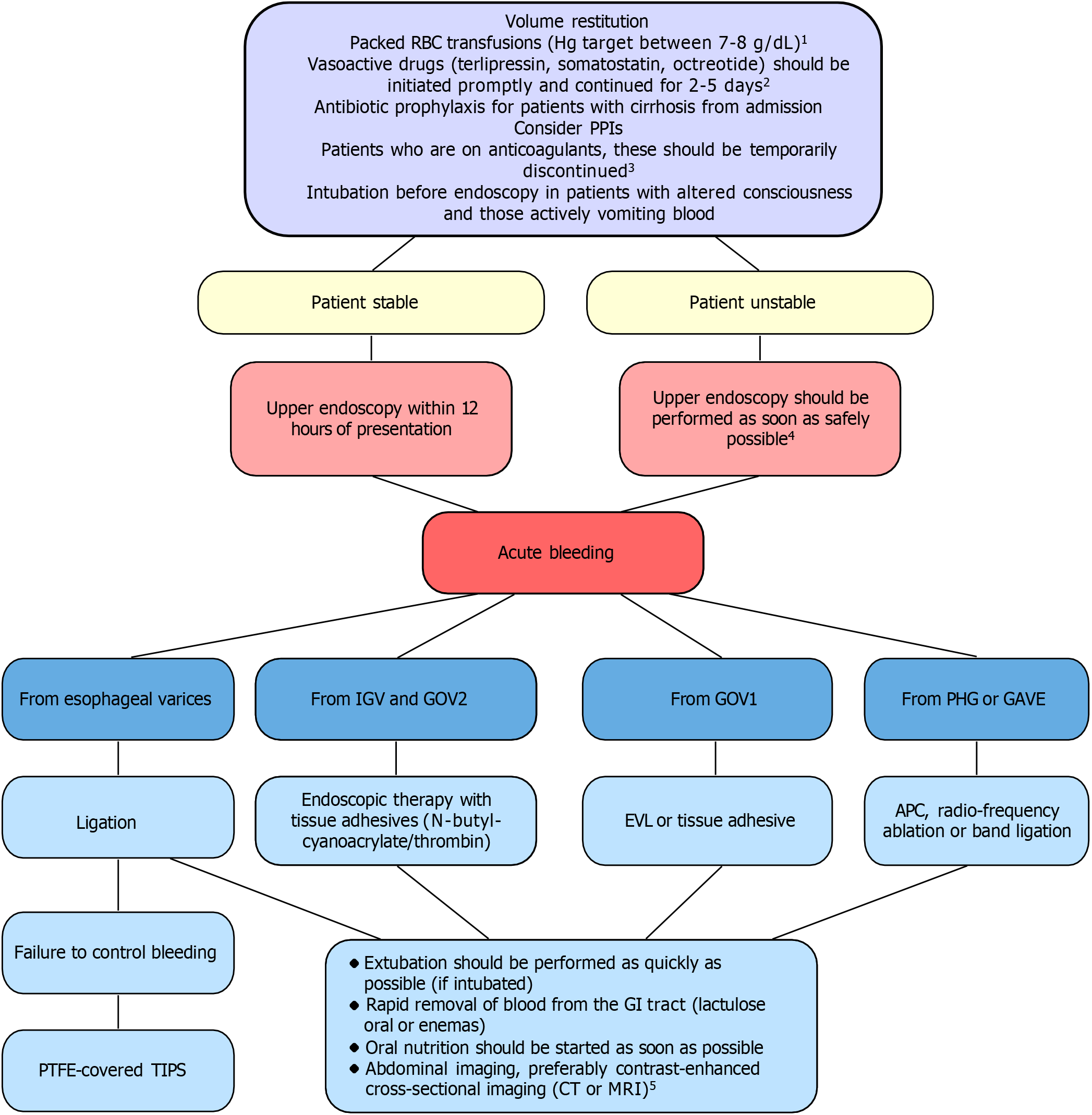
| VII[1], 2022 | As above; but patients with suspected AEVB should undergo upper endoscopy within 12 hours, If the patient is unstable, endoscopy should be performed as soon as safely possible; APC, radio-frequency ablation or EBL for PHG and GAVE bleeding | As above | As above but self-expandable metal stents self-expandable metal stents preferred due to safety | As above | As above | Absence of control of bleeding or by rebleeding within the first 5 days |
The second edition of the guidelines introduced significant improvements by defining the criteria for clinically significant bleeding and establishing two levels of bleeding control failure - within the first 6 hours and after that period[16]. An acute bleeding episode was defined as a 48-hour period from "zero time", with any bleeding after this time considered a recurrence. Endoscopic treatment remained the method of choice for esophageal variceal bleeding, although no specific technique was designated as the first choice. For gastric variceal bleeding, the use of tissue adhesives or thrombin was suggested, but confirmation of this method through research was needed. Regarding pharmacological treatment, terlipressin was recognized for its effectiveness, positively impacting survival rates. Somatostatin was also found to be equally effective, while there was insufficient data to make recommendations regarding octreotide. The guidelines also stated that vasopressin should only be used in combination with nitroglycerin. In emergency situations, when bleeding control with pharmacological and endoscopic treatments was ineffective, TIPS was recommended as a therapeutic option.
The Baveno III consensus retained most of the previously established definitions but placed special emphasis on the prognostic value of active bleeding during endoscopy as a predictor of bleeding control failure[17]. It was also emphasized that balloon tamponade should only be used in cases of massive bleeding as a temporary "bridge" to definitive treatment. The need to reassess certain hemodynamic criteria used to define treatment failure was also highlighted. The guidelines clearly confirmed the superiority of esophageal variceal EBL over sclerotherapy, establishing it as the first-line treatment. Pharmacological treatment with vasoactive drugs, started before endoscopy and continued for 5 days, was reinforced as a key element of early therapy. In the context of portal hypertensive gastropathy (PHG), it was noted that studies have shown the high effectiveness of vasoactive drugs in cases of acute bleeding. In cases of pharmacological therapy failure, emergency TIPS was recommended. The potential role of argon plasma coagulation in the treatment of PHG was considered to require further evaluation. For chronic bleeding associated with PHG, NSBBs were considered the first-line treatment. However, no consensus was reached on the optimal management of bleeding from gastric varices, and its efficacy continued to require validation through randomized controlled trials.
In Baveno IV, the definition of an acute bleeding episode was changed, extending its duration to 5 days[18]. New criteria for bleeding control failure were introduced, including: Fresh hematemesis 2 hours after treatment initiation, a hemoglobin drop of 3 g/dL (without blood transfusion), death, and an Adjusted Blood Requirement Index ≥ 0.75. Target transfusion values were also established, recommending maintaining hematocrit at 24% or hemoglobin levels at 8 g/dL, with the possibility of adjusting these values depending on comorbidities, patient age, hemodynamic status, and bleeding activity. Antibiotic prophylaxis was recognized as an integral component of therapy for patients with cirrhosis and variceal bleeding and should be started upon hospital admission. Vasoactive drugs were to be administered immediately upon suspicion of variceal bleeding, even before diagnostic endoscopy, and continued for 2 to 5 days. Early endoscopy within 12 hours of admission was recommended as a strategy to improve prognosis. Endoscopic treatment was deemed necessary for all patients with confirmed upper gastrointestinal bleeding caused by EVs, with EBL being the preferred method, while sclerotherapy remained an alternative option. For gastric variceal bleeding, endoscopic treatment with tissue adhesives, such as N-butylcyanoacrylate, was recommended. Balloon tamponade continued to be recommended only as a temporary solution in cases of massive bleeding until definitive treatment could be implemented. In the case of treatment failure despite pharmacological and endoscopic therapy, a second attempt at endoscopic treatment or TIPS was recommended.
The Baveno V consensus, held in 2010, introduced improvements to the previous guidelines while maintaining the 5-day duration for managing acute bleeding episodes[19]. The target hemoglobin level was modified to a range of 7-8 g/dL. The importance of antibiotic prophylaxis was emphasized, and the concept of early TIPS (within 72 hours) was intro
The sixth guidelines did not introduce changes regarding blood transfusion protocols[20]. The recommendation that antibiotic prophylaxis is an integral part of therapy for patients with cirrhosis and upper gastrointestinal bleeding was maintained, and it should be initiated upon hospital admission. The need for intubation in patients where clinically indicated was also emphasized. It was reiterated that in the case of suspected variceal bleeding, vasoactive drugs should be administered as soon as possible, even before endoscopy, and continued for a maximum of 5 days. Special attention was given to ensuring the availability of an on-call endoscopist 24/7, with experience in endoscopic hemostasis te
The latest guidelines expanded recommendations regarding airway protection, nutrition, and the use of proton pump inhibitors, highlighting that their use should be carefully reconsidered after endoscopy, with discontinuation recommended unless there is a specific indication to continue therapy[1]. A particularly important element was the new guidance on coagulation management, in which the transfusion of fresh frozen plasma and the use of recombinant factor VIIa were explicitly discouraged. Additionally, the guidelines also provided extended recommendations for managing specific clinical situations, such as ectopic varices and portal vein thrombosis. Detailed information on the management of active bleeding is provided in Figure 2.
Over the years, the guidelines for preventing recurrent bleeding from EVs varices have evolved significantly, reflecting advances in therapeutic options, a better understanding of risk stratification, and the results of clinical studies (Table 4).
| Baveno version (reference), year of publication | First-line therapy | Alternative therapy | Rescue therapy (failure of first-line) | Gastric varices | Special considerations |
| I[8], 1992 | Traditional NSBBs or sclerotherapy | No specific recommendation | Sclerotherapy, NSBBs, surgical shunt, and liver transplantation | Traditional NSBBs | Risk assessment debated |
| II[16], 1996 | Traditional NSBBs or EBL | Combined therapy proposed but untested | TIPS or surgical shunts | No specific recommendation | Consider transplantation for advanced cirrhosis |
| III[17], 2000 | Traditional NSBBs or EBL | EBL preferred in NSBB-intolerant patients; Combined therapy should be further investigated | TIPS or surgical shunts | No specific recommendation | Consider transplantation for advanced liver disease |
| IV[18], 2005 | Traditional NSBBs + EBL | EBL preferred in NSBB-intolerant patients | TIPS or surgical shunts for Child-Pugh A/B patients; Liver transplantation for Child-Pugh B/C patients | IGV1, GOV2: N-butyl-cyanoacrylate, TIPS or NSBBs; GOV1: N-butyl-cyanoacrylate, EBL, NSBBs | Start prophylaxis by day 6 |
| V[19], 2010 | Traditional NSBBs + EBL | EBL preferred in NSBB-intolerant patients; The addition of ISMN to NSBBs for hemodynamic non-responders; Those who are unable or unwilling to be treated with EBL: NSBBs + ISMN | TIPS preferred; Surgical shunt in Child-Pugh A and B pts if TIPS is unavailable; Transplantation should be considered | IGV1, GOV2: N-butyl-cyanoacrylate or TIPS; GOV1: N-butyl-cyanoacrylate, EBL, NSBBs | TIPS as bridge to transplantation |
| VI[20], 2015 | Traditional NSBBs + EBL | NSBB monotherapy if EBL contraindicated; EBL monotherapy only if intolerance/contraindications to NSBB | TIPS preferred | No specific recommendation | PHG has to be distinguished from GAVE because treatments are different |
| VII[1], 2022 | Traditional NSBBs (including carvedilol) + EBL | Any therapy alone if intolerance the combination therapy | TIPS preferred | No specific recommendation | Argon plasma coagulation or hemospray were introduced for recurrent bleeding in PHG |
In the initial guidelines, the necessity of preventing recurrent bleeding was recognized, but there was a lack of a clear treatment strategy due to limited available knowledge[8]. Among the therapeutic options listed were endoscopic sclerotherapy, NSBBs and surgical treatment, with the choice of method depending on the individual characteristics of the patient. In cases of failure of the primary strategy, alternative methods, such as repeated sclerotherapy, NSBBs, vascular shunts, or liver transplantation (LTx), could be considered. For the prevention of recurrent bleeding from gastric varices, NSBBs were considered the treatment of choice.
In Baveno II, a significant change occurred: Variceal EBL replaced sclerotherapy as the preferred endoscopic treatment for EVs[16]. The use of NSBBs was also recommended, and for the first time, the combination of both methods was suggested to improve treatment outcomes. Additionally, TIPS was considered for patients with recurrent bleeding despite optimal therapy, although the indications for this procedure still required further verification.
The third edition of the guidelines confirmed the equivalence of NSBBs and EBL as first-line therapies, while emphasizing the need to consider LTx in patients with advanced liver disease[17]. Further research into the effectiveness of combined therapy was encouraged. EBL was recognized as the preferred treatment for patients with contraindications to NSBBs or those who experienced recurrent bleeding despite their use. In cases of failure of first-line therapy, TIPS or surgical shunting were recommended.
In Baveno IV, it was recommended that secondary prophylaxis of bleeding should begin within six days after a hemorrhagic episode[18]. The preferred approach for EVs was the combination of NSBB with EBL, although the need for further research in this area was emphasized. EBL remained the main alternative for patients intolerant to NSBB. In cases of failure of both pharmacological and endoscopic therapies, TIPS or surgical shunting was recommended for patients with Child-Pugh A and B. LTx was considered an effective solution for patients with Child-Pugh B and C who ex
In Baveno V, it was confirmed that combination therapy (NSBB + banding) is more effective than monotherapy for secondary prophylaxis of bleeding from EVs[19]. The addition of isosorbide-5-mononitrate to NSBB was proposed for patients who did not achieve a hemodynamic response, as well as for those who could not or did not want to undergo EBL. TIPS was considered the preferred rescue therapy, especially when using polytetrafluoroethylene-covered stents. LTx remained the optimal long-term solution for eligible patients, with TIPS serving as a bridging therapy. Specific recommendations were made regarding gastric varices, emphasizing the role of cyanoacrylate injection, NSBB, and TIPS in preventing recurrent bleeding. In the case of PHG, NSBB remained the main therapeutic option for preventing recurrent bleeding episodes.
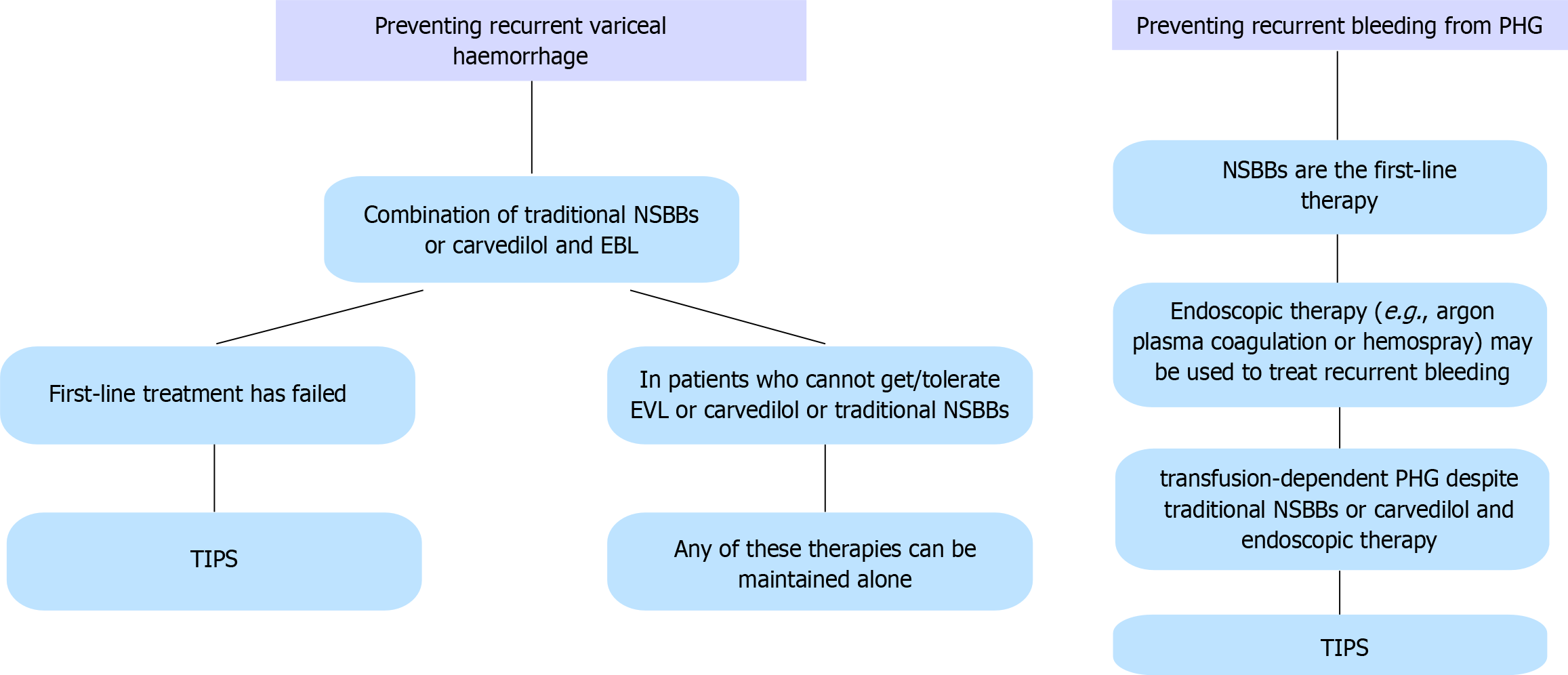
In the sixth edition of the Baveno guidelines, the importance of combination therapy with NSBBs and EBL as the first-line treatment was emphasized. Monotherapy with EBL was discouraged, unless there were contraindications to NSBBs[20]. Carvedilol was not recommended due to insufficient data. TIPS remained the preferred rescue therapy for patients in whom first-line treatment failed. The management of PHG was described in more detail, with NSBB recognized as the first-line therapy for preventing recurrent bleeding episodes. TIPS was proposed for patients who were dependent on transfusions and in whom NSBBs and/or endoscopic therapies proved ineffective.
In the latest Baveno guidelines, the effectiveness of combined therapy (NSBB + EBL) as first-line treatment for the prevention of recurrent bleeding from EVs was confirmed, and carvedilol was officially included as an equivalent option to traditional NSBBs[1]. A new recommendation was the allowance of monotherapy (NSBBs, including carvedilol, or EBL) for patients who do not tolerate combined treatment. TIPS remains the rescue therapy for patients in whom first-line treatment has failed. For PHG, NSBBs remain the first-choice therapy. Additionally, endoscopic treatment options such as argon plasma coagulation and hemospray were introduced for managing recurrent bleeding. TIPS was recommended for patients with PHG who are transfusion-dependent and do not respond to NSBBs and endoscopic treatment (Figure 3).
Recent developments in the management of PH reflect a dynamic shift toward more personalized and precise approaches. Several areas of innovation are likely to shape future editions of the Baveno guidelines.
First of all, artificial intelligence is rapidly emerging as a powerful tool in hepatology, particularly in the noninvasive diagnosis of CSPH. Machine learning algorithms applied to radiological data - such as ultrasound, computed tomography, and magnetic resonance imaging - can detect subtle imaging features associated with early hemodynamic changes[32]. Techniques like radiomics and vascularomics are being explored to extract high-dimensional data from standard imaging, allowing for accurate prediction of CSPH without the need for invasive procedures. Integration of artificial intelligence with multimodal clinical data holds promise for enhancing diagnostic accuracy and risk stratification[33,34].
In addition to established therapies, several drug classes are under investigation for their potential role in modifying portal pressure and improving outcomes. Among the most promising candidates are statins, which may have both antifibrotic and vascular benefits; anticoagulants, which could improve hepatic microcirculation; and phosphodiesterase inhibitors, with possible vasodilatory effects[35,36]. While early-phase studies show encouraging results, larger randomized controlled trials will be needed to confirm safety, efficacy, and clinical utility before these agents can be incorporated into standard practice.
The most recent Baveno research agenda highlights a strong focus on refining both invasive and noninvasive diagnostic tools, with particular attention to new imaging modalities, liver stiffness thresholds across etiologies, and circulating biomarkers[1]. Other key areas include individualization of therapy (e.g., HVPG-guided treatment), optimization of TIPS indications, and the role of gut microbiota modulation. Special interest is also directed toward identifying predictors of recompensation, improving management in patients with comorbidities or frailty, and developing strategies for those with portal vein thrombosis or refractory variceal bleeding. Together, these emerging trends underscore a transition from a uniform to a more tailored approach in PH, supported by technological innovation.
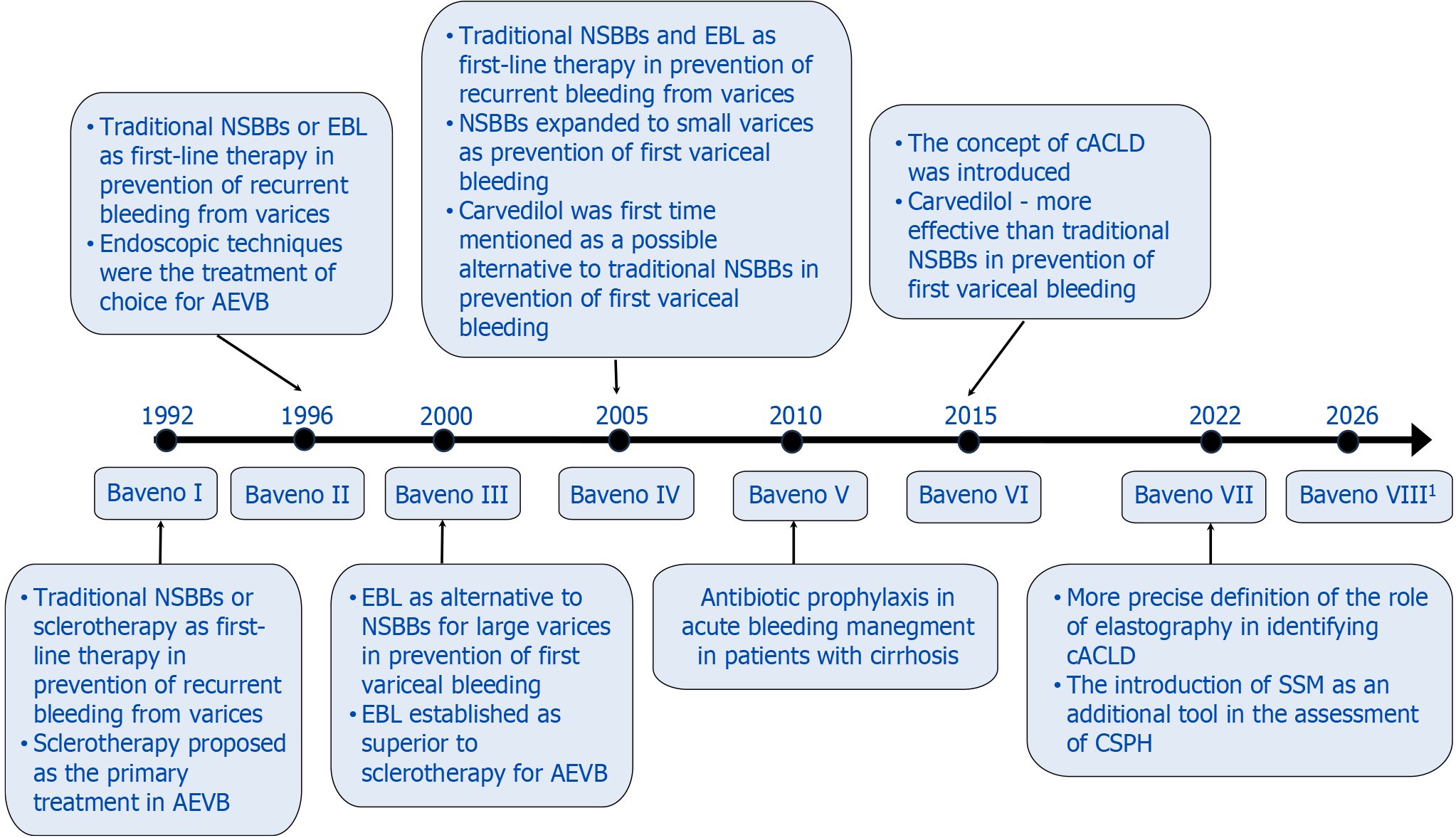
This review focuses on the evolution of the Baveno guidelines and the current state of management in PH. The most important changes across successive Baveno editions summarized in Figure 4. While efforts were made to include the most relevant and up-to-date literature, certain limitations should be acknowledged. First, the review is narrative in nature and not a systematic review, which may introduce selection bias in the choice of cited studies. Second, although the literature search covered publications from 2015 onward, earlier studies were also cited when necessary to reflect key historical recommendations or contextual milestones. Third, due to the rapid advancements in non-invasive diagnostics and therapeutic strategies, some emerging data may not yet be fully reflected in the current guideline updates. Finally, this review primarily focuses on adult patients with cirrhosis; data related to pediatric populations or non-cirrhotic PH are not comprehensively addressed.
Across successive editions, the guidelines have steadily progressed toward a more standardized, evidence-based approach to the diagnosis of PH and its complications, treatment, as well as primary and secondary prevention of variceal bleeding. The subsequent updates also emphasize the importance of more effective strategies for managing active bleeding. The evolution from Baveno I to Baveno VII reflects both advances in medical knowledge and technology, as well as an increasingly better understanding of the need for individualized therapy within standardized treatment protocols.
| 1. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1850] [Article Influence: 462.5] [Reference Citation Analysis (2)] |
| 2. | Tham EKJ, Tan DJH, Danpanichkul P, Ng CH, Syn N, Koh B, Lim RYZ, Wijarnpreecha K, Teng MLP, Nah BKY, Sim BKL, Cheng X, Zhang Z, Mitra K, Nakamura T, Takahashi H, Loomba R, Zheng MH, Muthiah M, Huang DQ. The Global Burden of Cirrhosis and Other Chronic Liver Diseases in 2021. Liver Int. 2025;45:e70001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol. 2020;26:6111-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (6)] |
| 4. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Fadlallah H, El Masri D, Bahmad HF, Abou-Kheir W, El Masri J. Update on the Complications and Management of Liver Cirrhosis. Med Sci (Basel). 2025;13:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 468] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Pfisterer N, Riedl F, Schwarz M, Simbrunner B, Dominik N, Kramer G, Jachs M, Hartl L, Putre F, Ritt L, Mandorfer M, Holzmueller P, Madl C, Trauner M, Reiberger T. Improved clinical outcomes of patients with cirrhosis and acute variceal bleeding over the last 2 decades. Gastrointest Endosc. 2025;S0016-5107(25)01639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, Groszmann R, Bosch J, Sauerbruch T, Soederlund C. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol. 1992;15:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Burroughs AK, editor. Methodology and reviews of clinical trials in portal hypertension: Proceedings of the International Workshop on Portal Hypertension. Elsevier, 1987. |
| 10. | World Health Organization. Coronavirus disease (COVID-19) pandemic. [cited 3 August 2025]. Available from: https://www.who.int/europe/emergencies/situations/covid-19. |
| 11. | Baveno Cooperation. Baveno VIII presymposium. [cited 3 August 2025]. Available from: https://baveno8.org/presymposium/. |
| 12. | Baveno Cooperation. Baveno VIII consensus workshop. [cited 3 August 2025]. Available from: https://baveno8.org/. |
| 13. | Pixinside. Baveno cooperation - summary. [cited 3 August 2025]. Available from: https://www.bavenocoop.net/. |
| 14. | Pixinside. Mission and aims. [cited 3 August 2025]. Available from: https://bavenocoop.net/about/mission-and-aims. |
| 15. | Lu Q, Leong S, Lee KA, Patel A, Chua JME, Venkatanarasimha N, Lo RH, Irani FG, Zhuang KD, Gogna A, Chang PEJ, Tan HK, Too CW. Hepatic venous-portal gradient (HVPG) measurement: pearls and pitfalls. Br J Radiol. 2021;94:20210061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | de Franchis R. Developing consensus in portal hypertension. J Hepatol. 1996;25:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. 2000;33:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 377] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 741] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 19. | de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 20. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2358] [Article Influence: 214.4] [Reference Citation Analysis (4)] |
| 21. | Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, Shiffman ML, Aguilar Schall R, Jia C, McColgan B, Djedjos CS, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Muir AJ, Afdhal NH, Bosch J, Goodman Z. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology. 2019;70:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 22. | Spina GP, Arcidiacono R, Bosch J, Pagliaro L, Burroughs AK, Santambrogio R, Rossi A. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, Gallego A, Bañares R, Puente A, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, Forns X, García-Pagán JC. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273-1283.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 24. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 873] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 25. | Mandorfer M, Abraldes JG, Berzigotti A. Non-invasive assessment of portal hypertension: Liver stiffness and beyond. JHEP Rep. 2025;7:101300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 26. | Brol MJ, Gödiker J, Uschner FE, Praktiknjo M, Trebicka J. Non-invasive Assessment of Clinically Significant Portal Hypertension. Curr Hepat Rep. 2023;22:206-215. [DOI] [Full Text] |
| 27. | Selicean S, Wang C, Guixé-Muntet S, Stefanescu H, Kawada N, Gracia-Sancho J. Regression of portal hypertension: underlying mechanisms and therapeutic strategies. Hepatol Int. 2021;15:36-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 28. | Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J; Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 29. | Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2020;2:100063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Lincoln Pharmaceuticals Limited. Prescribing Information (Summary of Product Characteristics) - Carvedilol. [cited 3 August 2025]. Available from: https://rwandafda.gov.rw/wp-content/uploads/2023/07/Carvilin-12.5-Carvedilol-12.5mg-tablets-SmPC.pdf. |
| 31. | Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Li Z, Sun X, Zhao Z, Yang Q, Ren Y, Teng X, Tai DCS, Wanless IR, Schattenberg JM, Liu C. A machine learning based algorithm accurately stages liver disease by quantification of arteries. Sci Rep. 2025;15:3143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Du Z, Yang L, He H, Wu X, Qi X, Zhang Y. Artificial intelligence for the noninvasive diagnosis of clinically significant portal hypertension. EngMedicine. 2025;2:100069. [DOI] [Full Text] |
| 34. | Reiniš J, Petrenko O, Simbrunner B, Hofer BS, Schepis F, Scoppettuolo M, Saltini D, Indulti F, Guasconi T, Albillos A, Téllez L, Villanueva C, Brujats A, Garcia-Pagan JC, Perez-Campuzano V, Hernández-Gea V, Rautou PE, Moga L, Vanwolleghem T, Kwanten WJ, Francque S, Trebicka J, Gu W, Ferstl PG, Gluud LL, Bendtsen F, Møller S, Kubicek S, Mandorfer M, Reiberger T. Assessment of portal hypertension severity using machine learning models in patients with compensated cirrhosis. J Hepatol. 2023;78:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Sakiani S, Heller T, Koh C. Current and investigational drugs in early clinical development for portal hypertension. Front Med (Lausanne). 2022;9:974182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Felli E, Nulan Y, Selicean S, Wang C, Gracia-Sancho J, Bosch J. Emerging Therapeutic Targets for Portal Hypertension. Curr Hepatol Rep. 2023;22:51-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/