Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.109808
Revised: June 24, 2025
Accepted: August 15, 2025
Published online: September 21, 2025
Processing time: 119 Days and 9.1 Hours
Cold exposure has traditionally been considered a pathological factor that can easily impair gastrointestinal (GI) digestion. Shihosogan-tang (ST), Yijung-tang (YT), and Pyeongwi-san (PS) are well-known herbal formulas frequently used to treat GI disorders in East Asia.
To compare the effects of these herbal formulas on GI motility and investigate their mechanisms of action using a cold stress (CS)-induced dyspepsia mouse model.
C57BL/6J mice were exposed to CS by immersion in cold water (10 ± 1 °C) while being restrained in conical tubes for 1 hour. This procedure was repeated six times over 2 weeks. Herbal formulas or mosapride (positive control) were administered orally five times per week over a 2-week period.
The pre-test results revealed that CS, rather than restraint stress, significantly delayed gut motility in mice. However, PS and ST notably improved gastric emptying and intestinal transit, surpassing YT. Additionally, PS and ST significantly reduced gastric potential of hydrogen and increased pepsin and lipase gene expression compared to CS. The observed mechanisms likely involved increased gastric acidity and enhanced levels of digestive enzymes, such as pepsin and lipase. Furthermore, PS administration elevated GI hormone levels and metabolites related to the gut microbiota (5-hydroxytryptamine and short-chain fatty acid) more effectively than ST and YT treatments.
PS more effectively alleviated CS-induced GI dysfunction than both YT and ST. These comparative findings offer valuable insights for clinical applications in the treatment of cold-related digestive disorders.
Core Tip: This study systematically compared the therapeutic effects of three classical herbal formulas [Pyeongwi-san (PS), Shihosogan-tang, and Yijung-tang] in a mouse model of cold stress-induced dyspepsia. Among them, PS was the most effective in restoring gastrointestinal motility, enhancing gastric acid secretion and upregulating digestive enzymes gene expression, and modulating serotonin, short-chain fatty acids, bile acid receptors, and glucagon-like peptide-1 signaling. These findings elucidate the mechanisms of PS’s superior efficacy and strongly support its clinical application in treating cold-induced gastrointestinal dysfunction.
- Citation: Wang JH, Han SY, Wu L, Han U, Cho SK, Park CW, Chin YW, Lim MY, Kim H. Comparative evaluation of three traditional herbal formulas on gastrointestinal motility in a mouse model of cold stress-induced dyspepsia. World J Gastroenterol 2025; 31(35): 109808
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/109808.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.109808
Dyspepsia is an extremely common gastrointestinal (GI) dysfunction characterized by various symptoms including epi
Cold stress (CS) is defined as a physiological condition in which exposure to low temperatures disrupts thermal ho
Meanwhile, traditional Chinese medicine theory classifies cold as one of the six external pathogens, as described in the book Yellow Emperor’s Inner Canon, also known as Huang-Di-Nei-Jing[14]. When cold invades the stomach, it disrupts the normal flow of Qi, causing it to ascend and leading to symptoms of dyspepsia, such as belching[15]. Therefore, kee
Therefore, these three herbal formulas were comparatively evaluated for their effects on ameliorating CS-induced GI dysfunction in a mouse model. In addition, we focused on digestive enzymes and gut microbiome-related metabolites, such as SCFA and serotonin, to explore the corresponding mechanisms. These findings may provide valuable evidence to enhance the scientific and clinical use of traditional herbal formulas for treating cold-induced dyspepsia.
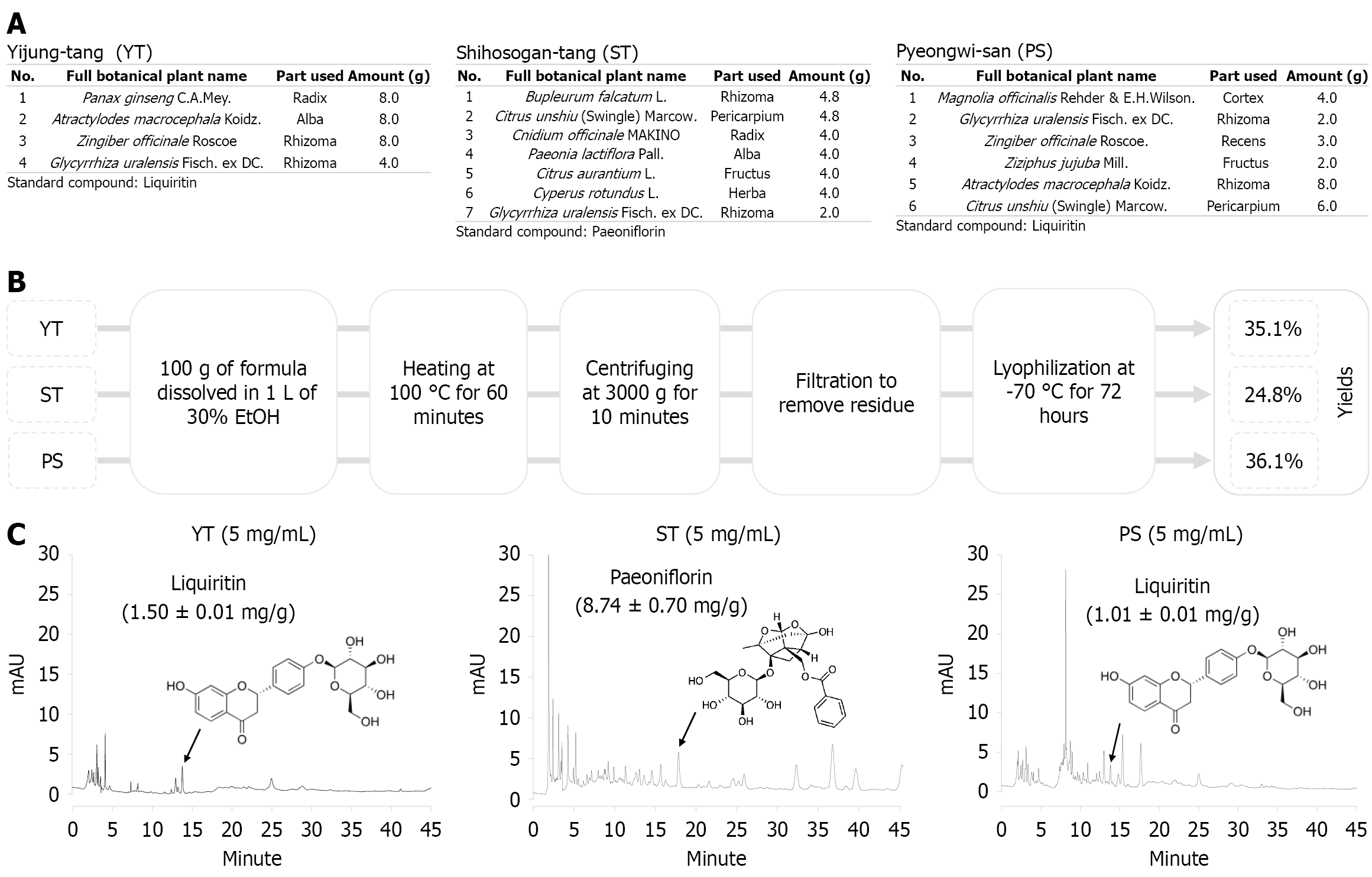
The Korean Pharmacopoeia-grade herbs used in the herbal formulas ST, YT, and PS were obtained from the Dongguk University Ilsan Medical Center (Goyang, Gyeonggi-do, Republic of Korea). The detailed components of the three herbal formulations and the proportions of each herb are shown in Figure 1A. Ethanol was used to extract all three herbal formulations. Briefly, each 100 g of powder was ground and then individually extracted with 1 L of 30% ethanol (Merck, Rahway, NJ, United States) by heating at 100 °C for 60 minutes. After centrifugation at 3000 × g for 10 minutes, the supernatants were filtered using filter paper (grade 4, Whatman, Kent, United Kingdom). The filtered extracts were evaporated by a rotary evaporator (EYELA N-1200A, Tokyo, Japan) and lyophilized at -70 °C, obtained yields of 24.8% for ST, 35.1% for YT, and 36.1% for PS, respectively (w/w, Figure 1B). The powders of herbal extract were stored at -20 °C for future animal experiments.
The three herbal formulas were fingerprinted using two-dimensional high-performance liquid chromatography (HPLC). ST, YT, and PS powders were dissolved in methanol to prepare 5 mg/mL solutions. Prior to analysis, the prepared solutions were sonicated for 30 minutes and filtered through a 0.2-μm syringe filter (Cytiva, Marlborough, MA, United States). Quantitative analysis was performed using a Dionex Ultimate 3000 Ultra-HPLC system equipped with a diode array detector (Thermo Fisher Scientific, Waltham, MA, United States) and Luna™ C18 column (250 mm × 4.6 mm, 5.0 μm) (Phenomenex, Torrance, CA, United States). The wavelength was set to 254 nm. The column was maintained at 30 °C, and injection volume was 10 μL. Paeoniflorin and liquiritin were used as reference standards for the three herbal formulas according to the Korean Herbal Pharmacopoeia (12th edition). Stock solutions of paeoniflorin or liquiritin were diluted to concentrations ranging from 500 to 31.25 μg/mL to establish calibration curves.
The mobile phase, composed of water containing 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B), was delivered at a flow rate of 1.0 mL/minute using step gradient elution (Supplementary Table 1) for ST and paeoniflorin (retention time: 17.66 minute). Additionally, a mobile phase consisting of solvents A and B was applied at a flow rate of 1.0 mL/minute using the step gradient method (Supplementary Table 2) for YT, PS, and liquiritin (retention time: 13.81 minute) (Figure 1C).
The animal experimental protocol was approved by the Institutional Animal Ethics Committee of Dongguk University (permit No. IACUC-2024-03260; approval date: 8 April 2024) and complied with the United States National Institutes of Health (NIH) guidelines for the humane care and use of laboratory animals. All sacrifices were conducted using an equal combination of Zoletil (tiletamine–zolazepam, Virbac, Carros, France) and Rompun (xylazine hydrochloride, Bayer, Leverkusen, Germany) for anesthesia, minimizing suffering as much as possible.
To determine whether restraint stress or CS had a more significant effect on GI motility, 20 male C57BL/6J mice (6 weeks old, Daehan Biolink, Eumsung, Republic of Korea) were randomly assigned to five groups of four mice each. All mice, except those in the normal group, were subjected to cold restraint stress under varying conditions: 10 °C for 30 minutes, 10 °C for 1 hour, 20 °C for 2 hours, and 20 °C for 4 hours (Supplementary Figure 1A). After 30 minutes of cold restraint stress, GI transit was assessed using the charcoal test.
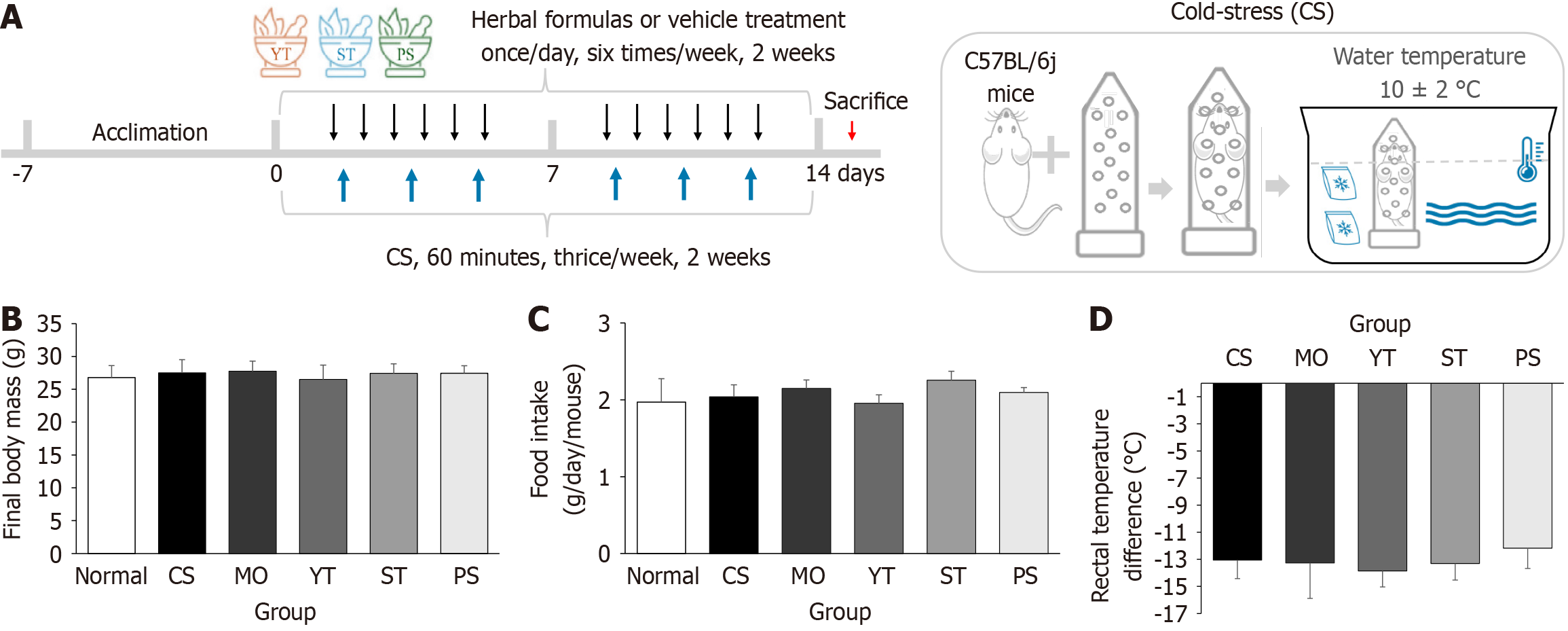
For the main experiment, 72 male C57BL/6J mice, 6 weeks old, were purchased from Daehan Biolink (Eumsung, Republic of Korea). After a 1-week acclimation in a room with a temperature of 22 °C ± 2 °C, humidity of 40% ± 10%, an equal light and dark cycle, chow diet, and free access to water, the mice were randomly divided into six groups [normal, CS, mosapride (MO), YT, ST, PS] with 12 animals in each group. Four mice were housed per cage, and food intake was measured once a week. The total amount consumed was then divided by the number of days and the number of mice to calculate the average daily intake per mouse. Except for the normal group, all mice were restrained in 50 mL Falcon conical tubes and immersed in 10 °C ± 2 °C water for 1 hour (Figure 2A). CS was induced six times, thrice a week for 2 weeks. During this period, the herbal formula extracts (10 mL/1000 mg/kg) and MO (10 mL/2 mg/kg, positive control) were administered intragastrically once daily for 2 weeks (Figure 2A). The same volume of distilled water was admini
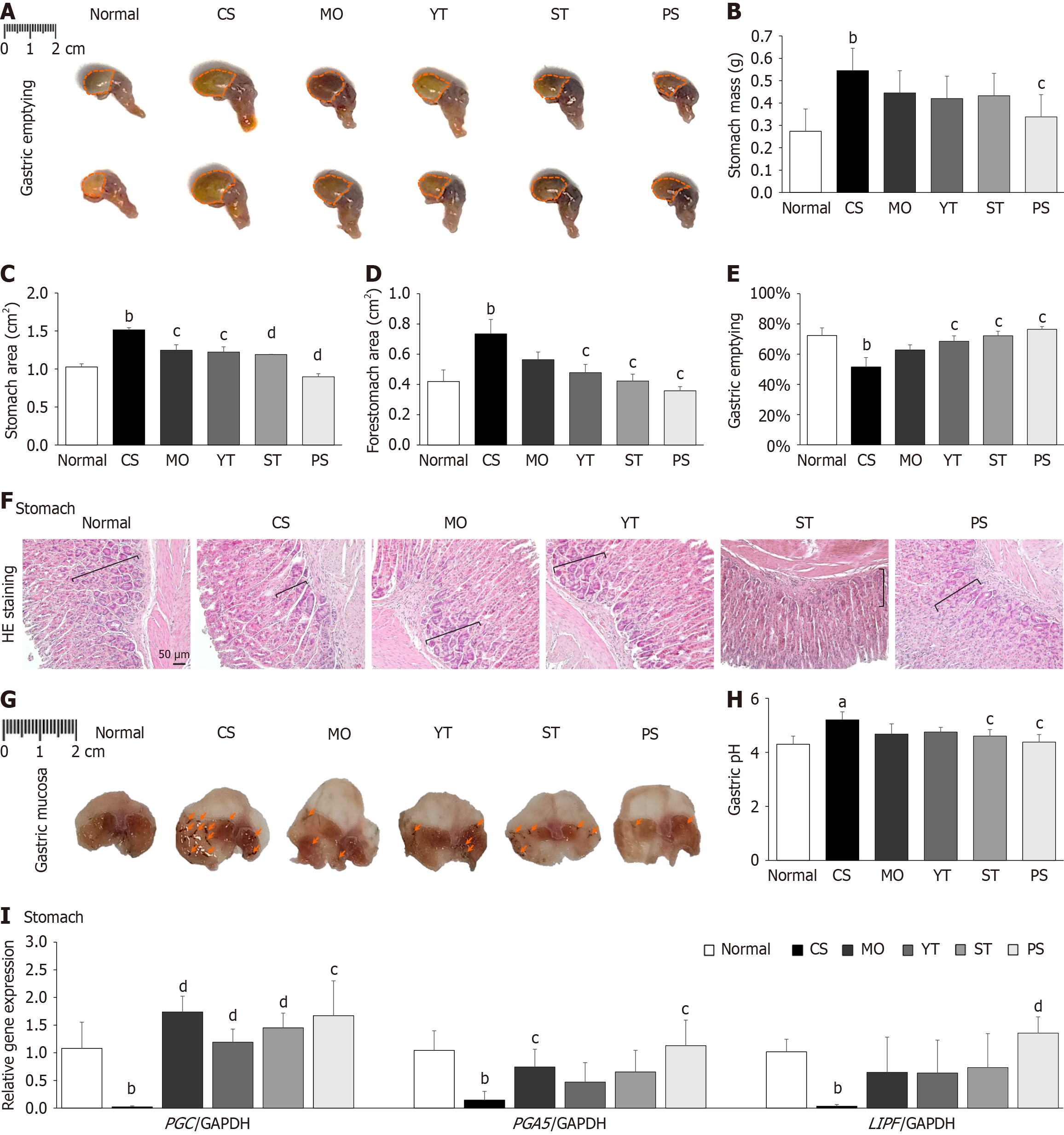
After 20 hours of fasting, mice were subjected to the last 1 hour of CS treatment, excluding the normal group. The mice were allowed to recover 30 minutes at room temperature, and then 400 μL of 0.05% phenol red dissolved in 1.5% carboxymethyl cellulose sodium (CMC) was orally gavaged as a nonabsorbable dye. After 30 minutes, the mice were sacrificed, and their stomachs were removed under anesthesia to evaluate the gastric emptying rate through gross findings, gastric weight, and measurement of the forestomach and stomach areas. The gastric emptying rate was represented as a percentage of the forestomach and stomach areas.
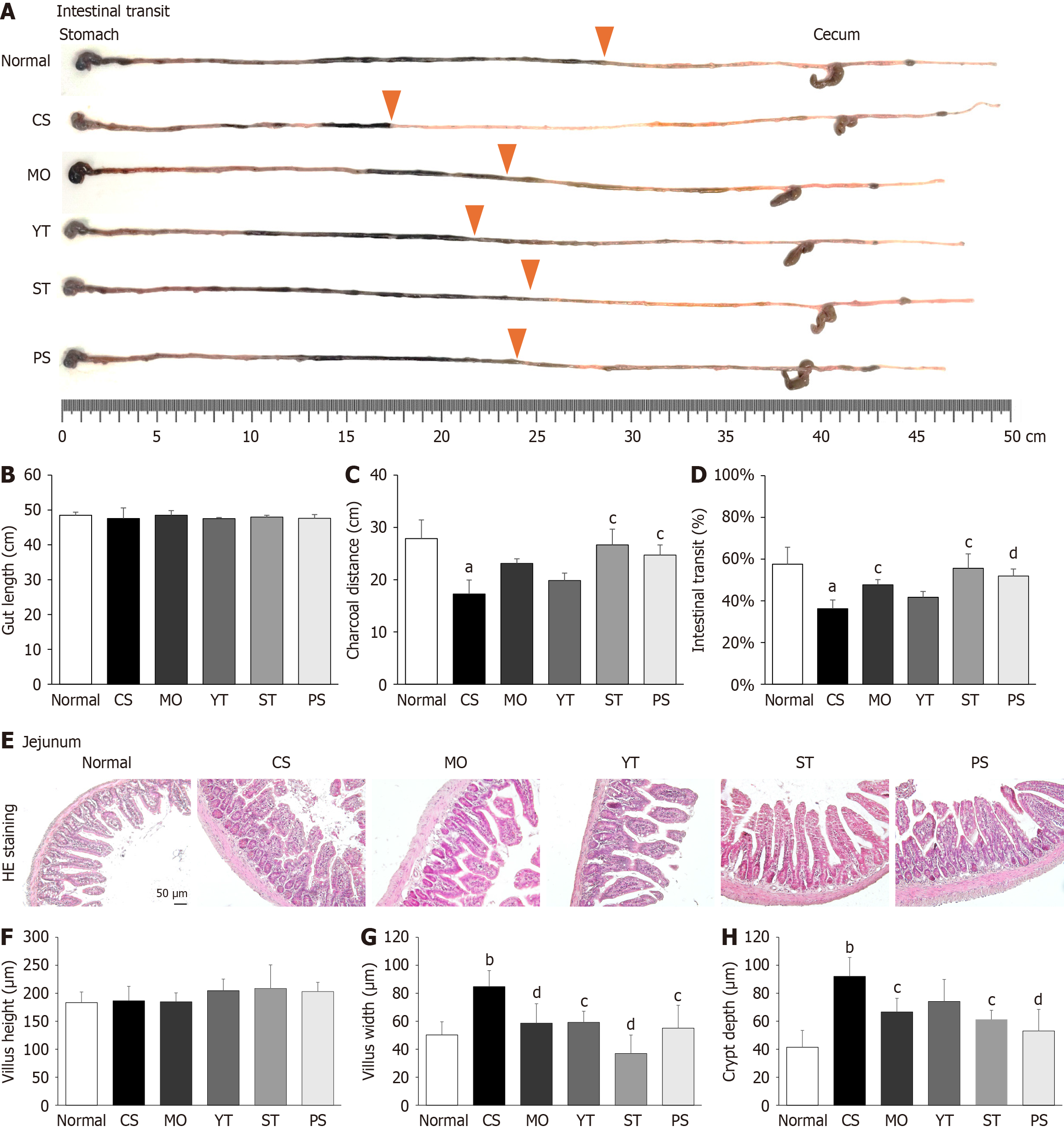
Similar to the gastric emptying experiment, the mice were orally administered 200 μL of 5% charcoal dissolved in 0.5% CMC after the final CS exposure to assess the intestinal transit rate. After 30 minutes, the mice were sacrificed, and their stomachs and intestines were removed to measure the distance traveled by the charcoal. The intestinal transit rate was calculated using the following formula: Intestinal transit rate (%) = (charcoal distance/gut length) × 100%. Herein, gut length was defined as the distance from the stomach to the cecum.
Stomach and jejunal tissues fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned into 4-μm-thick slices for histomorphological examination. After drying, tissue sections were deparaffinized, rehydrated, stained with hematoxy
Small intestine tissue (100 mg of jejunum) was homogenized in 1 mL of radioimmunoprecipitation assay buffer at 4 °C. After centrifugation at 5000 × g for 5 minutes, 100 μL of the supernatant was used to determine the concentrations of glucagon-like peptide-1 (GLP-1), (catalog No. CSB-E08118m), cholecystokinin (CCK), (catalog No. CSB-E08115m), and 5-hydroxytryptamine (5-HT) (5-HT/serotonin, catalog No. MBS723181) according to the user manual provided with the enzyme-linked immunosorbent assay kits (CUSABIO, Wuhan, Hubei Province, China and MyBioSource, San Diego, CA, United States).
The filtrates were transferred into 1.5-mL screw cap vials containing 100 μL inserts in preparation for HPLC analysis. The concentrations (mM/g of mouse feces) of lactate, formate, acetate, propionate, and butyrate were quantified using a Shimadzu HPLC system (Shimadzu, Japan) equipped with an LC-20AD pump, SIL-20A autosampler, and an SPD-20A UV/VIS detector. Analytes were separated using an Aminex HPX-87H column (300 mm × 7.8 mm, 9 μm particle size, Bio-Rad Laboratories, Hercules, CA, United States) at a constant flow rate of 0.6 mL/minute, with the column maintained at 50 °C. The HPLC system was operated in isocratic mode using 0.005 M sulfuric acid as the mobile phase, with detection at 210 nm. The injection volume was 10 μL, and the elution time for each run was 40 minutes. Standards for SCFAs were prepared at concentrations of 0.1-200 mmol/L, and calibration curves were generated for quantification, achieving an R2 value > 0.999.
Total RNA was extracted from frozen mouse stomachs and small intestines using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States). Complementary DNA was synthesized using AccuPower RT PreMix, which included M-MLV reverse transcriptase, reaction buffer, and RNase inhibitor (BIONEER, Daejeon, Republic of Korea). Real-time polymerase chain reaction (PCR) analysis was conducted on 10 related genes including glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene, following previously described protocols for PCR amplification. Detailed information and primer sequences are presented in Supplementary Table 3. Gene expression was analyzed using the LightCycler® 96 system (Roche, Basel, Switzerland). Quantification of relative gene expression was represented by 2–ΔCt calculations and then normalized by GAPDH.
All data are presented as mean ± SD, with sample sizes of either 4 or 8 per group, as indicated in the figure legends. Statistical comparisons among groups were performed using one-way analysis of variance to assess overall group diffe
A calibration curve was established by correlating the peak areas with the analyte concentrations. For paeoniflorin, the calibration equation was Y = 22.643 × X + 0.0487 with R2 > 0.999 and the limit of detection (LOD) and limit of quantitation (LOQ) were 2.66 μg/mL and 8.06 μg/mL, respectively. Paeoniflorin content was 0.87% (w/w) in ST (Figure 1C). Simi
The pre-test showed the comparison results of CS and restraint stress. None of the four cold restraint stress conditions affected body weight compared to normal conditions (P > 0.05) (Supplementary Figure 1B). Under the 10 °C condition, 1-hour water immersion significantly lowered rectal temperature compared to 30-minute water immersion (P < 0.01) (Supplementary Figure 1C). However, under the 20 °C condition, no significant difference in rectal temperature was observed between the 2-hour and 4-hour durations (P > 0.05).
By contrast, none of the four cold restraint stress conditions affected gut length compared to normal conditions (P > 0.05) (Supplementary Figure 1D and E). However, only the 10 °C condition and 1-hour exposure significantly reduced the charcoal distance and intestinal transit compared to normal conditions (P < 0.05) (Supplementary Figure 1D, F, and G). Therefore, the condition of 10 °C for 1 hour was used in the subsequent experimental studies.
After CS stimulation and herbal formula treatment, neither body weight nor food intake showed significant changes compared to that in normal conditions (P > 0.05) (Figure 2B and C). None of the herbal formulas exacerbated the CS-induced reduction in rectal temperature (P > 0.05) (Figure 2D). PS treatment slightly ameliorated the reduction in rectal temperature compared to CS treatment, rather than MO, ST, and YT treatments, although no significant differences were observed.
Cold exposure significantly increased stomach weight, size, and area compared to normal conditions owing to phenol red retention (P < 0.01) (Figure 3A-D). By contrast, PS treatment notably reduced stomach weight, area, and size compared to CS treatment (P < 0.05) (Figure 3A-D). Consequently, CS stimulation reduced gastric emptying rate by 28.8% compared to normal conditions (P < 0.01); however, the PS-treated group exhibited the highest gastric emptying rate among all the groups (P < 0.05) (Figure 3E).
Histopathological observations of the stomach revealed that cold exposure significantly reduced the number of parietal cells compared to the normal condition. However, treatment with any of the herbal formulas or MO notably increased the number of parietal cells compared to CS treatment (Figure 3F). Morphological examinations of the gastric mucosa also showed that cold exposure visibly induced severe hemorrhagic lesions in the gastric mucosa compared with the normal condition. Nevertheless, PS treatment effectively ameliorated gastric mucosal lesions, surpassing ST, YT, and MO (Figure 3G). Furthermore, cold exposure significantly elevated gastric pH compared to normal conditions (P < 0.05) (Figure 3H). Only the PS and ST treatments markedly reduced the gastric pH compared to CS treatment (P < 0.05), whereas the YT and MO treatments did not show any significant difference (Figure 3H).
Cold exposure drastically reduced the expression of the following three gastric digestive enzyme genes: PGC, PGA5, and LIPF, compared to normal conditions (P < 0.01; Figure 3I). PS treatment dramatically increased the expression of the aforementioned gastric digestive enzyme genes compared to CS treatment (P < 0.05, P < 0.01) (Figure 3I). PS treatment was more effective than ST and YT treatments at regulating the expression of gastric digestive enzyme genes (Figure 3I).
After cold exposure and drug treatment, gut length did not show notable changes compared to normal conditions (P > 0.05) (Figure 4A and B). Cold exposure significantly reduced charcoal distance and intestinal transit compared to normal conditions (P < 0.05) (Figure 4C and D). Both the PS and ST treatments, but not the MO and YT treatments, showed a marked recovery in charcoal distance and intestinal transit, similar to normal conditions (P < 0.05, P < 0.01) (Figure 4C and D).
Intriguingly, neither cold exposure nor drug treatment significantly affected jejunal villus height (Figure 4E and F). Histopathological observations of the jejunum showed that cold exposure significantly increased the villus width and crypt depth of the jejunum compared to normal conditions (P < 0.01) (Figure 4G and H). However, PS or ST treatment notably reduced the villus width of the jejunum compared to CS treatment, which was superior to the YT and MO treatments (P < 0.05, P < 0.01) (Figure 4G and H).
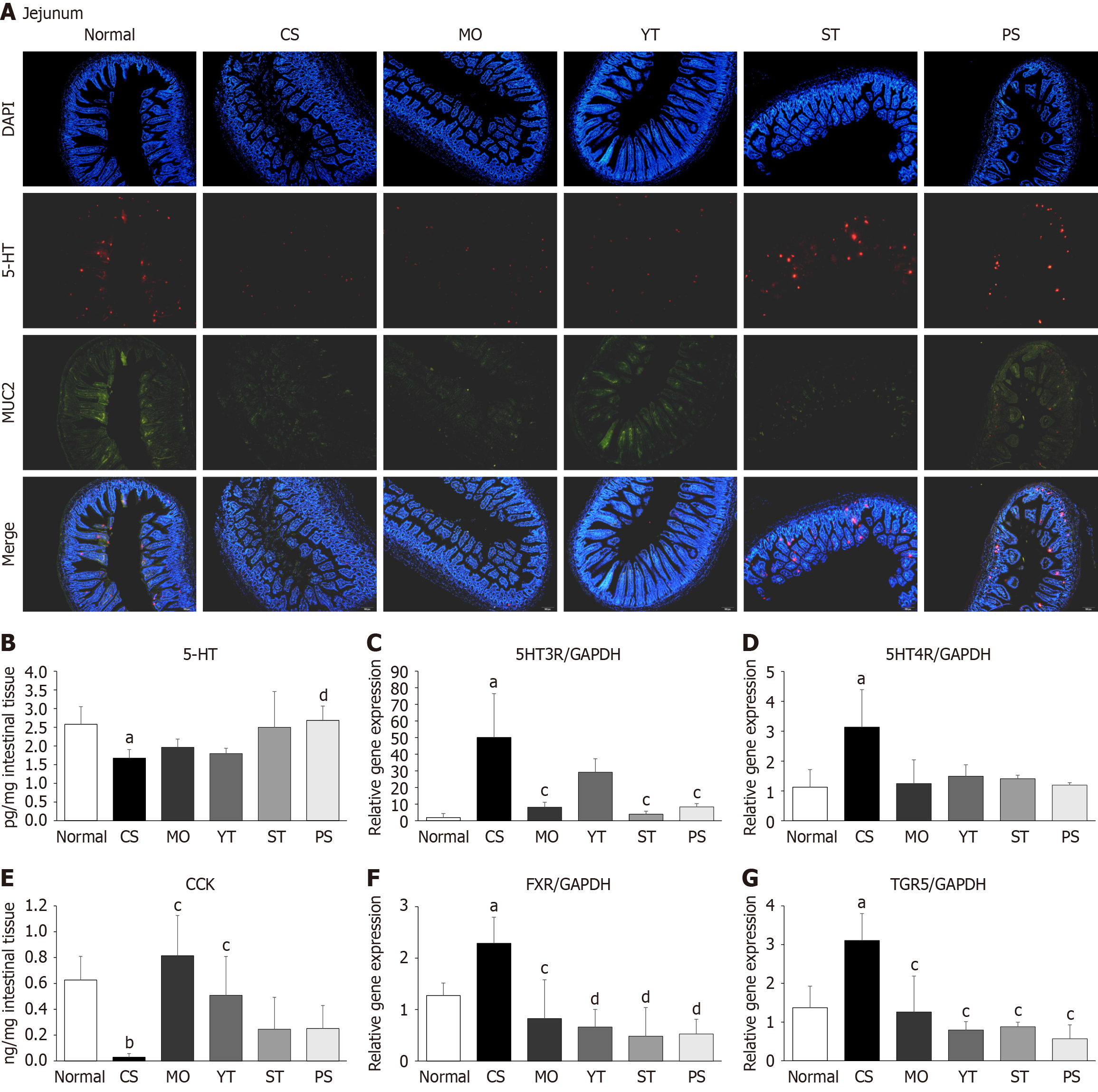
Double immunofluorescence staining showed that cold exposure reduced the fluorescence intensity of 5-HT (serotonin) and MUC2 antibodies in the jejunum compared to normal conditions (Figure 5A). By contrast, PS treatment significantly increased the fluorescence intensity of both antibodies compared to CS treatment, whereas MO treatment had no effect (Figure 5A). Interestingly, ST treatment increased the fluorescence intensity of the 5-HT antibody only, whereas YT treatment increased the fluorescence intensity of the MUC2 antibody only (Figure 5A).
Consistent with the immunofluorescence findings, cold exposure significantly reduced 5-HT concentration in the intestinal tissue compared to normal conditions (P < 0.05) (Figure 5B). However, only PS treatment noticeably increased the intestinal 5-HT concentration (P < 0.01) (Figure 5B). Although cold exposures significantly upregulated the 5-hydroxytryptamine type 3 receptor (5HT3R) and 5-hydroxytryptamine type 4 receptor (5HT4R) gene expressions, only PS and ST treatments significantly downregulated 5HT3R expression (P < 0.05) (Figure 5C and D). However, drug treatments did not increase 5HT4R gene expression.
Cold exposure significantly reduced the intestinal CCK concentration compared to normal conditions (P < 0.01) (Figure 5E). However, although YT and MO treatments significantly enhanced the intestinal CCK concentration (P < 0.05), neither PS nor ST treatment caused a significant increase compared to CS treatment (P > 0.05) (Figure 5E).
Farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5) are the nuclear and membrane bile acid receptors, respectively. Cold exposure markedly upregulated the expression of intestinal FXR and TGR5 compared to normal conditions (P < 0.05) (Figure 5F and G). All herbal formulas, as well as MO, drastically downregulated FXR and TGR5 expressions compared to CS (P < 0.05 and P < 0.01) (Figure 5F and G).
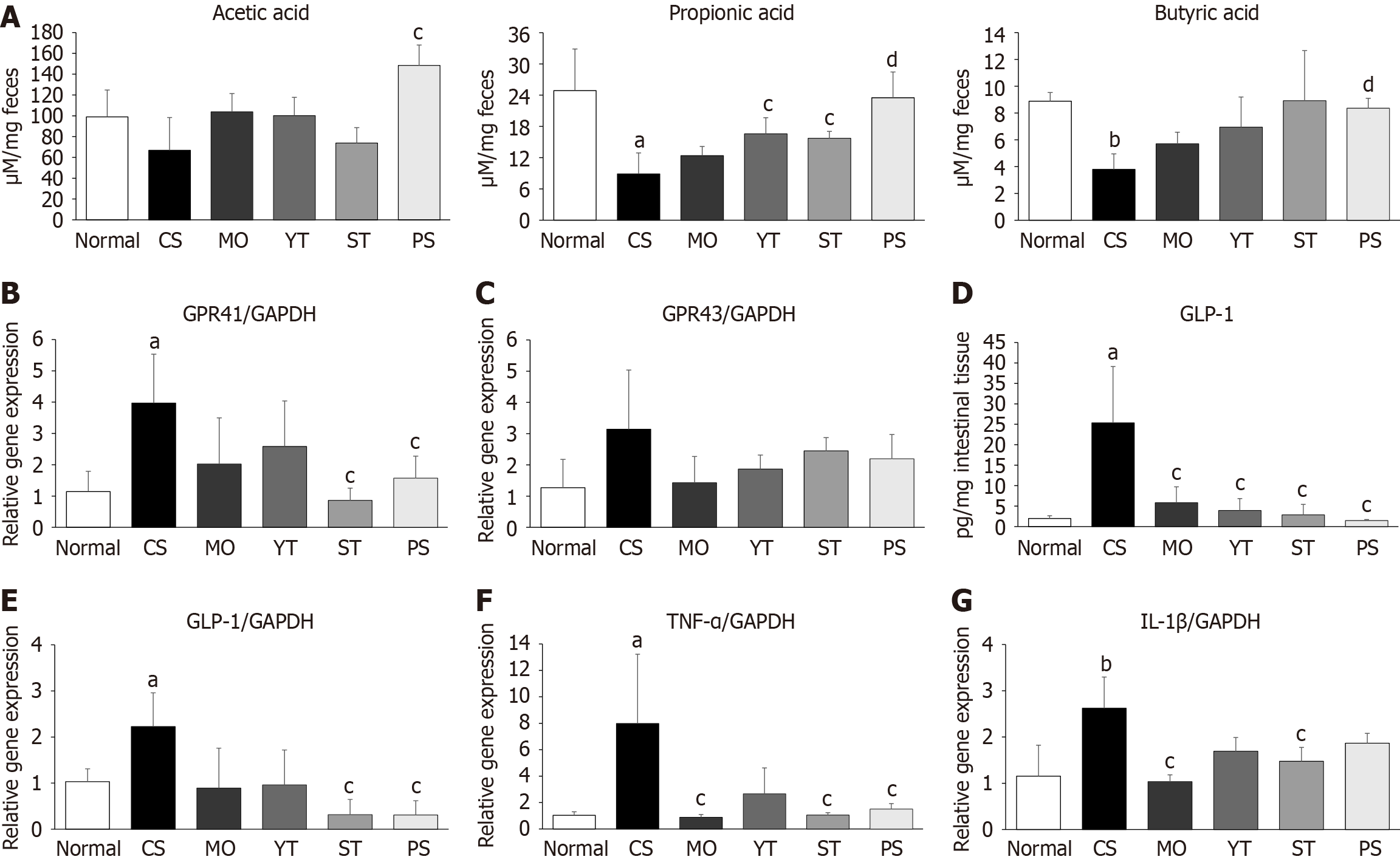
Cold exposure significantly reduced fecal propionic acid and butyric acid levels, but not acetic acid levels, compared to normal conditions (P < 0.05, P < 0.01) (Figure 6A). PS treatment dramatically increased fecal acetic acid, propionic acid, and butyric acid levels compared to CS treatment (P < 0.05 or P < 0.01) (Figure 6A). However, YT and ST treatments showed a relatively modest increase in fecal propionic acid levels compared to PS treatment (P < 0.05) (Figure 6A).
By contrast, cold exposure markedly upregulated the expression of G protein-coupled receptor 41 (GPR41) (P < 0.05) (Figure 6B), but not G protein-coupled receptor 43 (GPR43) (Figure 6C). PS and ST treatments notably downregulated GPR41 expression, whereas YT and MO treatments did not (P < 0.05) (Figure 6B). However, none of these drugs affected GPR43 expression in our study.
Cold exposure markedly elevated intestinal GLP-1 Levels compared to normal conditions (P < 0.05) (Figure 6D). Addi
Regarding intestinal inflammation, cold exposure notably upregulated the tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) gene expression levels in intestinal tissue compared to normal conditions (P < 0.05 or P < 0.01) (Figure 6F and G). However, MO treatment more effectively inhibited TNF-α and IL-1β gene expressions than herbal formula treatments. Among the three herbal formulas, ST exhibited the most potent inhibition of the inflammatory genes.
In traditional medical theory, cold is considered a critical pathological factor that directly contributes to GI disorders[15]. Cold restraint stress has been frequently used in animal models to investigate GI dysfunction because of its simplicity[24]. Numerous studies have demonstrated that both cold-water immersion and restraint stress can result in GI dysfunction, often leading to conditions such as dyspepsia[25,26]. A previous animal study has proved that gut motility in mice is temperature-dependent[27]. However, the dominant effects of cold and restraint stress on GI dysfunction remain unknown. Therefore, in preliminary experiments, we compared the relative sensitivity of gut motility to variations in temperature or restraint duration. We observed that 1-hour cold water-immersion apparently induced more sensitivity-induced intestinal transit than 4-hour restraint stress in normal-temperature water immersion (Supplementary Figure 1). Thus, CS was more effective than restraint stress in inhibiting intestinal motility.
Although cold exposure often leads to increased food intake and body weight as a compensatory mechanism to maintain core temperature and energy balance[28], our studies did not observe any evident changes in body mass, body temperature decline, and food intake following intermittent cold exposure (1 hour, 10 °C ± 2 °C, six times over 2 weeks) or co-treatment with any herbal formulas. Thus, alterations in basic metabolism and appetite can be ruled out as contri
Previous studies have indicated that CS can delay gastric emptying[29], with one human study reporting a dramatic decrease in total gastric emptying from 17% to 2% during CS[6]. To compare the effects of the herbal formulas on im
By contrast, the charcoal test is a straightforward and reliable method for assessing changes in intestinal motility, particularly in rodent models[33]. Our charcoal test results clearly demonstrate that cold exposure impairs intestinal motility. Both PS and ST effectively ameliorated this dysfunction, whereas YT had no effect. Moreover, intestinal histopathological findings showed that cold exposure predominantly induced intestinal villus width and crypt depth enlargement rather than length extension. Cold exposure generally reduced blood flow to peripheral tissues, including the intestines, thereby limiting the oxygen and nutrients needed for energy-intensive processes, such as villus elongation[34]. Meanwhile, increased crypt depth and villus width may represent a more adaptive response to CS, aimed at maintaining the structural integrity of the gut rather than solely enhancing nutrient absorption[35]. MUC2, the main mucin glycoprotein produced by intestinal epithelial cells, is mainly present in the mucus layer and is essential for the formation and protection of the intestinal barrier[36]. The decrease in the intestinal MUC2 levels supports the finding that cold exposure impairs the intestinal barrier. Consequently, the pathological findings suggest that the intestine may undergo compensatory epithelial remodeling following cold exposure. The increases in villus width and crypt depth may be part of a remodeling process that strengthens the intestine’s ability to adapt to CS. Thus, the inhibition of villus width and crypt depth expansion implied that PS and ST effectively alleviated CS-induced damage to intestinal motility.
Serotonin is an essential neurotransmitter, with approximately 90%-95% located in the gut, where it is principally produced by enterochromaffin cells and can be regulated by tryptophan metabolism through gut microbiota[37]. Peripheral serotonin plays a key role in the regulation of gut motility by acting as a signaling molecule that triggers peristaltic and secretory reflexes[38]. Several studies have shown that CS reduces serotonin levels and alters the structure of gut microbiota[35,38,39]. Our study also confirmed a reduction in intestinal serotonin levels following cold exposure. Interestingly, the gene expression of the two main serotonin receptors was simultaneously upregulated by CS. This inconsistency suggests a compensatory mechanism in the response to CS. Only PS treatment enhanced intestinal serotonin levels and reduced intestinal 5HT3R gene expression. Therefore, the direct or indirect reduction of intestinal serotonin may be one of the mechanisms by which PS treatment improves intestinal motility.
Many studies have reported that alterations in the gut microbiota are closely associated with the production of SCFAs and changes in the bile acid profile[40,41]. SCFAs are crucial for gut health, serving as an energy source for enterocytes and promoting gut motility by modulating intestinal muscle contraction, fluid absorption, and gut barrier function[42]. They also help regulate inflammation and support the overall health of the gut microbiome, thereby maintaining a balanced and functional digestive system[43]. Consistent with previous results, cold exposure visibly reduced SCFA production in the gut, particularly in the presence of butyric and propionic acids[44]. Meanwhile, GPR41 and GPR43, which are G protein-coupled receptors that are activated by SCFAs, are involved in immune and inflammatory responses[45]. In our study, low SCFA levels led to the compensatory upregulation of GPR41 and GPR43, thereby amplifying the inflammation process. Consequently, elevation of SCFA production and reduction of GPR41 and TNF-α levels present another potential mechanism illuminating how PS treatment improves intestinal function.
CS can upregulate the bile acid synthesis enzyme CYP7B1, increasing the liver and fecal bile acids[46]. In addition, both FXR (a nuclear receptor for bile acids) and TGR5 (a membrane receptor for bile acids) are important regulators of in
Finally, this study has several limitations that should be mentioned. First, only male mice were used in the experi
PS treatment was significantly more effective than either YT or ST treatment in alleviating GI dysfunction caused by intermittent CS. The observed benefits of PS may be closely associated with its ability to modulate key physiological pathways including enhancement of serotonin and SCFA levels, reduction of GLP-1 activity, and downregulation of intestinal bile acid receptors. This molecular and metabolic regulation may play a critical role in mitigating the adverse effects of CS on GI function. Furthermore, this comparative analysis not only highlights the distinct therapeutic advan
We sincerely thank Dr. Hwang SJ from the Institute of Bioscience and Integrative Medicine, Daejeon University, for his invaluable technical support in the evaluation of GI motility.
| 1. | Oustamanolakis P, Tack J. Dyspepsia: organic versus functional. J Clin Gastroenterol. 2012;46:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Lee K, Kwon CI, Yeniova AÖ, Koyanagi A, Jacob L, Smith L, Lee SW, Rahmati M, Shin JY, Shin JI, Cho W, Yon DK. Global prevalence of functional dyspepsia according to Rome criteria, 1990-2020: a systematic review and meta-analysis. Sci Rep. 2024;14:4172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 256] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (2)] |
| 4. | Ghoshal UC, Singh R, Chang FY, Hou X, Wong BC, Kachintorn U; Functional Dyspepsia Consensus Team of the Asian Neurogastroenterology and Motility Association and the Asian Pacific Association of Gastroenterology. Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction. J Neurogastroenterol Motil. 2011;17:235-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Talley NJ. Functional dyspepsia: new insights into pathogenesis and therapy. Korean J Intern Med. 2016;31:444-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Fone DR, Horowitz M, Maddox A, Akkermans LM, Read NW, Dent J. Gastroduodenal motility during the delayed gastric emptying induced by cold stress. Gastroenterology. 1990;98:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Li R, Amenyogbe E, Lu Y, Jin J, Xie R, Huang J. Effects of low-temperature stress on intestinal structure, enzyme activities and metabolomic analysis of juvenile golden pompano (Trachinotus ovatus). Front Mar Sci. 2023;10. [DOI] [Full Text] |
| 8. | Pujante IM, Díaz-lópez M, Mancera JM, Moyano FJ. Characterization of digestive enzymes protease and alpha-amylase activities in the thick-lipped grey mullet (Chelon labrosus, Risso 1827). Aquac Res. 2017;48:367-376. [DOI] [Full Text] |
| 9. | Muyan C, Xiumei Z, Tianxiang G, Chao C. Effects of temperature, pH and NaCl on protease activity in digestive tract of young turbot, Scophthalmus maximus. Chin J Ocean Limnol. 2006;24:300-306. [DOI] [Full Text] |
| 10. | Xing JQ, Zhou Y, Chen JF, Li SB, Fang W, Yang J. Effect of cold adaptation on activities of relevant enzymes and antioxidant system in rats. Int J Clin Exp Med. 2014;7:4232-4237. [PubMed] |
| 11. | Meng Y, Chen L, Lin W, Wang H, Xu G, Weng X. Exercise Reverses the Alterations in Gut Microbiota Upon Cold Exposure and Promotes Cold-Induced Weight Loss. Front Physiol. 2020;11:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Sun L, Zhu R, Zhang S, Liu S, Wang Y, Wu Y, Xing S, Liao X, Mi J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. NPJ Biofilms Microbiomes. 2022;8:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Bhattacharjee G, Khambhati K, Gohil N, Singh P, Gohil J, Gautam H, Maurya R, Chu DT, Ramakrishna S, Singh V. Gut microbiota in gastrointestinal diseases. Prog Mol Biol Transl Sci. 2022;191:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 14. | Curran J. The Yellow Emperor’s Classic of Internal Medicine. BMJ. 2008;336:777. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Fu W. The Yellow Emperor's Canon of Medicine: First Complete Summary of Ancient Chinese Medicine. Chin Med Cult. 2018;1:18-20. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Gasbarrini G, Candelli M, Graziosetto RG, Coccheri S, Di Iorio F, Nappi G. Evaluation of thermal water in patients with functional dyspepsia and irritable bowel syndrome accompanying constipation. World J Gastroenterol. 2006;12:2556-2562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yao Y, Habib M, Bajwa HF, Qureshi A, Fareed R, Altaf R, Ilyas U, Duan Y, Abbas M. Herbal therapies in gastrointestinal and hepatic disorders: An evidence-based clinical review. Front Pharmacol. 2022;13:962095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Hwang SJ, Wang JH, Lee JS, Lee HD, Choi TJ, Choi SH, Son CG. Yeokwisan, a Standardized Herbal Formula, Enhances Gastric Emptying via Modulation of the Ghrelin Pathway in a Loperamide-induced Functional Dyspepsia Mouse Model. Front Pharmacol. 2021;12:753153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Lim B. Korean medicine coverage in the National Health Insurance in Korea: present situation and critical issues. Integr Med Res. 2013;2:81-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Riedlinger JE, Tan PW, Lu W. Ping wei san, a Chinese medicine for gastrointestinal disorders. Ann Pharmacother. 2001;35:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Lee MC, Ha W, Park J, Kim J, Jung Y, Kim BJ. Effects of Lizhong Tang on gastrointestinal motility in mice. World J Gastroenterol. 2016;22:7778-7786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Qin F, Liu JY, Yuan JH. Chaihu-Shugan-San, an oriental herbal preparation, for the treatment of chronic gastritis: a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2013;146:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2295] [Cited by in RCA: 4341] [Article Influence: 434.1] [Reference Citation Analysis (0)] |
| 24. | Coskun T, Yeğen BC, Alican I, Peker O, Kurtel H. Cold restraint stress-induced gastric mucosal dysfunction. Role of nitric oxide. Dig Dis Sci. 1996;41:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, Tickle TL, Weingart G, Ren B, Schwager EH, Chatterjee S, Thompson KN, Wilkinson JE, Subramanian A, Lu Y, Waldron L, Paulson JN, Franzosa EA, Bravo HC, Huttenhower C. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. 2021;17:e1009442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 1492] [Article Influence: 298.4] [Reference Citation Analysis (0)] |
| 26. | Muraoka M, Mine K, Kubo C. A study of intestinal dysfunction induced by restraint stress in rats. Scand J Gastroenterol. 1998;33:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Han A, Hudson-Paz C, Robinson BG, Becker L, Jacobson A, Kaltschmidt JA, Garrison JL, Bhatt AS, Monack DM. Temperature-dependent differences in mouse gut motility are mediated by stress. Lab Anim (NY). 2024;53:148-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Lal NK, Le P, Aggarwal S, Zhang A, Wang K, Qi T, Pang Z, Yang D, Nudell V, Yeo GW, Banks AS, Ye L. Xiphoid nucleus of the midline thalamus controls cold-induced food seeking. Nature. 2023;621:138-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Lei Y, Chen J. Inhibitory effects of various types of stress on gastric tone and gastric myoelectrical activity in dogs. Scand J Gastroenterol. 2009;44:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Hara N, Hara Y, Natsume Y, Goto Y. Gastric hyperacidity and mucosal damage caused by hypothermia correlate with increase in GABA concentrations of the rat brain. Eur J Pharmacol. 1991;194:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Richter C, Tanaka T, Yada RY. Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem J. 1998;335 ( Pt 3):481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Chahinian H, Snabe T, Attias C, Fojan P, Petersen SB, Carrière F. How gastric lipase, an interfacial enzyme with a Ser-His-Asp catalytic triad, acts optimally at acidic pH. Biochemistry. 2006;45:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Marona HR, Lucchesi MB. Protocol to refine intestinal motility test in mice. Lab Anim. 2004;38:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Guo J, Hu H, Chen Z, Xu J, Nie J, Lu J, Ma L, Ji H, Yuan J, Xu B. Cold Exposure Induces Intestinal Barrier Damage and Endoplasmic Reticulum Stress in the Colon via the SIRT1/Nrf2 Signaling Pathway. Front Physiol. 2022;13:822348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Sun L, Wang X, Zou Y, He Y, Liang C, Li J, Li P, Zhang J. Cold stress induces colitis-like phenotypes in mice by altering gut microbiota and metabolites. Front Microbiol. 2023;14:1134246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Song C, Chai Z, Chen S, Zhang H, Zhang X, Zhou Y. Intestinal mucus components and secretion mechanisms: what we do and do not know. Exp Mol Med. 2023;55:681-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 128] [Reference Citation Analysis (1)] |
| 37. | Liu N, Sun S, Wang P, Sun Y, Hu Q, Wang X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int J Mol Sci. 2021;22:7931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 38. | Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Lv H, Xia S, He Y, Qiao C, Liu J, Guo J, Li S. Effect of chronic cold stress on gut microbial diversity, intestinal inflammation and pyroptosis in mice. J Physiol Biochem. 2024;80:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Fusco W, Lorenzo MB, Cintoni M, Porcari S, Rinninella E, Kaitsas F, Lener E, Mele MC, Gasbarrini A, Collado MC, Cammarota G, Ianiro G. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients. 2023;15:2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 648] [Reference Citation Analysis (0)] |
| 41. | Gao R, Meng X, Xue Y, Mao M, Liu Y, Tian X, Sui B, Li X, Zhang P. Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front Pharmacol. 2022;13:1027212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 42. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 3516] [Article Influence: 270.5] [Reference Citation Analysis (3)] |
| 43. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1899] [Article Influence: 316.5] [Reference Citation Analysis (2)] |
| 44. | Ichikawa N, Sasaki H, Lyu Y, Furuhashi S, Watabe A, Imamura M, Hayashi K, Shibata S. Cold Exposure during the Active Phase Affects the Short-Chain Fatty Acid Production of Mice in a Time-Specific Manner. Metabolites. 2021;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396-406.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 840] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 46. | Worthmann A, John C, Rühlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, Fischer M, Dandri M, Kremoser C, Scheja L, Franke A, Shaul PW, Heeren J. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 47. | Trabelsi MS, Lestavel S, Staels B, Collet X. Intestinal bile acid receptors are key regulators of glucose homeostasis. Proc Nutr Soc. 2017;76:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Zhou M, Wang D, Li X, Cao Y, Yi C, Wiredu Ocansey DK, Zhou Y, Mao F. Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer. Front Pharmacol. 2022;13:1016836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 49. | Chiang JY, Pathak P, Liu H, Donepudi A, Ferrell J, Boehme S. Intestinal Farnesoid X Receptor and Takeda G Protein Couple Receptor 5 Signaling in Metabolic Regulation. Dig Dis. 2017;35:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/