Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.109938
Revised: July 8, 2025
Accepted: August 5, 2025
Published online: September 7, 2025
Processing time: 98 Days and 16.3 Hours
Strictures in ulcerative colitis (UC) are relatively uncommon but are associated with increased risk of malignancy and complications. Until recently, fibrogenesis and strictures have remained largely unexplored in UC.
To investigate the incidence, long-term prognosis and risk factors of colorectal strictures in a large cohort of UC patients.
A total of 938 hospitalized UC patients at Peking Union Medical College Hospital were included from 2014 to 2024. Stricture was defined as a fixed localized narrowing of the colorectal lumen. Risk factors for stricture formation were identified by multivariable Cox regression. Prognosis was analyzed using the Kaplan-Meier or Fine-Gray method. Sensitivity analysis excluded malignant strictures due to their distinct pathophysiology.
The overall incidence of stricture was 12.4% over a median follow-up of 8.70 years, with a 10-year cumulative probability of 11.3%. Malignancy occurred in 8.6% of stricture cases. UC patients with strictures were at higher risk for intestinal complications, surgery and malignancy (P < 0.05). The 10-year cumulative probabilities of surgery and all-cause mortality were 37.6% and 1.6%, respectively. Age ≥ 40 years at diagnosis [hazard ratio (HR) = 2.197, 95% confidence interval (CI): 1.487-3.242] and extraintestinal manifestations (HR = 2.072, 95%CI: 1.326-3.239) were associated with higher stricture risk, while the use of biological agents such as vedolizumab (HR = 0.382, 95%CI: 0.203-0.720) was protective against strictures (P < 0.05). Sensitivity analysis on benign strictures showed consistent findings, with similar risk factors and worse long-term outcomes.
UC patients with strictures had worse long-term prognostic outcomes. Earlier endoscopic surveillance and biologic treatment should be considered in patients ≥ 40 years or those with extraintestinal manifestations.
Core Tip: Limited studies have focused on the long-term clinical outcomes and risk factors associated with strictures in ulcerative colitis (UC). This cohort study analyzed 938 UC patients with a median follow-up time of 8.70 years. The incidence of UC strictures was 12.4%, with 8.6% confirmed as malignant. UC patients with strictures had poor treatment efficacy and higher risks of surgery, intestinal complications and colorectal cancer. Age ≥ 40 years at diagnosis and extraintestinal manifestations were risk factors for stricture formation, while vedolizumab use was protective. Sensitivity analysis, excluding malignant strictures, showed consistent findings.
- Citation: Shao YP, Han TT, Lv H, Yang ST, Zhu QL, Li J, Li JN. Risk factors and long-term prognosis for colorectal strictures in ulcerative colitis. World J Gastroenterol 2025; 31(33): 109938
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/109938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.109938
Chronic intestinal inflammation can trigger progressive tissue damage and repair, culminating in intestinal fibrosis and strictures with severe complications in inflammatory bowel diseases (IBD)[1]. Ulcerative colitis (UC) has traditionally been regarded as a non- or minimally fibrotic disease with rare stricture formation due to the superficial nature of its inflammation. The reported incidence of UC strictures varied from 1% to 14.2%, influenced by differences in definitions and study heterogeneity[2-9]. However, Gordon et al[10] found that submucosal fibrosis could be detected in all involved colonic specimens of UC. Recent research also indicated that the fibrotic response in UC could extend beyond the mucosal and submucosal layer, potentially involving the whole bowel wall in advanced cases[11-13]. Additionally, the prevalence of UC strictures is sometimes comparable to that in colonic Crohn’s disease (CD)[4,7,14,15]. These findings suggest that fibrosis and strictures in UC have been largely underestimated in the past and warrant further investigation.
In addition to causing obstructive symptoms, strictures greatly limit endoscopic assessment of disease activity and dysplasia in the proximal colon. Several studies have identified a correlation between colonic strictures and colorectal cancer (CRC), with reported rates ranging from 1.9% to 40.0%[2-7,16-18]. In addition, UC patients with strictures were associated with poorer clinical outcomes[7]. Since the advent of biological therapies, research on the effects of these therapies on intestinal stricture and fibrosis has been limited. The available evidence, predominantly from CD patients, has demonstrated conflicting results: While some studies suggested that early anti-tumor necrosis factor-α (anti-TNF-α) treatment may delay surgical intervention in patients with strictures[19], others indicated that pre-operative anti-TNF-α therapy may increase fibrosis burden[20]. For newer biologics, such as vedolizumab (VDZ) or ustekinumab (UST), anti-fibrotic effects have been proposed based on mechanistic studies[21,22], but clinical evidence regarding their impact on strictures remains extremely scarce. Therefore, early identification of UC-related strictures and their associated risk factors, along with the exploration of potentially effective treatments, are crucial for optimizing clinical management.
The objectives of this study were to evaluate the incidence, risk factors and treatment of UC-related strictures, and compare clinical outcomes and long-term prognosis between UC patients with and without strictures.
UC patients hospitalized at Peking Union Medical College Hospital (PUMCH) from January 2014 to September 2024 were consecutively enrolled. Patients with a confirmed diagnosis of UC according to the European Crohn’s and Colitis Organization (ECCO) guidelines[23] and a follow-up duration ≥ 6 months were included. Additionally, patients were required to have at least two colonoscopies or cross-sectional imaging assessments. Exclusion criteria included: A change in diagnosis to CD or IBD unclassified, or a prior history of CRC or familial adenomatous polyposis. A colonic stricture was defined as a fixed, localized luminal narrowing identified by one or more of the following modalities: Endoscopy, cross-sectional imaging or surgical specimens. Endoscopic strictures were defined as fixed luminal narrowings that prevented (impassable strictures) or significantly resisted (passable strictures) the passage of a standard colonoscope. Radiologic strictures were characterized by segmental, fixed luminal narrowings with bowel wall thickening and/or upstream dilation, as identified on computed tomography, magnetic resonance imaging or ultrasonography. Surgical strictures were identified as localized narrowings visible on macroscopic examination of resected specimens, as documented in pathology reports. No strict quantitative threshold was applied due to the substantial variation in colonic caliber and potential influence of bowel preparation and distension on measurement reliability. Lumen narrowing caused by obvious polypoid lesions or inflammatory edema was excluded. Strictures were classified as benign or malignant based on histopathology from endoscopic biopsies or surgical specimens. Histologic assessment of fibrosis within strictures was not available in this study. Among patients with strictures identified by endoscopy or cross-sectional imaging who underwent at least one follow-up assessment using the same modality, stricture improvement was further evaluated. For endoscopic strictures, improvement was defined as resolution of luminal resistance during passage of a standard colonoscope or conversion from complete obstruction to successful intubation. For radiologic strictures, improvement was defined as a clear increase in luminal diameter at the affected segment on follow-up imaging compared to baseline. All improvements were assessed in the absence of surgical or endoscopic intervention. The study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. K24C3372).
The following clinical data were retrospectively collected from medical records: (1) Demographic and baseline characteristics: Sex, age at diagnosis, disease duration, follow-up time, disease extent at diagnosis (Montreal classification)[23], smoking status, family history of IBD and CRC, and history of appendectomy; (2) Clinical characteristics: Weight loss, extraintestinal manifestations (EIM) (including arthropathy, skin lesions, oral ulcers, ophthalmopathy, thrombosis and primary sclerosing cholangitis), laboratory results, and IBD-related treatments before and after stricture diagnosis, or until the end of follow-up in patients without strictures. Treatment data included 5-aminosalicylic (5-ASA), systemic glucocorticoids, immunomodulators, and biologics; (3) Stricture characteristics: Date and initial method of stricture identification, number and location of strictures, presence of symptomatic bowel obstruction, stricture improvement during follow-up, and association with CRC; and (4) Prognostic outcomes: UC-related surgery (colectomy or ileostomy), CRC, all-cause mortality, and severe intestinal complications (intestinal bleeding, perforation, obstruction, toxic megacolon, or fistula) during long-term follow-up, as well as clinical disease activity at the last follow-up. Clinical treatments and outcomes were assessed through medical records, with updates obtained through outpatient visits or phone follow-up. Complications were identified based on a combination of clinical symptoms, laboratory findings, and endoscopic or radiological results. Clinical disease activity was evaluated by the partial Mayo Clinic score. Clinical remission was defined as a partial Mayo Clinic score ≤ 2, with all sub-scores ≤ 1 (including stool frequency and rectal bleeding). Mild, moderate and severe disease activities were defined by partial Mayo Clinic score of 3-5, 6-10, and 11-12, respectively. Steroid dependence was defined as the inability to reduce prednisone to 10 mg/day within 3 months or disease relapse within 3 months after steroid cessation. Steroid resistance was defined as persistent disease activity > 4 weeks of treatment with prednisone at 0.75-1.0 mg/kg/day, after excluding infections.
Missing data were handled using multiple imputation, with a complete-case analysis performed as sensitivity analysis (missing data details are provided in Supplementary Table 1). Categorical variables are expressed as percentages and compared using the χ2 test or Fisher’s exact test. Continuous variables with a normal distribution are expressed as means ± SD and compared using the two-sample t-test. Non-normally distributed continuous variables are expressed as medians with interquartile range (IQR) and compared using the Wilcoxon rank-sum test. For time-to-event analyses, time-zero was defined as the date of UC diagnosis. Cumulative probabilities of stricture development and all-cause mortality were estimated using the Kaplan-Meier method with log-rank tests for group comparisons. The Fine-Gray sub-distribution hazard model was applied to assess the cumulative incidence of UC-related surgery (with death as competing event) and malignancy transformation (with death and total colectomy as competing events). Univariate Cox proportional hazards regression was used to identify potential risk factors for UC stricture development. Variables with a P value < 0.1 in univariate analysis, along with clinically relevant variables based on pathophysiology and existing literature, were included in a multivariate Cox regression model using stepwise (conditional) selection. Cox model assumptions, including proportional hazards and absence of multicollinearity, were formally tested and verified. Time-dependent Cox regression was used to identify risk factors for persistent UC strictures, accounting for immortal time bias.
Three additional sensitivity analyses were conducted: (1) Complete-case analysis to assess the impact of missing data on results; (2) Analysis restricted to benign strictures, excluding malignant cases due to their distinct pathophysiology; and (3) Analysis excluding external surgical referrals and early surgery cases (within 30 days of diagnosis) to minimize tertiary center selection bias.
SPSS version 23.0 (IBM Corporation, Chicago, IL, United States) and R 4.3.0 were used for statistical analysis. All statistical tests were two-sided, with a P value < 0.05 considered statistically significant.
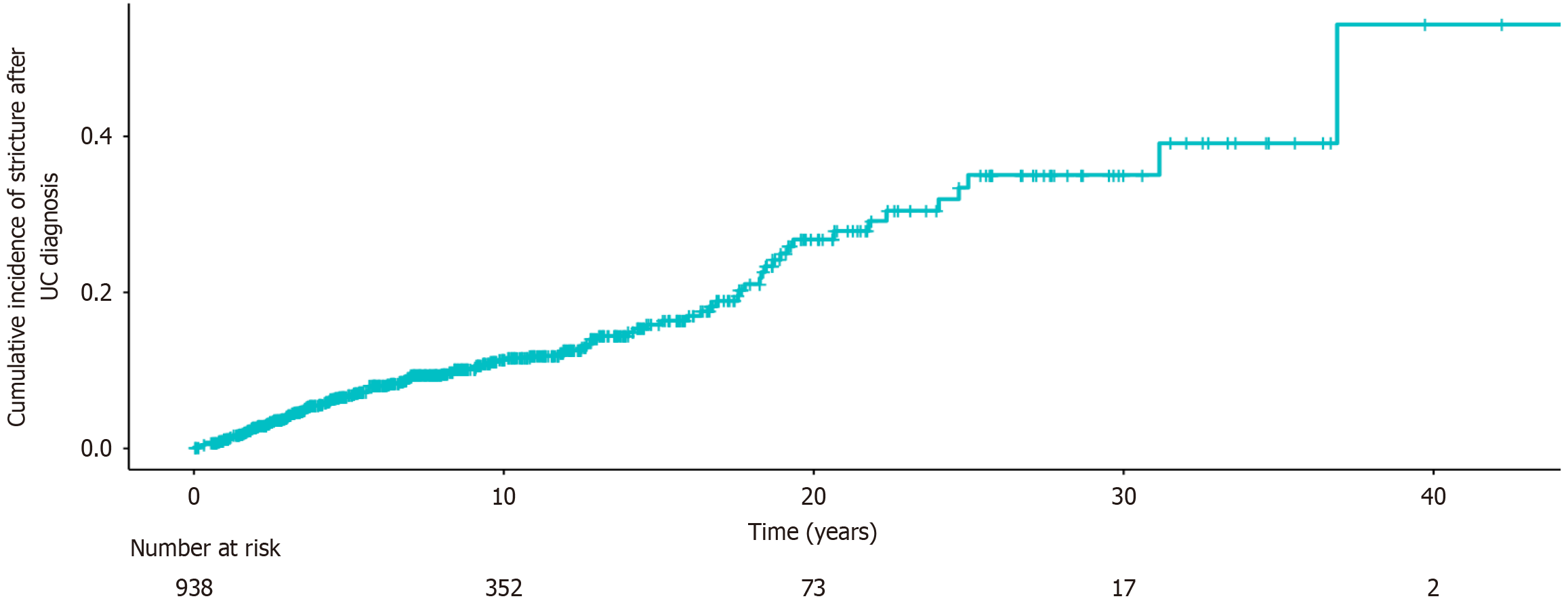
A total of 938 UC patients (532 males) were included, consisting of 116 patients with strictures (Table 1). The cumulative probability of stricture formation was 6.8% at 5 years, 11.3% at 10 years, 16.4% at 15 years, and 26.4% at 20 years after diagnosis (Figure 1). More than half of the patients had extensive disease (56.5%), while 13.5% had proctitis and 29.9% had left-sided colitis at diagnosis. The median age at diagnosis was higher in the stricture group than that in the non-stricture group (38.97 years, IQR: 30.52-49.39 years vs 34.50 years, IQR: 26.61-47.14 years, P = 0.020), and a greater proportion of patients in the stricture group were over 40 years old (49.1% vs 37.7%, P = 0.018). In addition, the disease duration was also longer in the stricture group (11.55 years, IQR: 7.13-18.74 years vs 8.96 years, IQR: 5.67-13.59 years, P < 0.001). Patients with strictures had a higher incidence of EIM (23.3% vs 13.6%, P = 0.006), particularly oral ulcers (10.3% vs 5.4%, P = 0.034) and venous thrombosis (7.8% vs 1.6%, P < 0.001). Hemoglobin levels at admission were significantly lower in the stricture group (103.78 ± 23.59 g/L vs 111.63 ± 26.28 g/L, P < 0.001). Before stricture formation, the proportion of patients treated with 5-ASA, systemic steroids, immunomodulators, and biologics was 95.7%, 84.5%, 27.6% and 21.6%, respectively. Steroid use was more frequent in the stricture group than in the non-stricture group (84.5% vs 74.3%, P = 0.017), whereas biologics use was significantly lower (21.6% vs 38.2%, P < 0.001).
| Stricture group | Non-stricture group | 1P value | 2P value | |||
| All stricture cases | Malignant stricture group | Benign stricture group | ||||
| Number of patients | 116 | 10 | 106 | 822 | ||
| Male | 58 (50.0) | 6 (60.0) | 52 (49.1) | 474 (57.7) | 0.119 | 0.092 |
| Age at diagnosis, year (median, IQR) | 38.97 (30.52-49.39) | 36.06 (32.19-42.39) | 40.69 (29.36-51.95) | 34.50 (26.61-47.14) | 0.020 | 0.016 |
| ≥ 40 | 57 (49.1) | 3 (30.0) | 54 (50.9) | 310 (37.7) | 0.018 | 0.009 |
| Disease duration, year (median, IQR) | 11.55 (7.13-18.74) | 25.39 (19.38-32.39) | 10.75 (6.81-15.69) | 8.96 (5.67-13.59) | < 0.001 | 0.005 |
| Follow-up time, year (median, IQR) | 9.85 (4.25-17.22) | 24.10 (19.17-32.37) | 7.95 (3.70-14.42) | 8.65 (4.87-12.82) | 0.373 | 0.660 |
| Montreal classification | 0.068 | 0.023 | ||||
| E1 (proctitis) | 23 (19.8) | 0 (0.0) | 23 (21.7) | 104 (12.7) | ||
| E2 (left-sided colitis) | 28 (24.1) | 3 (30.0) | 25 (23.6) | 253 (30.8) | ||
| E3 (pancolitis) | 65 (56.0) | 7 (70.0) | 58 (54.7) | 465 (56.6) | ||
| Smoking status | 0.736 | 0.961 | ||||
| Active smoker | 7 (6.0) | 0 (0.0) | 7 (6.6) | 58 (7.1) | ||
| Former smoker | 19 (16.4) | 0 (0.0) | 19 (17.9) | 154 (18.7) | ||
| Nonsmoker | 90 (77.6) | 10 (100.0) | 80 (75.5) | 610 (74.2) | ||
| Family history of IBD | 4 (3.4) | 1 (10.0) | 3 (2.8) | 39 (4.7) | 0.532 | 0.519 |
| Family history of CRC | 3 (2.6) | 0 (0.0) | 3 (2.8) | 33 (4.0) | 0.623 | 0.744 |
| History of appendectomy | 2 (1.7) | 0 (0.0) | 2 (1.9) | 16 (1.9) | 1.000 | 1.000 |
| Weight loss | 81 (69.8) | 6 (60.0) | 75 (70.8) | 544 (66.2) | 0.435 | 0.347 |
| Extraintestinal involvement | 27 (23.3) | 3 (30.0) | 24 (22.6) | 112 (13.6) | 0.006 | 0.013 |
| Arthropathy | 4 (3.4) | 0 (0.0) | 4 (3.8) | 40 (4.9) | 0.499 | 0.618 |
| Skin lesions | 7 (6.0) | 0 (0.0) | 7 (6.6) | 25 (3.0) | 0.165 | 0.108 |
| Oral ulcer | 12 (10.3) | 1 (10.0) | 11 (10.4) | 44 (5.4) | 0.034 | 0.039 |
| Ophthalmopathy | 1 (0.9) | 0 (0.0) | 1 (0.9) | 4 (0.5) | 0.484 | 0.455 |
| Thrombosis | 9 (7.8) | 1 (10.0) | 8 (7.5) | 13 (1.6) | < 0.001 | < 0.001 |
| PSC | 2 (1.7) | 1 (10.0) | 1 (1.2) | 10 (1.2) | 0.989 | 1.000 |
| Hemoglobin, g/L | 103.78 ± 23.59 | 108.10 ± 20.43 | 103.38 ± 23.91 | 111.63 ± 26.28 | < 0.001 | < 0.001 |
| CRP, mg/L (median, IQR) | 11.82 (2.20, 43.65) | 4.61 (2.57, 20.35) | 12.41 (2.10, 44.23) | 9.27 (2.07, 33.00) | 0.374 | 0.268 |
| ESR, mm/hour (median, IQR) | 23 (12, 46) | 15 (7, 16) | 24 (12, 49) | 20 (9, 40) | 0.171 | 0.097 |
| Treatment until stricture or the end of follow-up | ||||||
| 5-ASA | 111 (95.7) | 10 (100.0) | 101 (95.3) | 811 (98.7) | 0.053 | 0.034 |
| Systemic glucocorticoids | 98 (84.5) | 7 (70.0) | 91 (85.8) | 611 (74.3) | 0.017 | 0.009 |
| Immunomodulators | 32 (27.6) | 2 (20.0) | 30 (28.3) | 202 (24.6) | 0.483 | 0.404 |
| Biologics | 25 (21.6) | 0 (0.0) | 25 (23.6) | 314 (38.2) | < 0.001 | 0.003 |
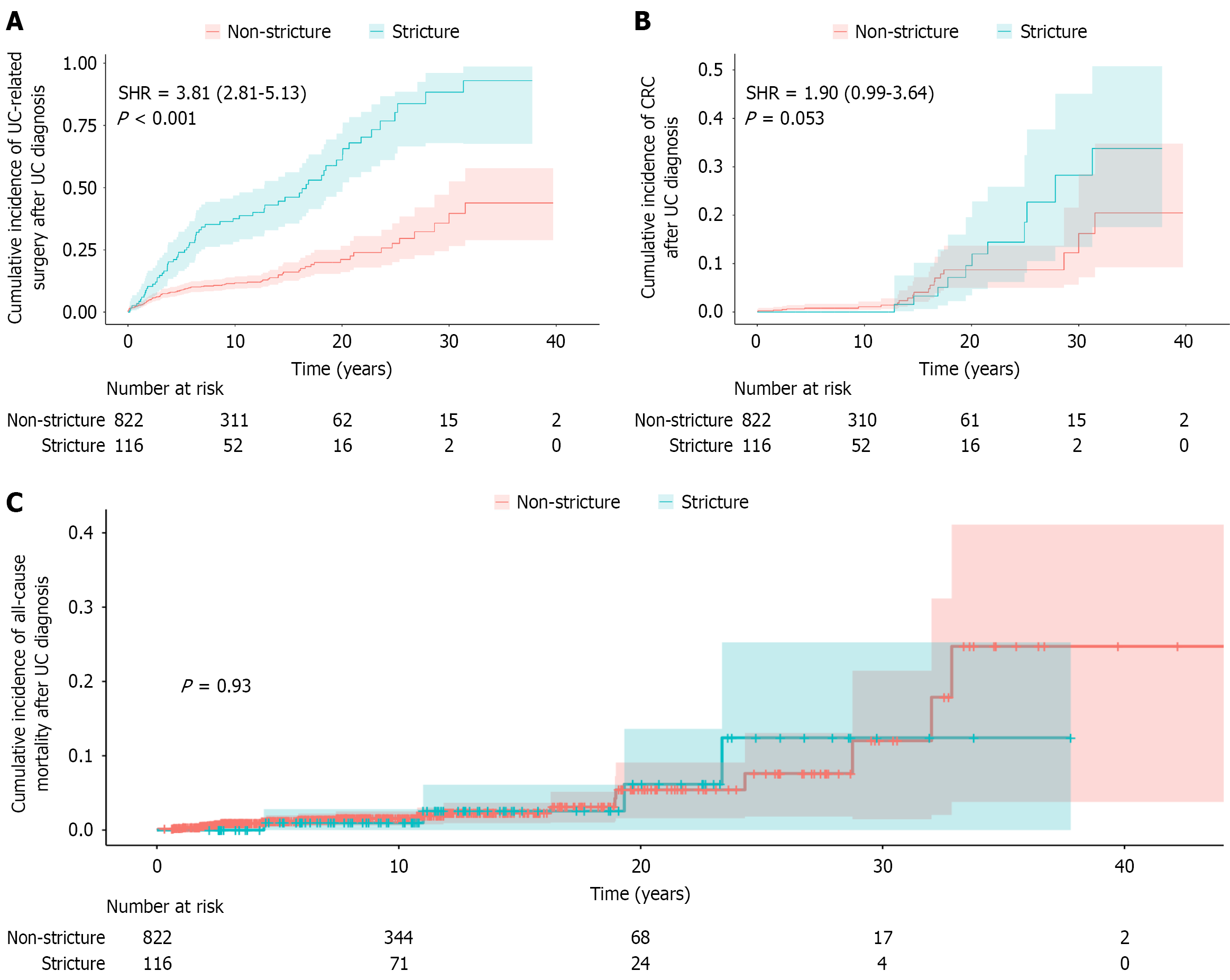
The median follow-up duration was 8.70 years (IQR: 4.80-13.30 years). Table 2 summarizes the clinical outcomes of the enrolled UC patients. Clinical remission, mild, moderate and severe activity until surgery or last follow-up were observed in 26.7%, 24.1%, 29.3% and 19.8% of patients in the stricture group, respectively, compared with 45.3%, 26.8%, 15.9% and 12.0% in the non-stricture group (P < 0.001). In addition, the stricture group also had higher rates of steroid dependence (24.1% vs 15.6%, P = 0.020) and steroid resistance (6.9% vs 3.0%, P = 0.066). UC-related surgery (55.2% vs 13.0%, P < 0.001), malignant transformation (8.7% vs 2.8%, P = 0.003), and serious intestinal complications (31.9% vs 6.4%, P < 0.001) were all significantly more common in the stricture group. Fine-Gray competing risk analysis (Figure 2) demonstrated a significantly higher risk of UC-related surgery in the stricture group [sub-distribution hazard ratio (SHR) = 3.81, 95% confidence interval (CI): 2.81-5.13, P < 0.001], and a trend toward increased CRC risk (SHR = 1.90, 95%CI: 0.99-3.64, P = 0.053). Kaplan-Meier analysis showed no difference in all-cause mortality between groups (P = 0.930). The 10-year cumulative probabilities of UC-related surgery, CRC and all-cause mortality in the stricture group were 37.6%, 0.0%, and 1.6%, respectively, compared with 11.7%, 1.0%, and 1.0% in the non-stricture group. At 20 years, these probabilities increased to 61.1%, 9.6% and 5.4% in the stricture group vs 21.1%, 8.7%, and 6.2% in the non-stricture group.
| Stricture group | Non-stricture group (n = 822) | 1P value | 2P value | |||
| All stricture cases (n = 116) | Malignant stricture group (n = 10) | Benign stricture group (n = 106) | ||||
| Clinical activity until surgery or the end of follow-up | < 0.001 | < 0.001 | ||||
| Clinical remission | 31 (26.7) | 3 (30.0) | 28 (26.4) | 372 (45.3) | ||
| Mild activity | 28 (24.1) | 6 (60.0) | 22 (20.8) | 220 (26.8) | ||
| Moderate activity | 34 (29.3) | 1 (10.0) | 33 (31.1) | 131 (15.9) | ||
| Severe activity | 23 (19.8) | 0 (0.0) | 23 (21.7) | 99 (12.0) | ||
| Steroid dependence | 28 (24.1) | 0 (0.0) | 28 (26.4) | 128 (15.6) | 0.020 | 0.005 |
| Steroid resistance | 8 (6.9) | 0 (0.0) | 8 (7.5) | 25 (3.0) | 0.066 | 0.038 |
| UC-related surgery | 64 (55.2) | 10 (100.0) | 54 (50.9) | 107 (13.0) | < 0.001 | < 0.001 |
| CRC | 10 (8.7) | 23 (2.8) | 0.003 | |||
| Severe intestinal complications | 37 (31.9) | 4 (40.0) | 33 (31.1) | 53 (6.4) | < 0.001 | < 0.001 |
| Bleeding | 8 (6.9) | 0 (0.0) | 8 (7.5) | 33 (4.0) | 0.155 | 0.157 |
| Perforation | 5 (4.3) | 1 (10.0) | 4 (3.8) | 9 (1.1) | 0.024 | 0.077 |
| Obstruction | 21 (18.1) | 2 (20.0) | 19 (17.9) | 6 (0.7) | < 0.001 | < 0.001 |
| Fistula | 3 (2.6) | 1 (10.0) | 2 (0.5) | 3 (0.4) | 0.028 | 0.103 |
| Toxic megacolon | 4 (3.4) | 0 (0.0) | 4 (3.8) | 4 (0.5) | 0.010 | 0.004 |
| All-cause mortality | 4 (3.4) | 2 (20.0) | 2 (1.9) | 20 (2.4) | 0.738 | 0.993 |
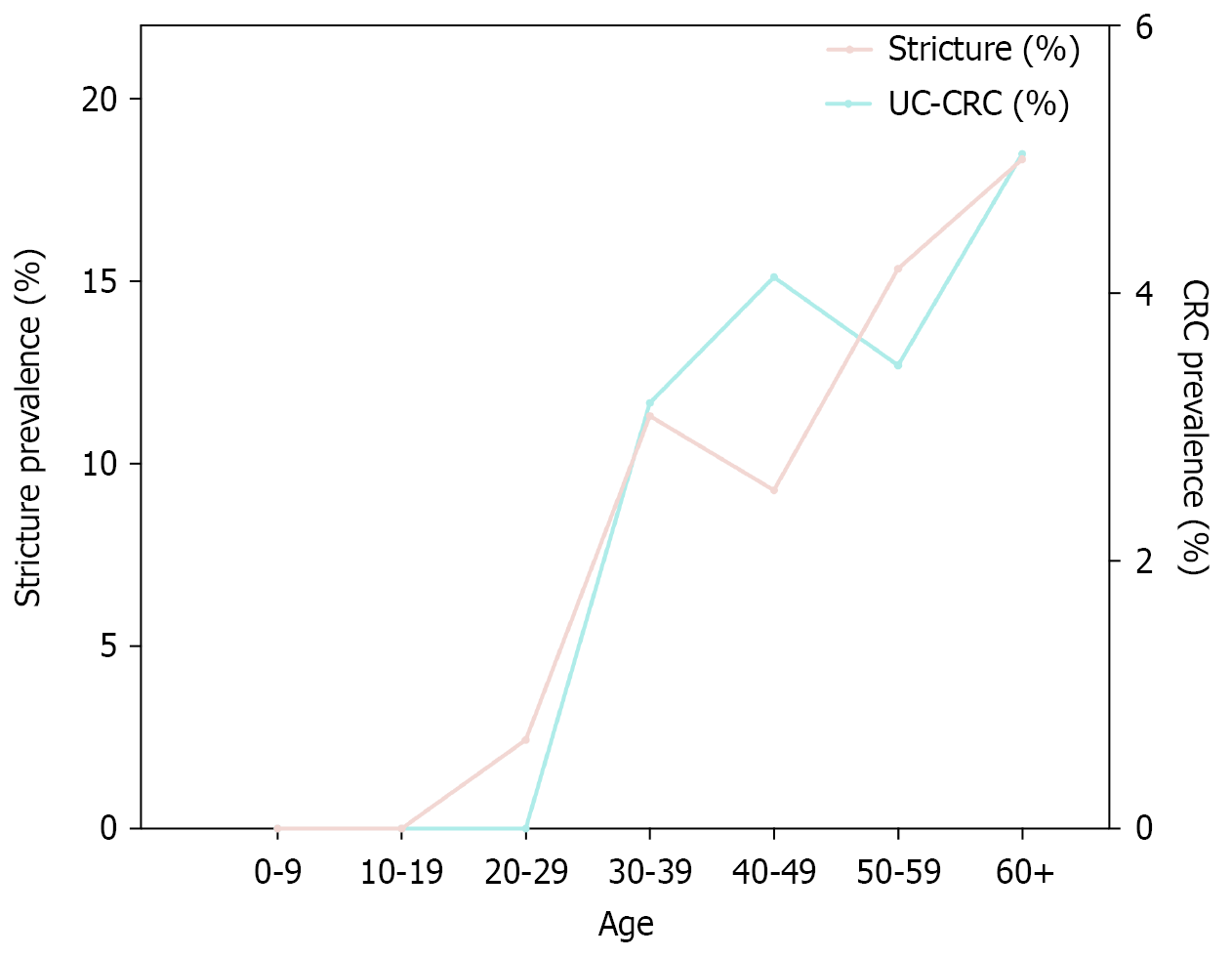
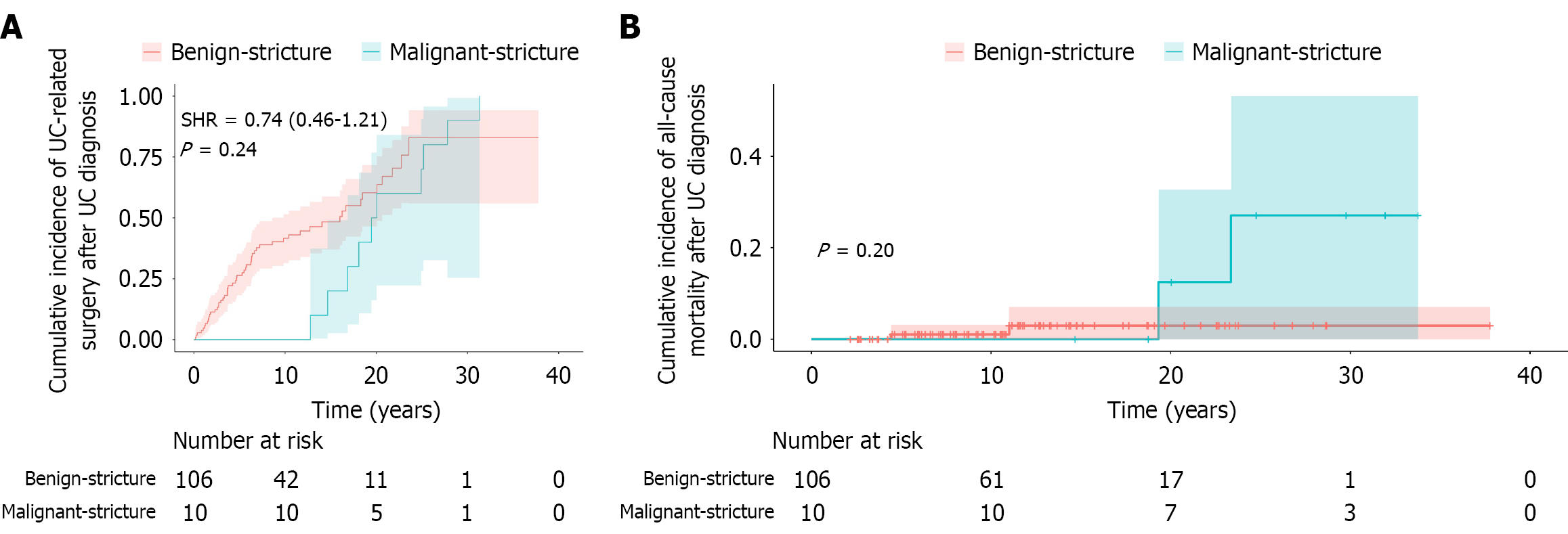
A total of 134 colorectal strictures were identified in 116 UC patients (Table 3). Malignancy was confirmed in 11 strictures from ten patients, all of which had preoperative biopsy evidence of high-grade dysplasia (HGD) (nine strictures) or CRC (two strictures). Notably, malignant transformation from initially benign strictures was observed in two patients during follow-up. Compared with benign strictures, patients with malignant strictures were associated with a significantly higher median age at stricture diagnosis (60.99 years, IQR: 50.58-63.38 years vs 46.08 years, IQR: 36.01-58.10 years, P = 0.042) and a longer median disease duration from UC diagnosis to stricture formation (18.88 years, IQR: 16.29-24.75 years vs 4.49 years, IQR: 2.27-9.64 years, P < 0.01). Additionally, both stricture and CRC prevalence demonstrated a roughly age-dependent increase (Figure 3). Excluding eight cases with unknown stricture locations, the most common sites were the sigmoid colon (46 cases, 34.3%) and the rectum (30 cases, 24.0%). At final follow-up, patients with malignant strictures had a higher proportion of mild disease activity (60.0% vs 20.8%). All malignant strictures required UC-related surgery, compared with 50.9% of benign strictures (P = 0.008). All-cause mortality was also significantly higher in the malignant group (20.0% vs 1.9%, P = 0.037). However, after adjustment for competing risks using the Fine-Gray model, there was no significant difference in the cumulative incidence of UC-related surgery (SHR = 0.74, 95%CI: 0.46-1.21, P = 0.240), and Kaplan-Meier analysis revealed no significant difference in overall survival between the groups (P = 0.200) (Figure 4).
| All stricture cases | Malignant stricture | Benign stricture | P value | |
| Number of patients | 116 | 10 | 106 | |
| Number of strictures | 0.409 | |||
| 1 | 99 (85.3) | 9 (90.0) | 90 (84.9) | |
| 2 | 16 (13.8) | 1 (10.0) | 15 (14.2) | |
| 3 | 1 (0.9) | 0 (0.0) | 1 (0.9) | |
| Male | 58 (50.0) | 4 (40.0) | 54 (50.9) | 0.822 |
| Age at diagnosis, year (median, IQR) | 38.97 (30.52-49.39) | 36.06 (32.19-42.39) | 40.69 (29.36-51.95) | 0.426 |
| Median age at diagnosis of stricture, year (median, IQR) | 47.83 (36.65-60.16) | 60.99 (50.58-63.38) | 46.80 (36.01-58.10) | 0.042 |
| Median duration from diagnosis to stricture formation, year (median, IQR) | 5.25 (2.45-12.76) | 18.88 (16.29-24.75) | 4.49 (2.27-9.64) | < 0.001 |
| Location of the stricture | 0.041 | |||
| Rectum | 31 (23.1) | 4 (36.4) | 27 (22.0) | |
| Left colon | 83 (61.9) | 3 (27.3) | 80 (65.0) | |
| Right colon | 20 (14.9) | 4 (36.4) | 16 (13.0) | |
| Clinical activity until surgery or the end of follow-up | 0.016 | |||
| Clinical remission | 31 (26.7) | 3 (30.0) | 28 (26.4) | |
| Mild activity | 28 (24.1) | 6 (60.0) | 22 (20.8) | |
| Moderate activity | 34 (29.3) | 1 (10.0) | 33 (31.1) | |
| Severe activity | 23 (19.8) | 0 (0.0) | 23 (21.7) | |
| Steroid dependence | 28 (24.1) | 0 (0.0) | 28 (26.4) | 0.139 |
| Steroid resistance | 8 (6.9) | 0 (0.0) | 8 (7.5) | 1.000 |
| UC-related surgery | 64 (55.2) | 10 (100.0) | 54 (50.9) | 0.008 |
| Severe intestinal complications | 37 (31.9) | 4 (40.0) | 33 (31.1) | 0.826 |
| Bleeding | 8 (6.9) | 0 (0.0) | 8 (7.5) | 1.000 |
| Perforation | 5 (4.3) | 1 (10.0) | 4 (3.8) | 0.368 |
| Obstruction | 21 (18.1) | 2 (20.0) | 19 (17.9) | 1.000 |
| Fistula | 3 (2.6) | 1 (10.0) | 2 (0.5) | 0.239 |
| Toxic megacolon | 4 (3.4) | 0 (0.0) | 4 (3.8) | 1.000 |
| All-cause mortality | 4 (3.4) | 2 (20.0) | 2 (1.9) | 0.037 |
In univariate Cox analysis (Table 4), age ≥ 40 years at diagnosis (P < 0.001), EIM (P < 0.001), elevated C-reactive protein (P = 0.003) or erythrocyte sedimentation rate (P = 0.028) and prior systemic steroid use (P = 0.013) were associated with an increased risk of stricture formation. Conversely, higher hemoglobin levels (P < 0.001) and prior use of 5-ASA (P = 0.053) or VDZ (P = 0.002) were associated with a lower risk of stricture. In multivariate Cox analysis, age ≥ 40 years at diagnosis (HR = 2.197, 95%CI: 1.487-3.242, P < 0.001), EIM (HR = 2.072, 95%CI: 1.326-3.239, P = 0.001) and prior steroid use (HR = 2.360, 95%CI: 1.325-4.203, P = 0.004) remained independent risk factors for stricture formation. In contrast, higher hemoglobin levels (HR = 0.988, 95%CI: 0.980-0.995, P = 0.001), prior 5-ASA use (HR = 0.214, 95%CI: 0.077-0.595, P = 0.003) and VDZ therapy (HR = 0.382, 95%CI: 0.203-0.720, P = 0.003) were independently associated with a reduced risk. The proportional hazards assumption was satisfied (global P = 0.274), and we did not identify multicollinearity (all variance inflation factors < 2.5).
| Variables | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Male | 0.896 (0.621-1.293) | 0.557 | ||
| Age at diagnosis ≥ 40 year | 2.273 (1.562-3.308) | < 0.001 | 2.197 (1.487-3.242) | < 0.001 |
| Montreal classification | 0.079 | 0.228 | ||
| E1 (proctitis) | 1 | 1 | ||
| E2 (left-sided colitis) | 0.555 (0.319-0.963) | 0.036 | 0.610 (0.347-1.073) | 0.087 |
| E3 (pancolitis) | 0.846 (0.525-1.362) | 0.491 | 0.780 (0.478-1.275) | 0.322 |
| Extraintestinal involvement | 2.077 (1.346-3.205) | < 0.001 | 2.072 (1.326-3.239) | 0.001 |
| Hemoglobin | 0.986 (0.979-0.992) | < 0.001 | 0.988 (0.980-0.995) | 0.001 |
| CRP | 1.007 (1.002-1.011) | 0.003 | 1.004 (0.999-1.010) | 0.145 |
| ESR | 1.008 (1.001-1.015) | 0.028 | 0.995 (0.986-1.004) | 0.301 |
| Treatment until stricture or the end of follow-up | ||||
| 5-ASA | 0.406 (0.163-1.012) | 0.053 | 0.214 (0.077-0.595) | 0.003 |
| Systemic glucocorticoids | 1.895 (1.145-3.135) | 0.013 | 2.360 (1.325-4.203) | 0.004 |
| Immunomodulators | 0.985 (0.654-1.482) | 0.942 | ||
| Anti-TNF-α | 0.764 (0.450-1.298) | 0.320 | 0.911 (0.517-1.604) | 0.746 |
| VDZ | 0.370 (0.199-0.689) | 0.002 | 0.382 (0.203-0.720) | 0.003 |
| UST | 0.049 (0.0-5887.28) | 0.614 | 0.000(0.0-2.4E+163) | 0.961 |
During follow-up, eight UC patients demonstrated stricture improvement without endoscopic or surgical intervention (Supplementary Table 2). The median time from stricture formation to improvement was 1.40 years (IQR: 0.51-2.22 years). Among these patients, four received steroids combined with biologics, one received steroids and immunosuppressants alone, and two patients were treated with 5-ASA with corticosteroids enemas. The median time from UC diagnosis to stricture formation was shorter in the improved group than the persistent group (2.54 years, IQR: 1.86-3.56 years vs 5.61 years, IQR: 2.86-12.76 years, P = 0.019). No patients in the improved group underwent UC-related surgery or developed malignancy during follow-up. Although the risks of severe disease activity and intestinal complications were lower in the improved group, the differences were not statistically significant. In time-dependent Cox analysis (Supplementary Table 3), a shorter interval from UC diagnosis to stricture formation tended to predict a higher likelihood of subsequent stricture resolution, although the association was not statistically significant.
Three sensitivity analyses were conducted to evaluate the robustness of the primary findings (Supplementary Tables 4-8). A complete-case analysis excluding patients with missing data, and an analysis excluding external surgical referrals and early surgery cases (within 30 days of diagnosis) both demonstrated results consistent with the primary analysis. Notably, in the stricture group, after excluding external surgical referrals and early surgery cases, the previously observed trend toward increased CRC risk was lost (Supplementary Figure 1). Additionally, we performed sensitivity analysis comparing benign strictures and non-stricture controls. Multivariable Cox regression assessing risk factors for benign strictures showed findings consistent with the primary analysis. The cumulative incidence of UC-related surgery remained significantly higher in the benign stricture group than in the control group (P < 0.001), whereas all-cause mortality was comparable between groups (P = 0.60) (Supplementary Figure 2).
Assessing the long-term prognosis and identifying risk factors are essential for optimizing clinical management in UC patients with strictures. In this large cohort, the incidence of UC strictures was 12.4%, with 8.6% confirmed as malignant over a median follow-up of 8.70 years. Patients with strictures had poorer treatment responses and significantly higher risks of UC-related surgery, intestinal complications, and CRC. Age ≥ 40 years at diagnosis, EIM, and steroid use were independent risk factors for stricture formation, whereas higher hemoglobin levels, 5-ASA and VDZ use were protective.
Due to the lack of a standardized definition, the reported incidence of colonic strictures in UC have considerably varied, ranging from 1% to 14%[2-9]. In our cohort, the incidence of strictures was 12.4%, comparable to that of a recent multicenter retrospective cohort study (14.2%)[7] but higher than those reported in other studies published since 2010 (1.06%-3.6%)[4,5,9]. This variation may be attributed to differences in study populations, diagnostic criteria, follow-up durations, and potential selection bias. As a tertiary referral center, PUMCH primarily manages patients with more advanced disease, including those referred specifically for colectomy. Additionally, in this study, strictures were identified based on findings from endoscopy, imaging or surgical evaluation, including cases where endoscopic passage was impeded but still passable. In contrast, some studies only included strictures that were completely impassable by endoscopy[4,9].
Stricture has been recognized as an independent risk factor for dysplasia and CRC[4,7,24]. In this study, the incidence of CRC in patients with strictures was 8.6%, similar to the 12.5% reported by Hunt et al[18]. According to the ECCO guidelines, annual colonoscopy surveillance is recommended for patients with strictures detected within the past 5 years[23]. Of the 11 malignant strictures identified in our cohort, nine had HGD confirmed on preoperative endoscopy. A multicenter retrospective study also demonstrated that the coexistence of a stricture and low-grade dysplasia significantly increased the risk of advanced neoplasia[25]. Notably, negative endoscopic biopsies cannot exclude malignancy in strictures. A national retrospective study reported that among 39 UC patients undergoing surgery for strictures without preoperative evidence of neoplasia, one (2.56%) had HGD and two (5.12%) had CRC. Therefore, identifying factors associated with malignant colonic strictures is critical to optimize endoscopic surveillance and guide surgical decision-making in high-risk patients. In this study, malignant strictures were diagnosed at an older age (60.99 years vs 46.8 years, P = 0.042) and after a longer disease duration (18.88 years vs 4.49 years, P < 0.001) than benign strictures. The incidence of stricture and CRC in the overall cohort also demonstrated a general upward trend with advancing age. Similarly, a nationwide retrospective study found that all cases of dysplasia or carcinoma occurred in patients over 59 years old and with a disease duration > 15 years[17]. A single-center retrospective study also reported that 61% of strictures in patients with a disease course > 20 years were malignant[3]. In addition, strictures with obstructive symptoms or located proximal to the splenic flexure were associated with a higher cancer risk[2]. However, the small number of malignant strictures in our cohort limited definitive conclusions.
During the follow-up, eight patients experienced stricture improvement without endoscopic or surgical intervention. As IBD-associated fibrosis is generally considered irreversible and the pathogenesis of UC-related strictures remains largely unknown, this improvement may reflect regression of inflammatory components rather than true remodeling of fibrosis. These patients had a shorter interval from UC diagnosis to stricture formation (2.54 years vs 5.61 years, P = 0.019) and were more likely to receive VDZ after stricture development (50.0% vs 23.9%, P = 0.233). Additionally, our study is the first to report an association between VDZ use and a reduced risk of stricture formation (HR = 0.382, P = 0.003). These findings suggest that in UC patients developing strictures within a relatively short time after diagnosis, timely initiation of VDZ may delay or even reverse stricture progression. Recent single-cell and spatial multi-omics analyses in UC patients have demonstrated the anti-fibrotic potential of VDZ, as evidenced by significant reductions in activated fibroblasts and fibrosis-related gene expression. VDZ also altered the trafficking and activation of mononuclear phagocytes (MNPs), which were key drivers of inflammation and fibrosis. Based on these results VDZ likely reduces fibrosis by inhibiting immune cell trafficking and attenuating fibroblasts and MNP activation[21]. A recent multicenter retrospective study found that UST achieved a higher steroid-free remission rate than infliximab in CD patients with intestinal stenosis[26]. UST may mitigate fibrosis and stenosis formation by suppressing myofibroblast proliferation or transformation[27]. However, our study failed to identify a correlation between the use of UST and the reduction or improvement of stricture. This may be explained by the small number of UST-treated patients (n = 7) and their exclusive distribution in the non-stricture group. The lack of anti-fibrotic effect with anti-TNF-α aligns with conflicting evidence in CD literature. While anti-TNF-α agents reduce mucosal inflammation, histological studies have paradoxically reported increased fibrosis in the submucosa and muscularis propria[28]. By interfering with the TNF-α regulation of transforming growth factor-β-mediated fibrogenesis, TNF-α antagonists may inadvertently promote the fibrotic pathway.
The distinction between passable and impassable strictures has important clinical implications for surveillance and management. Impassable strictures limit tissue acquisition and endoscopic access to the proximal colon, potentially delaying the timely detection of dysplasia or malignancy. However, approximately half of the patients achieving stricture improvement in our cohort initially had impassable strictures, indicating that endoscopic impassability does not uniformly signify irreversible fibrosis. A comprehensive assessment incorporating clinical disease activity is essential for optimal management. Impassable strictures during active disease may contain substantial inflammatory components and may benefit from intensive medical therapy. Those arising in clinical remission likely reflect predominant fibrosis and require early consideration of endoscopic dilation or surgical intervention.
Few studies have systematically explored risk factors for stricture formation in UC. In our analysis, 5-ASA use was associated with a reduced risk (HR = 0.214, P < 0.003), whereas steroid use significantly increased the risk (HR = 2.360, P = 0.004). These findings were consistent with observations from two multicenter retrospective studies by Laurain et al[5] and Xu et al[7]. However, these associations require cautious interpretation. As a cornerstone treatment for UC, 5-ASA is widely prescribed, which explains its high usage in both groups. Importantly, 5-ASA monotherapy is predominantly used for mild to moderate UC, while patients with more severe disease, who are at a higher risk for complications such as stricture formation, often require additional therapies like steroids. Therefore, the observed associations may be primarily driven by unmeasured disease characteristics rather than direct drug effects on fibrosis. Future prospective studies with standardized disease severity scoring, detailed treatment protocols, and potentially propensity score matching are needed to determine the actual effects. Additionally, later disease onset (≥ 40 years old) increased stricture risk (HR = 2.197, P < 0.001). Chen et al[29] recently found that cellular senescence score and senescence-associated secretory phenotype factors significantly increased in fibrostenotic tissues, indicating a correlation between intestinal senescence and fibrosis in chronic IBD. Differences in treatment intensity across age groups may also contribute to this finding. As older patients are more susceptible to treatment-related complications[30], the exposure to immunomodulators and biological agents is significantly lower in elderly-onset UC than in adult-onset UC[31].
There are limitations in our study. First, there is no standardized definition of colonic stricture in UC. Variability in diagnostic criteria may impact the interpretation of clinical outcomes and make it difficult to compare findings across studies. Second, the limited number of malignant stricture cases (n = 10) limited statistical power and precluded detailed analyses of risk factors or clinical differences between benign and malignant strictures. Additionally, interaction term analyses to access effect modification by stricture etiology were also infeasible due to numerical instability. Although our cohort was relatively large, this was a single-center retrospective study. Potential biases in patient selection and data collection may limit the generalizability of our findings. Future multicenter prospective studies are needed to validate these findings, evaluate the impact of biologics on stricture progression, and optimize endoscopic surveillance strategies in high-risk populations.
In this long-term cohort study, we evaluated the prognosis and risk factors associated with UC strictures. Timely initiation of endoscopic surveillance and biologic therapy are strongly recommended for patients with EIM, particularly those diagnosed at age 40 or older. For UC patients developing strictures within a short interval after diagnosis, early VDZ administration may help slow stricture progression.
The authors sincerely appreciate the participation of all patients in this study.
| 1. | Rieder F, Mukherjee PK, Massey WJ, Wang Y, Fiocchi C. Fibrosis in IBD: from pathogenesis to therapeutic targets. Gut. 2024;73:854-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 2. | Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci. 1990;35:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sonnenberg A, Genta RM. Epithelial Dysplasia and Cancer in IBD Strictures. J Crohns Colitis. 2015;9:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Laurain PA, Guillo L, D'Amico F, Netter P, Danese S, Baumann C, Luc A, Clerc-Urmes I, Sofos S, Peyrin-Biroulet L. Incidence of and Risk Factors for Colorectal Strictures in Ulcerative Colitis: A Multicenter Study. Clin Gastroenterol Hepatol. 2021;19:1899-1905.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | de Dombal FT, Watts JM, Watkinson G, Goligher JC. Local complications of ulcerative colitis. Stricture, pseudopolyps and cancer of the colon and rectum. Am J Proctol. 1967;18:198-201. [PubMed] |
| 7. | Xu W, Ding W, Gu Y, Cui L, Zhong J, Du P. Risk Factors of Colorectal Stricture Associated with Developing High-Grade Dysplasia or Cancer in Ulcerative Colitis: A Multicenter Long-term Follow-up Study. Gut Liver. 2020;14:601-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Edwards FC, Truelove SC. The Course and Prognosis of Ulcerative Colitis. Gut. 1963;4:299-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 411] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Yamagata M, Mikami T, Tsuruta T, Yokoyama K, Sada M, Kobayashi K, Katsumata T, Koizumi W, Saigenji K, Okayasu I. Submucosal fibrosis and basic-fibroblast growth factor-positive neutrophils correlate with colonic stenosis in cases of ulcerative colitis. Digestion. 2011;84:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Gordon IO, Agrawal N, Willis E, Goldblum JR, Lopez R, Allende D, Liu X, Patil DY, Yerian L, El-Khider F, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | de Bruyn JR, Meijer SL, Wildenberg ME, Bemelman WA, van den Brink GR, D'Haens GR. Development of Fibrosis in Acute and Longstanding Ulcerative Colitis. J Crohns Colitis. 2015;9:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015;19:62-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Ippolito C, Colucci R, Segnani C, Errede M, Girolamo F, Virgintino D, Dolfi A, Tirotta E, Buccianti P, Di Candio G, Campani D, Castagna M, Bassotti G, Villanacci V, Blandizzi C, Bernardini N. Fibrotic and Vascular Remodelling of Colonic Wall in Patients with Active Ulcerative Colitis. J Crohns Colitis. 2016;10:1194-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Goldberg HI, Caruthers SB Jr, Nelson JA, Singleton JW. Radiographic findings of the National Cooperative Crohn's Disease Study. Gastroenterology. 1979;77:925-937. [PubMed] |
| 15. | Yamazaki Y, Ribeiro MB, Sachar DB, Aufses AH Jr, Greenstein AJ. Malignant colorectal strictures in Crohn's disease. Am J Gastroenterol. 1991;86:882-885. [PubMed] |
| 16. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 304] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Fumery M, Pineton de Chambrun G, Stefanescu C, Buisson A, Bressenot A, Beaugerie L, Amiot A, Altwegg R, Savoye G, Abitbol V, Bouguen G, Simon M, Duffas JP, Hébuterne X, Nancey S, Roblin X, Leteurtre E, Bommelaer G, Lefevre JH, Brunetti F, Guillon F, Bouhnik Y, Peyrin-Biroulet L. Detection of Dysplasia or Cancer in 3.5% of Patients With Inflammatory Bowel Disease and Colonic Strictures. Clin Gastroenterol Hepatol. 2015;13:1770-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Hunt RH, Teague RH, Swarbrick ET, Williams CB. Colonoscopy in management of colonic strictures. Br Med J. 1975;3:360-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Rodríguez-Lago I, Hoyo JD, Pérez-Girbés A, Garrido-Marín A, Casanova MJ, Chaparro M, Fernández-Clotet A, Castro-Poceiro J, García MJ, Sánchez S, Ferreiro-Iglesias R, Bastón I, Piqueras M, Careda LEIB, Mena R, Suárez C, Cordón JP, López-García A, Márquez L, Arroyo M, Alfambra E, Sierra M, Cano N, Delgado-Guillena P, Morales-Alvarado V, Aparicio JC, Guerra I, Aulló C, Merino O, Arranz L, Hidalgo MA, Llaó J, Plaza R, Molina G, Torres P, Pérez-Galindo P, Romero MG, Herrera-deGuise C, Armesto E, Mesonero F, Frago-Larramona S, Benítez JM, Calvo M, Martín MDCL, Elorza A, Larena A, Peña E, Rodríguez-Grau MDC, Miguel-Criado J, Botella B, Olmos JA, López L, Aguirre U, Gisbert JP; Young GETECCU Group. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn's disease. United European Gastroenterol J. 2020;8:1056-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Gordon IO, Abushamma S, Kurowski JA, Holubar SD, Kou L, Lyu R, Rieder F. Paediatric Ulcerative Colitis Is a Fibrotic Disease and Is Linked with Chronicity of Inflammation. J Crohns Colitis. 2022;16:804-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Mennillo E, Kim YJ, Lee G, Rusu I, Patel RK, Dorman LC, Flynn E, Li S, Bain JL, Andersen C, Rao A, Tamaki S, Tsui J, Shen A, Lotstein ML, Rahim M, Naser M, Bernard-Vazquez F, Eckalbar W, Cho SJ, Beck K, El-Nachef N, Lewin S, Selvig DR, Terdiman JP, Mahadevan U, Oh DY, Fragiadakis GK, Pisco A, Combes AJ, Kattah MG. Single-cell and spatial multi-omics highlight effects of anti-integrin therapy across cellular compartments in ulcerative colitis. Nat Commun. 2024;15:1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Xu Y, Wang S, Ye Z, Zhang H. Identifying hub genes in response to ustekinumab and the impact of ustekinumab treatment on fibrosis in Crohn's disease. Front Immunol. 2024;15:1401733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1315] [Article Influence: 187.9] [Reference Citation Analysis (2)] |
| 24. | Zhan Y, Cheng X, Mei P, Wu J, Ou Y, Cui Y. Risk and incidence of colorectal stricture progressing to colorectal neoplasia in patients with inflammatory bowel disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2023;35:1075-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Cremer A, Demetter P, De Vos M, Rahier JF, Baert F, Moreels T, Macken E, Louis E, Ferdinande L, Fervaille C, Dedeurwaerdere F, Bletard N, Driessen A, De Hertogh G, Vermeire S, Franchimont D; Belgian Inflammatory Bowel Disease Research and Development (BIRD) Group. Risk of Development of More-advanced Lesions in Patients With Inflammatory Bowel Diseases and Dysplasia. Clin Gastroenterol Hepatol. 2020;18:1528-1536.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | He X, Wang Y, Sun J, Li Y, Ruan G, Li Y, Zheng W, Zhang X, Zhan R, Ding X, Liu A, Chen Y, Hu Y, Yang H, Qian J. Effectiveness comparison between ustekinumab and infliximab for Crohn's disease complicated with intestinal stenosis: a multicenter real-world study. Therap Adv Gastroenterol. 2024;17:17562848241290663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Abdulla M, Chew TS. Molecular targets and the use of biologics in the management of small bowel fibrosis in inflammatory bowel disease. Curr Opin Gastroenterol. 2021;37:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Schaeffer DF, Walsh JC, Kirsch R, Waterman M, Silverberg MS, Riddell RH. Distinctive histopathologic phenotype in resection specimens from patients with Crohn's disease receiving anti-TNF-α therapy. Hum Pathol. 2014;45:1928-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Chen J, Li G, He X, Chen X, Chen Z, Liu D, Guo S, Huang T, Lin Y, Lan P, Lian L, He X. ELMO1 ameliorates intestinal epithelial cellular senescence via SIRT1/p65 signaling in inflammatory bowel disease-related fibrosis. Gastroenterol Rep (Oxf). 2024;12:goae045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Singh S, Boland BS, Jess T, Moore AA. Management of inflammatory bowel diseases in older adults. Lancet Gastroenterol Hepatol. 2023;8:368-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 31. | Rozich JJ, Dulai PS, Fumery M, Sandborn WJ, Singh S. Progression of Elderly Onset Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Clin Gastroenterol Hepatol. 2020;18:2437-2447.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/