Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.109811
Revised: June 16, 2025
Accepted: August 13, 2025
Published online: September 7, 2025
Processing time: 102 Days and 5.3 Hours
Transperineal ultrasound (TPUS) is a non-invasive, real-time imaging technique increasingly utilized for the evaluation of anorectal and pelvic floor disorders in patients with inflammatory bowel disease (IBD). In ulcerative colitis, it enables accurate assessment of rectal wall thickness and vascularity, which correlate closely with both endoscopic and histological inflammation. This makes it a pra
Core Tip: Transperineal ultrasound (TPUS) is a non-invasive, bedside imaging tool for assessing anorectal inflammation and fistulizing disease in patients with inflammatory bowel conditions. It enables real-time evaluation of rectal inflammation in ulcerative colitis and offers reliable detection and monitoring of perianal fistulas and abscesses in Crohn’s disease. Its utility extends to children, pregnant women, and patients with pouch-related complications after surgery. Enhanced techniques-such as Doppler imaging, contrast administration, three-dimensional ultrasound, and computerized analysis-expand its diagnostic power. TPUS serves as a practical, repeatable, and cost-effective imaging option, especially as a first-line screening tool or when magnetic resonance imaging is unavailable, contraindicated, or impractical.
- Citation: Pal P, Mateen MA, Pooja K, Gupta R, Tandan M, Reddy DN. Transperineal ultrasound: Role in inflammatory bowel disease management. World J Gastroenterol 2025; 31(33): 109811
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/109811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.109811
Anorectal and perianal complications are among the most challenging manifestations of inflammatory bowel disease (IBD), particularly Crohn’s disease (CD) and ulcerative colitis (UC). These conditions often require repeated assessments to guide therapeutic decisions, detect complications, and monitor treatment response. While endoscopy and magnetic resonance imaging (MRI) remain the established standards for evaluation, they are limited by cost, accessibility, patient discomfort, and repeatability-particularly in scenarios such as pediatric care, pregnancy, or urgent outpatient settings. Transperineal ultrasound (TPUS), in contrast, offers a non-invasive, real-time, and bedside imaging solution that is repeatable, well-tolerated, and resource-efficient. In this context, TPUS has gained increasing attention as a non-invasive, real-time, and bedside tool capable of visualizing both structural and functional aspects of anorectal disease[1].
Despite its growing use, the role of TPUS in IBD remains under-recognized and inconsistently applied across clinical settings. Clinicians often lack clear guidance on its technical implementation, interpretation of findings, or how it compares with other imaging modalities such as MRI or endoscopic ultrasound (EUS)[2,3]. Moreover, the expanding applications of advanced features-such as use of microconvex probe, color Doppler, contrast enhancement, three-dimensional (3D) imaging, and computer-assisted quantification-have not been comprehensively reviewed in a single framework to support informed clinical decision-making[1,4,5].
In this review, we synthesize the current evidence and evolving applications of TPUS in IBD. We emphasize its potential as a frontline imaging modality in specific populations (children, pregnant women) and outpatient scenarios where rapid, repeatable, and radiation-free assessment is valuable. We cover its use in CD for the detection, classification, and monitoring of perianal fistulas and abscesses; in UC for assessing rectal inflammation; and in post-surgical pouch evaluation. We also highlight novel adjuncts such as Doppler imaging, contrast-enhanced ultrasound (CEUS), 3D reconstruction, and computer-aided interpretation. By contextualizing these findings within clinical practice, we aim to clarify the utility, limitations, and future directions of this promising imaging modality.
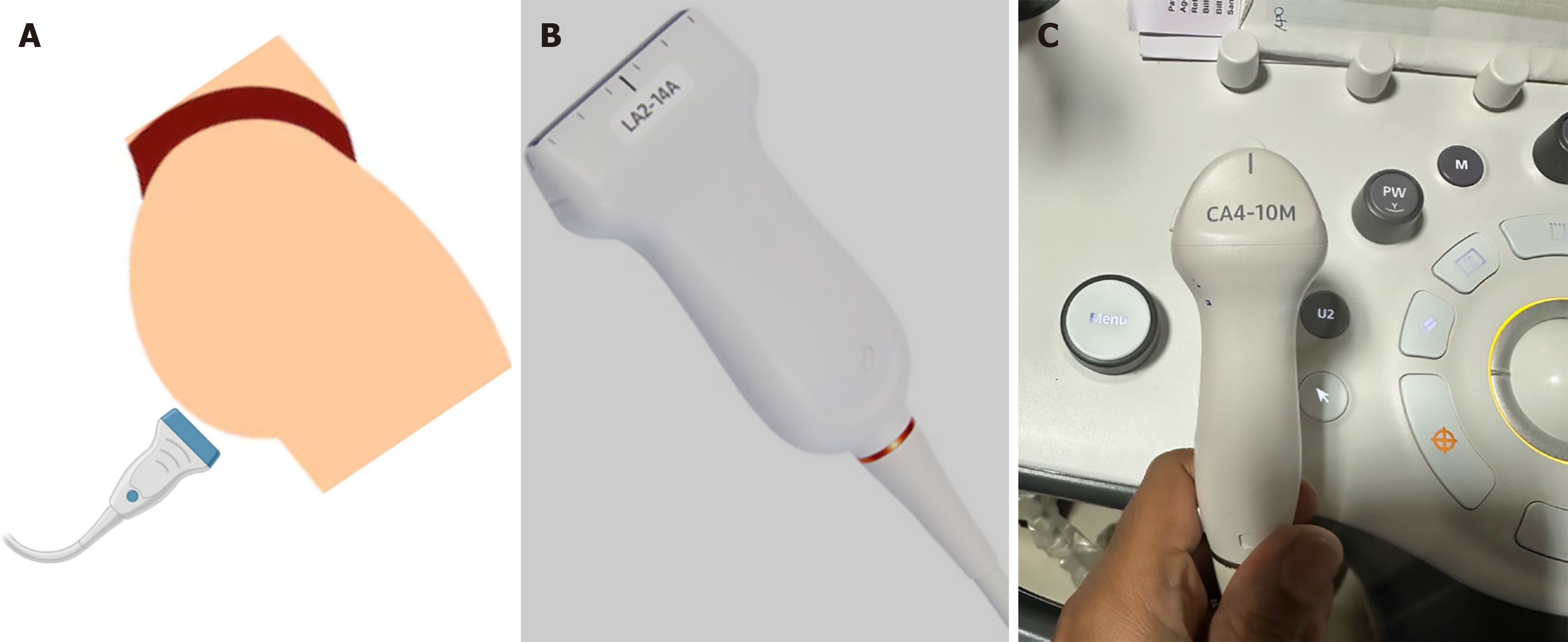
TPUS should begin with verbal consent, and the procedure is ideally performed in the presence of a female assistant when examining female patients. Bowel preparation is generally not required unless TPUS is scheduled on the same day as colonoscopy, in which case 1-2 L of polyethylene glycol solution may be used. To ensure patient comfort and modesty, disposable colonoscopy pants are recommended. The patient is positioned in the left lateral decubitus position with the right leg gently flexed at the hip and knee to allow full exposure of the perineum (Figure 1A)[1]. This standardized, reproducible positioning enhances both operator comfort and imaging consistency.
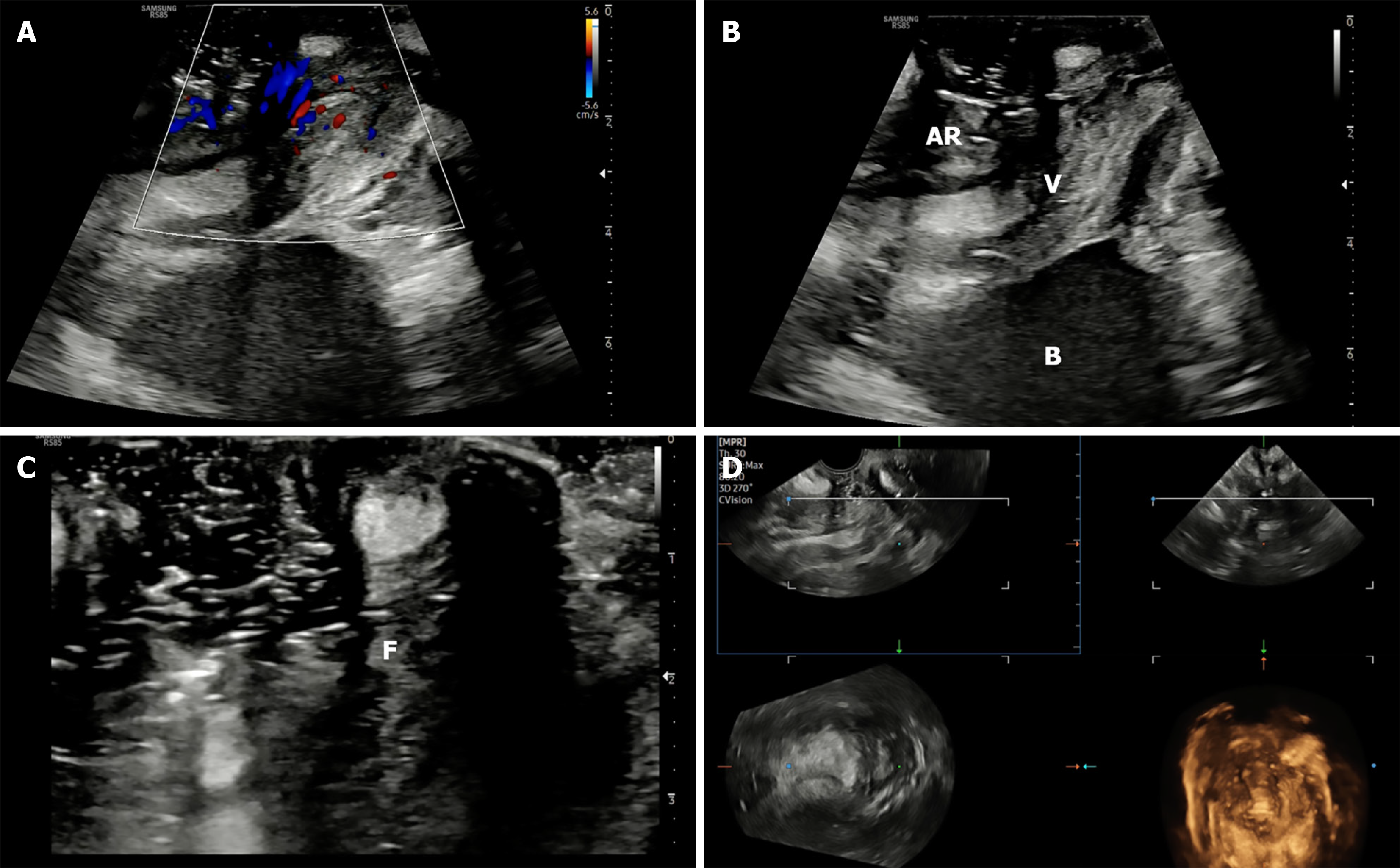
The examination can be performed using high-end ultrasound (US) systems, equipped with high-frequency linear (2-14 or 2-18 MHz) (Figures 1B and 2A), or microconvex (4-10 MHz) (Figures 1C and 2B-D). The advantage of micro convex probe is easy manoeuvrability in the anal cleft[1]. Harmonic imaging should be enabled for optimal resolution. The probe should be covered with a condom or saran wrap filled with coupling gel to prevent contamination. For color Doppler imaging, the gain should be set between 3-5 MHz with flow velocities calibrated between 5-8 m/second (Figures 2D and 3A). A low pulse repetition frequency (approximately 800 Hz) is ideal for detecting slow-flow signals associated with inflammatory activity in the perianal region[6]. Use of Doppler and CEUS should be guided by the clinical question, e.g., activity monitoring or detection of secondary tracts.
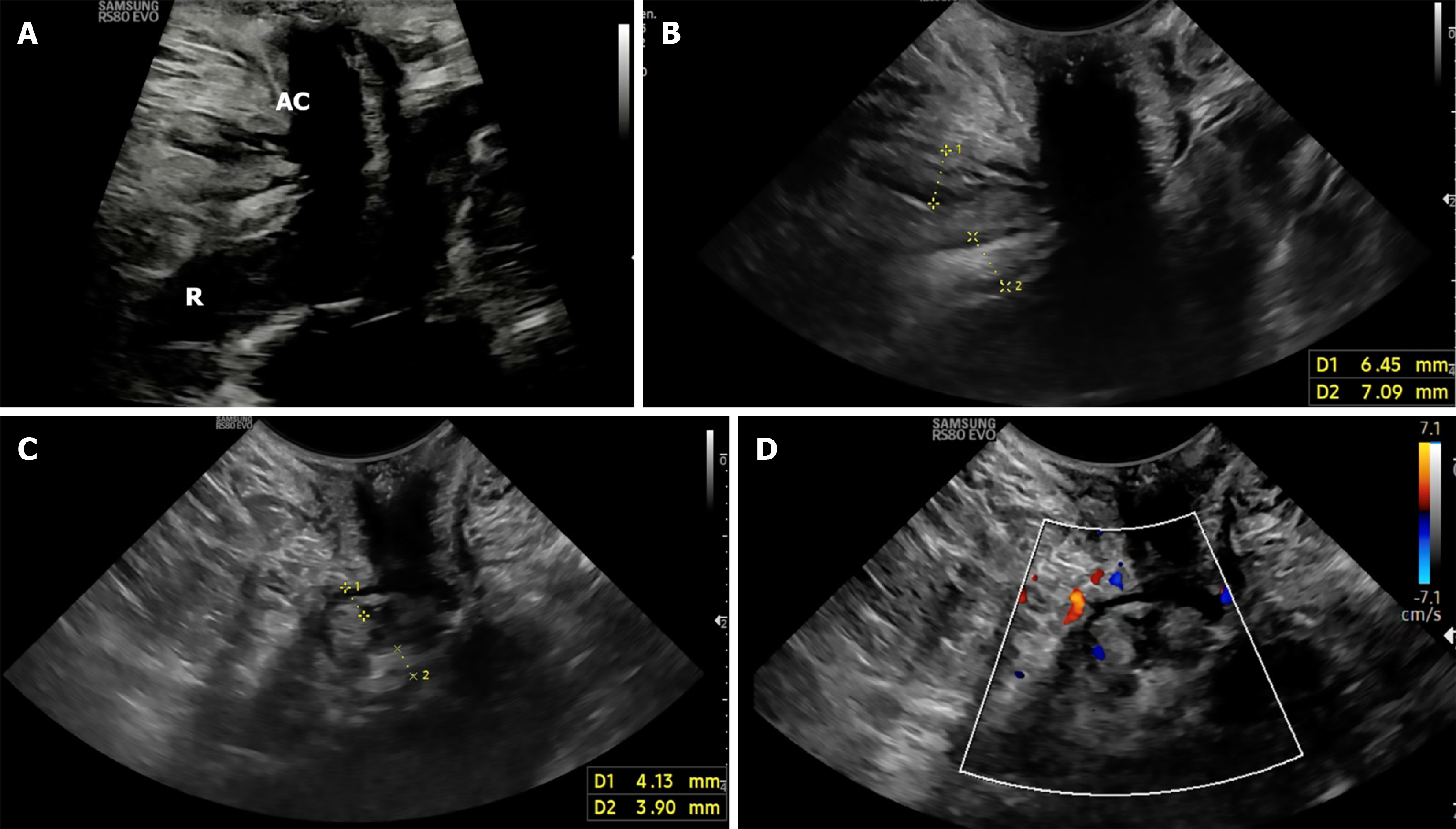
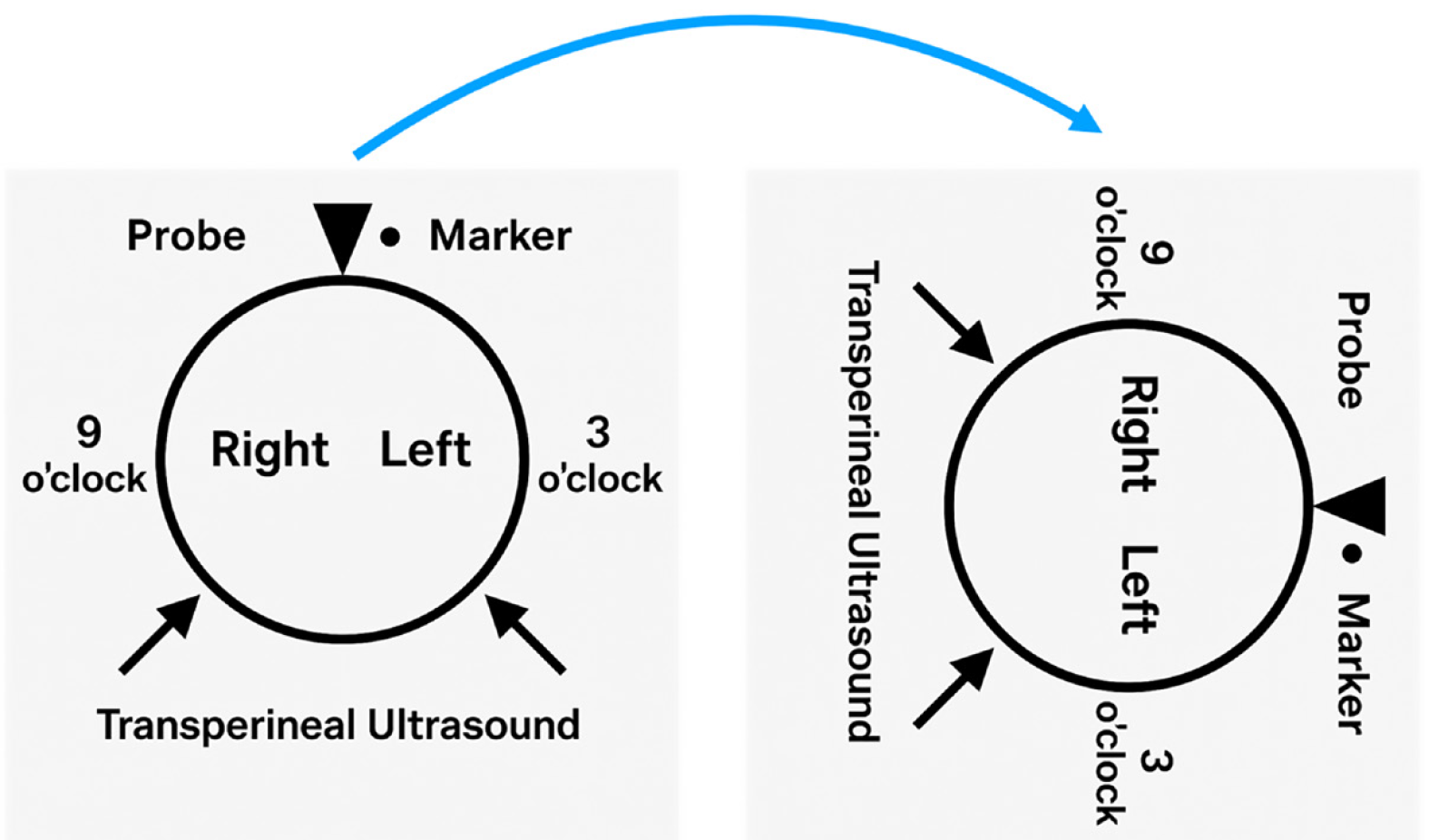
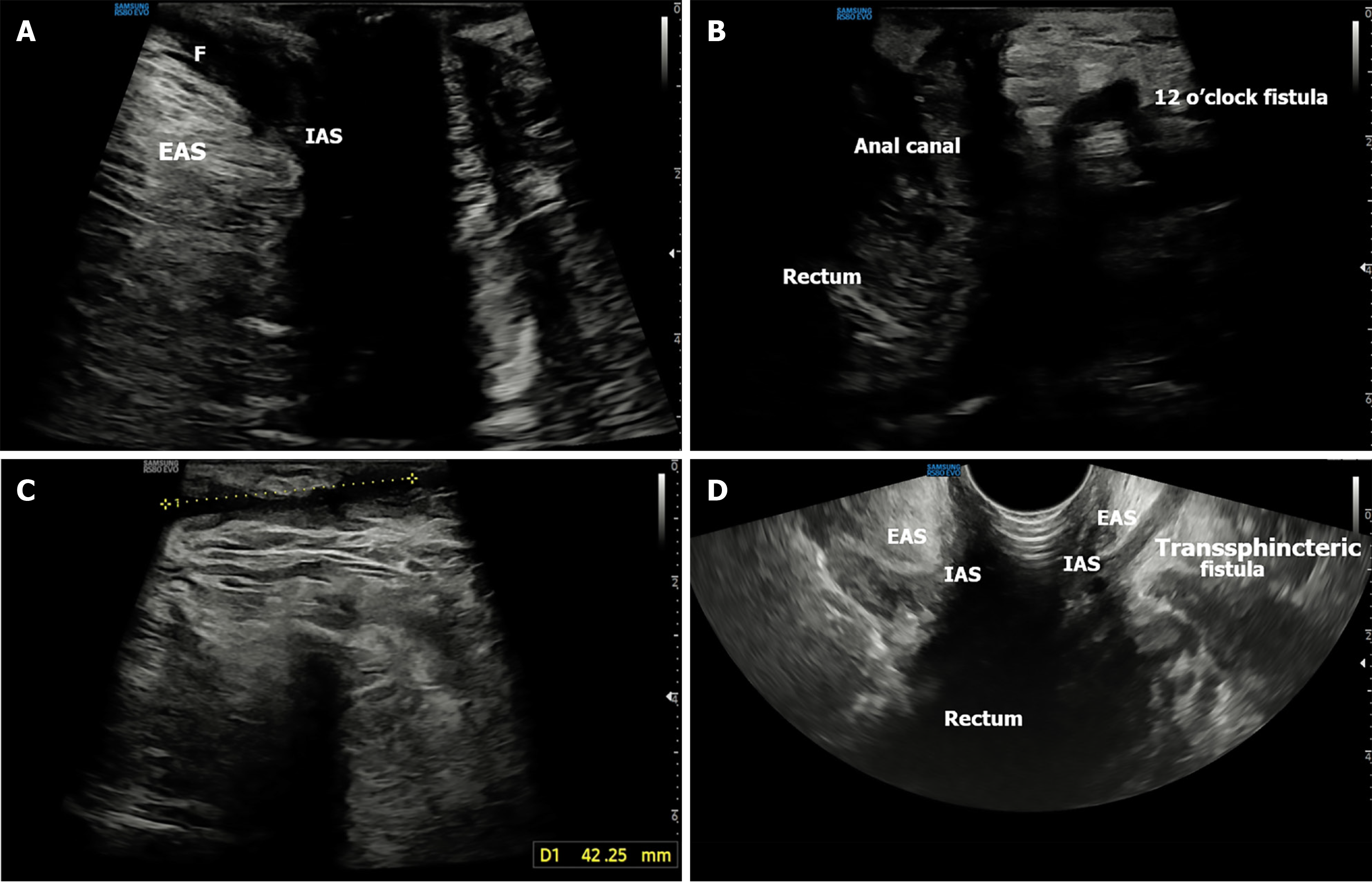
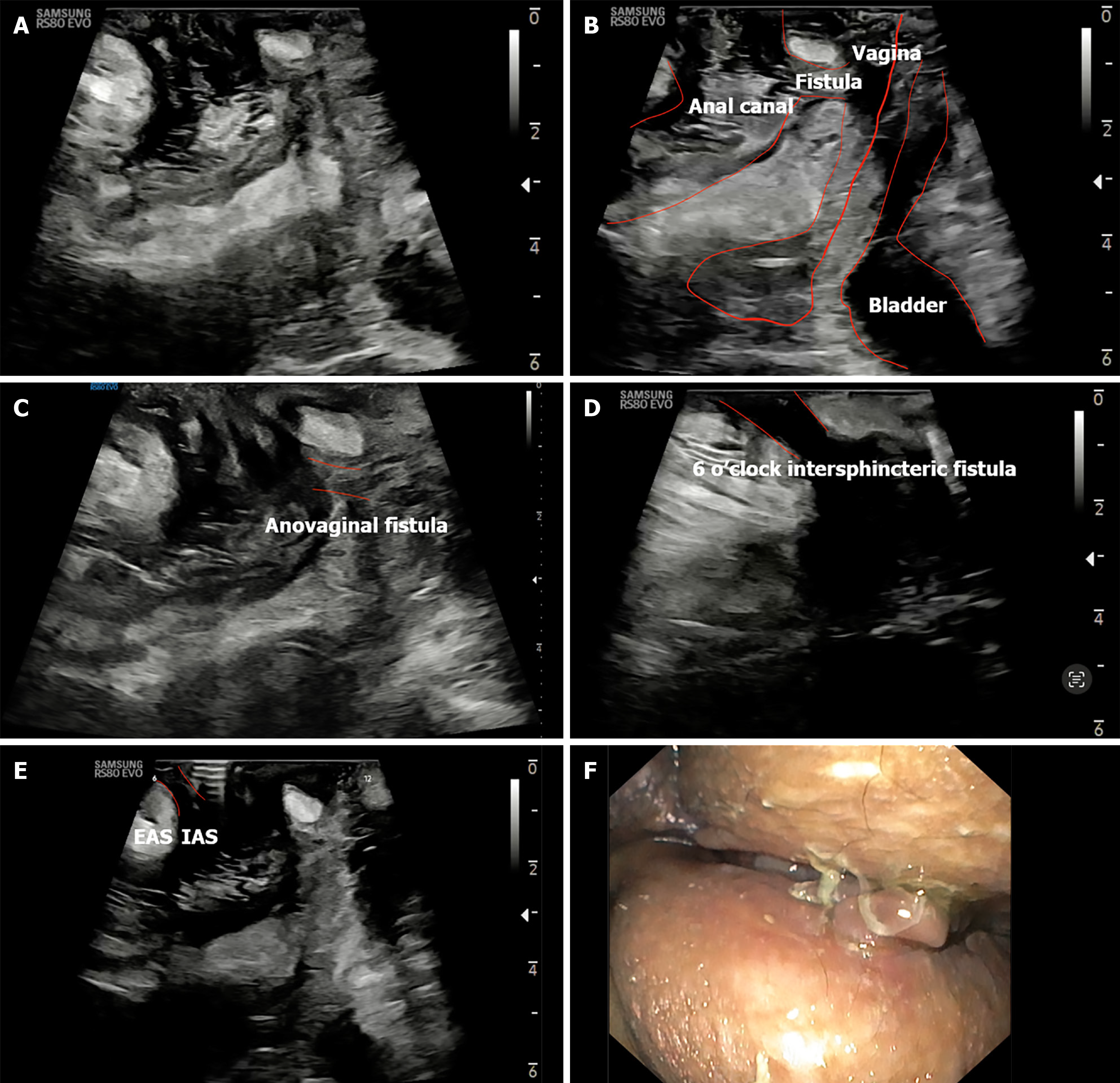
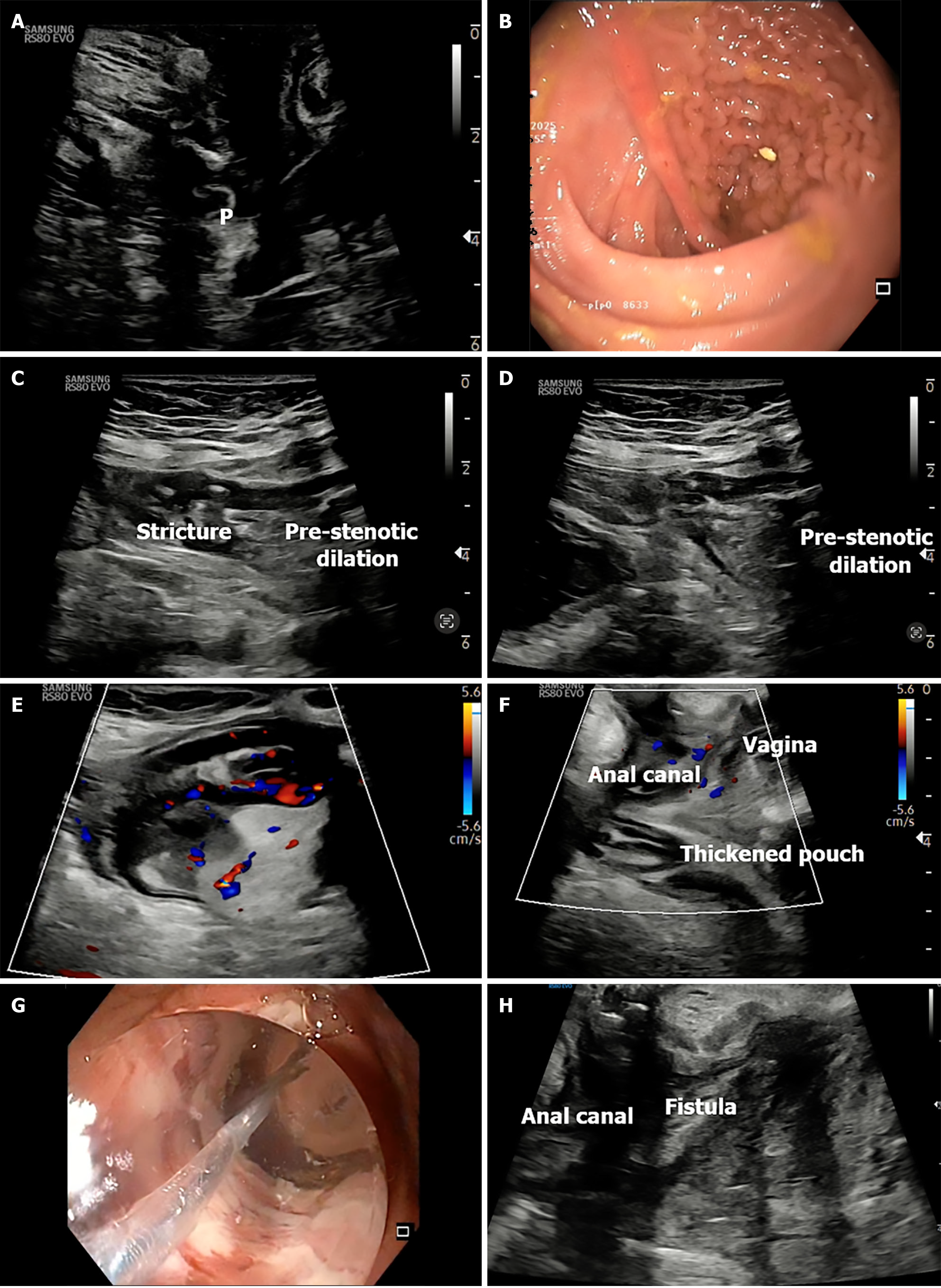
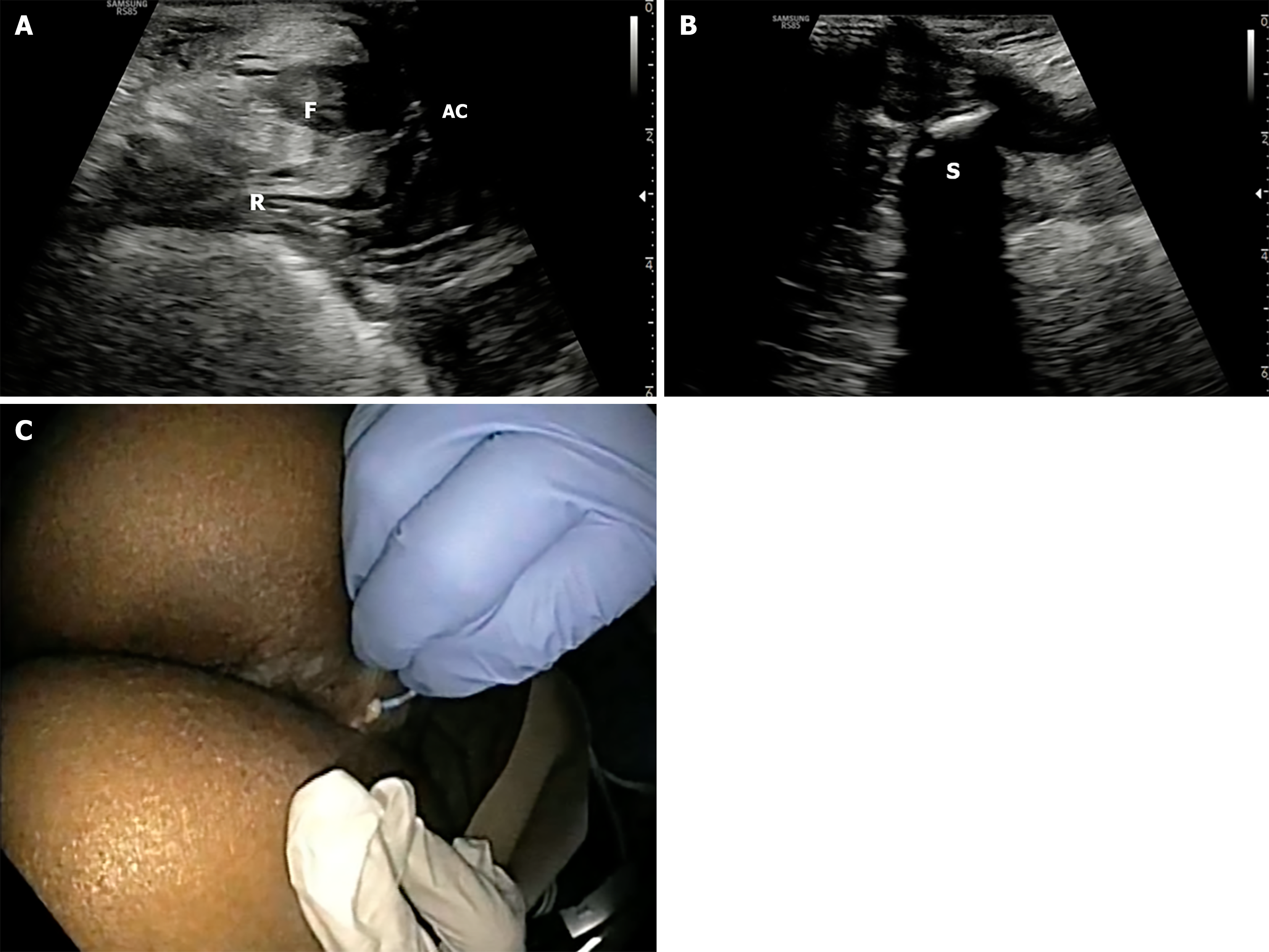
The probe is initially positioned horizontally anterior to the anus, with the vertical marker on probe oriented towards the operator. In this orientation, the sagittal section of the anorectum is visualised (Figure 3A and B). The superficial, vertical part of the anorectum is the anal canal (AC) and the horizontal, deeper part is the rectum (Figure 2A). The inner, hypoechoic part of the AC is the internal anal sphincter and the outer, hyperechoic part is the external anal sphincter (Figure 2B). In this section, the anterior structures like vagina and bladder with urethra are seen on the right of the screen anterior to anorectum (Figure 3B). To obtain coronal views, the probe can be rotated 90 degrees counterclockwise (Figure 2C). In this position, orienting the probe tip towards the coccyx can help obtain the axial section (Figure 2D). To simplify spatial orientation, the 12 o’clock position on a traditional clock is rotated to 3 o’clock in TPUS left lateral decubitus position (Figure 4), enhancing intuitive correlation with clinical examination. Tilting the probe anteriorly or posteriorly allows visualization of tracts extending toward the scrotum (12 o’clock) or coccyx (6 o’clock), respectively. Likewise, upward or downward tilting allows visualization of lateral extensions (3 o’clock or 9 o’clock on the clock face). Sometimes a gliding movement in perineum may be warranted to follow the external opening of a long fistula tract. High-frequency linear probes (e.g., 2-18 MHz) are especially useful for superficial tracts such as anovaginal or anovulvar fistulas (Figure 3C)[1]. The 3D probe can be used to obtain 3D image of the fistula keeping the cursor at the fistula level (Figure 3D).
Fistulas should be described using Parks classification (intersphincteric, transsphincteric, suprasphincteric, extrasphincteric), and clock-face orientation should be used to report internal and external openings. In left lateral position, the 12 o’clock reference corresponds to the anterior midline (3 o’clock with respect to operator)[1]. Routine parameters assessed include bowel wall thickness (BWT) (normal < 3 mm in colon and < 4 mm in rectum), wall stratification (preserved, obscure, or lost), presence of abscesses, and internal openings. Color Doppler signal is interpreted using the Modified Limberg score to grade vascularity[6]. When available, contrast agents like diluted hydrogen peroxide may be used to enhance visualization of fistulous tracts and identify secondary branches or cavities[7]. Reporting templates should include tract type, location, length, internal and external openings, associated abscess, and Doppler/CEUS findings.
Images should be obtained in both sagittal and axial planes. Dynamic clips or cine loops are encouraged to document tract extension and real-time features such as fluid motion or Doppler activity. All images should be stored in a archiving platform to facilitate surgical planning or follow-up comparisons. TPUS findings should always be interpreted in conjunction with clinical findings and may serve as a baseline for longitudinal imaging in chronic disease monitoring.
TPUS may have reduced accuracy in imaging supralevator or deeply located lesions due to limited probe penetration beyond 5-6 cm. Air artifacts from perineal skin folds may occasionally interfere with image quality. Operator experience plays a crucial role in interpretation; thus, training in standardized probe manipulation, anatomical correlation, and Doppler optimization is essential for consistent, high-quality TPUS assessments[8]. A structured learning curve and incorporation into point-of-care training modules are essential for reliable TPUS adoption in clinical practice.
Fistula detection: In CD, TPUS is valuable for screening, characterizing, serial follow up and guiding therapy in perianal fistulas and abscesses. TPUS is a highly accurate, non-invasive modality for detecting perianal fistulas. According to a meta-analysis by Maconi et al[9], TPUS achieved a pooled sensitivity of 98% and a positive predictive value (PPV) of 95% for fistula detection, with even higher sensitivity (99%) in CD-specific cohorts. Its point-of-care accessibility and minimal patient preparation requirements position it as an ideal first-line tool for bedside evaluation.
Fistula classification: TPUS is also effective in classifying fistulas according to Parks’ criteria. Correlation for fistula classification in reference to operative findings is 85.7% for both cryptoglandular and CD fistula[10]. Systematic review reported a pooled classification sensitivity of 92% (95%CI: 85%-97%) and PPV of 92%[9]. Classification accuracy was marginally lower when compared against MRI vs exam under anesthesia, highlighting the importance of multimodal correlation. TPUS is especially well-suited to identifying low and intermediate tracts, with reduced accuracy for complex, high, or branching configurations[9]. Integration with Doppler and contrast-enhanced imaging can improve delineation in borderline cases.
Most commonly, TPUS identifies intersphincteric (Figure 5A) and transsphincteric tracts (Figure 5B and C), which appear as hypoechoic linear structures between or across sphincters. These tracts may be visualized dynamically by tilting the linear probe in horizontal position. On tilting if they are going towards the right or left buttock they are around 9 o’clock and 3 o’clock respectively. Gliding the probe anteriorly and posteriorly may be required to trace the external opening towards the scrotum and coccyx respectively in the 12 o’clock and the 6 o’clock position. Trans-sphincteric fistula traverses both the sphincters after arising from the AC and draining into the external opening. While superficial fistulas are occasionally observed, extrasphincteric and suprasphincteric tracts are more challenging and may necessitate adjunctive MRI or transrectal US (Figure 5D) for full delineation[2,9].
Internal opening: Internal openings are a critical target for fistula mapping. TPUS demonstrated a pooled sensitivity of 91% (95%CI: 84%-97%) and a PPV of 87% (95%CI: 76%-95%) in detecting internal openings. In CD-specific studies, the sensitivity for internal opening detection remained robust at 87%, although these findings are more variable due to operator dependency and interference from air or fibrosis[9]. In cryptoglandular cases, abscesses with associated acoustic shadowing precluded identification of internal opening site[10]. Carefully guided probe positioning and experience are essential for confident identification. Structured training and probe manipulation techniques enhance accuracy.
For perianal abscesses, TPUS provides real-time identification of hypoechoic or anechoic fluid collections with posterior acoustic enhancement, often accompanied by peripheral hyperemia on color Doppler. The pooled sensitivity across studies is 86% (95%CI: 67%-99%) with a PPV of 90%, although slightly lower in MRI-validated cohorts[9]. TPUS is particularly effective in detecting low-lying or intersphincteric abscesses and is valuable in bedside monitoring after surgical drainage or seton placement. However, its diagnostic yield declines for deeper collections (e.g., ischiorectal or supralevator), where adjunctive MRI may be required[2].
TPUS is increasingly recognized as a valuable, well-tolerated imaging tool in children with perianal CD (PACD). In a preliminary study by Hwang et al[11], TPUS successfully identified fistulas and abscesses in pediatric patients with minimal discomfort, showing active disease in 47% of exams and abscesses in 19%, with good correlation to clinical symptoms. Lee et al[12] reported a sensitivity of 76% for fistulas and 56% for abscesses on TPUS compared to MRI, with better concordance than colonoscopy, especially for abscess detection. In a study comparing TPUS with MRI in treatment monitoring, Jung et al[13] found TPUS had excellent specificity (93.3%) and PPV (95.0%) for detecting treatment response, though with modest sensitivity (63.3%). Notably, superficial and thinner fistulas were more likely to be missed[13]. Together, these findings support the use of TPUS as a practical first-line and monitoring tool in pediatric PACD, particularly for intersphincteric and low transsphincteric disease, though MRI remains necessary for complex or supralevator tracts (Table 1).
| Ref. | Patients (n) | Main findings | Concordance or accuracy vs MRI |
| Hwang et al[11], 2014 | 43 | TPUS feasible in all; 47% with active fistula, 19% with abscess; well tolerated | No MRI comparison; pain severity correlated with activity |
| Lee et al[12], 2018 | 38 | TPUS sensitivity 76% (fistula), 56% (abscess); better concordance with MRI than colonoscopy | κ = 0.296 for fistula, 0.624 for abscess |
| Jung et al[13], 2022 | 29 | TPUS detected 80% of fistulas; excellent PPV (95%) and specificity (93%) for response | κ = 0.486; sensitivity 63%, accuracy 73% |
| Jung et al[13], 2022 (MRI/TPUS) analysis | 56 fistulas analyzed | TPUS more likely to miss superficial/thin tracts; intersphincteric better detected | Detection correlated with thickness (P = 0.009); 80.4% of MRI fistulas seen on TPUS |
Rectovaginal fistulas (RVFs) are a debilitating and often under-recognized complication of CD, typically requiring accurate anatomical delineation to guide therapy (Figure 6). In a prospective comparative study, Maconi et al[14] demonstrated that TPUS successfully detected all 9 RVFs and 2 anovulvar fistulas among 46 CD patients, with 84.9% sensitivity and 86.5% PPV compared to endoanal US (EUS) as the reference standard. TPUS provided clear visualization of the fistulous tract between the rectum and vagina (Figure 6A-C), without the need for invasive or painful procedures, making it especially advantageous in patients with anal stenosis or pain. While MRI and EUS remain standard for pre-surgical mapping, TPUS offers a practical, non-invasive alternative for screening and follow-up, particularly when access to cross-sectional imaging is limited.
Quantifying perianal fistula activity remains a clinical challenge, particularly for monitoring treatment response. Two studies by Caprioli et al[15] and Losco et al[4] demonstrated that computer-assisted analysis of anal US images, based on grayscale tone measurement, can objectively distinguish active from inactive fistulas in CD. Active tracts appear more hypoechoic, with a defined grayscale cutoff (mean < 117-118) discriminating inflammation with good diagnostic accuracy [area under the curve (AUC) approximately 0.87-0.90][4]. This approach improved agreement with clinical and MRI findings from fair (κ = 0.26-0.29) to good levels (κ = 0.61-0.77)[15]. While not yet routine in clinical practice, these studies highlight the potential role of computer-enhanced US as a reproducible, operator-independent tool for evaluating perianal disease activity, especially when combined with clinical indices such as pouchitis disease activity index (PDAI) or fistula drainage assessment.
Multiple studies have compared TPUS with MRI in assessing PACD, highlighting its strengths as a frontline, non-invasive imaging tool. In a prospective adult cohort, Maconi et al[2] reported excellent agreement between TPUS and MRI for fistula classification (κ = 0.78), with TPUS achieving 94% sensitivity and correctly classifying over 90% of fistulas. However, its sensitivity was reduced for suprasphincteric and extrasphincteric tracts and deep abscesses. Bor et al[16] similarly reported 100% sensitivity for both fistulas and abscesses using TPUS, outperforming MRI and transrectal US in a surgical validation study, recommending TPUS as a first-line tool in complex cases. Terracciano et al[17] further demonstrated strong agreement between TPUS and MRI using both Parks (κ = 0.67) and American Gastroenterological Association (κ = 0.83) classifications. Wedemeyer et al[18] validated the use of TPUS with conventional probes in 25 CD patients, achieving excellent agreement with MRI (κ > 0.83), and detecting 90% of fistulas and 86% of abscesses. Importantly, TPUS was feasible and well tolerated even in patients with anal stenosis, providing real-time, high-resolution imaging without the need for sedation or preparation.
In pediatric populations, Jung et al[13] observed moderate agreement between TPUS and MRI (κ = 0.49) for treatment response, with TPUS showing excellent specificity (93%) and PPV (95%). Lee et al[12] demonstrated that TPUS had better concordance with MRI than colonoscopy in detecting both fistulas and abscesses, though sensitivity remained modest.
Overall, these studies position TPUS as an effective modality for characterizing and monitoring low-lying perianal fistulas. While MRI remains superior for deep, supralevator, complex secondary, high tracts or horseshoe extensions, TPUS offers a rapid, accessible, and patient-friendly option for triaging and guiding treatment in PACD (Table 2).
| Ref. | No. of patients | Patient characteristics | Concordance rate (TPUS vs MRI or surgery) | Main findings |
| Maconi et al[2], 2013 | 59 | Adults with CD, prospective | κ = 0.78 (fistulae classification) | TPUS sensitivity 94%, PPV 93%; less accurate for extrasphincteric/suprasphincteric tracts and deep abscesses |
| Bor et al[16], 2016 | 23 | Adults with complicated CD | Not specified | TPUS 100% sensitivity for fistulae and abscesses; outperformed MRI and TRUS |
| Jung et al[13], 2022 | 29 | Pediatric CD on biologics | κ = 0.49 | TPUS had high specificity (93%) and PPV (95%) for assessing treatment response vs MRI |
| Lee et al[12], 2018 | 38 | Pediatric CD with PACD | κ = 0.30-0.62 | TPUS better concordance with MRI than colonoscopy; sensitivity 76% for fistulae, 56% for abscesses |
| Wedemeyer et al[18], 2004 | 25 | Adults with active PACD | κ > 0.83 | TPUS detected 90% of fistulas and 86% of abscesses; comparable to MRI; especially useful in anal stenosis |
| Terracciano et al[17], 2016 | 28 | IBD patients | Sensitivity 100% for RVF and superficial abscess; κ = 0.34 for deep abscess | TPUS highly concordant with MRI for Parks (κ = 0.67) and AGA (κ = 0.83) classifications; superior for RVF and superficial abscesses |
TPUS can also be used to assess rectal inflammation in CD, although its primary utility is in UC. Rectal wall thickening, increased vascularity on Doppler, and loss of stratification may be visualized[1]. TPUS can be used serially in patients on therapy to assess response, especially when sigmoidoscopy is not feasible. Its limited depth penetration necessitates caution in patchy or high rectal disease.
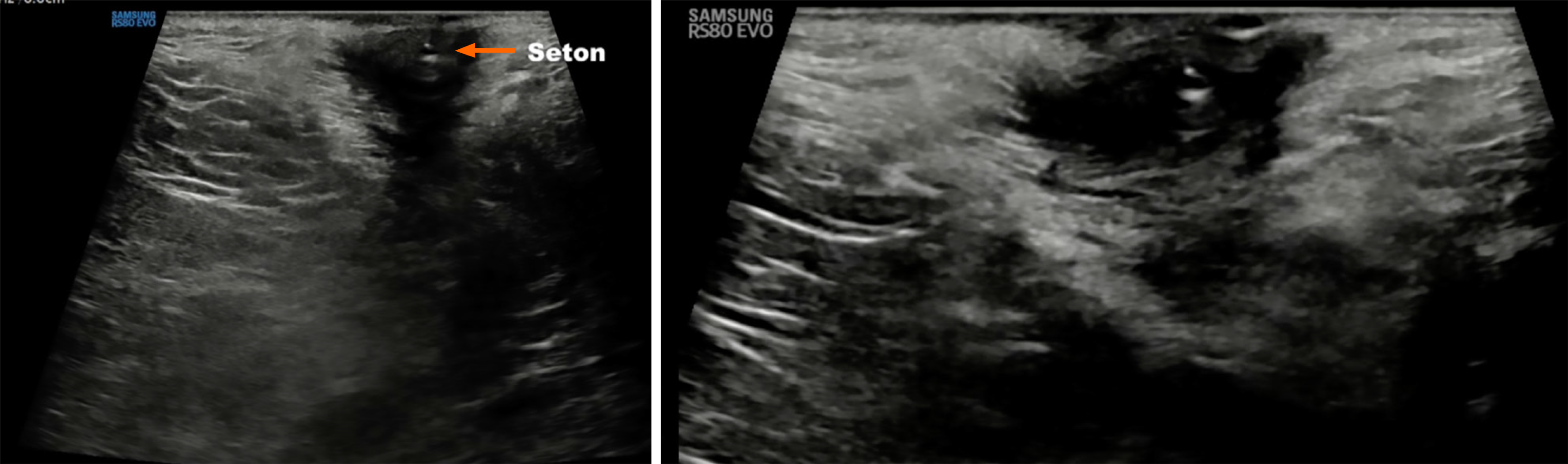
In CD patients with complex fistulas, TPUS offers a non-invasive method to monitor the position and effect of setons. The seton appears as a linear hyperechoic structure traversing the hypoechoic fistulous tract (Figure 7). TPUS can assess for residual tracts, early closure, secondary tracts, or ongoing inflammation around the seton, which may necessitate further intervention. It also allows assessment of epithelialization of fistulous tracts over time, guiding the decision for seton removal or escalation of biologic therapy[1]. It enables safe, serial follow-up without repeat MRI.
TPUS is now recognized as a robust, non-invasive tool for evaluating rectal inflammation in UC, with validated applications across disease activity monitoring, treatment response, and mucosal healing. Early prospective work demonstrated that a rectal BWT ≥ 4 mm predicted endoscopic and histological healing with > 90% accuracy, while reduction in BWT as early as one week into therapy predicted week 8 remission, outperforming C-reactive protein and fecal calprotectin[6,19].
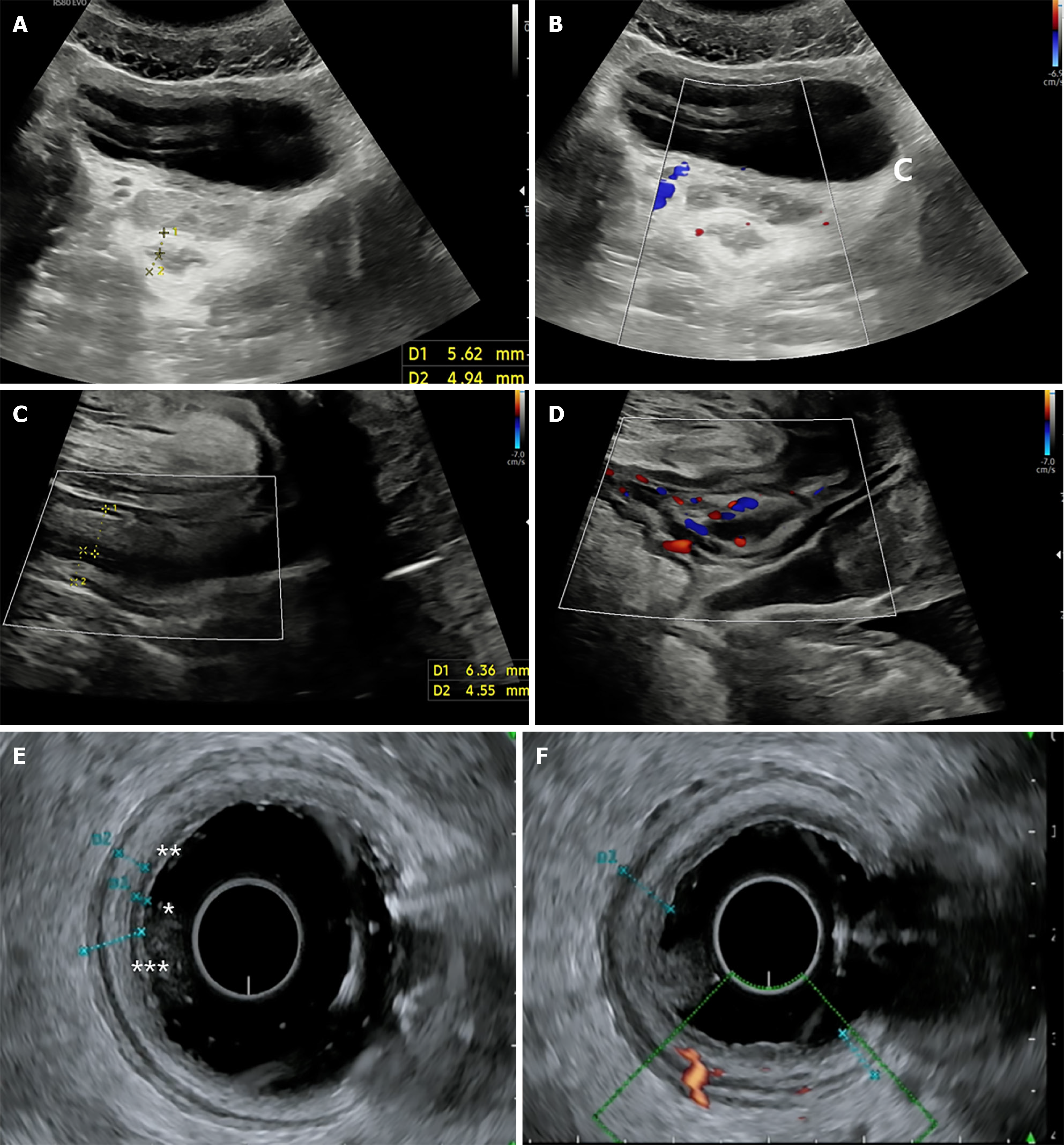
Building on this, the TRINITY study by Pal et al[3] compared TPUS, transabdominal US (TAUS), and EUS in a large cohort of 142 patients (Figure 8). While EUS showed the highest accuracy for predicting endoscopic and histologic remission, TPUS achieved moderate diagnostic performance (AUC 0.73 and 0.66, respectively). Notably, combining TAUS and TPUS improved performance modestly, highlighting the complementary value of a dual-modality approach in resource-constrained or endoscopy-limited settings[3]. These findings support TPUS as a frontline option in outpatient surveillance, especially in patients for whom EUS or repeated endoscopy is not feasible.
In pediatric UC, Tokushima et al[20] used superb microvascular imaging with TPUS and found that the combination of rectal wall thickening > 4.5 mm and microvascular signal at wall circumference achieved 100% sensitivity and specificity for detecting active disease. Meanwhile, in pregnancy, Greeve et al[21] confirmed TPUS as a feasible and well-tolerated modality, capable of influencing management even in the third trimester. These findings underscore TPUS’s unique value in vulnerable populations where endoscopy and MRI may be impractical.
Collectively, these studies establish TPUS as a practical, scalable, and repeatable tool for longitudinal UC care, particularly in distal colitis and vulnerable populations like children and pregnant women[20,21]. To fully standardize its use, further research should aim to validate segment-specific cutoffs, assess reproducibility across platforms, and define TPUS-guided therapeutic thresholds (Table 3).
| Ref. | No. of patients | Patient characteristics | Concordance rate | Main findings |
| Sagami et al[6], 2020 | 84 | UC patients undergoing induction therapy; correlation with endoscopy and histology | BWT ≥ 4 mm correlated with MES > 1; sensitivity > 90% | TPUS can predict mucosal and histological healing with high accuracy |
| Sagami et al[19], 2022 | 100 | UC patients on induction therapy; early TPUS response at week 1 vs remission at week 8 | ΔBWT at week 1 predictive of week 8 remission (AUC = 0.75) | ΔBWT ≥ 1 mm at 1 week predicted treatment success; more accurate than CRP/FC |
| Greeve et al[21], 2024 | 8 | Pregnant women with ulcerative proctitis | Feasibility and acceptability in pregnancy; no adverse events | TPUS feasible and safe during all pregnancy trimesters, including 3rd trimester |
| Tokushima et al[20], 2024 | 131 | Pediatric patients with UC (MES 0-3) and non-IBD proctitis | RWT > 4.5 mm and MSWC positive had 100% sensitivity and specificity for active UC vs proctitis | TPUS with mSMI can differentiate UC from non-IBD proctitis with accuracy similar to colonoscopy |
| Pal et al[3], 2025 | 142 | Adults with UC (proctitis, proctosigmoiditis, extensive colitis); compared TPUS, TAS, and EUS | TPUS AUC 0.73 (endoscopic remission), 0.66 (histologic remission) | TPUS moderately accurate for distal disease; TAS+TPUS improved diagnostic value but still inferior to EUS |
TPUS has emerged as a non-invasive, accessible tool to assess pouch-related inflammation in patients with an ileal pouch-anal anastomosis (IPAA) following colectomy for UC (Figure 9). In a prospective cross-sectional study of 44 pouch patients, Ardalan et al[22] demonstrated that TPUS had good diagnostic accuracy for pouchitis, with a pouch wall thickness ≥ 4.0 mm achieving 87% specificity and correlating strongly with endoscopic and histologic inflammation (AUC 0.79). A cutoff of < 3.0 mm reliably excluded pouchitis (88% sensitivity) and defined a state of "sonographic remission"[22].
Importantly, pouch wall thickness assessed via TPUS correlated with total PDAI (r = 0.51), endoscopic subscore (r = 0.45), and histological subscore (r = 0.52), suggesting TPUS can be used both for initial diagnosis and longitudinal response assessment[22]. While TPUS Doppler parameters showed limited discrimination-likely due to probe depth and pelvic vascular noise-the structural findings were reproducible, especially when using convex or microconvex probes.
The transabdominal approach was more effective for evaluating the pre-pouch ileum. In cases of moderate-severe pre-pouch ileitis, BWT ≥ 3.0 mm and Limberg score ≥ 1 provided AUCs of 0.78 and 0.79, respectively[22]. These sonographic changes were significantly more frequent in patients with chronic antibiotic-refractory or immune-mediated pouchitis. Therefore, combining transabdominal and transperineal gastrointestinal US allows localization of inflammation, particularly in complex or recurrent pouch dysfunction.
Faecal calprotectin (FC) served as a complementary test: < 100 μg/g ruled out inflammatory pouch disorders (sensitivity 89%), while ≥ 350 μg/g reliably ruled them in (specificity 100%). When combined with TPUS, this approach could have reduced the need for pouchoscopy by up to 70%, reserving it for equivocal or discordant cases[22].
In summary, TPUS-particularly when combined with TAUS and FC-offers a rapid, repeatable, and well-tolerated method to differentiate pouchitis, pre-pouch ileitis, and irritable pouch syndrome. Its use can streamline follow-up and reduce dependence on invasive procedures, provided operators are adequately trained and reporting frameworks are standardized.
The integration of color Doppler imaging into TPUS significantly enhances the evaluation of PACD by visualizing hypervascularity (Figure 3A) in and around fistulas and abscesses-serving as a marker of active inflammation. In a surgical validation study by Mallouhi et al[23], the addition of color Doppler to grayscale TPUS increased diagnostic confidence and overall accuracy for detecting perianal inflammatory disease (AUC improved from 0.86 to 0.89; P = 0.002). Hypervascularity at the lesion’s periphery was identified as an independent predictor of perianal inflammation, with an odds ratio of 8.5, supporting its value in distinguishing active from quiescent disease[23].
Color Doppler can differentiate active granulating tissue from fibrotic tracts and is particularly useful in guiding management during biologic therapy by identifying persistent subclinical inflammation or fluid collections not apparent on examination[1]. Routine use of color Doppler in longitudinal TPUS protocols can aid objective assessment of therapeutic response and early detection of recurrence.
To enhance detection and delineation, CEUS using diluted hydrogen peroxide has shown promising utility. When introduced into the external opening, the agent generates microbubbles that convert hypoechoic tracts to echogenic, thereby improving visualization of fistulous ramifications and small abscesses within inflammatory masses. This technique has been shown to be particularly helpful in mapping complex, ramified, or supralevator fistulas[1]. In a study by Sloots et al[7], Hydrogen peroxide ultarsound (HPUS) revealed that only 22% of Crohn’s fistulas were simple, while the remaining 78% were complex, including suprasphincteric, extrasphincteric, or anovaginal types. HPUS was superior to physical exam, computed tomography, and conventional US in demonstrating the full extent of fistula anatomy, emphasizing its value before surgery or biologic therapy. The safety profile, rapid acquisition, and bedside applicability of CEUS make it an attractive enhancement in routine TPUS-guided fistula characterization.
Sonoelastography is an emerging adjunctive technique that evaluates tissue stiffness. In the context of PACD, it may help differentiate between active, inflamed fistulas (which appear softer and more compressible) and fibrotic or sclerotic tracts (which are stiffer and more resistant to pressure)[1]. While early evidence is promising, larger validation studies are needed before widespread clinical adoption. Incorporation of sonoelastography into TPUS protocols holds promise for individualized monitoring of fibrosis progression and therapeutic response.
The 3D US has added significant value to the assessment of PACD by offering enhanced spatial resolution, multiplanar reconstruction, and improved delineation of complex fistula anatomy. Unlike conventional 2D imaging, 3D US allows for volumetric assessment of sphincter involvement, fistula branching, and associated abscesses, often revealing lesions that are clinically silent (Figure 3D).
In a prospective study of 95 patients with PACD, de la Portilla et al[5] demonstrated that 3D EUS influenced therapeutic decision-making in nearly half of all cases examined. Abscesses were detected in 17.7% of patients-many of which were unsuspected clinically-and the imaging findings correlated with surgical findings in 81.3% of cases. Importantly, 3D US identified sphincter defects and allowed accurate classification of fistulas using a novel sonographic scheme tailored for PACD. The study reported high inter-observer agreement (κ = 0.86), suggesting that 3D imaging is both reproducible and clinically impactful in guiding surgical timing, seton removal, or biologic therapy[5].
Beyond morphological assessment, 3D US has diagnostic specificity. Zawadzki et al[24] introduced the Crohn’s US fistula sign (CUFS)-a characteristic 3D US feature defined by a hypoechoic tract surrounded by a hyperechoic halo with a thin hypoechoic outer rim. In their study of 157 patients with anal fistulae, CUFS had a specificity of 98% and a PPV of 87% for CD, enabling differentiation from cryptoglandular fistulas. Remarkably, in 12 cases, CUFS prompted the diagnosis of CD prior to the appearance of intestinal symptoms[24].
Incorporating 3D US, especially with contrast enhancement (e.g., hydrogen peroxide), offers a non-invasive, bedside-compatible, and radiation-free modality to assess fistula complexity, guide interventions, and potentially identify CD in undiagnosed patients. While MRI remains the standard for deep-seated or supralevator disease, 3D TPUS/EAUS serves as a practical, first-line imaging strategy with growing diagnostic and prognostic utility in PACD.
TPUS can be used to guide therapeutic interventions, such as abscess drainage, seton placement or endoscopic balloon dilation, by providing real-time anatomical orientation (Figures 9G and 10). In patients on anti-tumor necrosis factor therapy, TPUS enables serial assessment to detect silent abscesses, confirm persistent tracts despite external closure, and guide the duration of medical therapy. It also facilitates precision-targeted interventions and minimizes procedural risks by delineating real-time anatomy at bedside.
TPUS has transitioned from an ancillary technique to a core imaging tool in the management of IBD. Its non-invasive nature, bedside feasibility, and expanding diagnostic applications-including rectal inflammation in UC, perianal fistulas in CD, and complications post-IPAA-make TPUS a versatile modality. Adjunctive tools such as color Doppler, CEUS, elastography, and 3D imaging enhance both anatomical and functional insights. TPUS compares favorably with MRI and EUS in many scenarios and excels as a point-of-care strategy, particularly in pediatrics, pregnancy, and resource-limited settings. With growing evidence and structured training, TPUS is poised for wider integration into IBD care algorithms-as a first-line, repeatable, and cost-effective modality. Standardized training programs, validated reporting templates, and multicenter reproducibility data will be crucial next steps to ensure consistent, high-quality implementation. Ultimately, TPUS offers the potential to transform disease monitoring from hospital-based imaging to real-time, bedside precision.
| 1. | Lavazza A, Maconi G. Transperineal ultrasound for assessment of fistulas and abscesses: a pictorial essay. J Ultrasound. 2019;22:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Maconi G, Tonolini M, Monteleone M, Bezzio C, Furfaro F, Villa C, Campari A, DellʼEra A, Sampietro G, Ardizzone S, de Franchis R. Transperineal perineal ultrasound versus magnetic resonance imaging in the assessment of perianal Crohn's disease. Inflamm Bowel Dis. 2013;19:2737-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Pal P, Mateen MA, Sekaran A, Pooja K, Kaleemuddin A, Rangineni T, Basha J, Gupta R, Tandan M, Lakhtakia S, Reddy DN. Can transperineal ultrasound replace endoscopic ultrasound in staging distal ulcerative colitis? Gut. 2025;gutjnl-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Losco A, Viganò C, Conte D, Cesana BM, Basilisco G. Assessing the activity of perianal Crohn's disease: comparison of clinical indices and computer-assisted anal ultrasound. Inflamm Bowel Dis. 2009;15:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | de la Portilla F, Durán V, Maestre MV, Díaz-Pavón JM, Vázquez-Monchul JM, Palacios C, Gollonet JL, Sánchez-Gil JM. Effectiveness of a three-dimensional anorectal ultrasound in perianal Crohn's disease: incompatibility with clinical and surgical examinations. Int J Colorectal Dis. 2015;30:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 6. | Sagami S, Kobayashi T, Aihara K, Umeda M, Morikubo H, Matsubayashi M, Kiyohara H, Nakano M, Ohbu M, Hibi T. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther. 2020;51:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Sloots CE, Felt-Bersma RJ, Poen AC, Cuesta MA, Meuwissen SG. Assessment and classification of fistula-in-ano in patients with Crohn's disease by hydrogen peroxide enhanced transanal ultrasound. Int J Colorectal Dis. 2001;16:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Nuernberg D, Saftoiu A, Barreiros AP, Burmester E, Ivan ET, Clevert DA, Dietrich CF, Gilja OH, Lorentzen T, Maconi G, Mihmanli I, Nolsoe CP, Pfeffer F, Rafaelsen SR, Sparchez Z, Vilmann P, Waage JER. EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound. Ultrasound Int Open. 2019;5:E34-E51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Maconi G, Greco MT, Asthana AK. Transperineal Ultrasound for Perianal Fistulas and Abscesses - A Systematic Review and Meta-Analysis. Ultraschall Med. 2017;38:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Nevler A, Beer-Gabel M, Lebedyev A, Soffer A, Gutman M, Carter D, Zbar AP. Transperineal ultrasonography in perianal Crohn's disease and recurrent cryptogenic fistula-in-ano. Colorectal Dis. 2013;15:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hwang JY, Yoon HK, Kim WK, Cho YA, Lee JS, Yoon CH, Lee YJ, Kim KM. Transperineal ultrasonography for evaluation of the perianal fistula and abscess in pediatric Crohn disease: preliminary study. Ultrasonography. 2014;33:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 12. | Lee EH, Yang HR, Kim JY. Comparison of Transperianal Ultrasound With Colonoscopy and Magnetic Resonance Imaging in Perianal Crohn Disease. J Pediatr Gastroenterol Nutr. 2018;66:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 13. | Jung JH, Ryu YJ, Kim JY, Yang HR. Transperineal ultrasonography for treatment response evaluation in children with perianal Crohn's disease. Ultrasonography. 2022;41:770-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G. Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in Crohn's disease. Am J Gastroenterol. 2007;102:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Caprioli F, Losco A, Viganò C, Conte D, Biondetti P, Forzenigo LV, Basilisco G. Computer-assisted evaluation of perianal fistula activity by means of anal ultrasound in patients with Crohn's disease. Am J Gastroenterol. 2006;101:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 16. | Bor R, Farkas K, Bálint A, Szűcs M, Ábrahám S, Milassin Á, Rutka M, Nagy F, Milassin P, Szepes Z, Molnár T. Prospective Comparison of Magnetic Resonance Imaging, Transrectal and Transperineal Sonography, and Surgical Findings in Complicated Perianal Crohn Disease. J Ultrasound Med. 2016;35:2367-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 17. | Terracciano F, Scalisi G, Bossa F, Scimeca D, Biscaglia G, Mangiacotti M, Valvano MR, Perri F, Simeone A, Andriulli A. Transperineal ultrasonography: First level exam in IBD patients with perianal disease. Dig Liver Dis. 2016;48:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 18. | Wedemeyer J, Kirchhoff T, Sellge G, Bachmann O, Lotz J, Galanski M, Manns MP, Gebel MJ, Bleck JS. Transcutaneous perianal sonography: a sensitive method for the detection of perianal inflammatory lesions in Crohn's disease. World J Gastroenterol. 2004;10:2859-2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 19. | Sagami S, Kobayashi T, Aihara K, Umeda M, Odajima K, Morikubo H, Asonuma K, Miyatani Y, Fukuda T, Matsubayashi M, Kiyohara H, Nakano M, Hibi T. Early improvement in bowel wall thickness on transperineal ultrasonography predicts treatment success in active ulcerative colitis. Aliment Pharmacol Ther. 2022;55:1320-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Tokushima K, Jimbo K, Suzuki M, Endo Y, Hibio M, Maruyama K, Kashiwagi K, Arai N, Sato M, Kudo T, Hoshino E, Ohtsuka Y, Shimizu T. Differentiation of Active Ulcerative Colitis vs Noninflammatory Bowel Disease Proctitis by Transperineal Superb Microvascular Imaging. Inflamm Bowel Dis. 2024;30:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Greeve T, Prentice RE, Shelton E, Lever F, Boyapati R, Burns M, Bell SJ. Feasibility of transperineal intestinal ultrasound in assessing ulcerative proctitis during pregnancy. JGH Open. 2024;8:e70035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (4)] |
| 22. | Ardalan ZS, Friedman AB, Con D, Chandran S, Gibson D, Pham A, De Cruz P, Tay K, Bell S, Rosella O, Sparrow MP, Gibson PR. Accuracy of Gastrointestinal Ultrasound and Calprotectin in the Assessment of Inflammation and its Location in Patients with an Ileoanal Pouch. J Crohns Colitis. 2022;16:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Mallouhi A, Bonatti H, Peer S, Lugger P, Conrad F, Bodner G. Detection and characterization of perianal inflammatory disease: accuracy of transperineal combined gray scale and color Doppler sonography. J Ultrasound Med. 2004;23:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 24. | Zawadzki A, Starck M, Bohe M, Thorlacius H. A unique 3D endoanal ultrasound feature of perianal Crohn's fistula: the 'Crohn ultrasound fistula sign'. Colorectal Dis. 2012;14:e608-e611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/