Published online Aug 28, 2025. doi: 10.3748/wjg.v31.i32.110333
Revised: July 2, 2025
Accepted: August 1, 2025
Published online: August 28, 2025
Processing time: 83 Days and 23.1 Hours
The association between diabetes mellitus (DM) and metabolic dysfunction-associated steatotic liver disease (MASLD) is well documented, with DM increasing the risk of developing MASLD and liver fibrosis.
To evaluate the cost-effectiveness and budget impact of transient elastography (TE) for detecting significant fibrosis in Thai patients with DM.
We conducted a lifetime cost-utility analysis from a societal perspective, combining a decision tree with a Markov model. Four alternatives were compared: Fibrosis-4 (FIB-4) index triage followed by TE, steatosis-associated fibrosis estimator (SAFE) score triage followed by TE, standalone TE, and no screening. Clinical probabilities, utilities, and costs came from previous studies and Siriraj Hospital data. Costs and quality-adjusted life-years (QALYs) were discounted 3% annually, and incremental cost-effectiveness ratios (ICERs) were judged against the 160000-Thai baht (THB; 4619 United States dollars [USD])/QALY threshold. A 5-year budget impact was evaluated from the payer perspective.
Among the screening methods evaluated, TE alone yielded the highest total lifetime costs of 200403 THB (5785 USD) and the highest QALYs of 12.81. Compared to no screening, all strategies demonstrated cost-effectiveness with ICERs of 75961, 80385, and 98965 THB (2193, 2321, and 2857 USD)/QALY gained for FIB-4 + TE, SAFE + TE, and TE alone, respectively. Extended dominance favored SAFE + TE, yet probabilistic analysis showed FIB-4 + TE had the highest cost-effectiveness probability and the smallest budget impact. Estimated annual budget impacts amounted to 470.7-755.8 million THB (13.6-21.8 million USD).
Implementing screening for significant fibrosis in patients with DM is cost-effective. In resource-limited settings, prioritizing the FIB-4 index as a triage tool before TE is recommended.
Core Tip: Transient elastography (TE) is a promising tool for liver fibrosis screening, but its cost-effectiveness data in low- and middle-income countries is limited. Screening for significant fibrosis in patients with diabetes mellitus is recommended, yet implementation faces resource challenges. Fibrosis-4 index followed by TE offers the highest probability of being cost-effective and the lowest budget impact in Thailand. Prioritizing scoring systems as a triage tool can optimize early detection and resource use in resource-limited settings.
- Citation: Kositamongkol C, Tantiyavarong P, Ratanatawan A, Sripongpun P, Mahawithitwong P, Kositamongkol P, Saokaew S, Phisalprapa P. Cost-effectiveness of transient elastography for liver fibrosis screening in Thai patients with diabetes mellitus. World J Gastroenterol 2025; 31(32): 110333
- URL: https://www.wjgnet.com/1007-9327/full/v31/i32/110333.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i32.110333
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common cause of chronic liver disease worldwide. Its prevalence is increased in people with diabetes mellitus (DM)[1]. Approximately one in four individuals in the general population has MASLD, but its prevalence rises up to 65.0% (95% confidence interval [CI]: 61.8%-68.2%) among patients with DM and 35.5% (95%CI: 19.6%-55.6%) of those had significant fibrosis (≥ F2)[1-3]. Despite its widespread prevalence, MASLD often remains underdiagnosed, particularly in its early stages. Many patients with early MASLD may present without symptoms or abnormal laboratory findings, making detection challenging. In fact, more than half of individuals with MASLD have normal liver enzyme levels, further contributing to its underdiagnosis[4].
MASLD patients with significant fibrosis face a markedly higher risk of both hepatic-related and non-hepatic events, alongside significantly increased mortality rates. Compared to those without fibrosis, the hazard ratios for all-cause mortality in patients with significant fibrosis range from 1.46 to 3.66[5]. Thus, screening for significant fibrosis is critically important due to its potential to enable early detection and treatment interventions. Early detection of fibrosis allows clinicians to encourage lifestyle modifications, including adopting a healthy diet, increasing physical activity, and achieving weight reduction, as recommended by international guidelines for MASLD management[6,7]. These interventions are supported by evidence demonstrating their ability to reverse or stabilize the progression of fibrosis[8].
Several screening methods are available for MASLD and liver fibrosis, including serum markers, ultrasonography, transient elastography (TE), computed tomography (CT), and magnetic resonance elastography (MRE)[6,9]. While liver biopsy is the gold standard, it is invasive and costly, making it unsuitable for population-level screening[6,7,9]. Advanced device-based techniques like CT and MRE offer high sensitivity but are expensive and less accessible for routine use. TE, a non-invasive tool capable of assessing both hepatic steatosis and fibrosis, has shown promise for identifying high-risk patients[10]. However, its relatively high cost and limited accessibility pose challenges for national-level implementation in resource-limited countries. In Thailand, TE are concentrated in urban hospitals. A stepwise screening approach using clinical scoring systems based on serum markers and demographic data to identify candidates for TE may offer a more feasible and cost-effective solution in low- and middle-income countries (LMICs). Supporting this concept, an Indian study proposed population-specific fibrosis-4 (FIB-4) cutoffs that improved the accuracy and feasibility of fibrosis screening[11].
In Thailand, MASLD poses significant health and economic burdens. An estimated 2.9 million people in Thailand had metabolic dysfunction-associated steatohepatitis with significant fibrosis in 2019, with total lifetime costs accounting for 15.2 billion United States dollars (USD) or about 3% of the country’s gross domestic product[12]. The significant burden of MASLD highlights the need for effective screening and early treatment intervention, particularly for individuals with DM who are at higher risk of severe disease progression and complications. Previous research in Thailand has shown that ultrasonography for MASLD screening in individuals with metabolic syndrome, and treatment with intensive weight reduction program, is a cost-effective strategy[13].
Despite the availability of various screening options and the growing global use of TE for screening, there is a lack of data on its cost-effectiveness in Thailand and other LMICs. Screening policies often contribute to increased healthcare costs, making economic evaluations crucial for informing policy decisions. Therefore, an economic evaluation is essential as part of the evidence for policy development.
This study conducted cost-utility and budget impact analyses of screening strategies involving TE for detecting significant fibrosis in patients with DM in Thailand.
This study was reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards 2022 Statement[14] (Supplementary Table 1) and was approved by the ethical committee of Faculty of Medicine Siriraj Hospital, Mahidol University (Certificate of Approval No. Si 990/2023) and the Human Research Ethics Committee of Thammasat University (Medicine) (Certificate of Approval No. 124/2024).
A cost-utility analysis was performed to estimate lifetime costs and health benefits of implementing screening policies for significant fibrosis detection in patients with DM. A decision tree and Markov model were adopted to capture outcomes through patient’s lifespan using societal perspective as recommended in Thai Health Technology Assessment guidelines[15]. The results were presented as life expectancy, total lifetime costs, total lifetime quality-adjusted life-years (QALYs). The incremental cost-effectiveness ratios (ICERs) in 2023 Thai baht (THB) and USD per QALY gained were calculated to determine the cost-effectiveness of each screening strategies compared to a no screening option. Additionally, the extended dominance among screening strategies was checked. The conversion rate used in this study was 34.64 THB = 1 USD. The willingness-to-pay (WTP) threshold of 160000 THB (4619 USD)/QALY gained was used to determine whether the options were cost-effective[14,15]. The economic analyses were conducted using Excel 365 (Microsoft Corporation).
This study conducted economic evaluation under the scope of patients with DM according to the American Diabetes Association[16]. The base-case analysis was conducted using the age of screening of 50 years. In addition, the scenario analyses were conducted by varying the age of screening from 30-80 years.
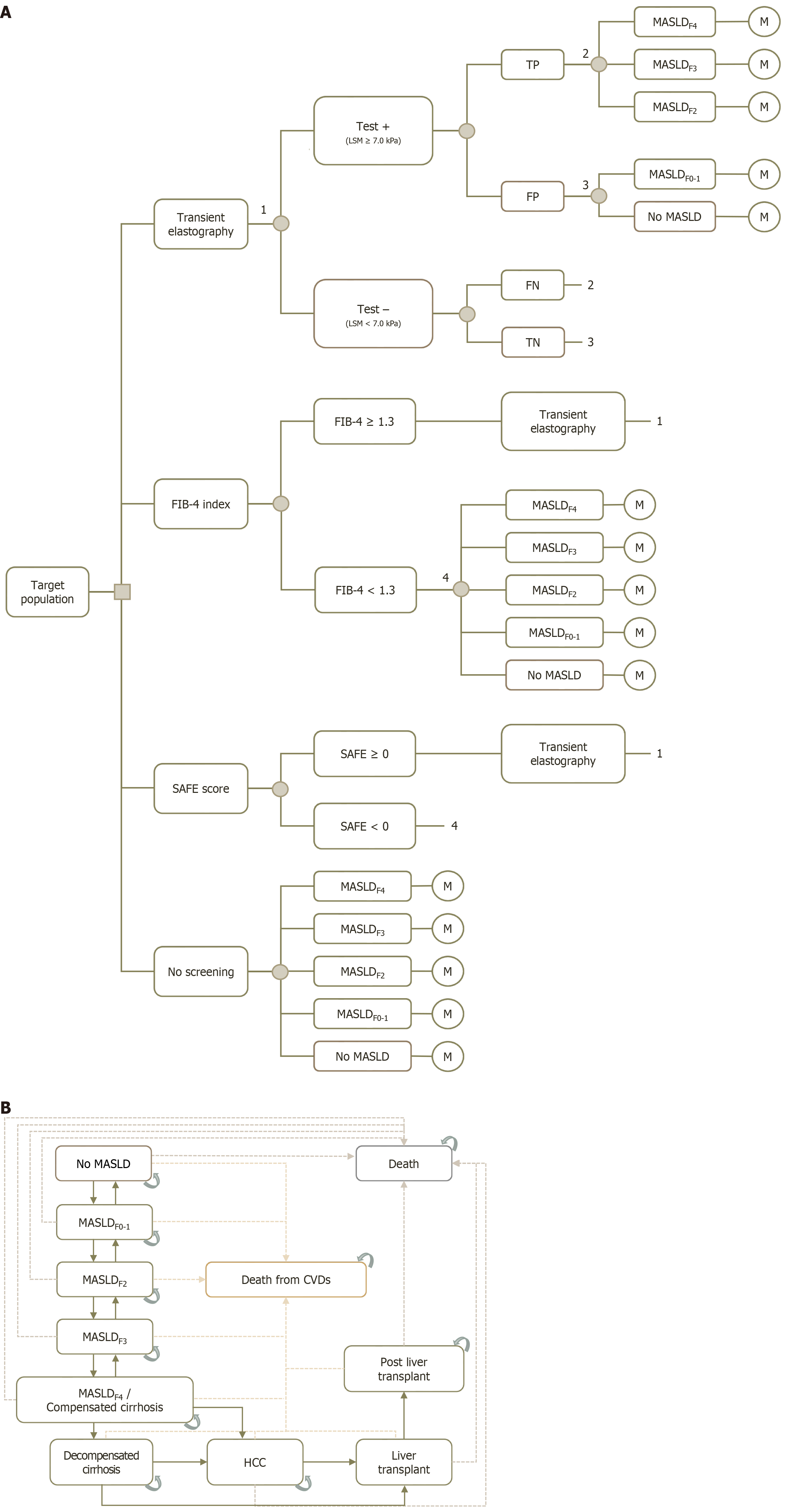
All screening strategies were modeled only once in a lifetime. The strategies evaluated included: (1) FIB-4 index followed by TE, referred to as “FIB-4 + TE”; (2) Steatosis-associated fibrosis estimator (SAFE) score followed by TE, referred to as “SAFE + TE”; (3) TE alone, referred to as “TE alone”; and (4) No screening, serving as the comparator in the base-case analysis.
The FIB-4 index was calculated using the following equation:
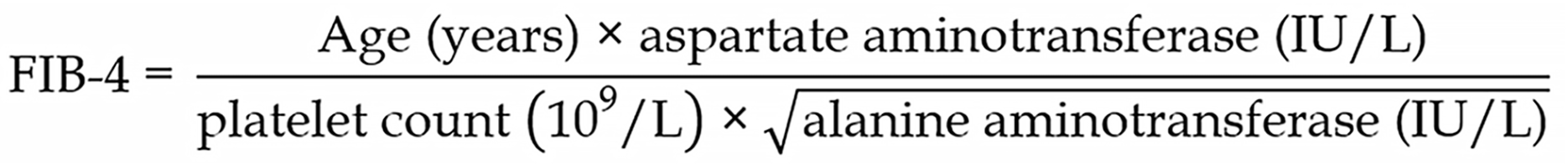
The SAFE score was calculated using the formula: SAFE score = [2.97 × age (year)] + [5.99 × body mass index (BMI) (kg/m2; if BMI > 40 set to 40)] + [62.85 × diabetes (0 if absent, 1 if present)] + [154.85 × ln(aspartate aminotransferase, IU/L)] - [58.23 × ln(alanine aminotransferase, IU/L)] + [195.48 × ln(globulin, g/dL)] - [141.61 × ln(platelet count, 109/L)] - 75.
The cutoff values for identifying significant fibrosis were set at ≥ 1.30 for FIB-4 index[6] and ≥ 0 for SAFE score[17].
The screening rates were assumed to be 90% for the screening strategies involved scoring systems, 80% for a TE alone strategy. For strategies involving scoring systems, it was assumed that 90% of patients with positive results would undergo TE for further investigation.
A decision tree and Markov model were employed to estimate the total costs and health outcomes over a patient’s lifetime. The decision tree (Figure 1A) was used to categorize patients into three different screening strategies, as mentioned above, and a no screening option. Patients were further stratified based on the results of these screening tests, either test-positive or test-negative. The model also accounted for false-positive and false-negative results, which were calculated using test performance derived from previous literature[10,18]. For screening strategies involving the FIB-4 index and SAFE score, patients with scores above the given cutoff values (including both true positives and false positives) would undergo further investigation by TE. Disease detection rates were calculated using primary data specific to Thai patients with DM. Conversely, patients with negative results from the scoring systems (i.e. FIB-4 < 1.3 or SAFE < 0) and those who underwent the no screening option were modeled to progress according to the natural course of MASLD.
The Markov model (Figure 1B) was used to simulate patient health states after the decision tree phase, capturing disease progression until death. This model was adapted from previously published models designed to represent MASLD-related events in the Thai population[12,13,19]. Our model consisted of 11 health states representing the natural course of MASLD. The model began with "no MASLD", after which patients could either remain in their current health state or progress to more severe health states over time. These health states included MASLD with fibrosis, compensated cirrhosis, decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), liver transplant (LT), and post-LT. For patients who had not yet transitioned to DC or more severe states, the model allowed them to regress to less severe health states. As we considered a lifetime time horizon, the death states were included as the absorbing heath states. These death states encompassed mortality from all causes, including cardiovascular diseases (CVDs), liver-related events, and other causes. The 1-year cycle length and annual discount rate of 3% were applied to both costs and outcomes in the model.
The input parameters (Table 1) for the model consisted of test performance, epidemiological data, treatment effectiveness, transitional probabilities (Tps) among health states, utilities, and direct costs. These parameters were derived from published literatures and primary data collected at Siriraj Hospital (Bangkok, Thailand), the largest university hospital in Thailand, which serves more than 3 million outpatient visits and 80000 inpatient admissions annually[20].
| Input parameter | Distribution | Base-case value | SE | Ref. |
| Test performance1 (%) | ||||
| FIB-4 index ≥ 1.3 | Alkhouri et al[18] | |||
| Sensitivity | Beta | 66.0 | 1.2 | |
| Specificity | Beta | 65.0 | 1.4 | |
| SAFE score ≥ 0 | Alkhouri et al[18] | |||
| Sensitivity | Beta | 87.3 | 0.8 | |
| Specificity | Beta | 35.1 | 1.4 | |
| TE, LSM ≥ 7 kPa | Selvaraj et al[10] | |||
| Sensitivity | Beta | 80.1 | 2.1 | |
| Specificity | Beta | 73.0 | 2.6 | |
| Epidemiological data | ||||
| Prevalence of MASLD | Phisalprapa et al[12] | |||
| Age 18-39.9 years | Beta | 0.353 | 0.013 | |
| Age 40-59.9 years | Beta | 0.348 | 0.002 | |
| Age ≥ 60 years | Beta | 0.244 | 0.004 | |
| Prevalence of MASLD with significant fibrosis | Primary data (Supplementary Table 2) | |||
| Age 30-39.9 years | Beta | 0.304 | 0.096 | |
| Age 40-49.9 years | Beta | 0.333 | 0.068 | |
| Age 50-59.9 years | Beta | 0.304 | 0.037 | |
| Age 60-69.9 years | Beta | 0.404 | 0.031 | |
| Age 70-79.9 years | Beta | 0.403 | 0.041 | |
| Age ≥ 80 years | Beta | 0.659 | 0.074 | |
| Incidence of MASLD (cases per 1000 patient-years) | Beta | 42.8 | 2.2 | Park et al[21] |
| Treatment effectiveness | ||||
| RRR of lifestyle modification | Log-normal | 0.204 | 0.124 | Vilar-Gomez et al[8] |
| Transitional probability | ||||
| No MASLD - MASLDF0 | Beta | 0.043 | 0.002 | Park et al[21] |
| MASLDF0 - no MASLD | Beta | 0.024 | 0.013 | Le et al[22] |
| MASLDF0 - MASLDF1 | Beta | 0.063 | 0.025 | |
| MASLDF1 - MASLDF0 | Beta | 0.025 | 0.017 | |
| MASLDF1 - MASLDF2 | Beta | 0.067 | 0.025 | |
| MASLDF2 - MASLDF1 | Beta | 0.045 | 0.022 | |
| MASLDF2 - MASLDF3 | Beta | 0.056 | 0.024 | |
| MASLDF3 - MASLDF2 | Beta | 0.058 | 0.024 | |
| MASLDF3 - MASLDF4/CC | Beta | 0.044 | 0.021 | |
| MASLDF4/CC - MASLDF3 | Beta | 0.046 | 0.022 | |
| MASLDF4/CC - DC | Beta | 0.043 | 0.041 | Gruneau et al[23] |
| MASLDF4/CC - HCC | Beta | 0.008 | 0.017 | |
| DC - HCC | Beta | 0.029 | 0.045 | |
| DC - LT (age ≤ 70 years) | Beta | 0.003 | 0.012 | |
| HCC - LT (age ≤ 70 years) | Beta | 0.012 | 0.027 | |
| Utilities | ||||
| DM | Beta | 0.753 | 0.116 | Deerochanawong et al[32] |
| MASLDF0-F3 | Beta | 0.753 | 0.116 | |
| MASLDF4/CC | Beta | 0.748 | 0.042 | Chongmelaxme et al[19] |
| DC | Beta | 0.603 | 0.022 | Prakongsai et al[24] |
| HCC | Beta | 0.380 | 0.015 | Levy et al[33] |
| LT | Beta | 0.570 | 0.015 | |
| Post LT | Beta | 0.683 | 0.015 | Prakongsai et al[24] |
| Costs | ||||
| Direct medical costs, 2023; THB (USD) | ||||
| FIB-4 index | Gamma | 271.0 (7.8) | NA2 | Riewpaiboon[34] |
| SAFE score | Gamma | 355.4 (10.3) | NA2 | |
| Transient elastography | Gamma | 2000.0 (57.7) | 500.0 (14.4) | Primary data |
| Lifestyle modification program | Gamma | 1426.8 (41.2) | 356.7 (10.3) | Riewpaiboon[34] |
| Treatment, THB (USD) per year | ||||
| DM without MASLD | Gamma | 9579.6 (276.6) | 2394.9 (69.1) | Primary data (Supplementary Table 4) |
| DM with MASLDF0-F3 | Gamma | 12340.9 (356.3) | 3085.2 (89.1) | Primary data (Supplementary Table 4) |
| DM with MASLDF4/CC | Gamma | 38746.5 (1118.6) | 9686.6 (279.6) | Primary data (Supplementary Table 4) |
| DC | Gamma | 151164.1 (4363.9) | 37791.0 (1091.0) | Chongmelaxme et al[19] and Thongsawat et al[35] |
| HCC | Gamma | 184822.2 (5335.6) | 46205.5 (1333.9) | |
| LT | Gamma | 683432.3 (19729.9) | 170858.1 (4932.5) | |
| Post LT | Gamma | 110287.1 (3183.9) | 27571.8 (796.0) | |
| Direct non-medical costs, THB (USD) per visit | ||||
| Secondary hospital | ||||
| Food | Gamma | 33.1 (1.0) | 4.0 (0.1) | Riewpaiboon[34] |
| Transportation | Gamma | 91.2 (2.6) | 5.2 (0.1) | |
| Tertiary hospital | ||||
| Food | Gamma | 66.2 (1.9) | 6.7 (0.2) | Riewpaiboon[34] |
| Transportation | Gamma | 179.7 (5.2) | 14.6 (0.4) | |
Performance characteristics of screening tests: The performance characteristics of screening tests for detecting significant fibrosis—specifically sensitivity and specificity—were derived from published literatures, using liver biopsy as the reference standard. For the FIB-4 index and SAFE score, a large multicenter study involving individual patient data from 3630 patients with biopsy-proven MASLD reported sensitivities of 63.6%-68.7% for the FIB-4 index and 80.1%-90.6% for the SAFE score at cutoff values of FIB-4 ≥ 1.3 and SAFE ≥ 0, respectively[18]. Sensitivity and specificity values for the base-case analysis were based on pooled data using a random-effects meta-analysis model which was performed in Stata Statistical Software, release 17.0 (StataCorp LLC, College Station, TX, United States; Supplementary Figures 1-4). Specificities were then calculated using the given prevalence, sensitivities, and negative predictive values. For TE, performance estimates for detecting significant fibrosis were obtained from a meta-analysis by Selvaraj et al[10]. They reported a pooled sensitivity and specificity of 80% (95%CI: 76%-83%) and 73% (95%CI: 68%-77%), respectively.
Epidemiological data: Prevalence and incidence data for MASLD and significant fibrosis were obtained from a previous economic evaluation in Thailand[12] and a meta-analysis by Park et al[21]. The meta-analysis, which pooled incidence rates from 32 primary studies involving over one million patients in South Korea, provided the primary estimate for incidence. Moreover, to capture age-related variations in the prevalence within our target population, we calculated age-specific prevalence proportions of MASLD with significant fibrosis using primary data from 670 patients with DM who underwent TE at Siriraj Hospital between 2018 and 2023 (Supplementary Table 2).
Tp: Tps for the Markov model, representing the likelihood of transitioning between health states, were obtained from the most recent systematic reviews and meta-analyses[22,23]. Progression and regression probabilities across MASLD fibrosis stages (F0 to F4) were calculated based on a meta-analysis by Le et al[22]. This meta-analysis pooled disease state Tp across the spectrum of MASLD from 54 primary studies conducted globally. The Tps for patients progressing to DC and beyond were derived from a systematic review by Gruneau et al[23], which reported the Tps used in 28 previous economic models. Additionally, liver transplantation was not considered for patients over 70 years of age. This assumption was made in consistent with Thai clinical practice context[24].
Treatment effectiveness: Base-case modeling assumed that every patient with a positive screening result for MASLD with significant fibrosis would begin a treatment regimen of lifestyle modification and structured weight-loss counseling. The effectiveness of this program was represented as a relative risk reduction in the progression rate from MASLDF2 through DC. This relative risk reduction was calculated based on the liver fibrosis outcomes associated with various levels of weight reduction, as well as the reported proportion of patients achieving different degrees of weight reduction, as detailed in the study by Vilar-Gomez et al[8]. The effect of treatment was assumed to persist until patients progressed beyond DC. We also tested how reduced adherence affected cost-effectiveness by lowering the adherence rate to as little as 60% in scenario analyses.
Mortality: Mortality rates specific to health states and causes of death (CVDs, liver-related events, and other causes) were derived from multiple sources: World Health Organization Life Tables[25], Krairittichai and Potisat[26], Gruneau et al[23], Taylor et al[27], Ng et al[5,28], and Tampi et al[29]. Mortality rates among patients with LTs were based on data from Thai cohorts[30,31]. Details on the mortality rates used in the analysis are provided in Supplementary Table 3.
All rates were converted to Tps using the following equation: Probability = 1 - exp(-rate × time).
Utilities: Utility values of patients with DM, with and without MASLD, were sourced from prior economic evaluations performed within the Thai context[19,32]. For patients with DC, HCC, and those who had undergone liver transplantation, utility values were derived from observational studies conducted in Thai patients[24,33].
Costs: A micro-costing method was employed to calculate the costs associated with the FIB-4 index and SAFE score[34]. However, because a standard cost for TE was not available, the TE cost adopted form Thai university hospitals (i.e. Siriraj Hospital and Songklanagarind Hospital). Additionally, primary data analysis from the electronic database of Siriraj Hospital, which included 1105 patients with DM who underwent TE, was conducted to summarize the costs of DM treatment and MASLD management (Supplementary Table 4). Both outpatient visit and inpatient admission-related costs were considered in the analysis. These costs comprised laboratory tests, procedures, medications, medical devices, services, and fees.
Costs for managing advanced-stage disease—including DC, HCC, and liver transplantation—were obtained from published local literatures[19,35]. To align with a societal perspective, direct medical costs were combined with direct non-medical costs (specifically, food and transportation). These direct non-medical costs were calculated using visit frequency data from the primary data analysis and unit costs obtained from the Standard Cost Lists for Health Technology Assessment in Thailand[34]. All costs were adjusted to 2023 values using the consumer price index[36].
All input parameters are shown in Table 1.
The models were tested for technical accuracy by verifying all calculations and algorithms. Face validity was established through expert review, ensuring that analysis framework, model structures and assumptions, input parameters, and outputs aligned with expected trends based on current knowledge. Internal validation was performed to ensure that mathematical calculations, algorithms, and programming were free from errors.
For external validation, model predictions were compared against real-world data related to MASLD and its consequences, particularly the HCC incidence, from previously published studies. Additionally, results were cross-referenced with findings from similar published models addressing MASLD screening cost-effectiveness in similar contexts to assess convergent validity.
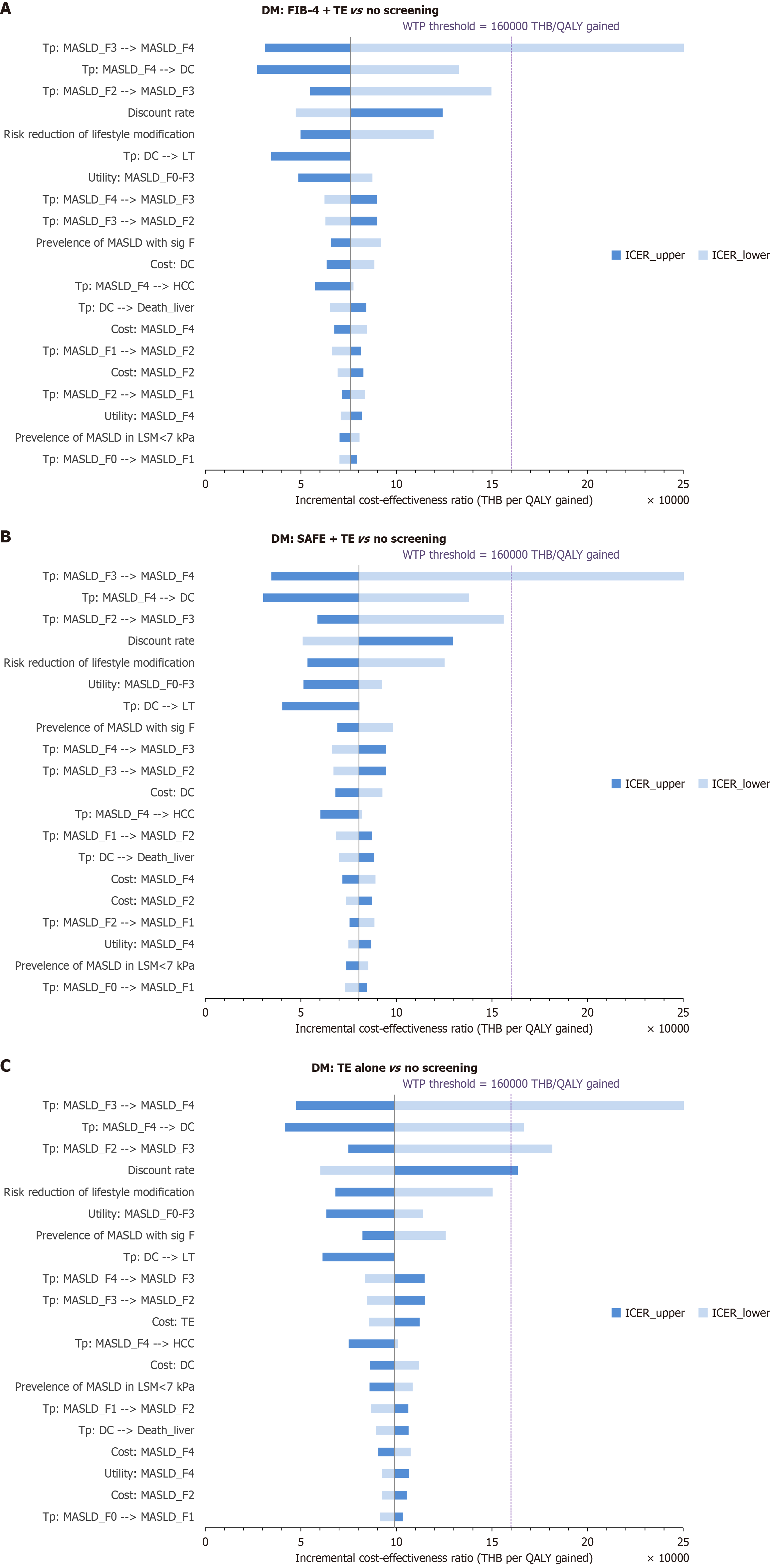
A one-way deterministic sensitivity analysis was performed to assess the impact of varying individual input parameters across their 95%CIs to determine their influence on the ICERs. We also varied the discount rate from 0% to 6% to evaluate its impact on the ICERs. Tornado diagrams were used to illustrate the results.
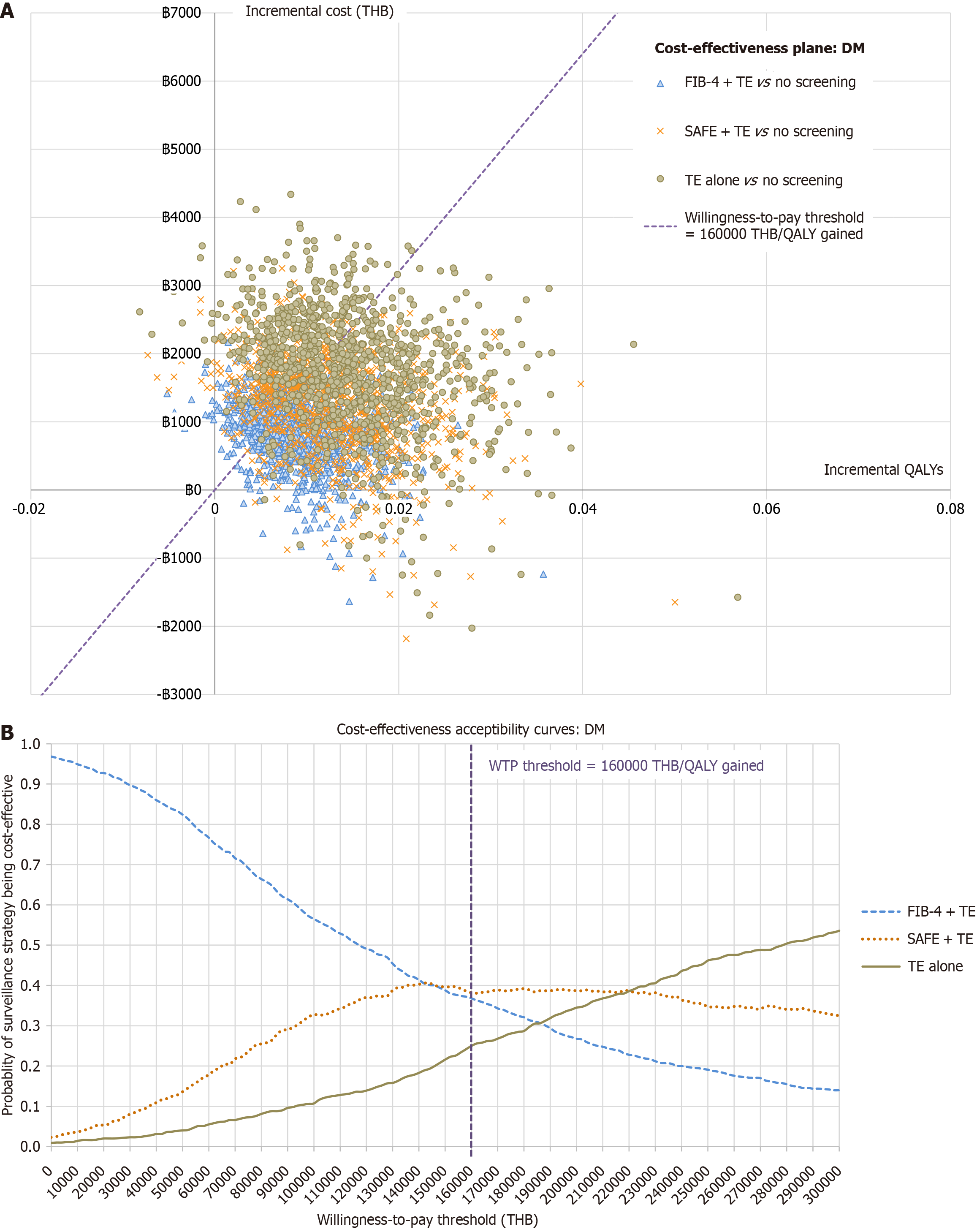
A probabilistic sensitivity analysis (PSA) was conducted using 1000 Monte Carlo simulations. Each input parameter was assigned a probability distribution based on the recommendation of the Health Technology Assessment guideline[15]. A beta distribution was used for test performance and Tps, while effectiveness of the treatment was assigned a log-normal distribution. Additionally, a gamma distribution was used for costs. The resulting distribution of ICERs is presented on cost-effectiveness planes and cost-effectiveness acceptability curves.
A budget impact analysis (BIA) was conducted from the payer perspective to estimate the potential financial implications of implementing a screening policy for significant fibrosis in patients with DM over a 5-year time horizon. The target population size was derived from the total Thai population[37], accounting for the proportion eligible for screening based on the prevalence and incidence of DM. Screening rates were assumed to be 80% when using the clinical scoring systems, with 90% of individuals with FIB-4 ≥ 1.3 or SAFE ≥ 0 undergoing subsequent TE. In a scenario where TE was used as a standalone screening tool, a screening rate of 80% was applied. The outcomes were presented as the average annual budget and the total 5-year budget in THB and USD required to implement the screening policy.
The cost-utility analysis demonstrated that all screening strategies for significant fibrosis detection were cost-effective compared to no screening, with ICERs below Thailand's WTP threshold of 160000 THB (4619 USD) per QALY gained. No screening option had the lowest cost and QALYs. FIB-4 + TE yielded the lowest ICER of 75961 THB (2193 USD) per QALY gained. TE alone provided the highest QALYs at the highest incremental cost, with an ICER of 98964 THB (2857 USD) per QALY gained. The details of total lifetime costs and QALYs are shown in Table 2 and in the cost-effectiveness frontier plot (Supplementary Figure 5).
| Outcome | No screening | FIB-4 + TE | SAFE + TE | TE alone |
| Life expectancy (years) | 25.30 | 25.33 | 25.33 | 25.34 |
| Total lifetime cost, THB (USD) | 198681.9 (5735.72) | 199481.6 (5758.8) | 199883.2 (5770.4) | 200402.6 (5785.4) |
| Total QALYs | 12.788 | 12.798 | 12.803 | 12.805 |
| Compared to no screening | ||||
| Incremental cost, THB (USD) | 799.7 (23.1) | 1201.3 (34.7) | 1720.7 (49.7) | |
| Incremental QALYs | 0.011 | 0.015 | 0.017 | |
| ICER, THB (USD) per QALY gained | 75961.0 (2192.9) | 80384.5 (2320.6) | 98964.5 (2857.0) | |
| Interpretation | Cost-effective1 | Cost-effective1 | Cost-effective1 | |
| Extended dominance analysis | ||||
| Incremental cost, THB (USD) | 799.7 (23.1) | 401.6 (11.6) | 519.3 (15.0) | |
| Incremental QALYs | 0.011 | 0.004 | 0.002 | |
| ICER, THB (USD) per QALY gained | 75961.0 (2192.9) | 90927.9 (2625.0) | 212678.2 (6139.8) | |
| Interpretation | Cost-effective1 | Cost-effective2 | Not cost-effective3 | |
However, in the extended dominance analysis, SAFE + TE would be the favored option because it provided the highest QALY with an ICER below the current WTP threshold. In contrast, the ICER of TE alone compared to SAFE + TE exceeds the threshold, making it not cost-effective (Table 2).
Scenario analyses, varying the age at screening from 30-80 years, indicated that screening becomes more cost-effective at younger ages. As compared to no screening, at a screening age of 70 years and older, TE alone would no longer be considered a cost-effective option. Furthermore, the screening policy involving non-invasive clinical scoring systems would not be cost-effective when applied in patients aged 80 years and older.
Even when adherence falls to 60%, all three screening strategies remain cost-effective. Threshold analyses showed that FIB-4 + TE, SAFE + TE, and TE alone cease to be cost-effective if adherence drops below 31.3%, 32.9%, and 48.2%, respectively. Supplementary Table 5 depicts how varying adherence alters ICERs.
The analysis framework, model structures and assumptions, input parameters, and outputs were validated by an expert in the gastrointestinal and hepatology fields who is also an economist. The extreme value testing and tracing method were employed to verify algorithmic accuracy in the Excel 365[15].
For external validation, predicted incidence rates of MASLD and HCC were compared against estimates from a published meta-analysis and a review. Our model projected a MASLD incidence rate of 45.6 cases per 1000 person-years, which is consistent with the rate of 42.8 cases per 1000 person-years (95%CI: 38.6-47.1) reported by Park et al[21]. The annual HCC incidence rate predicted by the model ranged from 1.08%-1.10% depending on the screening strategy. This range aligns with the HCC incidence rates of 0.50%-2.26% in patients with metabolic dysfunction-associated steatohepatitis cirrhosis from India and Japan, as reported by Huang et al[38].
One-way sensitivity analysis: One-way sensitivity analysis, examining the ICER of the screening strategies compared to no screening, revealed that the model was most sensitive to variations in the Tps from MASLDF3 to MASLDF4, from MASLDF4 to DC, and from MASLDF2 to MASLDF3, respectively. The discount rate and the effectiveness of lifestyle modification were identified as the fourth- and fifth-most influential parameters (Figure 2).
PSA: PSA demonstrated the overall robustness of the analyses. The cost-effectiveness plane (Figure 3A) showed a substantial proportion of simulations falling below the WTP threshold: FIB-4 + TE 68.4%, SAFE + TE 66.7%, and TE alone 60.2%. The percentages represent probability of each screening strategy being cost-effective compared to no screening. When compared among screening strategies, the cost-effectiveness acceptability curves (Figure 3B) showed that FIB-4 + TE and SAFE + TE had a similar probability of being cost-effective at the current WTP threshold. TE alone had a higher probability of being cost-effective when the WTP threshold rises and would become the most cost-effective option at a WTP threshold above 220000 THB (6351 USD) per QALY gained.
The BIA estimated the additional budget required for each screening strategy among Thai patients with DM aged 50-79 years. Given the five-year time horizon of the analysis, the eligible population was estimated to be approximately 2 million patients with DM, resulting in an annual budget impact ranging from 471-756 million THB (14-22 million USD). FIB-4 + TE was the strategy with the lowest budget impact. Unfortunately, SAFE + TE resulted in a higher budget impact than TE alone due to the large number of patients with positive SAFE scores requiring further evaluation. The estimated budget encompasses both the laboratory tests used to calculate the SAFE score and the cost of performing TE. The details are shown in Table 3.
| Parameter | Y1 | Y2 | Y3 | Y4 | Y5 | Total |
| Total population | 21348831 | 21348831 | ||||
| New population | 1028431 | 1011655 | 1033245 | 1046481 | 4119812 | |
| Patients with DM | 2113534 | 9331 | 9179 | 9375 | 9495 | 2150915 |
| Patients evaluated with scoring system | 1902181 | 8398 | 8261 | 8438 | 8546 | 1935824 |
| FIB-4 + TE | ||||||
| Patients with FIB-4 ≥ 1.3 | 1004193 | 2990 | 2941 | 3004 | 3042 | 1016170 |
| Patients undergo TE | 903774 | 2691 | 2647 | 2703 | 2738 | 914553 |
| Total BIA, THB (USD) | 2323021957 (67062996) | 7657269 (221057) | 7532362 (217451) | 7693112 (222091) | 7791661 (224936) | 2353696361 (67948531) |
| Average annual budget, THB (USD) | 470739272 (13589706) | |||||
| SAFE + TE | ||||||
| Patients with SAFE ≥ 0 | 1687835 | 7338 | 7219 | 7373 | 7467 | 1717232 |
| Patients undergo TE | 1519052 | 6604 | 6497 | 6635 | 6720 | 1545509 |
| Total BIA, THB (USD) | 3714212955 (107225095) | 16194000 (467502) | 15929840 (459876) | 16269803 (469691) | 16478221 (475707) | 3779084819 (109097872) |
| Average annual budget, THB (USD) | 755816964 (21819574) | |||||
| TE alone | ||||||
| Patients undergo screening | 1690827 | 7465 | 7343 | 7500 | 7596 | 1720732 |
| Total BIA, THB (USD) | 3381654830 (97624521) | 14930254 (431019) | 14686708 (423989) | 15000141 (433037) | 15192295 (438584) | 3441464227 (99351150) |
| Average annual budget, THB (USD) | 688292845 (19870230) | |||||
This comprehensive research significantly contributes to the evidence base for the cost-effectiveness of screening strategies for detecting significant fibrosis in patients with DM in Thailand. The screening policies involving TE for detecting significant fibrosis in patients with DM were found to be cost-effective.
Among the three screening strategies analyzed, TE alone provided the highest QALYs. However, its standalone application incurs an ICER of 212678 THB (6140 USD) per QALY gained relative to SAFE + TE, rendering it not cost-effective at the current Thai WTP threshold. As a result, SAFE + TE is considered the favored option with an ICER of 90928 THB (2625 USD) per QALY gained when compared to FIB-4 + TE. However, sensitivity analysis revealed that FIB-4 + TE had the highest probability of being cost-effective (68.4%) and produced the lowest budget impact. Although SAFE + TE and TE alone maximize QALYs, FIB-4 + TE appears more practical because its higher specificity lowers false-positive referrals and overall screening costs. If FIB-4 index precedes TE, national TE use falls by 47%, a critical advantage given Thailand’s limited TE capacity. There are only approximately 120 TE devices, mostly in university hospitals, tertiary care centers, and large private hospitals, making access especially limited in rural areas. These findings echo guidance from professional societies, such as the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases, which advocate for non-invasive scoring systems like FIB-4 index as an initial step before resource-intensive procedures like TE[6,7].
Previous research on the cost-effectiveness of TE has predominantly been conducted in high-income countries, where healthcare systems and economic conditions differ significantly from those in LMICs[39-41]. Studies from Europe and Asia have demonstrated that TE is a cost-effective screening tool for detecting liver fibrosis, particularly in populations at high risk for MASLD or alcohol-related liver disease. For example, TE was found to outperform fibrosis scores in terms of diagnostic accuracy and had ICERs ranging from 2570-6217 Euro (2800-7000 USD) per QALY gained in these populations[39,42]. However, the applicability of these findings to LMICs, including Thailand, requires careful consideration. Key considerations include the limited availability of equipment, variations in healthcare costs, and differences in other epidemiological parameters. Economic evaluations should reflect the healthcare infrastructure and budget constraints of the studied region. Based on the present study in Thailand, TE alone deems cost-effective for screening significant fibrosis in patients with DM. Yet, FIB-4 + TE may be the most appropriate strategy. This highlights the importance of prioritizing non-invasive clinical scoring systems like FIB-4 index in resource-limited settings.
Previous studies in Thailand were conducted to evaluate the cost-effectiveness of MASLD screening using ultrasonography and of various treatments for MASLD. Phisalprapa et al[13] previously assessed MASLD screening in Thailand, providing foundational evidence for the economic impact of the disease and the potential benefits of early detection. They reported that MASLD screening by ultrasonography is cost-effective. Nevertheless, they are also in support of the recommendation of utilizing non-invasive clinical scoring systems. Chongmelaxme et al[19] examined the cost-effectiveness of MASLD treatments in Thailand, focusing on pharmacotherapy and lifestyle interventions, and concluded that lifestyle modification is cost-effective. Additionally, the recent cost-utility analysis has shown that using non-invasive tests, particularly FIB-4 index and TE, to initiate HCC surveillance in MASLD patients is cost-effective[43]. These studies collectively underscore the importance and cost-effectiveness of screening strategies for liver fibrosis detection using non-invasive tests. Furthermore, it is observed that post-screening management plays a crucial role in enhancing the overall cost-effectiveness of these strategies.
Globally, TE has demonstrated promise as a cost-effective tool for liver fibrosis screening across various healthcare systems. However, adopting TE to LMICs poses challenges, including limited accessibility of the equipment and related healthcare personnel, along with its relatively high cost. In Thailand, FIB-4 + TE approach is estimated to decrease annual budget by approximately 217.5 million THB (6.3 million USD) per year, representing a 31.6% reduction compared to a TE alone screening strategy. Furthermore, the FIB-4 index, along with other clinical scoring systems typically calculated using demographic data and routine laboratory parameters, should not incur additional costs in clinical practice. If these parameters for FIB-4 index are excluded from the cost calculation, the annual budget drops further from 471 million THB (14 million USD) to 366 million THB (11 million USD). Moreover, real-world screening rates are likely lower than the assumed 80%-90%. If only 50% of patients access the clinical scoring system and 65% of those with scores above the cutoff value undergo further investigation by TE, the budget impact decreases to 206 million THB (6 million USD) annually. This scenario is likely to reflect the actual budget that would occur if the FIB-4 + TE screening policy were implemented. Additionally, expanded utilization of existing TE could improve its cost-effectiveness by lowering the unit cost per screening test through economies of scale. To further improve accessibility, especially in remote areas, the deployment of mobile TE screening units may be a practical and effective solution[44].
To the best of our knowledge, this study is the first in LMICs to evaluate the cost-effectiveness and estimate the budget impact of screening strategies involving TE for detecting significant fibrosis in patients with DM. This research provides a valuable contribution to the field of health economics, particularly in Thailand, by informing strategies for screening significant fibrosis in patients with DM. The strengths of this analysis lie in its comprehensive approach, which evaluates multiple screening strategies using up-to-date and context-specific input parameters derived from a thorough literature review. This study provides a holistic understanding of the disease's impact, encompassing both hepatic and non-hepatic-related consequences. By analyzing various common ages at screening in scenario analyses, the findings offer policymakers valuable information for the decision-making process. Notably, screening younger patients tends to be more cost-effective, although it would incur higher costs due to a larger eligible population. Policymakers must weigh these trade-offs carefully, balancing cost-effectiveness with budgetary constraints.
Despite its strengths, this research also has several limitations. Due to limited available evidence, the economic evaluations did not account for pharmacologic interventions for patients with significant fibrosis. Additionally, the effectiveness of lifestyle modifications used in the analysis was based on the study conducted outside Thailand, which may not fully reflect local contexts. However, sensitivity analyses indicated that even if the effectiveness of lifestyle modifications varied within a reasonable range, the overall findings would remain robust.
Our analysis evaluated the entire DM population rather than prespecified subgroups. Cost-effectiveness could differ in cohorts defined by glycemic control, disease duration, or comorbidity burden. Longitudinal data remain insufficient to construct separate models, yet deterministic sensitivity analyses indicate that faster fibrosis progression—likely in poorly controlled DM[45]—is associated with greater screening benefit. Moreover, glucose-lowering agents such as glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors improve MASLD outcomes[6,7]. These pharmacologic effects may alter the real-world value of fibrosis screening, emphasizing the need to integrate current treatment patterns when refining future models.
Our analysis framework considered only a one-time screening approach using the FIB-4 index, SAFE score, and TE, which may not capture the long-term cost-effectiveness of repeated or continuous screening strategies. Nevertheless, recommendations regarding optimal intervals for repeated screenings are yet to be established. Other non-invasive scores and imaging modalities were omitted due to limited availability and a lack of adequately validated data in Thailand. For example, MRE—a device with superior diagnostic accuracy compared to TE—was not included in this study because it requires greater resources, including longer examination times, higher equipment costs, and interpretation by a radiologist or trained specialist, which limits its accessibility in the country.
Although evidence suggests that non-cirrhotic patients can progress to HCC[46,47] at an incidence rate of 0.1-1.3 cases per 1000 patient-years[38], this pathway was not incorporated into our model. As a result, the analysis may slightly underestimate the health-related consequences of significant fibrosis. Finally, the generalizability of our findings may be limited by the use of single-center data for estimating some treatment costs. Nevertheless, since Siriraj Hospital is one of the largest university hospitals in Thailand, adopting its cost data should provide a valid and sufficiently accurate representation of the Thai context.
Further research is needed to evaluate repeated screening strategies, explore novel treatments, and validate the findings. Additionally, the analysis should be re-performed if there are any updates in any aspect related to the findings.
In conclusion, while implementing screening for significant fibrosis among patients with DM in Thailand is deemed cost-effective, the feasibility of national-level implementation remains a critical consideration. This research demonstrates that the FIB-4 + TE strategy offers a balanced approach, providing a high probability of cost-effectiveness with the lowest budget impact. Given the limited availability of TE, these findings support the adoption of the FIB-4 index as a practical and economically sound triaging tool for further investigation by TE. This approach aligns with the overarching goal of early disease detection and accessible treatment, ensuring that healthcare resources are optimized to improve patient outcomes in Thailand.
We would like to express our sincere appreciation to Euarat Mepramoon for her invaluable contributions to project coordination. During the preparation of this manuscript the authors used Perplexity AI and ChatGPT by OpenAI in order to enhance the readability of the first draft of this manuscript. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
| 1. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1631] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7921] [Article Influence: 792.1] [Reference Citation Analysis (8)] |
| 3. | En Li Cho E, Ang CZ, Quek J, Fu CE, Lim LKE, Heng ZEQ, Tan DJH, Lim WH, Yong JN, Zeng R, Chee D, Nah B, Lesmana CRA, Bwa AH, Win KM, Faulkner C, Aboona MB, Lim MC, Syn N, Kulkarni AV, Suzuki H, Takahashi H, Tamaki N, Wijarnpreecha K, Huang DQ, Muthiah M, Ng CH, Loomba R. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72:2138-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 4. | Harris R, Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol. 2017;2:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 5. | Ng CH, Lim WH, Hui Lim GE, Hao Tan DJ, Syn N, Muthiah MD, Huang DQ, Loomba R. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21:931-939.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 955] [Article Influence: 477.5] [Reference Citation Analysis (1)] |
| 7. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1573] [Article Influence: 524.3] [Reference Citation Analysis (1)] |
| 8. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1774] [Article Influence: 161.3] [Reference Citation Analysis (3)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (1)] |
| 10. | Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, Palaniyappan N, Liu CH, Aithal GP, Romero-Gómez M, Brosnan MJ, Tuthill TA, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M; LITMUS Investigators. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021;75:770-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (3)] |
| 11. | Deb R, Goswami S, Sengupta N, Baidya A, Khare VR, Datta J, Das M, Ray D. Redefining Liver Fibrosis Risk Assessment in Indians with Type 2 Diabetes: New FIB-4 Score Cutoff for Optimizing Sequential Assessment with Transient Elastography. Indian J Endocrinol Metab. 2025;29:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Phisalprapa P, Prasitwarachot R, Kositamongkol C, Hengswat P, Srivanichakorn W, Washirasaksiri C, Treeprasertsuk S, Charatcharoenwitthaya P, Chaiyakunapruk N. Economic burden of non-alcoholic steatohepatitis with significant fibrosis in Thailand. BMC Gastroenterol. 2021;21:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Phisalprapa P, Supakankunti S, Charatcharoenwitthaya P, Apisarnthanarak P, Charoensak A, Washirasaksiri C, Srivanichakorn W, Chaiyakunapruk N. Cost-effectiveness analysis of ultrasonography screening for nonalcoholic fatty liver disease in metabolic syndrome patients. Medicine (Baltimore). 2017;96:e6585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Husereau D, Drummond M, Augustovski F, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, de Bekker-Grob E, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu RF, Staniszewska S; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Guideline Development Working Group. Health Technology Assessment Guideline for Thailand 3rd edition. 2019. [cited 4 June 2025]. Available from: https://www.hitap.net/documents/186501. |
| 16. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1609] [Article Influence: 536.3] [Reference Citation Analysis (70)] |
| 17. | Li G, Lin H, Sripongpun P, Liang LY, Zhang X, Wong VWS, Wong GLH, Kim WR, Yip TCF. Diagnostic and prognostic performance of the SAFE score in non-alcoholic fatty liver disease. Liver Int. 2024;44:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Alkhouri N, Cheuk-Fung Yip T, Castera L, Takawy M, Adams LA, Verma N, Arab JP, Jafri SM, Zhong B, Dubourg J, Chen VL, Singal AK, Díaz LA, Dunn N, Nadeem R, Wai-Sun Wong V, Abdelmalek MF, Wang Z, Duseja A, Almahanna Y, Omeish HA, Ye J, Harrison SA, Cristiu J, Arrese M, Robert S, Lai-Hung Wong G, Bajunayd A, Shao C, Kubina M, Dunn W. ALADDIN: A Machine Learning Approach to Enhance the Prediction of Significant Fibrosis or Higher in Metabolic Dysfunction-Associated Steatotic Liver Disease. Am J Gastroenterol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Chongmelaxme B, Phisalprapa P, Sawangjit R, Dilokthornsakul P, Chaiyakunapruk N. Weight Reduction and Pioglitazone are Cost-Effective for the Treatment of Non-Alcoholic Fatty Liver Disease in Thailand. Pharmacoeconomics. 2019;37:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Mahidol University. Statistical Report 2023. [cited 4 June 2025]. Available from: https://si.mahidol.ac.th/th/division/mrst/admin/download_files/18_130_1YTG5TI.pdf. |
| 21. | Park J, Lee EY, Li J, Jun MJ, Yoon E, Ahn SB, Liu C, Yang H, Rui F, Zou B, Henry L, Lee DH, Jun DW, Cheung RC, Nguyen MH. NASH/Liver Fibrosis Prevalence and Incidence of Nonliver Comorbidities among People with NAFLD and Incidence of NAFLD by Metabolic Comorbidities: Lessons from South Korea. Dig Dis. 2021;39:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Le P, Payne JY, Zhang L, Deshpande A, Rothberg MB, Alkhouri N, Herman W, Hernandez AV, Schleicher M, Ye W, Dasarathy S. Disease State Transition Probabilities Across the Spectrum of NAFLD: A Systematic Review and Meta-Analysis of Paired Biopsy or Imaging Studies. Clin Gastroenterol Hepatol. 2023;21:1154-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 23. | Gruneau L, Ekstedt M, Kechagias S, Henriksson M. Disease Progression Modeling for Economic Evaluation in Nonalcoholic Fatty Liver Disease-A Systematic Review. Clin Gastroenterol Hepatol. 2023;21:283-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Prakongsai P, Pachanee K, Wongphan T. Economic evaluation of liver transplantation for moderate to severe liver cirrhosis patients in universal health coverage. 2016. [cited 4 June 2025]. Available from: https://kb.hsri.or.th/dspace/handle/11228/5204. |
| 25. | World Health Organization. Life Tables by Country: Thailand [Internet]. 2019. [cited 20 March, 2024]. Available from: https://apps.who.int/gho/data/view.searo.61640?lang=en. |
| 26. | Krairittichai U, Potisat S. Survival Rates and Mortality Risk Factors of Thai Patients with Type 2 Diabetes Mellitus. J Med Assoc Thai. 2017;100 Suppl 1:S8-15. [PubMed] |
| 27. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 798] [Article Influence: 133.0] [Reference Citation Analysis (1)] |
| 28. | Ng CH, Chan KE, Chin YH, Zeng RW, Tsai PC, Lim WH, Tan DJH, Khoo CM, Goh LH, Ling ZJ, Kulkarni A, Mak LL, Huang DQ, Chan M, Chew NW, Siddiqui MS, Sanyal AJ, Muthiah M. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2022;28:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Tampi RP, Wong VW, Wong GL, Shu SS, Chan HL, Fung J, Stepanova M, Younossi ZM. Modelling the economic and clinical burden of non-alcoholic steatohepatitis in East Asia: Data from Hong Kong. Hepatol Res. 2020;50:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Kositamongkol P, Sanphasitvong V, Sirivatanauksorn Y, Pongpaibul A, Limsrichamrern S, Mahawithitwong P, Asavakarn S, Tovikkai C, Dumronggittigule W. Outcome of Liver Transplantation in Hepatocellular Carcinoma Patients at Siriraj Hospital. Transplant Proc. 2017;49:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Sirivatanauksorn Y, Kongkaewpaisan N, Pongpaibul A, Limsrichamrern S, Mahawithitwong P, Kositamongkol P, Tovikkai C, Asavakarn S. Outcomes of orthotopic liver transplantation in non-malignant end-stage liver diseases. Transplant Proc. 2014;46:778-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Deerochanawong C, Krittayaphong R, Romano JGU, Rhee NA, Permsuwan U. Cost-Utility of Liraglutide Plus Standard of Care Versus Standard of Care in People with Type 2 Diabetes and Cardiovascular Risk in Thailand. Diabetes Ther. 2023;14:531-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, Bzowej N, Briggs AH. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Riewpaiboon A. Standard cost lists for health technology assessment [Internet]. Health Intervention and Technology Assessment Program (HITAP). 2011. [cited 20 February, 2024]. Available from: https://costingmenu.hitap.net/. |
| 35. | Thongsawat S, Piratvisuth T, Pramoolsinsap C, Chutaputti A, Tanwandee T, Thongsuk D. Resource Utilization and Direct Medical Costs of Chronic Hepatitis C in Thailand: A Heavy but Manageable Economic Burden. Value Health Reg Issues. 2014;3:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | World Bank Group. World Development Indicators. [cited 20 March, 2024]. Available from: https://databank.worldbank.org/source/2?series=FP.CPI.TOTL.ZG&country=THA&savedlg=1#. |
| 37. | The Bureau of Registration Administration (BORA). Official statistics registration systems, 2012-2022 [Internet]. 2019. [cited 20 March, 2024]. Available from: https://stat.bora.dopa.go.th/stat/statnew/statMONTH/statmonth/#/displayData. |
| 38. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1365] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 39. | van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, Brener S, Moher D, Thavorn K. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int. 2017;37:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Johansen P, Howard D, Bishop R, Moreno SI, Buchholtz K. Systematic Literature Review and Critical Appraisal of Health Economic Models Used in Cost-Effectiveness Analyses in Non-Alcoholic Steatohepatitis: Potential for Improvements. Pharmacoeconomics. 2020;38:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Younossi ZM, Paik JM, Henry L, Stepanova M, Nader F. Pharmaco-Economic Assessment of Screening Strategies for High-Risk MASLD in Primary Care. Liver Int. 2025;45:e16119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Serra-Burriel M, Graupera I, Torán P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, Fabrellas N, Arslanow A, Expósito C, Hernández R, Lai-Hung Wong G, Harman D, Darwish Murad S, Krag A, Pera G, Angeli P, Galle P, Aithal GP, Caballeria L, Castera L, Ginès P, Lammert F; investigators of the LiverScreen Consortium. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Decharatanachart P, Poovorawan K, Tangkijvanich P, Charatcharoenwitthaya P, Peeraphatdit T, Taychakhoonavudh S, Treeprasertsuk S, Chaiteerakij R. Cost-Utility Analysis of Non-Invasive Tests to Initiate Hepatocellular Carcinoma Surveillance in Metabolic Dysfunction-Associated Steatotic Liver Disease. Am J Gastroenterol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Nagappa B, Ramalingam A, Rastogi A, Dubey S, Thomas SS, Gupta E, Sarin SK. Number needed to screen to prevent progression of liver fibrosis to cirrhosis at primary health centers: An experience from Delhi. J Family Med Prim Care. 2021;10:1412-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Alfadda AA, Alqutub AN, Sherbeeni SM, Aldosary AS, Alqahtani SA, Isnani A, Gul R, Khaleel MS, Alqasim SM, Almaghamsi AM. Predictors of liver fibrosis progression in cohort of type 2 diabetes mellitus patients with MASLD. J Diabetes Complications. 2025;39:108910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 47. | Cespiati A, Cinque F, Meroni M, Lombardi R, Dongiovanni P, Fracanzani AL. An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians. Biomedicines. 2023;11:586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/