Published online Aug 28, 2025. doi: 10.3748/wjg.v31.i32.104277
Revised: March 27, 2025
Accepted: August 6, 2025
Published online: August 28, 2025
Processing time: 254 Days and 6.3 Hours
Hepatocellular carcinoma (HCC) is a global health concern, representing the second most common cause of malignancy-related mortality in the world. The primary cause of HCC in the United States is chronic infection with the hepatitis C virus (HCV). Clinical observations have established sex-based differences in HCV infection with the disease progressing more severely and more rapidly in males and postmenopausal females compared to premenopausal females, suggesting that estrogens and their receptors may play an important role in hepatic defenses and development of HCV-mediated HCC. However, the precise mechanism of estrogen protection and their effects on inflammation is poorly understood.
To determine whether estrogen receptor (ER) expression is correlated with the expression of tumor necrosis factor-alpha (TNF-α) in males and females with HCV-associated diseases.
The role of ERs in modulating innate immune responses was investigated using human liver tissues with HCV/cirrhosis and HCV/HCC. Messenger RNA (mRNA) and protein (nuclear and cytoplasmic) expression were measured for all markers of interest and compared to normal human liver tissue samples.
ERβ was reported for the first time to have a greater mRNA expression than ERα in normal liver (P ≤ 0.001). In addition, ERβ mRNA expression was found to be decreased in diseased livers (P ≤ 0.05), while TNF-α expression was increased (P ≤ 0.0001). Upon stratifying by sex within each disease group, ESR1 was found to be negatively correlated with ESR2 in females with HCV/cirrhosis (r = -0.84, P ≤ 0.001), whereas males with HCV/cirrhosis were found to have a significant positive correlation (r = 0.57, P ≤ 0.05). ESR2 mRNA expression had a significant positive correlation with TNF-α in both HCV/cirrhosis (r = 0.61, P ≤ 0.001) and HCV/HCC patients (r = 0.45, P ≤ 0.05).
All together, these findings indicate that changes in ERβ and TNF-α expression are associated with worsening disease, and may be part of the sex-dependent factors in HCC pathogenesis.
Core Tip: Our study, for the first time, demonstrates an increase in expression of estrogen receptor (ER) β compared to ERα in the liver of normal males and females. In addition, ERβ message RNA expression was found to be decreased in hepatitis C virus (HCV)/cirrhosis and HCV/hepatocellular carcinoma livers, while tumor necrosis factor-α expression was increased. Further analysis of the data revealed sex-specific correlations between ERα and ERβ. These findings suggest that changes in ERβ expression are associated with worsening HCV-related disease, and may be one of the sex-dependent factors in cirrhosis and hepatocellular carcinoma pathogenesis.
- Citation: Groover S, Addison S, Nicks S, Mwangi M, Brooks A, Kaul A, Kaul R. Sex based relative expression of estrogen receptors and tumor necrosis factor-alpha in liver affects hepatitis C virus viral pathogenesis. World J Gastroenterol 2025; 31(32): 104277
- URL: https://www.wjgnet.com/1007-9327/full/v31/i32/104277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i32.104277
Hepatocellular carcinoma (HCC) represents the second most common cause of malignancy related death in the world and its incidence is rapidly increasing, tripling in the last 4 decades[1]. In the United States alone, there are approximately 33000 new cases of HCC diagnosed per year, with 28000 people losing their life to HCC in that same time frame[2]. The most significant risk factor for development of HCC in the United States is chronic infection with hepatitis C virus (HCV)[1]. HCV is a global health concern that indiscriminately affects individuals in both underdeveloped and industrialized countries; over 2 million people in the United States alone are infected with the virus[3]. Persistent infection with HCV in the liver leads to a state of chronic inflammation, and over time can lead to problems such as fibrosis, cirrhosis, liver failure, and cancer, the most common of which is HCC[4]. HCV is a devastating cause of HCC worldwide, but especially in the United States where it represents the leading cause of HCC[1].
Interferon-based drug regimens were the foundation for HCV treatment for over 30 years, but are relatively unsuccessful at eliminating the virus and are associated with high rates of adverse side effects[4-6]. Direct-acting antivirals (DAAs) were developed in 2014 to address the shortcomings of interferon-based therapies; comparatively, DAAs have higher success rates and attenuated side effects[7]. Although the price of DAAs is decreasing as new drugs enter the market, a full treatment course can still range anywhere from 50000 Dollars to almost 100000 Dollars.
Due to the additional fact that there is currently no vaccine available for HCV, there is much interest in investigating host factors that can prevent or slow HCV-associated disease progression. Previous research has established that, compared to premenopausal females, both males and postmenopausal females are at a greater risk for developing HCV-associated diseases, and that their disease progression tends to be faster and more severe, suggesting that estrogens and their receptors may play an important role in HCV, and thereby progression to HCC[8-12].
Several epidemiological studies have reported that in females with HCV infection, lower circulating levels of estrogen are associated with a decreased response to treatment, increased expression of proinflammatory cytokines, more severe and faster rate of fibrosis, and greater risk of developing HCC[13-17]. Estrogen has also been shown to downregulate proinflammatory cytokine expression[18-20] and inhibit HCV entry and production[21-23], but, to date, causal factors linking estrogen to disease protection remain speculative. Estrogens exert their action primarily by activating nuclear estrogen receptors (ERs), of which there are two subtypes, ERα and ERβ. Previous studies in our lab have shown differential expression of ERα and ERβ in the livers of both males and females and across HCV-related disease severity[24]. Further, the diseased livers showed significantly higher expression of inflammatory marker nuclear factor kappa-B (NF-кB) and oncogenic marker cyclin D1. Given that HCV pathogenesis is almost entirely due to the ensuing chronic inflammation and not from cytotoxic factors from the virus itself, this paper reports the association between ERs and tumor necrosis factor-alpha (TNF-α) in HCV-infected subjects with cirrhosis and HCC.
TNF receptor 1 (TNFR1) plays the leading role in liver physiology[25]. TNF-α is a pleiotropic cytokine that is involved in a variety of functions in the liver such as proliferation, metabolic activation, inflammatory responses, and apoptosis. TNF-α facilitates the activation of several diverse signaling cascades, depending on its ligation to either TNFR1 or TNFR2. TNFR1 signaling can mediate the cleavage and activation of pro-apoptotic caspase-8; TNFR2 initiates the anti-apoptotic, pro-proliferative protein kinase B pathways[25,26]. Because TNF-α can initiate both cytoprotective and apoptotic responses, its role in HCV pathogenesis is unclear. Individuals with TNF-α genotypes that trigger increased production and secretion of TNF-α were associated with having an increased risk of fibrosis and cirrhosis[27], possibly due to the action of HCV proteins suppressing NF-кB activation and enhancing TNF-α-induced cell death[28].
Understanding the link between sex, ERs, and inflammation mediated by TNF-α during HCV pathogenesis will help identify new targets for therapy, further personalize treatment strategies, and recognize individuals who are at a higher risk for HCV-related HCC disease progression.
Explant liver tissues from deidentified patients with end stage liver disease due to either HCV-related cirrhosis or HCV-related HCC were obtained from the National Institutes of Health Liver Tissue and Cell Distribution System (LTCDS) at the University of Minnesota, Minneapolis, MN, United States. Normal liver tissues from LTCDS with no diagnosis of HCV or HCC obtained from cadaver donor livers not used for transplantation were included as controls. Exclusion criteria for both study and control samples included patients co-infected with human immunodeficiency virus or hepatitis B virus or with a history of alcohol consumption or drug use. Liver explants were aseptically collected and snap frozen in liquid nitrogen and stored at -80 °C until further use. The study received approval from the Institutional Review Board at the University of Minnesota under Exemption IV. The study was conducted at Oklahoma State University Center for Health Sciences under exempt Institutional Review Board guidelines.
Total RNA was extracted from all human liver tissues using TRIzol reagent (Cat No. 15596026; Invitrogen) and cleaned using the QIAGEN RNeasy mini kit (Cat No. 74106) per the manufacturer protocol. Approximately 2 μg of total RNA was reverse transcribed using Moloney-Murine Leukemia Virus Reverse Transcriptase (Cat No. M1701; Promega) and random primers per the manufacturer protocol. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed using custom primers from Invitrogen/Thermo Fisher Scientific. Reactions were carried out in triplicate with PowerUp SYBR Green Master Mix (Cat No. A25742; Applied Biosystems) on an Applied Biosystems 7500 real-time PCR system. Samples were analyzed using Applied Biosystems 7500 Software v.2.0.6. Relative differences in gene expression between groups were calculated using cycle threshold values (Ct). The expression of target genes in liver tissues was normalized to the expression of endogenous control genes beta-glucuronidase and serine and arginine-rich splicing factor 4[29-31]. The gene expression of the treatment group was further normalized to the experimental controls using the ΔΔCt method and expressed as fold change. Reaction efficiency for all targets was assessed to be 102.2% ± 4.0% (n = 2) by titrating samples and using the integrated tool of the Applied Biosystems software.
Protein concentrations were determined using the Pierce bicinchoninic acid protein assay kit (Cat No. 23225). Radio immunoprecipitation assay lysis buffer was used to prepare total protein extracts from cells. Representative liver tissues were selected from HCV/cirrhosis, HCV/HCC, and normal patient groups. Cytoplasmic, nuclear, and total protein fractions were prepared from liver tissues using the Thermo Fisher Scientific NE-PER Nuclear and Cytoplasmic Extraction Reagents (Cat No. 78833) or T-PER™ Tissue Protein Extraction Reagent (Cat No. 78510) as per the manufacturer’s instructions.
Equivalent protein (25-50 μg) per sample was loaded and separated on a 4%-12% gradient Bis-Tris polyacrylamide gels. Gels were then wet transferred to a nitrocellulose membrane, stained with REVERT total protein stain [Cat No. 926-11015; Odyssey CLx Near-Infrared Fluorescence Imaging System (LI-COR)] to verify complete transfer of proteins, and imaged for loading control. Blots were rinsed and blocked with intercept blocking buffer in the Bethesda system (Cat No. 927-60001, LI-COR). Blots were incubated overnight at 4 °C while shaking with the indicated primary antibody (Table 1). Blots were rinsed and incubated for 1 hour at room temperature while shaking with the appropriate secondary antibody. Blots were rinsed and imaged on a LI-COR. Image capture and densitometry were performed using Image Studio Lite v.5.2 software. Data were normalized to total protein[32,33]. Anti-histone H3 and anti-glyceraldehyde-3-phosphate dehydrogenase antibodies were used to confirm purity of cytoplasmic and nuclear fractions.
| Antibody | Catalog | Host/class/isotype | Concentration | Observed size (kDa) in human liver |
| Anti-ERβ | Abcam; ab3576 | Rabbit/polyclonal/IgG | 1:1000; (1:30000 2nd) | 70 |
| Anti-TNF-α | Abcam; ab1793 | Mouse/monoclonal/IgG1 | 1:1000; (1:10000 2nd) | 45 |
| Anti-ERα | Invitrogen; MA5-13191 | Mouse/monoclonal/IgG1 | 1:500; (1:10000 2nd) | 37 |
| Anti-Histone H3 | Abcam; ab1791 | Rabbit/polyclonal/IgG | 1:1000; (1:20000 2nd) | 15 |
| Anti-GAPDH (0411) | Santa Cruz; sc-47724 | Mouse/monoclonal/IgG1 | 1:1000; (1:10000 2nd) | 50 |
| Anti-rabbit IRDye® 800CW | LI-COR; 926-32213 | Donkey/IgG | Varies | |
| Anti-mouse IRDye® 800CW | LI-COR; 926-32212 | Donkey/IgG | Varies | |
| Recombinant human TNF-α | Abcam; ab55237 | 10 ng/well | 25 |
Data obtained from the experiments were analyzed statistically and graphed using GraphPad Prism version 10 (GraphPad Software Inc, La Jolla, CA, United States). Characteristics of the study population were compared using the χ2 test. Statistical significance between appropriate groups in in vitro data was determined by parametric one-way analysis of variance, and post-hoc analyses were performed using Dunnett’s multiple comparisons test. Ex vivo data were analyzed using non-parametric methods that included the Kruskall-Wallis test and Mann Whitney U test; post-hoc analyses were performed using Dunn’s Multiple Comparison test. Correlations between ER subtype expression and expression of inflammatory markers in liver tissues were analyzed by using the Spearman’s rank correlation test. P ≤ 0.05 was considered statistically significant.
A total of 55 cases (HCV/cirrhosis, n = 32; HCV/HCC, n = 23) and 36 normal controls were included in the analyses. Cases and controls did not differ significantly in the distribution of age, sex, or race (Table 2). Normal and cirrhosis males were slightly younger than their female counterparts, whereas HCC females were, on average, 10 years older than HCC males (Table 3). However, the medians were not significantly different between the groups.
| Characteristics | Normal (n = 36) | Cirrhosis (n = 32) | HCC (n = 23) | P value | |||
| n | (%) | n | (%) | n | (%) | ||
| Age (year) | |||||||
| < 49 | 10 | (27.8) | 12 | (37.5) | 6 | (26.1) | 0.2593 |
| 50-59 | 15 | (41.7) | 17 | (53.1) | 10 | (43.5) | |
| ≥ 60 | 11 | (30.6) | 3 | (9.4) | 7 | (30.4) | |
| Mean age (year) | 52.0 ± 14.6 | 52.2 ± 6.8 | 55.5 ± 7.1 | 0.3087 | |||
| Sex | |||||||
| Male | 18 | (50.0) | 17 | (53.1) | 18 | (78.3) | 0.0765 |
| Female | 18 | (50.0) | 15 | (46.9) | 5 | (21.7) | |
| Race | |||||||
| White | 18 | (50.0) | 22 | (68.8) | 15 | (65.2) | 0.4398 |
| African American | 2 | (5.6) | 1 | (3.1) | 2 | (8.7) | |
| Other/Unknown | 16 | (44.4) | 9 | (28.1) | 6 | (26.1) | |
| Mean AST (U/L) | 187.6 ± 389.3 | 114.2 ± 62.4 | 0.4542 | ||||
| Mean ALP (U/L) | 158.5 ± 150.1 | 189.3 ± 110.4 | 0.0369 | ||||
| Mean total bilirubin (mg/dL) | 8.6 ± 11.7 | 2.8 ± 6.8 | < 0.0001 | ||||
| Mean creatinine (mg/dL) | 1.8 ± 1.5 | 2.1 ± 2.9 | 0.3134 | ||||
| IFNL4-ΔG genotype | |||||||
| TT/TT | 11 | (30.6) | 11 | (34.4) | 7 | (30.4) | 0.8405 |
| ΔG/TT | 18 | (50.0) | 13 | (40.6) | 9 | (39.1) | |
| ΔG/ΔG | 6 | (16.7) | 5 | (15.6) | 6 | (26.1) | |
| Undetermined | 1 | (2.8) | 3 | (9.4) | 1 | (4.3) | |
| IFNL4-ΔG allele frequency (%) | 42.9 | 39.7 | 47.7 | ||||
| Mean age (year) | P value | |||
| Normal | Cirrhosis | HCC | ||
| Male | 50.8 ± 18.3 | 50.7 ± 5.0 | 53.3 ± 5.9 | 0.0716 |
| Female | 53.1 ± 10.4 | 54.0 ± 8.3 | 63.3 ± 5.1 | |
Information regarding total bilirubin, creatinine, and liver enzymes aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were available for most HCV/cirrhosis and HCV/HCC patients. AST and creatinine levels did not differ significantly between the groups, but ALP and total bilirubin levels were significantly different between HCV/cirrhosis and HCC groups (Table 2). When only HCV-positive samples were isolated and stratified by sex, males were found to have significantly higher levels of AST compared to females (Table 4).
| Characteristics | Female (n = 20) | Male (n = 35) | P value |
| Mean AST (U/L) | 79.0 ± 44.7 | 213.8 ± 390.7 | 0.0186 |
| Mean ALP (U/L) | 158.3 ± 112.7 | 182.1 ± 156.5 | 0.4255 |
| Mean total bilirubin (mg/dL) | 4.8 ± 9.3 | 7.7 ± 11.6 | 0.3179 |
| Mean creatinine (mg/dL) | 1.4 ± 1.0 | 1.5 ± 1.0 | 0.6716 |
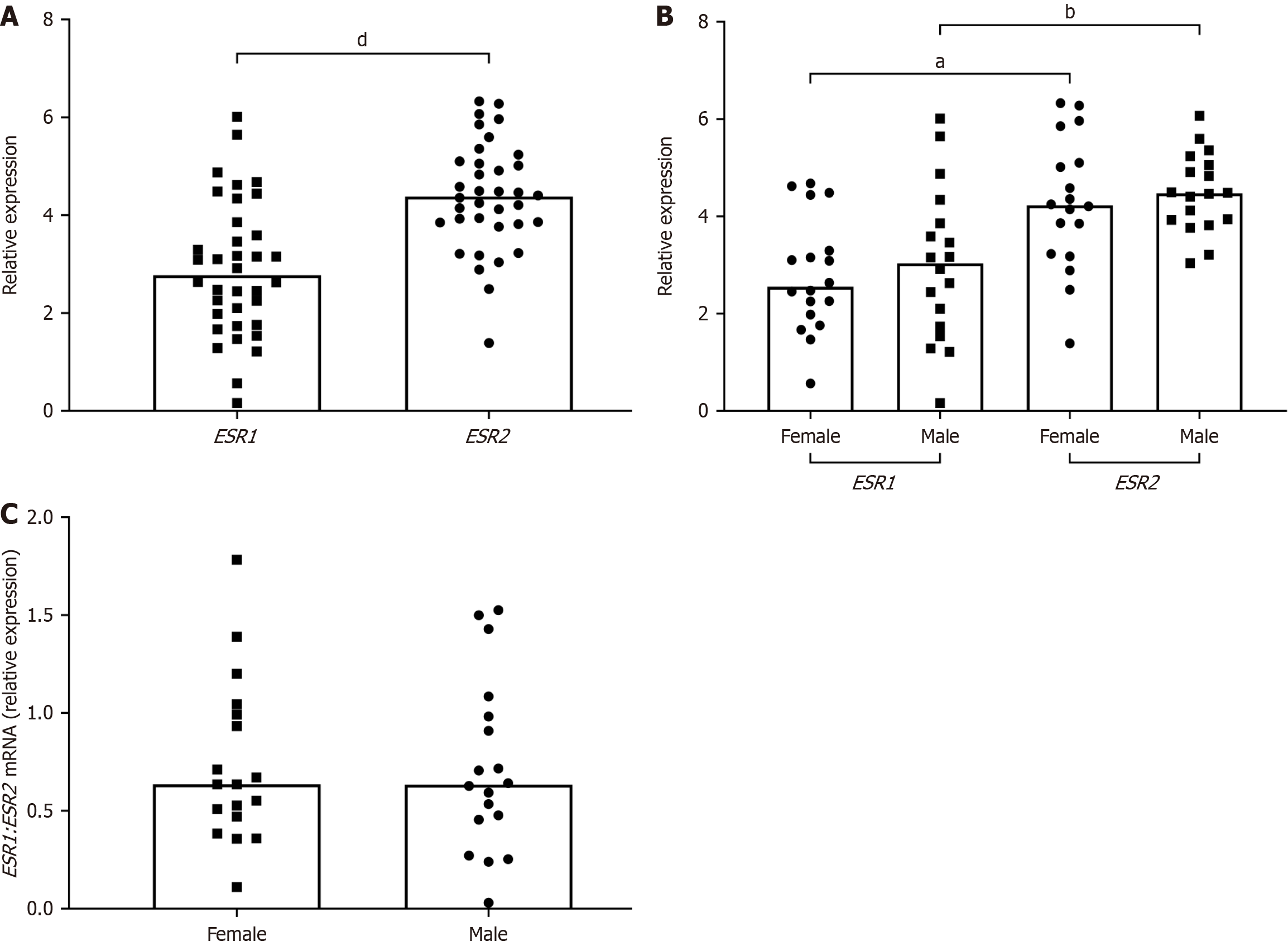
To investigate the role of ERs in HCV-associated diseases, the message RNA (mRNA) expression of ERα and ERβ was first measured in normal livers using RT-qPCR. ESR2 expression levels were significantly higher than ESR1 levels when the data were pooled (Figure 1A) and when they were segregated by sex (Figure 1B). Since ERα and ERβ are known co-regulators of physiological outcomes, the ESR1/ESR2 expression ratio was compared between males and females and it was found to be comparable (Figure 1C).
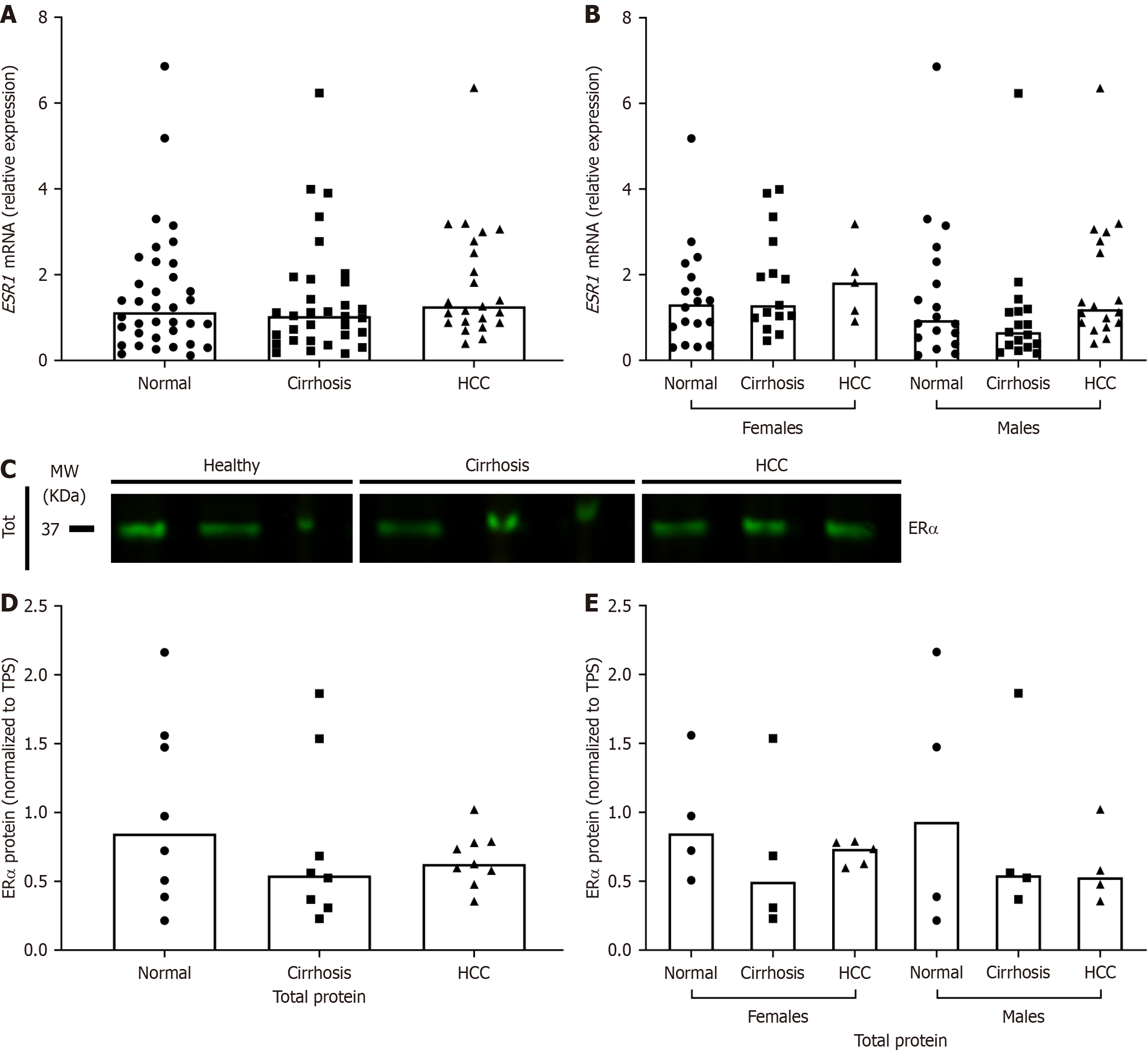
The expression of ER subtypes in HCV/cirrhosis and HCV/HCC liver explants was then measured and compared to normal using RT-qPCR and Western blotting. At the transcriptional level, there was an apparent increase in ESR1 expression in HCV/HCC livers, though the results were not statistically significant (Figure 2A and B). ERα protein expression decreased in HCV-related diseased livers, but again, the results were not statistically significant (Figure 2C-E).
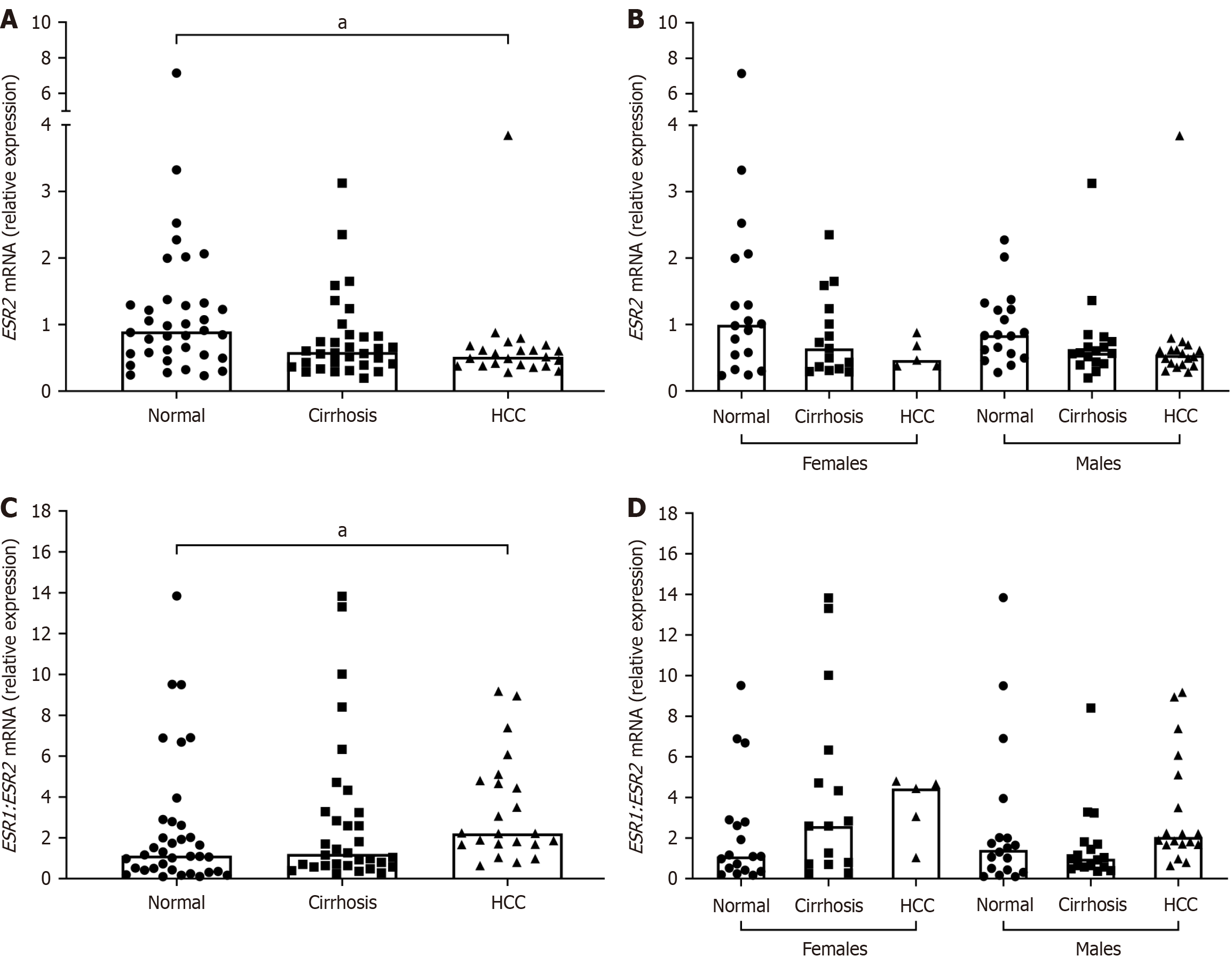
ESR2 mRNA expression was significantly lower in the HCV/HCC group as compared to normal (Figure 3). As expected, the ESR1/ESR2 ratio expression was significantly increased in HCV/HCC livers compared to normal (Figure 3). No significant differences in ESR2 expression or in ESR1/ESR2 ratio was found when results were stratified by both disease and sex (Figure 3). ESR1/ESR2 ratio in males with HCV/cirrhosis did show opposite trend of expression compared to females; however, it did not reach significance (Figure 3).
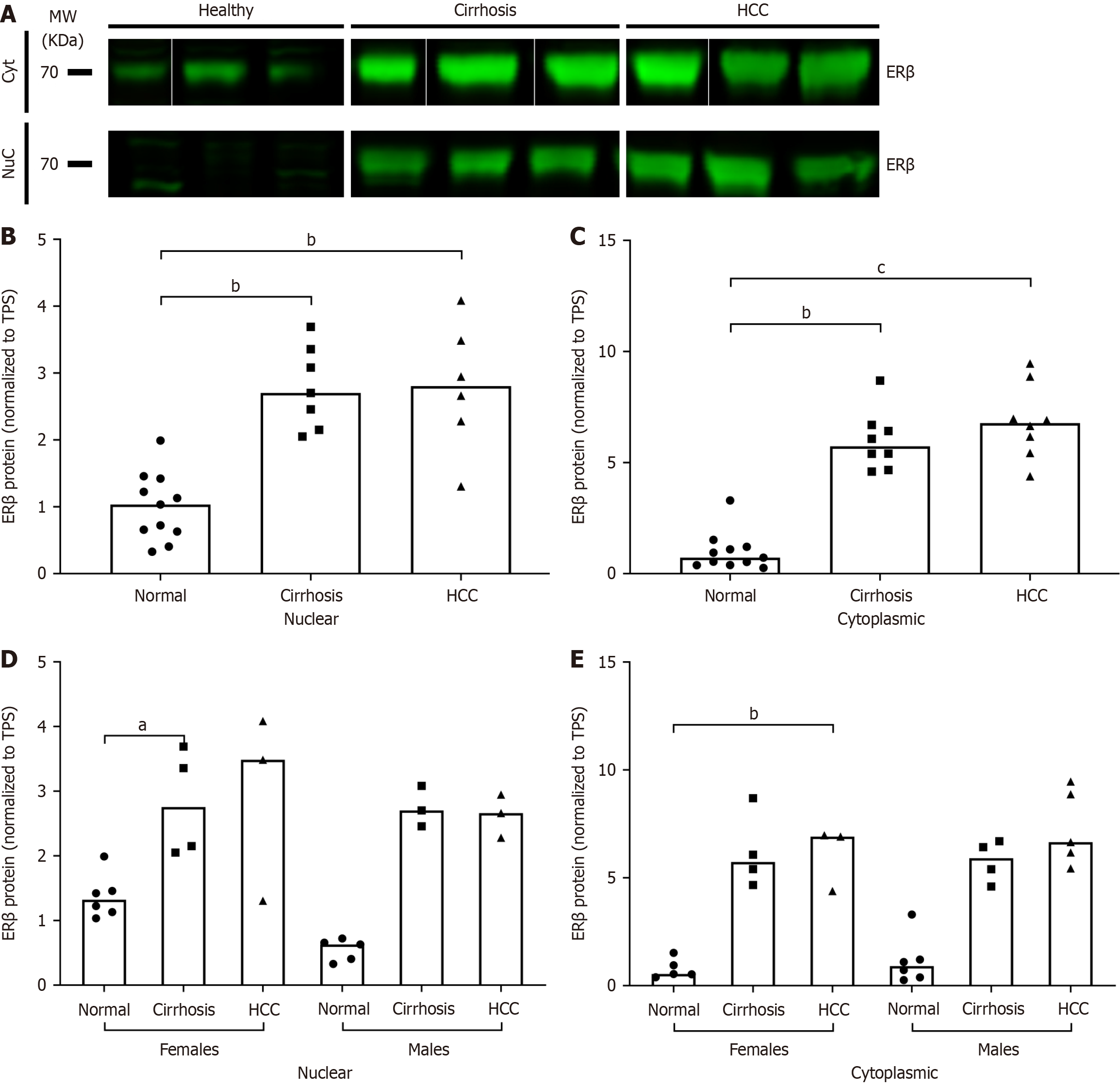
There was a significant increase in the liver protein expression of ERβ in HCV/cirrhosis and HCC as compared to normal in both nuclear and cytoplasmic fractions (Figure 4A-C). When results were stratified by sex, the increase in nuclear ERβ was only significant in cirrhosis females and the increase in cytoplasmic ERβ was only significant in HCC females (Figure 4D and E). Although no significant differences were found at the transcriptional level, at the translational level, when the ratio of ERα total protein expression to ERβ cytoplasmic protein expression was analyzed, there was a significant decrease in both HCV/cirrhosis and HCC subjects compared to normal (data not shown). These results suggest that HCV disease progression may alter the expression of ER subtypes, specifically ERβ.
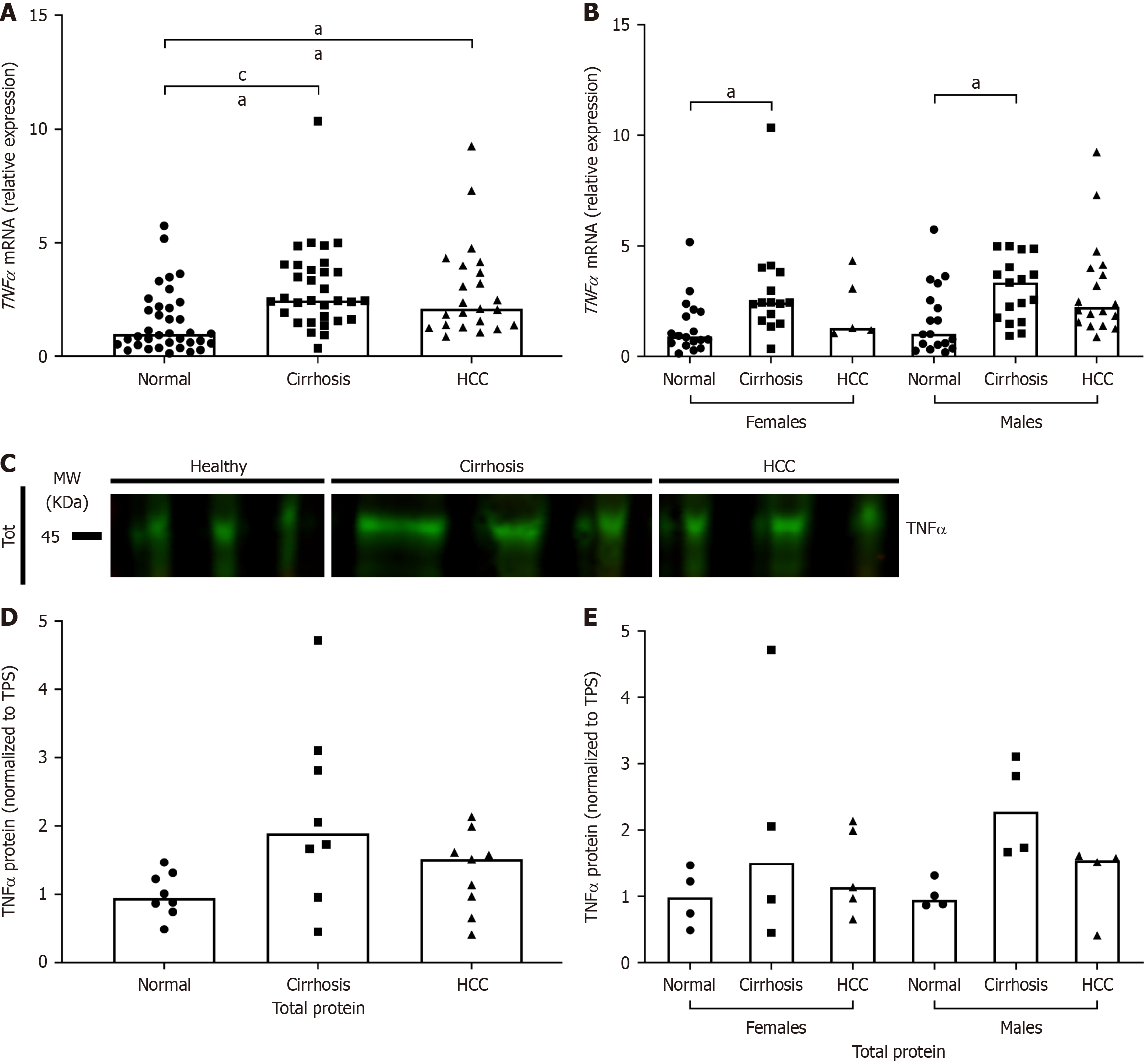
Because HCV pathogenesis relies primarily on inflammation to cause damage, the expression of proinflammatory cytokine TNF-α in this cohort was analyzed. TNF-α mRNA expression was significantly increased in both HCV/cirrhosis and HCV/HCC groups compared to normal (Figure 5A). When results were stratified by sex, TNF-α expression was only significantly higher in HCV/cirrhosis livers of both males and females compared to normal (Figure 5B). Although the results were not statistically significant, TNF-α protein expression followed a similar trend as mRNA expression (Figure 5C-E).
Spearman’s correlation coefficient was determined using RT-qPCR data from HCV/cirrhosis, HCV/HCC, and normal livers to compare the degree of association between the mRNA expression of ESR1, ESR2, ESR1/ESR2, and TNF-α (Table 5). Upon stratifying by sex within each disease group, ESR1 was found to be negatively correlated with ESR2 in females with HCV/cirrhosis (r = -0.84, P = 0.0002). Interestingly, males with HCV/cirrhosis were found to instead have a significant positive correlation between ESR1 and ESR2 (r = 0.57, P = 0.0197) (Table 5).
| Spearman’s correlations | ||||||||||
| Normal | ||||||||||
| Females (n = 18) | Males (n = 18) | Together (n = 36) | ||||||||
| ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ||
| ESR2 | ESR2 | ESR2 | ||||||||
| ESR1 | R value | 0.01 | -0.42 | -0.19 | ||||||
| P value | 0.9579 | 0.0861 | 0.2772 | |||||||
| TNF-α | R value | 0.05 | 0.54 | -0.38 | 0.34 | -0.11 | 0.35 | 0.2 | 0.18 | 0.01 |
| P value | 0.8293 | 0.022a | 0.119 | 0.1708 | 0.6508 | 0.16 | 0.2422 | 0.2972 | 0.9501 | |
| HCV/cirrhosis | ||||||||||
| Females (n = 15) | Males (n = 17) | Together (n = 32) | ||||||||
| ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ||
| ESR2 | ESR2 | ESR2 | ||||||||
| ESR1 | R value | -0.84 | 0.57 | -0.1 | ||||||
| P value | 0.0002c | 0.0197a | 0.6038 | |||||||
| TNF-α | R value | -0.3 | 0.64 | -0.57 | 0.42 | 0.58 | 0.07 | 0.09 | 0.61 | -0.19 |
| P value | 0.2708 | 0.0116a | 0.0286a | 0.0934 | 0.0162a | 0.802 | 0.6165 | 0.0002c | 0.3017 | |
| HCV/HCC | ||||||||||
| Females (n = 5) | Males (n = 23) | Together (n = 28) | ||||||||
| ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ESR1 | ESR2 | ESR1 | ||
| ESR2 | ESR2 | ESR2 | ||||||||
| ESR1 | R value | -0.1 | 0.14 | 0.08 | ||||||
| P value | 0.95 | 0.5814 | 0.7301 | |||||||
| TNF-α | R value | 0 | 0.3 | -0.1 | -0.11 | 0.6 | -0.56 | -0.21 | 0.45 | -0.57 |
| P value | > 0.99 | 0.6833 | 0.95 | 0.6627 | 0.0083b | 0.0151a | 0.3397 | 0.0293a | 0.0049b | |
ESR2 mRNA expression had a significant positive correlation with TNF-α in both HCV/cirrhosis (r = 0.61, P = 0.0002) and HCV/HCC patients (r = 0.45, P = 0.0293) (Table 5). When stratified by sex within each disease group, ESR2 was found to maintain the significant positive correlation with TNF-α in both HCV/cirrhosis males and females (r = 0.58, P = 0.0162; r = 0.64, P = 0.0116 respectively) but for HCV/HCC, only males had any significant correlation (r = 0.60, P = 0.0083) (Table 5). Interestingly, normal females also showed a significant positive correlation (r = 0.54, P = 0.0220). Despite the correlations found between ESR2 and TNF-α, the ratio of ESR1 to ESR2 was found to be correlated with TNF-α only in HCV/HCC patients (r = -0.57, P = 0.0049), but upon stratifying by sex, negative correlations were found between TNF-α and ESR1/ESR2 in HCV/HCC males (r = -0.56, P = 0.0151) and HCV/cirrhosis females (r = -0.57, P = 0.0286) (Table 5).
Chronic infection with HCV and the associated long-term inflammation causes affected individuals to be highly susceptible to liver complications such as fibrosis, cirrhosis, and HCC[34,35]. It is well established that male sex is a major risk factor for complications associated with chronic HCV infection[10,11,14,36,37]. Compared to females, males are three times more likely to develop liver cancer and four times more likely to die from cancer[38-40]. In HCC, estrogen has been shown to downregulate the production of key inflammatory markers as well as inhibit the activation of tumor-associated[41-43], which suggests that estrogen may directly contribute to the lower HCC incidence among females. However, there remains a huge gap in knowledge regarding the role of ER subtypes expression in HCV-associated disease, and the related contributions of inflammatory markers in these immune responses.
ERs play a pivotal role in modulating the immune system, particularly by influencing the behavior of macrophages and T cells, which are key players in immune responses. ERs are expressed on these immune cells and can modulate their functions in a ligand-dependent manner. In macrophages, estrogen signaling often results in an anti-inflammatory phenotype, reducing the secretion of pro-inflammatory cytokines like TNF-α and interleukin (IL)-6 while enhancing the release of anti-inflammatory cytokines like IL-10[20,44]. This modulation can create an environment that dampens excessive inflammation. Similarly, ERs on T cells influence their differentiation and activity; for instance, estrogen can promote the expansion of regulatory T cells, which help maintain immune tolerance, and modulate the activity of effector T cells, such as Th1 and Th17 cells, which are involved in inflammatory responses. In the context of HCV-related diseases, these estrogen-mediated effects could significantly impact disease progression. By reducing inflammation and promoting immune regulation, estrogen signaling might slow liver fibrosis and reduce the risk of cirrhosis or HCC[45,46]. Conversely, the immunosuppressive effects of estrogen might impair the clearance of HCV, potentially facilitating chronic infection. Understanding these dual roles highlights the complexity of estrogen's influence on immune responses in HCV-related diseases and underscores the need for tailored therapeutic approaches.
A major limitation of our study is due to the epidemiology of HCV-associated diseases; as previously stated, males are three times more likely to develop liver cancer compared to females. This results in a large discrepancy in our sample sizes between males and females, particularly in our HCC group (Table 2). Future studies should include more female patients as able, as well as a more diverse range of ethnicities, particularly individuals from Asian descent, so that the conclusions can be more broadly relevant.
Since ERβ was discovered more than a decade after ERα, there are limited data on the role of ERβ in the liver; however, the results from this study shows its importance in HCV pathogenesis. Previous work from our lab reported significantly increased expression of ESR2 mRNA in HCV-associated diseased livers compared to normal[24]. In contrast, Iavarone et al[47] reported a loss of ESR2 expression in patients with HCC compared to those with chronic liver disease. The present study found that while ESR2 mRNA expression was significantly lower in the HCV/HCC group compared to normals, cytoplasmic and nuclear protein expression was increased in both HCV/cirrhosis and HCV/HCC groups. The conflicting results observed at the translational level vs the transcriptional level may be attributed to the understanding that protein expression may not correspond linearly to its mRNA expression, due to possible disparities in translation efficiency or the half-life of the molecules[48]. Studies to further reveal the function of ERβ and its variants in HCV pathogenesis are warranted in order understand these differences in mRNA and protein expression.
Because all of the patient cases in this study were recipients of a liver transplant, it is important to understand the criteria to qualify for transplantation. Model for end-stage liver disease (MELD) scores are used to determine transplant candidacy in cirrhotic patients; in general, patients can qualify for liver transplantation once their MELD score is ≥ 15[49]. Patients with a MELD score < 15 may still become eligible if they have certain conditions or complications, such as HCC[49]. Patients with HCC can qualify for liver transplantation if they have either a single tumor ≤ 5 cm or up to three separate lesions all < 3 cm and no evidence of vascular invasion or metastasis. To summarize, in order to qualify for transplantation, the cirrhotic patients in our cohort needed to present with more advanced cirrhosis while the HCC patients were required to have less severe cancer. Furthermore, as our study only included patients with end-stage liver disease, we cannot draw conclusions regarding early disease pathways. In the future, samples from early stages of HCV-associated liver disease should be included to more comprehensively reveal the role that ERs play.
Expression of ERα is generally thought to promote carcinogenesis while ERβ acts as a tumor suppressor[50]; however, much of the work looking at ER expression in cancer has been in breast tissues. Furthermore, ER subtype expression can vary greatly depending on sex, age, disease conditions, and other variables. In our cohort, ESR2 transcript expression was greater than ESR1 expression in normal livers, and we also showed altered expression of ERβ, but not ERα, in patients with HCV-associated diseases. Our study was the first to report mRNA levels of ERα and ERβ in normal human livers from a United States population, and our results give evidence that ERβ may play a more important role than previous thought.
There is not yet a consensus on whether hepatic expression of either ER subtype is beneficial or detrimental in HCV pathogenesis. Some studies report a protective effect of ERα expression[51-53], others describe associations between ERα expression and worse prognosis[24], and still others report no correlations[54]. Although no significant differences in expression of ERα mRNA or protein was found in either HCV/cirrhosis or HCV/HCC groups compared to normals, it was determined that in this cohort, ESR1 is negatively correlated with AST and bilirubin (cirrhosis only) and with ALP (HCC only) (data not shown). These results support the conclusion that ESR1 expression is associated with better liver function. However, using primers/antibodies for only wild-type (wt) ERα and wtERβ only gives us partial data as there are several ER variants or isoforms with diverse tissue-specific expression patterns and functions. As liver function worsens, there is a decrease in wtERα expression and ERα variants (ERα46 and ERα36) are upregulated; this upregulation is associated with a worse prognosis for HCV-associated diseases[55-59]. In addition, this project only looked at the expression of markers in extratumoral tissues, as tumor biopsies were not available for all samples. Future work should include tumor samples from patients with HCC so that the role of ERs in the tumor microenvironment can be studied.
As expected, TNF-α expression levels were highest in cirrhosis and HCC livers, since these diseases are primarily driven by chronic inflammation and TNF-α is a potent proinflammatory cytokine. Notably, expression in HCC appears to be lower than in cirrhosis. This corresponds with other studies that reported a decrease in TNF-α protein expression in more advanced HCV-related disease[60]. Nevertheless, the role of TNF-α in HCV pathogenesis is decidedly complex.
Estrogen has been shown to be associated with a decrease in TNF-α expression[18,19,61], which is supported by an in vitro experiment we performed demonstrating that treatment with estradiol significantly downregulated expression of TNF-α mRNA in human hepatoma Huh7 cell line (data not shown). Estrogen downregulation of TNF-α may help explain the less severe disease presentation in females compared to males[62]. However, in human liver tissues, ESR2 was positively correlated with TNF-α in all disease/sex groups except for normal males and HCC females. While the lack of correlation in HCC females may be attributed to our small sample size (n = 5), the same cannot be said of the normal males. Although the correlation between ESR2 and TNF-α was demonstrated by our study, future studies should include in vitro models to further explore the functional mechanism.
In addition to looking at associations between ER subtypes and immune markers, another objective of the project was to investigate the relationship between hepatic ERα and ERβ and their distinctive roles in the liver. The relative expression of ERα and ERβ in a given environment is a significant determinant of their response to both endogenous and synthetic ligands, and a number of studies have observed ERβ-mediated modulation of ERα activity[63-67]. Upon binding with a ligand, ERs will dimerize prior to translocating to the nucleus, and relative concentrations of ERα and ERβ determine whether they will homodimerize or heterodimerize. ER homodimers regulate a different set of genes than that of ERαβ heterodimers[68].
These results as well as the data on TNF-α expression draw attention to the importance of considering time as a critical variable; in other words, the difference in outcomes may not be necessarily due to the distinct activity or interactions of the molecules, but rather in the regulation of immune pathways at different stages of HCV infection, namely fibrosis and HCC progression.
Genetics may very well play a role too; a polymorphism in a recently discovered type III interferon, IFNL4-ΔG/TT (rs368234815), has been strongly associated with HCV clearance and response to treatment[69]. Contrary to the typical antiviral function of interferons, it is individuals who carry the IFNL4-ΔG deletion allele, and therefore generate the full interferon-λ4 protein, who have impaired HCV clearance and response to treatment[69-72]. In the present study, we found that in patients with INFL4-ΔG/ΔG genotype, specifically female patients, hepatic expression of ESR2 was found to be significantly decreased (Supplementary Figure 1). This finding is consistent with the observation that ERβ expression is protective in some cancers[50]. Furthermore, we also found a positive correlation of ESR1 with ESR2 in cirrhosis males, and a negative correlation in cirrhosis females and IFNL4-ΔG allele females (data not shown). ESR1 and ESR2 or their isoforms are known to coregulate the activity of each other[73-76] and our results offer some evidence that coregulation may be, at least in part, dependent on sex.
This study further confirms the conclusion from our previous study[24] that differential ER subtype expression is observed in males and females in both the premalignant and malignant stages of HCV-related disease progression. Further research in other inflammatory, anti-inflammatory, and oncogenic markers is, therefore, an essential next step in elucidating the immunomodulation by ER subtypes in the liver. In addition, since the focus of this study was on nuclear ERα and β, more research is needed to determine if the sex-based differences in HCV infection outcome are also mediated G protein coupled estrogen receptor or by isoforms of the nuclear receptors.
Something that also needs to be considered is the possibility that while estrogen may have inherent protective functions, the differences in HCV pathogenesis and disease progression between males and females may be exacerbated by the inhibitory effects of testosterone and progesterone on immune response[77]. For that reason, evaluating the role of other sex steroids and the expression and function of their receptors would be a fruitful area for further work.
HCV remains a problem of scientific and clinical significance due to the genetic variability of the virus, lack of robust early detection methods, and challenges of vaccine development[78]. This project will be the basis for future work investigating the factors that are responsible for the sex-based differences in HCV disease pathogenesis. Studying the molecular pathways involved will lead to a better understanding of the reasons behind why only a fraction of individuals are able to clear the virus on their own, and will facilitate more personalized diagnosis and care.
Our study, for the first time, demonstrates an increase in expression of ERβ compared to ERα in the liver of normal males and females. In addition, ERβ mRNA expression was found to be decreased in HCV-positive livers, while TNF-α expression was increased. ERβ mRNA expression was also decreased in livers with the IFNL4-ΔG/ΔG genotype. Further analysis of the data revealed sex-specific correlations between ERα and ERβ. These findings suggest that changes in ERβ expression are associated with worsening HCV-related disease, and may be one of the sex-dependent factors in cirrhosis and HCC pathogenesis. Any changes in ER subtype ratio during progression of cirrhosis and cancer development in relation to TNF-α expression may provide crucial information especially in patients with IFNL4-ΔG/ΔG genotype. This information may also help us to monitor host therapeutic responses and viral clearance. Future studies can focus on signaling pathways in relation to ER subtype receptors that may intersect with TNF-α in the liver for development of sex-based novel therapeutics.
Much appreciation for the collaboration with Dr. Prokunina-Olsson L at the Laboratory of Translational Genomics, Division of Cancer Epidemiology and Genetics (NCI/NIH, Bethesda, MD, United States).
| 1. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 2. | United States Cancer Statistics. About the U.S. Cancer Statistics Data Visualizations Tool. [cited July 1, 2025]. Available from: https://www.cdc.gov/united-states-cancer-statistics/dataviz/index.html. |
| 3. | Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, Ryerson AB. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology. 2019;69:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 4. | Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61:S3-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M, Waggoner JG, Park Y, Jones EA. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 646] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2439] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 7. | Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol. 2015;21:11984-12002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Khan MH, Farrell GC, Byth K, Lin R, Weltman M, George J, Samarasinghe D, Kench J, Kaba S, Crewe E, Liddle C. Which patients with hepatitis C develop liver complications? Hepatology. 2000;31:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Corsi DJ, Karges W, Thavorn K, Crawley AM, Cooper CL. Influence of female sex on hepatitis C virus infection progression and treatment outcomes. Eur J Gastroenterol Hepatol. 2016;28:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, Ly KN, Cronin KA, Penberthy L, Kohler BA. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 705] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 12. | Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14:5945-5961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, Lee SD, Lin CL, Chen PJ, Lin SC, Chen CJ. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Codes L, Asselah T, Cazals-Hatem D, Tubach F, Vidaud D, Paraná R, Bedossa P, Valla D, Marcellin P. Liver fibrosis in women with chronic hepatitis C: evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut. 2007;56:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Villa E, Karampatou A, Cammà C, Di Leo A, Luongo M, Ferrari A, Petta S, Losi L, Taliani G, Trande P, Lei B, Graziosi A, Bernabucci V, Critelli R, Pazienza P, Rendina M, Antonelli A, Francavilla A. Early menopause is associated with lack of response to antiviral therapy in women with chronic hepatitis C. Gastroenterology. 2011;140:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 17. | Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, Phan AT, Hawk E, Javle M, Lee JS, Torres HA, Rashid A, Lenzi R, Hassabo HM, Abaza Y, Shalaby AS, Lacin S, Morris J, Patt YZ, Amos CI, Khaderi SA, Goss JA, Jalal PK, Kaseb AO. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2017;15:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Ralston SH, Russell RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Rogers A, Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Evans MJ, Lai K, Shaw LJ, Harnish DC, Chadwick CC. Estrogen receptor alpha inhibits IL-1beta induction of gene expression in the mouse liver. Endocrinology. 2002;143:2559-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Hayashida K, Shoji I, Deng L, Jiang DP, Ide YH, Hotta H. 17β-estradiol inhibits the production of infectious particles of hepatitis C virus. Microbiol Immunol. 2010;54:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Ulitzky L, Lafer MM, KuKuruga MA, Silberstein E, Cehan N, Taylor DR. A New Signaling Pathway for HCV Inhibition by Estrogen: GPR30 Activation Leads to Cleavage of Occludin by MMP-9. PLoS One. 2016;11:e0145212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Magri A, Barbaglia MN, Foglia CZ, Boccato E, Burlone ME, Cole S, Giarda P, Grossini E, Patel AH, Minisini R, Pirisi M. 17,β-estradiol inhibits hepatitis C virus mainly by interference with the release phase of its life cycle. Liver Int. 2017;37:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Iyer JK, Kalra M, Kaul A, Payton ME, Kaul R. Estrogen receptor expression in chronic hepatitis C and hepatocellular carcinoma pathogenesis. World J Gastroenterol. 2017;23:6802-6816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Liedtke C, Trautwein C. The role of TNF and Fas dependent signaling in animal models of inflammatory liver injury and liver cancer. Eur J Cell Biol. 2012;91:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 1001] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 27. | Dai CY, Chuang WL, Lee LP, Chen SC, Hou NJ, Lin ZY, Hsieh MY, Hsieh MY, Wang LY, Chang WY, Yu ML. Associations of tumour necrosis factor alpha promoter polymorphisms at position -308 and -238 with clinical characteristics of chronic hepatitis C. J Viral Hepat. 2006;13:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Park J, Kang W, Ryu SW, Kim WI, Chang DY, Lee DH, Park DY, Choi YH, Choi K, Shin EC, Choi C. Hepatitis C virus infection enhances TNFα-induced cell death via suppression of NF-κB. Hepatology. 2012;56:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Waxman S, Wurmbach E. De-regulation of common housekeeping genes in hepatocellular carcinoma. BMC Genomics. 2007;8:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Congiu M, Slavin JL, Desmond PV. Expression of common housekeeping genes is affected by disease in human hepatitis C virus-infected liver. Liver Int. 2011;31:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Yamaguchi H, Matsumoto S, Ishibashi M, Hasegawa K, Sugitani M, Takayama T, Esumi M. β-Glucuronidase is a suitable internal control gene for mRNA quantitation in pathophysiological and non-pathological livers. Exp Mol Pathol. 2013;95:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Moritz CP. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics. 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 33. | Kirshner ZZ, Gibbs RB. Use of the REVERT(®) total protein stain as a loading control demonstrates significant benefits over the use of housekeeping proteins when analyzing brain homogenates by Western blot: An analysis of samples representing different gonadal hormone states. Mol Cell Endocrinol. 2018;473:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3533] [Cited by in RCA: 3486] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 35. | Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2168] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 37. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [PubMed] |
| 38. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4300] [Article Influence: 226.3] [Reference Citation Analysis (2)] |
| 39. | Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 607] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 40. | Guo Y, Wu G, Yi J, Yang Q, Jiang W, Lin S, Yang X, Cai X, Mao L. Anti-Hepatocellular Carcinoma Effect and Molecular Mechanism of the Estrogen Signaling Pathway. Front Oncol. 2021;11:763539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 41. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1515] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 42. | Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y, Li L, Shen P. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs). J Biol Chem. 2012;287:40140-40149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Wei T, Chen W, Wen L, Zhang J, Zhang Q, Yang J, Liu H, Chen BW, Zhou Y, Feng X, Yang Q, Bai X, Liang T. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 2016;382:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Kilbourne EJ, Scicchitano MS. The activation of plasminogen activator inhibitor-1 expression by IL-1beta is attenuated by estrogen in hepatoblastoma HepG2 cells expressing estrogen receptor alpha. Thromb Haemost. 1999;81:423-427. [PubMed] |
| 45. | Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 46. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 47. | Iavarone M, Lampertico P, Seletti C, Francesca Donato M, Ronchi G, del Ninno E, Colombo M. The clinical and pathogenetic significance of estrogen receptor-beta expression in chronic liver diseases and liver carcinoma. Cancer. 2003;98:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Omoto Y, Kobayashi S, Inoue S, Ogawa S, Toyama T, Yamashita H, Muramatsu M, Gustafsson JA, Iwase H. Evaluation of oestrogen receptor beta wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur J Cancer. 2002;38:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Dove LM, Brown RS, Jr. Liver transplantation in adults: Patient selection and pretransplantation evaluation. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate, 2020. |
| 50. | Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Exp Hematol Oncol. 2018;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 51. | Cengiz M, Ozenirler S, Yılmaz G. Estrogen receptor alpha expression and liver fibrosis in chronic hepatitis C virus genotype 1b: a clinicopathological study. Hepat Mon. 2014;14:e21885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, Tsutsui A, Okamura S, Shibata H, Ito S. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001;21:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | O'Brien MH, Pitot HC, Chung SH, Lambert PF, Drinkwater NR, Bilger A. Estrogen Receptor-α Suppresses Liver Carcinogenesis and Establishes Sex-Specific Gene Expression. Cancers (Basel). 2021;13:2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Erkan G, Yilmaz G, Cengiz M, Degertekin CK, Akyol G, Ozenirler S. Lack of association of hepatic estrogen receptor-alpha expression with histopathological and biochemical findings in chronic hepatitis C. Pathol Res Pract. 2013;209:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498-500. [PubMed] |
| 56. | Villa E, Dugani A, Moles A, Camellini L, Grottola A, Buttafoco P, Merighi A, Ferretti I, Esposito P, Miglioli L, Bagni A, Troisi R, De Hemptinne B, Praet M, Callea F, Manenti F. Variant liver estrogen receptor transcripts already occur at an early stage of chronic liver disease. Hepatology. 1998;27:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Wang AG, Lee KY, Kim SY, Choi JY, Lee KH, Kim WH, Wang HJ, Kim JM, Park MG, Yeom YI, Kim NS, Yu DY, Lee DS. The expression of estrogen receptors in hepatocellular carcinoma in Korean patients. Yonsei Med J. 2006;47:811-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Miceli V, Cocciadiferro L, Fregapane M, Zarcone M, Montalto G, Polito LM, Agostara B, Granata OM, Carruba G. Expression of wild-type and variant estrogen receptor alpha in liver carcinogenesis and tumor progression. OMICS. 2011;15:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Wang R, Chen J, Yu H, Wei Z, Ma M, Ye X, Wu W, Chen H, Fu Z. Downregulation of estrogen receptor-α36 expression attenuates metastasis of hepatocellular carcinoma cells. Environ Toxicol. 2022;37:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Farinati F, Cardin R, Bortolami M, Guido M, Rugge M. Oxidative damage, pro-inflammatory cytokines, TGF-alpha and c-myc in chronic HCV-related hepatitis and cirrhosis. World J Gastroenterol. 2006;12:2065-2069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 570] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 62. | Zhang L, Wu J, Wu Q, Zhang X, Lin S, Ran W, Zhu L, Tang C, Wang X. Sex steroid axes in determining male predominance in hepatocellular carcinoma. Cancer Lett. 2023;555:216037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 63. | Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505-3512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 332] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 64. | Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970-4978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 65. | Lindberg MK, Movérare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a "ying yang" relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol. 2005;239:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Bakas P, Liapis A, Vlahopoulos S, Giner M, Logotheti S, Creatsas G, Meligova AK, Alexis MN, Zoumpourlis V. Estrogen receptor alpha and beta in uterine fibroids: a basis for altered estrogen responsiveness. Fertil Steril. 2008;90:1878-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19:1555-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I, Chen S, Brand N, Tarway M, Liu L, Sheikh F, Astemborski J, Bonkovsky HL, Edlin BR, Howell CD, Morgan TR, Thomas DL, Rehermann B, Donnelly RP, O'Brien TR. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 773] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 70. | Aka PV, Kuniholm MH, Pfeiffer RM, Wang AS, Tang W, Chen S, Astemborski J, Plankey M, Villacres MC, Peters MG, Desai S, Seaberg EC, Edlin BR, Strickler HD, Thomas DL, Prokunina-Olsson L, Sharp GB, O'Brien TR. Association of the IFNL4-ΔG Allele With Impaired Spontaneous Clearance of Hepatitis C Virus. J Infect Dis. 2014;209:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Meissner EG, Bon D, Prokunina-Olsson L, Tang W, Masur H, O'Brien TR, Herrmann E, Kottilil S, Osinusi A. IFNL4-ΔG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis. 2014;209:1700-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | O'Brien TR, Pfeiffer RM, Paquin A, Lang Kuhs KA, Chen S, Bonkovsky HL, Edlin BR, Howell CD, Kirk GD, Kuniholm MH, Morgan TR, Strickler HD, Thomas DL, Prokunina-Olsson L. Comparison of functional variants in IFNL4 and IFNL3 for association with HCV clearance. J Hepatol. 2015;63:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Zhao C, Matthews J, Tujague M, Wan J, Ström A, Toresson G, Lam EW, Cheng G, Gustafsson JA, Dahlman-Wright K. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67:3955-3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Lu W, Katzenellenbogen BS. Estrogen Receptor-β Modulation of the ERα-p53 Loop Regulating Gene Expression, Proliferation, and Apoptosis in Breast Cancer. Horm Cancer. 2017;8:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2321] [Cited by in RCA: 4403] [Article Influence: 440.3] [Reference Citation Analysis (0)] |
| 78. | Su L, Luo H, Yan Y, Yang Z, Lu J, Xu D, Du L, Liu J, Yang G, Chi H. Exploiting gender-based biomarkers and drug targets: advancing personalized therapeutic strategies in hepatocellular carcinoma. Front Pharmacol. 2024;15:1433540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/