Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.110582
Revised: July 8, 2025
Accepted: July 31, 2025
Published online: August 21, 2025
Processing time: 67 Days and 8.5 Hours
Postoperative gastrointestinal recovery affects hospital stay time and patient’s quality of life. Studies suggest that the use of dexmedetomidine during the perioperative period can promote post operational recovery of gastrointestinal function.
To evaluate the efficacy and safety of different doses of dexmedetomidine on postoperative gastrointestinal function recovery after laparoscopic colorectal surgery.
In this large-sample, retrospective study, 879 patients undergoing laparoscopic colorectal surgery were categorized into three groups: A control group receiving no dexmedetomidine (n = 281), a low-dose group receiving an intraoperative bolus of 0.5 μg/kg dexmedetomidine followed by a continuous infusion of 0.2 μg/kg/hour (n = 313), and a high-dose group receiving a 1.0 μg/kg bolus followed by a 0.5 μg/kg/hour infusion (n = 285). Time to postoperative first flatus, feces, and regular diet, and the intake, feeling nauseated, emesis, physical examination, and duration of symptoms score were evaluated.
Multiple linear regression analysis showed that age, gender, body mass index, American Society of Anesthesiologists classification, comorbidities and surgical site were not related to the time to first flatus (all P > 0.05). The times to postoperative first flatus, first feces, and regular diet were earlier in both dexmedetomidine groups than the control group (both P < 0.05). More patients in the control group experienced postoperative gastrointestinal intolerance (both P < 0.05). There was no significant difference between the high- and the low-dose groups (P > 0.05). The incidence of intraoperative bradycardia in the high-dose group was higher than that in the control group (19.15% vs 8.19%, P < 0.05).
Both low- and high-dose dexmedetomidine regimens enhance postoperative gastrointestinal recovery after laparoscopic colorectal surgery. The low-dose regimen demonstrates superior safety, supporting its integration into multimodal enhanced recovery pathways.
Core Tip: Both low-dose and high-dose dexmedetomidine enhance gastrointestinal recovery after laparoscopic colorectal surgery, significantly shortening time to first flatus, defecation, and oral intake vs controls. No dose-dependent benefit observed: High-dose dexmedetomidine (1.0 μg/kg + 0.5 μg/kg/hour) did not further accelerate gastrointestinal recovery compared to low-dose (0.5 μg/kg + 0.2 μg/kg/hour). Low-dose regimen demonstrated superior safety: High-dose dexmedetomidine significantly increased intraoperative bradycardia risk (19.15% vs 8.19% in controls, P < 0.05). Reduced opioid/sedative requirements: Dexmedetomidine groups required less propofol and remifentanil than controls, potentially mitigating opioid-induced gastrointestinal dysfunction. Clinical recommendation: Low-dose dexmedetomidine is optimal for enhancing gastrointestinal recovery while minimizing cardiovascular risks.
- Citation: Chen Y, Tang WL, Li CT, Zhao Y, Li B, Liao LM, Lin TH, Zhang LC. Efficacy and safety of different doses of dexmedetomidine on gastrointestinal function recovery after laparoscopic colorectal surgery. World J Gastroenterol 2025; 31(31): 110582
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/110582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.110582
Postoperative gastrointestinal recovery (POGIR) is a crucial factor affecting the rehabilitation of surgical patients. The recovery of gastrointestinal function is often a determinant of the overall recovery process, influencing the length of hospital stay and the patient’s quality of life[1]. Postoperative gastrointestinal dysfunction (POGD) is characterized by symptoms such as ileus, nausea, vomiting, and abdominal distension. POGD may prolong hospital stay and increase medical costs. In severe cases, POGD can even lead to reoperations, unplanned readmission, and increased mortality[2,3]. Therefore, promoting the recovery of postoperative gastrointestinal function has always been a hot topic in clinical research.
Dexmedetomidine, a highly selective α2-adrenergic receptor agonist, exerts effects including sedation, analgesia, anxiolysis, and sympathetic inhibition[4]. In recent years, studies have found that perioperative use of dexmedetomidine can promote the recovery of postoperative gastrointestinal function[5,6]. Intravenous administration of a loading dose of 0.5 μg/kg dexmedetomidine followed by a continuous infusion of 0.2 μg/kg/hour enhanced postoperative gastrointestinal motility function[7,8]. Studies have shown that an additional 1 μg/kg loading dose of dexmedetomidine is an independent protective factor for POGIR but may result in a lower heart rate[9]. However, in a study of 12 healthy volunteers, a loading dose of 1 μg/kg dexmedetomidine followed by a continuous infusion of 0.7 μg/kg markedly inhibited gastric emptying and gastrointestinal transit[10]. It seems different doses of dexmedetomidine may have different effects on postoperative gastrointestinal function.
However, existing literature primarily examines single-dose dexmedetomidine regimens. In these studies, dosing typically involves an initial intravenous bolus of 0.5 to 1 μg/kg administered over 10-15 minutes, followed by a continuous intravenous infusion maintained at 0.2 to 0.7 μg/kg/hour[7-9,11]. No studies have directly compared the efficacy of different doses of dexmedetomidine for laparoscopic colorectal surgery. In our hospital, the dosage selection of dexmedetomidine is generally based on the preferences of anesthesiologists. Some anesthesiologists follow the dosage recommended in the drug label, which the initial dosage is 1 μg/kg for intravenous infusion for 10 to 15 minutes, and then maintained at 0.2 to 0.7 μg/kg/hour by intravenous infusion, while others, based on clinical experience, reduce the dexmedetomidine dosage to mitigate cardiovascular adverse effects[7,8]. As addition of dexmedetomidine to promote post-operational gastrointestinal function recovery is not a standard practice, some anesthesiologists do not use it. Therefore, zero-dose, low-dose (load: 0.5 μg/kg; maintenance: 0.2 μg/kg/hour), and high-dose (load: 1.0 μg/kg; maintenance: 0.5 μg/kg/hour) regimens are administered within our hospital. Therefore, this retrospective study evaluated zero-dose, low-dose, and high-dose dexmedetomidine regimens to identify the optimal dosage for enhancing POGIR and reducing cardiovascular adverse reactions in patients undergoing laparoscopic colorectal surgery.
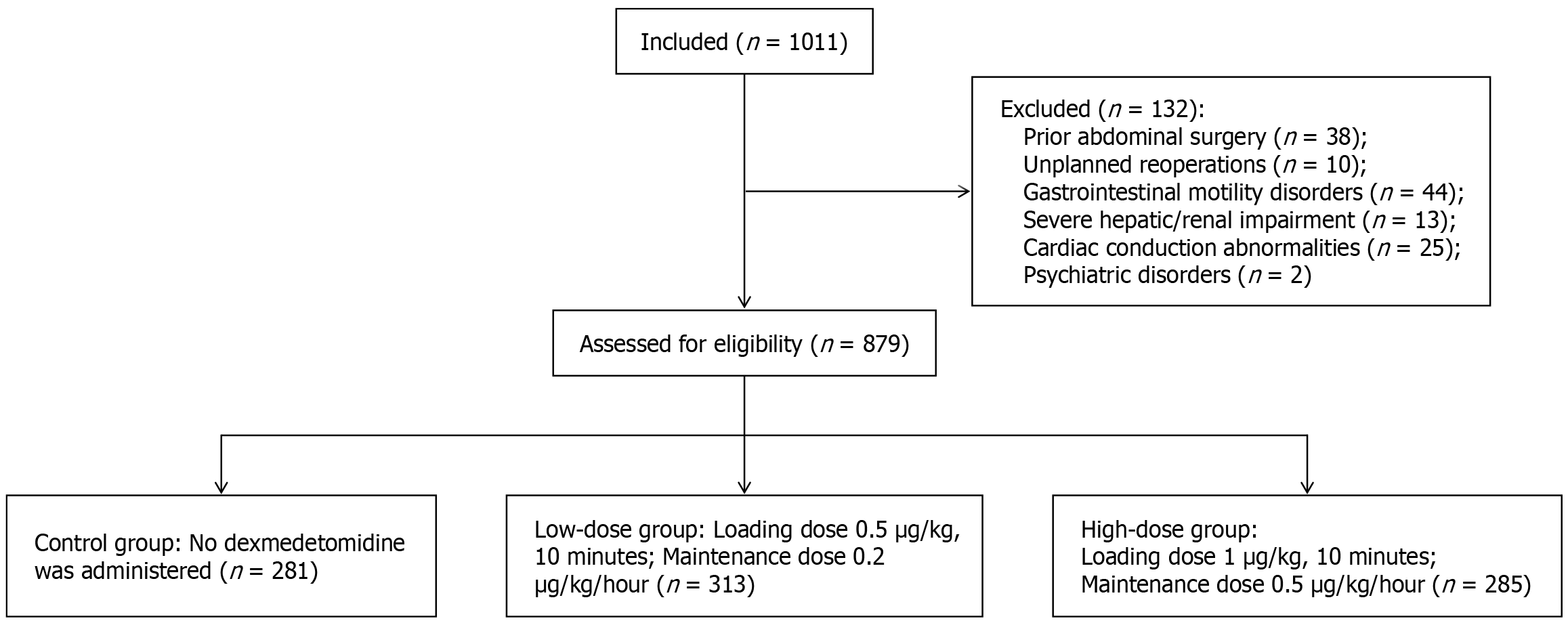
This retrospective study included adult patients who underwent laparoscopic colorectal surgery at Longyan First Hospital between January 2023 and December 2024. The study was approved by the Institutional Ethics Committee of our hospital (No. LYREC2025-k083-01). All participants were adults aged over 18 years, classified as American Society of Anesthesiologists (ASA) physical status I-III, and underwent elective laparoscopic colorectal procedures with surgical durations ranging from 1 to 6 hours. We excluded patients who had prior abdominal surgery, unplanned reoperations, or preexisting conditions such as gastrointestinal motility disorders, severe hepatic/renal impairment, cardiac conduction abnormalities (e.g., sick sinus syndrome, atrioventricular block, sinus bradycardia), allergies to dexmedetomidine or anesthetic agents, or psychiatric disorders (Figure 1).
Anesthesia was induced with intravenous sufentanil (0.2 to 0.3 μg/kg), propofol (1.0 to 2.5 mg/kg), and cisatracurium (0.2 mg/kg). Maintenance anesthesia was achieved through continuous intravenous infusion of propofol (50 to 100 μg/kg/minute), remifentanil (0.1 to 1.0 μg/kg/minute), and cisatracurium (1 to 2 μg/kg/minute), maintaining a Bispectral index value between 45-60. Standard intraoperative monitoring included electrocardiography, non-invasive blood pressure measurement, and pulse oximetry.
Patients were divided into three groups based on dexmedetomidine (Sichuan Meidakang Huakang Pharmaceutial Co., Ltd) administration: (1) Control group: No dexmedetomidine was administered; (2) Low-dose group: Received a dexmedetomidine loading dose of 0.5 μg/kg (infused over 10 minutes) prior to anesthesia induction, followed by continuous intravenous infusion at 0.2 μg/kg/hour until 30 minutes before surgical conclusion; and (3) High-dose group: Received a dexmedetomidine loading dose of 1.0 μg/kg (infused over 10 minutes) prior to anesthesia induction, followed by continuous intravenous infusion at 0.5 μg/kg/hour until 30 minutes before surgical conclusion.
All patients received 5 mg tropisetron intravenously for postoperative nausea and vomiting prophylaxis. Postoperative analgesia was maintained using a patient-controlled intravenous analgesia (PCIA) pump containing 100 μg sufentanil and 200 mg flurbiprofen axetil diluted in 150 mL normal saline. The PCIA protocol included a 3 mL loading dose, 3 mL/hour background infusion, and 2 mL patient-controlled bolus doses with a 30-minute lockout interval.
Surgical and anesthetic data were retrieved, including site of surgery, duration of surgery and anesthesia, time to tracheal extubation, and intraoperative doses of dexmedetomidine, sufentanil, remifentanil, and propofol.
The primary outcome measure was the time to first flatus. Secondary outcomes included time to first defecation, time to first oral intake, and the postoperative 7-day intake, feeling nauseated, emesis, physical examination, and duration of symptoms (I-FEED) score. The I-FEED score evaluates five domains (tolerance of oral intake, nausea, vomiting, abdominal distension, and symptom duration), each score 0-2. Patients were categorized into three groups: Normal (score: 0-2), postoperative gastrointestinal intolerance (POGI) (score: 3-5), and postoperative ileus (score: ≥ 6).
Intraoperative adverse events were retrieved, including bradycardia (heart rate < 43 bpm), tachycardia (heart rate > 120 bpm), hypertension (mean arterial pressure > 20% above baseline or systolic blood pressure > 160 mmHg), and hypotension (mean arterial pressure < 20% below baseline or systolic blood pressure < 80 mmHg). Serious cardiovascular or cerebrovascular events occurring within 30 days postoperatively were also recorded.
Data were analyzed using SPSS 20.0. Continuous variables with normal distribution are presented as mean ± SD and compared using independent t-tests (two groups) or one-way analysis of variance (three groups). The least significant difference test was used for multiple comparison adjusted in one way analysis of variance. Categorical variables are presented as frequency (%) and analyzed with χ2 tests. Multiple linear regression was used to analyze factors related to the time to first flatus. A P value < 0.05 was considered statistically significant.
Following screening, a total of 879 patients undergoing laparoscopic colorectal cancer surgery were enrolled, including 281 in the control group, 313 in the low-dose group, and 285 in the high-dose group. No statistically significant differences were observed in baseline demographic or clinical characteristics among the three groups (P > 0.05, Table 1).
| Item | Control (n = 281) | Low-dose (n = 313) | High-dose (n = 285) |
| Age (year) | 66.94 ± 8.68 | 65.91 ± 8.76 | 66.32 ± 9.14 |
| Gender | |||
| Male | 159 (56.58) | 175 (55.91) | 170 (59.65) |
| Female | 122 (43.41) | 138 (44.08) | 115 (40.35) |
| BMI (kg/m2) | 23.65 ± 3.03 | 23.78 ± 3.73 | 24.82 ± 3.44 |
| ASA grading | |||
| I | 8 (2.84) | 14 (4.47) | 11 (3.86) |
| II | 228 (81.14) | 265 (84.66) | 243 (85.26) |
| III | 45 (16.01) | 34 (10.86) | 31 (10.88) |
| Hypertension | 41 (14.59) | 32 (10.22) | 35 (12.28) |
| Diabetes | 14 (4.98) | 10 (3.19) | 17 (5.96) |
| Coronary heart disease | 23 (8.19) | 26 (8.31) | 20 (7.02) |
No statistically significant differences were observed among the three patient groups regarding surgical site, operative duration, or anesthesia time (P > 0.05 for all). Patients in the high-dose group demonstrated a significantly longer time to tracheal extubation during recovery compared to the control group (P < 0.05). Both low- and high-dose groups required lower propofol and remifentanil doses compared to the control group, while the high-dose group additionally showed reduced sufentanil requirements relative to controls (P < 0.05 for all, Table 2).
| Item | Control (n = 281) | Low-dose (n = 313) | High-dose (n = 285) |
| Site of surgery | |||
| Proctectomy | 88 (31.32) | 96 (30.67) | 102 (35.79) |
| Colectomy | 193 (68.68) | 217 (69.33) | 183 (64.21) |
| Right hemicolectomy | 122 (43.41) | 141 (45.05) | 113 (39.65) |
| Left hemicolectomy | 61 (21.71) | 63 (20.13) | 59 (20.70) |
| Total colectomy | 10 (3.56) | 13 (4.15) | 11 (3.86) |
| Operation time (minute) | 207.97 ± 22.35 | 210.42 ± 23.03 | 208.97 ± 22.79 |
| Anesthesia time (minute) | 283.80 ± 23.17 | 285.25 ± 24.06 | 286.60 ± 25.06 |
| Extubate time (minute) | 21.54 ± 4.95 | 22.29 ± 6.47 | 23.20 ± 6.65a,1 |
| Dose of dexmedetomidine (μg) | 0 | 69.97 ± 11.71 | 159.51 ± 27.71a,2 |
| Propofol (mg) | 1080.72 ± 197.13 | 1002.78 ± 184.6a,1 | 1005.08 ± 183.30a,1 |
| Sufentanil (μg) | 27.52 ± 5.41 | 26.74 ± 5.53 | 26.18 ± 5.12a,1 |
| Remifentanil (mg) | 3.28 ± 0.63 | 2.67 ± 0.51a,1 | 2.59 ± 0.50a,1 |
| Cisatracurium (mg) | 23.10 ± 2.52 | 22.89 ± 2.62 | 23.24 ± 2.73 |
The time to first flatus in both the low-dose and high-dose groups was significantly shorter than that in the control group (65.56 ± 5.15 hours and 64.80 ± 4.90 hours vs 76.21 ± 5.32 hours, respectively; P < 0.05 for both). However, no statistically significant difference was observed between the low-dose and high-dose groups (P > 0.05).
The time to first defecation and time to first oral intake in both the low-dose and high-dose groups were significantly shorter compared to the control group (P < 0.05 for both). Furthermore, the I-FEED score indicated a lower incidence of POGI in the low-dose and high-dose groups than in the control group (P < 0.05) (Table 3).
| Control (n = 281) | Low-dose (n = 313) | High-dose (n = 285) | Low-dose vs control MD (95%CI) | High-dose vs control MD (95%CI) | |
| Time to first flatus (hour) | 76.21 ± 5.32 | 65.56 ± 5.15a | 64.80 ± 4.90a | -10.658 (-11.484 to -9.831) | -11.410 (-12.255 to -15.565) |
| Time to first faces (hour) | 96.03 ± 5.14 | 83.68 ± 5.37a | 82.91 ± 5.47a | -12.356 (-13.215 to -11.496) | -13.134 (-14.013 to -12.255) |
| Time to first oral feeding (hour) | 85.02 ± 5.40 | 73.65 ± 4.95a | 72.88 ± 4.80a | -11.372 (-12.187 to -10.558) | -12.141 (-12.974 to -11.308) |
| I-FEED score | |||||
| Normal | 197 (70.11) | 250 (79.97)a | 221 (77.54)a | ||
| POGI | 65 (23.13) | 45 (14.48)a | 43 (15.09)a | ||
| POI | 19 (6.76) | 18 (5.75) | 21 (7.37) |
Using multivariate regression analysis, the regression equation was significant (P < 0.001). Among them, dexmedetomidine group were significantly correlated with the time to first flatus (β = -10.658, P < 0.001). However, age, gender, body mass index (BMI), ASA classification, comorbidities and surgical site not correlated with the time to first flatus (all P > 0.05). These variables explained 50.20% of the variance in time to first flatus (Table 4).
| Item | Simple linear regression | Multiple linear regression | ||||
| β value | t value | P value | β value | t value | P value | |
| Constant | 78.428 | 41.315 | < 0.001 | 76.214 | 249.397 | < 0.001 |
| Age | -0.019 | -0.925 | 0.355 | |||
| Gender | 0.009 | 0.026 | 0.979 | |||
| BMI | -0.039 | -0.76 | 0.447 | |||
| ASA grading | ||||||
| II | 0.126 | 0.138 | 0.890 | |||
| III | -0.582 | -0.52 | 0.603 | |||
| Comorbidities | 0.277 | 0.544 | 0.586 | |||
| Site of surgery | -0.207 | -0.559 | 0.577 | |||
| Groups | ||||||
| Dexmedetomidine | -10.707 | -25.234 | < 0.001 | -10.658 | -25.316 | < 0.001 |
| High-dose dexmedetomidine | -0.746 | -1.769 | 0.077 | -0.752 | -1.794 | 0.073 |
Subgroup analysis by gender revealed that female patients undergoing laparoscopic colorectal surgery who received high-dose dexmedetomidine experienced a significantly shorter time to first postoperative flatus (P < 0.05) (Table 5).
No statistically significant differences were observed in the intraoperative incidence of tachycardia, hypotension, or hypertension among the three groups (P > 0.05). However, the high-dose group exhibited a significantly higher incidence of bradycardia compared to the control group (19.15% vs 8.19%, P < 0.05). No major cardiovascular or cerebrovascular events occurred within 30 days postoperatively in any group (Table 6).
| Control (n = 281) | Low-dose (n = 313) | High-dose (n = 285) | |
| Bradycardia | 23 (8.19) | 41 (13.10) | 56 (19.65)a |
| Tachycardia | 16 (5.69) | 15 (4.79) | 13 (4.56) |
| Hypotension | 31 (11.03) | 39 (12.46) | 40 (14.18) |
| Hypertension | 35 (12.46) | 31 (9.90) | 25 (8.77) |
This retrospective study analyzed the effects of different dexmedetomidine dosages on POGIR in patients undergoing laparoscopic colorectal surgery. The results show that both low-dose and high-dose dexmedetomidine regimens significantly accelerated POGIR compared to the control group, as evidenced by reduced time to first flatus, defecation, and oral intake. The results align with prior studies demonstrating dexmedetomidine’s positive impact on postoperative gastrointestinal function[5,6]. Our study provides new insights into this area and supports the use of low-dose regimens.
Multivariate regression analysis showed that age, gender, BMI, ASA classification, comorbidities and surgical site were not significantly correlated with the time to first flatus. I-FEED score effectively evaluates postoperative gastrointestinal function recovery in patients undergoing laparoscopic colorectal surgery[12]. The results showed a lower incidence of POGI and a higher incidence of normal postoperative gastrointestinal function in both the low-dose and high-dose dexmedetomidine regimens compared with the control group. Subgroup analysis showed no significant difference in the time to first postoperative flatus between male patients receiving low-dose or high-dose dexmedetomidine. Although high-dose dexmedetomidine provided an additional reduction in the time to first postoperative flatus for female patients undergoing laparoscopic colorectal surgery compared to low-dose, the reduction was only 1 hour, a difference of limited clinical significance, while significantly compromising safety. Therefore, we recommend low-dose dexmedetomidine as the preferred regimen for both male and female patients.
As a selective α2-adrenoceptor agonist, dexmedetomidine enhances gastrointestinal motility by inhibiting cate
However, the high-dose regimen conferred no additional clinical benefits and was associated with a higher incidence of intraoperative bradycardia. These findings suggest that while dexmedetomidine facilitates POGIR, its effect is not dose dependent, supporting the preferential use of low-dose regimens in clinical practice. The absence of a dose-dependent relationship in dexmedetomidine’s gastrointestinal benefits suggests a potential “ceiling effect”, wherein higher doses fail to amplify therapeutic outcomes. This may reflect maximal sympathetic inhibition at lower doses, with additional α2-receptor stimulation beyond this threshold yielding no further improvement in gastrointestinal motility[17,18].
The significantly elevated bradycardia risk in the high-dose group underscores the importance of dose optimization. Bradycardia and hypotension represent the most common adverse effects associated with dexmedetomidine[19]. Dexmedetomidine induces bradycardia primarily through α2-mediated vagomimetic effects, which suppress sinoatrial node activity. This effect can be clinically significant, particularly in patients with preexisting conduction abnormalities[20]. Intravenous anticholinergic agents (e.g., atropine) can counteract this vagally mediated bradycardia. Several case reports describe dexmedetomidine-associated cardiac arrest during general or regional anesthesia, as well as cardiac conduction disorders including left anterior fascicular block and first-degree atrioventricular block[21,22]. Co-administration of amiodarone with dexmedetomidine has also been implicated as a potential contributing factor in cardiac arrest events[23]. Although no severe cardiovascular complications occurred in this study, the inherent risk of bradycardia necessitates caution when administering high-dose dexmedetomidine.
Strengths of this study include its large sample size (n = 879) and standardized postoperative analgesia protocols (PCIA pumps) across groups minimized confounding from different analgesic agents. Also, we only included patients who underwent laparoscopic colorectal surgery and minimized confounding from different operations.
The study has several limitations. As a retrospective study, there may be potential selection bias. Despite comparable baseline characteristics, some confounders (e.g., intraoperative fluid management, preoperative bowel preparation) could not be excluded. Additionally, the single-center design limits generalizability, and the absence of long-term follow-up precludes assessment of complications beyond 30 days.
Both low- and high-dose dexmedetomidine regimens enhance POGIR in laparoscopic colorectal surgery patients. The low-dose regimen demonstrates superior safety, supporting its integration into multimodal enhanced recovery pathways. Prospective studies are warranted to validate these finding.
We are grateful to the patients and their families for supporting the study.
| 1. | Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001;182:3S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Zhong Y, Cao Z, Baumer D, Ajmani V, Dukes G, Chen YJ, Ayad SS, Wischmeyer PE. Incidence and risk factors for postoperative gastrointestinal dysfunction occurrence after gastrointestinal procedures in US patients. Am J Surg. 2023;226:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Tevis SE, Carchman EH, Foley EF, Harms BA, Heise CP, Kennedy GD. Postoperative Ileus--More than Just Prolonged Length of Stay? J Gastrointest Surg. 2015;19:1684-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 803] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 5. | Tseng WC, Lin WL, Lai HC, Chen TW, Chiu YC, Chen PH, Wu ZF. Adjunctive dexmedetomidine infusion in open living donor hepatectomy: A way to enhance postoperative analgesia and recovery. Int J Clin Pract. 2021;75:e14002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Huang SS, Song FX, Yang SZ, Hu S, Zhao LY, Wang SQ, Wu Q, Liu X, Qi F. Impact of intravenous dexmedetomidine on postoperative bowel movement recovery after laparoscopic nephrectomy: A consort-prospective, randomized, controlled trial. World J Clin Cases. 2021;9:7762-7771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Lu Y, Fang PP, Yu YQ, Cheng XQ, Feng XM, Wong GTC, Maze M, Liu XS; POGF Study Collaborators. Effect of Intraoperative Dexmedetomidine on Recovery of Gastrointestinal Function After Abdominal Surgery in Older Adults: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2128886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Wu Y, Cai Z, Liu L, Wang J, Li Y, Kang Y, An N. Impact of intravenous dexmedetomidine on gastrointestinal function recovery after laparoscopic hysteromyomectomy: a randomized clinical trial. Sci Rep. 2022;12:14640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Sun W, Li F, Wang X, Liu H, Mo H, Pan D, Wen S, Zhou A. Effects of Dexmedetomidine on Patients Undergoing Laparoscopic Surgery for Colorectal Cancer. J Surg Res. 2021;267:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Iirola T, Vilo S, Aantaa R, Wendelin-Saarenhovi M, Neuvonen PJ, Scheinin M, Olkkola KT. Dexmedetomidine inhibits gastric emptying and oro-caecal transit in healthy volunteers. Br J Anaesth. 2011;106:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | He GZ, Bu N, Li YJ, Gao Y, Wang G, Kong ZD, Zhao M, Zhang SS, Gao W. Extra Loading Dose of Dexmedetomidine Enhances Intestinal Function Recovery After Colorectal Resection: A Retrospective Cohort Study. Front Pharmacol. 2022;13:806950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Alsharqawi N, Alhashemi M, Kaneva P, Baldini G, Fiore JF Jr, Feldman LS, Lee L. Validity of the I-FEED score for postoperative gastrointestinal function in patients undergoing colorectal surgery. Surg Endosc. 2020;34:2219-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 14. | Xu L, Li T, Chen Q, Liu Z, Chen Y, Hu K, Zhang X. The α2AR/Caveolin-1/p38MAPK/NF-κB axis explains dexmedetomidine protection against lung injury following intestinal ischaemia-reperfusion. J Cell Mol Med. 2021;25:6361-6372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Park JK, Cheong SH, Lee KM, Lim SH, Lee JH, Cho K, Kim MH, Kim HT. Does dexmedetomidine reduce postoperative pain after laparoscopic cholecystectomy with multimodal analgesia? Korean J Anesthesiol. 2012;63:436-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | De Ponti F, Giaroni C, Cosentino M, Lecchini S, Frigo G. Adrenergic mechanisms in the control of gastrointestinal motility: from basic science to clinical applications. Pharmacol Ther. 1996;69:59-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Cho JS, Kim HI, Lee KY, An JY, Bai SJ, Cho JY, Yoo YC. Effect of Intraoperative Dexmedetomidine Infusion on Postoperative Bowel Movements in Patients Undergoing Laparoscopic Gastrectomy: A Prospective, Randomized, Placebo-Controlled Study. Medicine (Baltimore). 2015;94:e959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Talke P, Li J, Jain U, Leung J, Drasner K, Hollenberg M, Mangano DT. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. The Study of Perioperative Ischemia Research Group. Anesthesiology. 1995;82:620-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 485] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Kim BJ, Kim BI, Byun SH, Kim E, Sung SY, Jung JY. Cardiac arrest in a patient with anterior fascicular block after administration of dexmedetomidine with spinal anesthesia: A case report. Medicine (Baltimore). 2016;95:e5278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ohmori T, Shiota N, Haramo A, Masuda T, Maruyama F, Wakabayashi K, Adachi YU, Nakazawa K. Post-operative cardiac arrest induced by co-administration of amiodarone and dexmedetomidine: a case report. J Intensive Care. 2015;3:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Fritock MD, Ing RJ, Twite MD. Cardiac Arrest in 2 Neonates Receiving Amiodarone and Dexmedetomidine. J Cardiothorac Vasc Anesth. 2017;31:2135-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/