Published online Jan 21, 2025. doi: 10.3748/wjg.v31.i3.99833
Revised: October 30, 2024
Accepted: November 15, 2024
Published online: January 21, 2025
Processing time: 141 Days and 17.1 Hours
C-X-C chemokine receptor type 5 (CXCR5)+CD8+ T cells represent a unique immune subset with dual roles, functioning as cytotoxic cells in persistent viral infections while promoting B cell responses. Despite their importance, the specific role of CXCR5+CD8+ T cells in chronic hepatitis B (CHB), particularly during interferon-alpha (IFN-α) treatment, is not fully understood. This study aims to elucidate the relationship between CXCR5+CD8+ T cells and sustained serologic response (SR) in patients undergoing 48 weeks of pegylated IFN-α (peg-IFN-α) treatment for CHB.
To elucidate the relationship between CXCR5+CD8+ T cells and sustained SR in patients undergoing 48 weeks of peg-IFN-α treatment for CHB.
This study enrolled 60 patients with hepatitis Be antigen (HBeAg)-positive CHB undergoing 48 weeks of peg-IFN-α treatment. Participants were assessed for eligibility based on criteria such as persistent HBsAg-positive status for at least six months, HBeAb-negative, hepatitis B virus DNA levels exceeding 2 × 104 co
Patients with CHB exhibited significantly lower levels of circulating CXCR5+CD8+ T cells compared to healthy controls (P < 0.01). Notably, CXCR5+CD8+ T cells were prominently expressed in patients who achieved sustained SR compared to non-SR (NSR). A significant correlation was observed between CXCR5 and PD-L1 expression (r = -0.189, P = 0.002). However, there was no significant correlation between serum IL-21 levels and CXCR5+CD8+ lymphocytes (r = -0.03, P = 0.625) or serum ALT levels (r = 0.026, P = 0.678).
The enhanced expression of CXCR5+CD8+ T cells in patients achieving HBeAg seroconversion during IFN-α treatment suggests that these cells play a crucial role in antiviral immune responses against hepatitis B. This study highlights the potential of CXCR5+CD8+ T cells as immune regulators in CHB, which may inform future thera
Core Tip: This study highlights the pivotal role of C-X-C chemokine receptor type 5 (CXCR5)+CD8+ T cells in chronic hepatitis B (CHB) during pegylated interferon-alpha treatment. Our findings revealed that patients who achieved sustained serologic response exhibited higher baseline and treatment levels of CXCR5+CD8+ T cells, suggesting a connection between CXCR5 expression and IFN-induced antiviral immune responses. This research emphasizes the importance of CXCR5+CD8+ T cells as a potential biomarker for monitoring treatment efficacy in CHB patients.
- Citation: Xu ZY, Dai ZS, Gong GZ, Zhang M. C-X-C chemokine receptor type 5+CD8+ T cells as immune regulators in hepatitis Be antigen-positive chronic hepatitis B under interferon-alpha treatment. World J Gastroenterol 2025; 31(3): 99833
- URL: https://www.wjgnet.com/1007-9327/full/v31/i3/99833.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i3.99833
Hepatitis B virus (HBV) establishes a persistent infection, leading to a significant health burden worldwide[1]. Approximately 240 million individuals are living with chronic HBV infection, and HBV-related liver diseases claim around 780000 lives annually[2]. Pegylated interferon-alpha (peg-IFN-α), which mediates both immunomodulatory and direct antiviral effects, represents a preferred treatment option for chronic hepatitis B (CHB), which includes both the innate and adaptive immune responses[3]. This activation enhances the synthesis of antiviral proteins that inhibit viral replication and facilitate the elimination of the virus.
The intracellular JAK/STAT signaling pathway is activated by interferon-alpha (IFN-α) through the IFN-α/β receptor complex, influencing the transcription of numerous genes known as IFN-stimulated genes. It is well-established that pattern recognition receptors in various cell types can efficiently activate interferon (IFN) production. A subset of CD8+ T lymphocytes expressing the chemokine receptor C-X-C chemokine receptor type 5 (CXCR5) plays a crucial role in regulating viral replication. Circulating CXCR5+CD8+ T cells maintain robust antiviral capabilities and are associated with treatment response in patients with CHB[4].
Furthermore, the expression of CXCR5 on the surface of CD8+ T cells serves as an indicator of memory stem cell-like properties in progenitor cells, enhancing responsiveness to immune checkpoint blockade therapy in cancer[5]. This phe
In contrast to other CXCR5 subsets, CD8+CXCR5+ T cells express higher levels of PD-1 and produce greater amounts of HBV-specific cytokines, such as IFN and interleukin-21 (IL-21)[6], and are associated with decreased levels of HBsAg and HBV-DNA. These CXCR5+CD8+ T cells can migrate into B-cell follicles and exhibit enhanced cytotoxicity. Moreover, they have demonstrated improved therapeutic responses when combined with anti- programmed death-ligand 1 (PD-L1) treatment. However, the role of CXCR5-expressing CD8+ T cells in the context of anti-HBV treatment using peg-IFN-α remains unclear. Therefore, this study aims to investigate the dynamic changes in CXCR5-expressing CD8+ T cells in hepatitis Be antigen (HBeAg)-positive patients receiving peg-IFN-α-2b treatment from baseline to 72 weeks. Additionally, we will examine the relationships between CXCR5+CD8+ T cells and PD-L1, as well as IL-21 in peripheral blood.
This trial was registered at http://clinicaltrials.govas (NCT01760122) on 03-01-2013 and China Drug Trials.org (ID: TB1211IFN). The study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of the Second Xiangya Hospital. All methods were carried out in accordance with relevant guidelines and regulations.
Sixty patients with HBeAg-positive CHB from The Second Xiangya Hospital of Central South University (Changsha, China) participated in a phase III, multi-center, open-label clinical trial of peg-IFN-α-2b (PegBeron®; Amoytop Biotechnology, Xiamen, China) at a dosage of 180 µg/week (Clinical trial registration: NCT03903796, registered on 03-01-2013 at (http://clinicaltrials.gov). Five milliliters of serum were collected for cytokine detection, and twenty milliliters of heparinized blood were obtained for flow cytometry analysis at The Second Xiangya Hospital. Samples were taken at baseline and at 12, 24, and 48 weeks after initiating peg-IFN treatment, with a treatment-free follow-up at 24 weeks. Samples were transported on dry ice and stored at -80 °C in the Central South University Liver Disease Research Center.
The study population was divided into two groups: A sustained seroconversion (SR) group, which showed HBeAg loss with anti-HBe antibody seroconversion by week 72, and a non-sustained seroconversion (NSR) group, in which HBeAg remained positive or HBeAb did not appear by week 72. Additionally, twenty HBsAg-negative individuals with normal alanine aminotransferase (ALT) levels from laboratory staff and students served as healthy controls (HC). This study was conducted in compliance with the Declaration of Helsinki and approved by the Ethics Committee of the Second Xiangya Hospital. Informed consent was obtained from each participant.
(1) Diagnosis of HBeAg positive CHB; (2) HBsAg positivity for at least six months prior to study enrollment; (3) HBV DNA > 2 × 104 copies/mL; (4) ALT levels between 2 and 10 times the upper limit of normal; and (5) Treatment naive to peg-IFN-α-2b.
(1) History of liver transplantation; (2) Coinfection with hepatitis C virus, hepatitis D virus, or human immunodeficiency virus (HIV); (3) Severe liver dysfunction or cirrhosis; (4) Presence of autoimmune or immune related disorders; (5) Pregnancy or breastfeeding; and (6) Current or recent use of immunosuppressive medications.
Blood samples were collected at baseline and at 12, 24, and 48 weeks after initiating peg-IFN treatment, with an addi
Participants were classified into two groups: The SR group, which demonstrated HBeAg loss with anti-HBe antibody seroconversion by week 72, and the NSR group, in which HBeAg remained positive or HBeAb did not appear by week 72. Additionally, twenty HBsAg-negative individuals with normal ALT levels from laboratory staff and students served as HCs.
Routine physical examinations, biochemical, and hematologic assessments were conducted at weeks 0, 12, 24, 48, and 72. The presence of HBsAg, HBeAg, anti-HBs, and anti-HBe was determined using Elecsys tests (Roche Diagnostics GmbH, Germany), with detection limits of 1 COI (cut-off index) for HBeAg and 0.05 IU/mL for HBsAg. HBV DNA levels were measured using the Roche Cobas® Amplicor HBV Test, version 2.0 (Roche Diagnostics, Germany), with a detection limit of 20 IU/mL.
Serum IL-21 concentrations were measured in duplicate using a commercial human IL-21 ELISA kit (Human ELISA kit ab100554, Abcam Inc., Cambridge, MN, United States), with a lower detection limit of 20 pg/mL.
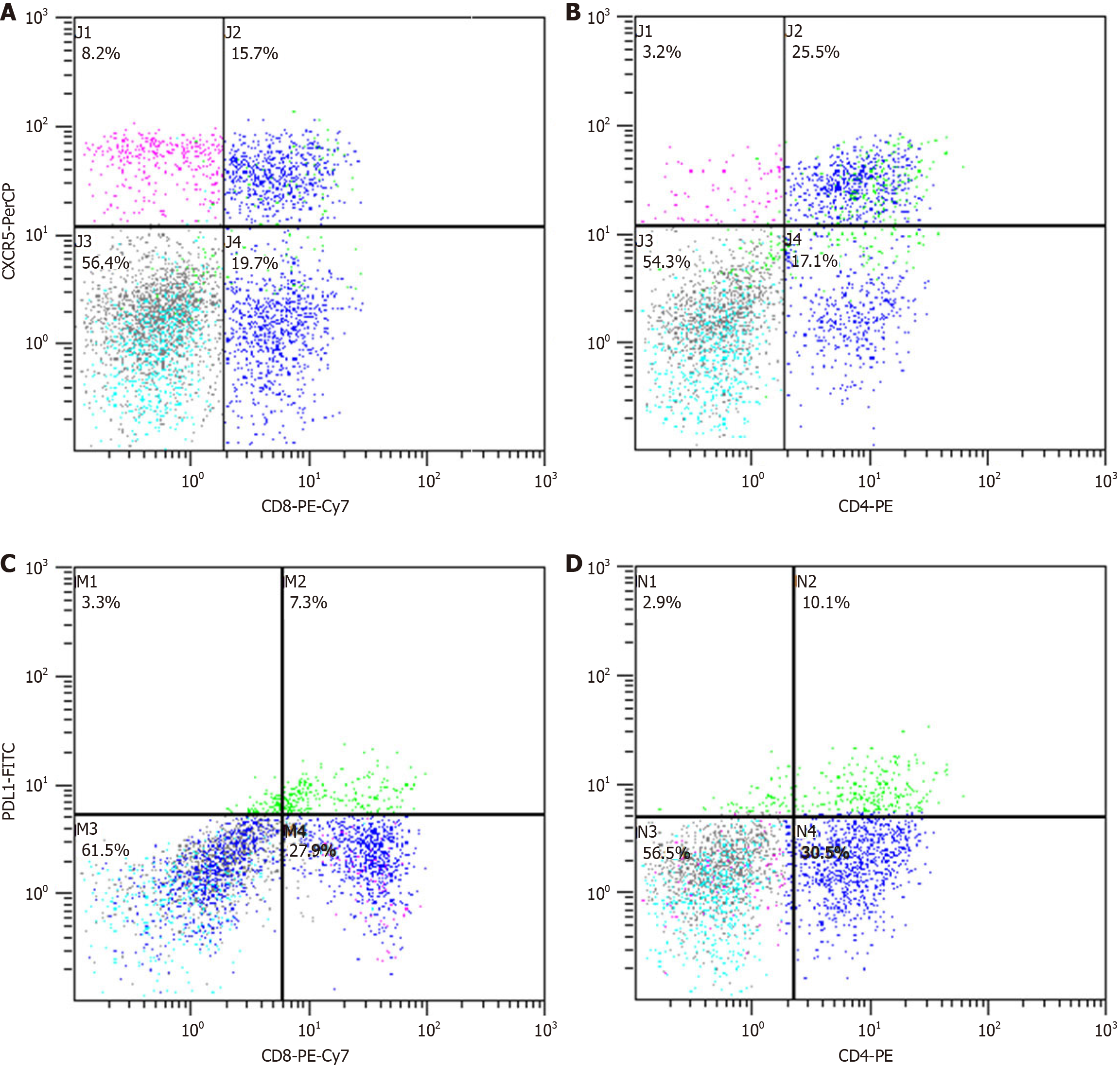
Peripheral blood mononuclear cells (PBMCs) were isolated and processed as previously described. A total of 1 × 106 PBMCs were stained with fluorochrome-conjugated antibodies: Anti-CD3-PE, anti-CD8-PE-CY7, anti-CD185 (CXCR5)-preCP-CY5.5, and anti-CD274 (PD-L1)-FITC for 30 minutes. Isotype controls were also included. Following staining, cells were washed twice and analyzed using a FACScan flow cytometer (Beckman Coulter, Inc., Brea, CA, United States) with Cell Quest software (version 5.1; BD Biosciences).
Statistical analyses were performed using IBM SPSS Statistics 23.0, with P values < 0.05 considered statistically significant in two-tailed tests. Continuous data are expressed as mean ± SE. Group comparisons utilized independent samples t-tests or one-way ANOVA with post-hoc least significant difference tests. χ2 tests were applied for categorical variables. Variance comparisons between groups employed a Folded F test. Spearman's rank correlation coefficient calculated associations among variables. Logistic binary regression analysis was conducted to identify factors associated with HBeAg seroconversion, reporting OR and 95%CI. Graphical representations and data visualizations were created using GraphPad Prism 9.
A total of 60 CHB patients receiving peg-IFN-α treatment (41.7% female, 58.3% male) and 20 HCs were studied (Figure 1). In the CHB cohort, 26.7% achieved sustained SR after 48 weeks of treatment, with baseline factors such as gender, HBeAg levels, and CXCR5+CD8+ T cells linked to HBeAg seroconversion (Table 1). Notably, CXCR5+CD8+ T cell levels were significantly higher in SR patients compared to non-SR (NSR), emphasizing their potential role in predicting response.
| HC (n = 20) | SR (n = 16) | NSR (n = 44) | P value | |
| Age (years) | 28.17 ± 0.25 | 26.00 ± 4.94 | 28.86 ± 8.32 | 0.260 |
| Gender (male/female) | 10/10 | 6/10 | 29/15 | 0.048a |
| Weight (kg) | 56.26 ± 4.20 | 57.54 ± 9.12 | 63.51 ± 11.03 | 0.097 |
| ALT (IU/mL) | 24.97 ± 6.47 | 205.55 ± 69.38 | 185.5 ± 103.61 | 0.530 |
| HBV DNA (log10 IU/mL) | n.d | 7.73 ± 0.61 | 7.94 ± 1.15 | 0.530 |
| HBsAg (log10 IU/mL) | Negative | 4.13 ± 0.46 | 4.44 ± 0.56 | 0.087 |
| Anti-HBs positive | 12 | 0 | 0 | n.d |
| HBeAg (log10 COI) | n.d | 2.90 ± 0.62 | 3.26 ± 0.46 | 0.044a |
| IL-21 (pg/mL) | 12.31 ± 5.42 | 91.27 ± 188.34 | 105.30 ± 379.75 | 0.901 |
| CXCR5+ (%) | 12.11 ± 4.00 | 17.39 ± 8.11 | 16.04 ± 8.68 | 0.639 |
| PD-L1+ (%) | 8.65 ± 1.94 | 12.57 ± 9.55 | 18.31 ± 10.97 | 0.113 |
| CXCR5+CD8+ (%) | 5.50 ± 2.43 | 3.41 ± 3.00 | 1.47 ± 1.34 | 0.042a |
| CXCR5+CD4+ (%) | 6.62 ± 1.06 | 14.43 ± 2.52 | 13.98 ± 1.39 | 0.867 |
| PDL1+CD8+ (%) | 7.75 ± 1.21 | 5.07 ± 3.22 | 8.59 ± 8.46 | 0.061 |
| PDL1+CD4+ (%) | 1.29 ± 0.23 | 7.95 ± 1.75 | 7.95 ± 1.40 | 0.812 |
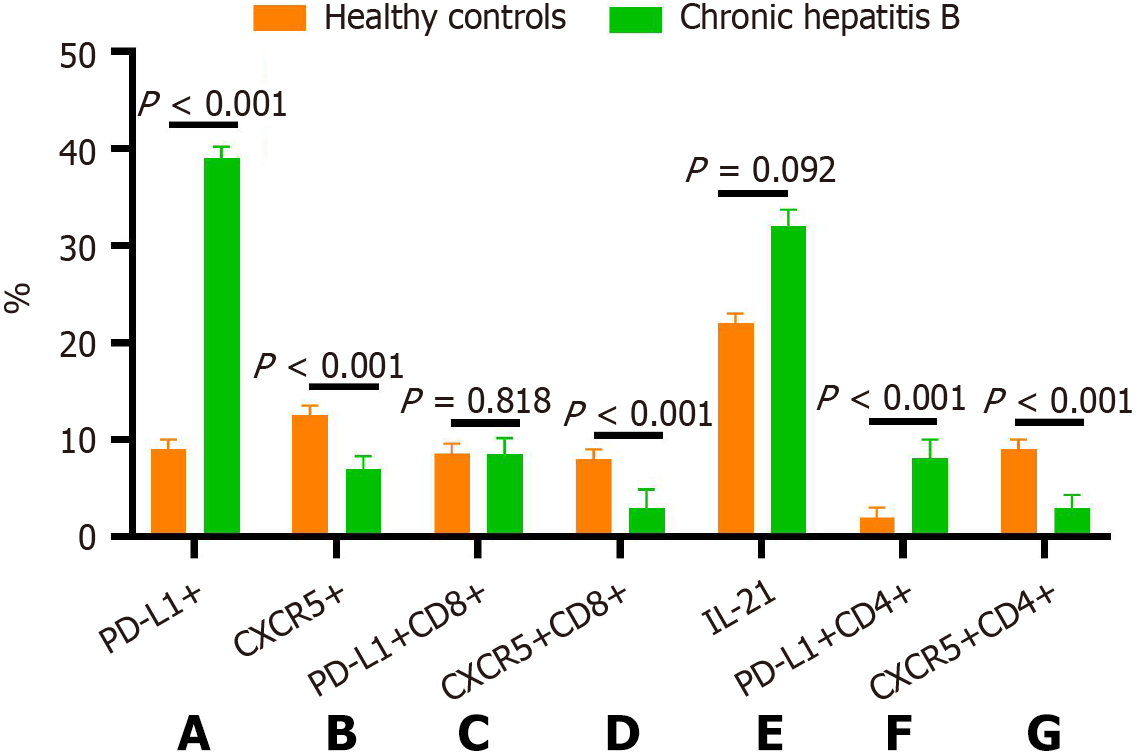
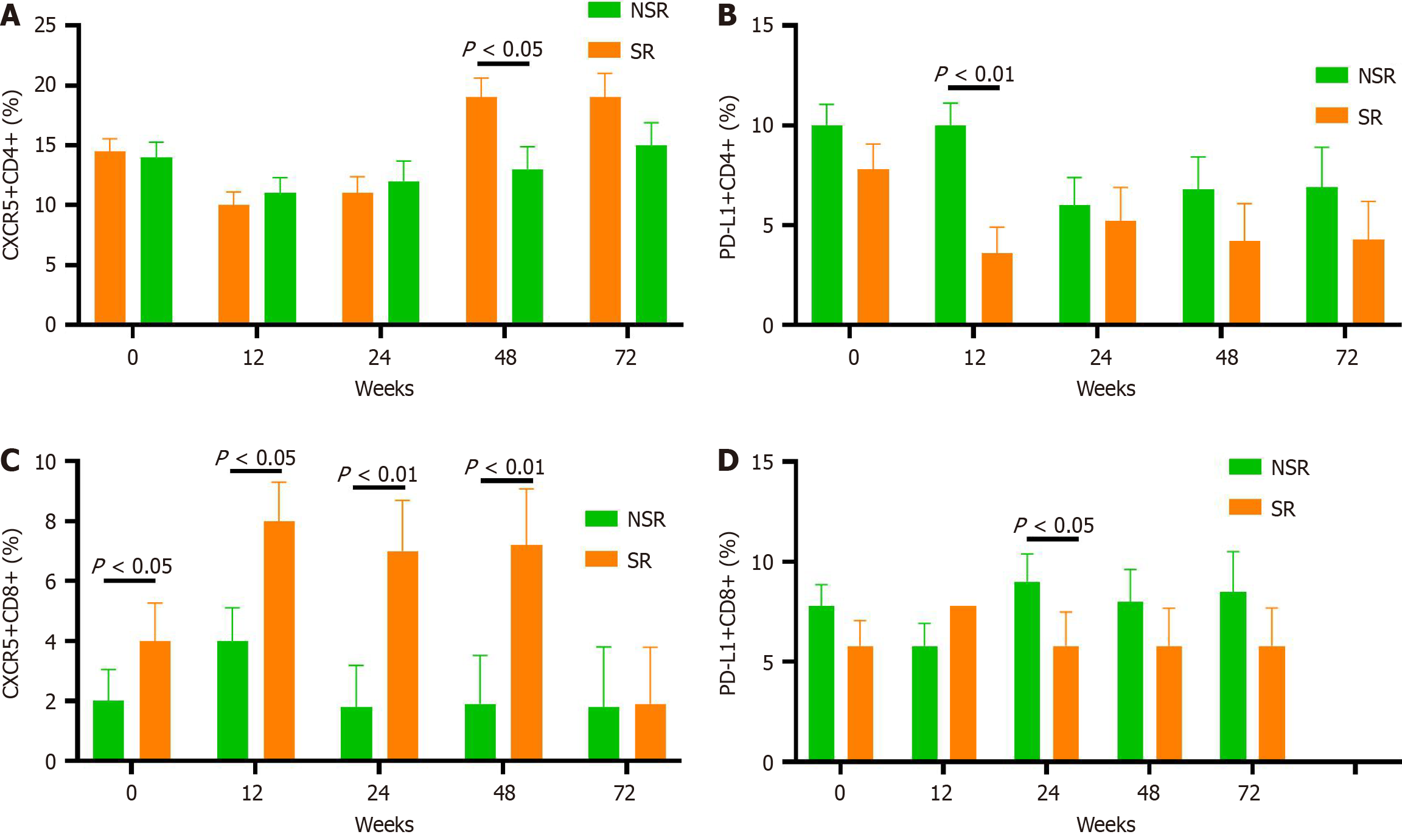
The frequency of CXCR5+ T cells in PBMCs and on CD8+/CD4+ T cells in CHB patients before peg-IFN treatment was significantly lower than in HCs (P < 0.001, P < 0.001, P = 0.008; Figures 2 and 3). In contrast, PD-L1 expression on PBMCs and CD4+ T cells in CHB patients was substantially higher than in HCs (P < 0.001). There was no difference in the frequency of CD8+PD-L1+ T cells between CHB patients and HCs. Although the CHB group had relatively higher levels of IL-21 compared to the HC group, the difference did not reach statistical significance (P = 0.092).
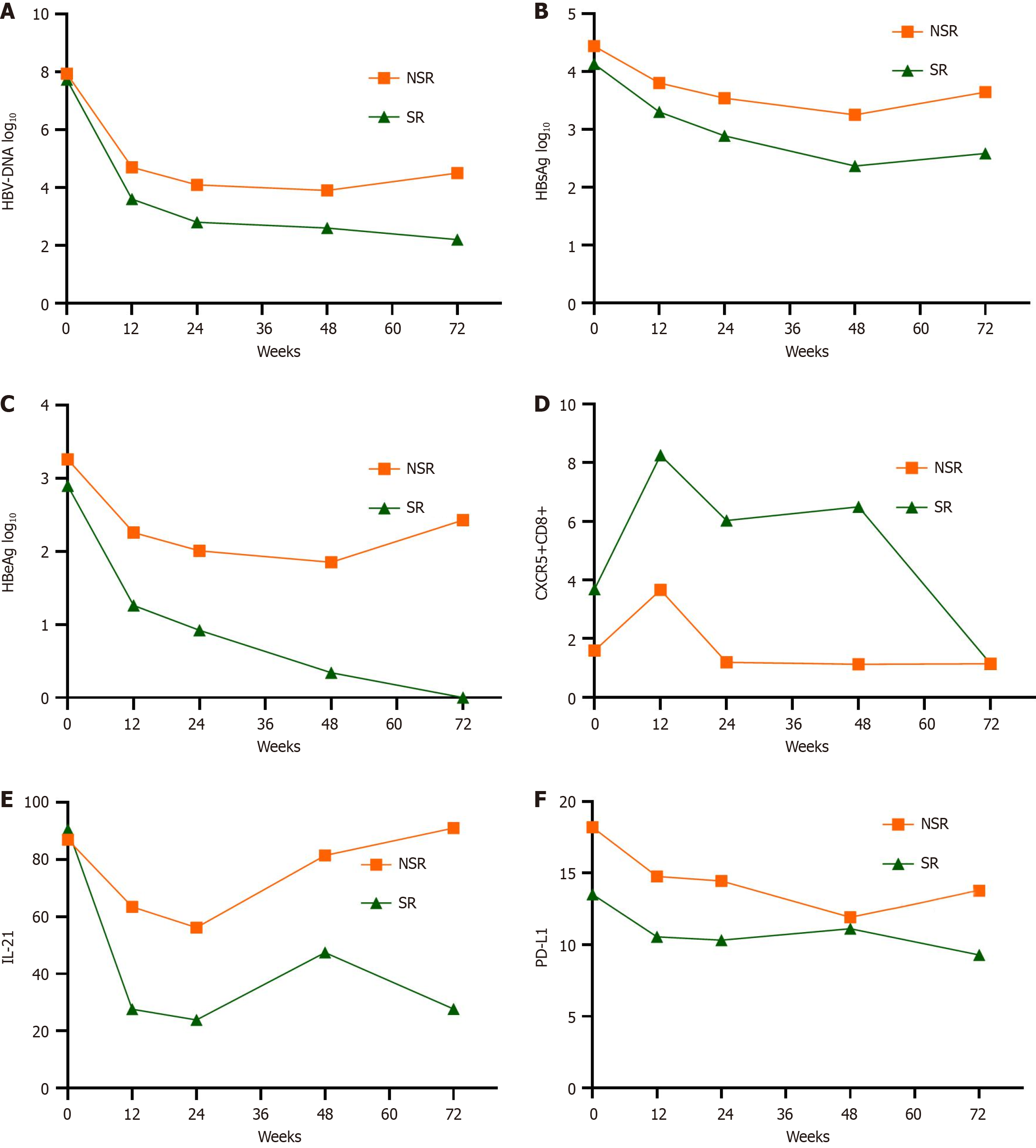
HBsAg, HBeAg, and HBV DNA levels gradually declined during peg-IFN-α treatment in both the SR and NSR groups. As shown in Figures 4 and 5.
At baseline, the percentage of CXCR5 expression on CD8+ lymphocytes was significantly higher in the SR group than the NSR group (Figure 4C, P = 0.042). This pattern persisted during the 48-week peg-IFN treatment (P = 0.013, P < 0.001, and P < 0.001 from week 12 to week 48, respectively). Only at week 48 did CXCR5 expression in CD4+ cells show a significant difference in the SR group.
Within the SR group, the percentage of CXCR5+CD8+ lymphocytes was significantly higher at weeks 12, 24, 48, and 72 compared to the baseline (Figure 5). After treatment, the expression of CXCR5 in CD8+ lymphocytes decreased to a significantly lower level at week 72 compared to the other time points. Concurrently, HBeAg levels gradually declined during treatment in the SR group. However, there was no significant difference in HBeAg levels between week 48 and 24 weeks post-treatment, although HBeAg continued to decrease after treatment. In contrast, in the NSR group, at week 12, the mean expression of CXCR5 in CD8+ lymphocytes increased with less than a 0.5 Log10 difference from baseline. However, CXCR5 expression in CD8+ lymphocytes decreased rapidly and remained at a low level at all time points after week 12. In addition, HBeAg levels fell at week 12 but exhibited a stalled decline at other time points.
At week 12, univariate regression analysis revealed that CXCR5+CD8+, PD-L1+CD4+ lymphocytes, and HBV DNA, HBsAg, and HBeAg were significantly associated with HBeAg seroconversion (P < 0.01). Multivariate regression analyses revealed a close relationship between HBeAg and CXCR5+CD8+ lymphocytes at week 12 with SR achievement (P < 0.05) (Table 2).
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| ALT | 1.002 | 0.998-1.002 | 0.383 | 1.008 | 0.999-1.014 | 0.063 |
| IL-21 | 0.999 | 0.994-1.004 | 0.645 | |||
| PDL1 | 0.957 | 0.891-1.029 | 0.236 | |||
| CXCR5 | 1.038 | 0.953-1.131 | 0.395 | 0.652 | 0.387-1.098 | 0.107 |
| PDL1+CD8+ | 1.070 | 0.959-1.193 | 0.225 | |||
| PDL1+CD4+ (%) | 0.85 | 0.733-0.984 | 0.018a | |||
| CXCR5+CD8+ | 1.459 | 1.125-1.892 | 0.004b | 4.820 | 1.081-21.487 | 0.039a |
| CXCR5+CD4+ | 0.952 | 0.853-1.062 | 0.375 | |||
| log10 HBV DNA | 0.412 | 0.241-0.705 | 0.001b | |||
| log10 HBsAg | 0.126 | 0.032-0.507 | 0.004b | |||
| log10 HBeAg | 0.300 | 0.144-0.626 | 0.001b | 0.009 | 0.001-0.490 | 0.021a |
This study highlights the significant role of CXCR5+CD8+ T cells in chronic HBV infection, particularly in the context of peg-IFN-α treatment. Our findings suggest that these cells may contribute to immune responses associated with HBeAg seroconversion, indicating potential therapeutic implications for chronic HBV management.
Chronic HBV infection affects approximately 2 billion people worldwide, particularly in Southeast Asia and China, where over 50% of hepatocellular carcinoma cases are linked to HBV infection[7]. Dysfunction of effector CD8+ T cells is often attributed to the immune system's failure to control viral replication. However, recent clinical and experimental studies on HIV and lymphocytic choriomeningitis virus (LCMV) infections challenge this notion, demonstrating that a subset of exhausted CD8+ T cells expressing CXCR5 plays a critical role in regulating viral replication[8,9].
CXCR5+CD8+ T cells are a unique subset with significant antitumor and antiviral activities, found in human tonsils and follicular lymphoma. They inhibit T (FH)-mediated B cell differentiation and show enhanced cytotoxicity, with adoptive transfer resulting in greater antitumor efficacy compared to CXCR5- cells. Research by Chu et al[5] identified 77 differentially expressed genes, with 33 linked to improved survival in follicular lymphoma. Additionally, these cells can be expanded using IL-23 and TGF-beta, highlighting their potential for therapeutic applications. Overall, CXCR5+CD8+ T cells play a critical role in suppressing B cell differentiation and enhancing antitumor responses.
Li et al[4] found that CXCR5+CD8+ T cells, particularly the intrahepatic CXCR5+ subset, retain effector functions characterized by enhanced production of HBV-specific IFN-γ and IL-21. Notably, PD-1 blockade and exogenous IL-21 enhance the production of IFN-γ from these cells. Furthermore, this subpopulation exhibits more potent antiviral activity than their CXCR5- counterparts, inhibiting HBsAg expression and supporting antibody production by B cells. This aligns with our findings that patients with higher CXCR5+CD8+ T cell expression during peg-IFN treatment exhibit stronger antiviral capabilities and a greater likelihood of achieving HBeAg seroconversion.
Notably, studies have shown that CXCR5+CD8+ T cells exhibit a phenotype similar to T follicular helper cells and reside within B cell follicles, suggesting they play a crucial role in mediating humoral responses. This resemblance indi
In pediatric HBV infection, recent literature has identified a potential antiviral role for CCR5+CD8+ T cells. A study by Tan et al[6] highlighted the diverse phenotypes and functions of CD8+ T cells in children with HBV, noting that the frequency of CCR5+CD8+ T cells correlated positively with patient age and CCR5+CD4+ T cells, while negatively corre
Successful IFN treatment in CHB patients exhibiting HBeAg seroconversion was associated with higher levels of CXCR5+CD8+ T cells and a sustained increase in these lymphocytes during peg-IFN-α therapy[11,12]. The CXCR5 expre
While previous studies reported significant differences in serum IL-21 concentrations between patients with sustained and non-SR[14,15], our study did not replicate these findings. Similar to our results, those studies indicated that IL-21 concentrations decreased over time during peg-IFN therapy in both groups. This aligns with evidence suggesting that IL-21 primarily participates in liver cirrhosis inflammation and fibrogenesis. We observed a positive association between IL-21 and CXCR5+CD4+ T cells, consistent with IL-21 being predominantly produced by CD4+ T cells.
PD-1 expression plays a unique role in CXCR5+CD8+ T cells. Although in a chronic LCMV infection model[16], PD-1 expression on CXCR5+CD8+ T cells was lower than on CXCR5−CD8+ T cells, other studies have shown that both cell types express PD-1, with CXCR5+ T cells displaying slightly higher levels[17,18]. In HIV infection, PD-1 may help localize CXCR5+CD8+ T cells to follicles rather than solely marking exhaustion[19]. Our findings show that PD-L1 expression on PBMCs significantly decreased at weeks 24 and 72 and on CD8+ lymphocytes at week 24, with no differences observed at other time points. Furthermore, we identified a negative correlation between PD-L1 and CXCR5 lymphocytes in peg-IFN-α-treated patients, indicating that PD-L1 may influence circulating CXCR5 expression in CD8+ lymphocytes, thereby impacting viral kinetics during treatment.
In summary, this study demonstrated that circulating CXCR5+CD8+ T cells may contribute to the immunotherapy mechanism of peg-IFN-α-2b in chronic HBV patients. The relationship between PD-L1 and CXCR5 was found to be inverse. Although CXCR5+CD8+ T cells did not produce most IL-21 in CHB patients, they interacted with CXCR5+CD4+ T cells. Future research could analyze how IFN, PD-L1, and CXCR5+CD8+ T cells interact to influence signaling path
This study has several limitations. The small sample size, especially when comparing the SR and NSR groups, may reduce statistical power and affect the reliability of the findings. Furthermore, discrepancies in demographic variables between the HC and patient groups could have introduced biases. Additionally, the study lacked transcriptional marker analysis and focused primarily on peripheral rather than liver-resident CCR5+CD8+ T cells.
The HBeAg seroconversion patients treated with IFN exhibited higher levels of CXCR5+CD8+ cell expression. The CXCR5 expression on CD8+ T cells is most likely related to IFN-induced antiviral immune responses against hepatitis B infections.
| 1. | Sun Y, Teng Y, Wang L, Zhang Z, Chen C, Wang Y, Zhang X, Xiang P, Song X, Lu J, Li N, Gao L, Liang X, Xia Y, Wu Z, Ma C. LINC01431 Promotes Histone H4R3 Methylation to Impede HBV Covalently Closed Circular DNA Transcription by Stabilizing PRMT1. Adv Sci (Weinh). 2022;9:e2103135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Zhao C, Wu X, Chen J, Qian G. The therapeutic effect of IL-21 combined with IFN-γ inducing CD4+CXCR5+CD57+T cells differentiation on hepatocellular carcinoma. J Adv Res. 2022;36:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Xun Z, Lin J, Yu Q, Liu C, Huang J, Shang H, Guo J, Ye Y, Wu W, Zeng Y, Wu S, Xu S, Chen T, Chen J, Ou Q. Taurocholic acid inhibits the response to interferon-α therapy in patients with HBeAg-positive chronic hepatitis B by impairing CD8+ T and NK cell function. Cell Mol Immunol. 2021;18:461-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Li Y, Tang L, Guo L, Chen C, Gu S, Zhou Y, Ye G, Li X, Wang W, Liao X, Wang Y, Peng X, Liu G, Zhang X, Sun J, Peng J, Hou J. CXCL13-mediated recruitment of intrahepatic CXCR5+CD8+ T cells favors viral control in chronic HBV infection. J Hepatol. 2020;72:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Chu F, Li HS, Liu X, Cao J, Ma W, Ma Y, Weng J, Zhu Z, Cheng X, Wang Z, Liu J, Jiang ZY, Luong AU, Peng W, Wang J, Balakrishnan K, Yee C, Dong C, Davis RE, Watowich SS, Neelapu SS. CXCR5+CD8+ T cells are a distinct functional subset with an antitumor activity. Leukemia. 2019;33:2640-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Tan A, He Y, Zhou Y, Peng X, Chang Y, Peng M, Ren H, Xu H. A potential antiviral role for CCR5+CD8+ T cells in children with hepatitis B. J Med Virol. 2024;96:e29661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, Ford N, Han Q, Lu Y, Bulterys M, Hutin Y. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:106-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 8. | He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, Xu L, Chen X, Hao Y, Wang P, Zhu C, Ou J, Liang H, Ni T, Zhang X, Zhou X, Deng K, Chen Y, Luo Y, Xu J, Qi H, Wu Y, Ye L. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 553] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 9. | Guo AL, Jiao YM, Zhao QW, Huang HH, Deng JN, Zhang C, Fan X, Xu RN, Zhang JY, Zhen C, Xie ZM, Qin YM, Xu JQ, Yang Y, Shi M, Huang L, Song JW, Wang FS. Implications of the accumulation of CXCR5+ NK cells in lymph nodes of HIV-1 infected patients. EBioMedicine. 2022;75:103794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Jensen O, Trivedi S, Meier JD, Fairfax KC, Hale JS, Leung DT. A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa. Sci Immunol. 2022;7:eabe8931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Li M, Sun F, Bi X, Lin Y, Yang L, Lu Y, Zhang L, Wan G, Yi W, Zhao L, Xie Y. Consolidation treatment needed for sustained HBsAg-negative response induced by interferon-alpha in HBeAg positive chronic hepatitis B patients. Virol Sin. 2022;37:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Zhou J, He X, Ou Y, Peng S, Li D, Zhou Q, Fu J, Long Y, Tan Y. Role of CXCR5+ CD8+ T cells in human hepatitis B virus infection. J Viral Hepat. 2023;30:638-645. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Khanam A, Tang LSY, Kottilil S. Programmed death 1 expressing CD8+ CXCR5+ follicular T cells constitute effector rather than exhaustive phenotype in patients with chronic hepatitis B. Hepatology. 2022;75:690-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Ragab HM, El Maksoud NA, Amin MA, Halim MH, Abdulla NA, Kamel A, Moussa SM. IL-21 as a Predictor of Sustained Virologic Response in Patients with Chronic Hepatitis C Virus Infection. Appl Biochem Biotechnol. 2018;185:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Wang X, Xu ZQ, Fu JJ, Cheng LW, Li Y, Li L, Pan XC. Role of interleukin-21 and interleukin-21 receptor polymorphisms in the treatment of HBeAg-positive chronic hepatitis B patients with peginterferon. Medicine (Baltimore). 2018;97:e10891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Raju S, Verbaro DJ, Egawa T. PD-1 Signaling Promotes Control of Chronic Viral Infection by Restricting Type-I-Interferon-Mediated Tissue Damage. Cell Rep. 2019;29:2556-2564.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Le KS, Amé-Thomas P, Tarte K, Gondois-Rey F, Granjeaud S, Orlanducci F, Foucher ED, Broussais F, Bouabdallah R, Fest T, Leroux D, Yadavilli S, Mayes PA, Xerri L, Olive D. CXCR5 and ICOS expression identifies a CD8 T-cell subset with T(FH) features in Hodgkin lymphomas. Blood Adv. 2018;2:1889-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams IR, Skinner PJ, Velu V, Amara RR. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A. 2017;114:1976-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Jiao YM, Yang HG, Huang HH, Tu B, Xing SJ, Mao L, Xia W, He R, Zhang JY, Xu RN, Jin L, Shi M, Xu Z, Qin EQ, Wang XC, Wu H, Ye L, Wang FS. Dichotomous Roles of Programmed Cell Death 1 on HIV-Specific CXCR5+ and CXCR5- CD8+ T Cells during Chronic HIV Infection. Front Immunol. 2017;8:1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/