Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.106895
Revised: April 15, 2025
Accepted: July 14, 2025
Published online: August 7, 2025
Processing time: 147 Days and 6.8 Hours
It is critical to explore effective therapeutic targets for improving the survival rate of patients with hepatocellular carcinoma (HCC). Although many studies have focused on flotillin-1 (FLOT1) as a lipid raft-associated protein that regulates the activation of some proteins or kinases to promote tumor cell survival and proliferation, few studies have explored the regulation of Golgi apparatus function.
To investigate the molecular mechanism through which FLOT1 activates the Golgi stress response downstream of transcription factor E3 (TFE3), thereby promoting the progression of HCC.
FLOT1 expression in HCC tissue, HCC cell lines, and nude mouse tumor models was assessed. The impact of FLOT1 silencing or its overexpression on the proliferation of HCC cells was studied. CCK-8, flow cytometry, and transwell assays were used to assess the proliferation, cell cycle, migration, and invasion abilities of HCC cells. A dual-luciferase reporter assay was used to study the effect of FLOT1 on the transcriptional activity of the downstream Golgi apparatus stress element promoter of TFE3. Western blotting, co-immunoprecipitation, and immunofluorescence staining were employed to detect relevant proteins.
High FLOT1 expression was correlated with a poor prognosis in patients with HCC. The knockdown of FLOT1 suppressed the proliferation, migration, and invasion of HCC cells and promoted their apoptosis. Xenograft assays revealed that FLOT1 knockdown inhibited HCC tumorigenesis in vivo. Mechanistically, FLOT1 inhibited the expression of mechanistic target of rapamycin complex 1/2 proteins through ubiquitination and downstream effector p-S6 kinase-T389, leading to the dephosphorylation and nuclear translocation of TFE3 and promotion of Golgi stress-mediated responses, ultimately resulting in HCC progression.
FLOT1 recruits and inhibits mechanistic target of rapamycin complex 1/2, causing dephosphorylation and TFE3 nuclear translocation, thereby activating the Golgi stress response and further promoting the proliferation, migration, and invasion capabilities of HCC cells. These results underscore the potential of FLOT1 as a promising therapeutic target for HCC.
Core Tip: Research has found a close correlation between Golgi apparatus function and the survival of cancer cells. This study elucidates that elevated expression of flotillin-1 (FLOT1) promotes the proliferation, migration, and invasion capabilities of hepatocellular carcinoma (HCC), as well as the mechanism by which FLOT1 inhibits mechanistic target of rapamycin complex 1/2 by ubiquitination, leading to the dephosphorylation and nuclear translocation of the transcription factor transcription factor E3, thereby activating the Golgi stress response and further promoting the malignant biological behavior of HCC. These findings underscore the potential of the lipid raft protein FLOT1 as a promising therapeutic target for HCC treatment in clinical practice.
- Citation: Zhang L, Bai CZ, Shan JY, Xue HL, Zheng SM, Chen YL, Tang SH. Flotillin-1 promotes the progression of hepatocellular carcinoma by activating TFE3-mediated Golgi stress response via inhibition of mTORC1/2. World J Gastroenterol 2025; 31(29): 106895
- URL: https://www.wjgnet.com/1007-9327/full/v31/i29/106895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i29.106895
Hepatocellular carcinoma (HCC) is a malignant neoplasm originating from hepatocytes and is the third leading cause of cancer-related mortality globally, accounting for 4.3% of all cancer cases and 7.8% of cancer deaths, surpassed only by lung and colorectal cancers[1]. It is estimated that by 2025, HCC will affect more than one million patients annually[2]. In China, liver cancer incidence and mortality rates rank among the top five, posing a significant threat to public health[1]. HCC is the predominant form of primary liver cancer and is frequently associated with viral hepatitis infections (including hepatitis B and C), liver cirrhosis, liver fibrosis, and nonalcoholic fatty liver disease, among other risk factors[3-7]. However, owing to its rapid progression and absence of early symptoms, most patients are diagnosed at advanced stages of the disease. The clinical course of HCC is typically aggressive, resulting in a particularly poor prognosis. Con
The Golgi apparatus is the main site for protein modification and activation within the cell and plays important roles as a stress sensor, a cell cycle trigger, a lipid/protein regulator, a mitotic checkpoint, and a mediator of malignant trans
The term “lipid raft” refers to a membrane domain rich in cholesterol, sphingolipids, and proteins, which serves as a crucial hub for signaling pathway transduction[22]. Flotillin (FLOT), a lipid raft-associated protein, is primarily expressed in the cytoplasm and Golgi apparatus of cells and plays a regulatory role in vesicular transport and protein modification and activation. FLOT consists of two highly conserved homologous proteins, FLOT1 and FLOT2[23]. Studies on the association of FLOT with tumors have shown that FLOT is upregulated in many cancers and can promote tumor cell survival and proliferation by modulating the activation of certain proteins or kinases, resulting in metastasis and poor prognosis[24-26]. Notably, FLOT can promote epithelial-mesenchymal transition, proliferation, and metastasis of HCC cells (HCCLM3 cells) by activating the protein kinase B/Wnt/β-catenin signaling pathway[27]. These findings indicate that FLOT primarily exerts a protumor effect by influencing protein expression and modification. However, whether this effect is related to the regulation of Golgi apparatus function is yet to be unambiguously established. This study aims to investigate the molecular mechanism through which FLOT1 activates the Golgi stress response downstream of TFE3 to promote the progression of HCC.
HCC tissue and paracancer tissue samples were collected from patients with HCC who received pathological liver resection at the General Hospital of Western Theater Command between January 2020 and December 2021. Written informed consent was obtained from all patients as per the protocols approved by the Institution Ethics Committee of the General Hospital of Western Theater Command.
HCC tissues were paraffin-embedded and sectioned. Immunohistochemistry (IHC) was performed using SAP kit (ZSGB-BIO, China). After dewaxing and hydration, the paraffin specimens of HCC tissues were placed in an autoclave containing 0.01-μM citrate buffer (pH = 6.0) for antigen retrieval. Then, the sections were blocked with 10% goat serum for 1 hour and incubated with FLOT1 antibody (Bioworld, China) overnight at 4 °C. The next day, the tissue sections were washed and incubated with the secondary antibody for 15 minutes. The immune-stained tissue sections were visualized using the 3,3’-diaminodbenzidine substrate. The immunochemistry results were independently evaluated by two pathologists under a microscope. Five representative staining fields of each HCC sample were analyzed.
The Kaplan-Meier Plotter Survival Analysis Database (http://www.kmplot.com/) was used to integrate The Cancer Genome Atlas (TCGA) data with microarray chips and used to analyze the relationship between gene expression levels and patient survival prognosis. This database was then utilized to analyze the relationship between the expression of FLOT1 and the overall survival time, disease-free stage, and different clinical characteristics of patients with HCC. The split cutoff of low and high expression was set in the automatic selection mode of the best truncation model; biased arrays were excluded. The log-rank test was used for computing P-value. P < 0.05 was regarded as significant.
The GSE14520 datasets were sourced from the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/), with the downloaded data in the MINiML format. R software (version v4.0.3) was used to analyze the expression of FLOT1 and TFE3 in the tumor tissue and non-tumor tissue, respectively. The results were considered statistically significant when the P-value was less than 0.05. The STAR-counts data and the corresponding clinical information for HCC were downloaded from the TCGA database (https://portal.gdc.cancer.gov). Subsequently, the data in the TPM format were extracted and normalized using log2(TPM + 1). After retaining samples with both RNAseq data and clinical information, 371 samples were obtained for further analysis. The ggstatsplot package in R software was utilized to generate correlation plots between two genes. Spearman’s correlation analysis was employed to describe the correlation between FLOT1 and TFE3. A significant correlation was considered to exist between the two variables if the P-value was less than 0.05. The correlation coefficient ranged from -1 to 1, where negative values indicated a negative correlation in gene expression and positive values indicated a positive correlation. The closer the absolute value of the correlation coefficient was to 1, the stronger the correlation between the two variables; conversely, the closer it was to 0, the weaker the correlation.
The human liver cancer cell line Huh-7 (No. iCell-h080) was purchased from Cellverse Bioscience Technology Co., Ltd., (Shanghai, China), and the human liver cancer cell line SNU-398 (No. SCSP-5206) was obtained from the Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank (Shanghai, China). The cells were cultured in the DMEM medium (No. PM150210, Procell Life Science & Technology Co., Ltd., China) supplemented with 10% fetal bovine serum (No. C04001-500, Vivacell, China) and 1% double antibody (S110JV, Shanghai BasalMedia Technologies Co., Ltd., China) in a 5% CO2 incubator at 37 °C. The cells were passaged at a ratio of 1:3.
FLOT1 overexpression plasmid (pCDH) and its control plasmid were transiently transfected into the liver cancer cell line Huh-7 using Lipofectamine. In brief, to prepare the transfection complex, two 1.5-mL centrifuge tubes were used, and 300 μL of Opti-MEMTM I reduced serum medium was added to each tube. To one of the tubes, 15 μg of plasmid was added, while 30 μL of Lipo 2000 transfection reagent was added to the other tube. After gentle mixing and allowing the mixture to stand for 5 minutes, the plasmid-containing medium was combined with the Lipo 2000-containing medium through gentle pipetting and the resulting solution was thoroughly mixed. The resulting mixture was allowed to stand for 10 minutes before adding sufficient medium to achieve the final volume of 6 mL. Huh-7 cells, at the logarithmic growth phase, were prepared with a cell density adjusted to 1 × 105 cells/mL. These cells were then seeded at 2 mL per well in a 6-well plate and incubated at 37 °C with 5% CO2. After the cells adhered to the wells, the supernatant was removed, and the transfection complex was added. The cells were further incubated at 37 °C with 5% CO2. After 6 hours, the medium was replaced with a complete culture medium, and the cells continued to be cultured.
Short-hairpin RNA (ShRNA) knockdown plasmid (psi-LVRU6GP) and its control plasmid were transfected into the liver cancer cell line SNU-387. The FLOT1 gene fragment was amplified by polymerase chain reaction (PCR) using the following primers: ShRNA-FLOT1: 5’-GGAAGACGGAGGCTGAGATTG-3’ and shRNA-NC: 5’-GTTCTCCGAACGTGTCACGT-3’. To create the transfection complex, 15 μL of shRNA was combined with 360 μL of 1 × riboFECTTM CP Buffer. Subsequently, 36 μL of riboFECTTM CP reagent was added to the mixture and gently pipetted to ensure thorough mixing. The resulting solution was incubated at room temperature for 15 minutes, allowing the formation of the riboFECTTM CP transfection complex with a final shRNA concentration of 50 nM. SNU-387 cells, also in the logarithmic growth phase, were adjusted to a density of 1 × 105 cells/mL. Subsequently, 2 mL of this cell suspension was transferred into each well of a six-well plate. The plate was then placed in an incubator set at 37 °C with an atmosphere containing 5% CO2 to maintain stable conditions during cultivation. For the transfection procedure, the prepared riboFECTTM CP transfection complex was added to the cell culture medium. This mixture was thoroughly combined before being introduced into the wells on the six-well plate, which was subsequently incubated under stable conditions at 37 °C and with 5% CO2.
Cell experiment 1: Huh-7 cells were divided into the OV-NC and OV-FLOT1 groups. SNU-387 cells were divided into the shRNA-NC and shRNA-FLOT1 groups.
Cell experiment 2: Huh-7 cells were divided into the OV-NC + H2O2 (0, 100, 200, 500, and 1000 μM), and OV-FLOT1 +
Cell experiment 3: SNU-387 cells were divided into the shRNA-NC, shRNA-NC + mTORC1/2 inhibitor (Torin1), shRNA-FLOT1, and shRNA-FLOT1 + mTORC1/2 inhibitor (Torin1) groups.
Huh-7 and SNU-387 cells, during their logarithmic growth phase, were seeded into a 96-well culture plate at a density of 5 × 104 cells per well (100 μL per well). The plate was then incubated in an environment with 5% CO2 at 37 °C to ensure optimal conditions for cell proliferation and maintenance. Following incubation for 24, 48, and 72 hours, CCK-8 solution (10 μL) was meticulously added to each well and kept at a stable temperature of 37 °C within the same CO2 atmosphere for 2 hours. The absorbance of each well was subsequently measured at 450 nm using a microplate reader (ELx800, BioTek® Instruments, VT, United States).
For cell migration assays, Huh-7 and SNU-387 cells in the logarithmic growth phase were adjusted to a density of 2 × 105 cells/mL and cultured in a CO2 incubator maintained at 37 °C for 48 hours. Following this, the cells were serum-starved in a serum-free medium for 24 hours, after which the cell density was adjusted to 1 × 105 cells/mL. Next, 200 μL of the cell suspension was added to the top compartment of each transwell chamber, while 600 μL of the medium containing 20% foetal bovine serum was added to the bottom compartment. The cells were then cultured for an additional 24 hours. Subsequently, the transwell chamber was carefully removed using tweezers, and the liquid in the upper chamber was aspirated and transferred to a well containing 800 μL of pre-cooled ethanol. An appropriate volume of 0.1% crystal violet staining solution was added, and the samples were allowed to stain at room temperature for 30 minutes. Finally, photographs were taken under a microscope, and cell counts were performed.
To prepare the transwell invasion chamber for cell invasion assays, BD Matrigel and serum-free medium were diluted at a ratio of 1:8 at 4 °C. Subsequently, 80 μL of the diluted mixture was added to the upper chamber of the transwell and incubated for 4-5 hours until it solidified into a gel. After this incubation period, the plate was removed, and 100 μL of a pre-warmed serum-free medium was added to the upper chamber. The chamber was then allowed to sit at room temperature for 15-30 minutes to facilitate the rehydration of the matrix gel. Other procedures were the same as those for cell migration.
Huh-7 and SNU-387 cells that were in the logarithmic growth phase were seeded into a 6-well culture plate. The density of cells in each well was 5 × 105 cells (2 mL/well). The plate was then placed in a CO2 incubator set at 37 °C for 48 hours to allow the cells to grow. After the 48-hour incubation period, the culture plates were removed from the incubator. The cells were harvested by centrifugation at 250 × g for 5 minutes. The supernatant was carefully discarded, and the cell pellet at the bottom of the tube was obtained. To fix the cells, 1 mL of pre-cooled 75% ethanol was added to the cell pellet. The cells were resuspended in ethanol and then incubated at 4 °C overnight. After overnight fixation at 4 °C, the cells were centrifuged at 350 × g for 5 minutes, the supernatant was aspirated and discarded, washed twice with phosphate-buffered saline, the fixative was washed off, and the cell precipitate was collected. To prepare the staining solution, RNase A and PI were mixed in a 1:9 volumetric ratio. The staining solution, which was freshly prepared, was added to the cell pellet, and 500 μL of PI/RNase A staining solution was added to resuspend the cells. After 30 minutes at room temperature in the dark, the cells were detected and analyzed using a flow analyzer (Cytoflex, Beckman).
In total, 24 specific pathogen-free-grade male Balb/c nude mice (4-5 weeks old) were purchased from Chengdu Yaokang Biotechnology Co., Ltd (Sichuan, China) [approval No. SCXK(Chuan)2020-0034]. The mice were acclimated to standard pathogen-free conditions for 1 week to adapt to the environment. The mice were kept under a normal 12 hours/12 hours light/dark cycle and had free access to water and food. The experimental procedures in this study were consistent with ARRIVE guidelines and were performed in accordance with the protocols approved by the Institution Ethics Committee of the General Hospital of Western Theater Command (Approval No. 2024EC5-ky039).
To construct the FLOT1 overexpression vector (pCDH) and the shRNA knockdown vector (psi-LVRU6GP), the vectors were first packaged into lentivirus and transfected into 293T cells. The supernatant containing the shRNA or overexpression virus was harvested and used to infect liver cancer cell lines (Huh-7 and SNU-387). Stable transfection of overexpressed (OV-FLOT1) and silenced (shRNA-FLOT1) liver cancer cell lines was established by selection with puromycin. The mice were randomly divided into the following groups: Huh-7 cells were divided into the OV-NC and OV-FLOT1 groups, while SNU-387 cells were divided into the shRNA-NC and shRNA-FLOT1 groups, with six mice in each group. A comparison of the two groups was conducted through a t-test. The sample size was calculated according to the G Power software 3.1.9.7 software (Franz Faul, Christian-Albrechts-Universität Kiel, Kiel, Germany) with an effect size of 0.80, α-err pro of 0.05, and power (1-β err pro) of 0.95. The total sample size was 10, with at least five mice in each group. Stable expressing/knockdown cells and control group cells were subjected to routine digestion and centrifugation, and were then resuspended and counted (3 × 105 cells per mouse). Next, 100-μL cell suspensions per mouse were injected under the armpits of each nude mouse using a microliter syringe. Tumor volumes were measured every three days, using the following formula: Tumor volumes (mm3) = longest diameter × (shortest diameter)2 × 1/2.
According to humane endpoints, nude mice were euthanized by cervical dislocation 24 days later. Their tumor volumes were calculated and weighed immediately. Tumor tissues were collected for IHC staining to detect Ki-67 expression. One mouse in the OV-NC group in which the tumor disappeared after 21 days was excluded. In addition, one mouse in the shRNA-FLOT1 group died on the 18th day. Photographs were taken of five mice in each group.
Huh-7 and SNU-387 cells were washed three times with phosphate-buffered saline, followed by the addition of 0.5% breaking liquid (No. G1204, Servicebio, China) for 10 minutes. Subsequently, 3% bovine serum albumin (No. GC305010, Servicebio, China) was added for 20 minutes. The cells were then incubated overnight at 4 °C with primary antibodies (anti-FLOT1, No. AG1967, 1:50, Beyotime, China; anti-TFE3, No. R382278, 1:100, ZenBioScience, China; anti-GM130, No. DF7556, 1:200, Affinity, China). This was followed by 30-minute incubation at 37 °C with secondary antibodies (FITC-labeled goat anti-rabbit, No. GB22303, 1:100, Servicebio, China; CY3-labeled goat anti-mouse, No. GB21301, 1:100, Servicebio, China). Next, 4’,6-diamidino-2-phenylindole was added and the solution was allowed to incubate for another 10 minutes at room temperature. Finally, images were acquired using the Image-J data analysis system from the National Institutes of Health in the United States. The 4’,6-diamidino-2-phenylindole-stained nuclei appeared blue, while positive expressions of TFE3 and GM130 exhibited green fluorescence; FLOT1-positive expressions were visualized as red.
Total proteins from Huh-7 and SNU-387 cells were extracted using RIPA lysis buffer (Servicebio, China). The resulting lysate was centrifuged at 4 °C for 10 minutes at 12000 rpm. Following centrifugation, the supernatant was collected, and the protein concentration was quantified using a BCA protein assay kit (No. P0009, Beyotime, China). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gel and subsequently transferred to polyvinylidene fluoride membranes for 2 hours. The membranes were then blocked with 5% skimmed milk for an additional 2 hours before being incubated overnight at 4 °C with primary antibodies. After three washes with Tris-buffered saline with Tween, the membranes were incubated with secondary antibodies for 2 hours at room temperature. Target bands were scanned and visualized using a chemiluminescent gel imager (Shanghai Tanon GIS 5200, China). Detailed information regarding the antibodies utilized is provided in Supplementary Table 1.
RNA extraction from Huh-7 and SNU-387 cells was initially performed using the Molpure® Cell/Tissue Total RNA Kit (No. 19221ES50, Yeasen Biotech, Shanghai, China). Reverse transcription was conducted with the PrimeScript RT Master Mix Kit (No. RR047A, Takara Bio, Tokyo, Japan). Subsequently, quantitative reverse transcription PCR was carried out to analyze the relative expression of target genes using TB GreenTM Premix Ex TaqTM II (Tli RNaseH Plus) (RR820A, Takara Bio, Tokyo, Japan), with 10.0 μL 2 × Real PCR EasyTM Mix-SYBR, 0.8 μL forward primer (10 μM), 0.8 μL reverse primer (10 μM), and 2.0 μL cDNA template. Quantitative reverse transcription PCR conditions: Pre-denaturation, 95 °C, 30 seconds; denaturation, 95 °C, 5 seconds; annealing, 55 °C, 30 seconds; full extension, fluorescence collection, 72 °C, 30 seconds, with 45 cycles. Relative expressions were quantified using the 2-ΔΔCt method with GAPDH as an endogenous control. The primer sequences are detailed as follows: GAPDH: F-TGACTTCAACAGCGACACCCA; R-CACCCTGTTGCTGTAGCCAAA; FLOT1: F-GCCTGAACCATGTTTTTCACTT; R-CATCTTTTTGGACTTGAGCTGT; TFE3: F-TAGAAAACGTCCTTGATCCTGA; R-TCGATGAAGAAGATGACGACAT.
Firstly, the h-GASE promoter plasmid and the h-TFE3 transcription factor plasmid were constructed. The vector was provided by Hanbio Biotechnology Co., Ltd (Shanghai, China). The pGL3-promoter vector DNA was digested with KpnI and XhoI, and the PT-h-Flot1 vector DNA was digested with EcoRI and ClaI. The following primer sequences of PT-4 × GASE-sh were used: PT-4 × GASE-sh-F, CACGTGGCACGTGGCACGTGGCACGTGGCC; PT-4 × GASE-sh-R, TCGAGGCCACGTGCCACGTGCCACGTGCCACGTGGTAC. The following primer sequences of PT-h-Flot1 were used: PT-h-Flot1-F, GCTTGGTACCGAGCTCGGATCCGAATTCGCCACCATGTTTTTCACTTG; PT-h-Flot1-R, ATCGCAGATCCTTACTAGTATCGATTCAGGCTGTTCTCAAAGGCTTGTG. Then, PCR amplification was performed according to the following system: 25 μL 2X PCR Buffer, 1 μL dNTP mix, 2 μL forward primer (10 μM), 2 μL reverse primer (10 μM), 1 μL DNA template (200 ng/μL), 18 μL ddH2O, and 1 μL Phanta Super-Fidelity DNA Polymerase. The PCR procedure followed the following sequence: Pre-degeneration, 95 °C, 5 minutes, 1 cycle; denaturation, 95 °C, 30 seconds, 27-35 cycles; annealing, 55-72 °C, 30 seconds, 27-35 cycles; elongation, 72 °C, 30-60 seconds/kb, 27-35 cycles; complete extension, 72 °C, 10 minutes, 1 cycle; the samples were stored at 12 °C. The target gene fragments and linearized vectors were ligated using HB infusionTM (Hanbio Biotechnology Co., Ltd, Shanghai, China) and transformed in DH5α competent cells [No. CB101-02, TIANGEN Biotech (Beijing) Co. Ltd., China]. The positive clones were sequenced. If the sequencing results were consistent with the target sequence, the target plasmid was successfully constructed. The plasmids were verified by QC with concentrations greater than 200 ng/μL and OD260:280 between 1.8 and 2.0. Subsequently, 293T cells were plated in 24-well plates with a cell density of 70%-80% in the DMEM medium. After 24 hours, the cells were grouped as follows: PGL3-basic + pCDNA3.1-EGFP + PRL, 4 × GASE-PGL3-pro + pCDNA3.1-EGFP + PRL, pGL3-basic + h-TFE3 + PRL, 4 × GASE-PGL3-pro + h-TFE3 + PRL, pGL3-basic + h-Flot1 + PRL, and 4 × GASE-PGL3-pro + h-Flot1 + PRL groups. Then, 0.2 μg of PRL, 0.4 μg of h-GASE promoter plasmid, and 0.6 μg of h-TFE3 transcription factor plasmid were mixed and transfected using 2.0 μL of 0.8 mg/mL LipoFiter transfection reagents (No. HB-LF-1000, Hanbio Biotechnology Co., Ltd, Shanghai, China) for 20 minutes. The medium was then discarded for a fresh medium, which was cultured at 37 °C, 5% CO2 for 48 hours. The luciferase activity [firefly luciferase/renilla luciferase (RLuc)] was detected using the Dual-Luciferase® Reporter Assay System (Promega Corporation, WI, United States).
To obtain a cell pellet, the cells were lysed by adding 100 μL of a pre-chilled Western and immunoprecipitation (IP) lysis buffer (No. BL509A, Biosharp, China) for every 106 cells. The lysate was subsequently placed on ice for 30 minutes at 4 °C and centrifuged at 12000 rpm for 10 minutes. The supernatant was collected for further analysis. Total protein concentration in the lysate was determined using the BCA protein concentration determination kit (No. P0009, Beyotime, China). Magnetic beads were gently resuspended through pipetting; an appropriate volume of beads was added to a clean centrifuge tube along with TBS to achieve a final volume of approximately 0.5 mL. The antibodies were diluted in TBS to prepare a working solution with the final concentrations ranging from 5 to 50 μg/mL. Protein A magnetic beads were then magnetically separated. The supernatant was removed and replaced with 500 μL of either antibody working solution or normal immunoglobulin G (IgG) working solution. The beads were subjected to incubation ranging from 15 minutes to 1 hour post-resuspension. Following the addition of another aliquot of TBS (500 μL), protein A magnetic beads were again gently resuspended by pipetting before discarding the supernatant and washing the beads three times with TBS prior to their final resuspension in TBS. The immunoprecipitation experiment was conducted using an immunoprecipitation kit (No. P2175S, Beyotime, China) and by employing protein A magnetic beads method. After incubating at 4 °C for 1 hour, protein A magnetic beads previously bound to normal IgG were magnetically separated from the sample for eliminating proteins that were non-specifically bound to normal IgG. Subsequently, the samples were incubated with either antibody-bound or normal IgG-bound protein A magnetic beads at a ratio of 20 μL of bead suspension to 500 μL of each protein sample volume, followed by overnight incubation at 4 °C. After removing the supernatant, lysis buffer containing inhibitors (0.5 mL) was added while gently resuspending the magnetic beads via pipetting. These solutions were washed three times using lysis buffer containing inhibitors. For each original bead volume equivalent to 20 μL, SDS-PAGE Sample Loading Buffer (1 ×) amounting to 100 μL was incorporated into the mixture, which was subsequently heated at 95 °C for 5 minutes before being placed on a magnetic rack for 10 seconds. Then the supernatant intended for SDS-PAGE gel electrophoresis or western blotting was collected. For western blotting analysis purposes, 10 μL of the IP sample was loaded onto an SDS-PAGE gel; any remaining IP sample was stored at -80 °C for future applications. The proteins underwent separation via SDS-PAGE followed by transfer onto polyvinylidene fluoride membranes and western blotting analysis was conducted by employing the following detection antibodies: Anti-FLOT1 (No. 15571-1-AP, 1:2000, Proteintech, China), anti-mTOR (No. 28273-1-AP, 1:10000, Proteintech, China), anti-TFE3 (No. 14480-1-AP, 1:2000, Proteintech, China), and HRP-conjugated Recombinant Rabbit Anti-Mouse IgG Kappa Light Chain (No. SA00001-19, 1:10000, Proteintech, China).
Statistical analyses were conducted using SPSS version 25.0 (SPSS Inc., Chicago, IL, United States). Data are presented as mean ± SD. Comparisons between the two groups were performed using the t-test. For comparisons among multiple groups, one-way analysis of variance (ANOVA) was employed, followed by the LSD test (homogeneity of variance) and Tamhane’s T2 test (heterogeneity of variance). P-value < 0.05 was considered statistically significant.
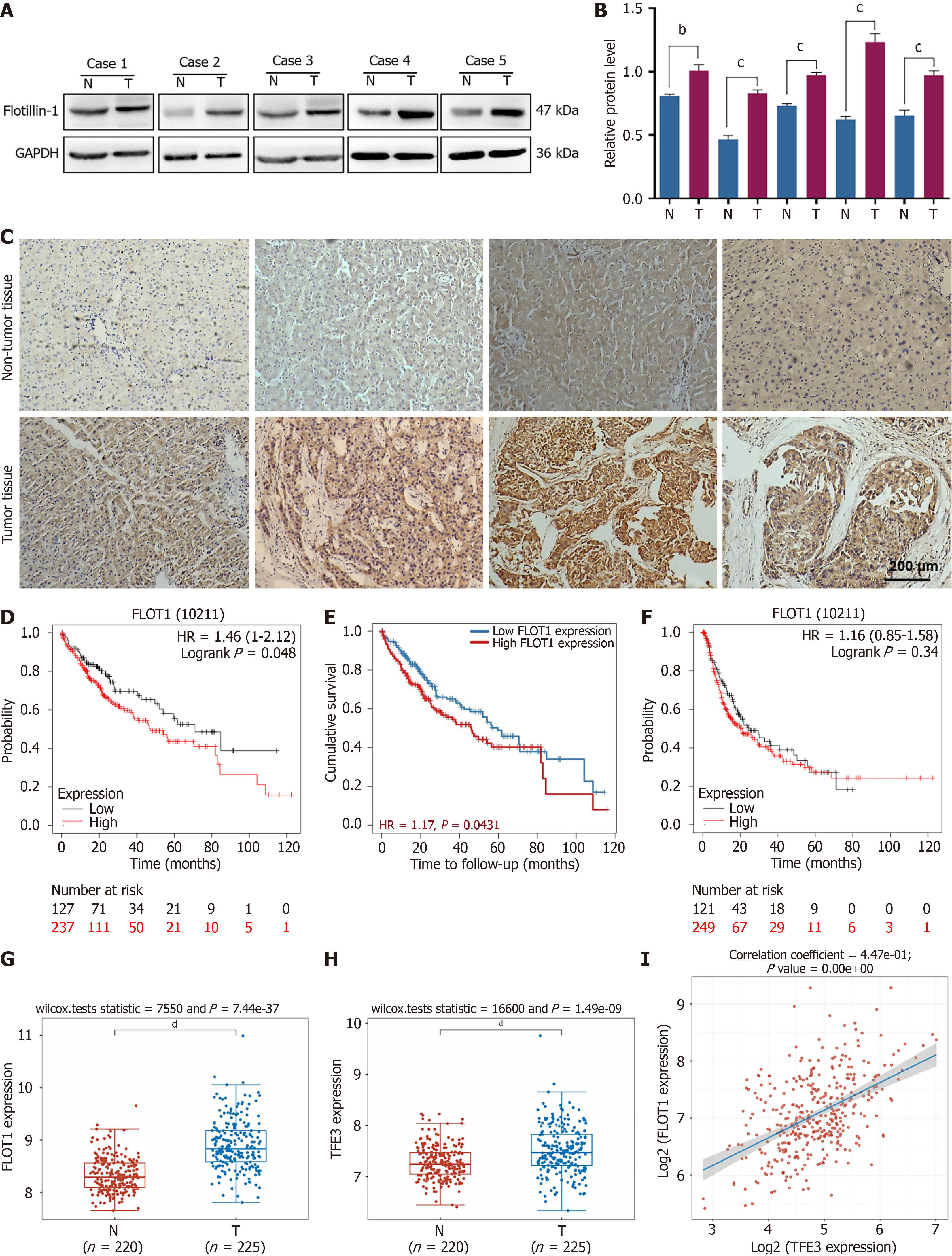
To further verify the high expression of FLOT1 in HCC, we collected surgical samples from five patients with HCC and extracted the total proteins from both HCC and adjacent normal tissues. The western blot results revealed that the expression level of FLOT1 in HCC tissues was significantly higher than that in adjacent normal tissue (both P < 0.01) (Figure 1A and B). High levels of FLOT1 were also detected in HCC tissues through IHC staining, whereas low levels of FLOT1 were detected in adjacent normal tissues. In addition, FLOT1 was primarily expressed in the cytoplasm of HCC cells (Figure 1C). Using the Kaplan-Meier Plotter database, we found that patients with high FLOT1 expression had shorter survival times than those with patients with low FLOT1 expression (Figure 1D). This result was also verified by using the Tumor Immune Estimating Resource data (Figure 1E). However, no significant difference in disease-free stage was detected (Figure 1F). Based on the GSE14520 dataset, we analyzed the expression of FLOT1 and TFE3 in tumor tissue and non-tumor tissue, respectively. The results, as shown in Figure 1G and H, indicated that the expressions of both FLOT1 and TFE3 were significantly elevated in tumor tissue compared to that in non-tumor tissue. Additionally, we analyzed the Spearman correlation of FLOT1-TFE3 using the TCGA dataset. The results revealed a correlation coefficient of 4.47e-01 and a P-value of 0.00e+00, suggesting a significant correlation between FLOT1 and TFE3 (Figure 1I). These results suggested that the high FLOT1 expression was correlated with a poor prognosis in patients with HCC.
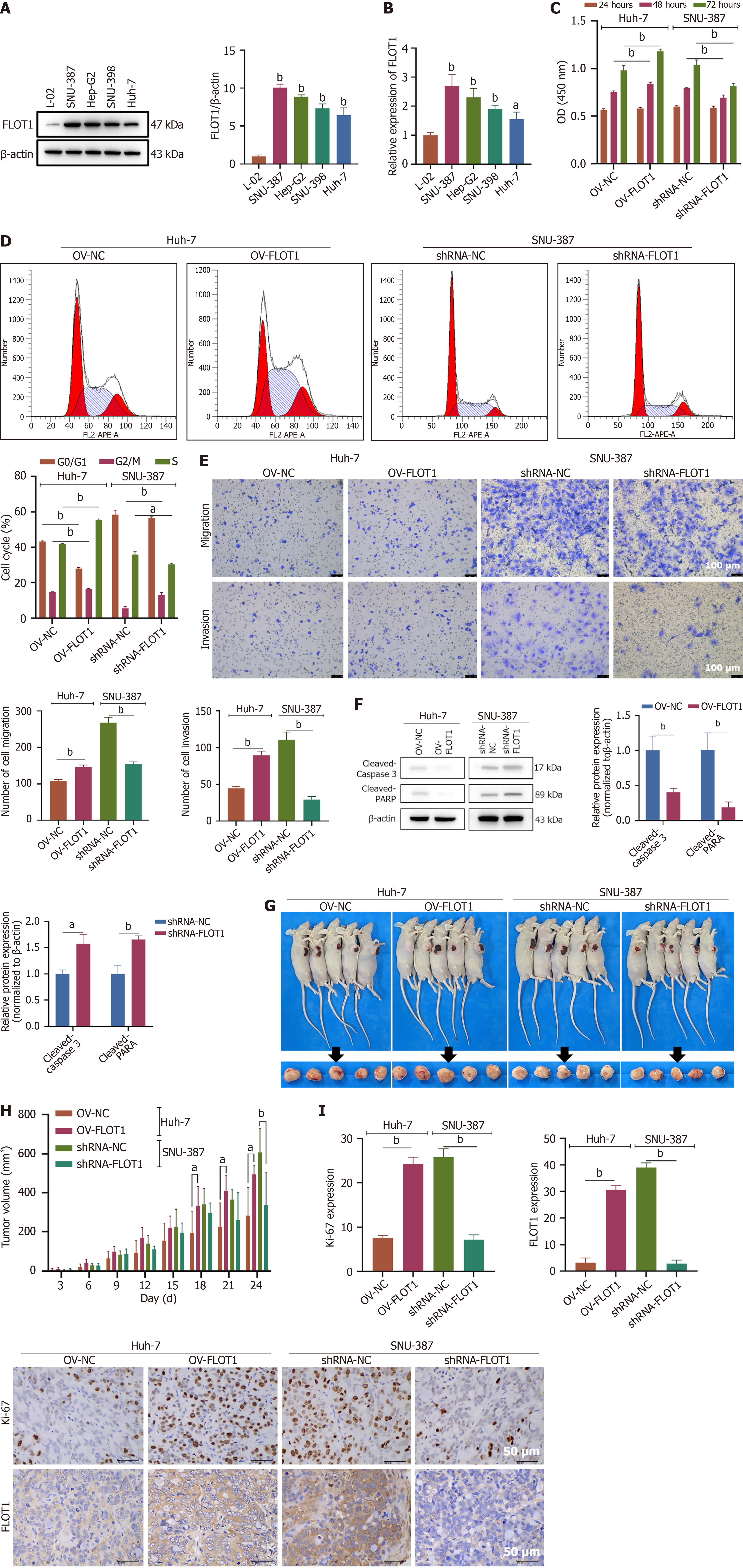
As shown in Figure 2A, the expression of FLOT1 was significantly elevated in HCC cells (SNU-387, Hep-G2, SNU-398, and Huh-7) compared to that in normal liver cells. Consistent results were obtained from both the PCR and western blot analyses (Figure 2B). To investigate the impact of FLOT1 expression on the proliferation, apoptosis, invasion, and migration of HCC cells, plasmids for FLOT1 overexpression and shRNA plasmids for FLOT1 knockdown were separately transfected into Huh-7 and SNU-387 cells, respectively. The CCK-8 results revealed that after 48 and 72 hours of culture, the viability of Huh-7 cells was significantly greater in the OV-FLOT1 group than it was in the OV-NC group (both P < 0.01); conversely, the viability of SNU-387 cells was significantly lower in the shRNA-FLOT1 group than it was in the shRNA-NC group (both P < 0.01) (Figure 2C). Cell cycle analysis revealed that, compared to the OV-NC group, the OV-FLOT1 group had a significantly shortened G0/G1 phase in Huh-7 cells, with prolonged G2/M and S phases (both P < 0.01). In contrast, compared to the shRNA-NC group, the shRNA-FLOT1 group had a significantly prolonged G2/M phase and a shortened S phase in SNU-387 cells (both P < 0.01), with no significant difference in the G0/G1 phase (Figure 2D). Moreover, OV-FLOT1 significantly promoted the migration and invasion abilities of Huh-7 cells (both P < 0.01), whereas shRNA-FLOT1 inhibited the migration and invasion abilities of SNU-387 cells (both P < 0.01) (Figure 2E). OV-FLOT1 significantly suppressed the apoptosis of Huh-7 cells by reducing the expression of cleaved-caspase 3 and cleaved-poly(ADP-ribose) polymerase (PARP) proteins (both P < 0.01), whereas shRNA-FLOT1 significantly induced the apoptosis of SNU-387 cells by increasing the expression of cleaved-caspase 3 and cleaved-PARP proteins (P < 0.05 and P < 0.01, respectively) (Figure 2F). Furthermore, we studied the effect of FLOT1 expression on the in vivo proliferation of HCC cells in a xenograft tumor mouse model (Figure 2G). Starting from day 18, OV-FLOT1 significantly increased the Huh-7 xenograft tumor volume (both P < 0.01), whereas on day 24, shRNA-FLOT1 significantly reduced the tumor volume of SNU-387 xenografts (P < 0.01) (Figure 2H). Additionally, IHC staining revealed that OV-FLOT1 significantly increased the expression of Ki-67 and FLOT1 in Huh-7 xenograft tissues (both P < 0.01), whereas shRNA-FLOT1 significantly decreased the expression of Ki-67 and FLOT1 in SNU-387 xenograft tissues (both P < 0.01) (Figure 2I). In summary, FLOT1 overexpression promoted the proliferation, migration, and invasion abilities of Huh-7 cells and inhibited the apoptosis of HCC cells both in vitro and in vivo. Conversely, FLOT1 knockdown suppressed the proliferation, migration, and invasion abilities of SNU-387 cells and induced the apoptosis of HCC cells both in vitro and in vivo.
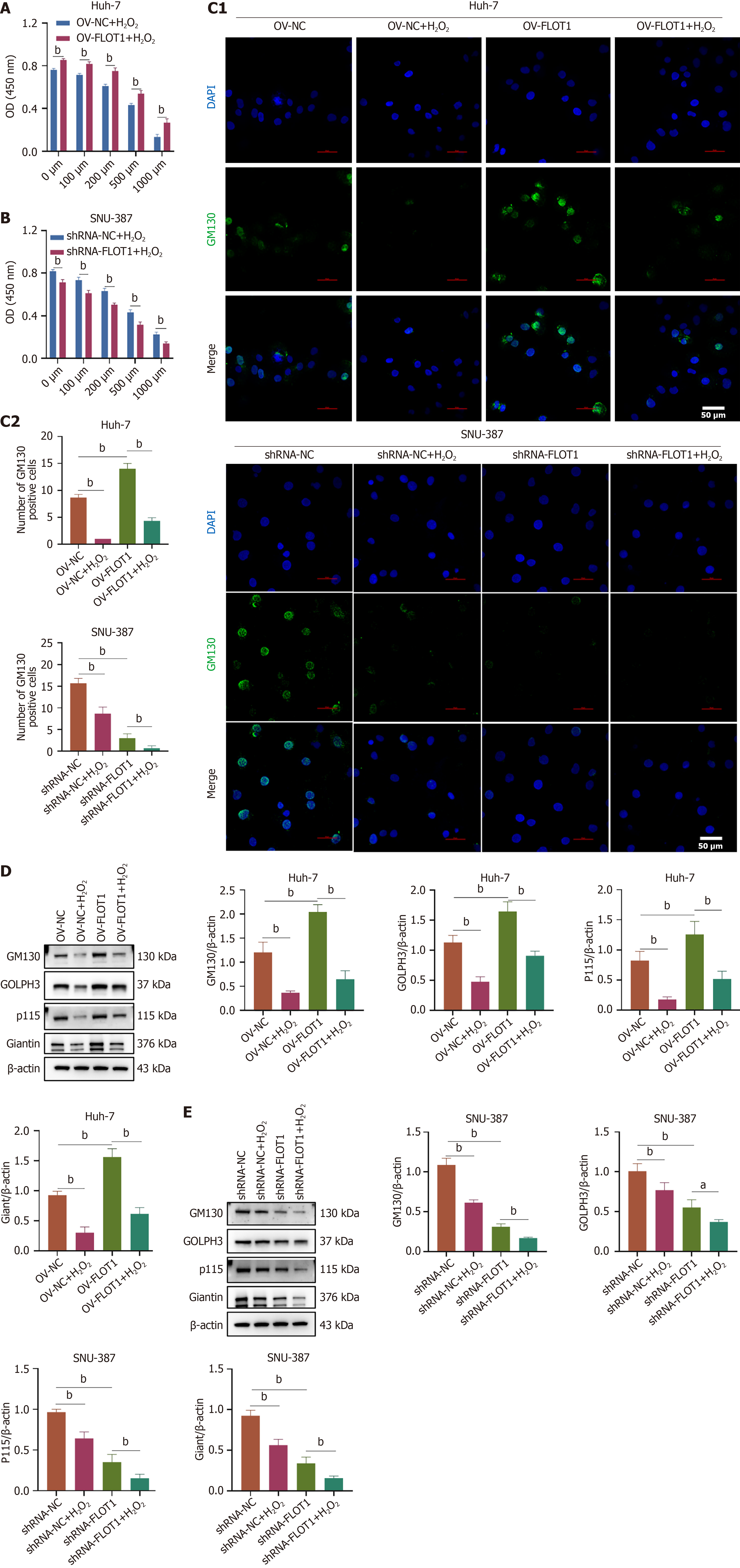
To study the regulatory role of FLOT1 expression on Golgi stress tolerance under H2O2 stimulation in HCC cells, Huh-7 cells were transfected with OV-FLOT1 and SNU-387 cells were transfected with shRNA-FLOT1, followed by H2O2 stimulation at 0, 100, 200, 500, and 1000 μM. The CCK-8 results revealed that OV-FLOT1 increased the viability of Huh-7 cells; however, this increase in cell viability was dose-dependently attenuated by H2O2 (both P < 0.01) (Figure 3A). Conversely, shRNA-FLOT1 reduced the viability of SNU-387 cells, and H2O2 further decreased cell viability in a dose-dependent manner (both P < 0.01) (Figure 3B). Furthermore, the impact of FLOT1 expression on Golgi stress-related protein expression in HCC was investigated. Immunofluorescence staining revealed that H2O2 significantly decreased the OV-FLOT1-induced increase in GM130 expression in Huh-7 cells (both P < 0.01), and H2O2 further downregulated the shRNA-FLOT1-induced reduction GM130 expression in SNU-387 cells (both P < 0.01) (Figure 3C). Moreover, western blot results confirmed that H2O2 significantly reversed the OV-FLOT-induced increase in the expression of GM130, giantin, p115, and GOLPH3 proteins in Huh-7 cells (both P < 0.01) (Figure 3D), whereas H2O2 further downregulated the shRNA-FLOT1-induced reduction in the expression of GM130, giantin, p115, and GOLPH3 proteins in SNU-387 cells (both P < 0.05) (Figure 3E). These results suggested that FLOT1 overexpression activated Golgi stress, leading to the upregulation of Golgi stress-related protein expression in Huh-7 cells, while FLOT1 knockdown inhibited Golgi stress in SNU-387 cells.
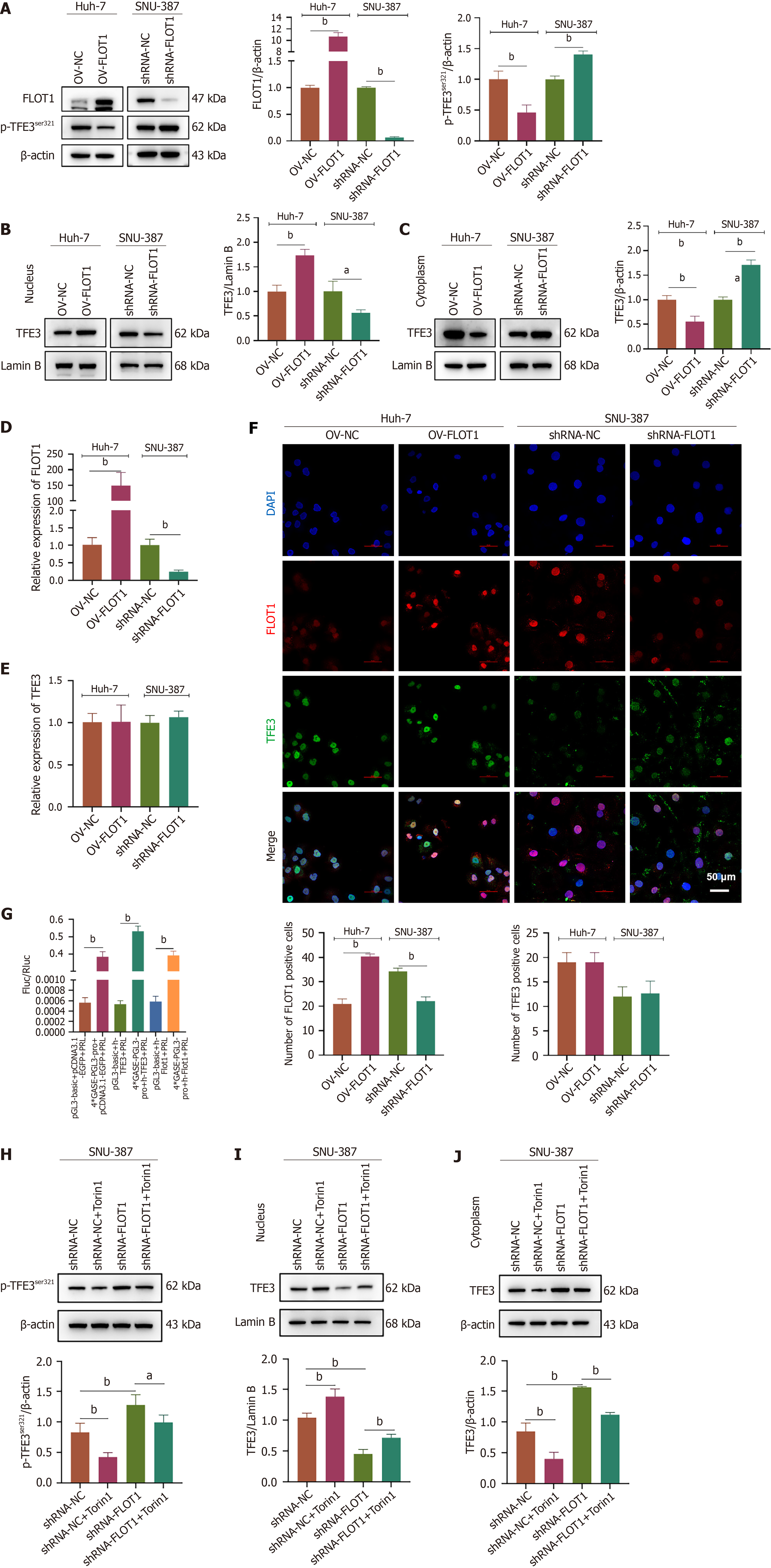
Western blotting, quantitative reverse transcription PCR, and immunofluorescence staining were performed to investigate the effects of FLOT1 expression on the expression and nuclear transfer of the Golgi stress transcriptional regulator TFE3. Western blot analysis revealed that OV-FLOT upregulated the protein expression of FLOT in Huh-7 cells (P < 0.01) and that shRNA-FLOT1 downregulated the protein expression of FLOT in SNU-387 cells (P < 0.01) (Figure 4A). In addition, OV-FLOT downregulated the phosphorylation of the TFE3Ser321 protein in Huh-7 cells (P < 0.01), whereas shRNA-FLOT1 upregulated the phosphorylation of the TFE3Ser321 protein in SNU-387 cells (P < 0.01) (Figure 4A). Furthermore, OV-FLOT upregulated the protein expression of TFE3 in the nucleus (P < 0.01, Figure 4B) and downregulated that of TFE3 in the cytoplasm of Huh-7 cells (P < 0.01, Figure 4C), whereas shRNA-FLOT1 downregulated the protein expression of TFE3 in the nucleus (P < 0.05, Figure 4B) and upregulated that of TFE3 in the cytoplasm of SNU-387 cells (P < 0.01, Figure 4C). The expression of FLOT1 detected by PCR was consistent with that detected by western blot (both P < 0.01) (Figure 4D), whereas the TFE3 mRNA levels remained stable (P > 0.05) (Figure 4E). Immunofluorescence double staining of FLOT1 and TFE3 revealed that FLOT1 was expressed mainly in the cytoplasm and the nucleus (Figure 4F). Cells with increased FLOT1 expression demonstrated an increase in the nuclear translocation of TFE3. A dual-luciferase reporter assay revealed that 4 × GASE-PGL3-pro had promoter activity; 4 × GASE-PGL3-pro interacted with h-TFE3. However, no interaction between 4 × GASE-PGL3-Pro and h-Flot1 was detected (both P < 0.01) (Figure 4G), indicating that FLOT1 expression plays a role in modulating TFE3 to initiate the transcription of genes containing GASE promoters. Furthermore, western blot analysis confirmed that treatment with the mTORC1/2 inhibitor Torin1 reduced the shRNA-FLOT1-induced increase in the phosphorylation of TFE3Ser321 (both P < 0.05) (Figure 4H). Torin1 treatment increased the shRNA-FLOT1-induced reduction in TFE3 expression in the nucleus (both P < 0.01) (Figure 4I), but decreased the shRNA-FLOT1-induced increase in TFE3 expression in the cytoplasm (both P < 0.01) (Figure 4J). These results suggested that FLOT1 overexpression could facilitate the nuclear entry of TFE3 and be associated with mTORC1/2.
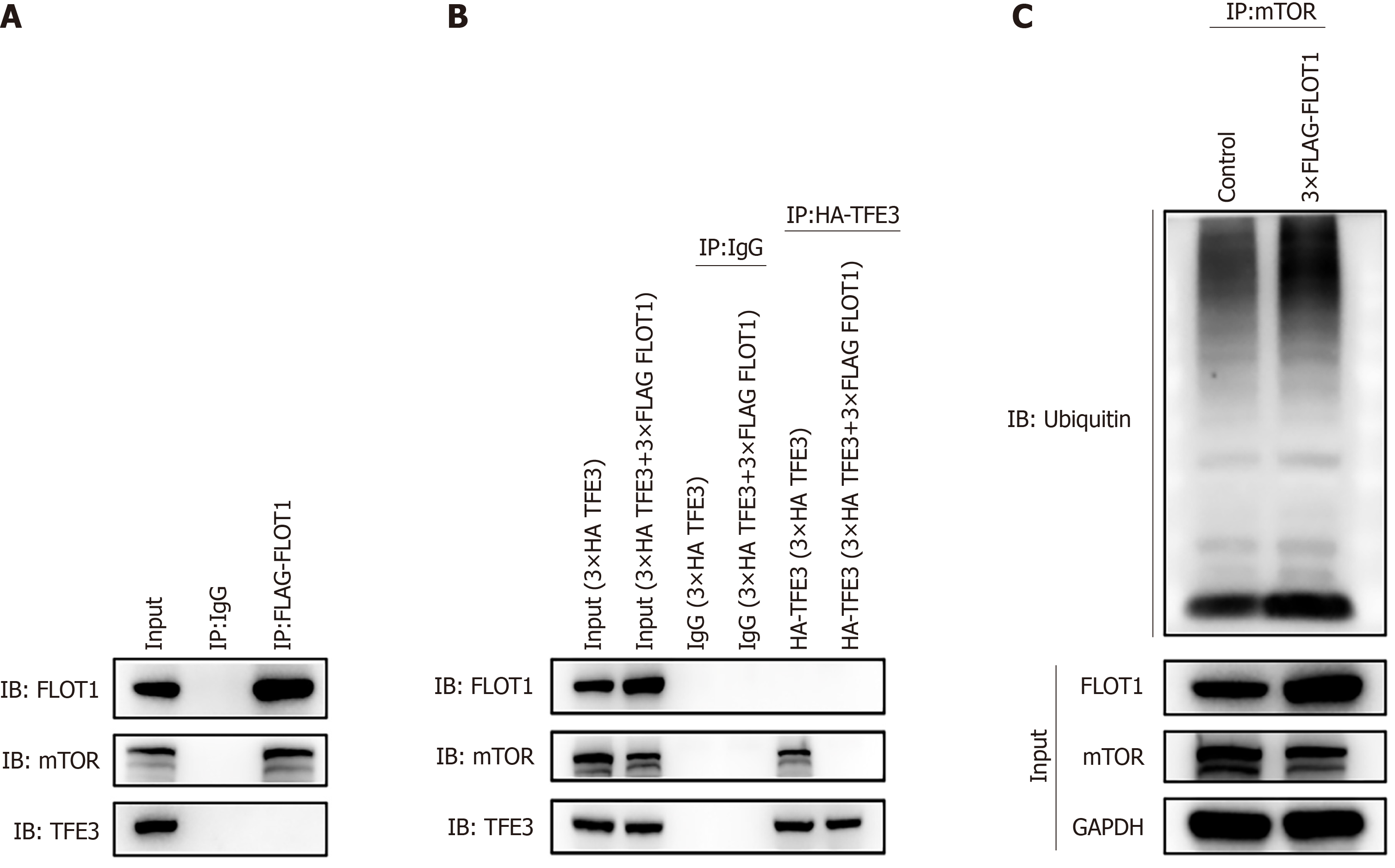
To analyze the binding of FLOT1 with the TFE3 and mTORC1/2 proteins, 3 × FLAG FLOT1 was transfected into 293T cells, and whole-cell lysates were immunoprecipitated with a flag-FLOT1 antibody and then subjected to western blotting with TFE3 and mTOR antibodies to pull down the FLAG-FLOT1 protein. As shown in Figure 5A, in the 293T cell FLAG-FLOT1 group, enriched FLOT1 protein was found to interact with the mTOR protein, but not with the TFE3 protein.
To analyze whether the expression of FLOT1 affects the binding of TFE3 to mTORC1/2, 3 × HA TFE3 and 3 × FLAG FLOT1 were transfected separately or in combination into 293T cells. In addition, whole-cell lysates were immunoprecipitated with an HA-TFE3 antibody and then subjected to western blotting with an mTOR antibody to pull down the HA-TFE3 protein. As shown in Figure 5B, the 293T cell HA-TFE3 group did not have any FLOT1 protein that bound to the enriched TFE3 protein, although it contained mTOR protein that bound to the enriched TFE3 protein. The 293T cell HA-TFE3 (3 × HA TFE3 + 3 × FLAG FLOT1) group had no mTOR protein bound to the enriched TFE3 protein; successful enrichment of the TFE3 protein was achieved after immunoprecipitation of TFE3 in 293T cells.
To assess the FLOT1-induced addition of ubiquitin (Ub) to mTORC1/2 proteins, 3 × FLAG FLOT1 was transfected into Huh-7 cells, and untransfected cells were used as a control. Whole-cell lysates were immunoprecipitated with an mTORC1/2 antibody and subjected to western blotting with an anti-Ub antibody to pull down the mTORC1/2 protein. As shown in Figure 5C, western blotting detection of immunoprecipitated mTOR revealed the obvious expression of Ub proteins, indicating the presence of Ub protein in the mTOR protein.
These results confirmed that FLOT1 could bind to mTORC1/2, but not to TFE3. Additionally, TFE3 could bind to mTORC1/2. However, FLOT1 competed with TFE3 for binding to mTORC1/2. Therefore, FLOT1 could inhibit mTORC1/2 and promote the ubiquitination of mTORC1/2 proteins, thereby increasing the dephosphorylation and nuclear translocation of TFE3.
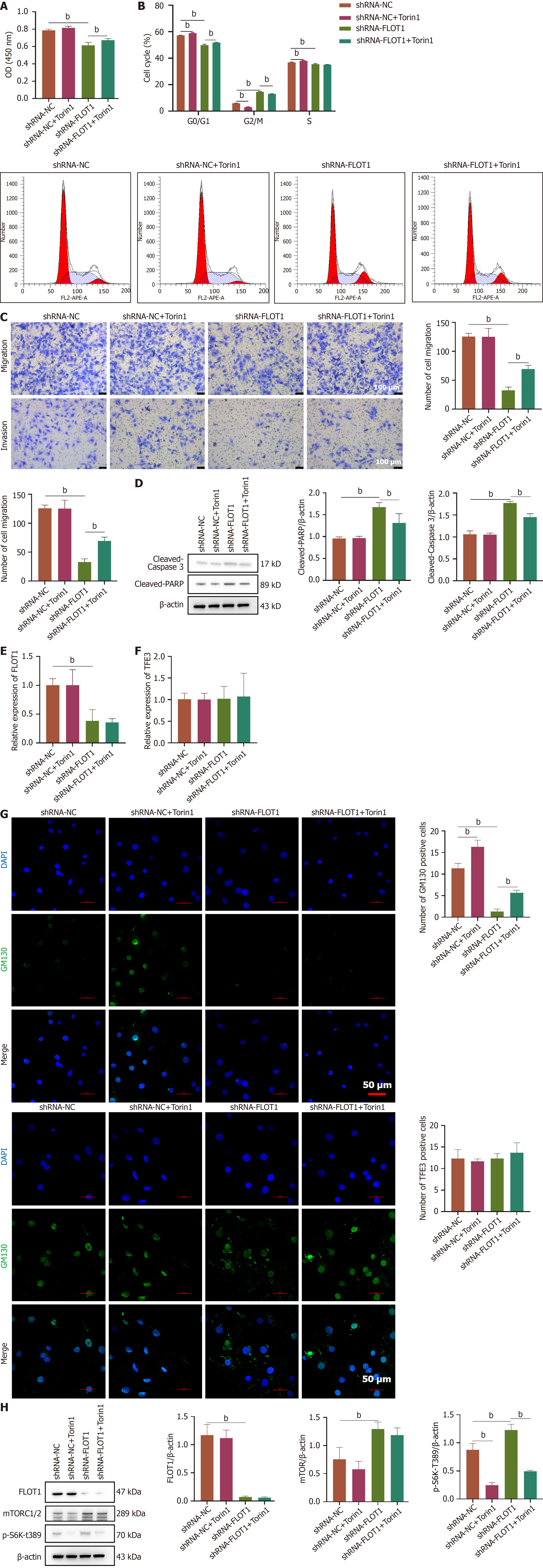
To investigate the molecular mechanism by which FLOT1 regulates the endoplasmic reticulum stress-mediated survival of liver cancer cells, SNU-387 cells were transfected with shRNA-FLOT1 and treated with the mTORC1/2 inhibitor Torin1. The CCK-8 assay results demonstrated that Torin1 reversed the decrease in cell viability induced by shRNA-FLOT1 (P < 0.01) (Figure 6A). As shown in Figure 6B, cell cycle analysis revealed that shRNA-FLOT1 prolonged the G2/M phase and shortened the G0/G1 and S phases, whereas the changes in the G0/G1 and G2/M phases were reversed by treatment with Torin1 (both P < 0.01). Additionally, Torin1 reversed the decrease in cell migration and invasion induced by shRNA-FLOT1 (both P < 0.01) (Figure 6C). Furthermore, shRNA-FLOT1 increased the protein expression of cleaved-caspase 3 and cleaved-PARP proteins, which were reversed by Torin1 treatment (both P < 0.01) (Figure 6D). PCR analysis confirmed a significant decrease in FLOT1 mRNA expression after FLOT1 was knocked down (P < 0.01). However, the addition of Torin1 did not affect FLOT1 mRNA expression (P > 0.05) (Figure 6E). Similarly, the TFE3 mRNA levels remained unchanged (P > 0.05) (Figure 6F). Immunofluorescence staining revealed that Torin1 reversed the decrease in the number of GM130-positive cells induced by shRNA-FLOT1 (P < 0.01), whereas the number of TFE3-positive cells remained stable (P > 0.05) (Figure 6G). Furthermore, western blot analysis revealed a significant decrease in FLOT1 protein expression after FLOT1 was knocked down (P < 0.01) and a significant increase in mTORC1/2 expression (P < 0.01). However, Torin1 treatment did not affect the protein expression of FLOT1 or mTORC1/2 (P > 0.05) (Figure 6H). Interestingly, Torin1 treatment significantly reversed the increase in p-S6 kinase (S6K)-T389 expression induced by shRNA-FLOT1 (P < 0.01) (Figure 6H). These results suggested that the knockdown of FLOT1 promoted the expression of mTORC1/2 proteins and the downstream effector p-S6K-T389, leading to the phosphorylation and translocation of TFE3 from the nucleus to the cytoplasm, the inhibition of Golgi stress-mediated responses downstream of TFE3, and, consequently, the suppression of HCC cell proliferation, migration, and invasion.
HCC continues to pose a significant threat to human health, necessitating the identification of novel therapeutic targets to improve patient outcomes. Lipid rafts, which are dynamic assemblies of proteins and lipids capable of accommodating receptors and regulatory molecules, serve as critical platforms for signal transduction. Recent studies have highlighted their pivotal role in tumor regulation[28], with FLOT1 frequently serving as a marker for these lipid rafts. FLOT1 is a 47-kDa protein encoded by 13 exons and belongs to the stomatin, prohibitin, FLOT, and HflK/C (SPFH) domain-containing protein family; it also possesses a unique FLOT domain and is prone to heteromer formation[29,30]. As an essential lipid raft protein involved in signal transduction, FLOT1 represents a promising new target for cancer therapy. Elevated levels of FLOT1 may disrupt membrane domain stability during tumorigenesis, thereby influencing tumor progression[24,31]. The structures of proteins dictate their function. Their expression levels and localization are crucial determinants of their biological activity. This study demonstrated, through IHC staining, that FLOT1 expression was positively correlated with HCC tissues. Furthermore, FLOT1 Levels were significantly higher in HCC tissues than they were in adjacent noncancer tissues. Notably, high FLOT1 expression correlated with reduced overall survival in patients with HCC; those exhibiting elevated FLOT1 expression had shorter survival times than their low-expression counterparts. However, no significant differences were observed regarding disease-free stages. Additional investigations revealed that FLOT1 was upregulated in HCC cells; FLOT1 overexpression promoted proliferation, migration, and invasion of Huh-7 cells, whereas FLOT1 knockdown inhibited these characteristics of malignancy in SNU-387 cells. These observations were further supported by using a mouse tumor xenograft model.
Caspase-3 is the most critical terminal splice enzyme in cellular apoptosis and plays a pivotal role in the mechanisms underlying cell death. PARP is typically cleaved by executioner caspases and serves as a significant indicator of apoptosis[32]. In our study, overexpression of FLOT1 decreased the levels of apoptosis-related proteins, specifically those of cleaved-caspase-3 and cleaved-PARP. Conversely, FLOT1 knockdown enhanced the apoptosis in HCC cells, indicating that FLOT1 knockdown promoted apoptosis through the activation of caspase-3 and subsequent cleavage of PARP. Apoptotic signaling mediated by clusters of apoptotic signaling molecule-enriched rafts (CASMERs) is closely associated with membrane rafts[33,34]. Our finding suggests that HCC cells may exhibit elevated levels of the raft protein FLOT1 compared with those in normal cells, thereby increasing their propensity to form CASMERs. Consequently, FLOT1 has emerged as a potential therapeutic target for modulating apoptotic signaling pathways in HCC treatment. However, the precise mechanism by which FLOT1 influences HCC cell apoptosis via CASMER regulation remains to be further investigated.
This study also investigated the role of Golgi stress in HCC cells. Over the past decade, it has become increasingly apparent that alterations in the Golgi structure and function are critical to tumor initiation and progression. The most characteristic Golgi proteins include vesicle docking proteins such as p115 and GM130 and the transmembrane protein giantin. GOLPH3 is a highly conserved cisternal protein within the Golgi apparatus that plays essential roles in maintaining its structure, facilitating protein transport from the Golgi to other organelles or cell membranes, participating in glycosylation processes, regulating cellular stress responses for protection, and contributing to cell cycle dynamics and mitosis[35]. GM130 functions as a scaffold protein that directly recruits RNA and associated RNA-binding proteins to the Golgi membrane, establishing a tight association with it[36]. P115 primarily mediates vesicular transport between the endoplasmic reticulum and the Golgi apparatus. Studies have demonstrated that this transport is facilitated by both p115 and cis-Golgi protein GM130[37]. Additionally, golgins represent a family of long coiled-coil proteins located within the Golgi that form complexes with p115, Rab1, and GM130 to support normal vesicular function within this organelle[38]. Recent investigations have shown that the Golgi apparatus significantly influences tumor cell migratory capabilities. Furthermore, golgin-related proteins can modify tumor cell behavior toward increased aggressiveness, thereby pro
TFE3 is a member of the basic helix-loop-helix-leucine zipper transcription factor MiT family and is the major transcriptional regulator in the Golgi stress response[41]. TFE3 is localized in both the nucleus and cytoplasm. However, during the progression of HCC, it translocates from the cytoplasm to the nucleus. Research has shown that TFE3 is phosphorylated by mTORC1 inside the cell, and this phosphorylated form is retained in the cytoplasm through its binding with 14-3-3 molecular chaperone[19]. When the cells are in a starvation state, TFE3 rapidly undergoes dephosphorylation, translocates to the nucleus, and binds to the coiled-coil domain[40]. This phenomenon promotes the occurrence of cellular autophagy and the biogenesis of lysosomes, thereby aiding in cellular survival. Furthermore, during Golgi stress, TFE3 translocates into the nucleus and binds to the promoters of genes for glycosylating enzymes, vesicular transport components, and Golgi-associated secretory proteins to increase the functionality of the Golgi apparatus[42]. Therefore, mTORC1-mediated TFE3 expression is crucial for controlling tumor cell proliferation and survival. This study explored the impact of FLOT1 expression on the expression and nuclear translocation of the Golgi stress-regulated transcription factor TFE3. The overexpression of FLOT1 inhibited TFE3 dephosphorylation by suppressing mTORC1/2, promoting its translocation from the cytoplasm to the nucleus, and leading to its accumulation in the nucleus upon mTORC1 inactivation. Knocking down FLOT1, on the other hand, promotes TFE3 phosphorylation by activating mTORC1/2, facilitating its translocation from the nucleus to the cytoplasm. Co-immunoprecipitation also confirmed the underlying mechanism, suggesting that FLOT1 might recruit and inhibit the mTORC1/2 protein and promote its ubiquitination, thereby increasing TFE3 dephosphorylation and nuclear translocation. mTORC1 mainly promotes protein synthesis by phosphorylating two key effectors, the p70 ribosomal protein S6K and the eukaryotic initiation factor 4E-binding protein[43,44]. Our study demonstrated that FLOT1 knockdown promoted the expression of mTORC1/2 proteins and their downstream effector molecules p-S6K-T389, resulting in TFE3 phosphorylation and translocation from the nucleus to the cytoplasm, thus inhibiting the downstream Golgi stress-mediated response of TFE3 and suppressing HCC cell proliferation, migration, and invasion. This process could be reversed by using an mTORC1/2 inhibitor, Torin1, which decreased the expression of mTORC1/2 and p-S6K-T389.
The oncogenic mechanism of FLOT1 exhibits significant tumor type heterogeneity. In gastric cancer, FLOT1 promotes tumor progression and metastasis through the BCAR1/extracellular signal-regulated kinase signaling pathway[45]. High expression of FLOT1 in lung cancer tissues activates FLOT1 transcription, which promotes immune evasion, further enhances macrophage recruitment and M2 polarization, increases programmed death ligand-1 expression, and reduces ferroptosis[46]. FLOT1 also facilitates the growth, invasion, and migration of lung adenocarcinoma cells, inhibits apoptosis, induces epithelial-mesenchymal transition, and regulates the cell cycle via activation of the extracellular signal-regulated kinase/protein kinase B signaling pathway[47]. In the HCC model of this study, FLOT1 degrades mTORC1/2 through ubiquitination and activates TFE3-dependent Golgi stress. This study is the first to report that FLOT1 regulates Golgi morphology to support cell survival in HCC through the mTORC1/2-TFE3 axis, rather than directly driving cell migration, invasion, or immune evasion in the tumor microenvironment. The specific differences in the actions of FLOT1 can be attributed to the higher demand for organelle homeostasis due to the unique metabolic stresses (such as lipid overload) present in HCC cells[48]. Although the Golgi stress response is associated with cancer progression[40], our findings indicate that FLOT1 serves as a novel regulatory hub distinct from classical Golgi stress regulators such as HSP47 and CREB3, which rely on endoplasmic reticulum-Golgi trafficking mechanisms[49,50]. FLOT1 establishes a molecular link between the cell membrane and Golgi homeostasis, offering a new perspective on inter-organelle communication. Previous studies have reported the crucial role of mTORC1/2 kinase activity regulation in Golgi stress[51]. However, this study is the first to untangle how FLOT1 specifically activates the Golgi stress pathway by regulating the ubiquitination and degradation of mTORC1/2. It also underscores the central role of TFE3 in maintaining Golgi homeostasis. These results present a fresh viewpoint for the precision treatment of liver cancer, demonstrating the importance, uniqueness, and innovative contributions of this study.
However, this study had several limitations. First, lipid rafts, lipid structures that can regulate cell signaling and function, reportedly have elevated levels of membrane lipid rafts and cholesterol in cancer cells. Future research should further investigate the role of FLOT1 in regulating lipid metabolism and glycosylation modifications in HCC. Second, although our study confirmed the effect of the combination of sh-FLOT1 and Torin1 on the expression, subcellular localization of TFE3 and its downstream molecule mTORC1/2, the absence of overexpression experiments may have obscured whether FLOT1-mediated inhibition of mTORC1/2 is the primary driver of TFE3 activation and its functional consequences. Third, the potential pleiotropy of H2O2 as a broad-spectrum oxidative stress inducer and its nonspecific effects on multiple organelles, such as mitochondria and endoplasmic reticulum, also limit the applications of this study. Finding safe and specific Golgi inducers or inhibitors remains a pressing scientific challenge. Future research should aim to further enhance the specificity of the mechanism.
Although this study indicates that FLOT1 is a potential target for HCC therapy, its clinical translation still faces multiple challenges. Firstly, the development of small molecule inhibitors targeting FLOT1 is necessary as a highly specific intervention tool for lipid raft proteins, similar to how Filipin III targets lipid raft cholesterol for tumor-targeted therapy[52]. Secondly, studies have reported the successful use of a GalNAc-modified small interfering RNA delivery platform for targeted liver therapy[53], suggesting that a FLOT1 small interfering RNA delivery system could be used for targeted HCC therapy. Additionally, preclinical studies are needed to validate the synergistic effect of FLOT1 inhibition and TFE3 targeting, evaluate the impact of FLOT1-targeted therapy on the tumor microenvironment, and verify the sensitivity of FLOT1-targeted therapy to avoid variations in treatment efficacy due to tumor heterogeneity.
This study examined the effects of both FLOT1 silencing and overexpression on the proliferation, apoptosis, and in vivo growth of liver cancer at the clinical, cellular, and animal levels. The study results revealed that elevated FLOT1 exp
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12706] [Article Influence: 6353.0] [Reference Citation Analysis (6)] |
| 2. | Childs A, Aidoo-Micah G, Maini MK, Meyer T. Immunotherapy for hepatocellular carcinoma. JHEP Rep. 2024;6:101130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 3. | Lin CL, Kao JH. Development of hepatocellular carcinoma in treated and untreated patients with chronic hepatitis B virus infection. Clin Mol Hepatol. 2023;29:605-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 4. | Müller M, Bird TG, Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. 2020;72:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Baglieri J, Brenner DA, Kisseleva T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int J Mol Sci. 2019;20:1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 6. | Alqahtani SA, Chan WK, Yu ML. Hepatic Outcomes of Nonalcoholic Fatty Liver Disease Including Cirrhosis and Hepatocellular Carcinoma. Clin Liver Dis. 2023;27:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Cao M, Xia C, Cao M, Yang F, Yan X, He S, Zhang S, Teng Y, Li Q, Tan N, Wang J, Chen W. Attributable liver cancer deaths and disability-adjusted life years in China and worldwide: profiles and changing trends. Cancer Biol Med. 2024;21:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Yan T, Yu L, Zhang N, Peng C, Su G, Jing Y, Zhang L, Wu T, Cheng J, Guo Q, Shi X, Lu Y. The advanced development of molecular targeted therapy for hepatocellular carcinoma. Cancer Biol Med. 2022;19:802-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Wei C, Yang X, Liu N, Geng J, Tai Y, Sun Z, Mei G, Zhou P, Peng Y, Wang C, Zhang X, Zhang P, Geng Y, Wang Y, Zhang X, Liu X, Zhang Y, Wu F, He X, Zhong H. Tumor Microenvironment Regulation by the Endoplasmic Reticulum Stress Transmission Mediator Golgi Protein 73 in Mice. Hepatology. 2019;70:851-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Dai T, Zhang D, Cai M, Wang C, Wu Z, Ying Z, Wu J, Li M, Xie D, Li J, Song L. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating the NF-κB pathway. J Pathol. 2015;235:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Liu H, Wang X, Feng B, Tang L, Li W, Zheng X, Liu Y, Peng Y, Zheng G, He Q. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma progression by activating mTOR signaling pathway. BMC Cancer. 2018;18:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Liu M, Duan Y, Dong J, Zhang K, Jin X, Gao M, Jia H, Chen J, Liu M, Wei M, Zhong X. Early signs of neurodegenerative diseases: Possible mechanisms and targets for Golgi stress. Biomed Pharmacother. 2024;175:116646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Sasaki K, Yoshida H. Organelle autoregulation-stress responses in the ER, Golgi, mitochondria and lysosome. J Biochem. 2015;157:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Paul BD. Signaling Overlap between the Golgi Stress Response and Cysteine Metabolism in Huntington's Disease. Antioxidants (Basel). 2021;10:1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Pastore N, Vainshtein A, Klisch TJ, Armani A, Huynh T, Herz NJ, Polishchuk EV, Sandri M, Ballabio A. TFE3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol Med. 2017;9:605-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Gao J, Gao A, Liu W, Chen L. Golgi stress response: A regulatory mechanism of Golgi function. Biofactors. 2021;47:964-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Jamaludin MI, Wakabayashi S, Taniguchi M, Sasaki K, Komori R, Kawamura H, Takase H, Sakamoto M, Yoshida H. MGSE Regulates Crosstalk from the Mucin Pathway to the TFE3 Pathway of the Golgi Stress Response. Cell Struct Funct. 2019;44:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Taniguchi M, Nadanaka S, Tanakura S, Sawaguchi S, Midori S, Kawai Y, Yamaguchi S, Shimada Y, Nakamura Y, Matsumura Y, Fujita N, Araki N, Yamamoto M, Oku M, Wakabayashi S, Kitagawa H, Yoshida H. TFE3 is a bHLH-ZIP-type transcription factor that regulates the mammalian Golgi stress response. Cell Struct Funct. 2015;40:13-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Paquette M, El-Houjeiri L, C Zirden L, Puustinen P, Blanchette P, Jeong H, Dejgaard K, Siegel PM, Pause A. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy. 2021;17:3957-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | Nardone C, Palanski BA, Scott DC, Timms RT, Barber KW, Gu X, Mao A, Leng Y, Watson EV, Schulman BA, Cole PA, Elledge SJ. A central role for regulated protein stability in the control of TFE3 and MITF by nutrients. Mol Cell. 2023;83:57-73.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Dang TT, Back SH. Translation Inhibitors Activate Autophagy Master Regulators TFEB and TFE3. Int J Mol Sci. 2021;22:12083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 3556] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 23. | Riento K, Frick M, Schafer I, Nichols BJ. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J Cell Sci. 2009;122:912-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Gauthier-Rouvière C, Bodin S, Comunale F, Planchon D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020;39:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Kumar R, Pereira RS, Niemann J, Azimpour AI, Zanetti C, Karantanou C, Minka W, Minciacchi VR, Kowarz E, Meister M, Godavarthy PS, Maguer-Satta V, Lefort S, Wiercinska E, Bonig H, Marschalek R, Krause DS. The differential role of the lipid raft-associated protein flotillin 2 for progression of myeloid leukemia. Blood Adv. 2022;6:3611-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Li J, Zuo X, Shi J, Zhang J, Duan X, Xu G. Flotillin 1 is differentially expressed in human epithelial ovarian tumors. Neoplasma. 2018;65:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zhang N, Li H, Qin C, Ma D, Zhao Y, Zhu W, Wang L. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J Cancer Res Clin Oncol. 2019;145:895-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Zhang S, Zhu N, Li HF, Gu J, Zhang CJ, Liao DF, Qin L. The lipid rafts in cancer stem cell: a target to eradicate cancer. Stem Cell Res Ther. 2022;13:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 29. | Yokoyama H, Matsui I. The lipid raft markers stomatin, prohibitin, flotillin, and HflK/C (SPFH)-domain proteins form an operon with NfeD proteins and function with apolar polyisoprenoid lipids. Crit Rev Microbiol. 2020;46:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Ma C, Wang C, Luo D, Yan L, Yang W, Li N, Gao N. Structural insights into the membrane microdomain organization by SPFH family proteins. Cell Res. 2022;32:176-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 31. | Banning A, Tomasovic A, Tikkanen R. Functional aspects of membrane association of reggie/flotillin proteins. Curr Protein Pept Sci. 2011;12:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Zhang N, Chen Y, Jiang R, Li E, Chen X, Xi Z, Guo Y, Liu X, Zhou Y, Che Y, Jiang X. PARP and RIP 1 are required for autophagy induced by 11'-deoxyverticillin A, which precedes caspase-dependent apoptosis. Autophagy. 2011;7:598-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Mollinedo F, Gajate C. Lipid rafts, death receptors and CASMERs: new insights for cancer therapy. Future Oncol. 2010;6:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Mollinedo F, Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J Lipid Res. 2020;61:611-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 35. | Bucurica S, Gaman L, Jinga M, Popa AA, Ionita-Radu F. Golgi Apparatus Target Proteins in Gastroenterological Cancers: A Comprehensive Review of GOLPH3 and GOLGA Proteins. Cells. 2023;12:1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Seemann J. RNA scaffolds the Golgi ribbon by forming condensates with GM130. Nat Cell Biol. 2024;26:1139-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Radulescu AE, Mukherjee S, Shields D. The Golgi protein p115 associates with gamma-tubulin and plays a role in Golgi structure and mitosis progression. J Biol Chem. 2011;286:21915-21926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Koreishi M, Gniadek TJ, Yu S, Masuda J, Honjo Y, Satoh A. The golgin tether giantin regulates the secretory pathway by controlling stack organization within Golgi apparatus. PLoS One. 2013;8:e59821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Wang X, Wang Z, Zhang Y, Wang Y, Zhang H, Xie S, Xie P, Yu R, Zhou X. Golgi phosphoprotein 3 sensitizes the tumour suppression effect of gefitinib on gliomas. Cell Prolif. 2019;52:e12636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Bajaj R, Warner AN, Fradette JF, Gibbons DL. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells. 2022;11:1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Wei S, Testa JR, Argani P. A review of neoplasms with MITF/MiT family translocations. Histol Histopathol. 2022;37:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Lang XP, Pan J, Yang CX, Chen P, Shi CC, Hong Y, Wang J, Xiao S. A renal cell carcinoma with EWSR1-TFE3 fusion gene. Genes Chromosomes Cancer. 2020;59:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Yellen P, Chatterjee A, Preda A, Foster DA. Inhibition of S6 kinase suppresses the apoptotic effect of eIF4E ablation by inducing TGF-β-dependent G1 cell cycle arrest. Cancer Lett. 2013;333:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Sakai M, Fukumoto M, Ikai K, Ono Minagi H, Inagaki S, Kogo M, Sakai T. Role of the mTOR signalling pathway in salivary gland development. FEBS J. 2019;286:3701-3717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Wang R, Huang W, Cai K, Xiao S, Zhang W, Hu X, Guo J, Mao L, Yuan W, Xu Y, Chen Z, Chen Z, Lai C. FLOT1 promotes gastric cancer progression and metastasis through BCAR1/ERK signaling. Int J Biol Sci. 2023;19:5104-5119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 46. | Tao Y, Ji H, Hu W, Jiang G, Yang F, Peng X, Zhang X, Yin Y, Yuan Z, Chen D. SMARCC1 promotes M2 macrophage polarization and reduces ferroptosis in lung cancer by activating FLOT1 transcription. J Mol Med (Berl). 2025;103:453-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Correction to "FLOT1 promotes tumor development, induces epithelial-mesenchymal transition, and modulates the cell cycle by regulating the Erk/Akt signaling pathway in lung adenocarcinoma". Thorac Cancer. 2023;14:1905-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Wu K, Lin F. Lipid Metabolism as a Potential Target of Liver Cancer. J Hepatocell Carcinoma. 2024;11:327-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 49. | Taniguchi M, Yoshida H. TFE3, HSP47, and CREB3 Pathways of the Mammalian Golgi Stress Response. Cell Struct Funct. 2017;42:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Sampieri L, Di Giusto P, Alvarez C. CREB3 Transcription Factors: ER-Golgi Stress Transducers as Hubs for Cellular Homeostasis. Front Cell Dev Biol. 2019;7:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 51. | Kaeser-Pebernard S, Vionnet C, Mari M, Sankar DS, Hu Z, Roubaty C, Martínez-Martínez E, Zhao H, Spuch-Calvar M, Petri-Fink A, Rainer G, Steinberg F, Reggiori F, Dengjel J. mTORC1 controls Golgi architecture and vesicle secretion by phosphorylation of SCYL1. Nat Commun. 2022;13:4685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 52. | Lee SY, Ko SH, Shim JS, Kim DD, Cho HJ. Correction to "Tumor Targeting and Lipid Rafts Disrupting Hyaluronic Acid-Cyclodextrin-Based Nanoassembled Structure for Cancer Therapy". ACS Appl Mater Interfaces. 2018;10:44197-44198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Wan X, Ge Y, Zhu W, Zhang J, Pan W, Li N, Tang B. GalNAc-functionalized metal-organic frameworks for targeted siRNA delivery: enhancing survivin silencing in hepatocellular carcinoma. Biomater Sci. 2025;13:2704-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/