Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.105210
Revised: June 5, 2025
Accepted: July 15, 2025
Published online: August 7, 2025
Processing time: 146 Days and 0.5 Hours
Core-fucosylated low-molecular-weight kininogen (LMWK-Fc) levels are significantly elevated in patients with liver fibrosis and cirrhosis.
To assess the value of LMWK-Fc as a diagnostic biomarker for liver fibrosis.
Our study included 132 healthy people and 132 patients with liver fibrosis. The LMWK-Fc level was measured based on the principle of chemiluminescence. Fibrosis stage and inflammatory activity were assessed by liver biopsy. Com
LMWK-Fc had an area under the curve of 0.871, which indicates a good level of performance in distinguishing between patients with and without fibrosis. Furthermore, the LMWK-Fc level had a certain correlation with the liver stiffness value, which reached 0.5789.
LMWK-Fc could be used as a non-invasive marker of liver fibrosis. Further studies are needed to evaluate the usefulness of this marker.
Core Tip: Liver fibrosis is a common outcome of chronic liver disease, and early detection and diagnosis of liver fibrosis are crucial. Traditional biopsy-based liver fibrosis diagnosis has limitations such as invasiveness. Core-fucosylated low-molecular-weight kininogen (LMWK-Fc) refers to the presence of fucose residues connected to the core of N-linked polysaccharide structures. Changes in LMWK-Fc levels may reflect the evolution of liver fibrosis. This study addressed this gap by studying the potential role of LMWK-Fc levels as a biomarker for predicting liver fibrosis, finding that LMWK-Fc may be a potential biomarker for screening and diagnosing liver fibrosis.
- Citation: Gao GF, Yu J, Tong L, Jiang D. Predictive value of core-fucosylated low-molecular-weight kininogen levels in patients with liver fibrosis: A prospective study. World J Gastroenterol 2025; 31(29): 105210
- URL: https://www.wjgnet.com/1007-9327/full/v31/i29/105210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i29.105210
Liver fibrosis is a common consequence of chronic liver diseases, such as hepatitis, alcohol-related liver disease, and non-alcoholic fatty liver disease[1,2]. It represents a critical stage in the progression of liver pathology, as it may eventually lead to cirrhosis and liver failure[3,4]. Early detection and accurate assessment of liver fibrosis are essential for appropriate clinical management and therapeutic intervention[5,6]. Traditional diagnostic methods, including liver biopsy, are invasive and associated with several limitations, such as sampling variability and the risk of complications[7]. However, the inaccuracy of biopsy sampling can result in missed diagnoses of cirrhosis in up to 20% of patients[8]. For this reason, liver biopsy is not a recommended as a tool for screening individuals at high risk of progressive liver fibrosis. Thus, there is a pressing need for non-invasive biomarkers that can reliably predict the presence and severity of liver fibrosis.
Glycans, including glycoproteins, play a pivotal role in cellular communication and signaling processes[9,10]. Core fucosylation, a specific glycosylation modification, has emerged as an intriguing focus of investigation in liver disease[11]. One glycoprotein that has gained prominence in this context is low-molecular-weight kininogen (LMWK)[12]. LMWK is a glycoprotein involved in the regulation of blood coagulation and fibrinolysis[13], and alterations in its glycosylation pattern, specifically core fucosylation[14], have been linked to various pathophysiological conditions[15].
Core-fucosylated LMWK (LMWK-Fc) refers to the presence of a fucose sugar residue attached to the core of the N-linked glycan structure. This modification has been implicated in the regulation of the kinin-kallikrein system, which plays a vital role in inflammation and fibrosis[16]. The level of LMWK-Fc has been confirmed to be closely related to pulmonary interstitial fibrosis and renal interstitial fibrosis[17,18]. Changes in LMWK-Fc levels may reflect the evolving state of liver fibrosis, making it a potential non-invasive biomarker for assessing liver fibrosis. Our study aims to address this gap by investigating the potential role of LMWK-Fc levels as a predictive biomarker for liver fibrosis. We found that LMWK-Fc could be a potential biomarker for the screening and diagnosis of liver fibrosis.
This prospective study involved 132 patients diagnosed with liver fibrosis, and 132 age- and gender-matched healthy controls. Peripheral venous blood samples were evaluated for the presence of hepatitis B virus surface antigen as well as the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and albumin. The platelet (PLT) count was also determined. The patients were diagnosed with liver disease, liver fibrosis, or liver cirrhosis based on transient elastography examination and clinical diagnosis. As for the 132 healthy controls, there was no indication for liver biopsy; their liver and renal function tests were normal, they had negative serology for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus, and their abdominal ultrasonography findings were within normal limits. These healthy controls had no history of hepatitis in any form, no exposed to hepatotoxic drugs, and record of alcohol abuse. Laboratory parameters, including hemoglobin, PLT count, ALT, AST, ALP, GGT, and albumin, were evaluated in all patients on the day of the clinical assessment. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. All patients provided written informed consent for their participation in the study.
We used the LMWK-Fc assay kit (chemiluminescence method, Fosun Diagnostics Co., Ltd., Shanghai, China) to measure the levels of LMWK-Fc in serum samples from patients with liver fibrosis and healthy controls. This assay kit is based on the principle of chemiluminescence, which utilizes specific antibodies to detect and quantify LMWK-Fc levels in biological samples. The assay kit has a detection range of 1.3%-40% and a detection limit of 1.3%. The assay procedure consists of the following steps. The LMWK in the sample binds to the magnetic bead-anti-LMWK antibody complex to form a magnetic bead-antibody antigen complex. Magnetic beads are separated from unbound components through a magnetic separator, and lectin ALP is added. The lectin specifically binds to LMWK-Fc to form a magnetic bead-antibody antigen-lectin enzyme complex. The magnetic separator is used again to separate the magnetic beads and remove any unbound components. By adding a chemiluminescent substrate, the ALP that has been bound to the solid phase prompts the substrate to emit photons, and its luminescence intensity is proportional to the level of LMWK-Fc in the sample. The level of LMWK-Fc in the sample was calculated through the calibration curve. The LMWK-Fc concentration of each sample was calculated by using a standard curve generated from the standard solutions. The intra- and inter-assay coefficients of variation of the assay kit were less than 10% and 15%, respectively.
To measure liver stiffness (LSM), a probe was placed in the intercostal space over the patient’s right hepatic lobe. Patients fasted for 3 hours prior to the examination. LSM results with at least 10 valid readings and an interquartile range of less than 30% of the median LSM value were included in the study. In both the healthy cohort and the liver disease cohort, the absence of fibrosis was defined as an LSM ≤ 7.0 kPa, while liver cirrhosis was defined as an LSM ≥ 14.0 kPa. Patients with a LSM between 7.0 and 13.0 kPa were classified as having an intermediate hepatic fibrosis.
Baseline characteristics are shown as mean ± SD, median (interquartile range), or number (percentage). We used SPSS software, version 15 (SPSS Inc., Chicago, IL, United States) for the statistical analysis. We assessed the normality the data distribution with the Kolmogorov-Smirnov test. We compared the patient and healthy control groups with the t-test or the Mann-Whitney U test. We calculated correlations with the Spearman rank test or Kendall’s tau-b test. A P value of less than 0.05 was considered to indicate statistical significance. We assessed the predictive accuracy of each non-invasive index by calculating the area under the receiver operating characteristic curve (AUROC). We calculated the sensitivity, specificity, positive predictive value, and negative predictive value of LMWK-Fc by using receiver operating characteristic (ROC) curves.
Table 1 shows the descriptive statistics of the sample, including the number of participants, the mean ± SD of the continuous variables, and the frequency and percentage of the categorical variables. The sample consisted of 132 participants in each group, with a mean age of 41.06 ± 11.91 years in the normal group and 41.89 ± 11.50 years in the fibrosis group. There was no significant difference in age between the two groups (P = 0.552). The normal group had a higher proportion of female participants (33.33%) than the fibrosis group (22.73%), but the difference was not statistically significant (P = 0.055). The fibrosis group had a significantly lower PLT count; higher levels of AST, ALT, ALP, GGT, total bilirubin, and LMWK; and a higher liver stiffness value and aminotransferase-to-PLT ratio index (APRI) than the normal group (P < 0.05 for all).
| Normal, n = 132 | Fibrosis, n = 132 | P value | |
| Age | 41.06 ± 11.91 | 41.89 ± 11.50 | 0.552 |
| Sex, female [n (%)] | 44 (33.33) | 30 (22.73) | 0.055 |
| PLT | 226.07 ± 64.92 | 144.72 ± 81.79 | < 0.001 |
| AST | 25.84 ± 19.28 | 62.56 ± 148.45 | < 0.001 |
| ALT | 35.68 ± 85.95 | 87.04 ± 264.16 | < 0.001 |
| ALB | 48.81 ± 16.15 | 47.41 ± 9.69 | 0.936 |
| GGT | 43.52 ± 65.89 | 81.70 ± 179.03 | < 0.001 |
| ALP | 79.34 ± 26.81 | 102.54 ± 64.99 | 0.009 |
| TB | 12.21 ± 6.27 | 27.82 ± 58.26 | 0.002 |
| LMWK | 7.17 ± 1.88 | 11.05 ± 3.63 | < 0.001 |
| Liver stiffness value | 4.41 ± 1.35 | 8.75 ± 5.12 | < 0.001 |
| APRI | 13.63 ± 10.72 | 80.37 ± 108.07 | < 0.001 |
The LMWK-Fc level had a strong positive correlation with the liver stiffness value (r = 0.5789, P < 0.05), which is a direct measure of liver fibrosis by transient elastography. The LMWK-Fc level had a moderate negative correlation with the PLT count (r = -0.4404, P < 0.05) and a weak positive correlation with the ALP level (r = 0.3052, P < 0.005), which are indirect indicators of liver fibrosis and portal hypertension. The LMWK-Fc level had a weak positive correlation with the APRI and the GGT and AST levels, which are markers of liver inflammation and damage. The LMWK-Fc level had no significant correlation with the ALT level (r = 0.0936, P = 0.1353) or the albumin level (r = -0.0315, P = 0.6177), which are markers of liver function (Table 2).
| Fibrosis indicator | Correlation, r | P value |
| Liver stiffness value | 0.5789 | 0.0000 |
| PLT | -0.4404 | 0.0001 |
| ALP | 0.3052 | 0.0000 |
| APRI | 0.2180 | 0.1133 |
| GGT | 0.1487 | 0.0232 |
| AST | 0.1366 | 0.0367 |
| ALT | 0.0936 | 0.1353 |
| ALB | -0.0315 | 0.6177 |
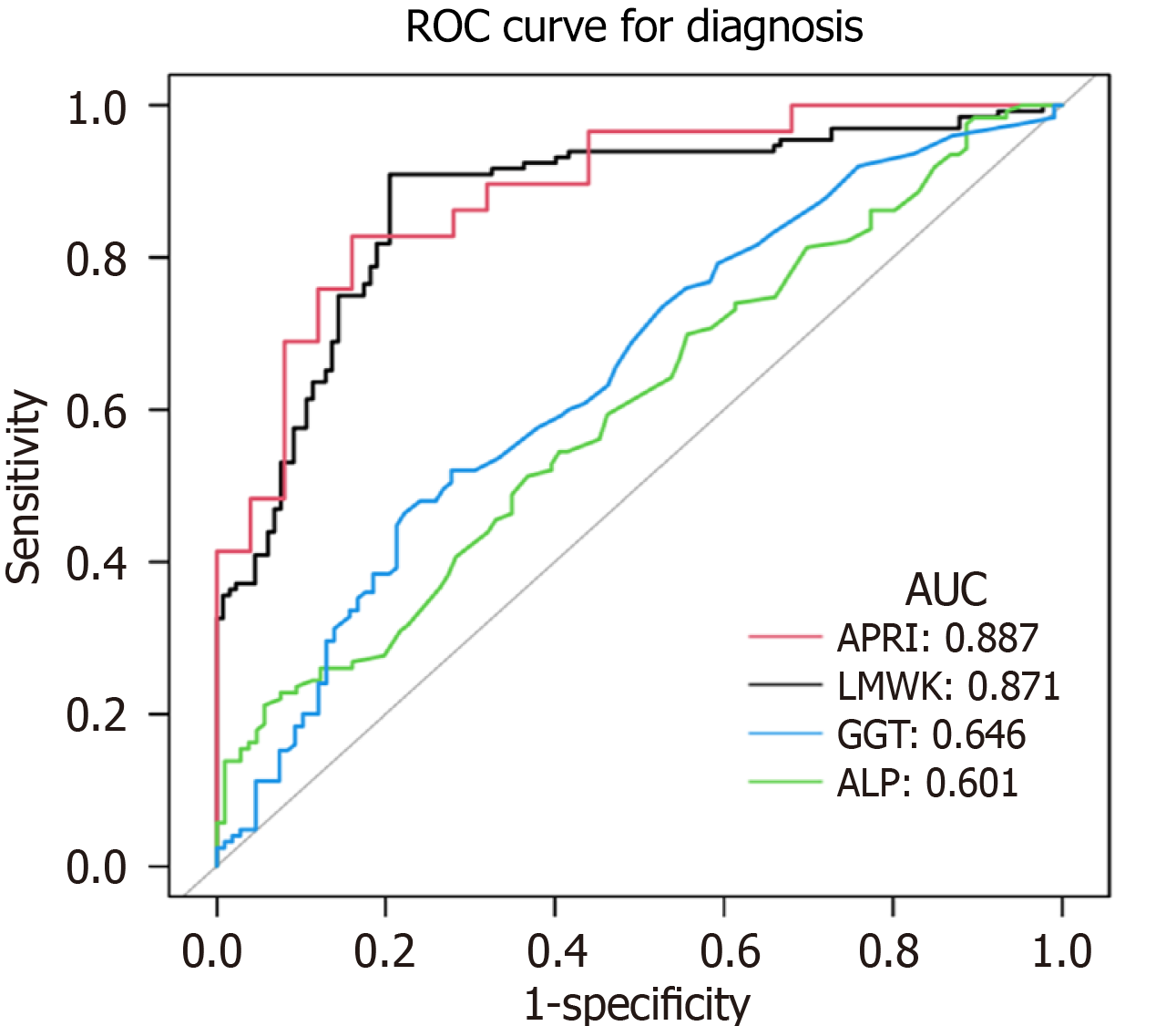
As shown in Figure 1, a ROC curve was created for the diagnosis of a medical condition using three different tests: LMWK-Fc, GGT, and ALP. The figure shows that LMWK-Fc had an area under the curve (AUC) of 0.871, which indicates a good level of performance in distinguishing between the normal and fibrosis groups. Meanwhile, the AUC of APRI, GGT, and ALP was 0.887, 0.646, and 0.601, respectively.
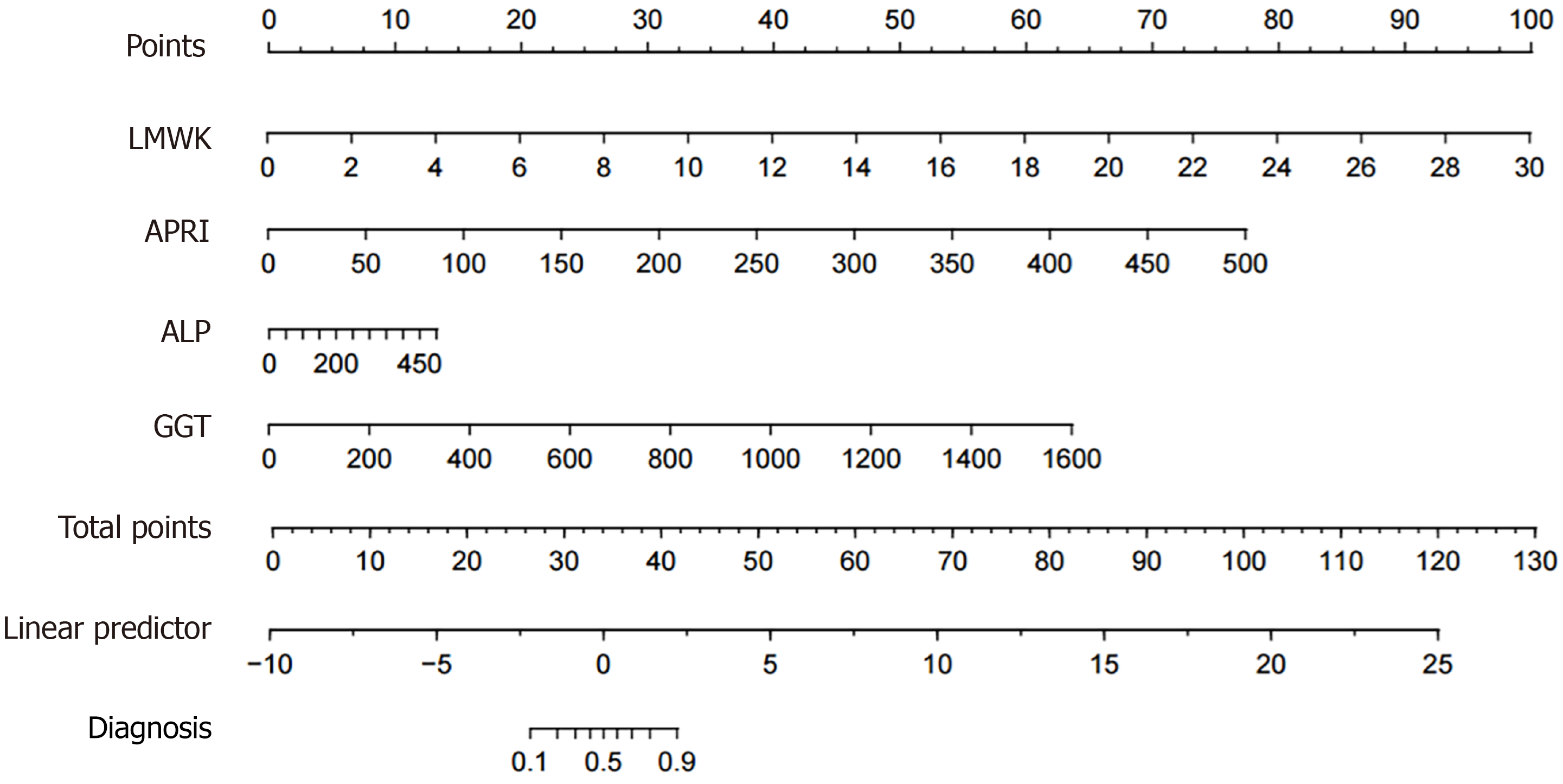
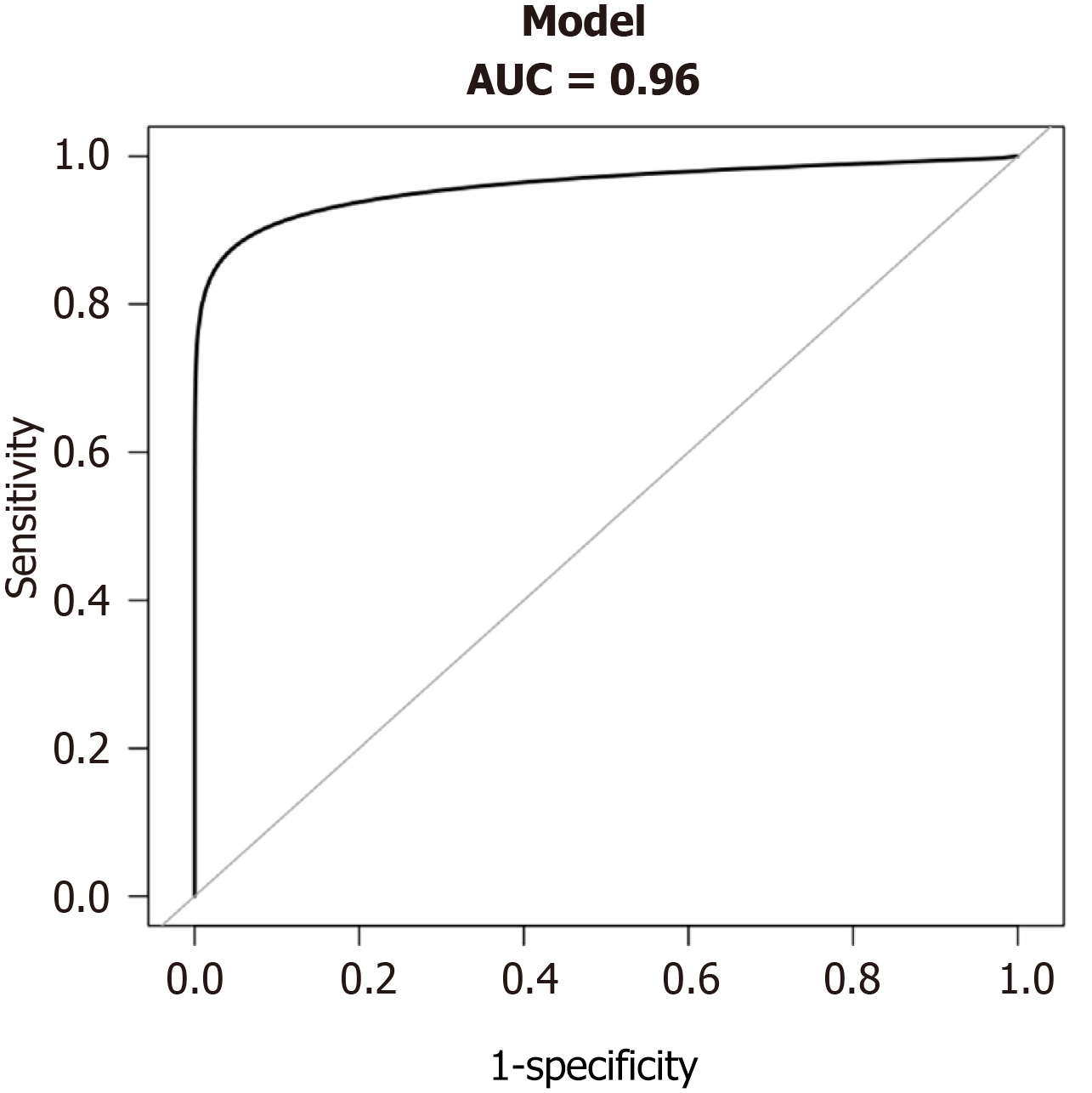
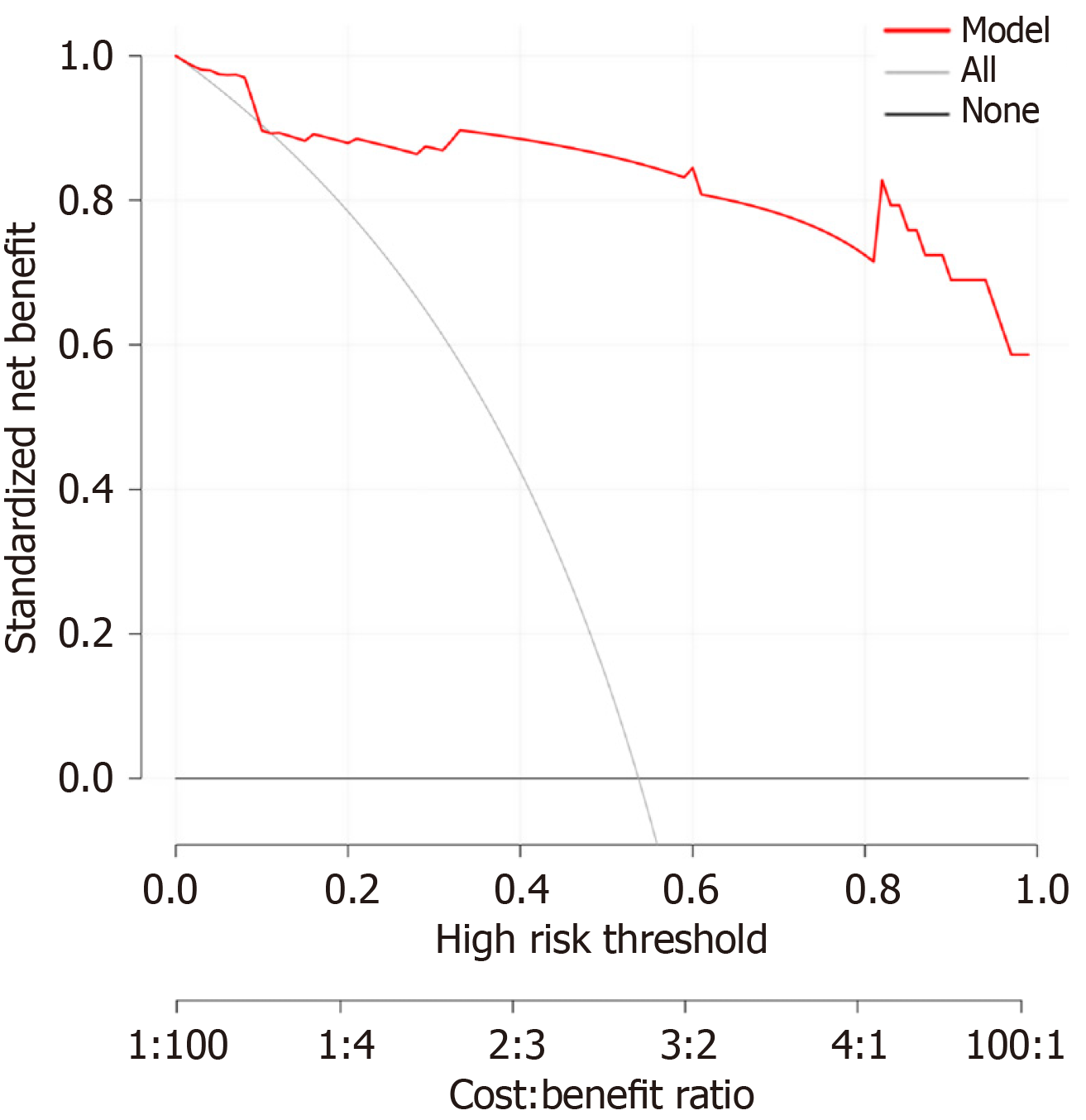
We constructed a nomogram that incorporated LMWK-Fc, APRI, ALP, and GGT as predictors of liver fibrosis (Figure 2). The nomogram assigned different weights to each predictor based on their contribution to the diagnosis of liver fibrosis. We evaluated the performance of the nomogram using the AUROC. The nomogram had a high AUC of 0.96 (Figure 3), indicating that it had high diagnostic accuracy for liver fibrosis. We also performed a clinical decision curve analysis to compare the net benefit of the nomogram with that of the existing tests (Figure 4). The clinical decision curve analysis showed that the nomogram had a superior net benefit across a wide range of threshold probabilities, indicating that it had high clinical value and applicability for liver fibrosis.
The present study aimed to investigate the predictive value of LMWK-Fc levels in patients diagnosed with liver fibrosis. Our findings suggest that LMWK-Fc levels have the potential to serve as a useful predictive biomarker for the progression of liver fibrosis. Our results indicate a significant positive correlation between LMWK-Fc levels and the severity of liver fibrosis, as determined by non-invasive imaging techniques. This finding supports the hypothesis that LMWK-Fc may play a role in the pathogenesis of liver fibrosis and points to its potential as an indicator of progression of the disease. Furthermore, the predictive value of LMWK-Fc levels is underscored by their association with clinical parameters commonly used to assess liver function, such as serum markers of liver injury and fibrosis, as well as non-invasive imaging modalities. These associations further highlight the potential utility of LMWK-Fc levels as a non-invasive predictive tool for monitoring the progression of liver fibrosis in clinical settings.
One of the main advantages of LMWK-Fc as a biomarker for liver fibrosis is its non-invasive nature, which only requires a blood draw from the patient. This is in contrast to other methods of liver fibrosis assessment, such as liver biopsy, which is invasive, costly, and prone to complications and sampling errors[19]. Moreover, LMWK-Fc is a simple and convenient test that can be easily performed in routine clinical laboratories, without the need for specialized equipment or personnel.
Therefore, LMWK-Fc can offer a more accessible and affordable alternative to other diagnostic methods for liver fibrosis. Another advantage of LMWK-Fc as a biomarker for liver fibrosis is its high diagnostic efficacy, which is reflected by its high AUROC value. The AUC is a measure of the overall performance of a binary classifier, ranging from 0 to 1. A higher AUC indicates a better classifier, while a lower AUC indicates a worse classifier. In our study, LMWK-Fc had an AUC of 0.871, which indicates a good level of performance in distinguishing between the normal and fibrosis groups. This is comparable to or even higher than the AUC for other biomarkers for liver fibrosis, such as the aspartate APRI, which had an AUC of 0.887 in our study, and the FibroScan, which had an AUC of 0.845 in a previous study[20]. Therefore, LMWK-Fc can offer a more accurate and reliable diagnosis of liver fibrosis than other biomarkers.
Previous studies have shown that core fucosylation of N-glycans is involved in the progression of various diseases, including fibrosis[21,22]. The mechanism underlying the association between LMWK-Fc levels and liver fibrosis remains unclear. However, it has been suggested that LMWK-Fc levels may reflect the activation of hepatic stellate cells, which are key players in the development of liver fibrosis[23]. Further studies are needed to elucidate the exact mechanism underlying the association between LMWK-Fc levels and liver fibrosis. However, it is essential to acknowledge the limitations of our study. The sample size of our cohort was relatively small, and the generalizability of our findings to larger patient populations needs validation through multicenter studies. Additionally, longitudinal studies are warranted to elucidate the dynamic changes in LMWK-Fc levels throughout the course of liver fibrosis and its potential role in predicting clinical outcomes.
Our study provides evidence that LMWK-Fc levels may be a useful biomarker for the diagnosis and prognosis of liver fibrosis. Further studies are needed to validate these findings and elucidate the underlying mechanism.
We are grateful to all participants, especially the study members. We are also obliged to the sample testers, who performed the measurements.
| 1. | Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 462] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 2. | Man S, Deng Y, Ma Y, Fu J, Bao H, Yu C, Lv J, Liu H, Wang B, Li L. Prevalence of Liver Steatosis and Fibrosis in the General Population and Various High-Risk Populations: A Nationwide Study With 5.7 Million Adults in China. Gastroenterology. 2023;165:1025-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 3. | Berumen J, Baglieri J, Kisseleva T, Mekeel K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech Dis. 2021;13:e1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Zhang CY, Liu S, Yang M. Treatment of liver fibrosis: Past, current, and future. World J Hepatol. 2023;15:755-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 5. | Liedtke C, Nevzorova YA, Luedde T, Zimmermann H, Kroy D, Strnad P, Berres ML, Bernhagen J, Tacke F, Nattermann J, Spengler U, Sauerbruch T, Wree A, Abdullah Z, Tolba RH, Trebicka J, Lammers T, Trautwein C, Weiskirchen R. Liver Fibrosis-From Mechanisms of Injury to Modulation of Disease. Front Med (Lausanne). 2021;8:814496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Virarkar M, Morani AC, Taggart MW, Bhosale P. Liver Fibrosis Assessment. Semin Ultrasound CT MR. 2021;42:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Angelini G, Panunzi S, Castagneto-Gissey L, Pellicanò F, De Gaetano A, Pompili M, Riccardi L, Garcovich M, Raffaelli M, Ciccoritti L, Verrastro O, Russo MF, Vecchio FM, Casella G, Casella-Mariolo J, Papa L, Marini PL, Rubino F, le Roux CW, Bornstein S, Mingrone G. Accurate liquid biopsy for the diagnosis of non-alcoholic steatohepatitis and liver fibrosis. Gut. 2023;72:392-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 8. | Rockey DC. Advances in hepatology: 2015. Curr Opin Gastroenterol. 2015;31:173-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Varki A. Biological roles of glycans. Glycobiology. 2017;27:3-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1742] [Cited by in RCA: 1842] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 10. | Kram M. Galectin-3 inhibition as a potential therapeutic target in non-alcoholic steatohepatitis liver fibrosis. World J Hepatol. 2023;15:201-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
| 11. | Sanda M, Ahn J, Kozlik P, Goldman R. Analysis of site and structure specific core fucosylation in liver cirrhosis using exoglycosidase-assisted data-independent LC-MS/MS. Sci Rep. 2021;11:23273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Motta G, Juliano L, Chagas JR. Human plasma kallikrein: roles in coagulation, fibrinolysis, inflammation pathways, and beyond. Front Physiol. 2023;14:1188816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Cugno M, Scott CF, Salerno F, Lorenzano E, Müller-Esterl W, Agostoni A, Colman RW. Parallel reduction of plasma levels of high and low molecular weight kininogen in patients with cirrhosis. Thromb Haemost. 1999;82:1428-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Wang M, Shen J, Herrera H, Singal A, Swindell C, Renquan L, Mehta A. Biomarker analysis of fucosylated kininogen through depletion of lectin reactive heterophilic antibodies in hepatocellular carcinoma. J Immunol Methods. 2018;462:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Zhu J, Warner E, Parikh ND, Lubman DM. Glycoproteomic markers of hepatocellular carcinoma-mass spectrometry based approaches. Mass Spectrom Rev. 2019;38:265-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Chao J, Bledsoe G, Chao L. Kallikrein-kinin in stem cell therapy. World J Stem Cells. 2014;6:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Zhang T, Zhang M, Yang L, Gao L, Sun W. Potential targeted therapy based on deep insight into the relationship between the pulmonary microbiota and immune regulation in lung fibrosis. Front Immunol. 2023;14:1032355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Hu X, Shen N, Liu A, Wang W, Zhang L, Sui Z, Tang Q, Du X, Yang N, Ying W, Qin B, Li Z, Li L, Wang N, Lin H. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol Ther. 2022;30:763-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Lai M, Afdhal NH. Liver Fibrosis Determination. Gastroenterol Clin North Am. 2019;48:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Ueda N, Kawaoka T, Imamura M, Aikata H, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Hayes CN, Yokozaki M, Chayama K. Liver fibrosis assessments using FibroScan, virtual-touch tissue quantification, the FIB-4 index, and mac-2 binding protein glycosylation isomer levels compared with pathological findings of liver resection specimens in patients with hepatitis C infection. BMC Gastroenterol. 2020;20:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wang M, Sanda M, Comunale MA, Herrera H, Swindell C, Kono Y, Singal AG, Marrero J, Block T, Goldman R, Mehta A. Changes in the Glycosylation of Kininogen and the Development of a Kininogen-Based Algorithm for the Early Detection of HCC. Cancer Epidemiol Biomarkers Prev. 2017;26:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Mehta A, Block TM. Fucosylated glycoproteins as markers of liver disease. Dis Markers. 2008;25:259-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Li X, Zhang Q, Wang Z, Zhuang Q, Zhao M. Immune and Metabolic Alterations in Liver Fibrosis: A Disruption of Oxygen Homeostasis? Front Mol Biosci. 2021;8:802251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/