Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.106166
Revised: June 12, 2025
Accepted: June 16, 2025
Published online: July 21, 2025
Processing time: 146 Days and 21 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a prevalent chronic liver disorder driven by obesity and metabolic dysfunction. MASLD progresses to metabolic dysfunction-associated steatohepatitis, which is characterized by inflammation, hepatocyte injury, and fibrosis, increasing the risk of cirrhosis and liver failure. Recent studies suggest that neutrophil extracellular traps (NETs) and extracellular DNA (ecDNA) contribute to liver inflammation and fibrogenesis. However, their role in MASLD pathogenesis remains incom
To investigate the dynamics of circulating NETs and ecDNA as potential bio
Using three complementary mouse models, thioacetamide (TAA)-induced fibrosis, choline-deficient L-amino acid-defined (CDAA) diet-induced metabolic dysfunction-associated steatohepatitis, and cafeteria (CAF) diet-induced MASLD, we assessed the association between NET-related markers and liver damage. Blood samples were collected biweekly to analyze ecDNA and NET markers, including myeloperoxidase (MPO) and MPO-DNA complexes, using ELISA and real-time PCR. Liver histo
The TAA and CDAA models exhibited significant liver injury, characterized by increased plasma alanine aminotransferase and aspartate aminotransferase levels, hepatocellular damage, and fibrosis. Elevated circulating NET markers (MPO and ecDNA) were observed in these models, with a strong correlation between NET formation and liver pathology. The CAF diet model induced steatosis but failed to elicit significant liver fibrosis or an increase in NET markers, suggesting that NETosis is associated with more severe liver damage. Notably, ecDNA and MPO levels correlated with neutrophil infiltration and fibrosis scores, indicating their potential as biomarkers of MASLD progression.
NETosis and ecDNA levels reflect liver injury severity in MASLD. NET markers and liver fibrosis were strongly associated in TAA and CDAA models, whereas CAF model showed minimal NET involvement.
Core Tip: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a growing global health concern with limited therapeutic options. This study demonstrated that extracellular DNA (ecDNA) and neutrophil extracellular traps (NETs) serve as potential biomarkers of liver injury severity in MASLD. Using three distinct animal models, we showed that NET markers, including myeloperoxidase and ecDNA, correlated with liver fibrosis and inflammation. These findings highlighted NETs and ecDNA as promising targets for early diagnosis and intervention in MASLD, offering new insights into disease progression and potential therapeutic strategies.
- Citation: Feješ A, Belvončíková P, Bečka E, Strečanský T, Pastorek M, Janko J, Filová B, Babál P, Šebeková K, Borbélyová V, Gardlík R. Myeloperoxidase, extracellular DNA and neutrophil extracellular trap formation in the animal models of metabolic dysfunction-associated steatotic liver disease. World J Gastroenterol 2025; 31(27): 106166
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/106166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.106166
Metabolic dysfunction-associated steatotic liver disease (MASLD) [formerly known as nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease (MAFLD)] is the most common chronic liver condition, with an increasing worldwide prevalence along with the escalating global obesity problem[1]. In Europe the prevalence of MASLD exceeds 25% in the adult population and more often affects individuals with comorbidities of metabolic syndrome components[2]. Realizing the interconnection between MASLD and metabolic syndrome, recent shifts in liver disease nomenclature have led to new terminology. Steatotic liver disease encompasses the multi-etiological presence of steatosis in the liver. This can be further subcategorized as MASLD if an individual meets criteria for at least one of the cardiometabolic risk factors [elevated body mass index (BMI) or waist circumference; abnormal glucose metabolism or type 2 diabetes or its treatment; hypertension or antihypertensive medication use; elevated triglycerides; and low high-density lipoprotein cholesterol levels or lipid-lowering treatment]. MASLD, accompanied by inflammation, progresses into metabolic dysfunction-associated steatohepatitis (MASH) [formerly named nonalcoholic steatohepatitis (NASH)] and later fibrosis[3].
The accumulation of lipids in the liver is responsible for steatosis and may cause initial inflammation in MASH, leading to cell membrane disruption and hepatocyte death[4]. Innate immune cells such as polymorphonuclear leukocytes, primarily represented by neutrophils, are recruited in response to this inflammation. These cells act as a first-line response to bacterial and sterile stimuli (e.g., DNA from disintegrated hepatocytes). The activation of neutrophils induces various processes against pathogens, including phagocytosis, degranulation, the generation of reactive oxygen species, and specific cell death, called NETosis. NETosis is characterized by the release of web-like structures, neutrophil extracellular traps (NETs) composed of neutrophil DNA, and nuclear, granular, and cytosolic proteins, such as myeloperoxidase (MPO), neutrophil elastase (NE), and other compounds. During NETosis, decondensed chromatin is expelled outside the cell, releasing extracellular DNA (ecDNA)[5].
Currently, there are no established treatment therapies for MASH, possibly due to the limited understanding of its pathogenesis. Previous clinical studies have shown that ecDNA plays a role in systemic autoimmune inflammatory diseases, such as rheumatoid arthritis[6]. Elevated concentrations of ecDNA were described in patients with MASLD as well. In addition, this ecDNA correlated with disease severity[7]. Correspondingly, increased markers of NETosis, specifically serum concentrations of MPO-associated DNA, have been reported in patients with MASH[8]. Immunogenic properties of ecDNA could potentiate the progression of MASLD to MASH. Long-term persistent inflammation can further stimulate the immune system, amplify inflammatory cascades, and damage additional tissue[5]. The formation of NETs in the liver is triggered by the presence of damage-associated molecular patterns, including released ecDNA and nuclear proteins from the damaged hepatocytes[9]. Early liver neutrophil infiltration and NET formation have already been observed in the experimental MASH model induced by a high-fat diet and neonatal streptozotocin[8].
However, fewer studies focus on the dynamics of circulating ecDNA and the NET production in the pathogenesis of MASLD, leading to initial inflammation and progression of MASLD to MASH. Gaining insight into the dynamics of immune system activation on the periphery is vital for a better understanding of the pathogenesis and developing effective prevention strategies. Targeting circulating ecDNA and NETs could emerge as a promising early marker and therapeutic objective in the future.
Preclinical models of liver damage allow us to study the dynamics of ecDNA and NET formation during liver disease. Thioacetamide (TAA) is a compound broadly used to mimic liver fibrosis. Its hepatotoxicity stems from the molecule bioactivation and the formation of reactive oxygen metabolites such as TAA-S-oxide and TAA-S-dioxide, triggering lipid peroxidation of hepatocyte membranes. This process results in inflammation and extensive fibrosis and can potentially lead to the development of hepatocellular carcinoma later on[10]. These pathological changes could be visible 6 weeks after intraperitoneal administration of TAA in rodent models, making it a suitable model for studying the dynamics of circulating ecDNA and peripheral NET production[11].
A different model is based on administering a choline-deficient L-amino acid-defined (CDAA) diet supplemented with 1% cholesterol, which promotes oxidative damage. The CDAA diet is designed to induce liver steatosis, inflammation, and fibrosis within 6 weeks, making it valuable for studying liver diseases like MASLD[12]. Dietary choline deficiency leads to insufficient phosphatidylcholine production and impaired very low-density lipoprotein synthesis, which results in the accumulation of triglycerides in the liver and steatosis[13].
Lastly, the cafeteria (CAF) model could mimic spontaneous-diet-induced MASLD. It has previously been shown that the Western-style CAF diet mimics unhealthy dietary habits in the human population[14,15]. The CAF diet contains several food items that are highly sweet, fatty, and salty[16]. The high intake of fats and carbohydrates in the human population can lead to the development of MASLD[17]. Excessive caloric intake can result in obesity and metabolic syndrome, which may have various pathophysiological consequences, including MASLD, characterized by a high accumulation of cholesterol and triacylglycerols in the liver. This accumulation can further promote liver damage and impaired glucose metabolism, resulting in insulin resistance[18]. Rodents fed with a CAF diet show impairment in lipid and glucose metabolism, leading to the development of obesity and metabolic syndrome with hepatic steatosis[19]. Furthermore, the CAF diet model might be suitable for investigating low-grade inflammation and immune responses in metabolic pathologies such as MASLD and MASH[20].
Using various animal models of liver damage associated with fibrosis or steatohepatitis might aid in understanding the potential role of NET formation linked to the severity of liver pathologies. The main aim of this study was to investigate the dynamics of circulating ecDNA and peripheral NET formation as potential predictors of liver injury and possible therapeutic targets in mouse models of liver damage associated with fibrosis or steatohepatitis.
Female C57BL/6J mice (n = 50) were used at 6 months of age (C57BL/6J, Strain #000664; Jackson Laboratory supplied by Charles River Laboratories, Sulzfeld, Germany). Animals were group-housed (3-5 mice per cage) in polyethylene cages (36.5 cm × 20.5 cm × 14 cm) and kept in a controlled environment of 24 ± 2 °C and 55% ± 10% humidity with ad libitum access to food and water on a 12-hour light/dark cycle (lights on at 7 am and off 7 pm). Researchers handled animals daily.
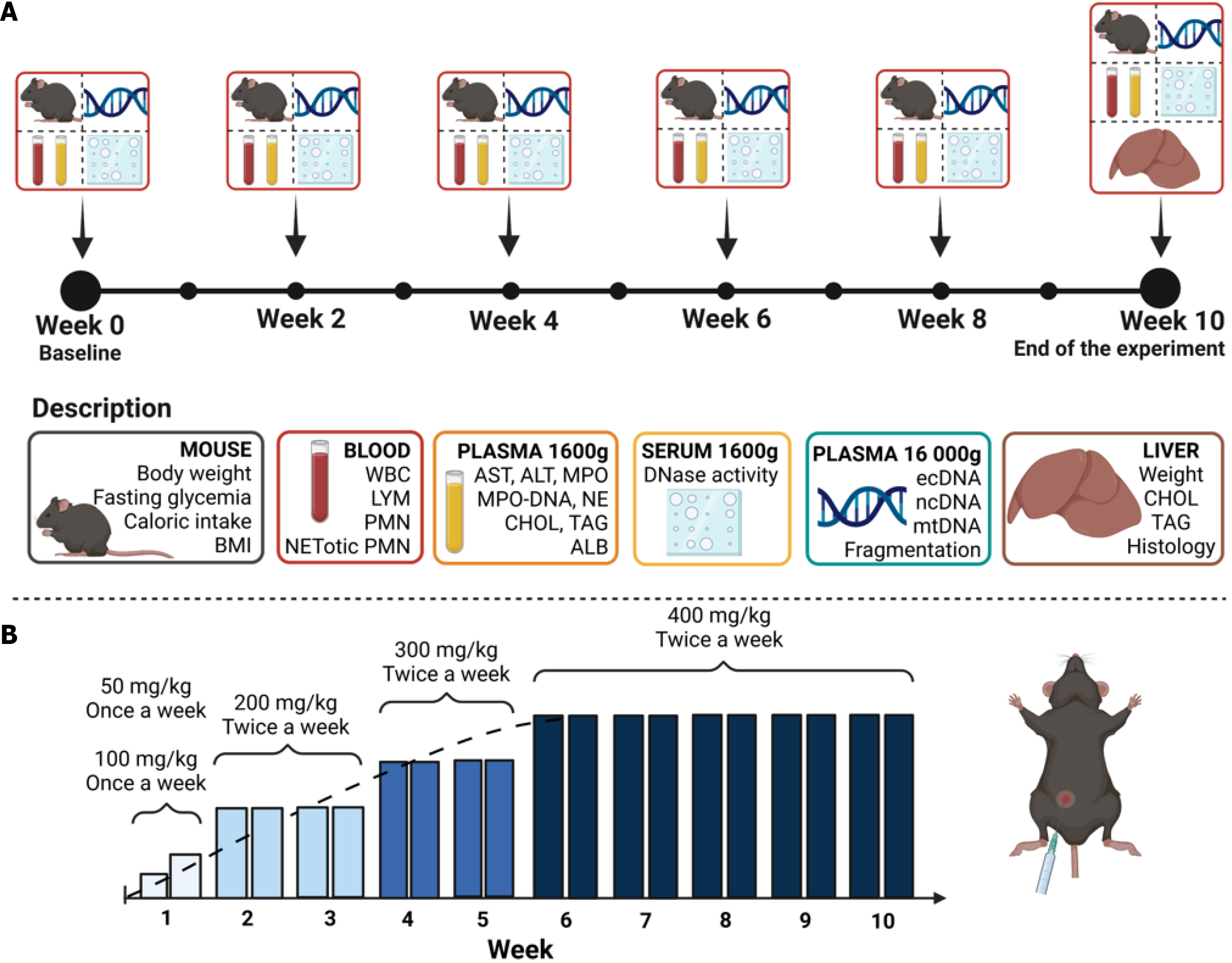
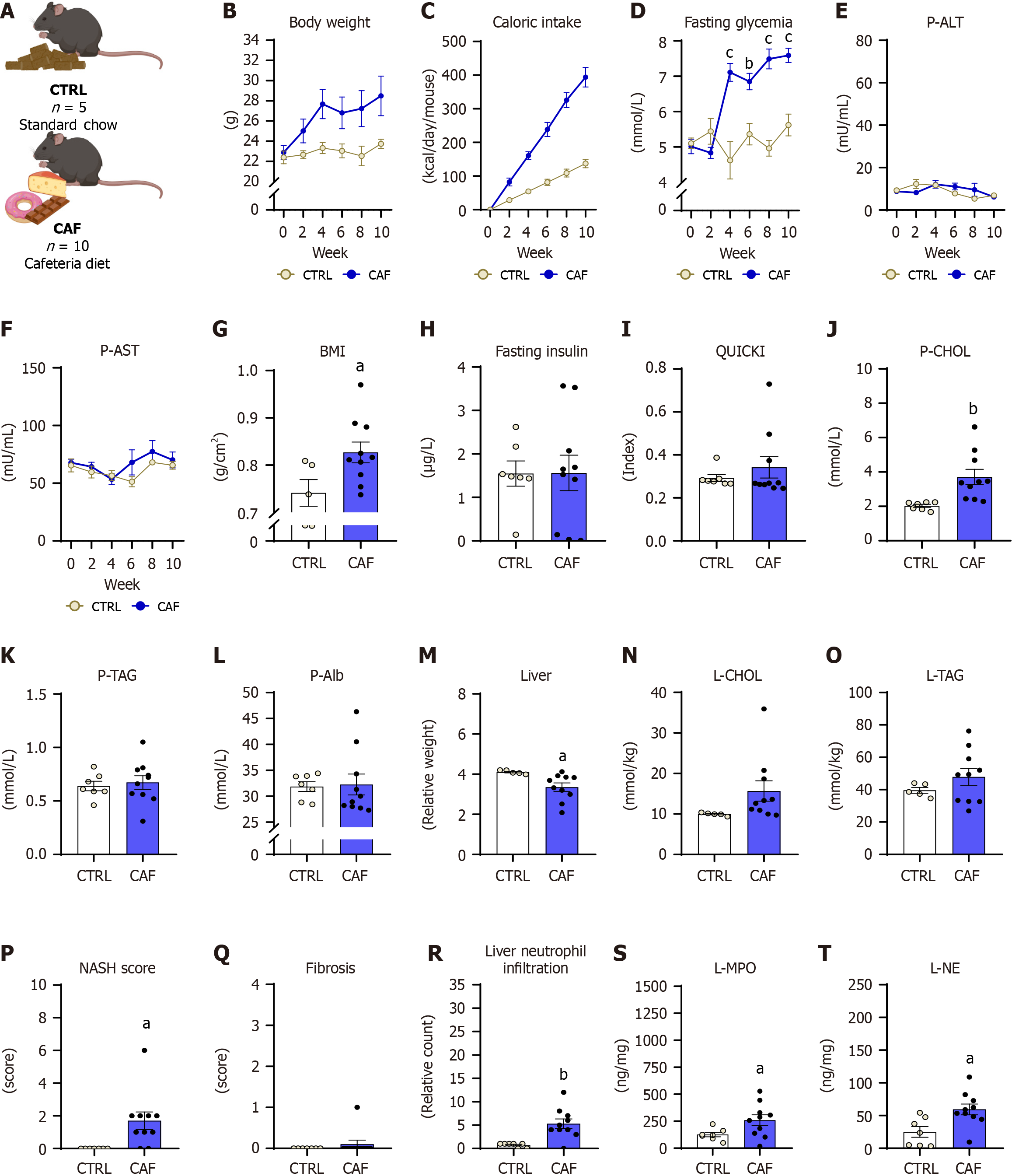
Three animal models, one chemically induced and two induced through a specific diet, were employed to investigate circulating ecDNA and peripheral NET production dynamics in MASLD and MASH. Each experimental protocol spanned 10 weeks, during which body weight, cumulative caloric intake, and fasting glycemia were monitored every second week (Figure 1A). Additionally, blood was regularly collected (over 2 weeks).
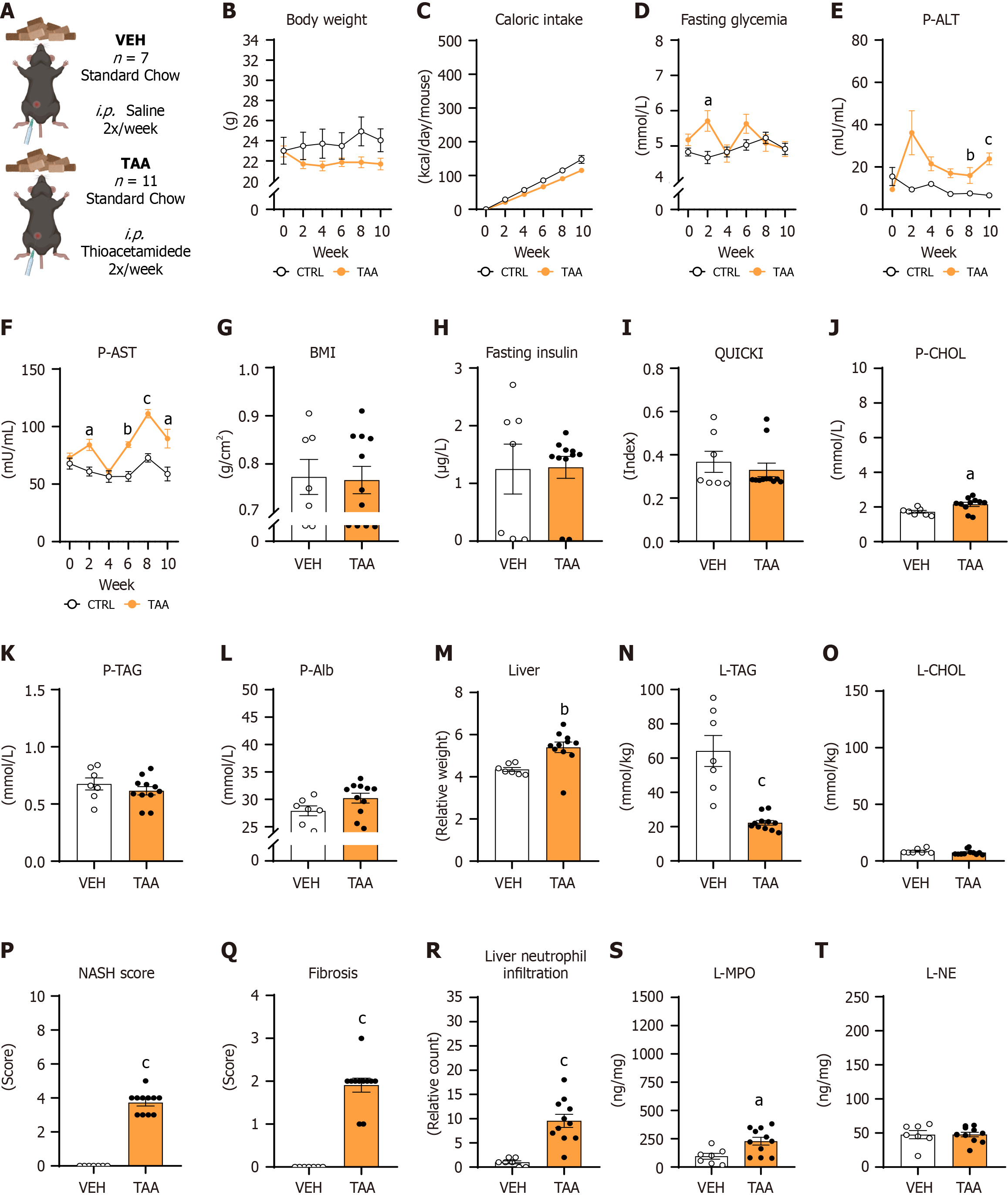
This model aimed to induce liver fibrosis using the hepatotoxic substance TAA (Merck & Co., Rahway, NJ, United States), based on the principle of the covalent bond of reactive species to proteins and lipids in the liver[10]. Female mice were divided into the control group (CTRL VEH, n = 7) and the experimental group (TAA, n = 11). The TAA group was intraperitoneally administered TAA twice a week in the volume of 100 µL with a gradually increasing dose over time. Doses started at 50 mg/kg and 100 mg/kg in the first week, 200 mg/kg in the second and third week, 300 mg/kg in the fourth and fifth week, and 400 mg/kg in the sixth through tenth week of the experiment (Figure 1B)[21]. The CTRL VEH group received saline in the corresponding volume and time intervals. Both groups were fed with a standard chow (Catalog No. E15051-04; ssniff Spezialdiäten GmbH, Soest, Netherlands). Mice were sacrificed 2 days after the last TAA application.
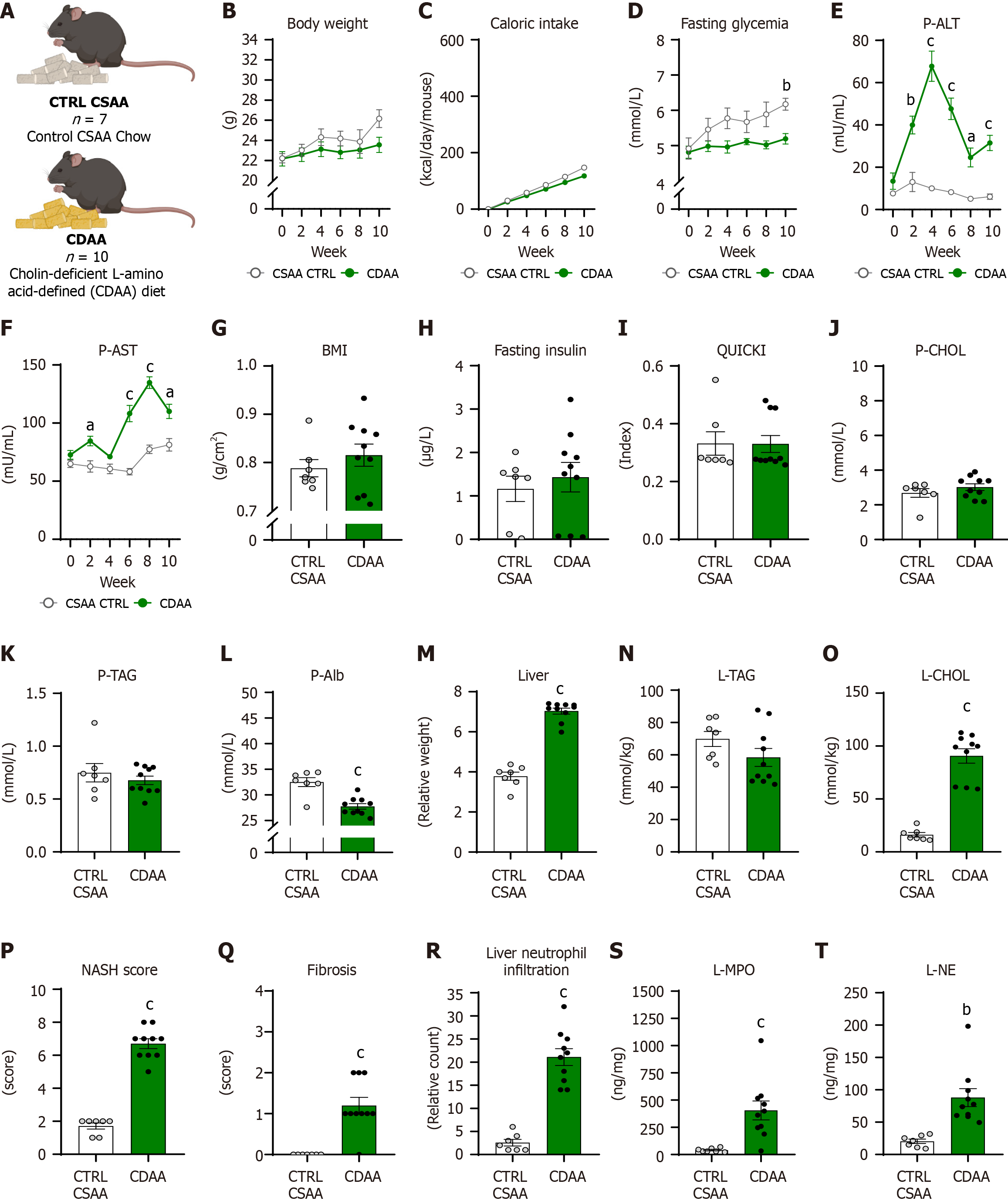
The current animal model aimed to induce experimental MASH using a CDAA diet with a low methionine content supplemented with 1% cholesterol. These components are crucial for reducing oxidative stress and homeostasis of lipid metabolism in the liver[22]. Female mice were divided into a control group [CTRL choline-sufficient L-amino acid-defined control (CSAA), n = 7] fed with the CSAA diet (Catalog No. E15668-04; ssniff Spezialdiäten GmbH), and the experimental group (CDAA, n = 10) fed with the CDAA diet (Catalog No. E15666-94; ssniff Spezialdiäten GmbH).
The CAF diet model was used to mimic dietary habits and spontaneous diet-induced MASLD[16]. Female mice were divided into control diet-fed (CTRL, n = 5) and CAF-fed (CAF, n = 10) groups. The CTRL group received standard chow (Catalog No. E15051-04; ssniff Spezialdiäten GmbH). The CAF comprised freely available food items purchased from grocery stores. The diet was divided into two menus and changed in 2-day intervals to ensure a novelty effect[16]. The first menu contained caramel biscuit snacks, salami, chocolate donuts, and salty sticks. The second menu included a chocolate biscuit with vanilla cream, edam cheese (fat-30%), cheese crackers, and chocolate cream (Supplementary Table 1).
At week 10 and before terminal sampling, all animals were anesthetized using 3% isoflurane (1000 mg/g; Vetpharma Animal Health, Barcelona, Spain) anesthesia. The nose-to-anus length was measured in the supine position. All measurements were performed using scale and thread[23]. The BMI was calculated from terminal body weight and nose-to-anus length[24].
Blood was collected from the retro-orbital plexus under inhalation anesthesia [3% isoflurane (1000 mg/g; Vetpharma Animal Health), 97% oxygen] into EDTA collection tubes (Sarstedt AG & Co. KG, Nümbrecht, Germany) for plasma analysis and into Eppendorf tubes for serum analysis. Blood count was determined with the hemoanalyzer Abacus VET 5 (Diatron MI ZRT, Budapest, Hungary). Plasma and serum were centrifuged at 1600 g for 10 minutes at 4 °C and stored until further analysis at -20 °C. After 8 hours of food deprivation in the dark phase, fasting glycemia from the tail vein was measured using a glucometer Accu Chek Performa (Roche LTD, Basel, Switzerland). The fasting insulin concentration was determined using an ELISA kit according to the manufacturer’s protocol (Mouse Insulin ELISA; Mercodia, Uppsala, Sweden). The quantitative index of insulin sensitivity was calculated. Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzyme activities were determined using commercially available assay kits and performed according to the manufacturer’s protocols (Merck & Co.). The concentrations of plasma cholesterol, triacylglycerols, and albumin were measured using a biochemical analyzer (Vitros 250; Ortho Clinical Diagnostics, Raritan, NJ, United States). NE and MPO in the plasma were measured using the ELISA method and performed following the manufacturer’s protocols (R&D Systems, Inc, Minneapolis, MN, United States). The level of MPO-DNA in plasma was measured using an adapted ELISA assay. Briefly, a high-binding 96-well plate (Sarstedt AG & Co. KG) was coated overnight at 4 °C with 0.5 µg/mL anti-MPO antibody (0400-0002; Bio-Rad, Hercules, CA, United States) in 0.1 M carbo
Nonspecific DNase activity was determined from serum using a single radial enzyme dispersion assay with the green-fluorescent dye GoodView™ (SBS Genetech, Beijing, China). Samples were loaded onto a 1% agarose gel containing 0.5 M Tris-HCl, 10 mmol/L CaCl2, 10 mmol/L MgCl2, pH 7.5, and 0.5 mg/mL DNA isolated from chicken livers. The gel with loaded samples was incubated at 37 °C for 17 hours in the dark. Dilutions of DNase I (Qiagen GmbH, Hilden, Germany) were used to construct a calibration curve. The diameter of hydrolyzed DNA circles (reflecting DNase activity) was measured using ImageJ software (National Institutes of Health, Bethesda, MD, United States)[25,26].
Plasma samples for ecDNA isolation were acquired by centrifugation of blood samples at 1600 g at 4 °C for 10 minutes and then at 16000 g at 4 °C for 10 minutes. The isolation of ecDNA was performed using the QiaAmp DNA Blood Mini Kit (Qiagen GmbH) according to the manufacturer’s instructions with the modified elution volume of 50 µL. Isolated ecDNA was quantified using a Qubit 3.0 fluorometer and the Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA, United States). The isolated ecDNA was stored at -20 °C until subsequent analysis.
A real-time PCR method using nuclear-specific and mitochondrial-specific primers was used to analyze the subcellular origin of ecDNA. The sequences of primers applied for nuclear DNA (ncDNA) analysis were forward 5´-TGTCAGATATGTCCTTCAGCAAGG- 3´ and reverse 5´-TGCTTAACTCTGCAGGCGTATG- 3´ and for mitochondrial DNA (mtDNA) analysis were forward 5´-CCCAGCTACTACCATCATTCAAGT- 3´ and reverse 5´-GATGGTTTGGGAGATTGGTTGATGT- 3´. Each primer was added in a final concentration of 250 nM per reaction. For the PCR reaction, SsoAdvanced Universal IT SYBRGreen SuperMix (Bio-Rad Laboratories) and real-time thermal cycler qTOWER³ Series (Analytik Jena GmbH + Co, Jena, Germany) were used. PCR reaction conditions included an initial denaturation step at 95 °C for 15 minutes, followed by 40 cycles of denaturation at 94 °C for 15 seconds, annealing at 60 °C for 30 seconds, and polymerization at 72 °C for 30 seconds. An additional annealing step at 65 °C for 15 seconds was performed, concluding with a melt curve analysis at 95 °C. The quantification of ncDNA and mtDNA was determined as gene equivalents per mL of plasma.
For analysis 10-20 µL of isolated ecDNA were run on QSep 1+ (Bioptic, Taipei, Taiwan, China) by High Sensitivity Cartridge N1 (Bioptic). Injection of the samples was set up for 20 seconds at 8 kV. Samples were analyzed for 200 seconds. Subsequently, all data were processed as follows: Baseline was set up to 10000; peak smoothing to 3; peak threshold to 10; and peak definition to 3. For each sample, two zones for smear analysis were defined: Zone 1 was set from 20 bp to 250 bp (targeting mononucleosomal peak fragments); and Zone 2 was set from 250 bp to 1000 bp (targeting oligonucleosomal peak fragments). The ratio of the small vs large fragments (20-250 bp and 250-1000 bp) was calculated from the area under the curve from Zone 1 and Zone 2.
For flow cytometry 50 µL of peripheral blood was lysed in ice-cold isotonic ammonium chloride buffer (150 mmol/L NH4Cl, 10 mmol/L KHCO3, 0,1 mmol/L EDTA) for 15 minutes, followed by centrifugation at 400 g at 4 °C for 10 minutes. The pellet was resuspended in 100 µL FACS buffer (RPMI + 1% FBS) with the addition of 2.5 µg/sample of Alexa Fluor®647 anti-mousse Ly-6G antibody (127610; Biolegend, San Diego, CA, United States), 0.1 µg/sample of primary rabbit monoclonal anti-histone H3 (citrulline R17) antibody (ab219407; Abcam, Cambridge, United Kingdom), followed by 0.025 µg/sample of secondary Brilliant Violet 510™ Donkey anti-rabbit IgG antibody (406419; Biolegend) antibody in a combination of 200 nM of SYTOX™ Green Nucleic Acid Stain (S7020; Invitrogen, Eugene, OR, United States). Samples were stained for 15 minutes at room temperature in the dark and immediately assayed on the DxFlex flow cytometer (Beckman Coulter, Brea, CA, United States) and analyzed by FCSExpress 6.0 software (De Novo Software, Pasadena, CA, United States). NETotic neutrophils were identified as Ly-6G-positive events, with ecDNA acid and citrullinated histone H3 double positivity, and their number was recalculated per 1 mL of blood (Supplementary Figure 1).
Animals were anesthetized using isoflurane anesthesia, and blood was collected as described above. All animals underwent cardiac perfusion with saline; consequently, cervical dislocation was performed. The liver was gently removed and weighed, and liver samples were taken from the same liver lobules. The liver samples were analyzed for the concentration of liver cholesterol and triacylglycerols[27,28]. Subsequently, 10% homogenate (1 × PBS) of 100 mg of liver tissue was used to determine the protein content (BCA Protein Assay kit; SERVA Electrophoresis GmbH, Heidelberg, Germany) and concentration of L-MPO (R&D Systems, Inc) and L-NE (R&D Systems, Inc).
Liver samples for histological evaluation were taken from the same lobules and fixed in 4% formaldehyde for 48 hours. Consequently, all liver samples were processed into paraffin, cut into 4 μm slices using a microtome (Hyrax M40; Zeiss, Oberkochen, Germany), and stained with hematoxylin and eosin. Pathologies were determined using a standard protocol[29]. Briefly, all liver sections were scored for steatosis (score 0-3), ballooning of hepatocytes (score 0-2), and lobular inflammation (score 0-3). The NASH score was calculated as a sum of all three scores (steatosis, ballooning of hepa
GraphPad Prism version 8.0.1 (GraphPad Software, Inc, La Jolla, CA, United States) was used for the statistical analysis and visualization of the results. Time-dependent parameters were analyzed using repeated measures of two-way analysis of variance (independent factors: Time and treatment/diet) with a Bonferroni post-hoc correction. The independent t-test was used for the terminal parameters (at week 10). Pearson’s correlation was performed at weeks 8 and 10. All statistical test values less than 0.05 were considered statistically significant. Graphical abstract and figures created by BioRender.com.
The body weight of TAA mice (Figure 2A) decreased over 10 weeks but without significant differences compared with the CTRL VEH group [treatment: P = not significant (NS), time: P < 0.05; interaction: P < 0.001; Figure 2B]. Mice that were injected with TAA showed decreased caloric intake over the weeks compared with vehicle-injected mice, while significant differences within the individual periods of measurements were not detected (treatment: P = NS; time: P < 0.01; time × treatment interaction: P < 0.01; Figure 2C). Fasting glycemia was affected by neither TAA treatment nor time, while the interaction of both factors was significant (treatment: P = NS, time: P = NS; interaction: P < 0.05; Figure 2D). The increase of fasting glycemia was detected in the second week in the TAA group compared with mice that received vehicle (week 2: 5.7 ± 0.3 mmol/L vs 4.7 ± 0.2 mmol/L, P < 0.05; Figure 2D).
The activity of ALT in plasma increased from baseline to the tenth week in female mice receiving TAA injections compared with the CTRL VEH group (treatment: P < 0.01, time: P = NS; interaction: P < 0.05; Figure 2E). A significant increase of plasma ALT activity in TAA-injected mice compared with CTRL VEH-injected mice was detected in weeks 6 and 10 (TAA vs CTRL VEH: Week 6: 17 ± 2.1 mU/mL vs 7.3 ± 0.4 mU/mL; P < 0.05; week 10: 23.9 ± 2.8 mU/mL vs 6.6 ± 0.9 mU/mL; P < 0.05; Figure 2E). Similarly, the activity of AST in plasma after TAA injections increased over 10 weeks in the TAA females compared with the CTRL VEH group of females (treatment: P < 0.001; time: P < 0.001; interaction: P < 0.001; Figure 2F). Significantly increased AST activity was measured in TAA-treated females compared to CTRL VEH-injected females in weeks 2, 6, 8, and 10 (TAA vs CTRL VEH: Week 2: 84.1 ± 4.9 mU/mL vs 60.9 ± 3.9 mU/mL; P < 0.05; week 6: 84.3 ± 2.3 mU/mL vs 56.6 ± 4.3 mU/mL; P < 0.01; week 8: 111.2 ± 3.7 mU/mL vs 72.8 ± 3.6 mU/mL; P < 0.001; week 10: 89.6 ± 8.1 mU/mL vs 58.8 ± 6.1 mU/mL; P < 0.05; Figure 2F).
The BMI and fasting insulin concentrations did not differ between TAA and vehicle-injected females (P = NS; Figure 2G and H). Correspondingly, with fasting glycemia and insulin concentrations, the quantitative insulin sensitivity check index was comparable between TAA and CTRL VEH females (P = NS; Figure 2I). Mice injected with TAA had higher plasma cholesterol concentrations than vehicle-injected mice (TAA vs CTRL VEH: 2.2 ± 0.4 mmol/L vs 1.7 ± 0.2 mmol/L, P < 0.05; Figure 2J). Plasma concentrations of triacylglycerols did not differ in the TAA model (P = NS; Figure 2K). Moreover, TAA females did not show hypoalbuminemia compared to CTRL VEH females (P = NS; Figure 2L). The relative weight of the liver was 19.5% greater in TAA-injected mice compared to CTRL VEH-injected mice (P < 0.01; Figure 2M).
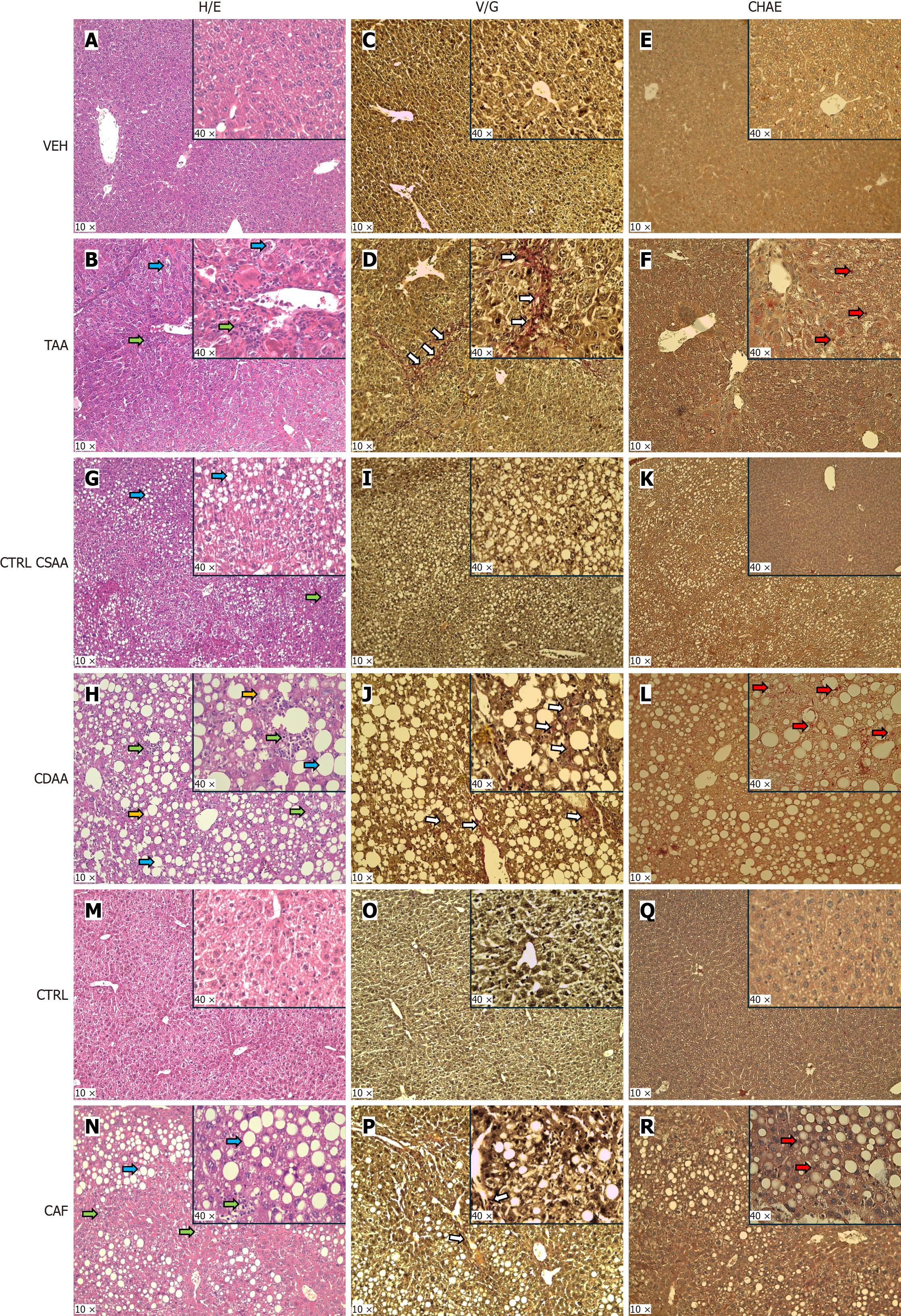
Moreover, the content of triacylglycerols in the liver was lower in the TAA group compared with the CTRL VEH group of females (P < 0.001; Figure 2N), while cholesterol content did not differ between groups (P = NS; Figure 2O). TAA-injected females showed lobular inflammation (P < 0.001) and steatosis (P < 0.001) in the liver compared with vehicle-injected females, while ballooning of hepatocytes were not present (Supplementary Figure 2A; Figure 3A and B). The NASH score was significantly higher in TAA-injected female mice than in vehicle-injected ones (P < 0.001; Figure 2P).
Fibrosis was highly spread in the TAA-injected female mice compared with vehicle-injected females (Figure 3C and D), represented by a higher fibrosis score in the TAA group than the CTRL VEH group (P < 0.001; Figure 2Q). TAA-injected females showed more neutrophils infiltrating the liver tissue than vehicle-injected females (P < 0.001; Figure 2R, Figure 3E and 3F). Additionally, TAA-injected mice showed higher MPO concentration in the liver compared with CTRL VEH-injected mice (P < 0.05; Figure 2S), while NE concentration in the liver did not differ between groups (Figure 2T).
The body weight in the CDAA model (Figure 4A) increased over the 10 weeks with no difference between the CDAA and CTRL CSAA groups (diet: P = NS, time: P < 0.001; interaction: P < 0.05; Figure 4B). Cumulative caloric intake in both CDAA and CTRL CSAA groups increased over time with the main effect of diet (diet: P < 0.05, time: P < 0.001; interaction: P < 0.001; Figure 4C) but without any differences in individual weeks. The fasting glycemia increased in the CTRL CSAA group over 10 weeks of model induction, while in the CDAA experimental group it did not (diet: P < 0.01, time: P < 0.01; interaction: P = NS; Figure 4D). The CDAA group showed increased fasting glycemia after 10 weeks of model induction compared with the CTRL CSAA mice (5.2 ± 0.5 mmol/L vs 6.2 ± 0.2 mmol/L, P < 0.01; Figure 4D).
The activity of ALT in plasma increased over weeks of diet administration with significant differences between groups (diet: P < 0.001, time: P < 0.001, interaction: P < 0.001; Figure 4E): The CDAA group showed an increased ALT activity in week 2, with a peak in week 4 and a decrease in weeks 6, 8, and 10, but it was still significantly higher compared with CTRL CSAA mice (CDAA vs CTRL CSAA: Week 2: 40 ± 5.5 mU/mL vs 14.5 ± mU/mL; P < 0.01; week 4: 67.8 ± 9.4 mU/mL vs 10.1 ± 1.1 mU/mL; P < 0.001; week 6: 47.6 ± 6.6 mU/mL vs 8.3 ± 1.2 mU/mL; P < 0.001; week 8: 24.6 ± 4.9 mU/mL vs 5.1 ± 0.7 mU/mL; P < 0.05; week 10: 31.5 ± 4.6 mU/mL vs 6.1 ± 1.3 mU/mL; P < 0.001; Figure 4E).
Plasma activity of AST increased over the 10 weeks, while the CDAA group differed from CTRL CSAA (diet: P < 0.001, time: P < 0.001; interaction: P < 0.001; Figure 4F) and showed increased plasma AST activity in week 2, 6, 8, and 10 compared with CSAA control mice, with a peak at week 8 of CDAA diet administration (CDAA vs CTRL CSAA: Week 2: 84.6 ± 9 mU/mL vs 62.7 ± 3.9mU/mL; P < 0.05; week 6: 108.2 ± 12.3 mU/mL vs 58.2 ± 2.8 mU/mL; P < 0.001; week 8: 134.8 ± 13.7 mU/mL vs 77.4 ± 3.7 mU/mL; P < 0.001; week 10: 110.1 ± 12 mU/mL vs 81.3 ± 5.4 mU/mL; P < 0.05; Figure 4F).
The BMI in CDAA mice did not differ from CTRL CSAA mice (P = NS; Figure 4G). The fasting insulin concentrations did not differ between CDAA and CTRL CSAA mice (P = NS; Figure 4H). Correspondingly, with fasting insulin concentrations, the quantitative index of insulin sensitivity (QUICKI) also did not differ in the CDAA model (P = NS; Figure 4I).
Plasma concentrations of cholesterol and triacylglycerols did not differ in the CDAA model (P = NS; Figure 4J and K). Mice fed with a CDAA diet had lower plasma albumin concentrations than CTRL CSAA mice (CDAA vs CTRL CSAA: 27.7 ± 1.7 mmol/L vs 32.5 ± 2.4 mmol/L, P < 0.001; Figure 4L). The relative liver weight was 89% greater in the CDAA mice compared to CSAA control mice (P < 0.001; Figure 4M). The triacylglycerol content in the liver did not differ between groups (P = NS; Figure 4N), while CDAA-fed females showed 5.6-fold more cholesterol content in the liver compared with CTRL CSAA mice (P < 0.001; Figure 4O). CDAA mice compared with the CTRL CSAA mice showed higher NASH scores (P < 0.001; Figure 4P), ballooning of hepatocytes (P < 0.001; Supplementary Figure 2B), and lobular inflammation (P < 0.001; Supplementary Figure 2B). Other pathologies, such as steatosis and lobular inflammation, were also present in the CTRL CSAA mice (Supplementary Figure 2B; Figure 3G and H). In the CDAA mice, mild fibrosis was detected (Figure 3I and J) but not in the CTRL CSAA females. The fibrosis score was higher in the CDAA group than in CTRL CSAA females (P < 0.001; Figure 4Q). The count of neutrophil infiltration in the liver was greater in the CDAA females compared with the CTRL CSAA group (P < 0.001; Figure 3K and L, Figure 4R). The concentration of MPO (P < 0.01) and NE (P < 0.01) in the livers of CDAA mice was higher than in CTRL CSAA mice (Figure 4S and T).
The body weight in CAF mice (Figure 5A) increased over 10 weeks of CAF diet administration, while significant differences were absent compared with control diet-fed mice (diet: P = NS, time: P < 0.01; interaction: P < 0.05; Figure 5B). The cumulative caloric intake in the CAF model increased throughout the experiment with the main effect of diet (diet: P < 0.05, time: P < 0.01; interaction: P < 0.001; Figure 5C). The CAF group showed higher caloric intake at weeks 2, 4, 6, 8, and 10 compared with control diet-fed mice (all: P < 0.05; Figure 5C).
Fasting glycemia increased in CAF females over the 10 weeks of diet administration (diet: P < 0.01, time: P < 0.001; interaction: P < 0.001; Figure 5D). CAF females showed increased glycemia from week 4 until the end of the experiment (CAF vs CTRL: Week 4: 7.1 ± 0.7 mmol/L vs 4.6 ± 1.1 mmol/L; P < 0.001; week 6: 6.9 ± 0.7 mmol/L vs 5.4 ± 1.2 mmol/L; P < 0.01; week 8: 7.5 ± 0.8 mmol/L vs 5 ± 1.1 mmol/L; P < 0.001; week 10: 7.6 ± 0.7 mmol/L vs 5.6 ± 1.3 mmol/L; P < 0.001; Figure 5D).
Plasma activity of both ALT and AST were comparable between CAF females and CTRL females (ALT: Diet: P = NS, time: P = NS, interaction: P = NS; Figure 5E; AST: Diet: P = NS, time: P = NS, interaction: P = NS; Figure 5F). The BMI was higher in the CAF diet-fed females compared with CTRL diet-fed females (10.3%, P < 0.05; Figure 5G). Despite the high fasting glycemia concentrations in CAF diet-fed mice, the fasting insulin concentrations and the QUICKI index in CAF females did not differ from that of CTRL females (P = NS; Figure 5H and I).
CAF females showed higher plasma cholesterol concentrations than CTRL mice (CAF vs CTR: 3.7 ± 1.4 mmol/L vs 3.1 ± 0.2 mmol/L; P < 0.001; Figure 5J), while circulating concentrations of triacylglycerols and albumin did not differ between groups of females (P = NS; Figure 5K and L). The relative liver weight was 22.4% lower in CAF females compared with CTRL females (P < 0.05; Figure 5M). The liver triacylglycerols and cholesterol content did not differ between CAF-fed and CTRL diet-fed mice (P = NS; Figure 5N and O). The CAF diet-fed mice had a higher NASH score than the control group (P < 0.05; Figure 5P).
Moreover, lobular inflammation (P < 0.01) and steatosis (P < 0.05) were present in the CAF group while not identified in the control group (Supplementary Figure 2C; Figure 3M and N). Fibrosis was detected in one animal in the CAF group (Figure 3O and P), with no significant differences in the fibrotic score between the CAF and CTRL groups (P = NS; Figure 5Q). Neutrophil infiltration in the liver was higher in the CAF diet-fed mice compared with the CTRL diet-fed mice (P < 0.01; Figure 3Q and R, Figure 5R). The CAF model showed a higher concentration of MPO (P < 0.05) and NE (P < 0.05) in the liver than in CTRL mice (Figure 5S and T).
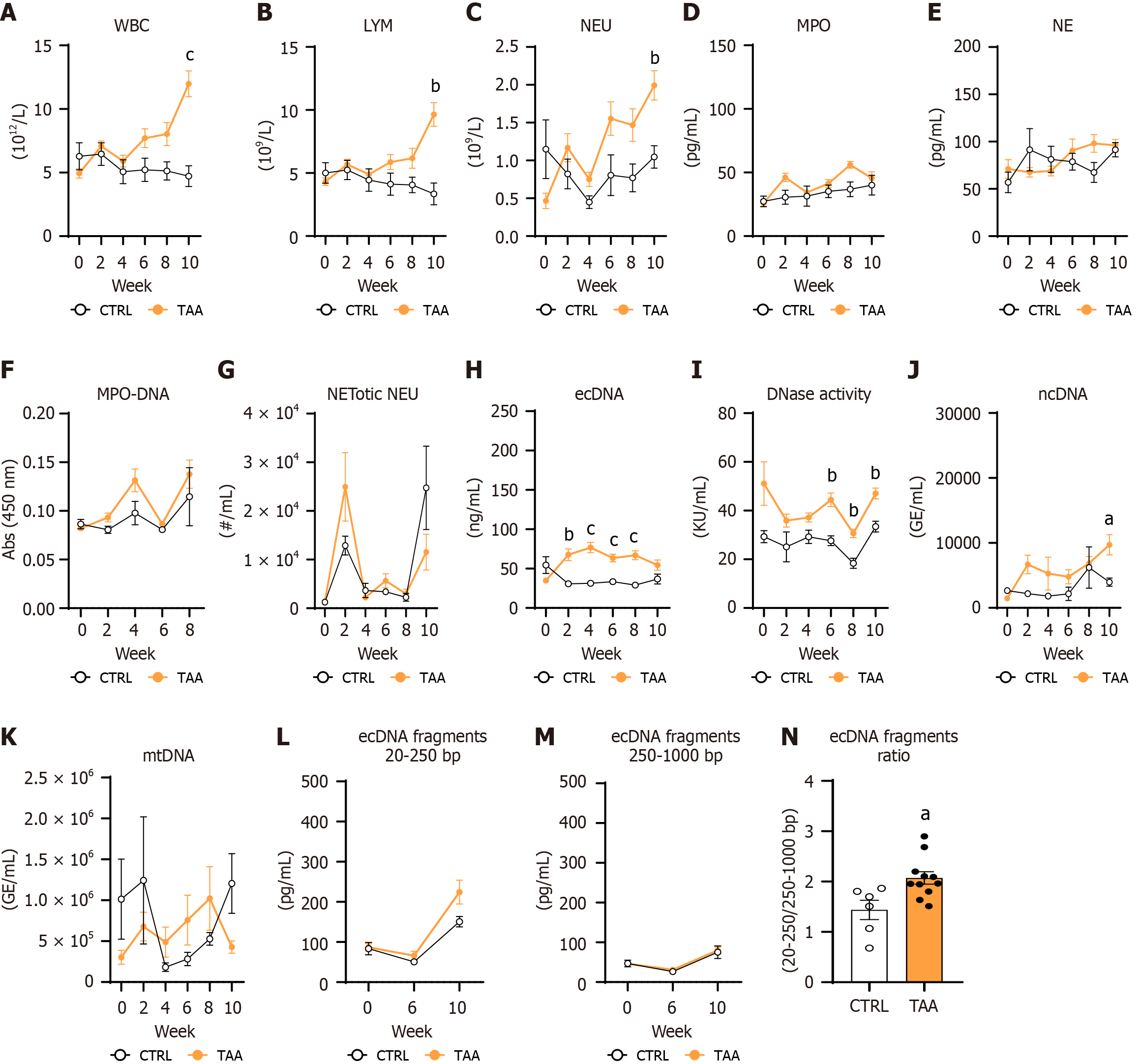
The white blood cell (WBC) increased in TAA-injected mice over 10 weeks of treatment (treatment: P < 0.05; time: P < 0.01; interaction: P < 0.001; Figure 6A). WBC counts were significantly higher in the TAA group compared with vehicle-injected mice at the end of the experiment (60%, P < 0.001; Figure 6A). The lymphocyte count in blood was affected by treatment, time, and interaction of both factors (treatment: P < 0.05; time: P < 0.05; interaction: P < 0.001; Figure 6B). Lymphocyte count was up to 66% higher in TAA-injected mice compared with vehicle-injected mice at week 10 of the experiment (P < 0.01; Figure 6B).
The neutrophil count increased over the experiment duration with the effect of treatment (treatment: P < 0.05; time: P < 0.01; interaction: P < 0.001; Figure 6C) peaking at week 10 in TAA females compared with CTRL females (48%, P < 0.01; Figure 6C). MPO concentrations in plasma changed over the duration of the TAA model with a non-significant trend toward the effect of TAA injection and the interaction of both factors (treatment: P = 0.06; time: P < 0.01; interaction: P = 0.06; Figure 6D). No effect of TAA treatment and time on plasma NE concentrations was detected (treatment: P = NS; time: P = 0.06; interaction: P = 0.05; Figure 6E).
The MPO-DNA complex absorbance changed over the duration of the TAA model with no group differences (treatment: P = NS; time: P < 0.01; interaction: P = NS; Figure 6F). The number of NETotic neutrophils in circulation changed over 10 weeks of the TAA model in both groups, while TAA injections did not affect the proportion of NETotic neutrophils in the blood (treatment: P = NS; time: P < 0.001; interaction: P < 0.05; Figure 6G). The concentration of ecDNA in the TAA model was affected by TAA injections but not time, while the interaction of both factors made a robust effect (treatment: P < 0.001; time: P = NS; interaction: P < 0.001; Figure 6H). The TAA-injected mice displayed increased ecDNA concentrations compared with vehicle-injected mice at weeks 2, 4, 6, and 8, while at the end of the experiment, this increase disappeared (TAA vs CTRL VEH: Week 2: 67.5 ± 7.6 ng/mL vs 30.6 ± 2.3 ng/mL; P < 0.01; week 4: 76.8 ± 6.6 ng/mL vs 31.4 ± 2.8 ng/mL; P < 0.001; week 6: 63.4 ± 4.8 ng/mL vs 33.4 ± 2.3 ng/mL; P < 0.001; week 8: 66.9 ± 5.7 ng/mL vs 29 ± 1.5 ng/mL; P < 0.001; week 10: 54.6 ± 6.6 ng/mL vs 36.8 ± 6.3 ng/mL; P = NS; Figure 6H).
The activity of the DNase enzyme was highly dependent on TAA injections and less on time (treatment: P < 0.001; time: P < 0.05; interaction: P = NS; Figure 6I). The significant differences between the TAA and vehicle-injected mice in the DNase activity were detected from weeks 6 to 10 of the experiment (week 6: P < 0.01; week 8: P < 0.01; week 10: P < 0.01; Figure 6I) and were not changed from the baseline measurements (week 0) up to week 4 of TAA treatment.
The ncDNA concentration was affected by TAA treatments but not the time or interaction of these two factors (treatment: P < 0.01; time: P = 0.051; interaction: P = NS; Figure 6J). The increase in ncDNA was detected at week 10 in TAA-injected mice compared with control vehicle-injected mice (59.2%, P < 0.05; Figure 6J). Statistical differences in the mtDNA fraction were not detected in the TAA model (treatment: P = NS; time: P = NS; interaction: P = 0.08; Figure 6K). Fragmentation of the ecDNA, specifically the concentration of fragments in the range from 20 to 250 bp, increased from the baseline to the week 10 of the TAA model induction, while statistical differences between the groups were not detected (treatment: P = NS; time: P < 0.001; interaction: P = NS; Figure 6L). The concentration of large fragments in the range of 250-1000 bp increased from week 0 until week 10 in the TAA model, with no group differences (treatment: P = NS; time: P < 0.001; interaction: P = NS; Figure 6M). The area ratio under the peak plot curve of 20-250 bp and 250-1000 bp was higher in the TAA-injected mice than in CTRL vehicle-injected mice (30.6%, P < 0.05; Figure 6N). This suggests that TAA-injected females showed more fragmented ecDNA than the CTRL females.
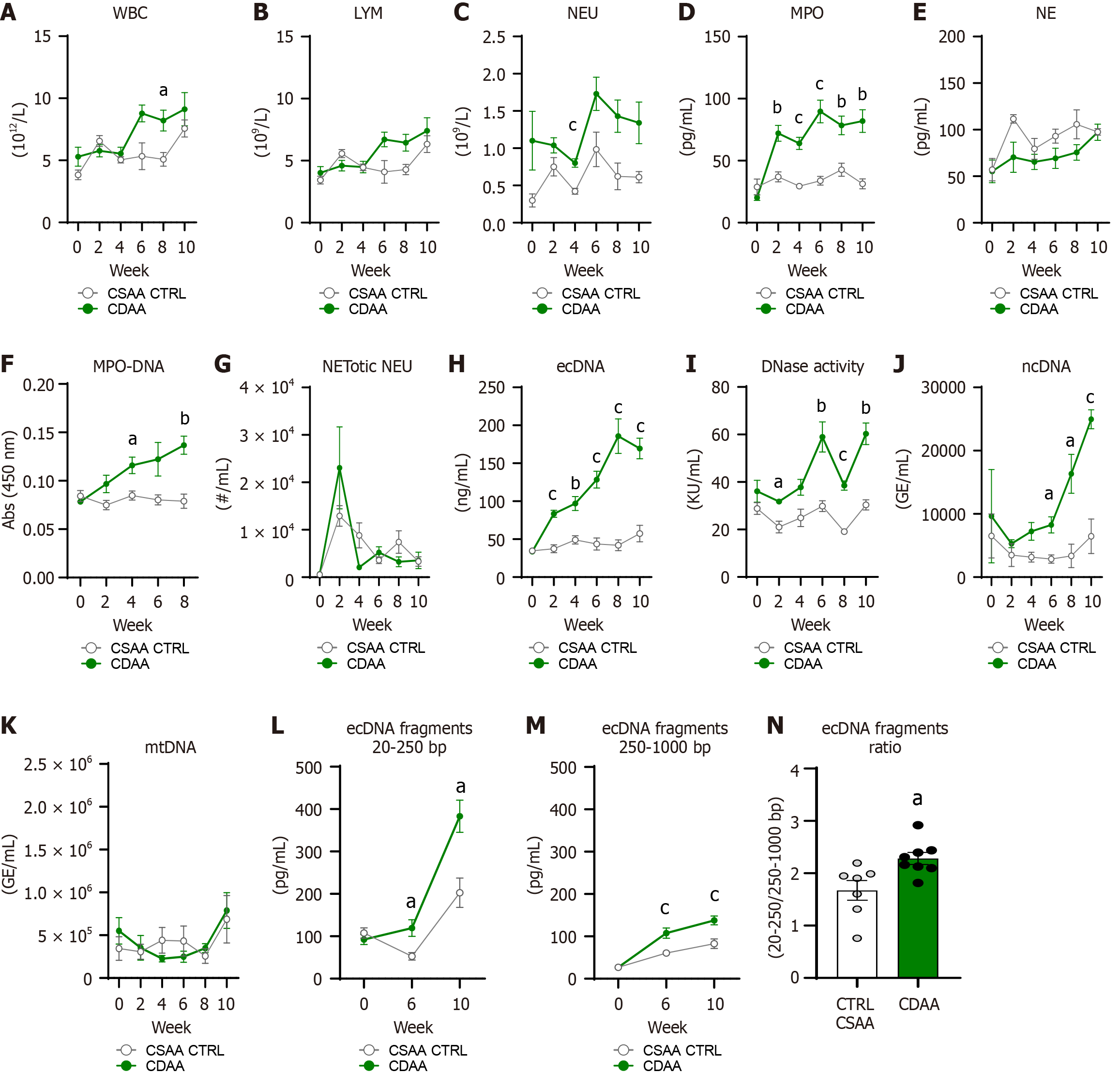
The WBC count in the blood in the CDAA model changed during the experiment, with a main effect of the CDAA diet on WBC (diet: P < 0.05, time: P < 0.01; interaction: P < 0.05; Figure 7A). The CDAA diet-fed mice showed higher WBC count at week 8 of the experiment compared with CSAA controls (17.1%, P < 0.05; Figure 7A). In addition, CSAA controls showed an increase in WBC count at week 10 with no difference compared with CDAA (Figure 7A).
The lymphocyte count increased in both CDAA and CSAA mice with no difference between groups in separate weeks (diet: P = NS, time: P < 0.01; interaction: P < 0.05; Figure 7B). CDAA diet consumption led to an increase of neutrophil counts in blood over the experiment (diet: P < 0.01, time: P < 0.05; interaction: P = NS; Figure 7C) with a significant difference detected in week 4 when CDAA females showed greater neutrophil abundance in blood than CSAA females (47.1%: P < 0.001; Figure 7C).
Robust effects of CDAA diet, time, and interaction of these factors were present in the analysis of the circulating concentration of MPO (diet: P < 0.001, time: P < 0.001; interaction: P < 0.001; Figure 7D). The higher plasmatic concentrations of MPO were detected in the CDAA mice from week 2 up to the end of the model duration (CDAA vs CTRL CSAA: Week 2: 72.1 ± 9.2 pg/mL vs 37 ± 4 pg/mL; P < 0.01; week 4: 63.9 ± 7.7 pg/mL vs 29.4 ± 1.4 pg/mL; P < 0.001; week 6: 89.7 ± 12.2 pg/mL vs 33.8 ± 3.5 pg/mL; P < 0.001; week 8: 78.4 ± 10.4 pg/mL vs 42.7 ± 5.2 pg/mL; P < 0.01; week 10: 82 ± 11.7 pg/mL vs 31.3 ± 4 pg/mL; P < 0.01; Figure 7D).
The concentration of NE in plasma was affected by time factor and a non-significant trend of the effect of CDAA diet was also present (diet: P = 0.05, time: P < 0.01; interaction: P = NS; Figure 7E). However, significant differences in specific timepoints between groups of females were not observed. The absorbance of MPO-DNA complex increased in the CDAA-fed mice over the model induction compared with CTRL CSAA females (diet: P < 0.001, time: P = 0.06; interaction: P < 0.05; Figure 7F). CDAA-fed females showed higher absorbance of MPO-DNA complex compared to CTRL CSAA-fed females at weeks 4 and 8 of the CDAA model (week 4: P < 0.05; week 8: P < 0.01; Figure 7F).
The amount of NETotic neutrophils in the circulation was affected by the time factor. However, CDAA diet and interaction of these factors were not (diet: P = NS; time: P < 0.05; interaction: P = NS; Figure 7G); therefore, group differences were absent. Circulating concentrations of ecDNA increased over the model induction with the robust effect of CDAA diet administration (diet: P < 0.001, time: P < 0.001; interaction: P < 0.001; Figure 7H). The CDAA mice showed increased ecDNA concentrations in circulation at week 2, 4, 6, 8, and 10 of the experiment compared with CSAA control mice (CDAA vs CTRL CSAA: Week 2: 83.4 ± 9.1 ng/mL vs 37.5 ± 5.2 ng/mL; P < 0.001; week 4: 97 ± 12.7 ng/mL vs 49 ± 5.5 ng/mL; P < 0.01; week 6: 128.5 ± 16.1 ng/mL vs 43.6 ± 8.1 ng/mL; P < 0.001; week 8: 185.8 ± 27.9 ng/mL vs 42 ± 7.1 ng/mL; P < 0.001; week 10: 169.5 ± 20.7 ng/mL vs 57.4 ± 11 ng/mL; P < 0.001; Figure 7H).
The DNase activity in circulation increased because of the significant effect of diet, time, and their interaction on its activity (diet: P < 0.001, time: P < 0.001; interaction: P < 0.01; Figure 7I). The CDAA females showed greater DNase activity at weeks 2 and 6 and then a slight decrease in week 8 with a continuous increase up to week 10 compared with CSAA females (week 2: 33.9%; P < 0.05; week 6: 49.5%; P < 0.01; week 8: 50.4%; P < 0.001; week 10: 49.6%; P < 0.01; Figure 7I). The ncDNA in the CDAA model showed a robust increase in line with the model duration with the main effect of diet factor (diet: P < 0.01, time: P = 0.06; interaction: P = 0.06; Figure 7J). The increase of the ncDNA fraction was detected in the CDAA mice compared with CSAA control mice at week 6, 8, and 10 (week 6: 65.3%; P < 0.05; week 8: 79.5%; P < 0.05; week 10: 74.1%; P < 0.001; Figure 7J). On the contrary, there were no effects of the CDAA diet, time, and interaction of these factors on the plasma mtDNA fractions (diet: P = NS; time: P = NS; interaction: P = NS; Figure 7K).
The concentrations of the small fragments of ecDNA in the range of 20-250 bp were higher in the CDAA-fed mice and increased over time of the CDAA diet consumption (diet: P < 0.01, time: P < 0.001; interaction: P < 0.01; Figure 7L). The CDAA mice showed higher concentrations of small fragments of ecDNA at weeks 6 and 10 compared with CSAA control mice (week 6: 55.6%; P < 0.05; week 10: 47%; P < 0.05; Figure 7L). The large fragments in the range of 250-1000 bp of circulating ecDNA increased in the CDAA-fed mice over the 10 weeks (diet: P < 0.001, time: P < 0.001; interaction: P < 0.05; Figure 7M). The large fragments of ecDNA were significantly higher at weeks 6 and 10 in the CDAA-fed mice compared with the CSAA control mice (week 6: 43.7%; P < 0.01; week 10: 59,2%; P < 0.001; Figure 7M). The ratio of the small and large fragments of ecDNA was higher in the CDAA-fed females compared with the control females (26.7%; P < 0.05; Figure 7N). This indicates a higher amount of the small fragments after MAFLD induction using the CDAA model.
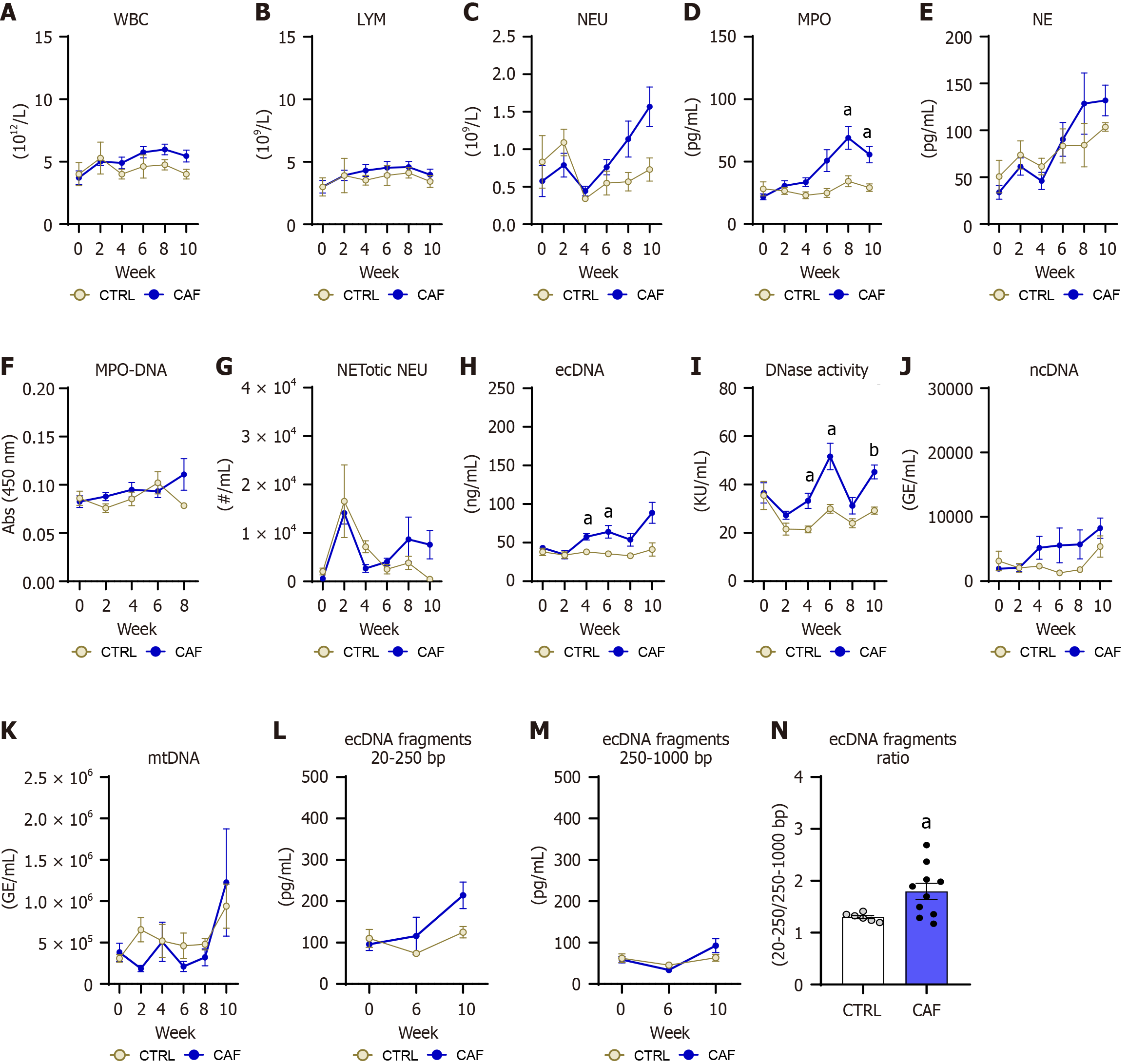
WBC count in the CAF diet model was affected by the time factor but not the diet or interaction (diet: P = NS, time: P < 0.05; interaction: P = NS; Figure 8A). The lymphocyte count in the CAF diet model did not differ in diet or time frame (diet: P = NS, time: P = NS; interaction: P = NS; Figure 8B). The abundance of neutrophils in the peripheral blood increased during the CAF diet model (diet: P = NS, time: P < 0.05; interaction: P < 0.05; Figure 8C), but group differences were not observed over time. The plasma concentrations of the MPO increased in the CAF diet-fed mice (diet: P < 0.05, time: P < 0.01; interaction: P < 0.01; Figure 8D) and significantly differed from the CTRL mice at week 8 and 10 of this model (CAF vs CTRL: Week 8: 69.1 ± 10.9 pg/mL vs 34.3 ± 8.3 pg/mL; P < 0.05; 55.7 ± 8.3 pg/mL vs 29.2 ± 6-9 pg/mL; P < 0.05; Figure 8D). The NE increased in both groups, with no differences between groups (diet: P = NS, time: P < 0.01; interaction: P = NS; Figure 8E). The absorbance of MPO-DNA complex in plasma in the CAF diet model did not change over the diet consumption, with no statistical difference between groups (diet: P = NS, time: P = NS; interaction: P = NS; Figure 8F).
The abundance of the NETotic neutrophils in circulation did not differ in the CAF diet model induction (diet: P = NS, time: P = NS; interaction: P = NS; Figure 8G). The concentration of ecDNA in circulation increased with the CAF diet consumption (diet: P < 0.05, time: P < 0.05; interaction: P < 0.05; Figure 8H). The mice fed with the CAF diet exhibited higher concentrations of circulating ecDNA compared with control mice at week 4 and 6 and tended to have higher ecDNA concentrations at week 10 of CAF diet consumption (CAF vs CTRL: Week 4: 57.6 ± 6.8 ng/mL vs 37.9 ± 8.9 ng/mL; P < 0.05 week 6: 64.1 ± 9.9 ng/mL vs 35.5 ± 8.4 ng/mL; P < 0.05; week 10: 88.7 ± 15.4 ng/mL vs 41.1 ± 11.4 ng/mL; P = 0.06; Figure 8H).
The plasma DNase activity has changed with the course of the CAF diet consumption (diet: P < 0.01, time: P < 0.01; interaction: P = NS; Figure 8I). The CAF diet-fed mice showed increased DNase activity compared with CTRL mice at week 4, 6, and 10 of the experiment (week 4: 35.4%; P < 0.05; week 6: 42%; P < 0.05; week 10: 35.4%: P < 0.01; Figure 8I). Both ncDNA and mtDNA were comparable between the CAF and CTRL females over the 10-week experiments (diet: P = NS, time: P = NS; interaction: P = NS; Figure 8J and K). The concentrations of the small fragments of ecDNA increased over the CAF diet consumption, while the main effect of the diet was lacking (diet: P = NS, time: P < 0.05; interaction: P = NS; Figure 8L), with a significant trend of a higher number of small fragments in the CAF diet-fed mice compared with CTRL females (P = 0.07). Correspondingly, the large fragment concentrations of ecDNA also increased alongside diet consumption, while group differences were not detected (diet: P = NS, time: P < 0.05; interaction: P = NS; Figure 8M). Moreover, the ratio calculated from small and long fragments of ecDNA was higher in the CAF diet-fed mice compared with CTRL diet-fed mice (27.6%, P < 0.05; Figure 8N).
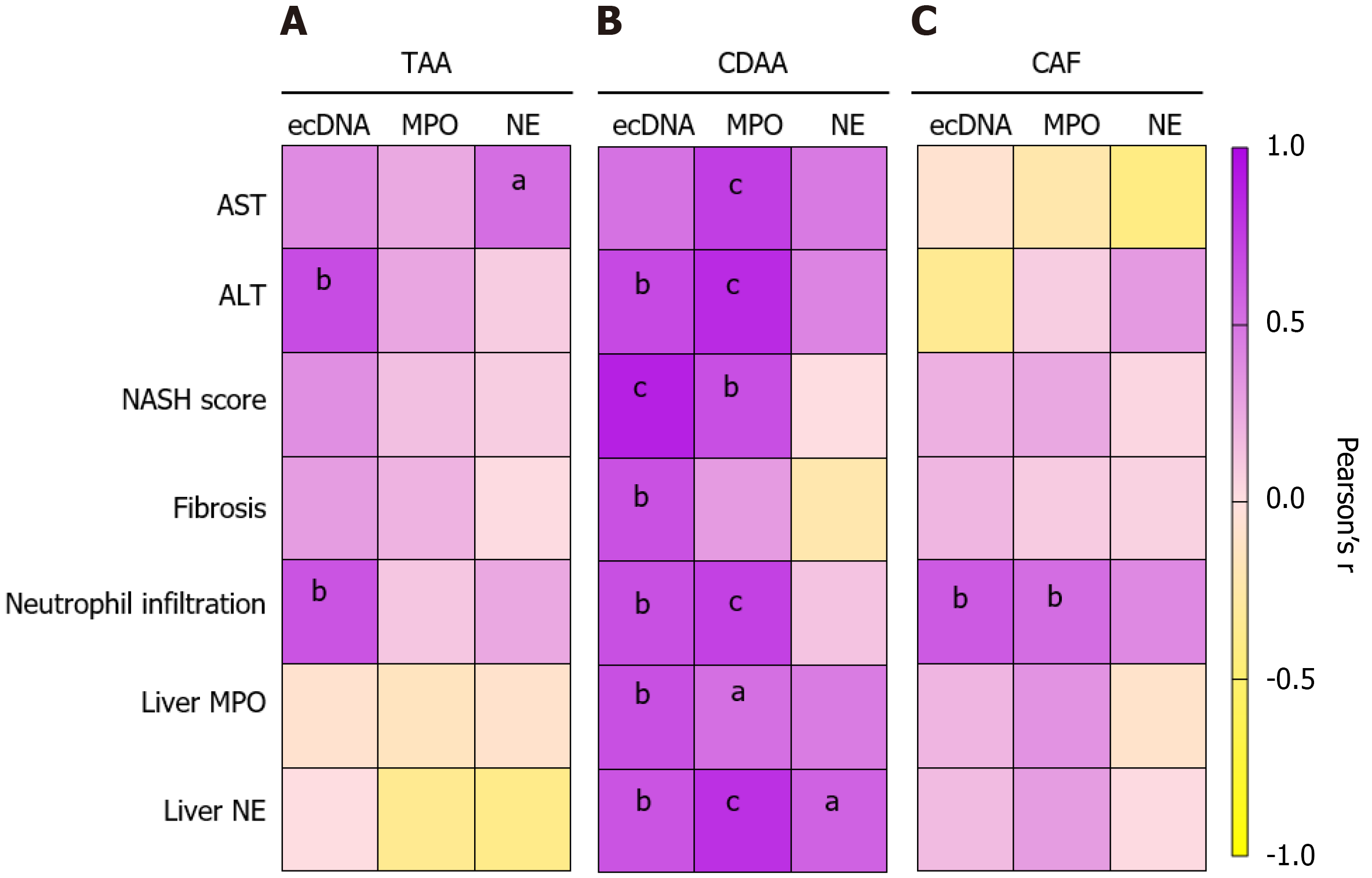
The higher AST activity in plasma in the chemically induced liver damage TAA model was not associated with the ecDNA and MPO concentrations (ecDNA: r = 0.393; P = NS, MPO: r = 0.255; P = NS; Figure 9A), but a positive correlation was found between AST and NE (r = 0.523, P < 0.01; Figure 9A). In addition, the higher activity of ALT correlated with the higher concentrations of ecDNA (r = 0.677, P < 0.01; Figure 9A), while no correlation with increased MPO and NE concentrations were observed (MPO: r = 0.263, NE: r = 0.292; both: P = NS; Figure 9A).
The severity of the liver damage in the TAA model represented by the NASH score and fibrosis was not associated with the peripheral markers of NETs formation (ecDNA, MPO, NE). The neutrophil infiltration into the liver in the TAA model was positively associated with the higher ecDNA concentration in plasma (r = 0.641, P < 0.01; Figure 9A), while MPO and NE concentrations were not associated. The peripheral markers of NET formation (ecDNA, MPO, NE) in the TAA-liver fibrosis model did not correlate with the local (i.e. liver concentrations) of MPO and NE (Figure 9A).
The CDAA model showed higher AST activity in week 10 of the model, and this correlated with higher concentrations of ecDNA and MPO (ecDNA: r = 0.504, P < 0.01; MPO: r = 0.744, P < 0.001; Figure 9B). Moreover, AST activity showed a trend towards correlation with NE (r = 0.468, P = 0.058; Figure 9B). Comparably, the higher activity of ALT in the CDAA model correlated with the concentrations of ecDNA and MPO (ecDNA: r = 0.692, P < 0.001; MPO: r = 0.835, P < 0.001; Figure 9B) but not with concentrations of NE (r = 0.422, P = NS; Figure 9B).
More severe liver damage represented by the NASH score in CDAA model correlated with the higher concentrations of ecDNA (r = 0.884, P < 0.001) and MPO (r = 0.667, P < 0.01) but not NE (r = 0.09, P = NS; Figure 9B). The fibrotic score in the CDAA model was positively associated with the ecDNA concentrations (r = 0.658, P < 0.01; Figure 9B) but not with the MPO or NE peripheral concentrations. Additionally, the neutrophil infiltration into the liver in the CDAA model was positively associated with the ecDNA (r = 0.662, P < 0.01; Figure 9B) and MPO (r = 0.730, P < 0.001; Figure 9B) peripheral concentrations and not with the NE plasma concentrations. The higher liver concentrations of MPO were positively associated with the peripheral ecDNA (r = 0.663, P < 0.01) and MPO (r = 0.52, P < 0.01) concentrations in the CDAA model (Figure 9B). Moreover, the CDAA model showed a positive association between liver NE concentrations and peripheral ecDNA (r = 0.645, P < 0.01), MPO (r = 0.807, P < 0.001) and NE concentrations (r = 0.577, P < 0.05; Figure 9B).
In the CAF diet model of MAFLD, the higher activities of AST and ALT and the NASH score, a direct liver marker of liver damage and fibrosis, were not associated with the higher concentrations of ecDNA, MPO, and NE (Figure 9C). The neutrophil infiltration into the liver in the CAF model was positively associated with ecDNA (r = 0.617, P < 0.01; Figure 9C) and MPO (r = 0.529, P < 0.01; Figure 9C) concentrations in plasma but not with NE concentrations. Local liver markers of NET formation were not associated with the peripheral NET formation markers (Figure 9C).
In addition, the MPO-DNA complex count in week 8 showed no correlation with AST or ALT activities in the TAA model (P = NS; Supplementary Figure 2D). In the CDAA model the MPO-DNA positively correlated with the AST activity (r = 0.701, P < 0.01), while no correlation was shown with ALT activity (Supplementary Figure 2E). MPO-DNA complex count in the CAF diet model positively correlated with AST (r = 0.531, P < 0.05) and ALT activities (r = 0.607, P < 0.01; Supplementary Figure 2F). Interestingly, the higher fragmentation of ecDNA represented by the ratio of fragments size 20-250 bp to 250-1000 bp in the TAA and CDAA liver damage models were not associated with DNase activities (P = NS; Supplementary Figure 2G and H). On the contrary, fragmentation of ecDNA correlated with higher DNase activity in the CAF model (r = 0.691, P < 0.01; Supplementary Figure 2I).
The present study showed that TAA injections led to liver damage, represented by several plasma and tissue markers and morphological pathologies, with negligible effects on body weight, food intake, and circulating glycemic or lipid changes. Overall, an increase in AST and ALT concentrations in plasma points to continuous liver damage in line with the TAA injections. Corresponding with the plasma markers, the histopathological changes, mainly the lobular inflammation, infiltration of immune cells (neutrophils) into the liver tissue, and increased fibrosis, are also characteristic of the TAA model. In line with the liver damage, TAA injections caused an elevated number of total WBC in both neutrophils and lymphocyte populations, indicating ongoing chronic inflammation. While the MPO and NE were not increased throughout the model, ecDNA (an immunogenic molecule) increased. The DNase activity changes were also detected, with no clinical fold change from the baseline measurements. No marginal subcellular origin shift of ecDNA was observed, while TAA induced more fragmented ecDNA. Additionally, the TAA injections resulted in a higher concentration of MPO in the liver, while NE concentrations did not differ.
The MASH model was induced using the CDAA diet, which was represented by increased liver enzyme activity (ALT, AST) and increased cholesterol accumulation in the liver. Additionally, morphological changes in the liver, represented by NASH scores, fibrosis scores, and liver neutrophil infiltration, also proved MASH-like abnormalities. Interestingly, the marginal effect of CDAA on body weight, food intake, and glycemic or lipidic plasma markers was not changed. The liver damage associated with MASH was also supported by low plasma albumin concentrations, which suggests the impaired ability of the liver related to its synthetic properties. The abundance of immune cells in the circulation in animals with CDAA liver damage increased with the robust involvement of MPO and ecDNA. In parallel with the ecDNA increase during the CDAA diet challenge, a nuclear fraction of ecDNA also increased. Notably, CDAA induced higher concentrations of MPO and NE in the liver compared with controls. Moreover, both small and large fragments of ecDNA increased, with more mononucleosomal fragments.
In the CAF diet model, which mimics the consequences of unbalanced diet consumption that represents the “physiological” development of hepatic steatosis, liver damage was minimally present. Histopathological abnormalities were present in mice fed with the CAF diet, with mild NASH development, no fibrosis, and relatively low neutrophil infiltration into the liver tissue, which reflects the liver enzyme activity. Conversely, the CAF diet-fed mice increased body weight and caloric intake, resulting in hyperglycemia but no insulin resistance. Moreover, the higher concentrations of plasmatic cholesterol indicated the development of dyslipidemia. The immune cell profile in the CAF diet model did not show a relevant increase, while the peripheral MPO concentrations arose. Other related NET-associated proteins or ecDNA concentrations and ncDNA or mtDNA were not relevantly increased. The NET-associated proteins such as MPO and NE in the liver were higher compared with the controls. Despite no significant rise in ecDNA and subcellular origins, the CAF diet-fed mice showed more fragmented DNA.
More importantly, associations of NET-associated protein markers and concentrations of ecDNA were found with liver injury, peripheral parameters, and histopathological properties. These associations seem to be model-dependent. In the TAA model, plasmatic ALT was associated with the ecDNA concentration, and NE was related to the AST. The neutrophil infiltration into the damaged liver tissue was also associated with the ecDNA concentration in the TAA model. Notably, the CDAA model showed associations of all plasma markers (AST, ALT), the NASH score, fibrosis, and neutrophil infiltration into the liver with the peripheral ecDNA and MPO concentration. Surprisingly, no associations of peripheral NETs markers (ecDNA, MPO, and NE) were found with the plasmatic liver damage markers (AST, ALT), NASH score, and fibrosis. Interestingly, the neutrophil infiltration into the liver was associated with the ecDNA and MPO levels in circulation. Additionally, the NET-associated proteins of the liver, such as MPO and NE, were positively related to peripheral markers just in the CDAA model.
In the present study, we successfully induced liver injury with intraperitoneal injections of TAA over 10 weeks, which has been described already[31-33]. Moreover, we observed decreased body weight from week 2 of the TAA injections, with no change in food intake. The body weight and food intake decrease as well as the increased activities of AST and ALT were previously described over 6 weeks (TAA dose: 100 mg/kg)[31] and 7 weeks (50-400 mg/kg, weekly)[33]. It was previously reported that TAA injections could cause glucose metabolism impairment, leading to insulin resistance, which is linked to ovarian damage[34]. In our experiment we did not observe any glucose metabolism dysfunction (Figure 2D, H, and I).
Corresponding with the ovarian damage caused by long-term TAA injections, we observed a decrease in relative uterus weight and length, which can indicate that not just the liver was affected (data not shown). It is essential to point out that TAA is a toxic compound to the liver tissue. The liver is the main organ for lipid metabolism. Relatively increased cholesterol concentrations in plasma (Figure 2J) suggest impairment in cholesterol metabolism. On the other hand, cholesterol accumulation in the liver was not affected. However, the liver triacylglycerol storage of TAA-injected mice was decreased, with no increased peripheral secretion, which could point to the defect in the lipid export from the liver to the bloodstream by very low-density lipoprotein[35].
The CDAA diet successfully induced MAFLD and that aligns with the previously published 8-week[36] and 24-week[37] consumption of the CDAA diet. We observed the change in body weight or caloric intake, while Kucsera et al[38] described no body weight differences across CDAA-fed 10-month-old female and male C57BL/6J mice and control mice fed with standard chow for 8 weeks. The liver injury represented by AST and ALT activities in circulation was detected in the CDAA murine model with different activity peak times. In contrast with the study by Yasuda et al[39], where the CDAA diet decreased serum triglycerides, cholesterol levels, and body weight, we showed no change in the present parameters. Moreover, they showed an increase in liver triglycerides content, while we showed no change. Importantly, we detected a high accumulation of cholesterol in the liver. This discrepancy in the results might be because Yasuda et al[39] used the CDAA diet in combination with a high-fat diet, while in our study we used just CDAA. These interesting opposite results might indicate the different mechanisms and hepatic disease progression using high-fat diet as an additional property. Choline-diet deficiency leads to impaired metabolic pathways, specifically the impairment of synthetic properties by hepatocytes, while lower albumin concentrations prove the mechanism[40].
It has been previously shown that body weight, plasma cholesterol, and the development of hyperglycemia increased in the CAF diet model in both sexes of C57BL/6 mice[41]. In our 10-week CAF diet setting, we did not observe changes in liver damage markers such as AST and ALT. On the other hand, Ceylani et al[42] showed that 7 weeks of CAF diet consumption led to a decrease in AST and ALT activities, pointing to liver damage. The development of liver steatosis was reported using the CAF diet[42,43], with lobular inflammation and macrovesicular steatosis observed, while the NASH score in our study did not reach significance. In line with the increase in body weight, the body mass also increased and that points to the development of abdominal obesity. More importantly, the fasting glycemia continuously increased with model duration, while insulin secretion successfully rescued animals from the development of insulin resistance represented by the QUICKI index.
On the other hand, insulin resistance using the CAF diet developed after 22 weeks[19], 12 weeks[44], and 10 weeks[45]. We observed a higher concentration of plasma cholesterol after 10 weeks of diet challenge, with no change in triacylglycerols. Dyslipidemia in the CAF diet models are described as elevated concentrations of plasmatic cholesterol and triacylglycerols. Notably, it was recently shown that the development of metabolic complications and liver damage by the CAF diet are highly sex- and age-dependent[41,46]. Due to this, the mild liver damage in our experiment might be related to this.
Using several models, we successfully induced three different hepatic pathologies (TAA: Hepatic fibrosis; CDAA: Hepatic steatohepatitis; CAF: Hepatic steatosis) that might suit NET involvement research. Notably, the local liver markers of NET formation, such as MPO and NE, were higher in the CDAA and CAF models, while the most prominent influx was in the CDAA model. In the TAA model, we observed only the MPO increase. As described, the TAA model represents the fibrosis of the liver, while our experimental setting suggests that neutrophils undergo degranulation with less prominent NET formation. On the other hand, the diet-induced liver steatosis model showed elevation of both MPO and NE in the liver, which might suggest that NET formation is involved in the metabolic remodeling of the liver function in the liver damage conditions related to steatohepatitis.
Liver damage is highly linked to the inflammatory process. Due to liver damage, immune cell infiltration into damaged tissue, and cytokine production, local inflammation might promote a systemic response. In the last week of the TAA model, we observed an increase in WBC, lymphocytes, and neutrophils during the induction of liver injury by TAA. These observations were published previously[47,48]. Additionally, no marginal increase in immune cells in circulation was observed in CDAA and CAF models. The infiltration of immune cells, specifically neutrophils, into the liver was observed in patients with MASLD[49,50].
The role of neutrophil increase in the bloodstream and infiltration into the fibrotic liver tissue is still controversial, as the elevated expression of IL-17, derived from neutrophils and mast cells, is a standard marker of advanced liver fibrosis[51,52]. Moreover, neutrophils produce reactive oxygen species, which can promote the polarizing of macrophages toward an alternative or reparative and anti-inflammatory phenotype in paracetamol-induced and CCL-4-mediated liver fibrosis.
The main structure of the NETs is the so-called ecDNA. The ecDNA concentration was elevated in the TAA model of liver injury with no change in ncDNA and mtDNA. The rise of cell-free DNA was observed in the experiment using acetaminophen-induced liver injury. Moreover, patients with moderate and severe drug liver injury also showed high plasma concentrations of cell-free DNA that is strongly associated with the AST and ALT liver damage markers[53].
Interestingly, in the CDAA setting but not in the CAF model, we observed the marginal rise of ecDNA concentrations and the nuclear fraction of ecDNA. The ecDNA and nuclear fraction of ecDNA in plasma seem to be good predictors for liver damage, while the pathophysiological role is still unclear[53]. A recent study showed that peripheral NET-dsDNA was elevated in patients with MASLD compared with healthy controls[54]. Notably, high levels of cell-free DNA were also observed in patients with MAFLD[55], which was associated with AST activity. DNA outside of cells acts as a danger-associated molecular pattern that potentially promotes the inflammatory process at the systemic level[55]. We can only speculate on the origin of the rise in the ecDNA and ncDNA, yet it points to damaged/ fibrotic hepatocytes or the formation of NETs.
There is a need to point out that the ecDNA plays an essential role in the different liver pathologies, while the precise mechanism needs to be explored. The compounds of the NETs, such as MPO and NE or CitH3, were observed to be elevated in patients suffering from liver disease[56]. It was previously shown that NETs play a crucial role in the end stages of liver disease, such as cirrhosis or hepatocellular carcinoma[57].
We showed that the aggressiveness of liver damage represented by different models, especially the CDAA model, is strongly linked to the elevation of the peripheral NET-associated markers. We showed that the plasmatic concentrations of MPO and NE and the abundance of MPO-DNA complexes increased with the progression of steatohepatitis in the CDAA model. The increase in citrullinated histone abundance was also observed in the CDAA model from the day 7 to day 28, with a decrease after 7 weeks of diet consumption[58].
Arelaki et al[52] noted that NETs in the circulation are strongly associated with NASH and nonalcoholic fatty liver disease scores in patients with MASLD. Additionally, the NETs were associated with the hypercoagulable state in patients with NASH[59]. On the other hand, we did not observe any elevation of peripheral NET-associated markers using the TAA model of liver injury and the CAF model for diet-induced hepatic steatosis. In patients with obesity and MALSD, elevated NE concentrations have been observed[60]. We thus suggest that once the liver is damaged, the neu
One strategy for degrading NETs is via DNases. DNases are potent enzymes that cleave/break the ecDNA into smaller fragments[62]. With the ecDNA cleavage by DNases, the immunogenicity of ecDNA might be diminished. We showed that the highest DNase activity along the experiment duration occurred in the CDAA model. The DNase activity in mice that received TAA was different statistically but not clinically. On the other hand the DNase activity in the CAF model rises with no change in the ecDNA concentration. Putting together the three hepatic pathologies, our results indicated that the DNase activity was associated with the stage of the hepatic injury. Golonka et al[63] showed that the activity of DNase I is a strong predictor for the development of liver cancer. Notably, the decreased mortality of animals in acute liver failure is strongly associated with the high activity of DNases[64]. We must point out that we did not have any deaths among our experimental mouse models. We can speculate that the physiological role of the DNase activity might help with the response of the immune system to the different hepatic pathologies.
It was previously shown that treatment by DNase I reduces the signs of liver damage[53,65], yet we found that the physiological role of DNase is not sufficient for hepatic injury and is model-dependent. We recently showed that DNase I treatment is sufficient to ameliorate acute alcohol-induced liver injury[66]. Wu et al[67] showed that the DNase I treat
In all studied models the ratio between short and large fragments was significantly increased compared with associated control groups. The increase of mononucleosomal DNA fragments may be caused by necrosis[69]. At the same time apoptosis produces distinct and conserved ecDNA fragmentation profiles[70]. In observed research models more severe inflammation was associated with a higher abundance of small ecDNA fragments (20-250 bp) and a higher small-to-large fragments ratio. These results suggest increased necrosis with an increasing small-to-large fragments ratio. This increase does not correlate with the plasma DNase activity except for the relatively small CAF model correlation. These results follow those from a study from Cheng et al[71]. They had found that despite a significant difference in DNase I activity between wildtype and DNase I knockout mice, there was no difference in the concentration or fragmentation patterns of circulating cell-free DNA, suggesting that DNase I does not play a significant role in the fragmentation of cell-free DNA.
Our correlation analysis showed that the NET-associated markers (ecDNA and MPO) in circulation are highly linked to AST and ALT liver damage markers and the NASH score in a model-dependent manner. While no relevant association was found in the CAF model, and mild associations were found in the TAA model, the CDAA model, which represents steatohepatitis, was highly associated with the peripheral markers. Surprisingly, the NE concentrations in plasma did not change in any of our models of liver damage, resulting in no clinically relevant associations with the liver damage markers. This finding suggests that the peripheral concentration of a specific NET protein (NE) is not a good marker. It was previously shown that NE might be inhibited[72] or self-cleaved[73] to diminish additional degranulation of neutrophils. Our results indicated that the NET markers in circulation depend highly on the stage and progression of liver disease.
In our study, we identified several limitations according to the methodology. The prevalence of MASLD is higher in males in general, while females of fertile ages are at lower risk. After menopause the prevalence becomes comparable between males and females[74]. Several mainstream research organizations suggest the use of both sexes in preclinical research. However, to conduct our experiment we used only female mice as female mice are more resistant to the development of liver injury than males. Experiments conducted on males are more prominent in the field. The present study focused on the peripheral NET markers in different models of liver disease. Primarily, liver damage is characterized as a tissue injury. In this study, we did not focus on the formation of NETs in the liver tissue since blood markers are more readily obtained than liver biopsies in humans. However, more frequent sampling and NET markers’ measurement would further improve and clarify results on the dynamics and role of NETs in MASLD.
We successfully characterized three different hepatic pathologies using the TAA, CDAA, and CAF models. We suggest that the CDAA model represents the worst liver pathologies from the presented data, yet it does not reflect the human pathophysiological process for developing steatohepatitis. Peripheral markers of NET formation were highly involved in the CDAA model, but not in the TAA and CAF models. We hypothesized that the formation of NETs is involved in liver pathologies. In contrast, the breakpoint of secretions of NET structures into the bloodstream from damaged liver tissue depends on the injury level. Further studies are required to investigate the formation and secretion of NETs from the damaged liver tissue into the circulation in different animal models that would mimic several liver diseases.
The authors would like to thank Emília Klincová for processing the samples for histological analyses and MSc Ivana Feješ Slivková for helping analyze the histopathology of the liver slides. The authors are also grateful to Dr. Zuzana Rausová and Dr. Oľga Uličná for biochemical markers measurement.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7901] [Article Influence: 790.1] [Reference Citation Analysis (2)] |
| 2. | Cholongitas E, Pavlopoulou I, Papatheodoridi M, Markakis GE, Bouras E, Haidich AB, Papatheodoridis G. Epidemiology of nonalcoholic fatty liver disease in Europe: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:404-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Narro GEC, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2024;29:101133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 399] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 4. | Mooli RGR, Ramakrishnan SK. Liver Steatosis is a Driving Factor of Inflammation. Cell Mol Gastroenterol Hepatol. 2022;13:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Dömer D, Walther T, Möller S, Behnen M, Laskay T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front Immunol. 2021;12:636954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 6. | Macáková K, Illésová J, Mlynáriková V, Lesayová A, Konečná B, Vlková B, Celec P, Šteňová E. The dynamics of extracellular DNA associates with treatment response in patients with rheumatoid arthritis. Sci Rep. 2022;12:21099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Karlas T, Weise L, Kuhn S, Krenzien F, Mehdorn M, Petroff D, Linder N, Schaudinn A, Busse H, Keim V, Pratschke J, Wiegand J, Splith K, Schmelzle M. Correlation of cell-free DNA plasma concentration with severity of non-alcoholic fatty liver disease. J Transl Med. 2017;15:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM, Minervini MI, Huang H, Simmons RL, Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 9. | Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, Tsung A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 10. | Ezhilarasan D. Molecular mechanisms in thioacetamide-induced acute and chronic liver injury models. Environ Toxicol Pharmacol. 2023;99:104093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 11. | Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology. 2011;140:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Denda A, Kitayama W, Kishida H, Murata N, Tsutsumi M, Tsujiuchi T, Nakae D, Konishi Y. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res. 2002;93:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 457] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | de la Garza AL, Martínez-Tamez AM, Mellado-Negrete A, Arjonilla-Becerra S, Peña-Vázquez GI, Marín-Obispo LM, Hernández-Brenes C. Characterization of the Cafeteria Diet as Simulation of the Human Western Diet and Its Impact on the Lipidomic Profile and Gut Microbiota in Obese Rats. Nutrients. 2022;15:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Kendig MD, Westbrook RF, Morris MJ. Pattern of access to cafeteria-style diet determines fat mass and degree of spatial memory impairments in rats. Sci Rep. 2019;9:13516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Lalanza JF, Snoeren EMS. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci Biobehav Rev. 2021;122:92-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Orliacq J, Pérez-Cornago A, Parry SA, Kelly RK, Koutoukidis DA, Carter JL. Associations between types and sources of dietary carbohydrates and liver fat: a UK Biobank study. BMC Med. 2023;21:444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Carli F, Della Pepa G, Sabatini S, Vidal Puig A, Gastaldelli A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep. 2024;6:101185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 19. | Mašek T, Barišić J, Micek V, Starčević K. Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex. Nutrients. 2020;12:3339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring). 2011;19:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 21. | Thi Thanh Hai N, Thuy LTT, Shiota A, Kadono C, Daikoku A, Hoang DV, Dat NQ, Sato-Matsubara M, Yoshizato K, Kawada N. Selective overexpression of cytoglobin in stellate cells attenuates thioacetamide-induced liver fibrosis in mice. Sci Rep. 2018;8:17860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Freitas I, Boncompagni E, Tarantola E, Gruppi C, Bertone V, Ferrigno A, Milanesi G, Vaccarone R, Tira ME, Vairetti M. In Situ Evaluation of Oxidative Stress in Rat Fatty Liver Induced by a Methionine- and Choline-Deficient Diet. Oxid Med Cell Longev. 2016;2016:9307064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Borbélyová V, Šarayová V, Renczés E, Čonka J, Janko J, Šebeková K, Štefíková K, Ostatníková D, Celec P. The effect of long-term hypogonadism on body composition and morphometry of aged male Wistar rats. Physiol Res. 2021;70:S357-S367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Gargiulo S, Gramanzini M, Megna R, Greco A, Albanese S, Manfredi C, Brunetti A. Evaluation of growth patterns and body composition in C57Bl/6J mice using dual energy X-ray absorptiometry. Biomed Res Int. 2014;2014:253067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Janíková M, Pribulová N, Kmeťová K, Macáková K, Dobišová A, Kopčová M, Bucová M, Vlková B, Celec P. Extracellular DNA and Deoxyribonuclease Activity as Prognostic Markers in Sepsis. Biomedicines. 2024;12:2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Macáková K, Borbélyová V, Tekeľová M, Janko J, Pastorek M, Hokša R, Moravanský N, Šteňová E, Vlková B, Celec P. Effects of exogenous deoxyribonuclease I in collagen antibody-induced arthritis. J Inflamm (Lond). 2024;21:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Abel LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357-366. [PubMed] |
| 28. | Jover A. A technique for the determination of serum glycerides. J Lipid Res. 1963;4:228-230. [PubMed] |
| 29. | Review Team, LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM, Ramos JF, Sarin S, Stimac D, Thomson AB, Umar M, Krabshuis J, LeMair A; World Gastroenterology Organisation. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 30. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8551] [Article Influence: 407.2] [Reference Citation Analysis (8)] |
| 31. | Tsai MY, Yang WC, Lin CF, Wang CM, Liu HY, Lin CS, Lin JW, Lin WL, Lin TC, Fan PS, Hung KH, Lu YW, Chang GR. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules. 2021;26:1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Lin YY, Hu CT, Sun DS, Lien TS, Chang HH. Thioacetamide-induced liver damage and thrombocytopenia is associated with induction of antiplatelet autoantibody in mice. Sci Rep. 2019;9:17497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Dwivedi DK, Jena GB. Simultaneous Modulation of NLRP3 Inflammasome and Nrf2/ARE Pathway Rescues Thioacetamide-Induced Hepatic Damage in Mice: Role of Oxidative Stress and Inflammation. Inflammation. 2022;45:610-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Lee DH, Park H, You JH, Seok J, Kwon DW, Kim YR, Kim GJ. Increased IGFBP2 Levels by Placenta-Derived Mesenchymal Stem Cells Enhance Glucose Metabolism in a TAA-Injured Rat Model via AMPK Signaling Pathway. Int J Mol Sci. 2023;24:16531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Chen J, Fang Z, Luo Q, Wang X, Warda M, Das A, Oldoni F, Luo F. Unlocking the mysteries of VLDL: exploring its production, intracellular trafficking, and metabolism as therapeutic targets. Lipids Health Dis. 2024;23:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Wei G, An P, Vaid KA, Nasser I, Huang P, Tan L, Zhao S, Schuppan D, Popov YV. Comparison of murine steatohepatitis models identifies a dietary intervention with robust fibrosis, ductular reaction, and rapid progression to cirrhosis and cancer. Am J Physiol Gastrointest Liver Physiol. 2020;318:G174-G188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Kucsera D, Tóth VE, Gergő D, Vörös I, Onódi Z, Görbe A, Ferdinandy P, Varga ZV. Characterization of the CDAA Diet-Induced Non-alcoholic Steatohepatitis Model: Sex-Specific Differences in Inflammation, Fibrosis, and Cholesterol Metabolism in Middle-Aged Mice. Front Physiol. 2021;12:609465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Yasuda D, Torii H, Shimizu R, Hiraoka Y, Kume N. Reduced Serum Cholesterol and Triglyceride Levels in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet (CDAHFD)-Induced Mouse Model of Non-alcoholic Steatohepatitis (NASH). Biol Pharm Bull. 2020;43:616-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 41. | Bazhan N, Kazantseva A, Dubinina A, Balybina N, Jakovleva T, Makarova E. Age of Cafeteria Diet Onset Influences Obesity Phenotype in Mice in a Sex-Specific Manner. Int J Mol Sci. 2024;25:12436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Ceylani T, Önlü H, Keskin S, Allahverdi H, Teker HT. SCD Probiotics mitigate cafeteria diet-induced liver damage in Wistar rats during development. J Gastroenterol Hepatol. 2023;38:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Zeeni N, Dagher-Hamalian C, Dimassi H, Faour WH. Cafeteria diet-fed mice is a pertinent model of obesity-induced organ damage: a potential role of inflammation. Inflamm Res. 2015;64:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Higa TS, Spinola AV, Fonseca-Alaniz MH, Evangelista FS. Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. Int J Physiol Pathophysiol Pharmacol. 2014;6:47-54. [PubMed] |
| 45. | Brandimarti P, Costa-Júnior JM, Ferreira SM, Protzek AO, Santos GJ, Carneiro EM, Boschero AC, Rezende LF. Cafeteria diet inhibits insulin clearance by reduced insulin-degrading enzyme expression and mRNA splicing. J Endocrinol. 2013;219:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | De Leon ER, Brinkman JA, Fenske RJ, Gregg T, Schmidt BA, Sherman DS, Cummings NE, Peter DC, Kimple ME, Lamming DW, Merrins MJ. Age-Dependent Protection of Insulin Secretion in Diet Induced Obese Mice. Sci Rep. 2018;8:17814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Nishi K, Yagi H, Ohtomo M, Nagata S, Udagawa D, Tsuchida T, Morisaku T, Kitagawa Y. A thioacetamide-induced liver fibrosis model for pre-clinical studies in microminipig. Sci Rep. 2023;13:14996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Kuramochi M, Izawa T, Kuwamura M, Yamate J. Involvement of neutrophils in rat livers by low-dose thioacetamide administration. J Vet Med Sci. 2021;83:390-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016;150:1704-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 50. | Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, Shasthry SM, Maiwall R, Trehanpati N, Singh TP, Sarin SK. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology. 2017;65:631-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, Bilodeau M, Shoukry NH. Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci Immunol. 2018;3:eaar7754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Arelaki S, Koletsa T, Sinakos E, Papadopoulos V, Arvanitakis K, Skendros P, Akriviadis E, Ritis K, Germanidis G, Hytiroglou P. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 2022;481:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Sun M, Chen P, Xiao K, Zhu X, Zhao Z, Guo C, He X, Shi T, Zhong Q, Jia Y, Tao Y, Li M, Leong KW, Shao D. Circulating Cell-Free DNAs as a Biomarker and Therapeutic Target for Acetaminophen-Induced Liver Injury. Adv Sci (Weinh). 2023;10:e2206789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Xu M, Xu H, Ling YW, Liu JJ, Song P, Fang ZQ, Yue ZS, Duan JL, He F, Wang L. Neutrophil extracellular traps-triggered hepatocellular senescence exacerbates lipotoxicity in non-alcoholic steatohepatitis. J Adv Res. 2025;S2090-1232(25)00175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 55. | Mihm S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int J Mol Sci. 2018;19:3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 56. | Meijenfeldt FAV, Jenne CN. Netting Liver Disease: Neutrophil Extracellular Traps in the Initiation and Exacerbation of Liver Pathology. Semin Thromb Hemost. 2020;46:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Zenlander R, Havervall S, Magnusson M, Engstrand J, Ågren A, Thålin C, Stål P. Neutrophil extracellular traps in patients with liver cirrhosis and hepatocellular carcinoma. Sci Rep. 2021;11:18025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 58. | Zhao X, Yang L, Chang N, Hou L, Zhou X, Yang L, Li L. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020;11:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Du J, Zhang J, Chen X, Zhang S, Zhang C, Liu H, Li Y, Li M, Wu X, Xiang M, Wang C, Liu L, Wang C, Fang S, Shi J. Neutrophil extracellular traps induced by pro-inflammatory cytokines enhance procoagulant activity in NASH patients. Clin Res Hepatol Gastroenterol. 2022;46:101697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Mirea AM, Toonen EJM, van den Munckhof I, Munsterman ID, Tjwa ETTL, Jaeger M, Oosting M, Schraa K, Rutten JHW, van der Graaf M, Riksen NP, de Graaf J, Netea MG, Tack CJ, Chavakis T, Joosten LAB. Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Mol Med. 2019;25:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Tang J, Yan Z, Feng Q, Yu L, Wang H. The Roles of Neutrophils in the Pathogenesis of Liver Diseases. Front Immunol. 2021;12:625472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 62. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1493] [Cited by in RCA: 1921] [Article Influence: 274.4] [Reference Citation Analysis (0)] |
| 63. | Golonka RM, Yeoh BS, Petrick JL, Weinstein SJ, Albanes D, Gewirtz AT, McGlynn KA, Vijay-Kumar M. Deoxyribonuclease I Activity, Cell-Free DNA, and Risk of Liver Cancer in a Prospective Cohort. JNCI Cancer Spectr. 2018;2:pky083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Janovičová Ľ, Kmeťová K, Pribulová N, Janko J, Gromová B, Gardlík R, Celec P. Endogenous DNase Activity in an Animal Model of Acute Liver Failure. Int J Mol Sci. 2023;24:2984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Vokálová L, Lauková L, Čonka J, Melišková V, Borbélyová V, Bábíčková J, Tóthová L, Hodosy J, Vlková B, Celec P. Deoxyribonuclease partially ameliorates thioacetamide-induced hepatorenal injury. Am J Physiol Gastrointest Liver Physiol. 2017;312:G457-G463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Belvončíková P, Feješ A, Gromová B, Janovičová Ľ, Farkašová A, Babál P, Gardlík R. Therapeutic Effects of DNase I on Peripheral and Local Markers of Liver Injury and Neutrophil Extracellular Traps in a Model of Alcohol-Related Liver Disease. Int J Mol Sci. 2025;26:1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Wu J, Zhang C, He T, Zhang S, Wang Y, Xie Z, Xu W, Ding C, Shuai Y, Hao H, Cao L. Polyunsaturated fatty acids drive neutrophil extracellular trap formation in nonalcoholic steatohepatitis. Eur J Pharmacol. 2023;945:175618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 68. | Xia Y, Wang Y, Xiong Q, He J, Wang H, Islam M, Zhou X, Kim A, Zhang H, Huang H, Tsung A. Neutrophil extracellular traps promote MASH fibrosis by metabolic reprogramming of HSC. Hepatology. 2025;81:947-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 69. | Ungerer V, Bronkhorst AJ, Van den Ackerveken P, Herzog M, Holdenrieder S. Serial profiling of cell-free DNA and nucleosome histone modifications in cell cultures. Sci Rep. 2021;11:9460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Zhu D, Wang H, Wu W, Geng S, Zhong G, Li Y, Guo H, Long G, Ren Q, Luan Y, Duan C, Wei B, Ma J, Li S, Zhou J, Mao M. Circulating cell-free DNA fragmentation is a stepwise and conserved process linked to apoptosis. BMC Biol. 2023;21:253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 71. | Cheng THT, Lui KO, Peng XL, Cheng SH, Jiang P, Chan KCA, Chiu RWK, Lo YMD. DNase1 Does Not Appear to Play a Major Role in the Fragmentation of Plasma DNA in a Knockout Mouse Model. Clin Chem. 2018;64:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Janciauskiene S, Wrenger S, Immenschuh S, Olejnicka B, Greulich T, Welte T, Chorostowska-Wynimko J. The Multifaceted Effects of Alpha1-Antitrypsin on Neutrophil Functions. Front Pharmacol. 2018;9:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Dau T, Sarker RS, Yildirim AO, Eickelberg O, Jenne DE. Autoprocessing of neutrophil elastase near its active site reduces the efficiency of natural and synthetic elastase inhibitors. Nat Commun. 2015;6:6722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Cherubini A, Della Torre S, Pelusi S, Valenti L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol Med. 2024;30:1126-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/