Published online Jul 7, 2025. doi: 10.3748/wjg.v31.i25.105518
Revised: March 27, 2025
Accepted: June 10, 2025
Published online: July 7, 2025
Processing time: 147 Days and 23.1 Hours
In metabolic dysfunction-associated steatotic liver disease (MASLD) the identification of patients at high risk of evolution to metabolic dysfunction-associated steatohepatitis (MASH) is challenging.
To investigate the performance of different ultrasound (US)-based techniques for the non-invasive assessment of liver fibrosis, steatosis, and inflammation in these patients.
We collected data from consecutive patients who underwent liver biopsy for suspected MASLD between January 2019 and December 2021. Two-dimensional shear-wave elastography, sound speed plane-wave US, attenuation plane-wave US, viscosity plane-wave US (Vi.PLUS) using Aixplorer MACH 30 system, and transient elastography and controlled attenuation parameter from FibroScan were measured before biopsy.
A total of 120 participants were enrolled. Both transient elastography and two-dimensional shear-wave elasto
Multiparametric US allows the non-invasive assessment of steatosis, inflammation, and fibrosis in patients with MASLD. Liver viscosity improved the capability of non-invasively identifying patients with MASH.
Core Tip: This article provided further insights into the performance of different ultrasound-based techniques for non-invasive multiparametric assessment of liver fibrosis, steatosis, and inflammation in patients with metabolic dysfunction-associated steatotic liver disease. We found that among the analyzed parameters, liver viscosity was associated with the degree of inflammation and ballooning and allows together with ultrasound attenuation and biochemical parameters the identification of patients with metabolic dysfunction-associated steatohepatitis with good reliability. This could help to distinguish patients at high risk from those at low risk, reducing the number of liver biopsies in patients at low risk of metabolic dysfunction-associated steatohepatitis.
- Citation: Liguori A, Ainora ME, Di Gialleonardo L, Viceconti N, Petrucci L, Esposto G, Giustiniani MC, Mignini I, Borriello R, Galasso L, Paratore M, Garcovich M, Riccardi L, Pompili M, Grieco A, Gasbarrini A, Miele L, Zocco MA. Multiparametric ultrasound for non-invasive assessment of liver steatosis, fibrosis, and inflammation in metabolic dysfunction-associated steatotic liver disease. World J Gastroenterol 2025; 31(25): 105518
- URL: https://www.wjgnet.com/1007-9327/full/v31/i25/105518.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i25.105518
Metabolic dysfunction-associated steatotic liver disease (MASLD) is today universally considered the most common liver disease worldwide[1]. MASLD ranges from simple steatosis to progressive metabolic dysfunction-associated steatohepatitis (MASH), which can advance to cirrhosis[2]. As liver fibrosis (LF) and inflammatory activity in MASLD are the most significant predictors of liver-related complications and mortality, non-invasive methods for assessing hepatic steatosis (HS), LF, and inflammation can be of critical importance in clinical practice[3]. Liver biopsy is still considered the gold standard for staging LF and grading disease activity (lobular inflammation and ballooning)[4]. However, it is an invasive procedure with some drawbacks such as high cost, sampling errors, interobserver variability and risks of complications. For these reasons, many non-invasive tests (NITs) for the assessment of liver disease severity have been developed in the last decades[5].
Among imaging techniques ultrasound (US)-based liver elastography has become the method of choice for non-invasive LF assessment[6,7]. Transient elastography (TE) is the first and most validated technique, followed by shear wave elastography (SWE)[8-10]. Two-dimensional SWE (2D-SWE) allows a real-time tissue elasticity evaluation by mea
The first diagnosis challenge in patients with MASLD is the objective quantification of HS[15]. The non-invasive assessment of HS using US has already been investigated. The controlled attenuation parameter (CAP) embedded in the FibroScan device was the first US-based quantitative tool developed for HS assessment. It analyzes the US attenuation through the liver and has demonstrated a good correlation with histological steatosis grades in numerous studies[16-18].
Other US-based technologies that can quantify fibrosis and steatosis with the same device were developed recently[19,20]. Among them attenuation imaging can quantify the degree of fatty infiltration by measuring the attenuation coefficient of the US beam in the liver parenchyma[21]. Similarly, sound speed estimation allows HS assessment by estimating the intrahepatic speed of sound throughout a fixed region of interest. In recent studies both methods demonstrated a good diagnostic performance in assessing the presence of moderate-to-severe steatosis[22,23].
Together with LF and HS, necroinflammation is the third important aspect that should be evaluated in patients with MASLD since it plays an essential role in the diagnosis of MASH and in the progression of liver disease[24]. However, both non-invasive stratification of disease activity and MASH diagnosis remain an unmet need so far. A non-invasive tool capable of identifying patients at increased risk of MASH would limit liver biopsy to a smaller number of better-selected patients while reducing the costs and the risks associated with such a procedure in patients at low risk of MASH.
Recently, it has been demonstrated that multiparametric US examinations have the potential to provide a comprehensive estimation of the main components of MASLD compared with TE[25] or liver biopsy[26]. Preclinical studies highlighted shear-wave dispersion imaging as a promising technique for assessment of liver viscosity. Sugimoto et al[27] showed that an increase in necroinflammatory changes in the rat liver led to an increase in viscosity[27]. Association between viscosity and liver inflammation in humans, particularly in patients with MASLD, is still controversial. Deffieux et al[28] showed that viscosity is linked to LF, but it is not a good predictor of liver inflammation. On the other hand, Sugimoto et al[29] showed that the dispersion slope of the shear wave, an indirect index of tissue viscosity, was directly linked to histological inflammation grade in patients with MASLD[29,30]. This difference can primarily be attributed to the different types of patients included in the studies. Whereas the study by Sugimoto et al[30] included patients with MASLD, the study by Deffieux et al[28] included patients with various liver diseases. It is well known that liver inflammation features differ depending on etiology, such as hepatitis C virus infection, HBV infection, or MASH, even when the fibrosis stage is the same. Etiology-specific studies could give a deeper insight into the role of viscosity in the assessment of liver inflammation, hopefully allowing its larger use for the benefit of patients.
This study aimed to evaluate the performance of different US-based techniques for the non-invasive assessment of LF, steatosis, and disease activity using liver biopsy as the reference method. The secondary aim was to assess whether a combination of US parameters may be useful for the non-invasive identification of patients with MASH.
This is a single center, prospective, cross-sectional study performed during a 2-year interval (January 2019 to December 2021) in a tertiary Department of Gastroenterology and Hepatology in Italy. All consecutive patients with clinical suspicion of MASLD/MASH referred to the Liver Disease Unit of the Fondazione Policlinico Universitario A. Gemelli in Rome for percutaneous liver biopsy according to current guidelines were prospectively enrolled in this study[5,31].
Inclusion criteria were age > 18 years, fatty changes of the liver observed by US, platelet count of at least 80 × 109/L, international normalized ratio of less than 1.5, and signed informed consent.
Subjects with increased alcohol consumption (ethanol intake > 3 alcohol units per day for males and > 2 alcohol units per day for females) were excluded. Other exclusion criteria were chronic liver disease of clear etiology (viral hepatitis, autoimmune hepatitis, primary biliary cholangitis, or primary sclerosing cholangitis), clinical signs of portal hypertension (ascites, gastroesophageal varices, hepatic encephalopathy, portosystemic shunts), biliary obstruction, oncological history, and heart failure.
On the same date of the liver biopsy, all patients underwent US-based measurements of liver stiffness, steatosis, and viscosity in the same session, using two distinctive systems. 2D-SWE, viscosity plane-wave US (Vi.PLUS), sound speed plane-wave US (SSp.PLUS), and attenuation plane-wave US (Att.PLUS) were performed using the Aixplorer MACH 30 system (Hologic, Aix-en-Provence, France), while TE and CAP measurements were performed with the FibroScan system (EchoSens, Paris, France).
At the time of US examination, a blood sample was obtained for evaluation of complete blood counts, international normalized ratio, thrombocytes, total bilirubin concentrations, aminotransferases levels, gamma-glutamyl transferase, and albumin. Body mass index (BMI), fibrosis 4 score, nonalcoholic fatty liver disease fibrosis score, and Agile 3 + score were calculated for each patient. Finally, comorbidities (diabetes, arterial hypertension, dyslipidemia) were collected from medical records.
The study protocol was approved by the Ethical Review Board of Fondazione Policlinico Universitario “A. Gemelli” IRCCS and conformed to the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent before inclusion.
The primary outcome of the study was to determine the diagnostic performance of different US-based techniques for the non-invasive assessment of liver steatosis, fibrosis, and inflammation compared with histological evaluation. The secondary outcome was the development of a statistical model to predict the histologic features of MASH based on these imaging markers.
All US examinations, 2D-SWE, Vi.PLUS, SSp.PLUS, and Att.PLUS were conducted with the Aixplorer MACH 30 system (Hologic, Aix-en-Provence, France), equipped with a wideband C1-6 MHz curvilinear probe by two trained operators (Ainora ME and Liguori A with 12 years and 6 years of experience in liver US, respectively). They were unaware of the clinical data and the results of histopathological assessment. Each examination was performed twice, once by each operator, and the median values were used for the analysis.
The LS evaluation was performed with the 2D-SWE technique according to the World Federation for Ultrasound in Medicine and Biology and European Federation of Societies for Ultrasound in Medicine and Biology guidelines[6,7]. The measurement box was placed at least 1.5 cm below the liver capsule, avoiding large vessels or bile ducts and rib shadows. Measurements were obtained by positioning a 15-mm-diameter region of interest in the center of a color map with complete and homogeneous filling, obtained during a breath hold. Each SWE measurement was acquired at a stability index of at least 90% to guarantee the spatial and temporal stiffness stability within the circular region of interest. The median value of three successful LS measurements for each operator (six measurements in total) was obtained from each patient with an interquartile range to the median ratio (IQR/M) < 30% as a measurement reliability criterion[7]. The results were expressed in kilopascals (kPa).
Simultaneously, the machine also provided the Vi.PLUS value, which is an index of tissue shear wave dispersion or viscosity. The median value of three successful viscosity measurements for each operator (six measurements in total) was obtained from each patient. The changes of shear wave propagation velocity at different frequencies were qualitatively represented in a color-coded image and quantitatively expressed in Pa.s[32].
Both Att.PLUS and SSp.PLUS were determined in one acquisition using the same US transducer. Measurements were obtained from the right hepatic lobe through intercostal spaces with the probe axis orthogonal to the liver capsule. The operator selected the most appropriate area in the right liver lobe (usually 5th or 6th segment) with the upper part of the biggest box on the liver capsule and the smallest box free of large vessels. Reliable measurements were defined as the median values of three measurements with an IQR/M < 30%.
The results were expressed in m/s for SSp.PLUS and in dB/cm/MHz for Att.PLUS over a range of values (from 1450 to 1600 m/s and from 0.2 to 1.6 dB/cm/MHz, respectively)[33].
TE and CAP measurements were performed with the FibroScan Compact 530 system equipped with the standard M (3.5 Hz frequency) probe or the XL (2.5 Hz frequency) probe. All patients were evaluated in fasting conditions (at least 6 h) by a single qualified operator (Liguori A with 6 years of experience in TE), according to the European Federation of Societies for Ultrasound in Medicine and Biology and World Federation for Ultrasound in Medicine and Biology guidelines[6,7]. Reliable results, representing the median value of 10 valid measurements, with an IQR/M < 30%, were expressed in kPa for fibrosis (range 2.5-75.0 kPa) and in dB/m for steatosis (range 100-400 dB/m)[12].
Immediately after the imaging procedures, liver biopsy was performed under real-time US guidance using a 17-gauge or 18-gauge core needle biopsy kit. Tissue specimens were fixed in formalin and stained with hematoxylin-eosin and Masson trichrome.
All liver specimens were examined by two senior pathologists (Vecchio FM, Giustiniani MC) with more than 15 years of experience in hepatic pathological assessment. They were unaware of the results of imaging techniques and laboratory tests. The degree of HS, hepatocyte ballooning, lobular inflammatory activity, and LF stage was assessed according to the histological scoring system for MASLD[34].
The nonalcoholic fatty liver disease activity score was also calculated. MASH was defined as nonalcoholic fatty liver disease activity score with at least 1 point for steatosis, ballooning, and lobular inflammation[34].
Numerical variables were presented as mean ± SD (in cases of normal distribution) or median value and 1st-3rd quartiles (in cases of non-normal distribution). Categorical variables were presented as numbers and percentages. Parametric tests (t-test or analysis of variance) and non-parametric test (Kruskal-Wallis) were used to assess differences between variables with normal and non-normal distribution, respectively. The χ2 test was used to determine significant differences between categorical variables.
The overall diagnostic performance of the imaging parameters was estimated according to the area under the receiver operating characteristic curve (AUROC) together with the 95% confidence interval. AUROC comparisons were performed using the Delong test. The cutoff values were set to maximize Youden index for staging LF, HS and inflammatory activity as compared with histopathology scores. For these cutoff values the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were reported. The following summarizes the sample size according to logistic regression model analysis. The assumed prevalence of MASH in patients who underwent liver biopsy in a secondary/tertiary care setting was estimated at 60% according to data from local historical data. Considering alpha error = 0.05, power of the analysis 80%, expected odds ratio 2.0 (for a one standard deviation increase in the continuous explanatory variable, viscosity), approximately 100 patients were expected to be enrolled.
The imaging parameters were combined with clinical and biochemical data into a multiple logistic regression model to predict MASH with a backward stepwise selection procedure to select the optimal parameters. The TRIPOD guidelines[35] were followed to report the development and validation of the prediction model. The following predictor variables were considered: Age (years); sex; diabetes; arterial hypertension; BMI (kg/m2); aspartate aminotransferase (AST) (IU/L); alanine aminotransferase (ALT) (IU/L); platelets (cell × 109/L); gamma-glutamyl transferase (IU/L); SSp.PLUS; Att.PLUS; and Vi.PLUS. Considering that MASH diagnosis is based upon liver steatosis grade, inflammation grade, and ballooning grade, we built a score for MASH diagnosis with non-invasive US and laboratory parameters that assessed those histopathological features and that significantly associated with MASH at univariate logistic regression analysis (P < 0.05). For score construction we considered either AST or ALT in multivariate regression analysis because of their known collinearity.
Internal validation of the model was performed with the bootstrapping technique (number of samples = 1000). Performances of the score were assessed by the AUROC and goodness of fit analysis. Decision curve analysis was performed to assess the impact of false positive and false negatives rates and to measure the net clinical benefit of the model.
The statistical analysis was performed using SPSS 20.0, R and STATA14 software, and statistical significance was defined as a P value below 0.05.
During the study period 135 patients with suspected MASLD were referred for liver biopsy. Among them 7 patients were excluded for unreliable or invalid measurements with at least one imaging technique (2 for 2D-SWE, 2 for TE, 3 for both), and 8 patients were excluded for different etiologies of liver disease. Overall, 120 patients were included in the final analysis (mean age 49 ± 11, 56.7% male). Demographic, biochemical, histological, and imaging characteristics of the patients are reported in Table 1. According to histopathological evaluation, 81 patients (67.5%) were diagnosed with MASH, 57 patients (47.5%) had fibrotic MASH (MASH and significant fibrosis), and 34 patients (28.4%) had advanced fibrosis.
| Characteristic | Entire cohort (n = 120) | No MASH (n = 39) | MASH (n = 81) | P value |
| Sex (male) | 68 (56.7) | 24 (61.5) | 44 (54.3) | NS |
| Age (years) | 49 ± 11 | 50 ± 10 | 49 ± 11 | NS |
| BMI (kg/m2) | 33.2 ± 7.1 | 31.7 ± 6.9 | 34.0 ± 7.1 | NS |
| Obesity | 72 (60.0) | 19 (48.7) | 53 (65.4) | 0.08 |
| Diabetes | 35 (29.1) | 7 (17.9) | 28 (34.5) | 0.06 |
| Hypertension | 54 (45.0) | 14 (35.9) | 40 (49.4) | NS |
| Biochemical profile | ||||
| Platelet count (× 106/mL) | 230.9 ± 68.2 | 230.6 ± 59.0 | 231.0 ± 72.6 | NS |
| AST (IU/L) | 43.7 ± 38.9 | 32.3 ± 14.3 | 49.3 ± 45.3 | 0.02 |
| ALT (IU/L) | 59.2 ± 50.2 | 42.4 ± 23.9 | 67.2 ± 57.2 | 0.01 |
| GGT (IU/L) | 65.7 ± 56.2 | 68.6 ± 73.2 | 64.3 ± 47.3 | NS |
| ALP (IU/L) | 74.7 ± 37.1 | 85.7 ± 52.6 | 69.6 ± 25.9 | 0.03 |
| Albumin (g/L) | 40.9 ± 3.5 | 41.6 ± 3.2 | 40.5 ± 3.5 | NS |
| Triglycerides (mg/dL) | 157.9 ± 90.6 | 137.5 ± 53.7 | 168.0 ± 103.1 | NS |
| Total cholesterol (mg/dL) | 196.3 ± 44.2 | 195.9 ± 42.0 | 196.5 ± 45.5 | NS |
| HDL (mg/dL) | 44.7 ± 14.3 | 50.1 ± 22.5 | 42.7 ± 8.8 | 0.04 |
| FIB4 | 1.54 ± 2.67 | 1.20 ± 0.61 | 1.71 ± 3.22 | NS |
| NFS | -1.24 ± 1.71 | -1.54 ± 1.48 | -1.10 ± 1.80 | NS |
| Agile3 + | 0.14 (0.06-0.53) | 0.11 (0.05-0.33) | 0.23 (0.06-0.60) | 0.02 |
| Histological findings | ||||
| Steatosis grade | < 0.01 | |||
| 1 | 47 (39.2) | 39 (100) | 8 (9.9) | |
| 2 | 49 (40.8) | 0 (0) | 49 (60.5) | |
| 3 | 24 (20.0) | 0 (0) | 24 (29.6) | |
| Lobular inflammation grade | < 0.01 | |||
| 0 | 4 (3.3) | 4 (10.2) | 0 (0) | |
| 1 | 90 (75.0) | 35 (89.8) | 55 (67.9) | |
| 2 | 24 (20.0) | 0 (0) | 24 (29.6) | |
| 3 | 2 (1.7) | 0 (0) | 2 (2.5) | |
| Hepatocyte ballooning grade | < 0.01 | |||
| 0 | 13 (10.8) | 13 (33.3) | 0 (0) | |
| 1 | 94 (78.4) | 26 (66.6) | 68 (84.2) | |
| 2 | 13 (10.8) | 0 (0) | 13 (15.8) | |
| Fibrosis stage | 0.01 | |||
| 0 | 3 (2.5) | 3 (7.7) | 0 (0) | |
| 1 | 43 (35.8) | 19 (48.8) | 24 (29.6) | |
| 2 | 40 (33.3) | 12 (30.7) | 28 (34.6) | |
| 3 | 30 (25.0) | 4 (10.2) | 26 (32.1) | |
| 4 | 4 (3.4) | 1 (2.6) | 3 (3.7) | |
| Elastographic measurements | ||||
| LS by TE (kPa) | 7.9 ± 5.0 | 6.2 ± 3.2 | 8.7 ± 5.3 | 0.01 |
| 2D-SWE (kPa) | 7.34 ± 3.16 | 6.61 ± 3.02 | 7.70 ± 3.19 | 0.07 |
| CAP (dB/m) | 302.9 ± 67.2 | 250.5 ± 74.5 | 328.2 ± 45.9 | < 0.01 |
| Att.PLUS (dB/cm/mHz) | 0.52 ± 0.11 | 0.55 ± 0.12 | 0.51 ± 0.10 | 0.06 |
| SSp.PLUS (m/s) | 1530 (1505-1550) | 1545 (1525-1570) | 1525 (1500-1545) | < 0.01 |
| Vi.PLUS (Pa.s) | 2.24 ± 0.51 | 2.04 ± 0.54 | 2.34 ± 0.48 | 0.03 |
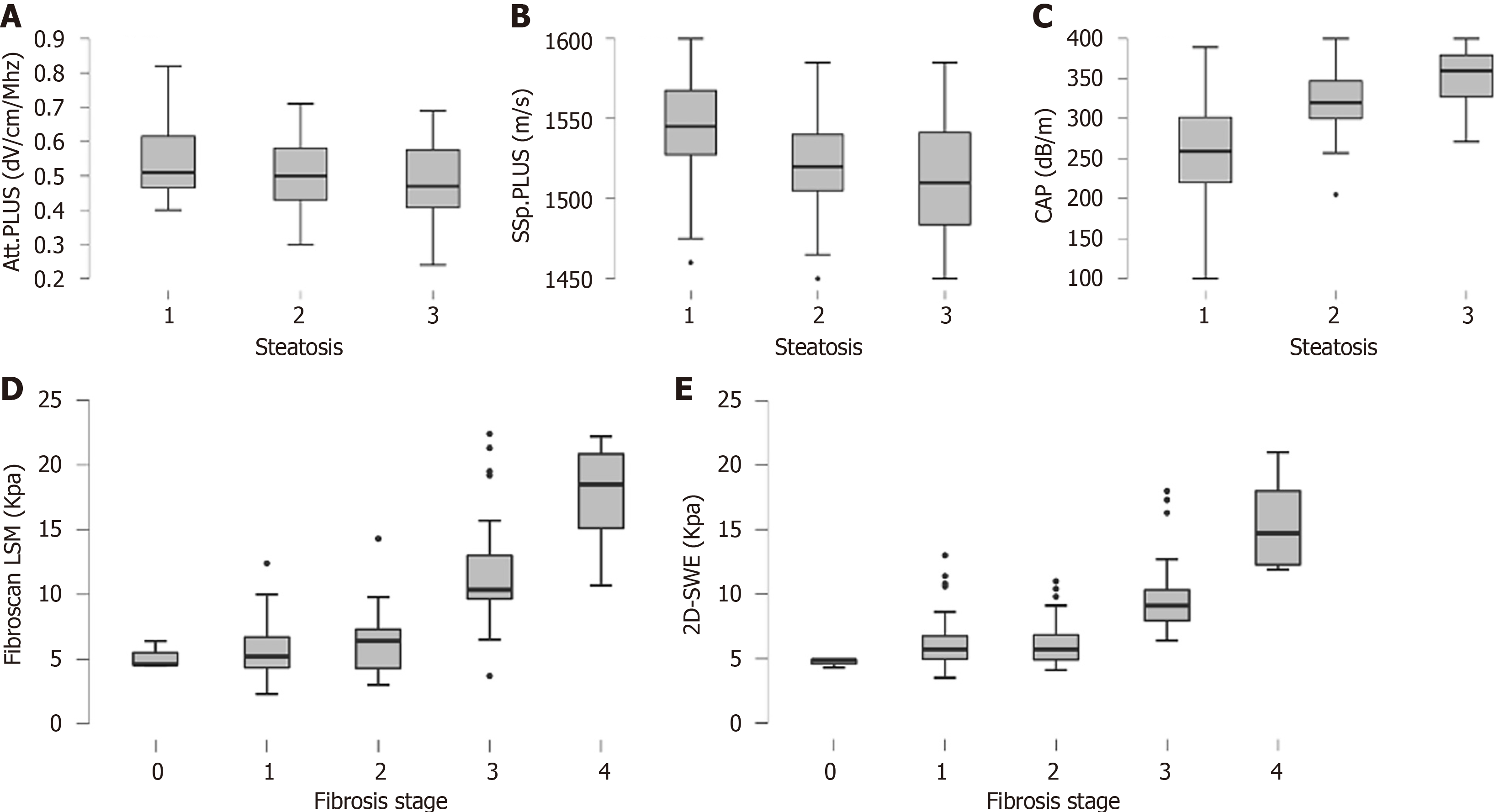
The distribution of CAP, Att.PLUS, and SSp.PLUS values according to histologically assessed steatosis grade is shown in Figure 1. CAP and SSp.PLUS were significantly different among different steatosis grades (P < 0.01), whereas Att.PLUS did not vary significantly (P = 0.15).
SSp.PLUS and CAP showed fair-to-optimal performance for the diagnosis of moderate-to-severe steatosis with AUROCs of 0.71 and 0.83, respectively (Table 2). The optimal CAP, Att.PLUS, and SSp.PLUS cutoff values for predicting severe steatosis and their corresponding sensitivity, specificity, NPV, and PPV are summarized in Table 2.
| Variable | Cutoff value | AUC (95%CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P value |
| Steatosis ≥ 2 | |||||||
| CAP (dB/m) | 275 | 0.83 (0.75-0.91) | 94.5 | 66.0 | 81.2 | 88.6 | Reference |
| CAP (dB/m) | 3011 | 78.0 | 74.5 | 82.6 | 68.6 | ||
| Att.PLUS (dB/cm/mHz) | 0.44 | 0.60 (0.50-0.70) | 89.4 | 31.5 | 45.6 | 82.1 | < 0.01 |
| SSp.PLUS (m/s) | 1535 | 0.71 (0.61-0.81) | 70.2 | 65.7 | 56.9 | 77.4 | 0.02 |
| Inflammation ≥ 1 and ballooning ≥ 1 | |||||||
| Vi.PLUS (Pa.s) | 2.3 | 0.72 (0.59-0.86) | 52.8 | 92.8 | 98.2 | 20.6 | Reference |
| MASH | |||||||
| Vi.PLUS (Pa.s) | 2.1 | 0.68 (0.58-0.79) | 69.1 | 64.1 | 80.0 | 50.0 | Reference |
| VAS-MASH-US score | 0.60 | 0.75 (0.65-0.84) | 79.0 | 66.7 | 83.1 | 60.4 | 0.14 |
| 0.48 | 90.0 | 43.6 | 76.6 | 65.4 | |||
| 0.84 | 30.9 | 90.0 | 86.2 | 38.4 | |||
| Fibrosis ≥ 2 | |||||||
| TE (kPa) | 6.4 | 0.75 (0.65-0.83) | 72.9 | 71.7 | 80.6 | 62.3 | Reference |
| 2D-SWE (kPa) | 6.4 | 0.69 (0.60-0.79) | 59.5 | 73.9 | 78.6 | 53.1 | 0.25 |
| FIB-4 | 1.07 | 0.65 (0.55-0.75) | 62.2 | 58.7 | 70.8 | 49.1 | 0.10 |
| NFS | -0.97 | 0.69 (0.59-0.79) | 55.4 | 78.3 | 80.4 | 52.2 | 0.40 |
| Vi.PLUS (Pa.s) | 2.3 | 0.64 (0.53-0.74) | 59.5 | 71.7 | 77.2 | 52.4 | 0.04 |
| Fibrosis ≥ 3 | |||||||
| TE (kPa) | 81 | 91.2 | 83.7 | 68.9 | 96.0 | Reference | |
| 8.4 | 0.93 (0.87-0.99) | 85.3 | 91.9 | 80.5 | 94.0 | ||
| 121 | 35.3 | 97.7 | 84.6 | 78.5 | |||
| 2D-SWE (kPa) | 7.2 | 0.90 (0.85-0.95) | 88.2 | 81.4 | 61.7 | 93.1 | 0.43 |
| FIB-4 | 1.26 | 0.80 (0.71-0.89) | 70.6 | 74.4 | 52.2 | 86.5 | 0.01 |
| NFS | -1.12 | 0.83 (0.75-0.90) | 88.2 | 73.2 | 56.6 | 94.0 | 0.03 |
| Agile3 + | 0.42 | 0.93 (0.88-0.98) | 85.3 | 89.5 | 76.3 | 93.9 | 0.99 |
| Vi.PLUS (Pa.s) | 2.1 | 0.81 (0.74-0.89) | 100 | 58.1 | 48.6 | 100 | < 0.01 |
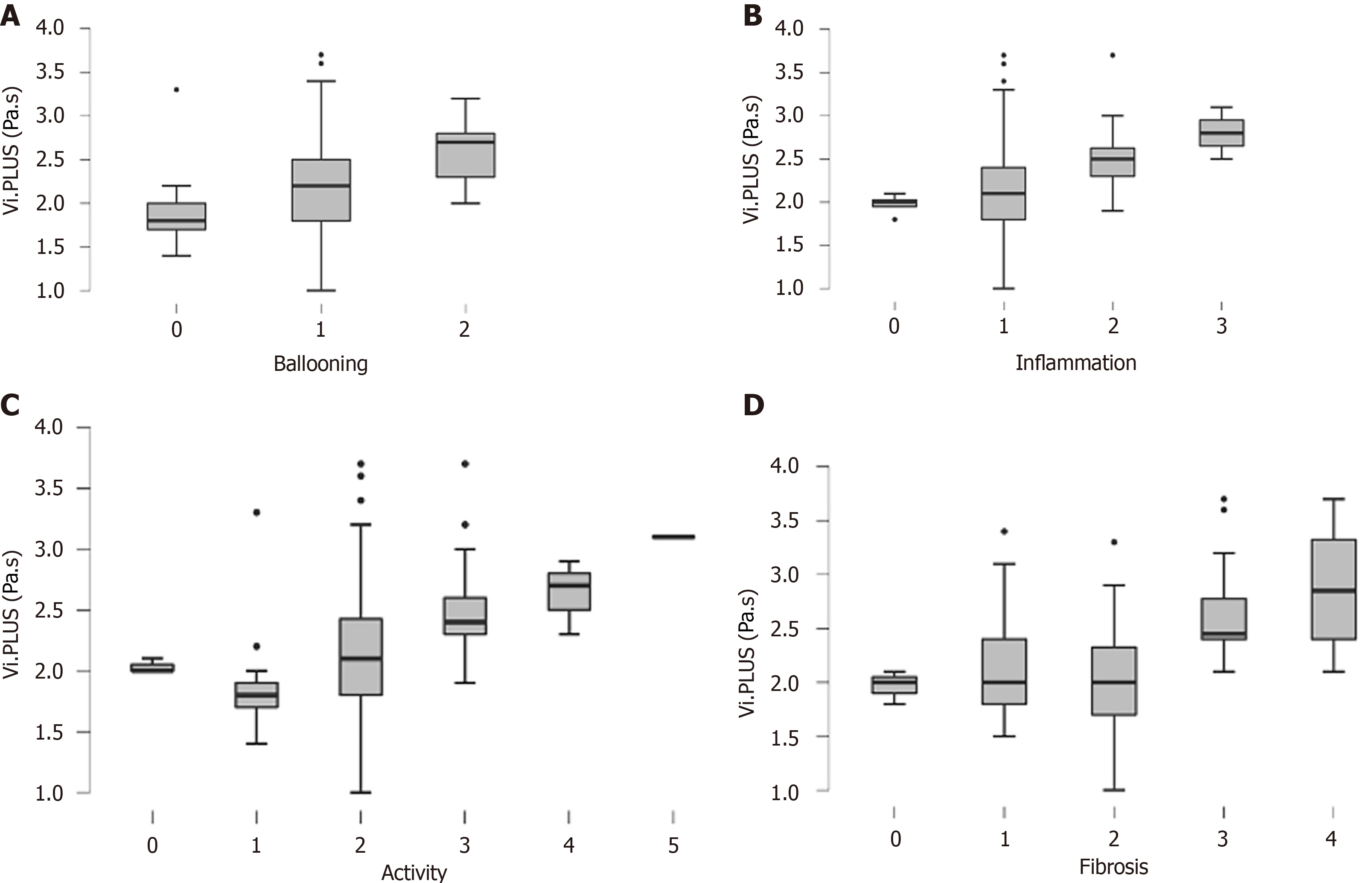
The distribution of Vi.PLUS values according to lobular inflammation grade, ballooning grade, disease activity, and fibrosis is shown in Figure 2. Vi.PLUS increased progressively with the degree of lobular inflammation and ballooning (P < 0.01). The diagnostic performance of Vi.PLUS for the diagnosis of both ballooning grade 1 and lobular inflammation 1 was good with an AUROC of 0.72 (Table 2). Vi.PLUS was significantly higher in patients with advanced fibrosis compared with patients with lower stages of fibrosis (F < 3) (Figure 2).
LS values from both 2D-SWE and TE progressively and significantly increased according to fibrosis stages (P < 0.01) (Figure 1). Both TE and 2D-SWE showed good performance for the diagnosis of significant fibrosis (F ≥ 2, AUROC = 0.75 and 0.69, respectively) and advanced fibrosis (F ≥ 3, AUROC = 0.93 and 0.90, respectively) (Figure 1 and Table 2). The optimal cutoff values determined by the Youden index and their corresponding sensitivity, specificity, NPV and PPV in identifying F ≥ 2 and F ≥ 3 are detailed in Table 2.
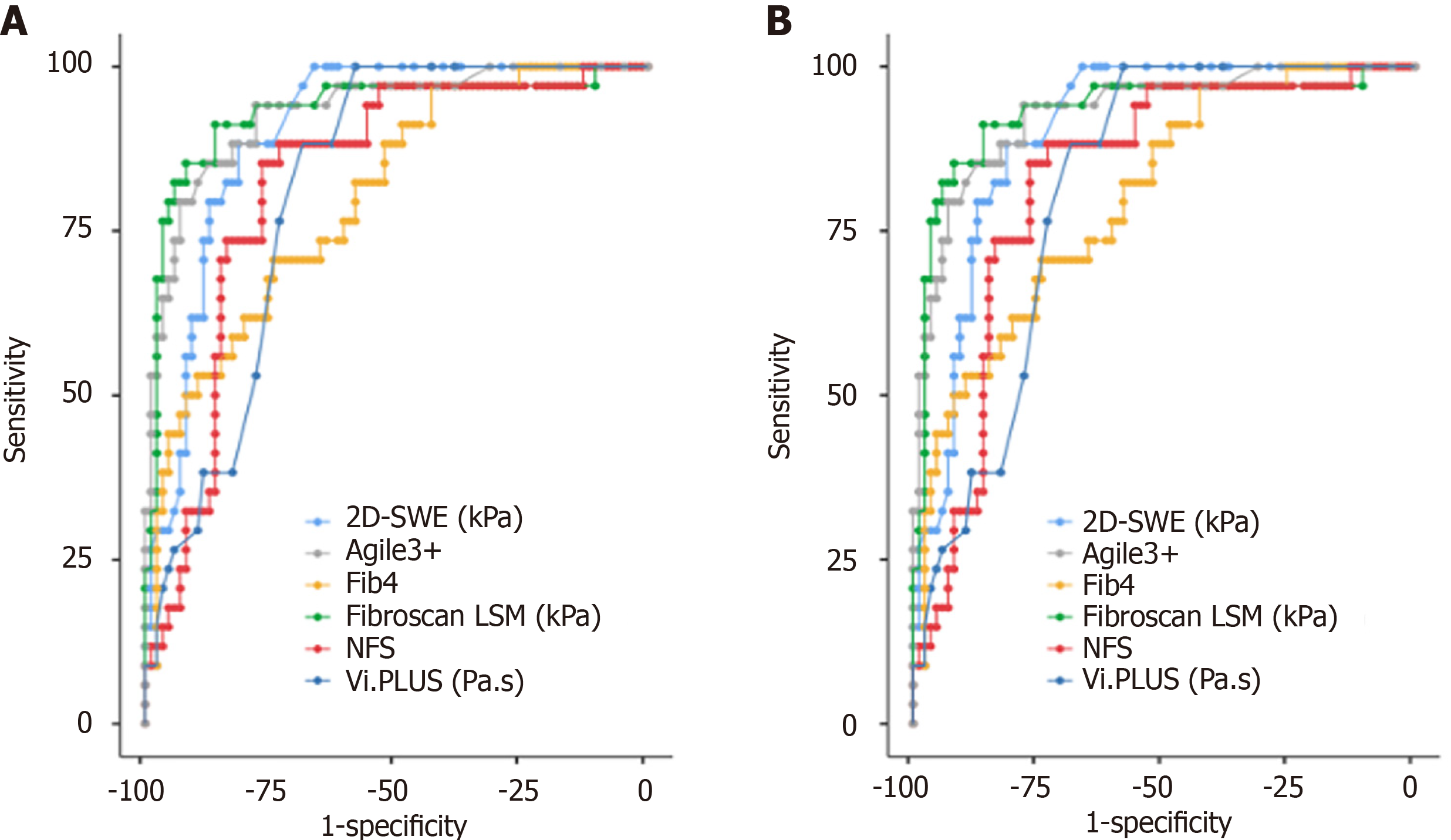
Among all NITs, TE had the best diagnostic performance for the diagnosis of both significant fibrosis and advanced fibrosis, with an AUROC of 0.75 and 0.93, respectively, and optimal cutoff values of 6.4 (sensitivity 72.9%, specificity 71.7%) and 8.4 (sensitivity 85.3%, specificity 91.9%), respectively. The diagnostic performance of 2D-SWE was also high with an AUROC of 0.90 (cutoff 7.2 kPa, sensitivity 88.2%, specificity 81.4%) for F ≥ 3 (Figure 3 and Table 2).
Univariate logistic regression models were built to examine the relationships between the presence of MASH and the following parameters: Age; sex; AST; ALT; BMI; diabetes; hypertension; Att.PLUS values; SSp.PLUS values; and Vi.PLUS values (Table 3). According to the univariate analysis, Vi.PLUS values (P < 0.01), SSp.PLUS values (P < 0.01), ALT (P = 0.01), and AST (P = 0.01) were all associated with MASH (Table 3). A multivariate logistic regression analysis was carried out to assess the influence of each parameter for the diagnosis of MASH. Three independent predictors were selected: Vi.PLUS; AST; and SSp.PLUS. A score based on those variables had a good accuracy for the diagnosis of MASH, with an AUROC of 0.75. The predictor equation for MASH would be:
| Variable | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex (male) | 0.74 (0.34-1.62) | 0.45 | ||
| Age | 0.98 (0.95-1.02) | 0.48 | ||
| Att.PLUS | 0.03 (0.01-1.06) | 0.06 | ||
| SSp.PLUS | 0.98 (0.97-0.99) | < 0.01 | 0.98 (0.97-0.99) | < 0.01 |
| CAP | 1.02 (1.01-1.03) | < 0.01 | ||
| Vi.PLUS | 3.59 (1.47-8.78) | < 0.01 | 3.12 (1.20-8.16) | 0.02 |
| BMI (kg/m2) | 1.05 (0.99-1.11) | 0.11 | ||
| Triglycerides | 1.00 (0.99-1.01) | 0.11 | ||
| Total cholesterol | 1.00 (0.99-1.01) | 0.94 | ||
| Diabetes | 2.42 (0.95-6.17) | 0.06 | ||
| Hypertension | 1.74 (0.79-3.82) | 0.16 | ||
| ALT | 1.02 (1.01-1.03) | 0.01 | 1.03 (1.01-1.06) | 0.05 |
| AST | 1.03 (1.01-1.06) | 0.02 | ||
X = 23.532 + (1.141 × Vi.PLUS) + (0.027 × AST) + (-1.716 × SSp.PLUS/100).
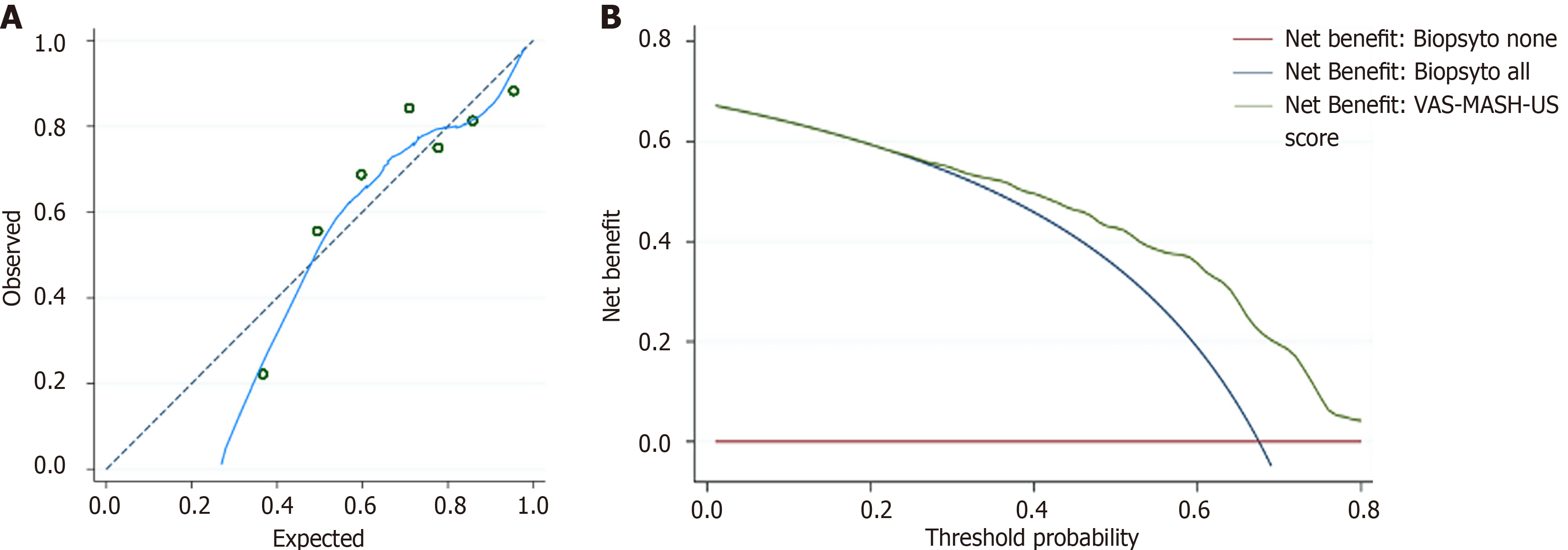
This score was named viscosity-AST-speed of sound MASH US score (VAS-MASH-US). The calibration line was close to the ideal calibration that showed a good goodness of fit in predicted probability of MASH (Hosmer-Lemeshow test, P = 0.34) (Figure 4). According to ROC analysis the optimal cutoff value for MASH diagnosis was 0.60 (sensitivity 79.0%, specificity 66.7%). When we applied the dual cutoff stratification analysis, we found that the cutoff with high sensitivity (> 90.0%) was to 0.48, and the cutoff with high specificity (> 90.0%) was 0.84. According to this method 42 patients were correctly classified as MASH (35%), 13 patients were wrongly classified as MASH (10.8%), and 65 patients fell in the indeterminate area (54.1%). Decision curve analysis suggested that it was a good option compared with performing liver biopsy in all patients or no patients since it had the highest net benefit across the range of threshold probabilities (Figure 4).
Identifying reliable non-invasive biomarkers for the diagnosis and classification of MASLD is a major challenge in hepatology. In the last two decades, the development of elastography techniques has allowed a leap forward in the non-invasive assessment of fibrosis stage in patients with various liver diseases[5]. This is particularly true for patients with MASLD, in which scores or imaging techniques have also been developed and validated to reliably assess the degree of liver steatosis[36]. Despite this development the need for patients with MASLD to undergo liver biopsy has not significantly decreased in the same period. This is probably related to the lack of non-invasive parameters that can identify MASH with the same reliability.
In this scenario our study had two ambitious aims: To evaluate the ability of a new imaging parameter (viscosity) to discriminate the degree of ballooning and inflammation and consequently to build a model able to identify patients with MASH based on multiparametric US evaluation.
To our knowledge this is the first study comparing the results of viscosity measurement with Aixplorer MACH30 with the histopathological features of MASLD. We found that viscosity (Vi.PLUS) was associated with MASH and particularly with the degree of inflammation and ballooning. Our results are in line with the findings obtained by Sugimoto et al[27] with a different software (Canon Aplio i800) and support the hypothesis that necroinflammatory parameters have a significative impact on the viscoelastic characteristics of liver tissue in MASLD, as the shear wave dispersion can be influenced by cellular edema, necrosis, and extracellular matrix changes[26,27].
On the other hand, in the milestone study by Deffieux et al[28], viscosity was a poor predictor of disease activity in patients with various chronic liver diseases. Considering our results and those from previous studies, the association between viscosity and necroinflammatory activity seems to be particularly strong in MASLD compared with other chronic liver diseases. We can hypothesize that the inflammatory processes of each type of liver disease have a different impact on the viscoelastic properties of liver tissue. In addition, it is already known that the process of hepatic fibro
Steatosis, together with lobular inflammation and ballooning, is a main determinant of MASH diagnosis. In our study CAP showed the best performance for non-invasive assessment of steatosis with an AUROC of 0.83 for the diagnosis of severe steatosis. SSp.PLUS showed a good performance for stratifying liver steatosis compared with histopathological assessment, whereas Att.PLUS did not display statistically significant differences among different steatosis grades. These results are in contrast with that obtained by other authors who demonstrated a good discrimination ability of attenuation[39,40]. However, this discrepancy could be related to the different US equipment. Larger studies comparing Att.PLUS with liver steatosis grade are needed to better define its ability to stratify HS.
Notably, SSp.PLUS has a good diagnostic performance for severe steatosis with an AUC of 0.71. Interestingly, the technology is an innovative approach for HS quantification. In fact, the two main scores proposed for identifying patients with a high risk of MASH, namely Fibroscan-AST score and LAD-MASH score, consider the attenuation parameter from Fibroscan (CAP) and the attenuation coefficient from Canon Aplioi800 for steatosis assessment[27,40].
Considering the possibility to measure both the degree of steatosis and the severity of inflammation in the same examination session, we tried to combine these features into a non-invasive predictive model easily applicable in clinical practice for identifying patients with MASH (the VAS-MASH-US score). This score showed good performance for MASH diagnosis with an AUROC of 0.75 and a goodness-of-fit testifying a low discrepancy between observed and expected patients with MASH diagnosis. The cutoff of 0.6, obtained maximizing the Youden index, showed a good sensitivity (79.0%) but a suboptimal specificity (66.7%). On the other hand, the dual cutoff strategy was able to improve the risk stratification ability of the score with 35% of correctly classified patients and only 10% of wrongly classified patients. Unfortunately, even with this approach, more than 50% of patients fell in the indeterminate area of the score requiring liver biopsy for correct disease classification. These limited diagnostic performances, in our opinion, do not allow the replacement of liver biopsy in the diagnosis of MASH. However, given the good sensitivity of the score, we propose using it for a better selection of patients to undergo liver biopsy. As shown by the decision curve analysis, applying the score to identify candidates for liver biopsy would maintain the same number of histological MASH diagnoses while reducing unnecessary biopsies, leading to significant cost savings.
Current guidelines recommend histological examination for all patients with suspected MASH or advanced fibrosis based on LS measurement, comorbidities, and altered liver function tests. However, among 120 patients who underwent liver biopsy in our center, only two-thirds had a diagnosis of MASH.
Our score aimed to identify high-risk patients, thereby minimizing unnecessary biopsies. The highest sensitivity (90.0%) was achieved with a VAS-MASH-US score of 0.48 (AUC = 0.75, confidence interval: 0.65-0.84), below which MASH is unlikely. Thus, the score could help optimize the risk/benefit ratio by ensuring only patients with a high pre-test probability undergo biopsy, reducing both costs and procedural risks.
Some limitations were associated with our study. First, this was a single center study with a relatively small sample size. This limitation does not allow the assessment of clinical relevance of the VAS-MASH-US score in different patient populations with MASLD (e.g., obese vs non-obese, diabetic vs non-diabetic). Second, the risk score for MASH estimated in our study was not validated with an independent validation cohort. Although we used internal validation methods for the development of the score according to Tripod guidelines, the lack of an external validation cohort limits the broader application of the score.
The development of a risk scoring system based on multiparametric US assessment, including viscosity and sound speed evaluation, is a cheap, widely-available, and easy-to-use method for an appropriate selection of candidates for liver biopsy for a definitive histological diagnosis of MASH. Further large population-based cohort studies are warranted to validate these results and to evaluate the performance of this US score in different populations and clinical settings.
We would like to acknowledge Ministero della Salute-Ricerca corrente 2024 and Fondazione Roma for the invaluable support for scientific research. We would like to thank Prof. Vecchio FM for his contribution to this study.
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4012] [Article Influence: 501.5] [Reference Citation Analysis (2)] |
| 2. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 798] [Article Influence: 133.0] [Reference Citation Analysis (1)] |
| 3. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1506] [Article Influence: 167.3] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3279] [Article Influence: 327.9] [Reference Citation Analysis (6)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1292] [Article Influence: 258.4] [Reference Citation Analysis (1)] |
| 6. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 394] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 7. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 606] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 8. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1119] [Article Influence: 159.9] [Reference Citation Analysis (1)] |
| 9. | Foncea CG, Popescu A, Lupusoru R, Fofiu R, Sirli R, Danila M, Sporea I. Comparative study between pSWE and 2D-SWE techniques integrated in the same ultrasound machine, with Transient Elastography as the reference method. Med Ultrason. 2020;22:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 10. | Moga TV, Sporea I, Lupușoru R, Popescu A, Popa A, Bota S, Șirli R, Danilă M, Schlesinger A, Tzschätzsch H. Performance of a Noninvasive Time-Harmonic Elastography Technique for Liver Fibrosis Evaluation Using Vibration Controlled Transient Elastography as Reference Method. Diagnostics (Basel). 2020;10:653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 361] [Article Influence: 45.1] [Reference Citation Analysis (2)] |
| 12. | Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, Castain C, Le Bail B, Chermak F, Foucher J, Laurent F, Montaudon M, De Ledinghen V. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J Hepatol. 2014;61:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 13. | Popa A, Șirli R, Popescu A, Bâldea V, Lupușoru R, Bende F, Cotrău R, Sporea I. Ultrasound-Based Quantification of Fibrosis and Steatosis with a New Software Considering Transient Elastography as Reference in Patients with Chronic Liver Diseases. Ultrasound Med Biol. 2021;47:1692-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 14. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C; Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 15. | Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, Xin Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564-3573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 16. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 924] [Article Influence: 102.7] [Reference Citation Analysis (2)] |
| 17. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 1061] [Article Influence: 151.6] [Reference Citation Analysis (1)] |
| 18. | Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, de Ledinghen V, Trinchet JC, Beaugrand M. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 19. | Fujiwara Y, Kuroda H, Abe T, Ishida K, Oguri T, Noguchi S, Sugai T, Kamiyama N, Takikawa Y. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med Biol. 2018;44:2223-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Sporea I, Bâldea V, Lupușoru R, Bende F, Mare R, Lazăr A, Popescu A, Șirli R. Quantification of Steatosis and Fibrosis using a new system implemented in an ultrasound machine. Med Ultrason. 2020;22:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 21. | Miele L, Zocco MA, Pizzolante F, De Matthaeis N, Ainora ME, Liguori A, Gasbarrini A, Grieco A, Rapaccini G. Use of imaging techniques for non-invasive assessment in the diagnosis and staging of non-alcoholic fatty liver disease. Metabolism. 2020;112:154355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 22. | Bae JS, Lee DH, Lee JY, Kim H, Yu SJ, Lee JH, Cho EJ, Lee YB, Han JK, Choi BI. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol. 2019;29:6499-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (2)] |
| 23. | Paige JS, Bernstein GS, Heba E, Costa EAC, Fereirra M, Wolfson T, Gamst AC, Valasek MA, Lin GY, Han A, Erdman JW Jr, O'Brien WD Jr, Andre MP, Loomba R, Sirlin CB. A Pilot Comparative Study of Quantitative Ultrasound, Conventional Ultrasound, and MRI for Predicting Histology-Determined Steatosis Grade in Adult Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2017;208:W168-W177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 24. | Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (3)] |
| 25. | Basavarajappa L, Baek J, Reddy S, Song J, Tai H, Rijal G, Parker KJ, Hoyt K. Multiparametric ultrasound imaging for the assessment of normal versus steatotic livers. Sci Rep. 2021;11:2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Jang JK, Lee ES, Seo JW, Kim YR, Kim SY, Cho YY, Lee DH. Two-dimensional Shear-Wave Elastography and US Attenuation Imaging for Nonalcoholic Steatohepatitis Diagnosis: A Cross-sectional, Multicenter Study. Radiology. 2022;305:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 27. | Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Yoshimasu Y, Kasai Y, Itoi T. Clinical utilization of shear wave dispersion imaging in diffuse liver disease. Ultrasonography. 2020;39:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, Tripon S, Terris B, Mallet V, Sogni P, Tanter M, Pol S. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol. 2015;62:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 29. | Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Abe M, Yoshimasu Y, Kasai Y, Sakamaki K, Hara T, Itoi T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology. 2020;296:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 30. | Sugimoto K, Lee DH, Lee JY, Yu SJ, Moriyasu F, Sakamaki K, Oshiro H, Takahashi H, Kakegawa T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Choi BI, Itoi T. Multiparametric US for Identifying Patients with High-Risk NASH: A Derivation and Validation Study. Radiology. 2021;301:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 31. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1797] [Article Influence: 599.0] [Reference Citation Analysis (2)] |
| 32. | Popa A, Bende F, Șirli R, Popescu A, Bâldea V, Lupușoru R, Cotrău R, Fofiu R, Foncea C, Sporea I. Quantification of Liver Fibrosis, Steatosis, and Viscosity Using Multiparametric Ultrasound in Patients with Non-Alcoholic Liver Disease: A "Real-Life" Cohort Study. Diagnostics (Basel). 2021;11:783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 33. | Dioguardi Burgio M, Imbault M, Ronot M, Faccinetto A, Van Beers BE, Rautou PE, Castera L, Gennisson JL, Tanter M, Vilgrain V. Ultrasonic Adaptive Sound Speed Estimation for the Diagnosis and Quantification of Hepatic Steatosis: A Pilot Study. Ultraschall Med. 2019;40:722-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8559] [Article Influence: 407.6] [Reference Citation Analysis (9)] |
| 35. | Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2833] [Cited by in RCA: 3566] [Article Influence: 324.2] [Reference Citation Analysis (0)] |
| 36. | Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 37. | Karsdal MA, Detlefsen S, Daniels SJ, Nielsen MJ, Krag A, Schuppan D. Is the Total Amount as Important as Localization and Type of Collagen in Liver Fibrosis Attributable to Steatohepatitis? Hepatology. 2020;71:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 294] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 39. | Ferraioli G, Barr RG, Berzigotti A, Sporea I, Wong VW, Reiberger T, Karlas T, Thiele M, Cardoso AC, Ayonrinde OT, Castera L, Dietrich CF, Iijima H, Lee DH, Kemp W, Oliveira CP, Sarin SK. WFUMB Guidelines/Guidance on Liver Multiparametric Ultrasound. Part 2: Guidance on Liver Fat Quantification. Ultrasound Med Biol. 2024;50:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 40. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 610] [Article Influence: 101.7] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/