Published online Jun 21, 2025. doi: 10.3748/wjg.v31.i23.106949
Revised: April 23, 2025
Accepted: May 26, 2025
Published online: June 21, 2025
Processing time: 101 Days and 5.7 Hours
The role of NLR family pyrin domain containing 3 (NLRP3) in post-endoscopic submucosal dissection (ESD) esophageal stricture remains incompletely un
To explore the effect of CEL on the prevention of esophageal stricture in rats.
NLRP3, interleukin (IL)-1β, and IL-18 mRNA levels were measured in patients’ tissues after esophageal ESD. NLRP3 expression in esophageal fibroblasts was determined using immunohistochemistry and immunofluorescence staining. Lentiviral transfection was used to induce NLRP3 overexpression and thioredoxin reductase 1 (TXNRD1) silencing. The CCK8 assay was used to determine the optimal CEL concentration. Reactive oxygen species (ROS) generation was detected via fluorescence and flow cytometry. Masson’s trichrome staining and barium esophagography were performed to assess collagen deposition and esophageal stenosis.
The mRNA levels of NLRP3 and IL-1β were higher in human tissues from the ESD resection bed than in normal esophageal mucosa. NLRP3 overexpression in primary rat esophageal fibroblasts led to high collagen 1 expression. Thus, NLRP3 participated in esophageal inflammation and tissue repair after ESD. Comparable to prednisolone, CEL significantly inhibited NLRP3 activation in vitro and in vivo, and esophageal strictures were markedly alleviated. Mechanistically, CEL upregulated TXNRD1 expression and reduced ROS production, thereby inhibiting NLRP3 expression. This effect was reversed by TXNRD1 silencing. Furthermore, TXNRD1 interacted with NLRP3 and promoted its ubiquitination.
CEL is a promising alternative therapeutic agent for the prevention of post-ESD esophageal strictures.
Core Tip: NLR family pyrin domain containing 3 (NLRP3) and interleukin-1β are increased in the endoscopic submucosal dissection resection bed of patients with esophageal neoplasms. Celastrol (CEL) prevented esophageal stricture in rats, and the effects were comparable to the positive control, prednisolone. Thioredoxin reductase 1 (TXNRD1) was increased by CEL in lipopolysaccharide plus adenosine triphosphate stimulated primary rat esophageal fibroblasts. Independent of its effect on reactive oxygen species, TXNRD1 interacts with NLRP3 and promotes its ubiquitination.
- Citation: Zhang MX, Wu C, Feng XX, Tian W, Zhao NH, Lu PP, Ding Q, Liu M. Celastrol alleviates esophageal stricture in rats by inhibiting NLR family pyrin domain containing 3 activation. World J Gastroenterol 2025; 31(23): 106949
- URL: https://www.wjgnet.com/1007-9327/full/v31/i23/106949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i23.106949
Esophageal strictures negatively affect patients' quality of life and may lead to severe complications[1]. With the development of endoscopic technology, endoscopic submucosal dissection (ESD) has become the standard therapy for superficial esophageal neoplasms in clinical practice[2]. Consequently, the incidence of esophageal strictures after endoscopic treatment for early esophageal cancer has increased, posing a major clinical challenge.
Following endoscopic therapy, the esophageal wall is prone to severe inflammation and ulceration due to irritation from food passage and exposure to digestive fluids, such as saliva, and gastric acid reflux[3]. Inflammation potently induces transforming growth factor-β signaling, activates fibroblasts, and induces tissue fibrosis[4]. Excessive fibrosis and scar formation during the healing process are the most commonly recognized causes of esophageal strictures[5-7]. To prevent esophageal strictures, inflammation at mucosal defects should be minimized to limit damage to the muscularis propria and prevent excessive fibrosis[8].
Many inflammation-associated molecules are involved in the process of injury repair. When tissue injury occurs, innate immune cells express damage-associated molecular patterns (DAMPs); DAMPs are recognized by inflammasomes and activate subsequent cascade pathways, triggering the inflammatory response[9]. NLR family pyrin domain containing 3 (NLRP3) is the most widely recognized inflammasome[10]. It has been reported that NLRP3 promotes esophageal stricture formation following ulcer healing in rats[11]. However, no studies have confirmed the expression of NLRP3 in human esophageal tissues after ESD.
Celastrol (CEL), a pentacyclic triterpenoid derived from Tripterygium wilfordii Hook F, has shown anti-inflammatory effects in several inflammatory diseases[12]. However, CEL has not been applied in esophageal stricture prevention. Here, we examined NLRP3, interleukin (IL)-1β, and IL-18 mRNA in human tissues after esophageal ESD procedure. Then, the effect of CEL was investigated in vitro and in vivo.
The protocols were approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (No. S167). From November 2023 to May 2024, 21 consecutive patients were included. Inclusion criteria was as follows: (1) Adult patients aged 18 to 60 years; (2) Willingness to comply with all study procedures; (3) Provision of written informed consent; (4) Initial endoscopic diagnosis suggesting early esophageal cancer requiring ESD treatment; and (5) Discontinuation of antiplatelet and anticoagulant medications for at least 1 week, absence of cardiovascular diseases, diabetes, hepatic or renal insufficiency, and good tolerance to anesthesia.
The ESD procedure has been described previously[13]. Following a previously published method[14], after completing the resection, three mucosal biopsies were collected from the resection bed, and from healthy mucosa which is 2 cm proximal to the resection bed, respectively, with the patients’ written informed consent, under the precondition of no obvious bleeding in the resection bed. During the first month following the resection, all patients took a double-dose proton pump inhibitors. Clinical characteristics of the patients are shown in Table 1. All esophageal biopsies were immediately fixed in 10% formalin for histological analysis or stored at -80 °C for further RNA extraction.
| Label | Gender | Age, years | Lesion number | Longitudinal diameter (cm) | Circumferential diameter (cm) | Histopathology |
| 1 | Female | 53 | 3 | 11 | 4 | Carcinoma in situ |
| 2 | Female | 46 | 4 | 3 | 2 | Carcinoma in situ |
| 3 | Male | 54 | 2 | 9 | 2 | HGIN |
| 4 | Female | 53 | 1 | 2 | 2 | HGIN |
| 5 | Female | 47 | 5 | 9 | 5 | Squamous carcinoma (M2) |
| 6 | Male | 55 | 1 | 6 | 4 | Squamous carcinoma (M2) |
| 7 | Male | 56 | 1 | 6 | 5 | Squamous carcinoma (M2) |
| 8 | Male | 45 | 1 | 3 | 4 | carcinoma in situ |
| 9 | Male | 57 | 1 | 3 | 3 | carcinoma in situ |
| 10 | Male | 56 | 1 | 3 | 3 | HGIN |
| 11 | Female | 55 | 1 | 3 | 3 | Squamous carcinoma (M3) |
| 12 | Male | 53 | 3 | 3 | 2 | Squamous carcinoma (M2) |
| 13 | Female | 57 | 1 | 3 | 2.5 | Squamous carcinoma (M2) |
| 14 | Male | 65 | 1 | 2 | 2.5 | HGIN |
| 15 | Male | 67 | 1 | 3 | 2 | HGIN |
| 16 | Male | 62 | 2 | 4 | 3 | Squamous carcinoma (M3) |
| 17 | Male | 71 | 1 | 3 | 2 | Squamous carcinoma (M2) |
| 18 | Male | 56 | 1 | 3 | 3 | carcinoma in situ |
| 19 | Male | 62 | 1 | 5 | 3 | Squamous carcinoma (M2) |
| 20 | Male | 61 | 1 | 5 | 2 | Squamous carcinoma (M3) |
| 21 | Male | 73 | 1 | 3 | 2 | Squamous carcinoma (M2) |
Paraffin-embedded human and rat tissue sections complete antigen retrieval and blocking. Then, sections were incubated with primary antibodies (NLRP3, SAB5700723, Sigma-Aldrich) overnight and secondary antibody for 1 hour. Sub
Paraffin-embedded human and rat tissue sections were dewaxed and hydrated. After antigen retrieval, the sections were further incubated overnight with primary antibodies against vimentin (SAB5700070, Sigma-Aldrich) and NLRP3 (SAB5700723, Sigma-Aldrich), and then stained with Alexa Fluor 647 and Alexa Fluor 488-conjugated secondary antibodies. DAPI was used for nuclear counterstaining.
Differences in colocalization between the control and model groups were analyzed by comparing the Pearson correlation coefficient for each two-channel comparison[15]. It is calculated using Image J software.
Colocalization of thioredoxin reductase 1 (TXNRD1) (sc-28321, Santa Cruz Biotechnology) and NLRP3 (SAB5700723, Sigma-Aldrich) in primary rat esophageal fibroblasts (REFs) was also detected by immunofluorescence (IF) staining.
Hematoxylin-eosin (HE) staining and Masson trichrome staining were used to detect the presence of esophageal fibroblasts and the degree of fibrosis. Rat esophageal tissues on day 9 were embedded in paraffin and sectioned at a thickness of 3 μm for HE and Masson trichrome staining. For HE staining, the fibroblast cell count was determined using Image J software. For Masson’s staining, the degree of fibrosis in the stained sections was quantitatively evaluated using Image-Pro Plus 6.0. All quantitative analyses were performed by at least two experimenters who were blinded to the subgroup details.
The sample size was identified by resource equation. Due to our study design, 4-6 rats were needed in each group. Rats were numbered and divided into 5 groups according to a randomized blocked design (assigned using R package “randomizr”).
Specific-pathogen-free male Sprague-Dawley rats (200-230 g, 6 weeks old) (Shulaibao, Wuhan, China) were fasted for 12 hours and subjected to laparotomy under inhalation anesthesia with isoflurane (4.0 L/minutes for induction, and 2.0 L/minutes for maintenance). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Huazhong University of Science and Technology (No. 4016).
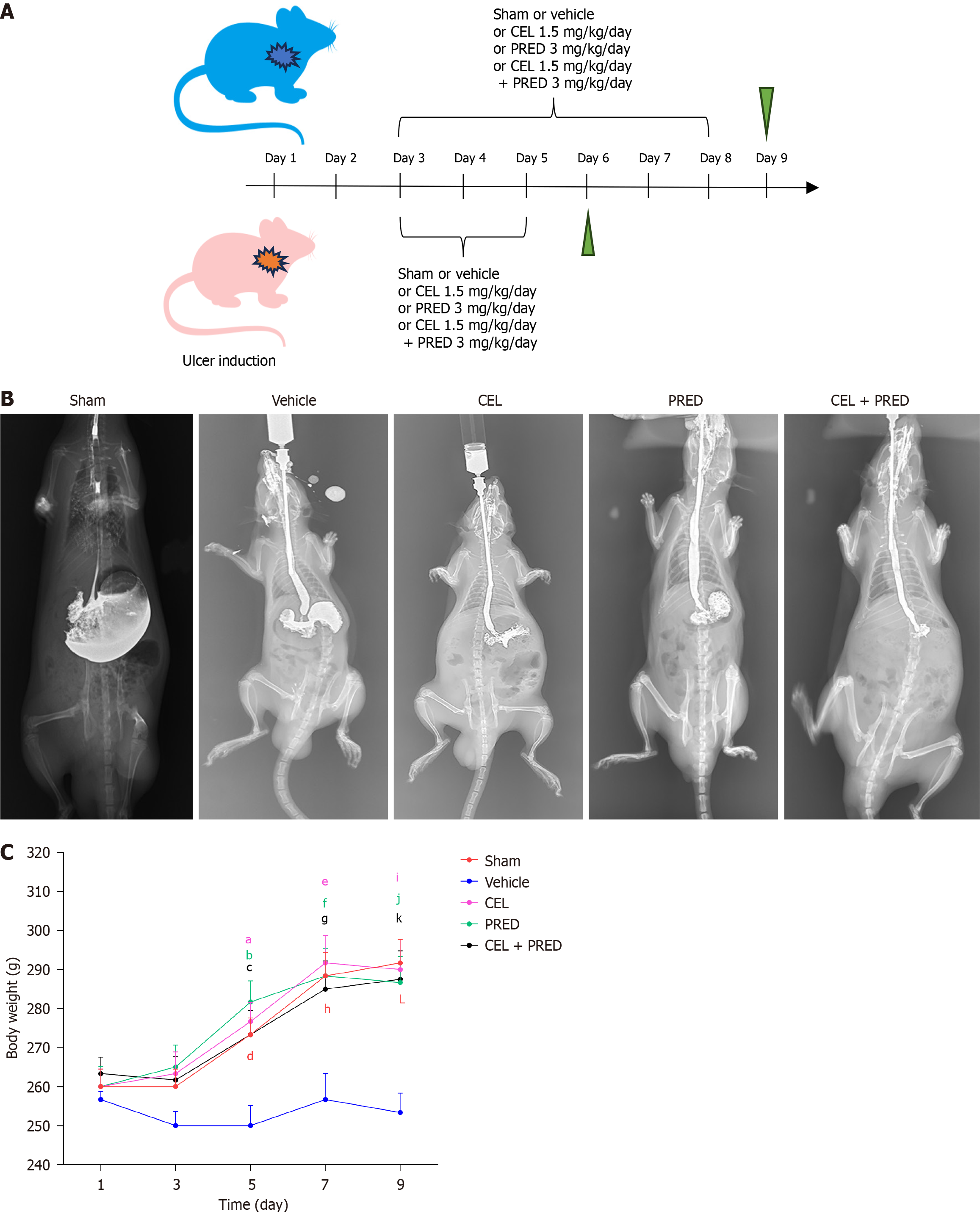
Esophageal ulcers were induced as previously described[11]. Rats that underwent laparotomy but not induction of esophageal ulcers were used as negative controls for analysis. The rats were divided into five groups and given the following reagents via gastric lavage: (1) Sham group: 0.5% sodium carboxymethyl cellulose; (2) Vehicle group: 0.5% sodium carboxymethyl cellulose; (3) CEL group: CEL (1.5 mg/kg/day; S1290, Selleck) suspended in vehicle; (4) Prednisolone (PRED) group (as the positive control): PRED (3 mg/kg/day; HY-17463, MedChemExpress) suspended in vehicle; and (5) Combination therapy group: CEL (1.5 mg/kg/day) plus PRED (3 mg/kg/day) suspended in vehicle. All reagents were administered once daily starting on day 3. The rats were euthanized on day 6 and day 9, to analyze inflammation and fibrosis, respectively.
Body weight was recorded on days 1, 3, 5, 7, and 9. To assess esophageal stricture, barium esophagography was performed on day 9 under intraperitoneal anesthesia with 2% tribromoethanol by a radiologist who was blinded to the subgroup details.
Every effort was made to minimize their suffering. Rats were acclimatized to laboratory conditions for 2 weeks prior to experimentation. Food and water were sufficient. Intragastric gavage administration was carried out with No. 16 straight gavage needles. All rats were euthanized by 2% tribromoethanol overdose for tissue collection.
Primary REF cultures were established from Sprague-Dawley rats (3-5 days old), as previously described[16]. Immunohistochemistry (IHC) was performed to evaluate the expression of vimentin (SAB5700070, Sigma-Aldrich) and cytokeratin 19 (ab220193, Abcam). Passages 3-5 of primary esophageal fibroblasts were used in subsequent experiments.
REFs were divided into five groups: (1) Control group: PBS was used as negative control; (2) NLRP3 activation group: The NLRP3 inflammasome primer, lipopolysaccharide (LPS) (1 μg/mL, L2630, Sigma-Aldrich) for 6 hours and followed by the activator, adenosine triphosphate (ATP) (5 mmol/L, HY-B2176, MedChemExpress) for 30 min to ensure activation of the NLRP3 inflammasome; (3) CEL group: CEL (200 nM, S1290, Selleck) for 1 hour followed by the LPS plus ATP stimulation; (4) PRED group: PRED (1 μM, HY-17463, Medchemexpress) for 1 hour followed by the LPS plus ATP stimulation; and (5) Combination therapy group: CEL (200 nM, S1290, Selleck) and PRED (1 μM, HY-17463, MedChemExpress) for 1 hour followed by the LPS plus ATP stimulation.
To assess the effect of CEL on primary REFs, CCK8 (HY-K0301, MedChemExpress) assay was performed. Cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM) containing PBS, dimethyl sulfoxide, or CEL (200 nM, 1 μM, 5 μM, and 10 μM) for 24, 48, and 72 hours. Subsequently, the CCK8 solution was added to each well for 4 hours, and absorbance at 450 nm was measured.
NLRP3 overexpression was achieved via lentivirus transfection (pLent-EF1a-FH-CMV-GFP-P2A-Puro, purchased from Weizhen Biology, Wuhan). TXNRD1 knockdown (HBLV-r-TXNRD1-shRNA1-ZsGreen-Puro) and overexpression (HBAD-Adeasy-r-TXNRD1-3xflag-EGFP) were achieved via lentivirus transfection (purchased from Hanheng Biology, Wuhan). Primary REFs were seeded and incubated with either lentiviral particles or control lentiviral particles along with polybrene in the DMEM medium (multiplicity of infection = 150) when the cells reached 30% confluence. Transfection efficiency was verified after 72 hours by fluorescence. In the primary REFs, the aforementioned lentivirus showed a transfection efficiency of over 90%.
Reactive oxygen species (ROS) analysis kit (HY-D0940, MedChemExpress) was used to measure the ROS levels in each group after different treatments. Primary REFs were divided into three groups: (1) Control group: PBS as negative control; (2) LPS group: LPS (1 μg/mL) for 6 hours and followed by ATP (5 mmol/L) for 30 minutes; and (3) CEL + LPS group: CEL (200 nM) for 1 hour followed by the LPS plus ATP stimulation. Cells were incubated with DCFH-DA and observed under a fluorescence microscope. The fluorescence intensity of ROS in each group was quantified. For flow cytometry analysis, after incubation with DCFH-DA, cells were digested and resuspended in PBS.
TXNRD activity was measured using the thioredoxin reductase assay kit (BC1155, Solarbo) following the manufacturer’s protocol. Primary REFs were divided into three groups: (1) Control group: PBS as negative control; (2) LPS group: LPS (1 μg/mL) for 6 hours and followed by ATP (5 mmol/L) for 30 minutes; and (3) CEL + LPS group: CEL (200 nM) for 1 hour followed by the LPS plus ATP stimulation. TXNRD activity was measured through the absorbance at 412 nm.
Protein samples were transferred onto a PVDF membrane. The membrane was blocked for 1 hour. After washing with Tris-buffered saline containing Tween-20, the membrane was incubated overnight at 4 °C with the following primary antibodies: NLRP3 (ab263899, Abcam), IL-1β (ab283818, Abcam), Caspase1 (2225, Cell Signaling Technology), nuclear factor erythroid 2-related 2 (Nrf2) (A21176, ABclonal), TXNRD1 (11117-1-AP, Proteintech), GAPDH (ET1601-4, Huabio) and Collagen 1 (COL1) (14695-1-AP, Proteintech).
Total RNA was extracted from tissues and cultured cells using TRIzol reagent, followed by reverse transcription (RR037A, TaKaRa). PCR was conducted in a reaction volume of 20 μL using PCR mix (RR820A, TaKaRa), and PCR amplification was performed using a PCR cycler (Thermofisher). Primer sequences are listed in Table 2.
| Gene name | Forward primer | Reverse primer | |
| Rat | ACTB | TTCGCCATGGATGACGATATC | TAGGAGTCCTTCTGACCCATAC |
| NLRP3 | GACTGCCGTCTATGTCTTTTTC | GAAGCTGTAAAATCTCTCGCAG | |
| IL1β | AACTGTGAAATAGCAGCTTTCG | CTGTGAGATTTGAAGCTGGATG | |
| COL1 | AAAGATGGACTCAACGGTCTC | CAGGAAGCTGAAGTCATAACCA | |
| Human | ACTB | CCAGAGACAGTTATGCGAATTG | TTCTGGGAATTCGGGAACATAA |
| NLRP3 | GGCAAATTCGAAAAGGGGTATT | CTGATTTGCTGAGAGATCTTGC | |
| IL1β | GCCAGTGAAATGATGGCTTATT | AGGAGCACTTCATCTGTTTAGG | |
| IL18 | GCTGAAGATGATGAAAACCTGG | CAAATAGAGGCCGATTTCCTTG |
Cells in the control and TXNRD1-overexpression groups were collected and lysed with lysis buffer. The supernatant fractions were collected and incubated with control immunoglobulin G or primary antibody overnight, followed by incubation with resuspended Protein A/G beads (HY-K0202, MedChemExpress) to pull down bound proteins. Immunoprecipitated proteins were separated by SDS-PAGE after western blotting. The following primary antibodies were used: Anti-ubiquitin (3936S, Cell Signaling Technology), anti-NLRP3 (ab263899, Abcam), anti-TXNRD1 (sc-28321, Santa Cruz Biotechnology), anti-GFP (AE078, Abclonal) and anti-GAPDH (ET1601-4, Huabio).
Statistical significance was determined using the Student’s t-test (two groups) or One-way ANOVA with Dunnett’s multiple comparisons as a post hoc test (three or more groups) for data with a normal distribution. For data without normal distribution or where there was a violation of the assumption of homogeneity of variance, the Mann-Whitney U test (unpaired samples) or the Wilcoxon signed-rank test (paired samples) was performed; for three or more groups, the Kruskal-Wallis nonparametric ANOVA was used. Differences with P values < 0.05 were considered significant. The statistical methods of this study were reviewed by a biomedical statistician from the School of Public Health, Tongji Medical College, Huazhong University of Science and Technology. Statistical analysis was performed in GraphPad Prism 10.0.2 software.
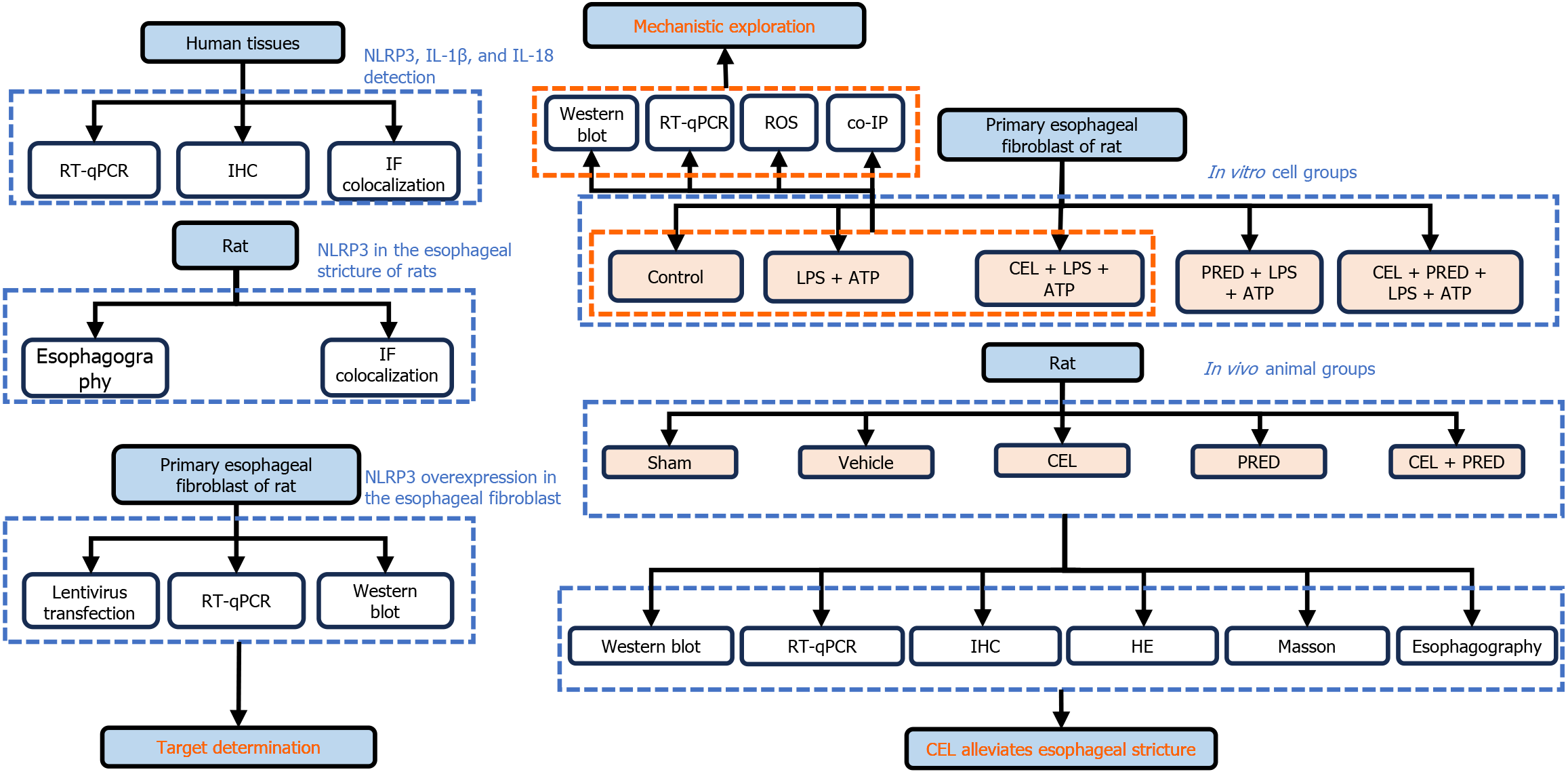
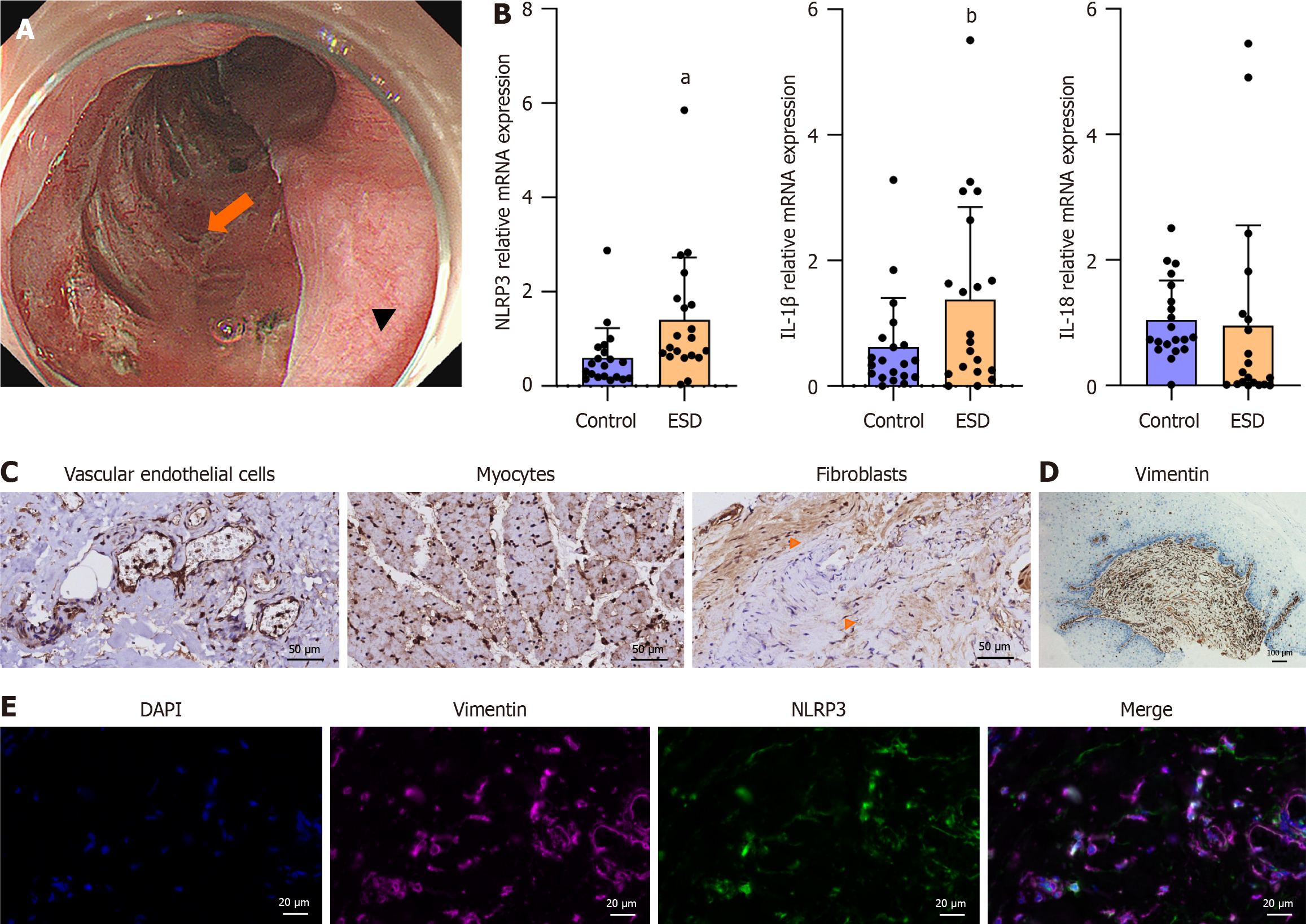
The study design was shown in Figure 1. Between November 2023 and May 2024, 21 patients who underwent planned esophageal endoscopic resection for superficial esophageal neoplasms agreed to participate in the study. Tissues were acquired as Figure 2A. The clinical and histological characteristics of the patients are presented in Table 1. The mRNA levels of NLRP3 and its downstream target IL-1β and IL-18 were measured. The results demonstrated that the mRNA levels of NLRP3 and IL-1β were higher in the ESD resection bed than in the healthy mucosa, but IL-18 was not elevated in the ESD resection bed (Figure 2B).
Owing to the mucosal loss status of the ESD resection bed, NLRP3 was detected in fibroblasts, myocytes, and vascular endothelial cells (Figure 2C). Activated fibroblasts play an important role in esophageal strictures[3,5,6]. Consistent with this finding, fibroblast (vimentin-positive spindle-like cell) is the main component in the submucosa of a patient with post-ESD esophageal stricture (Figure 2D). The expression of NLRP3 in fibroblasts was further confirmed by the colocalization of NLRP3 and vimentin in human tissues (Figure 2E).
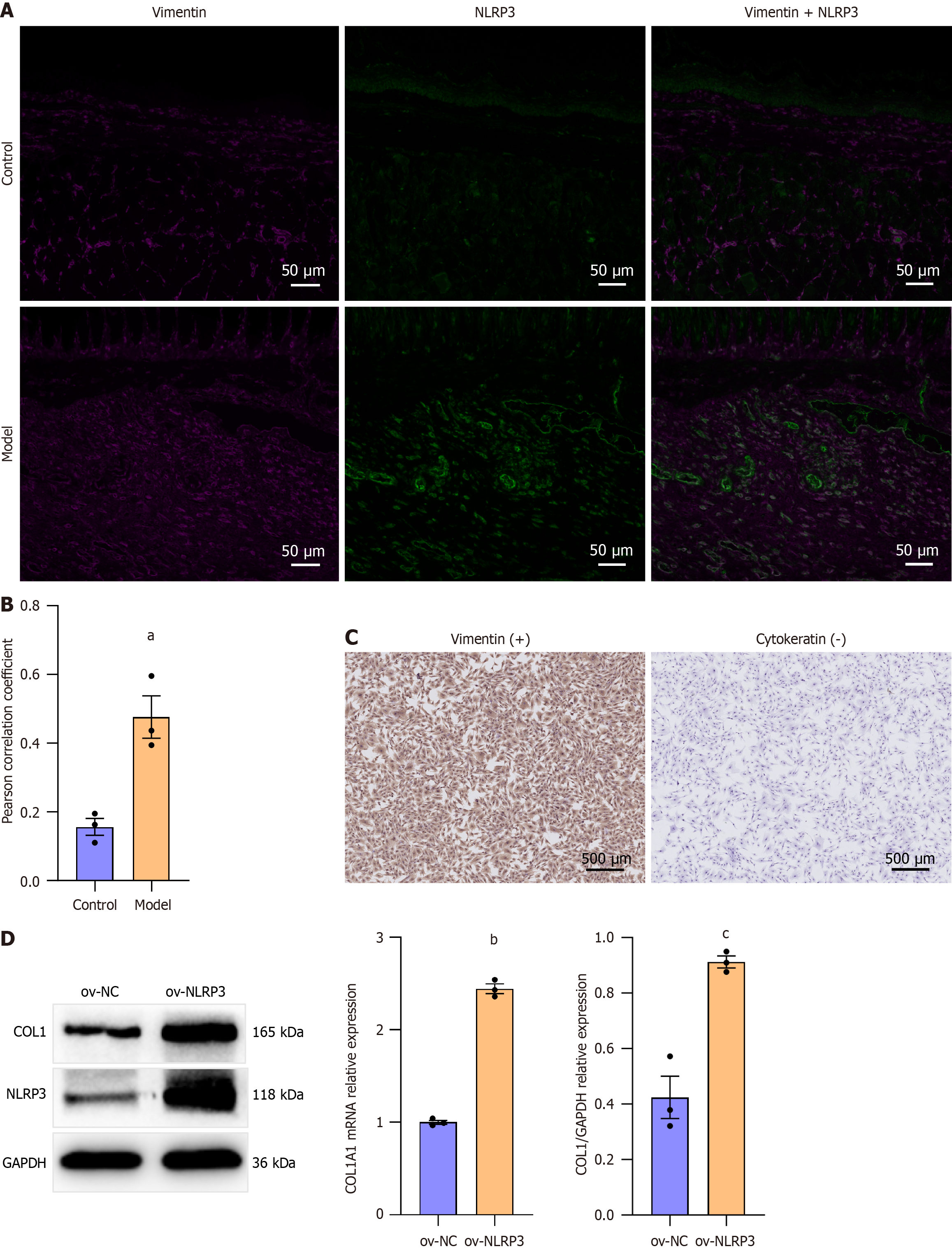
To determine the role of NLRP3 in esophageal stricture formation, an esophageal stricture rat model was established as described in Method. The Pearson correlation coefficient revealed significantly increased colocalization of NLRP3 and vimentin in esophageal scar tissue after ulcer healing compared to the normal esophagus in rats (Figure 3A and B). Then, we isolated primary REFs and achieved NLRP3 overexpression by lentivirus transfection. IHC identified the cells as fibroblasts, not epithelial cells[16] (Figure 3C). NLRP3 overexpression significantly increased the mRNA and protein levels of COL1 in primary REFs (Figure 3D), indicating that NLRP3 activation in esophageal fibroblasts promotes fibrosis. Recently, the NLRP3 inflammasome was shown to connect a persistent proinflammatory cytokine milieu with the progression of structural remodeling by activated fibroblasts[17]. These results suggest that NLRP3 could be a potential target for post-ESD esophageal stricture prevention.
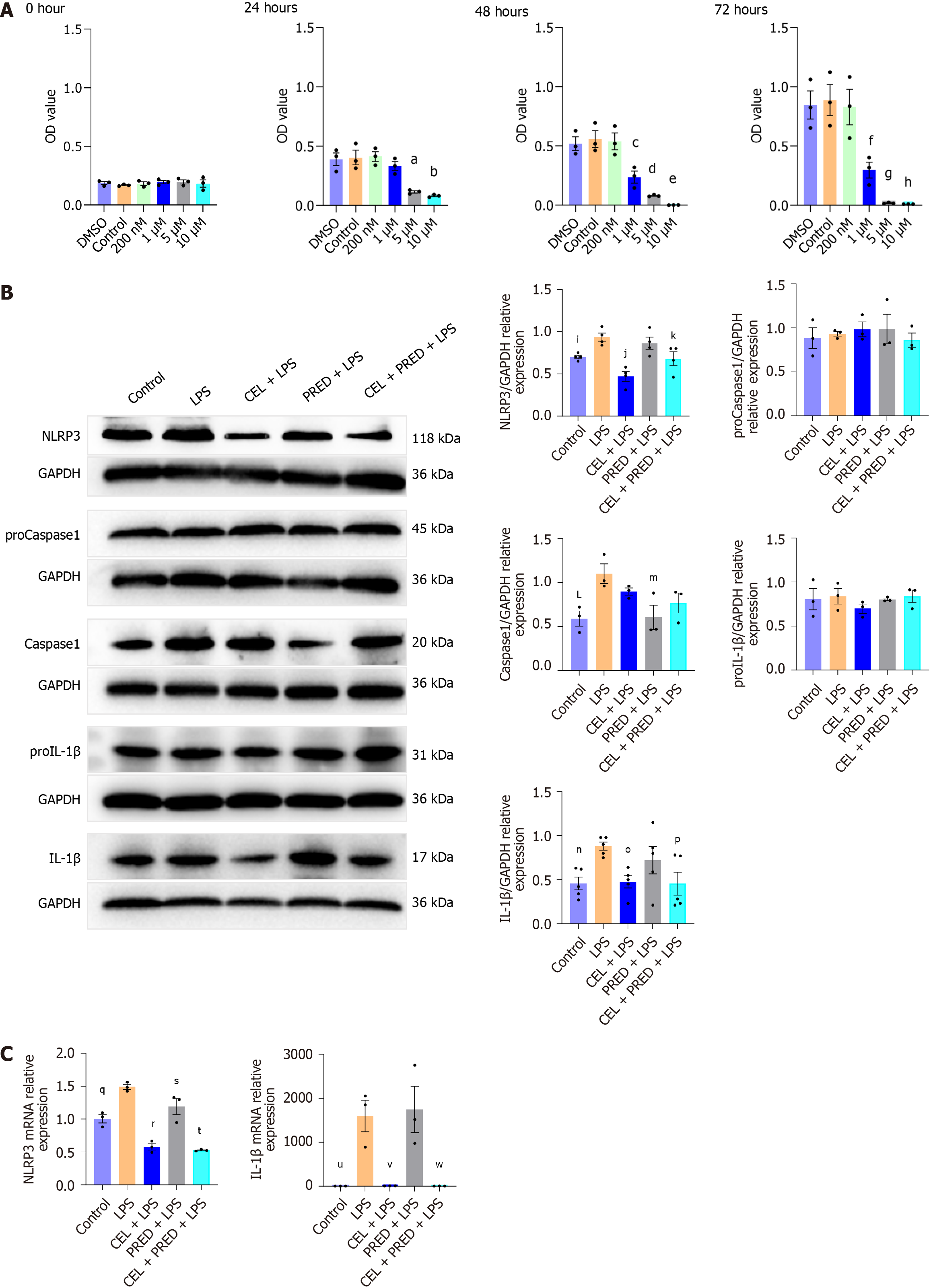
LPS and ATP were used to initiate NLRP3 inflammasome activation. PRED is identified as a positive control as the standard prophylaxis for esophageal strictures. First, the results of the CCK-8 assay showed that 200 nM CEL did not induce any obvious cytotoxicity until 72 horus (Figure 4A). The optimal PRED dose was determined in a preliminary experiment. The two-step challenge (LPS followed by ATP) significantly increased the protein levels of NLRP3, caspase-1 p20, and cleaved IL-1β. Pretreatment with CEL remarkably inhibited the increased protein levels of NLRP3 and cleaved IL-1β. As a positive control, PRED exhibited limited inhibitory effects on NLRP3 and cleaved IL-1β, but its efficacy was enhanced in the combination treatment (Figure 4B). The mRNA levels of NLRP3 and IL-1β were also reduced by CEL and combination treatment (Figure 4C). Thus, CEL has shown an excellent effect on NLRP3 in vitro.
Targeting NLRP3, the effect of CEL on esophageal stricture prevention was explored in vivo. Based on the results of our pretreatment experiments, we used an animal experimental procedure similar to that described previously[11] (Figure 5A). Barium esophagography was performed to evaluate the extent of esophageal stricture on day 9. It revealed esophageal stenosis with a bird’s beak sign in the vehicle group compared to the sham group, with significant alleviation in the drug-treatment group (representative image shown in Figure 5B).
The body weight of the rat was recorded until day 9, correlating with the extent of esophageal stricture. The analysis showed that body weight in the vehicle group decreased significantly compared to those of the sham group from day 5 to day 9. CEL, PRED, and CEL + PRED treatment significantly mitigated body weight loss associated with esophageal strictures during this period, suggesting that drug treatment improves food intake restriction in rats with esophageal strictures. On day 9, the average body weight of the CEL group was the largest of the three drug-treatment groups (Figure 5C).
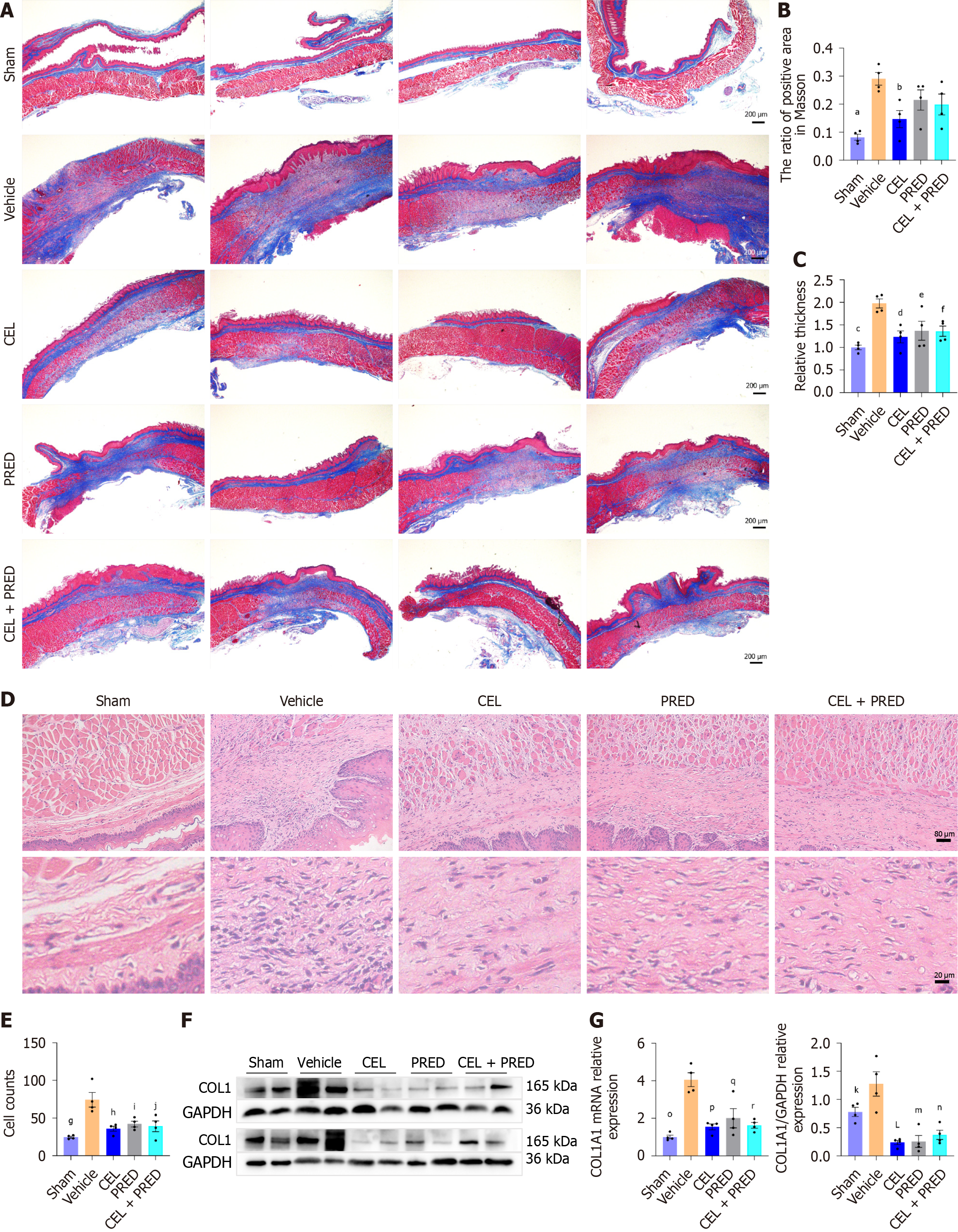
Masson’s trichrome staining showed that in the sham group, the submucosal layer was thin with a soft wall and a linear distribution of collagen fibers. However, in the vehicle group, the esophageal wall was thickened and stiff with numerous collagen fibers in the submucosal and muscularis layers. Stiffness and collagen deposition were improved in the drug-treatment group (CEL, PRED, and CEL + PRED) (Figure 6A).
Quantitative analysis of collagen deposition showed significant increase in the vehicle group than the sham group, consistent with the worse stenosis on the X-ray. There was marked reduction in the CEL group compared with the vehicle group. Although PRED and CEL + PRED also exhibited inhibitory effects, these were not statistically significant (Figure 6B). CEL also reduced the esophageal wall thickness more significantly than the PRED and CEL + PRED groups (Figure 6C).
HE staining showed that fibroblasts in the drug treatment groups were distributed more sparsely than those in the vehicle group (Figure 6D and E). Besides, the mRNA and protein levels of COL1 were significantly reduced by the drug treatments (Figure 6F and G).
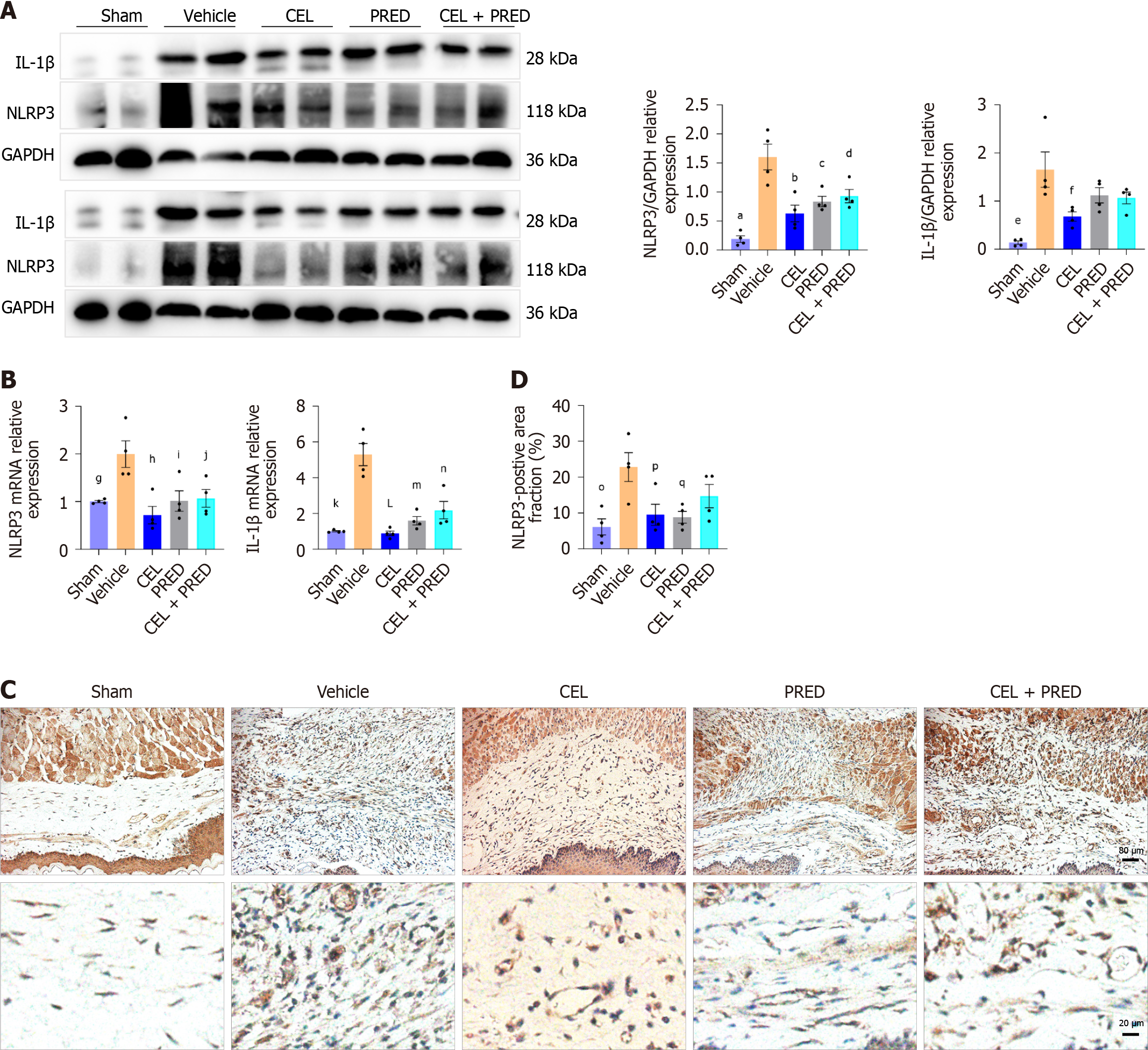
The suppression of inflammation contributed to fibrosis alleviation. To assess the inhibitory effect of CEL on NLRP3 activation in vivo, the protein and mRNA levels of NLRP3 and its downstream effector IL-1β were examined in esophageal ulcer tissues on day 6. As shown in Figure 7A and B, mRNA and protein levels of NLRP3 and IL-1β were significantly higher in the vehicle group than in the sham group, whereas the treatment of CEL significantly reduced the mRNA and protein levels. PRED and CEL + PRED exhibited weaker inhibitory effects than CEL. In addition, CEL and PRED treatment significantly reduced the NLRP3-positive area compared to the vehicle group (Figure 7C and D).
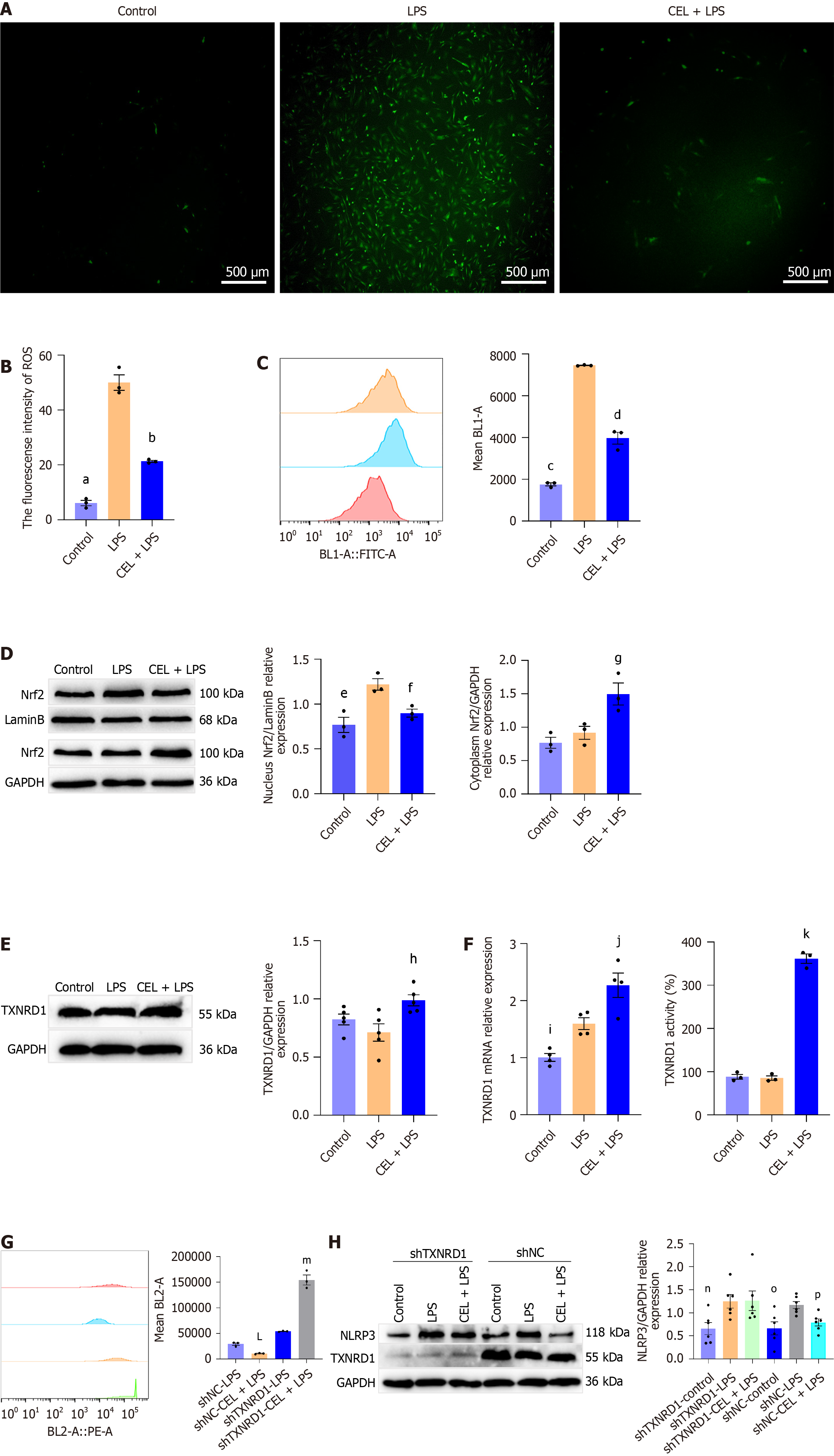
Then we investigated the mechanism of CEL on NLRP3 activation. ROS, byproducts of oxidative stress, act as secondary signals that promote NLRP3 activation[18]. ROS fluorescence was performed and fluorescence intensity was remarkably lower in the CEL-treated group than in the LPS + ATP-induced group (Figure 8A and B). These findings were further supported by flow cytometry analysis (Figure 8C).
Subsequently, we examined whether CEL counteracted ROS generation by activating the Nrf2 antioxidant pathway in primary REFs. We found that LPS plus ATP induced a two-fold increase in Nrf2 nuclear translocation, whereas CEL pretreatment (200 nM for 1 hour) did not further enhance Nrf2 nuclear translocation (Figure 8D).
Considering the top 20 differentially expressed genes in CEL reported previously[19] and negative feedback effect, we investigated the mRNA and protein levels of one of the downstream targets of Nrf2, TXNRD1. The results showed that CEL significantly increased TXNRD1 mRNA and protein expression compared with those of the LPS plus ATP group. Besides, the TXNRD1 activity was higher in the CEL group than in others (Figure 8E and F).
To determine whether TXNRD1 plays a key role in CEL-mediated ROS alleviation and NLRP3 inhibition, we performed rescue experiments using TXNRD1 shRNA. TXNRD1 inhibition abrogated the CEL-induced reduction in ROS levels (Figure 8G) and NLRP3 protein expression (Figure 8H).
In addition to regulating ROS production, TXNRD1 altered protein stability by catalyzing the modification of protein substrates[20]. Thus, the interaction between TXNRD1 and NLRP3 in primary REFs was investigated.
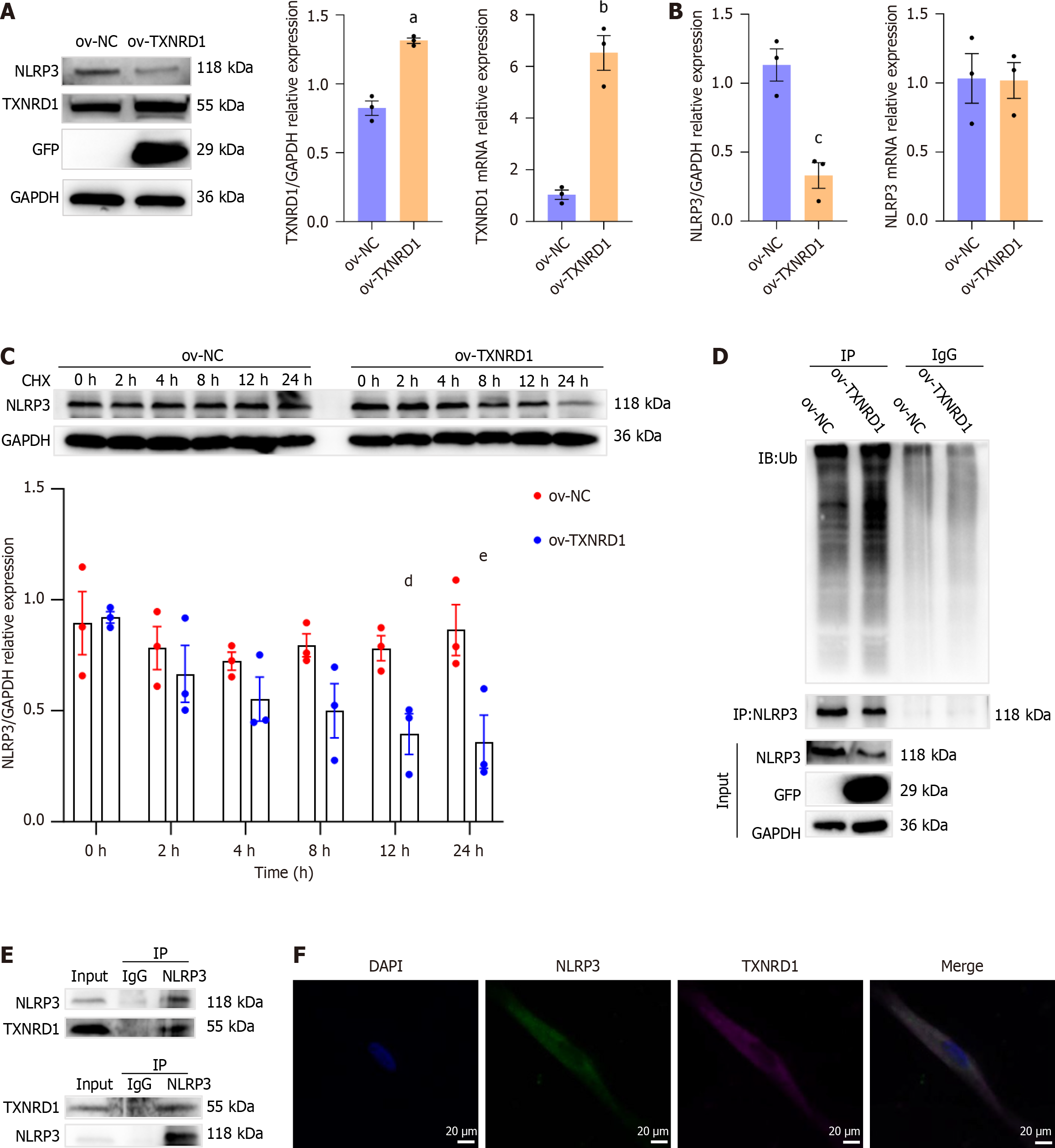
TXNRD1 overexpression was successfully induced in primary REFs using lentiviral particles (Figure 9A). We first examined NLRP3 mRNA and protein levels in control and TXNRD1-overexpressing cells. The results showed that while TXNRD1 overexpression did not affect NLRP3 mRNA levels, it significantly reduced NLRP3 protein levels (Figure 9B). Following cycloheximide treatment for 12 hours and 24 hours, NLRP3 protein levels in TXNRD1-overexpressing cells were significantly lower than those in the control group (Figure 9C), indicating that TXNRD1 overexpression promoted NLRP3 degradation.
Ubiquitination facilitates NLRP3 degradation and inhibits NLRP3 activation[21]. We further explored whether CEL-induced TXNRD1 upregulation could suppress NLRP3 levels via the ubiquitin-proteasome system. We then investigated NLRP3 ubiquitination following MG132 treatment and found that TXNRD1 overexpression accelerated NLRP3 ubiquitination (Figure 9D). To identify the interaction between TXNRD1 and NLRP3, co-immunoprecipitation was performed and the results demonstrated that TXNRD1 co-immunoprecipitated with NLRP3 (Figure 9E). IF staining confirmed that TXNRD1 was colocalized with NLRP3 (Figure 9F). These results indicate an interaction between TXNRD1 and NLRP3.
Consequently, TXNRD1 upregulation not only inhibits ROS production but also enhances NLRP3 ubiquitination, thereby suppressing NLRP3 activation.
Esophageal strictures associated with submucosal fibrosis often develop during the healing of post-ESD ulcers, particularly when the resection area extends to approximately 75% of the circumference[2]. Owing to its dual anti-inflammatory effects, oral or local steroid injection has been recommended to prevent esophageal stenosis following ESD. However, severe complications, such as perforation, immunosuppression, delayed ulcer healing, and osteoporosis, often occur[22], and the incidence of stenosis remains between 10% and 45% despite prophylactic measures[2]. Therefore, alternative treatments should be explored.
Inflammation at mucosal defects should be minimized to prevent excessive fibrosis and stricture formation[8]. To clarify the inflammatory response in the esophagus at an early stage after endoscopic treatment, we examined the activation of inflammatory pathways in the resection bed immediately after ESD for superficial esophageal neoplasms. Increased NLRP3 and IL-1β mRNA levels were detected in the submucosal tissues. In contrast, IL-18 mRNA level was not significantly different compared to normal mucosa. Differences in the expression trends of IL-18 and IL-1β have been reported previously[23,24], but the mechanisms have not been elucidated.
IL-18 has been reported to present dual functions in chronic gut inflammation[25,26]. In a mouse model of acute colitis, IL-18 had an anti-inflammatory role, promoting epithelial barrier repair and maintaining intestinal homeostasis[27]. Butyrate-producing gut bacteria may aid in mucosal healing in inflammatory bowel disease by increasing IL-18 expression[28]. A previous study reported that IL-18 signaling activity is lower in patients with Barrett’s esophagus and esophageal adenocarcinoma[29]. However, the role of IL-18 in esophageal diseases has not been clarified due to the scarcity of relevant studies. Given the protective effect of IL-18 against epithelial damage, we hypothesized that IL-18 may affect more than the single synergistic pro-inflammatory effect with IL-1β in esophageal tissues after ESD. Further exploration is warranted on this topic.
Upon exposure to digestive juices and thermal injury, we observed positive NLRP3 expression in multiple cell types in the submucosal space. Fibroblasts are central to scarring and fibrosis in post-ESD esophageal strictures[3,5,7]. However, their potential role in sensing tissue damage by pattern recognition via the inflammasome in the ESD resection bed remains unexplored. NLRP3 activation in fibroblasts has been reported in the pathology of several fibrotic diseases[30,31]. In the context of esophageal fibrosis, our study demonstrated that NLRP3 overexpression in primary REFs increased collagen deposition in vitro and that NLRP3 colocalization with fibroblasts was elevated in the rat model of esophageal stricture. These findings establish a link between NLRP3 activation and esophageal stricture. Thus, targeting NLRP3 activation in esophageal fibroblasts may offer a potential strategy for preventing stricture formation.
CEL alleviates fibrosis through NLRP3 inhibition in several fibrotic diseases[32,33]. Based on this, we evaluated the suppressive effects of CEL on NLRP3 activation both in vitro and in vivo. Our results showed that CEL significantly inhibited NLRP3 activation and IL-1β secretion in primary REFs. Moreover, CEL significantly alleviated NLRP3 acti
However, despite its great efficacy, the clinical application of CEL is limited due to its poor oral utilization and water solubility[33]. It also has a rather narrow dosage window, with severe toxicity reported in a rat arthritis model at doses higher than 2.5-5 mg/kg/day[34]. In our study pre-test, we found that CEL had significant toxicity when administered intraperitoneally at 1 mg/kg/day, whereas gastric gavage markedly attenuated this toxicity in rats, similar to a previous report[35]. As the duration of CEL treatment was only 10 days in this study, it is unclear whether a longer duration would induce significant cumulative toxicity in vivo. Therefore, although CEL has great potential as an alternative treatment for patients who cannot tolerate steroid treatment, the preventive effect of CEL must be validated in large animal models (e.g. porcine or canine models of esophageal ESD). Whether CEL toxicity can be ameliorated by topical application or combination therapy requires further investigation.
Inhibition of ROS production partially inhibits the ROS-dependent activation of NLRP3[36]. Among the numerous ROS-associated signaling pathways, the Nrf2 signaling pathway has garnered most attention[37]. We found that LPS induced an increase in Nrf2 nuclear translocation, but CEL pretreatment did not enhance this effect. Interestingly, TXNRD1 expression and activity were increased by CEL.
TXNRD1 is an endogenous antioxidant enzyme that regulates redox homeostasis and protects DNA from oxidative stress-associated damage[38]. TXNRD1 knockdown using shRNA blocked CEL-induced ROS reduction and NLRP3 inhibition, confirming that TXNRD1 is essential for CEL-mediated ROS suppression and NLRP3 inhibition. ROS could switch on and off diverse signaling pathways including the Nrf2/Keap1, PI3K/AKT, and MAPK pathways[39]. Thus, the observed reduction in Nrf2 nuclear translocation under CEL treatment may be a secondary effect resulting from ROS suppression.
Independent of its enzymatic activity, we identified a novel regulatory function of TXNRD1 in NLRP3 ubiquitination and, for the first time, demonstrated ubiquitin-proteasome degradation of NLRP3 in primary REFs. We hypothesized that TXNRD1 alters the protein stability of NLRP3 by their interaction, but the specific mechanism requires further explo
This study demonstrates that CEL has significant potential for preventing post-ESD esophageal strictures by reducing collagen deposition and esophageal fibrosis in rats. It may serve as an alternative treatment for patients in whom steroid prophylaxis is ineffective. In addition, our study provides a more nuanced understanding of the interplay between TXNRD1 and ubiquitination.
We thank the Experimental Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China for their support.
| 1. | Huang Q, Zhong J, Yang T, Li J, Luo K, Zheng Y, Yang H, Fu J. Impacts of anastomotic complications on the health-related quality of life after esophagectomy. J Surg Oncol. 2015;111:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 281] [Reference Citation Analysis (0)] |
| 3. | Honda M, Nakamura T, Hori Y, Shionoya Y, Nakada A, Sato T, Yamamoto K, Kobayashi T, Shimada H, Kida N, Hashimoto A, Hashimoto Y. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy. 2010;42:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Iwata K, Mikami Y, Kato M, Yahagi N, Kanai T. Pathogenesis and management of gastrointestinal inflammation and fibrosis: from inflammatory bowel diseases to endoscopic surgery. Inflamm Regen. 2021;41:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Nonaka K, Miyazawa M, Ban S, Aikawa M, Akimoto N, Koyama I, Kita H. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013;13:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Honda M, Kobayashi H, Nakayama Y, Kawamura H, Todate Y, Matsunaga R, Yamaguchi H, Hamada K. The mechanism of esophageal stricture after endoscopic resection: Histological and biomechanical evaluation in a canine model. Ann Cancer Res Ther. 2017;25:30-37. [DOI] [Full Text] |
| 7. | Nonaka K, Ban S, Ryozawa S. Strictures after endoscopic submucosal dissection of the esophagus: Are the histopathological findings the same between human and porcine models? Dig Endosc. 2019;31:106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Duan Y, Jia W, Liang Y, Zhang X, Yang Z, Yang Q. Progress in the treatment and prevention of esophageal stenosis after endoscopic submucosal dissection. Clin Res Hepatol Gastroenterol. 2024;48:102290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 10. | Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 2625] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 11. | Hirano S, Higashimori A, Nagami Y, Nadatani Y, Tanigawa T, Ominami M, Fukunaga S, Otani K, Hosomi S, Tanaka F, Kamata N, Taira K, Watanabe T, Fujiwara Y. Pirfenidone prevents esophageal stricture by inhibiting nucleotide binding oligomerization domain like receptor protein 3 inflammasome activation. J Gastroenterol Hepatol. 2022;37:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Astry B, Venkatesha SH, Laurence A, Christensen-Quick A, Garzino-Demo A, Frieman MB, O'Shea JJ, Moudgil KD. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol. 2015;157:228-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Nagami Y, Ominami M, Shiba M, Sakai T, Fukunaga S, Sugimori S, Otani K, Hosomi S, Tanaka F, Taira K, Kamata N, Yamagami H, Tanigawa T, Watanabe T, Ishihara T, Yamamoto K, Fujiwara Y. Prediction of esophageal stricture in patients given locoregional triamcinolone injections immediately after endoscopic submucosal dissection. Dig Endosc. 2018;30:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Barret M, Doridot L, Le Gall M, Beuvon F, Jacques S, Pellat A, Belle A, Abou Ali E, Dhooge M, Leblanc S, Camus M, Nicco C, Coriat R, Chaussade S, Batteux F, Prat F. Mechanisms of esophageal stricture after extensive endoscopic resection: a transcriptomic analysis. Endosc Int Open. 2023;11:E149-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Wennström M, Schultz N, Gallardo PM, The Netherlands Brain Bank, Serrano GE, Beach TG, Bose S, Hansson O. The Relationship between p-tau217, p-tau231, and p-tau205 in the Human Brain Is Affected by the Cellular Environment and Alzheimer's Disease Pathology. Cells. 2024;13:331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Hong C, Zhuang H, Cai B, Chen J, Huang S, Fang T. β-Elemene Attenuates Fibrosis after Esophageal Endoscopic Submucosal Dissection via Modulating the HIF-1α/HK2/p38-MAPK Signaling Axis. ACS Biomater Sci Eng. 2021;7:3399-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68-69:106-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 18. | Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-R462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3416] [Cited by in RCA: 4880] [Article Influence: 443.6] [Reference Citation Analysis (4)] |
| 19. | Xu H, Zhao H, Ding C, Jiang D, Zhao Z, Li Y, Ding X, Gao J, Zhou H, Luo C, Chen G, Zhang A, Xu Y, Zhang H. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct Target Ther. 2023;8:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |
| 20. | Saei AA, Beusch CM, Sabatier P, Wells JA, Gharibi H, Meng Z, Chernobrovkin A, Rodin S, Näreoja K, Thorsell AG, Karlberg T, Cheng Q, Lundström SL, Gaetani M, Végvári Á, Arnér ESJ, Schüler H, Zubarev RA. System-wide identification and prioritization of enzyme substrates by thermal analysis. Nat Commun. 2021;12:1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Xu J, Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 398] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 22. | Qiu Y, Shi R. Roles of Steroids in Preventing Esophageal Stricture after Endoscopic Resection. Can J Gastroenterol Hepatol. 2019;2019:5380815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Manfrere KCG, Torrealba MP, Ferreira FM, de Sousa ESA, Miyashiro D, Teixeira FME, Custódio RWA, Nakaya HI, Ramos YAL, Sotto MN, Woetmann A, Ødum N, Duarte AJDS, Sanches JA, Sato MN. Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. Int J Mol Sci. 2023;24:4674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Baazm M, Ghafarizadeh AA, Noshad Kamran AR, Beyer C, Zendedel A. Presence of The NLRP3 Inflammasome Components in Semen of Varicocele Patients. Int J Fertil Steril. 2020;14:46-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50:778-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 796] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 26. | Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3⁺ Treg cell function in the intestine. Mucosal Immunol. 2015;8:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 27. | Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 840] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 28. | Fagundes RR, Bravo-Ruiseco G, Hu S, Kierans SJ, Weersma RK, Taylor CT, Dijkstra G, Harmsen HJM, Faber KN. Faecalibacterium prausnitzii promotes intestinal epithelial IL-18 production through activation of the HIF1α pathway. Front Microbiol. 2023;14:1298304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Babar M, Ryan AW, Anderson LA, Segurado R, Turner G, Murray LJ, Murphy SJ, Johnston BT, Comber H, Reynolds JV, McManus R. Genes of the interleukin-18 pathway are associated with susceptibility to Barrett's esophagus and esophageal adenocarcinoma. Am J Gastroenterol. 2012;107:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Artlett CM. The Role of the NLRP3 Inflammasome in Fibrosis. Open Rheumatol J. 2012;6:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Weber S, Sitte S, Voegele AL, Sologub L, Wilfer A, Rath T, Nägel A, Zundler S, Franchi L, Opipari AW, Sonnewald S, Reid S, Hartmann A, Eichhorn P, Handtrack C, Weber K, Grützmann R, Neufert C, Schellerer VS, Naschberger E, Ekici AB, Büttner C, Neurath MF, Atreya R. NLRP3 Inhibition Leads to Impaired Mucosal Fibroblast Function in Patients with Inflammatory Bowel Diseases. J Crohns Colitis. 2024;18:446-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Sun Z, Li Y, Qian Y, Wu M, Huang S, Zhang A, Zhang Y, Jia Z. Celastrol attenuates ox-LDL-induced mesangial cell proliferation via suppressing NLRP3 inflammasome activation. Cell Death Discov. 2019;5:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Fan J, Ren M, Chen W, Wang H, He Y. Celastrol relieves myocardial infarction-induced cardiac fibrosis by inhibiting NLRP3 inflammasomes in rats. Int Immunopharmacol. 2023;121:110511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 34. | Cascão R, Carvalho T, Goncalves J, Moita L, Fonseca J. AB0096 Efficacy and safety of oral administration of pure celastrol in aia rats. Ann Rheum Dis. 2017;76:1080. [DOI] [Full Text] |
| 35. | Shan WG, Wang HG, Chen Y, Wu R, Wen YT, Zhang LW, Ying YM, Wang JW, Zhan ZJ. Synthesis of 3- and 29-substituted celastrol derivatives and structure-activity relationship studies of their cytotoxic activities. Bioorg Med Chem Lett. 2017;27:3450-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 993] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 37. | Tu W, Wang H, Li S, Liu Q, Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 38. | Lee D, Xu IM, Chiu DK, Leibold J, Tse AP, Bao MH, Yuen VW, Chan CY, Lai RK, Chin DW, Chan DF, Cheung TT, Chok SH, Wong CM, Lowe SW, Ng IO, Wong CC. Induction of Oxidative Stress Through Inhibition of Thioredoxin Reductase 1 Is an Effective Therapeutic Approach for Hepatocellular Carcinoma. Hepatology. 2019;69:1768-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 39. | Milkovic L, Cipak Gasparovic A, Cindric M, Mouthuy PA, Zarkovic N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells. 2019;8:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/