Published online May 28, 2025. doi: 10.3748/wjg.v31.i20.107451
Revised: April 12, 2025
Accepted: May 12, 2025
Published online: May 28, 2025
Processing time: 65 Days and 18.2 Hours
Infected necrotizing pancreatitis (INP) remains a life-threatening complication of acute pancreatitis. Despite advancements such as endoscopic ultrasound (EUS)-guided drainage, lumen-apposing metal stents, and protocolized step-up strate
Core Tip: Endoscopic therapy serves as the first-line treatment for infected necrotizing pancreatitis (INP), effectively reducing mortality and complication rates. Standardizing INP endoscopic management relies on multidisciplinary collaboration, advanced imaging techniques (e.g., three-dimensional reconstruction, endoscopic ultrasound-guided procedures), and biomarker-driven strategies (C-reactive protein, procalcitonin) to tailor interventions, optimize timing, and minimize complications. Addressing the heterogeneity in endoscopic management remains crucial, requiring clarification of optimal intervention windows, selection of appropriate stents, implementation of combined therapies for complex lesions, and strategies to reduce endoscopic debridement sessions and complication risks, thereby guaranteeing individualized therapeutic demands with clinical efficacy assurance.
- Citation: Zeng Y, Zhang JW, Yang J. Endoscopic management of infected necrotizing pancreatitis: Advancing through standardization. World J Gastroenterol 2025; 31(20): 107451
- URL: https://www.wjgnet.com/1007-9327/full/v31/i20/107451.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i20.107451
Acute pancreatitis, a typical gastrointestinal emergency, progresses to necrotizing pancreatitis in approximately 20% of cases, with infection of necrotic tissue [infected necrotizing pancreatitis (INP)] occurring in 30%–70% of these patients[1-4]. INP carries a mortality rate of up to 30%, necessitating timely and effective intervention[5,6]. Traditional open surgical necrosectomy, while life-saving, is associated with significant morbidity, including organ failure, pancreaticocutaneous fistulas, and prolonged hospitalization[7].
The advent of minimally invasive endoscopic techniques, including endoscopic ultrasound (EUS)-guided transmural drainage, direct endoscopic necrosectomy, and lumen-apposing metal stents (LAMS), has revolutionized INP manage
Despite these advances, the clinical practice of INP management remains heterogeneous. Variability in procedural timing, choice of devices (e.g., plastic stents vs LAMS), adjunctive irrigation methods, and antimicrobial protocols con
This review synthesizes advances in the endoscopic management of INP while incorporating real-world expertise from the authors' tertiary referral center, with the dual objectives of improving patient outcomes and promoting consistency across varied clinical environments.
The 2012 Revised Atlanta Classification established standardized definitions for INP and categorized acute pancreatitis complications and severity. However, due to its publication timeline, this consensus did not address the evolving role of endoscopic management, particularly the application of EUS in treating INP[19]. Prioritizing computed tomography (CT) as the primary imaging modality demonstrates significant clinical utility for diagnosing INP in resource-limited settings, leading to widespread global adoption. While early contrast-enhanced CT (CECT) (performed within 72–96 hours) remains the gold standard for differentiating interstitial edematous pancreatitis from necrotizing pancreatitis, its speci
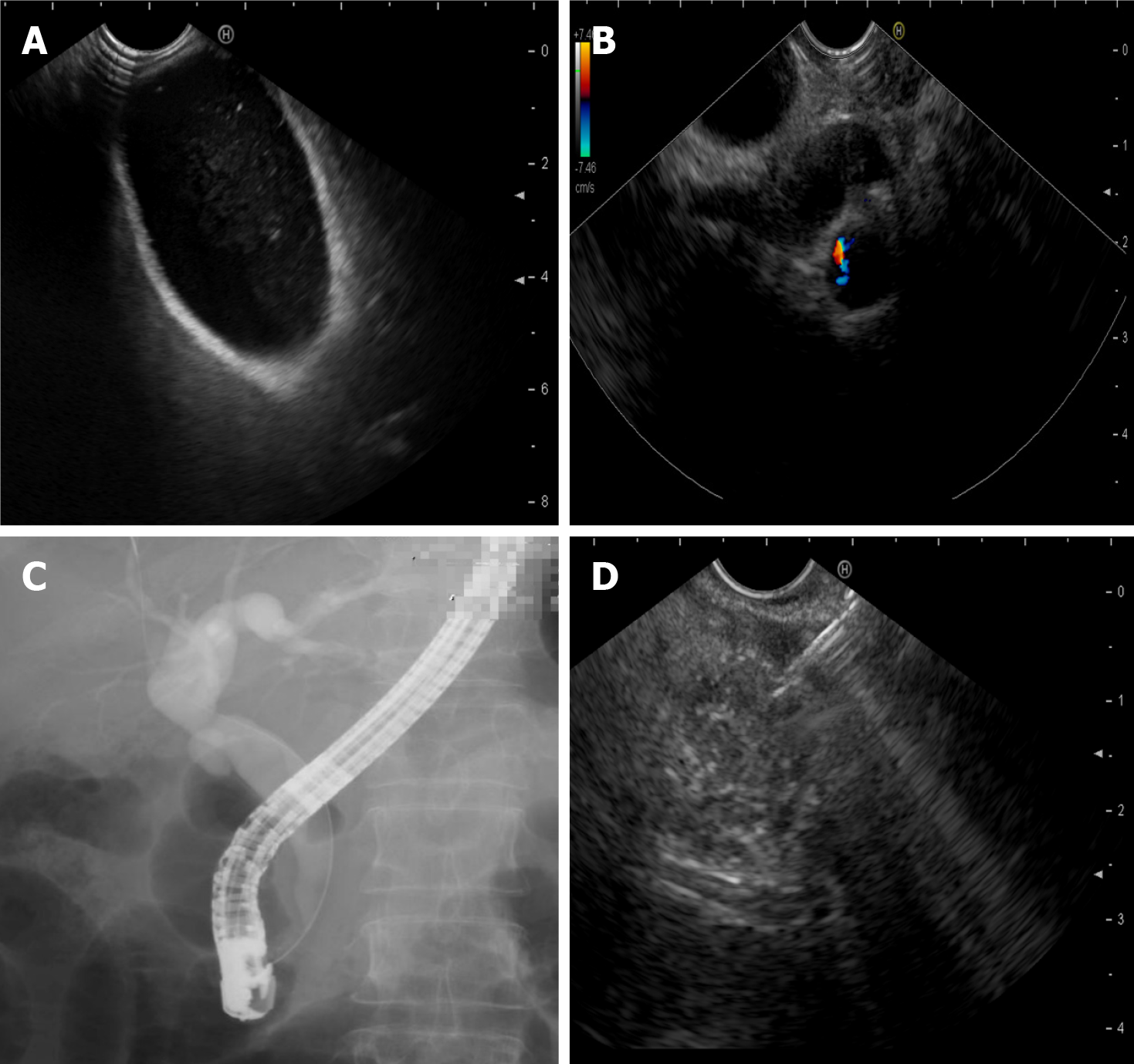
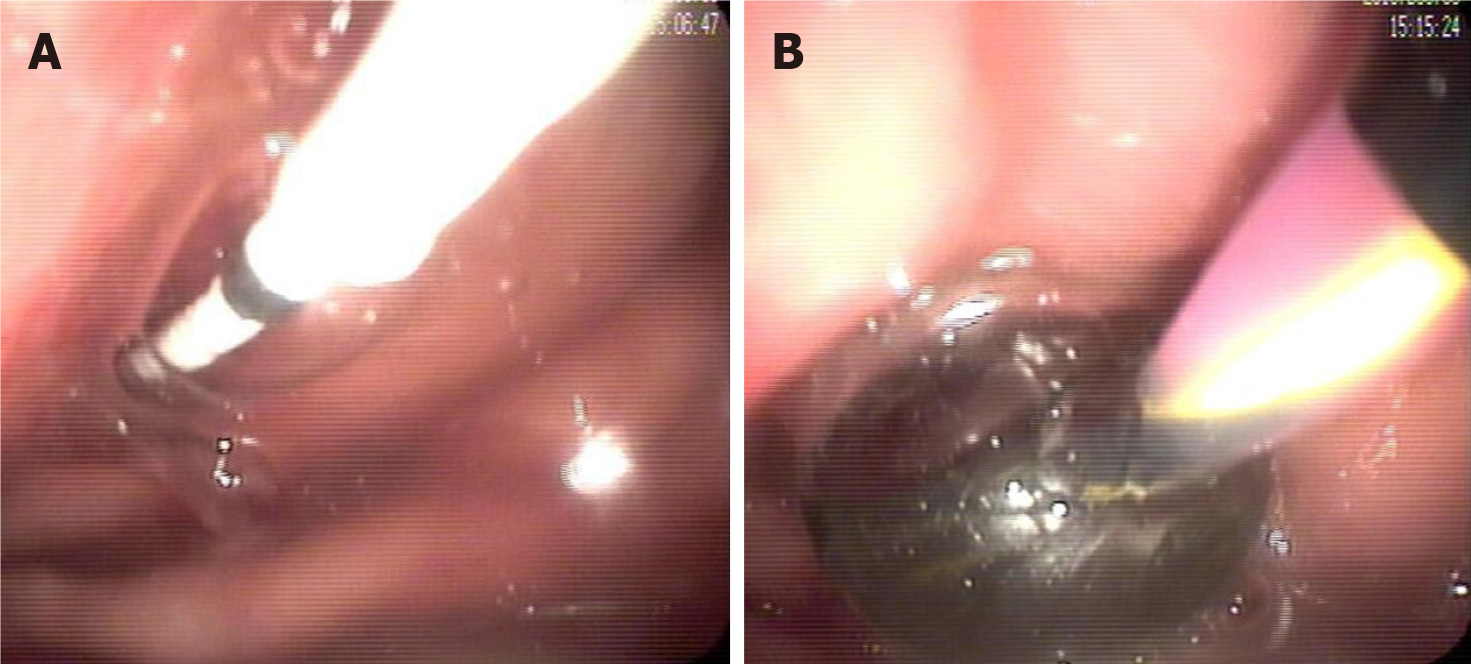
Endoscopic evaluation, especially EUS, should be considered in specific clinical scenarios[26]. Endoscopic techniques are pivotal in diagnosing pancreatitis, demonstrating superior diagnostic accuracy compared to magnetic resonance cholangiopancreatography (MRCP), particularly in patients with idiopathic or recurrent pancreatitis[27,28]. EUS and endoscopic retrograde cholangiopancreatography (ERCP) enable early identification of underlying etiologies that may evade detection by conventional imaging[29-31]. These include, but are not limited to, biliary sludge or microlithiasis (sub-5 mm stones), pancreaticobiliary maljunction, sphincter of Oddi dysfunction, small pancreatic cancers (less than 20 mm in size), and communication between pancreatic lesions and the main pancreatic duct (MPD) (Figure 1)[32-35]. Such findings are critical for guiding timely therapeutic interventions. For instance, EUS provides high-resolution imaging to confirm ductal continuity or identify DPDS, and ERCP allows direct therapeutic actions, such as biliary sphincterotomy or stent placement, to alleviate obstructions[36-38]. Furthermore, delineating ductal anatomy helps determine whether transpapillary drainage via the duodenal papilla should be integrated with conventional transmural drainage (transga
Therefore, our team advocates for early EUS (within 72 hours of symptom onset) in patients with acute pancreatitis of unclear etiology in specialized endoscopy centers, aiming to identify underlying pathologies that may be missed by CT or MRCP. Furthermore, during EUS, assessing pancreatic cystic lesions adjacent to the MPD warrants evaluation for DPDS, a critical determinant of persistent leaks and recurrent collections that may influence endoscopic therapeutic planning[41]. While the diagnosis of INP relies on clinical manifestations (e.g., fever, organ dysfunction) and imaging findings (e.g., the "gas within necrosis" sign on CT), the necessity of obtaining microbiological evidence via EUS-guided fine-needle aspiration (EUS-FNA) remains debated[22,42-44]. Our team advocates that EUS-FNA, combined with culture or metage
While encouraged by the progress in the endoscopic treatment of INP, endoscopists should also confront the reality that surgical management of INP has already achieved a high degree of standardization, with minimal variability among research groups across major centers worldwide. In stark contrast, endoscopic treatment remains highly non-stan
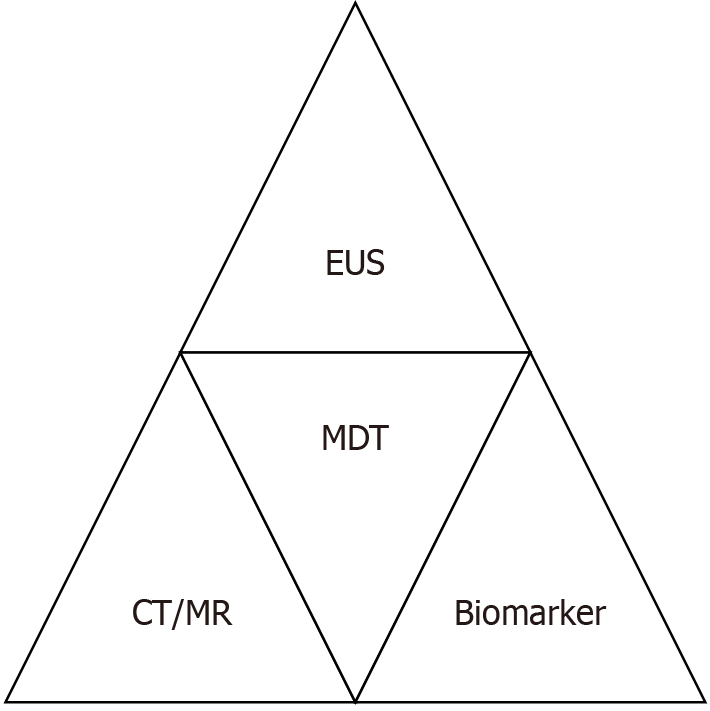
A structured pre-procedural evaluation is critical to optimize therapeutic outcomes and minimize risks (Figure 2). Key components include contrast-enhanced CT/MRI, biomarker-driven risk stratification, EUS reassessment, and multidisciplinary consensus.
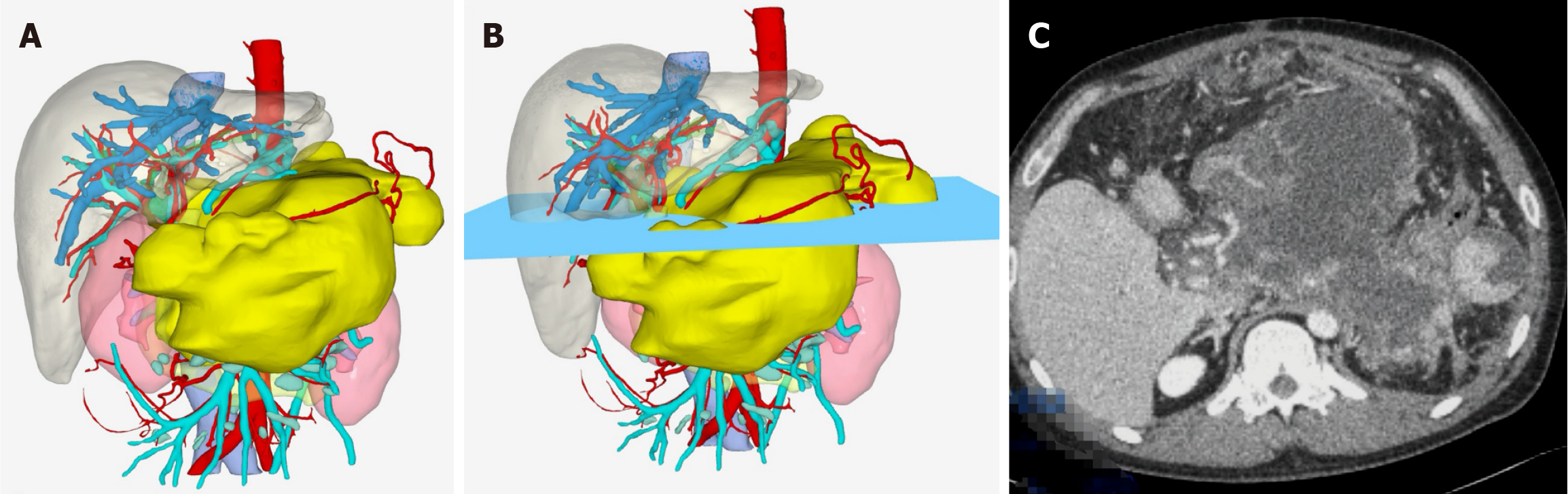
First, contrast-enhanced CT/MRI and MRCP are frequently utilized in the preoperative assessment of endoscopic procedures for INP. The former is primarily used to assess the necrosis extent (e.g., < 30% vs > 30%), identify local complications, and detect signs of infection (e.g., air bubble signs)[23,54]. The latter is mainly used to evaluate biliary and pancreatic duct abnormalities and the potential connection between pancreatic pseudocysts and the MPD[55]. In most cases, imaging modalities, typically performed before EUS, confirm the presence of infection (e.g., gas within necrosis), evaluate lesion size, and assess wall integrity and definition. These findings, integrated with clinical symptoms such as fever or persistent abdominal pain, guide the decision for endoscopic drainage and necrosectomy, ensuring adherence to evidence-based intervention criteria[43]. Our team has utilized contrast-enhanced CT imaging to construct three-dimensional (3D) reconstruction models of necrotic lesions in INP patients. This model has been applied to guide endoscopic debridement of walled-off necrosis (WON) in cases with extensive necrosis or complicated patients (Figure 3). Preliminary results have demonstrated promising clinical utility, and we anticipate sharing these data with the broader community soon. In addition to imaging modalities, widely used scoring systems (e.g., the Ranson score, acute phy
Second, biomarker-driven risk stratification is also integral. Elevated PCT (> 1.8 ng/mL) and IL-8 (> 112 pg/mL), and increased lactate (> 2 mmol/L) serve as robust indicators of severe infection necessitating prompt intervention[57,58]. These biomarkers enable noninvasive prediction of infected necrosis and inform perioperative strategies for endoscopic or surgical debridement[59,60]. Persistently elevated PCT and CRP levels beyond 72 hours post-onset exhibit greater diagnostic accuracy for INP compared to early-phase measurements, underscoring the need for serial biomarker monitoring in dynamic risk assessment[46]. Our team's single-center study revealed that the endoscopic debridement interval showed no significant correlation with the patient's biochemical markers (e.g., white blood cell count, neutrophil percentage, CRP). In contrast, statistically significant associations were observed with pre-intervention modified CT severity index (MCTSI) and elevated CRP levels[61].
Third, EUS assessment is crucial to evaluate the wall thickness of the collection, the proximity of adjacent vessels, and the presence of solid necrotic debris content, guiding the selection of access routes and devices[62]. Notably, contrast-enhanced CT is less sensitive than EUS in identifying the proportion of solid necrosis within INP[42,63,64]. Thus, EUS evaluations were systematically performed to quantify the extent of solid necrotic debris and assess peripancreatic vasculature, including the splenic vein and collateral vessels. This approach aimed to minimize bleeding risks during transmural drainage by avoiding vascular structures, particularly in patients with sinistral portal hypertension[65]. Studies have demonstrated that more significant solid necrotic debris observed on EUS is associated with increased requirement for necrosectomy, higher rates of stent occlusion, and prolonged hospitalization[66]. Our team's research further revealed that while the location and size of WON on EUS showed no significant correlation with the interval between endoscopic debridement sessions, they were significantly associated with the solid necrosis ratio within WON during EUS evaluation[61]. Consequently, the quantification of solid necrosis content should be prioritized in EUS assessments. Moreover, under Doppler imaging guidance, EUS evaluates the presence of sinistral portal hypertension and gastric varices (GV) secondary to INP[67,68]. This evaluation informs the design of puncture pathways and drainage strategies to mitigate perioperative complications such as intraoperative and postoperative stent-related bleeding, thereby underscoring the dual diagnostic and therapeutic utility of EUS in this context. Data from multiple studies indicate that perioperative bleeding rates associated with EUS-guided drainage of pancreatic cystic lesions are higher than those of standard EUS examinations, with particular attention needed for the risk of delayed bleeding following LAMS placement[69,70]. However, in patients with preexisting GV, the perioperative bleeding rates of EUS-guided variceal therapies are comparable to those of conventional endoscopic variceal therapies[71]. Therefore, based on our institutional experience in a high-volume endoscopic center, GV should not preclude EUS-guided INP management but necessitate meticulous preprocedural planning, including Doppler evaluation and multidisciplinary consultation. During Doppler assessment, clinicians must evaluate vasculature adjacent to the intended puncture and drainage pathways and anticipate potential vascular risks due to post-drainage changes in lesion size and vascular proximity along necrosectomy trajectories. This comprehensive vascular mapping minimizes bleeding risks and ensures endoscopic procedural safety, particularly in patients with sinistral portal hypertension.
Ultimately, while endoscopic management has been recommended as first-line treatment for INP patients meeting indications, the treatment plan and timing must be determined through multidisciplinary team (MDT) collaboration involving gastroenterologists, endoscopists, radiologists, intensivists, and surgeons to confirm the INP diagnosis, assess severity, and establish intervention timing[43,55,72]. The ongoing debate over early intervention (within 4 weeks of onset) vs delayed intervention (more than 4 weeks of onset) remains unresolved, primarily due to heterogeneity in patient conditions and institutional expertise[53,73,74]. Notably, studies have demonstrated that endoscopic interventions per
This review is based on the multidisciplinary clinical experience from our tertiary referral center with evidence-based analyses conducted using Reference Citation Analysis, a unique AI system for evaluating citations in biomedical litera
An alternative non-endoscopic approach for drainage is PCD, typically employed in early disease stages or clinical settings lacking endoscopic expertise or equipment. PCD is also frequently utilized in critically ill INP patients requiring intensive care. However, our center's experience demonstrates that in patients with extensive necrotic lesions, percuta
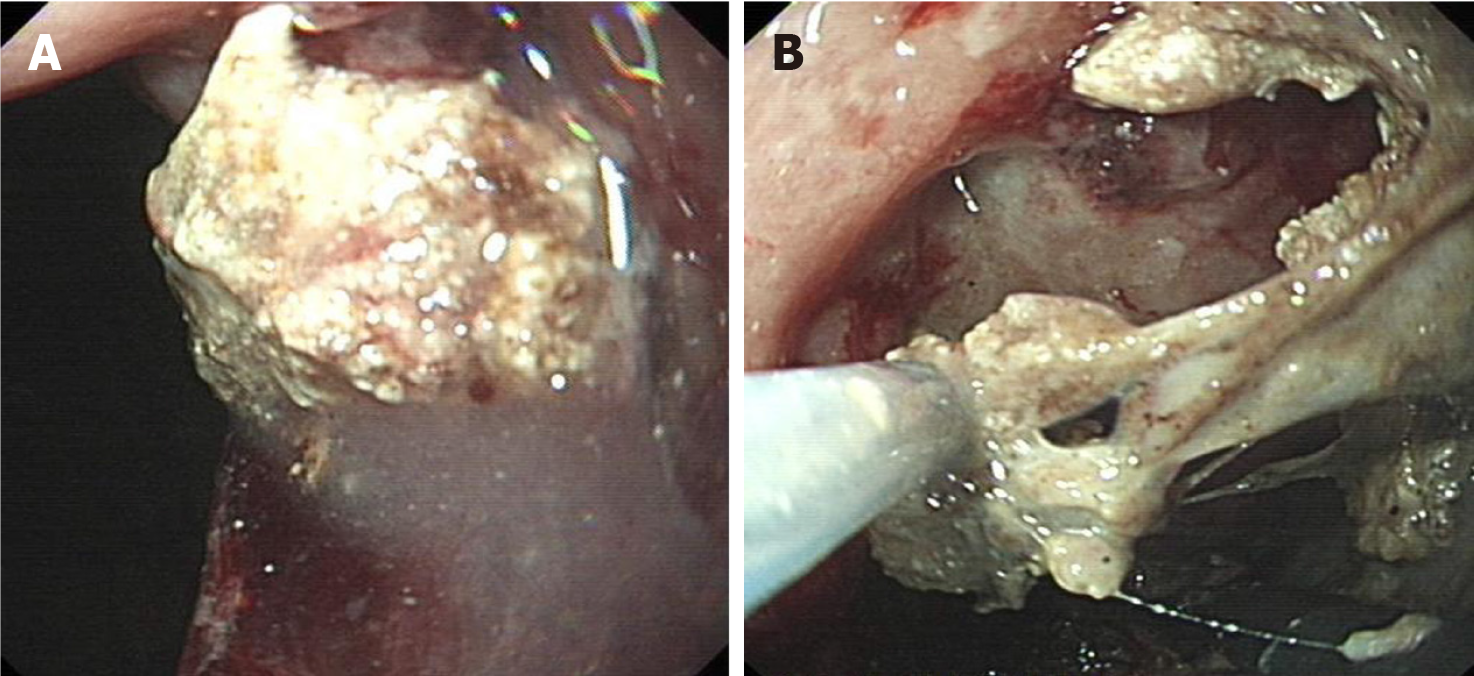
When endoscopic management of INP is indicated, a debridement and drainage tract should ideally be created under EUS guidance[101]. A 19-gauge needle and 0.035-inch guidewire are commonly employed for initial access[76,102]. However, when using LAMS with a single-step deployment system, the need for a needle and guidewire may be obviated[103,104]. For needle-based access, subsequent tract dilation typically requires bougies, balloons, or cystotomes. Given that the needle trajectory may form an acute angle with the necrotic cavity surface, complicating bougie or cystotome advancement and increasing the risk of procedural failure, our center advocates for graded balloon dilation as the preferred and safer method for transmural tract creation (Figure 5)[105]. This approach facilitates controlled tract expansion and allows immediate hemostasis during transmural tract formation by balloon compression, minimizing the technical challenges and risks associated with repeated device exchanges for bleeding control[106,107].
Stent selection continues to generate debate, particularly between LAMS and plastic stents. LAMS, with their wide lumens (15–20 mm), are favored for large (> 6 cm) WON due to facilitated direct necrosectomy and reduced reintervention rates, albeit at the cost of higher bleeding risks as highlighted in recent studies[108-111]. However, comparative studies have demonstrated that LAMS does not reduce the need for endoscopic debridement in INP patients compared to double-pigtail plastic stents[112]. While some retrospective studies have found that plastic stents are associated with reduced overall bleeding events, including pseudoaneurysm bleeding, compared to LAMS, multicenter prospective cohort studies and meta-analyses have demonstrated comparable technical success rates [P = 0.986; relative risk (RR) = 1.00; 95% confidence interval (CI): 0.93–1.08], similar clinical success rates (P = 0.139; RR = 1.063; 95%CI: 0.98–1.15], lower complication rates in LAMS-treated patients (P = 0.009; RR = 0.746; 95%CI: 0.60–0.93], comparable mortality (P = 0.640), and similar odds of bleeding complications requiring reintervention (RR = 0.44; 95%CI: 0.16–1.17)[109,110,112]. Therefore, step-up staged approaches, including staged PCD-endoscopic strategies, along with single or multiple plastic stent drainage, remain practical and cost-effective treatment options for smaller INP lesions or in low-resource settings where limitations exist in EUS equipment availability, LAMS accessibility, procedural training resources, or EUS expertise. At the same time, in well-resourced settings, optimal stent configuration—such as single vs multiple plastic stents or hybrid LAMS-plastic combinations—lacks consensus[52,64]. While some studies suggest that coaxial plastic stent placement within LAMS may reduce stent occlusion rates, a recent systematic review and meta-analysis concluded that adjunctive stent placement does not significantly differ from LAMS alone in terms of adverse events, including infection, stent migration, occlusion, or reintervention rates[113-115]. Our team posits that for INP lesions with a solid necrosis content < 30%, the therapeutic efficacy of LAMS and multiple double-pigtail plastic stents is comparable in achieving drainage. However, we strongly advocate LAMS as the first-line option for lesions with solid necrosis content ≥ 30%. In resource-limited settings where plastic stents are utilized, we recommend deploying ≥ 3 double-pigtail plastic stents to facilitate subsequent endoscopic debridement, thereby eliminating the need for balloon dilation and reducing procedural com
The optimization of endoscopic debridement management in INP to improve procedural efficiency remains a dynamic and debated field. Early necrosectomy, though controversial, has demonstrated associations with improved clinical outcomes and reduced healthcare costs, necessitating careful patient selection under MDT guidance to balance risks and benefits[116]. Current recommendations for debridement intervals (mean 6.23 days ± 4.71 days, range 3–21 days) are primarily based on aggregated global endoscopic expertise rather than standardized protocols[117]. Our single-center research identified five predictive parameters—EUS-measured solid necrosis ratio, MCTSI, postoperative fever, elevated CRP, and pre-interventional fever—as critical determinants of optimal timing for EUS-guided necrosectomy in WON[61]. At the same time, emerging ML and DL models show promise in refining intervention timing by integrating radiomic and clinical data. Retrospective analyses have demonstrated that ML models using support vector machines and random forest algorithms outperform conventional statistical models. ML models have also identified IL-6, infected necrosis, febrile episodes, and CRP as significant determinants for surgical intervention timing (< 4 weeks or ≥ 4 weeks) in INP patients[118]. An individualized and rational endoscopic debridement schedule not only facilitates expedited recovery in INP patients but also enables optimized management of complications such as stent occlusion, migration, embedment, and stent-related bleeding, thus adhering to the principle that preventive strategies (early detection and intervention) yield superior outcomes with minimal poor prognosis impact compared to reactive measures (Figure 6). Innovations in endoscopic devices are also significantly enhancing debridement efficiency. Examples include the waterjet necrosectomy device, which has demonstrated preliminary efficacy and safety in preclinical animal studies, the powered endoscopic debridement system, capable of simultaneously resecting and removing solid necrotic debris, and the over-the-scope grasper, designed to remove large necrotic fragments that are challenging to extract with conventional tools[119-121]. Moreover, intracavitary irrigation strategies in INP have shown the potential to enhance debridement effi
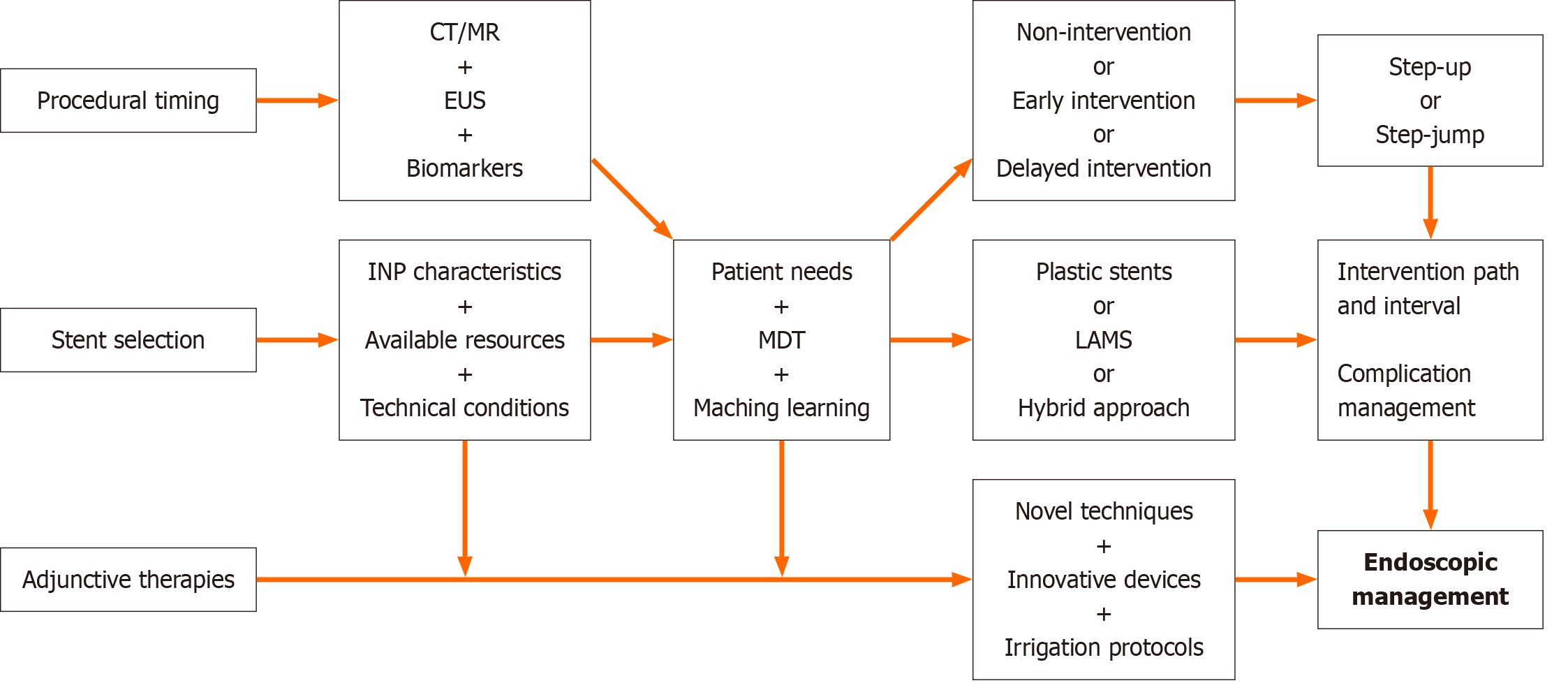
While standardizing endoscopic management protocols for INP ensures consistent and high-quality care, it is equally important to recognize individual patients' unique clinical presentations and needs. Thus, pursuing standardized protocols must not eclipse the imperative for patient-specific adaptations. We propose a hybrid model integrating standardized decision-making frameworks with MDT-adjusted flexibility to resolve this realistic dilemma. This approach would allow for applying evidence-based guidelines while accommodating patient-specific factors, such as comorbidities, prior treatment responses, and personal preferences. The cornerstone of this approach is to commence with a systematic pre-procedural assessment. At the same time, patient-specific strategies (e.g., step-up vs step-jump), stent selection, debridement pathway establishment, debridement interval optimization, efficiency-enhancing adjuncts, and perioperative complication management require MDT evaluation to achieve patient-tailored adaptations within standardized therapeutic frameworks (Figure 7).
Endoscopic management of INP has transformed care by prioritizing minimally invasive, patient-centered strategies over traditional surgical approaches. Yet, persistent heterogeneity in endoscopic strategies, device selection, and adjunctive techniques underscores the urgent need for protocol standardization. Critical debates persist, including early vs delayed intervention, step-up vs step-jump, the role of hybrid strategies, personalized debridement intervals, and optimal irrigation agents, highlighting the necessity of balancing innovation with standardized, evidence-based protocols. Harmonizing endoscopic techniques through rigorous comparative studies and AI-driven algorithms will reduce future practice variability, optimize resource utilization, and ensure equitable access to high-quality INP care globally.
| 1. | Hollemans RA, Timmerhuis HC, Besselink MG, Bouwense SAW, Bruno M, van Duijvendijk P, van Geenen EJ, Hadithi M, Hofker S, Van-Hooft JE, Kager LM, Manusama ER, Poley JW, Quispel R, Römkens T, van der Schelling GP, Schwartz MP, Spanier BWM, Stommel M, Tan A, Venneman NG, Vleggaar F, van Wanrooij RLJ, Bollen TL, Voermans RP, Verdonk RC, van Santvoort HC; Dutch Pancreatitis Study Group. Long-term follow-up study of necrotising pancreatitis: interventions, complications and quality of life. Gut. 2024;73:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Lv C, Zhang ZX, Ke L. Early prediction and prevention of infected pancreatic necrosis. World J Gastroenterol. 2024;30:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology. 2019;156:1994-2007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 277] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 4. | Lu JD, Cao F, Ding YX, Wu YD, Guo YL, Li F. Timing, distribution, and microbiology of infectious complications after necrotizing pancreatitis. World J Gastroenterol. 2019;25:5162-5173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (8)] |
| 5. | Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC, Bruno MJ; Dutch Pancreatitis Study Group. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 6. | Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 7. | van Brunschot S, Hollemans RA, Bakker OJ, Besselink MG, Baron TH, Beger HG, Boermeester MA, Bollen TL, Bruno MJ, Carter R, French JJ, Coelho D, Dahl B, Dijkgraaf MG, Doctor N, Fagenholz PJ, Farkas G, Castillo CFD, Fockens P, Freeman ML, Gardner TB, Goor HV, Gooszen HG, Hannink G, Lochan R, McKay CJ, Neoptolemos JP, Oláh A, Parks RW, Peev MP, Raraty M, Rau B, Rösch T, Rovers M, Seifert H, Siriwardena AK, Horvath KD, van Santvoort HC. Minimally invasive and endoscopic versus open necrosectomy for necrotising pancreatitis: a pooled analysis of individual data for 1980 patients. Gut. 2018;67:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Li P, Zhang Z, Wang S, Jin Z, Du Y, Yang A, Feng Y, Zou X, Wang L, Wang X, Tian L, Zhou P, Zhang Y, Liu J, Ding Z, Zhang J, Yang J, Sun S, Zhang S. A Chinese prospective multicenter cohort study evaluating EUS-guided drainage of pancreatic fluid collections using the Hot AXIOS system. Endosc Ultrasound. 2023;12:259-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 9. | Nzenwa IC, Panossian VS, DeWane MP, Albutt KH, Hernandez-Barco YG, Fernandez-Del Castillo CF, Lillemoe KD, Warshaw AL, Fagenholz PJ, Luckhurst CM. Volume Matters: Examining The Management Of Necrotizing Pancreatitis In The United States. Ann Surg. 2025. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Tran Z, Xu J, Verma A, Ebrahimian S, Cho NY, Benharash P, Burruss S. National trends and clinical outcomes of interventional approaches following admission for infected necrotizing pancreatitis in the United States. J Trauma Acute Care Surg. 2023;94:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, Feranec N, Wilcox CM, Tharian B, Hawes RH, Varadarajulu S. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1027-1040.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 12. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 506] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 13. | Tang P, Ali K, Khizar H, Ni Y, Cheng Z, Xu B, Qin Z, Zhang W. Endoscopic versus minimally invasive surgical approach for infected necrotizing pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2023;55:2276816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Zeng Y, Zhang JW, Yang J. Efficacy and Safety of Anhydrous Ethanol-Assisted Endoscopic Ultrasound-Guided Transluminal Necrosectomy in Infected Necrotizing Pancreatitis. Dig Dis Sci. 2024;69:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 15. | Moon JH, Park SW, Lee YN, Lee SH, Kim SH, Lee DW, Cho CM, Kim SB, Park CH. A comparison of novel electrocautery-enhanced lumen-apposing metal stents and plastic stents in endoscopic ultrasound-guided drainage of infected walled-off necrosis: a multicenter randomized study. Endoscopy. 2024;56:926-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Van Veldhuisen CL, Sissingh NJ, Boxhoorn L, van Dijk SM, van Grinsven J, Verdonk RC, Boermeester MA, Bouwense SAW, Bruno MJ, Cappendijk VC, van Duijvendijk P, van Eijck CHJ, Fockens P, van Goor H, Hadithi M, Haveman JW, Jacobs MAJM, Jansen JM, Kop MPM, Manusama ER, Mieog JSD, Molenaar IQ, Nieuwenhuijs VB, Poen AC, Poley JW, Quispel R, Römkens TEH, Schwartz MP, Seerden TC, Dijkgraaf MGW, Stommel MWJ, Straathof JWA, Venneman NG, Voermans RP, van Hooft JE, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Long-Term Outcome of Immediate Versus Postponed Intervention in Patients With Infected Necrotizing Pancreatitis (POINTER): Multicenter Randomized Trial. Ann Surg. 2024;279:671-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Bang JY, Lakhtakia S, Thakkar S, Buxbaum JL, Waxman I, Sutton B, Memon SF, Singh S, Basha J, Singh A, Navaneethan U, Hawes RH, Wilcox CM, Varadarajulu S; United States Pancreatic Disease Study Group. Upfront endoscopic necrosectomy or step-up endoscopic approach for infected necrotising pancreatitis (DESTIN): a single-blinded, multicentre, randomised trial. Lancet Gastroenterol Hepatol. 2024;9:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Wang ZJ, Song YH, Li SY, He ZX, Li ZS, Wang SL, Bai Y. Endoscopic management of pancreatic fluid collections with disconnected pancreatic duct syndrome. Endosc Ultrasound. 2023;12:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4708] [Article Influence: 362.2] [Reference Citation Analysis (48)] |
| 20. | Hu JX, Zhao CF, Wang SL, Tu XY, Huang WB, Chen JN, Xie Y, Chen CR. Acute pancreatitis: A review of diagnosis, severity prediction and prognosis assessment from imaging technology, scoring system and artificial intelligence. World J Gastroenterol. 2023;29:5268-5291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (5)] |
| 21. | Badat N, Millet I, Corno L, Khaled W, Boulay-Coletta I, Zins M. Revised Atlanta classification for CT pancreatic and peripancreatic collections in the first month of acute pancreatitis: interobserver agreement. Eur Radiol. 2019;29:2302-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | van Baal M, Bollen T, Bakker O, van Goor H, Rijkers G, Boermeester M, Dejong C, van Eijck C, Gooszen H, van der Harst E, van Santvoort H, Besselink M. Diagnosing infected necrotizing pancreatitis: Clinical signs, gas bubbles or routine fine-needle aspiration? Pancreatology. 2012;12:574. [DOI] [Full Text] |
| 23. | Li J, Lin C, Ning C, Wei Q, Chen L, Zhu S, Shen D, Huang G. Early-onset emphysematous pancreatitis indicates poor outcomes in patients with infected pancreatic necrosis. Dig Liver Dis. 2022;54:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Bagherzadeh S, Jabbari N, Khalkhali HR. Radiation dose and cancer risks from radiation exposure during abdominopelvic computed tomography (CT) scans: comparison of diagnostic and radiotherapy treatment planning CT scans. Radiat Environ Biophys. 2021;60:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, Khorasani R. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 731] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 26. | Rana SS. Evaluating the role of endoscopic ultrasound in pancreatitis. Expert Rev Gastroenterol Hepatol. 2022;16:953-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 27. | Wan J, Ouyang Y, Yu C, Yang X, Xia L, Lu N. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:1180-1188.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Suzuki M, Sekino Y, Hosono K, Yamamoto K, Kawana K, Nagase H, Kubota K, Nakajima A. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for the diagnosis of computed tomography-negative common bile duct stone: Prospective randomized controlled trial. Dig Endosc. 2022;34:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Umans DS, Rangkuti CK, Sperna Weiland CJ, Timmerhuis HC, Bouwense SAW, Fockens P, Besselink MG, Verdonk RC, van Hooft JE; Dutch Pancreatitis Study Group. Endoscopic ultrasonography can detect a cause in the majority of patients with idiopathic acute pancreatitis: a systematic review and meta-analysis. Endoscopy. 2020;52:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Wilcox CM, Seay T, Kim H, Varadarajulu S. Prospective Endoscopic Ultrasound-Based Approach to the Evaluation of Idiopathic Pancreatitis: Causes, Response to Therapy, and Long-term Outcome. Am J Gastroenterol. 2016;111:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Kaw M, Brodmerkel GJ Jr. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc. 2002;55:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Żorniak M, Sirtl S, Beyer G, Mahajan UM, Bretthauer K, Schirra J, Schulz C, Kohlmann T, Lerch MM, Mayerle J; LMU Microlithiasis Expert Survey Team. Consensus definition of sludge and microlithiasis as a possible cause of pancreatitis. Gut. 2023;72:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Kitano M. History of pancreato-hepatobiliary endoscopy: Endoscopic ultrasound diagnosis. Dig Endosc. 2022;34 Suppl 2:102-106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Hanada K, Amano H, Abe T. Early diagnosis of pancreatic cancer: Current trends and concerns. Ann Gastroenterol Surg. 2017;1:44-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Zeng JQ, Deng ZH, Yang KH, Zhang TA, Wang WY, Ji JM, Hu YB, Xu CD, Gong B. Endoscopic retrograde cholangiopancreatography in children with symptomatic pancreaticobiliary maljunction: A retrospective multicenter study. World J Gastroenterol. 2019;25:6107-6115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 36. | Wang L, Elhanafi S, Storm AC, Topazian MD, Majumder S, Abu Dayyeh BK, Levy MJ, Petersen BT, Martin JA, Chari ST, Vege SS, Chandrasekhara V. Impact of disconnected pancreatic duct syndrome on endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endoscopy. 2021;53:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Meng Y, Ding J, Tian C, Wang M, Tang K. Endoscopic transpapillary drainage for walled-off pancreatic necrosis with complete main pancreatic duct disruption by metallic stent placement: A retrospective study. Front Med (Lausanne). 2022;9:1064463. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Timmerhuis HC, van Dijk SM, Verdonk RC, Bollen TL, Bruno MJ, Fockens P, van Hooft JE, Voermans RP, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Various Modalities Accurate in Diagnosing a Disrupted or Disconnected Pancreatic Duct in Acute Pancreatitis: A Systematic Review. Dig Dis Sci. 2021;66:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Jagielski M, Smoczyński M, Adrych K. The role of endoscopic treatment of pancreatic duct disruption in patients with walled-off pancreatic necrosis. Surg Endosc. 2018;32:4939-4952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Chen Y, Jiang Y, Qian W, Yu Q, Dong Y, Zhu H, Liu F, Du Y, Wang D, Li Z. Endoscopic transpapillary drainage in disconnected pancreatic duct syndrome after acute pancreatitis and trauma: long-term outcomes in 31 patients. BMC Gastroenterol. 2019;19:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Li H, Feng X, Gao F, Chen Q, Linghu E. Diagnostic value of EUS-guided SF6 pancreatography for pancreatic cystic lesions on cyst communication with the pancreatic duct. Endosc Ultrasound. 2023;12:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | van Baal MC, Bollen TL, Bakker OJ, van Goor H, Boermeester MA, Dejong CH, Gooszen HG, van der Harst E, van Eijck CH, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. The role of routine fine-needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery. 2014;155:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (3)] |
| 44. | Hong D, Wang P, Xu Y, Xu S, Yu L, Tong Z, Li W, Qin K, Ke L; Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG). Metagenomic Next-Generation Sequencing-Based Fine-Needle Aspiration in Patients With Suspected Infected Pancreatic Necrosis. Clin Transl Gastroenterol. 2024;15:e00726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | George N, Perisetti A, Al-shoha M, Siddique S, Raghavapuram S, Banerjee D, Garcia-saenz-de-sicilia M, Tharian B. Pancreatic Cysts in Pancreatitis: Does EUS-FNA Add Value Over Baseline EUS or MRI? AM J Gastroenterol. 2017;112:S1450. [DOI] [Full Text] |
| 46. | Tarján D, Szalai E, Lipp M, Verbói M, Kói T, Erőss B, Teutsch B, Faluhelyi N, Hegyi P, Mikó A. Persistently High Procalcitonin and C-Reactive Protein Are Good Predictors of Infection in Acute Necrotizing Pancreatitis: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2024;25:1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 47. | Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev. 2017;4:CD012645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Wiese ML, Urban S, von Rheinbaben S, Frost F, Sendler M, Weiss FU, Bülow R, Kromrey ML, Tran QT, Lerch MM, Schauer B, Aghdassi AA. Identification of early predictors for infected necrosis in acute pancreatitis. BMC Gastroenterol. 2022;22:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 49. | Huang B, Gao Y, Wu L. Assessment of body composition and prediction of infectious pancreatic necrosis via non-contrast CT radiomics and deep learning. Front Microbiol. 2024;15:1509915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Li J, Chen Z, Li L, Lai T, Peng H, Gui L, He W. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front Cell Infect Microbiol. 2022;12:933221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Rizzatti G, Rimbaş M, Larghi A. Endoscopic Ultrasound-Guided Drainage for Infected Necrotizing Pancreatitis: Better Than Surgery But Still Lacking Treatment Protocol Standardization. Gastroenterology. 2019;157:582-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Binda C, Fabbri S, Perini B, Boschetti M, Coluccio C, Giuffrida P, Gibiino G, Petraroli C, Fabbri C. Endoscopic Ultrasound-Guided Drainage of Pancreatic Fluid Collections: Not All Queries Are Already Solved. Medicina (Kaunas). 2024;60:333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 53. | Zeng Y, Yang J, Zhang JW. Endoscopic transluminal drainage and necrosectomy for infected necrotizing pancreatitis: Progress and challenges. World J Clin Cases. 2023;11:1888-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (19)] |
| 54. | Zhao K, Adam SZ, Keswani RN, Horowitz JM, Miller FH. Acute Pancreatitis: Revised Atlanta Classification and the Role of Cross-Sectional Imaging. AJR Am J Roentgenol. 2015;205:W32-W41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 56. | Ning C, Ouyang H, Xiao J, Wu D, Sun Z, Liu B, Shen D, Hong X, Lin C, Li J, Chen L, Zhu S, Li X, Xia F, Huang G. Development and validation of an explainable machine learning model for mortality prediction among patients with infected pancreatic necrosis. EClinicalMedicine. 2025;80:103074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 57. | Pavlidis ET, Pavlidis TE. Management of infected acute necrotizing pancreatitis. World J Clin Cases. 2023;11:482-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 58. | Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Rau B, Steinbach G, Baumgart K, Gansauge F, Grünert A, Beger HG. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med. 2000;26 Suppl 2:S159-S164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Cho IR, Do MY, Han SY, Jang SI, Cho JH. Comparison of Interleukin-6, C-Reactive Protein, Procalcitonin, and the Computed Tomography Severity Index for Early Prediction of Severity of Acute Pancreatitis. Gut Liver. 2023;17:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Liu Q, Yang J, Zhang J. Factors affecting the time interval of endoscopic ultrasound-guided endoscopic necrosectomy of walled-off pancreatic necrosis: A retrospective single-center study in China. Pancreatology. 2024;24:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Oh CH, Song TJ, Lee JK, Park JS, Lee JM, Son JH, Jang DK, Choi M, Byeon JS, Lee IS, Lee ST, Choi HS, Kim HG, Chun HJ, Park CG, Cho JY. Clinical Practice Guidelines for the Endoscopic Management of Peripancreatic Fluid Collections. Gut Liver. 2021;15:677-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 63. | Lakhtakia S. The endoscopic ultrasound features of pancreatic fluid collections: appearances can be deceptive! Endoscopy. 2022;54:563-564. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Zhu H, Du Y, Wang K, Li Z, Jin Z. Consensus guidelines on the diagnosis and treatment of pancreatic pseudocyst and walled-off necrosis from a Chinese multiple disciplinary team expert panel. Endosc Ultrasound. 2024;13:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 65. | Stecher SS, Simon P, Friesecke S, Glitsch A, Kühn JP, Lerch MM, Mayerle J. Delayed severe bleeding complications after treatment of pancreatic fluid collections with lumen-apposing metal stents. Gut. 2017;66:1871-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Ding L, Li XY, Tan JX, Xia L, He WH, Xiong HF, Zhu Y, Liu P, Shu X, Liu ZJ, Zhu Y, Chen YX, Lu NH. Association between morphological features of necrotizing pancreatitis on endoscopic ultrasound and outcomes of the endoscopic transmural step-up approach. J Dig Dis. 2022;23:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Laleman W, Vanderschueren E, Van der Merwe S, Chang KJ. The use of endoscopic ultrasound in the diagnosis and management of portal hypertension. Best Pract Res Clin Gastroenterol. 2022;60-61:101811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Hammoud GM, Ibdah JA. Utility of endoscopic ultrasound in patients with portal hypertension. World J Gastroenterol. 2014;20:14230-14236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | ASGE Standards of Practice Committee; Forbes N, Coelho-Prabhu N, Al-Haddad MA, Kwon RS, Amateau SK, Buxbaum JL, Calderwood AH, Elhanafi SE, Fujii-Lau LL, Kohli DR, Pawa S, Storm AC, Thosani NC, Qumseya BJ. Adverse events associated with EUS and EUS-guided procedures. Gastrointest Endosc. 2022;95:16-26.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 70. | Lyu Y, Li T, Wang B, Cheng Y, Chen L, Zhao S. Comparison Between Lumen-Apposing Metal Stents and Plastic Stents in Endoscopic Ultrasound-Guided Drainage of Pancreatic Fluid Collection: A Meta-analysis and Systematic Review. Pancreas. 2021;50:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Mohan BP, Chandan S, Khan SR, Kassab LL, Trakroo S, Ponnada S, Asokkumar R, Adler DG. Efficacy and safety of endoscopic ultrasound-guided therapy versus direct endoscopic glue injection therapy for gastric varices: systematic review and meta-analysis. Endoscopy. 2020;52:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 72. | Nemoto Y, Attam R, Arain MA, Trikudanathan G, Mallery S, Beilman GJ, Freeman ML. Interventions for walled off necrosis using an algorithm based endoscopic step-up approach: Outcomes in a large cohort of patients. Pancreatology. 2017;17:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, Beilman G, Flanagan S, Freeman ML, Mallery S. Early (<4 Weeks) Versus Standard (≥ 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol. 2018;113:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 74. | Niu CG, Zhang J, Zhu KW, Liu HL, Ashraf MF, Okolo PI 3rd. Comparison of early and late intervention for necrotizing pancreatitis: A systematic review and meta-analysis. J Dig Dis. 2023;24:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Rana SS, Gupta R. Early transluminal drainage of pancreatic necrotic collections. Gastrointest Endosc. 2020;92:1136. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Hocke M, Burmeister S, Braden B, Jenssen C, Arcidiacono PG, Iglesias-Garcia J, Ignee A, Larghi A, Möller K, Rimbas M, Siyu S, Vanella G, Dietrich CF. Controversies in EUS-guided treatment of walled-off necrosis. Endosc Ultrasound. 2022;11:442-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Dronov OI, Kovalska IO, Horlach AI, Shchyhel IA. Prediction of External Pancreatic Fistula Development in Patients With Acute Infected Necrotising Pancreatitis. Wiad Lek. 2023;76:2365-2371. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Liepert AE, Ventro G, Weaver JL, Berndtson AE, Godat LN, Adams LM, Santorelli J, Costantini TW, Doucet JJ. Decreasing use of pancreatic necrosectomy and NSQIP predictors of complications and mortality. World J Emerg Surg. 2022;17:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Luo Z, Shi J, Fang Y, Pei S, Lu Y, Zhang R, Ye X, Wang W, Li M, Li X, Zhang M, Xiang G, Pan Z, Zheng X. Development and evaluation of machine learning models and nomogram for the prediction of severe acute pancreatitis. J Gastroenterol Hepatol. 2023;38:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 80. | Liu F, Yao J, Liu C, Shou S. Construction and validation of machine learning models for sepsis prediction in patients with acute pancreatitis. BMC Surg. 2023;23:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 81. | Yasuda I, Takahashi K. Endoscopic management of walled-off pancreatic necrosis. Dig Endosc. 2021;33:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 82. | Bai R, Sui Y, Lu T, Chen H, Wang G, Kong R, Tan H, Wang Y, Li G, Sun B. Effect of the Step-Jump Approach in Infected Pancreatic Necrosis: A Propensity Score-Matched Study. J Inflamm Res. 2024;17:6005-6021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Purschke B, Bolm L, Meyer MN, Sato H. Interventional strategies in infected necrotizing pancreatitis: Indications, timing, and outcomes. World J Gastroenterol. 2022;28:3383-3397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 84. | Zheng Z, Lu J, Cao F, Ding Y, Guo Y, Mei W, Qu Y, Liu S, Sun H, Cui Y, Li A, Li F. "One-step" approach versus "Step-up" approach minimally invasive treatment for infected pancreatic necrosis: a study protocol for a single-center, prospective, randomized controlled trial. BMC Gastroenterol. 2022;22:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, Poley JW, van Ramshorst B, Vleggaar FP, Boermeester MA, Gooszen HG, Weusten BL, Timmer R; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 511] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 86. | Hines OJ, Donald GW. Endoscopic transgastric necrosectomy for infected necrotizing pancreatitis. JAMA. 2012;307:1084-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Chahal P, Papachristou GI, Baron TH. Endoscopic transmural entry into pancreatic fluid collections using a dedicated aspiration needle without endoscopic ultrasound guidance: success and complication rates. Surg Endosc. 2007;21:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Omelchuk N. Minimally-invasive methods of acute pancreatic postnecrotic pseudocysts treatment. Pancreatology. 2019;19:S148. [DOI] [Full Text] |
| 89. | Jagielski M, Smoczyński M, Adrych K. The role of transpapillary drainage in management of patients with pancreatic fluid collections and pancreatic duct disruption as a consequences of severe acute pancreatitis. Pancreatology. 2017;17:30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Ni J, Peng K, Yu L, Xie H, Luo S, Xu K, Xia Y, Xie N, Lu J, Lu L, Hu D, Wan X, Li B. Transpapillary Stenting Improves Treatment Outcomes in Patients Undergoing Endoscopic Transmural Drainage of Ductal Disruption-Associated Pancreatic Fluid Collections. Am J Gastroenterol. 2023;118:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 91. | Muangkaew P, Kamalaporn P, Mingphruedhi S, Rungsakulkij N, Suragul W, Vassanasiri W, Tangtawee P. Outcomes of delayed endoscopic retrograde cholangiopancreatography in patients with acute biliary pancreatitis with cholangitis. Asian J Surg. 2020;43:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 205] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 93. | Lee SY, Park SH, Do MY, Lee DK, Jang SI, Cho JH. Increased ERCP-related adverse event from premature urgent ERCP following symptom onset in acute biliary pancreatitis with cholangitis. Sci Rep. 2024;14:13663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 94. | Gorris M, van der Valk NP, Fockens P, Jacobs MA, Montazeri NSM, Voermans RP, Wielenga MC, van Hooft JE, van Wanrooij RL. Does same session EUS-guided tissue acquisition and ERCP increase the risk of pancreatitis in patients with malignant distal biliary obstruction? HPB (Oxford). 2022;24:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 95. | Fusaroli P, Lisotti A. EUS and ERCP in the Same Session for Biliary Stones: From Risk Stratification to Treatment Strategy in Different Clinical Conditions. Medicina (Kaunas). 2021;57:1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Keshavarz P, Azrumelashvili T, Yazdanpanah F, Nejati SF, Ebrahimian Sadabad F, Tarjan A, Bazyar A, Mizandari M. Percutaneous catheter drainage of pancreatic associated pathologies: A systematic review and meta-analysis. Eur J Radiol. 2021;144:109978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Rana SS, Verma S, Kang M, Gorsi U, Sharma R, Gupta R. Comparison of endoscopic versus percutaneous drainage of symptomatic pancreatic necrosis in the early (< 4 weeks) phase of illness. Endosc Ultrasound. 2020;9:402-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 98. | Mangiafico S, Bertani H, Pigò F, Russo S, Lupo M, Cocca S, Grande G, Germani U, Manta R, Conigliaro R. A New Step-Up Dual Endoscopic Approach for Large-Size Infected Pancreatic Necrosis: Percutaneous Endoscopic Necrosectomy Followed by Transluminal Endoscopic Drainage/Necrosectomy. Surg Laparosc Endosc Percutan Tech. 2024;34:156-162. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 99. | Binda C, Sbrancia M, La Marca M, Colussi D, Vizzuso A, Tomasoni M, Agnoletti V, Giampalma E, Ansaloni L, Fabbri C. EUS-guided drainage using lumen apposing metal stent and percutaneous endoscopic necrosectomy as dual approach for the management of complex walled-off necrosis: a case report and a review of the literature. World J Emerg Surg. 2021;16:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Shionoya K, Matsunami Y, Itoi T. Endoscopic ultrasound-guided transmural pancreatic cyst drainage using an electrocautery lumen-apposing metal stent without fluoroscopy for critically ill patients in the intensive care unit setting. Dig Endosc. 2024;36:244-245. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 101. | Iwashita T, Iwata K, Hamada T, Saito T, Shiomi H, Takenaka M, Maruta A, Uemura S, Masuda A, Matsubara S, Mukai T, Takahashi S, Hayashi N, Isayama H, Yasuda I, Nakai Y. Supportive treatment during the periprocedural period of endoscopic treatment for pancreatic fluid collections: a critical review of current knowledge and future perspectives. J Gastroenterol. 2023;58:98-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | van Wanrooij RLJ, Bronswijk M, Kunda R, Everett SM, Lakhtakia S, Rimbas M, Hucl T, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Pérez-Miranda M, van Hooft JE, van der Merwe SW. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022;54:310-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 103. | Mussetto A, Fugazza A, Fuccio L, Triossi O, Repici A, Anderloni A. Current uses and outcomes of lumen-apposing metal stents. Ann Gastroenterol. 2018;31:535-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 104. | Yi H, Liu Q, He S, Zhong L, Wu SH, Guo XD, Ning B. Current uses of electro-cautery lumen apposing metal stents in endoscopic ultrasound guided interventions. Front Med (Lausanne). 2022;9:1002031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Salame M, Gleeson FC, Chandrasekhara V, Law RJ, Rajan E, Iyer PG, Bofill-Garcia A, Ghanem OM, Abu Dayyeh BK, Ravi A, Storm AC, Vargas EJ. Safety of EUS latex balloon use in patients with a latex allergy. Gastrointest Endosc. 2024;99:1032-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 106. | Braden B, Hocke M, Selvaraj E, Kaushal K, Möller K, Ignee A, Vanella G, Arcidiacono PG, Teoh A, Larghi A, Rimbas M, Hollerbach S, Napoleon B, Dong Y, Dietrich CF. Mishaps with EUS-guided lumen-apposing metal stents in therapeutic pancreatic EUS: Management and prevention. Endosc Ultrasound. 2023;12:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Wang BH, Xie LT, Zhao QY, Ying HJ, Jiang TA. Balloon dilator controls massive bleeding during endoscopic ultrasound-guided drainage for pancreatic pseudocyst: A case report and review of literature. World J Clin Cases. 2018;6:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Parsa N, Nieto JM, Powers P, Mitsuhashi S, Abdelqader A, Hadzinakos G, Anderloni AA, Fugazza A, James TW, Arlt A, Ellrichmann M, Aparicio JR, Trindade AJ, Stevens TK, Chahal P, Shah SL, Messallam AA, Lang G, Fejleh MP, Benias PC, Sejpal DV, Jones J, Mir FF, Aghaie Meybodi M, Ichkhanian Y, Vosoughi K, Novikov AA, Irani SS, Pawa R, Ahmed AM, Sedarat A, Hsueh W, Hampe J, Sharaiha RZ, Berzin TM, Willingham FF, Kushnir VM, Brewer Gutierrez OI, Ngamruengphong S, Huggett MT, Baron TH, Repici A, Adler DG, Nasr JT, Kowalski TE, Kumbhari V, Singh VK, Khashab MA. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20-mm versus 15-mm lumen-apposing metal stents: an international, multicenter, case-matched study. Endoscopy. 2020;52:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Guzmán-Calderón E, Chacaltana A, Díaz R, Li B, Martinez-Moreno B, Aparicio JR. Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: A systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2022;29:198-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 110. | Brimhall B, Han S, Tatman PD, Clark TJ, Wani S, Brauer B, Edmundowicz S, Wagh MS, Attwell A, Hammad H, Shah RJ. Increased Incidence of Pseudoaneurysm Bleeding With Lumen-Apposing Metal Stents Compared to Double-Pigtail Plastic Stents in Patients With Peripancreatic Fluid Collections. Clin Gastroenterol Hepatol. 2018;16:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 111. | Oh D, Lee JH, Song TJ, Song KB, Hwang DW, Kim JH, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Clinical outcomes of EUS-guided transluminal drainage with a novel lumen-apposing metal stent for postoperative pancreatic fluid collection after pancreatic surgery. Gastrointest Endosc. 2022;95:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Boxhoorn L, Verdonk RC, Besselink MG, Boermeester M, Bollen TL, Bouwense SA, Cappendijk VC, Curvers WL, Dejong CH, van Dijk SM, van Dullemen HM, van Eijck CH, van Geenen EJ, Hadithi M, Hazen WL, Honkoop P, van Hooft JE, Jacobs MA, Kievits JE, Kop MP, Kouw E, Kuiken SD, Ledeboer M, Nieuwenhuijs VB, Perk LE, Poley JW, Quispel R, de Ridder RJ, van Santvoort HC, Sperna Weiland CJ, Stommel MW, Timmerhuis HC, Witteman BJ, Umans DS, Venneman NG, Vleggaar FP, van Wanrooij RL, Bruno MJ, Fockens P, Voermans RP; Dutch Pancreatitis Study Group. Comparison of lumen-apposing metal stents versus double-pigtail plastic stents for infected necrotising pancreatitis. Gut. 2023;72:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 113. | Shamah SP, Sahakian AB, Chapman CG, Buxbaum JL, Muniraj T, Aslanian HA, Villa E, Cho J, Haider HI, Waxman I, Siddiqui UD. Double pigtail stent placement as an adjunct to lumen-apposing metal stentsfor drainage of pancreatic fluid collections may not affect outcomes: A multicenter experience. Endosc Ultrasound. 2022;11:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Vanek P, Falt P, Vitek P, Zoundjiekpon V, Horinkova M, Zapletalova J, Lovecek M, Urban O. EUS-guided transluminal drainage using lumen-apposing metal stents with or without coaxial plastic stents for treatment of walled-off necrotizing pancreatitis: a prospective bicentric randomized controlled trial. Gastrointest Endosc. 2023;97:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 115. | Giri S, Harindranath S, Afzalpurkar S, Angadi S, Sundaram S. Does a coaxial double pigtail stent reduce adverse events after lumen apposing metal stent placement for pancreatic fluid collections? A systematic review and meta-analysis. Ther Adv Gastrointest Endosc. 2023;16:26317745231199364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Ali H, Inayat F, Jahagirdar V, Jaber F, Afzal A, Patel P, Tahir H, Anwar MS, Rehman AU, Sarfraz M, Chaudhry A, Nawaz G, Dahiya DS, Sohail AH, Aziz M. Early versus delayed necrosectomy in pancreatic necrosis: A population-based cohort study on readmission, healthcare utilization, and in-hospital mortality. World J Methodol. 2024;14:91810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 117. | Guo J, Saftoiu A, Vilmann P, Fusaroli P, Giovannini M, Mishra G, Rana SS, Ho S, Poley JW, Ang TL, Kalaitzakis E, Siddiqui AA, De La Mora-Levy JG, Lakhtakia S, Bhutani MS, Sharma M, Mukai S, Garg PK, Lee LS, Vila JJ, Artifon E, Adler DG, Sun S. A multi-institutional consensus on how to perform endoscopic ultrasound-guided peri-pancreatic fluid collection drainage and endoscopic necrosectomy. Endosc Ultrasound. 2017;6:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 118. | Lan L, Guo Q, Zhang Z, Zhao W, Yang X, Lu H, Zhou Z, Zhou X. Classification of Infected Necrotizing Pancreatitis for Surgery Within or Beyond 4 Weeks Using Machine Learning. Front Bioeng Biotechnol. 2020;8:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Brand M, Hofmann N, Ho CN, Meining A. The over-the-scope grasper (OTSG). Endoscopy. 2021;53:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Stassen PMC, de Jonge PJF, Bruno MJ, Koch AD, Trindade AJ, Benias PC, Sejpal DV, Siddiqui UD, Chapman CG, Villa E, Tharian B, Inamdar S, Hwang JH, Barakat MT, Andalib I, Gaidhane M, Sarkar A, Shahid H, Tyberg A, Binmoeller K, Watson RR, Nett A, Schlag C, Abdelhafez M, Friedrich-Rust M, Schlachterman A, Chiang AL, Loren D, Kowalski T, Kahaleh M. Safety and efficacy of a novel resection system for direct endoscopic necrosectomy of walled-off pancreas necrosis: a prospective, international, multicenter trial. Gastrointest Endosc. 2022;95:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 121. | Yachimski P, Landewee CA, Campisano F, Valdastri P, Obstein KL. The waterjet necrosectomy device for endoscopic management of pancreatic necrosis: design, development, and preclinical testing (with videos). Gastrointest Endosc. 2020;92:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Werge M, Novovic S, Roug S, Knudsen JD, Feldager E, Gluud LL, Schmidt PN. Evaluation of local instillation of antibiotics in infected walled-off pancreatic necrosis. Pancreatology. 2018;18:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 123. | Bhargava V, Gupta R, Vaswani P, Jha B, Rana SS, Gorsi U, Kang M, Gupta R. Streptokinase irrigation through a percutaneous catheter helps decrease the need for necrosectomy and reduces mortality in necrotizing pancreatitis as part of a step-up approach. Surgery. 2021;170:1532-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 124. | Bhargava MV, Rana SS, Gorsi U, Kang M, Gupta R. Assessing the Efficacy and Outcomes Following Irrigation with Streptokinase Versus Hydrogen Peroxide in Necrotizing Pancreatitis: A Randomized Pilot Study. Dig Dis Sci. 2022;67:4146-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/