Published online May 28, 2025. doi: 10.3748/wjg.v31.i20.104891
Revised: March 29, 2025
Accepted: May 6, 2025
Published online: May 28, 2025
Processing time: 141 Days and 23.4 Hours
The diagnosis of primary biliary cholangitis (PBC) remains challenging, particularly in cases where anti-mitochondrial antibody (AMA), anti-mitochondrial E2 subunit antibody (AMA-M2), anti-glycoprotein 210 (anti-gp210), and anti-spec
To identify additional plasma biomarkers for non-invasive diagnostic methods of PBC.
We utilized the Sengenics KREX™ immunome protein array to identify potential biomarkers for the diagnosis of PBC. Subsequently, we validated the predictive capability of the RPL30 antibody through an ELISA and retro
In our study we observed that RPL30 demonstrated the highest fold-change difference in PBC, with a penetrance frequency of 40% and a penetrance fold change of 38.30147. The analysis of anti-RPL30 optical density values between patients with AMA/AMA-M2/anti-gp210/anti-Sp100-negative PBC (autoantibody-negative PBC) and healthy controls using a receiver operating characteristic curve yielded an area under the curve of 0.853. This analysis established an optimal cutoff value of 0.0708, achieving 100% specificity and 75% sensitivity. The com
Our study highlighted the potential of anti-RPL30 as a promising biomarker for diagnosing PBC, particularly in autoantibody-negative cases.
Core Tip: The diagnosis of autoantibody-negative primary biliary cholangitis (PBC) remains challenging and is typically confirmed through liver needle biopsy. Using the immunome protein array, RPL30 was identified as a novel biomarker for PBC. Validation of the RPL30 autoantibody through ELISA analysis showed a higher positivity rate in autoantibody-negative PBC cases, including anti-mitochondrial antibody, anti-mitochondrial E2 subunit antibody, anti-glycoprotein 210, and anti-speckled protein 100-negative cases compared with the healthy control group. Anti-RPL30 improved the diagnostic rate of antibody-negative PBC and correlated with hepatic damage, poorer synthetic function, and higher Model for End-Stage Liver Disease scores.
- Citation: Zeng ZY, Huang ZX, Wang YR, Xie LK, Lin YP, Liang Y, Liu ZY, Li DL, Zhang XY. Anti-RPL30 as a novel biomarker for enhanced diagnosis of autoantibody-negative primary biliary cholangitis. World J Gastroenterol 2025; 31(20): 104891
- URL: https://www.wjgnet.com/1007-9327/full/v31/i20/104891.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i20.104891
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease characterized by progressive, non-suppurative inflammation of the intrahepatic small bile ducts. It predominantly affects females aged 40 to 60 years and if left untreated often advances to liver fibrosis and cirrhosis. Notably, during the 1980s, half of the patients diagnosed with PBC exhibited one or more symptoms, with the disease having already progressed to cirrhosis in some cases[1]. After 2000 a study conducted in Japan disclosed that 75%-80% of patients with PBC were diagnosed while asymptomatic and without evidence of cirrhosis[2]. Hence, early diagnosis and appropriate treatment are pivotal in effectively managing PBC.
However, the challenge lies in the fact that many patients with PBC primarily present with common liver function abnormalities, making accurate diagnosis elusive. Recognized as an immune-mediated disorder, the diagnosis of PBC heavily relies on the detection of autoantibodies along with abnormal liver enzymes (cholestasis markers). Approximately 10%-15% of patients with PBC do not test positive for anti-mitochondrial antibody (AMA)[3,4]. The positivity rates of anti-glycoprotein 210 (anti-gp210) and anti-speckled protein 100 (anti-Sp100) are relatively low. The epidemiology of PBC in cases where autoantibodies are completely negative remains unknown. Autoantibody-negative or anti-mitochondrial E2 subunit antibody (AMA-M2) negative PBC may indeed be underestimated due to the significant challenges in accurate diagnosis. Therefore, there is an urgent need to explore additional plasma biomarkers that could enable the non-invasive diagnosis of autoantibody-negative PBC.
In this study, we utilized Sengenics KREX™ technology to identify high-value, true-positive biomarkers. We validated their sensitivity and specificity using an ELISA and analyzed their association with clinical features.
The study encompassed two independent cohorts of patients. Cohort 1 comprised 5 patients diagnosed with PBC, another 5 patients with autoimmune hepatitis (AIH), and another 5 patients with chronic hepatitis B (CHB) as well as 2 healthy donors. These samples were utilized for the immunome protein array analysis. Cohort 2 included 17 subjects who tested negative for PBC-specific autoantibodies, such as AMA, AMA-2, anti-gp210, and anti-Sp100 autoantibodies. These individuals were screened from 62 cases of PBC and diagnosed based on pathological findings combined with either cholestasis or the presence of positive antibodies. Cohort 2 consisted of 20 normal controls. In both cases, patient diag
In accordance with international standards, the diagnosis of PBC in this study was guided by the European Association for the Study of the Liver Clinical Practice Guidelines. A confirmed diagnosis required meeting at least two of the following three criteria: (1) Biochemical evidence of cholestasis (alkaline phosphatase > 1.5 × upper limit of normal for more than 6 months); (2) Presence of AMA ≥ 1:40 or PBC-specific anti-nuclear antibodies (anti-gp210 or anti-Sp100); and (3) Histopathological features consistent with PBC. The inclusion criteria were strictly defined as patients meeting at least two of the aforementioned diagnostic criteria accompanied by complete serological and biochemical data. The exclusion criteria encompassed cases with incomplete liver biopsy records, insufficient clinical documentation, or unavailability of serum samples.
Consecutive eligible patients were recruited at the hepatology clinic of the 900th Hospital of the PLA Joint Logistic Support Force between January 2002 and December 2022. For the retrospective analysis cohort, clinicopathological data from 62 patients with PBC were collected from electronic medical records. The collected data included gender, age, immunoglobulin levels, antinuclear antibody titer, AMA, AMA-2, anti-gp210, anti-Sp100, liver function tests, renal function tests, routine blood indices, coagulation indicators, and Model for End-Stage Liver Disease (MELD) scores. The data were selected from the most recent records corresponding to the time of serum sample collection. All remaining serum samples from patients with PBC and controls were obtained from a biobank at the 900th Hospital of the PLA, which had accumulated over nearly two decades. Written informed consent was obtained from all participants recruited for the study (Protocol 2022-054), which was approved by the ethical committee of the 900th Hospital of the PLA. To ensure confidentiality, all clinical information was recorded anonymously and coded.
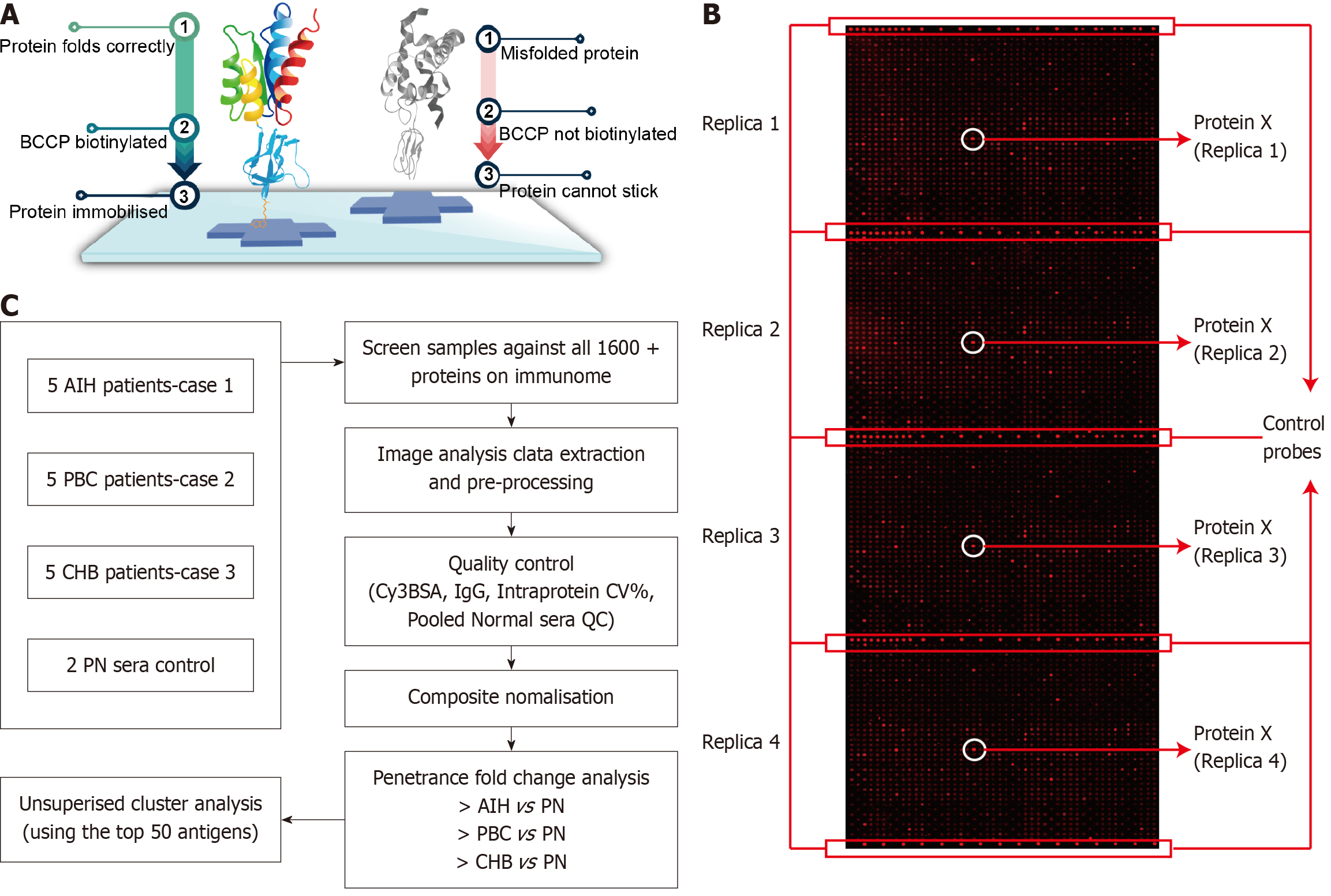
The immunome protein array, a critical component of this technology, employs KREX proteins that are cloned alongside biotin carboxyl carrier protein (BCCP), which functions as a folding marker and solubility enhancer. Biotin is then introduced into the cells where it binds to correctly folded proteins via cellular biotin ligases. Misfolded proteins, which can cause co-translational misfolding of BCCP, are washed away during the process, leading to the loss of BCCP function. Only correctly folded proteins remain attached through the binding interaction involving BCCP (Figure 1A).
By employing this method, we discovered the RPL30 autoantibody in patients with PBC, and we further validated this finding through an ELISA assay. This discovery underscored RPL30 as a new biomarker for diagnosing PBC, thereby improving diagnostic efficiency. Human peripheral blood samples were collected via venipuncture, and plasma samples were isolated through centrifugation and subsequently stored at -80 °C. Plasma samples from cohort 1 were subsequently sent to Sengenics for analysis using the immunome protein array. This array features over 1600 KREX™ proteins spotted in quadruplicate, offering picogram-level sensitivity and fully quantitative arrays with exceptional consistency (Figure 1B). All samples successfully met the quality control parameters, which evaluated quantitative metrics pertaining to the array and assay quality as well as the consistency of the results.
Notably, the results demonstrated exceptional data consistency. The coefficient of variance for the intra-protein, intra-slide, and inter-array measurements for all proteins and control probes was below the quality control limit of 15% (Figure 1C). Subsequently, an in-depth, statistically rigorous analysis was conducted on all data to verify the reproducibility of each array. Frequency analysis was utilized to identify any proteins exhibiting differences in immunoglobulin binding between the case and control groups. The data were then normalized to yield a normally distributed dataset. Using the penetrance fold change method from Sengenics, significant protein biomarkers were identified for comparison between the case and control groups.
A qualitative ELISA assay was conducted to analyze anti-RPL30 antibodies in plasma. Plates were coated with RPL30 protein (source: Abnova, characteristics: RPL30 Recombinant Protein, dilution status: 1 μg/mL), which served as the capture antigen, and incubated overnight at 4 °C. After washing, the plates were blocked with 5% BSA at 37 °C for 1 h. Plasma samples, pre-diluted 10-fold with PBS, were subsequently added to the plates and incubated at 37 °C for 2 h. Following this, the secondary antibody, horseradish peroxidase-conjugated anti-human IgG, was introduced, and the plates were incubated for 45 min at room temperature. Finally, the substrate, TMB, was added and incubated for 15 min. Following this incubation period, the reaction was halted using H2SO4, and the absorbance was measured at an optical density of 450 nm. The autoimmune bodies of AMA, AMA-2, anti-gp210, and anti-Sp100 were detected using ELISA kits (Oumeng Diagnostics, Peking, China).
Continuous variables were presented as either mean ± SD or as median with interquartile range. Com
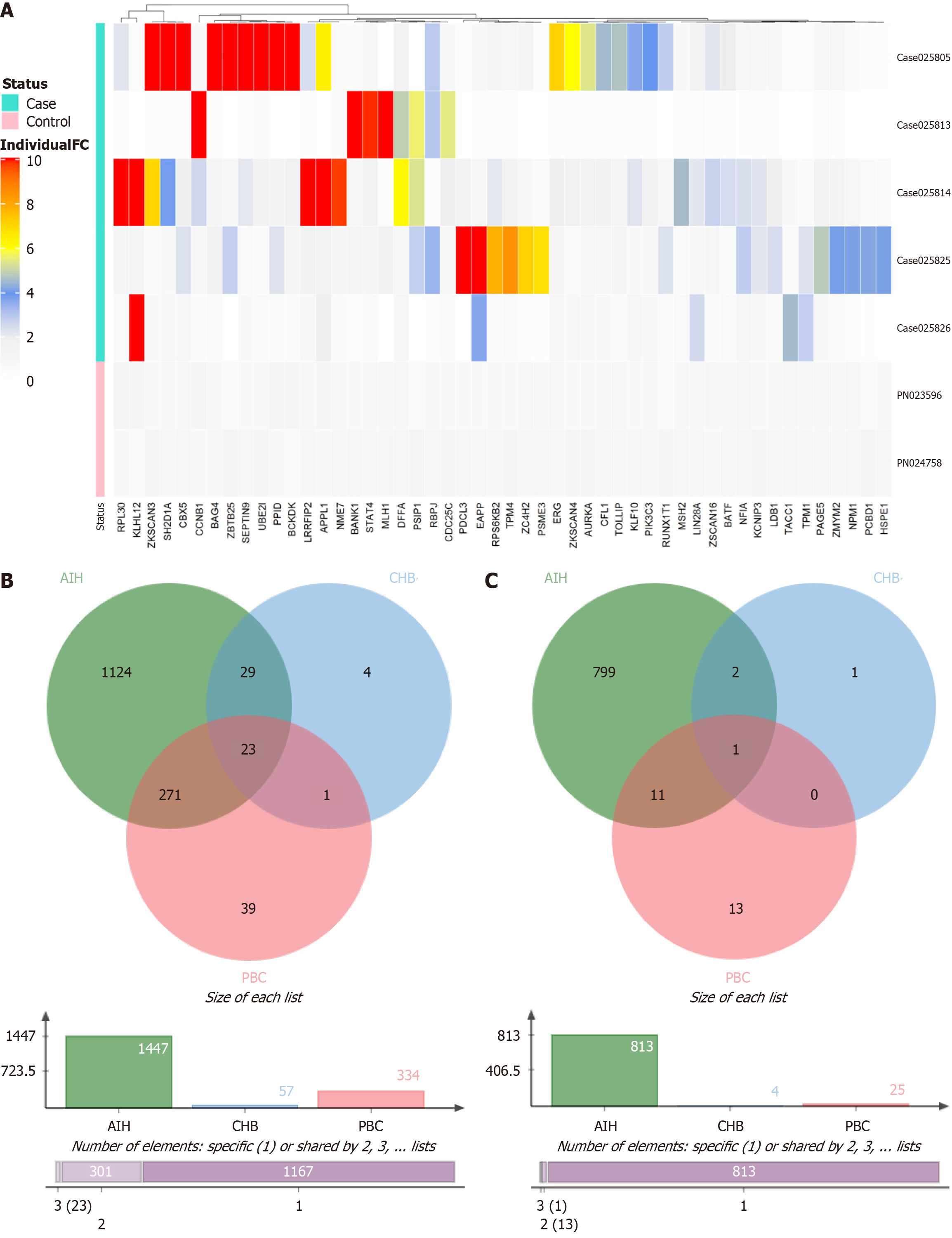
To pinpoint a dependable biomarker for streamlining PBC diagnosis using serum samples, we conducted a self-antigen assay with the immunome protein array including 5 patients each with PBC, AIH, and CHB as well as two healthy donors. The analysis was performed using a penetrance fold change-based method with criteria set at penetrance fold change ≥ 2.0 and penetrance frequency ≥ 20%. This approach identified the top 50 antigens with high autoantibody titers PBC, AIH, and CHB (Supplementary Figure 1).
Subsequently, penetrance fold change was analyzed in comparison with normal samples, resulting in the identification of the top 50 self-antigens (Supplementary Table 1, Figure 2A). Unsupervised clustering of these top 50 biomarker hits across patients with AIH, PBC, and CHB was conducted for further screening. Patients with AIH and CHB (5 patients/cohort) were enrolled for crosschecking to enhance the specificity of PBC biomarkers. The analysis revealed that out of the 334 molecular markers, 271 were double-positive in patients with AIH, 1 was positive in patients with CHB, and 23 were triple-positive. Consequently, only 39 markers (with penetrance frequency ≥ 20%) and 13 markers (with penetrance frequency ≥ 30%) were identified exclusively in PBC samples (Figure 2B and 2C).
The disease association of the top 50 antigens for AIH, PBC, and CHB with elevated autoantibody responses was established through a literature and data mining approach using the open targets platform. Comparing the top 50 biomarkers identified through penetrance fold change analysis across patients with AIH, PBC, and CHB (Supplementary Table 2) revealed that RPL30 had the highest penetrance fold change difference value, indicating that the RPL30 autoantibody may serve as a biomarker with a relatively high sensitivity and specificity for PBC diagnosis.
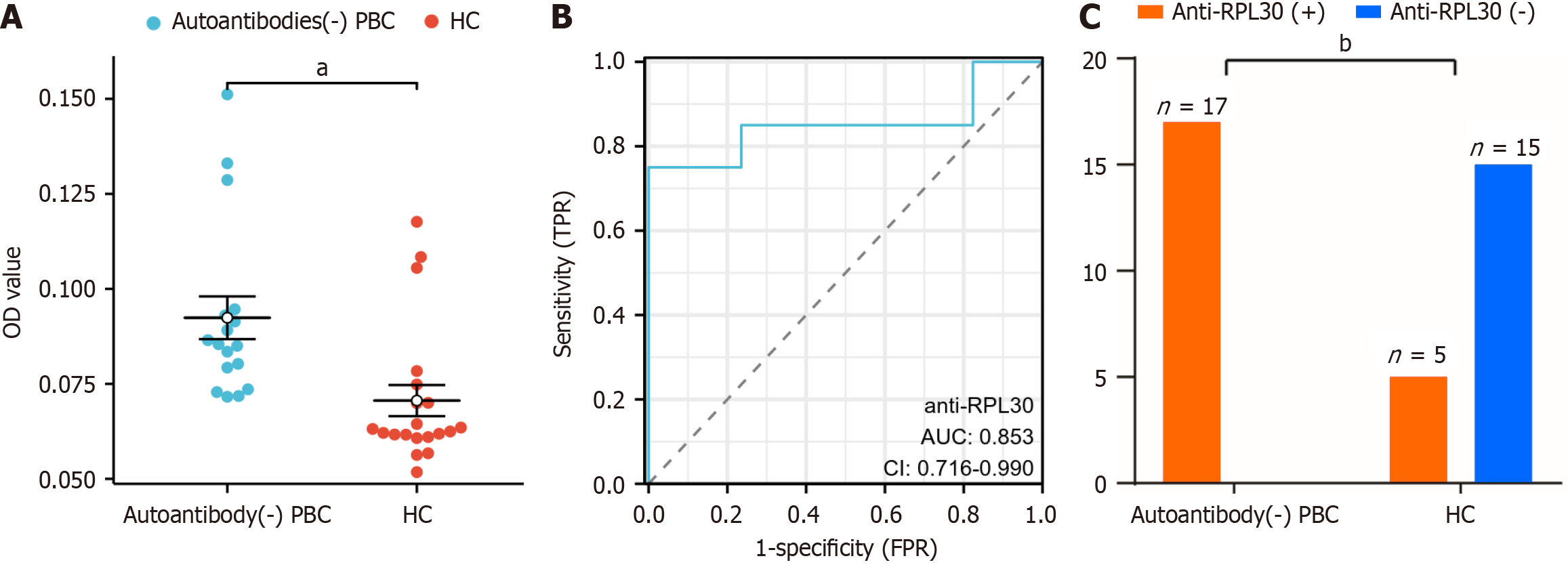
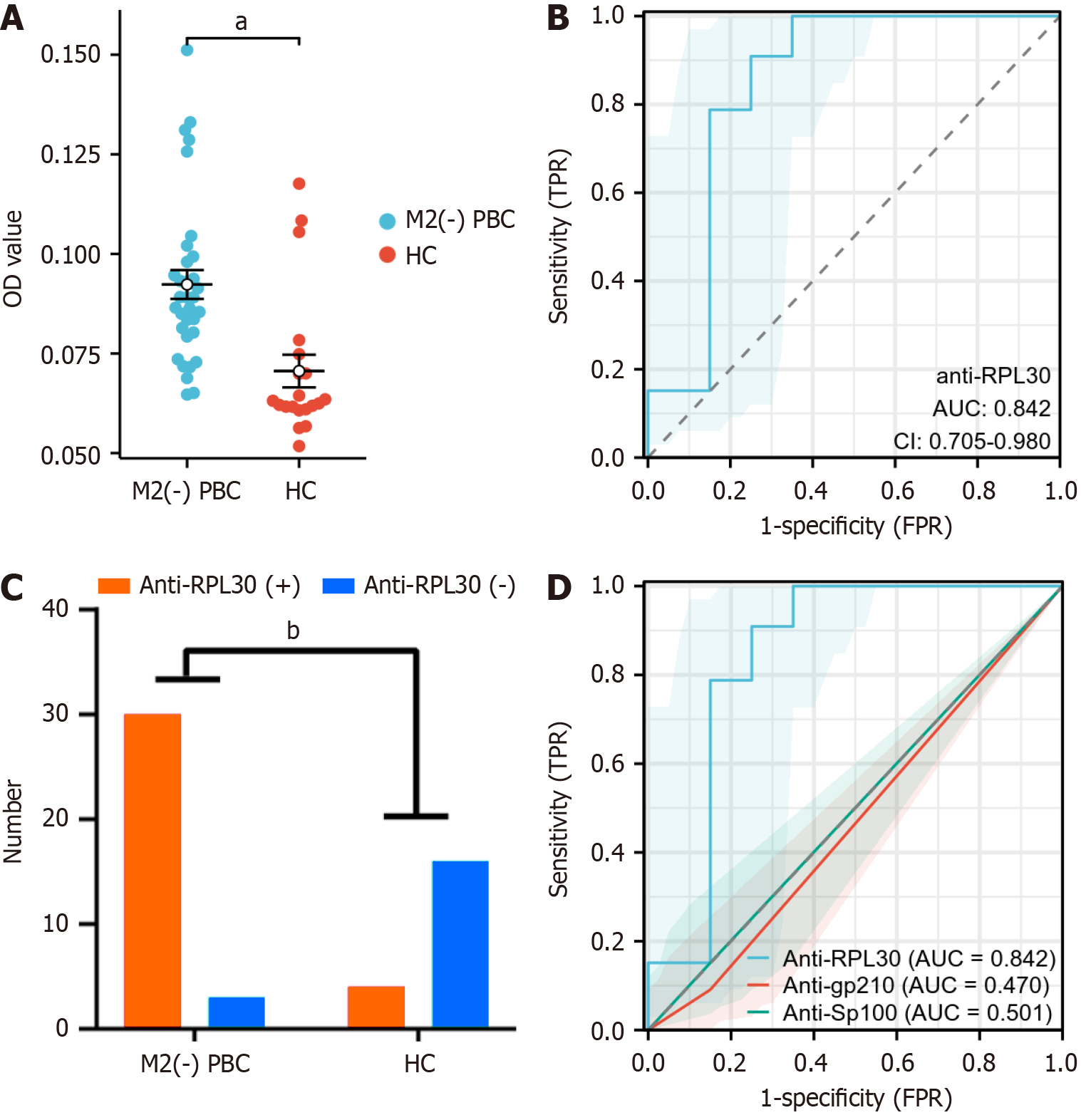
To validate the predictive role of anti-RPL30 in PBC, we developed an ELISA assay using RPL30 as the capturing antigen. The ELISA results showed that the levels of autoantibodies specific to RPL30 in autoantibody-negative PBC and AMA-M2-negative PBC samples were significantly higher than those in healthy controls (HC; Figure 3A and 4A). Furthermore, ROC curve analysis demonstrated the high sensitivity of the anti-RPL30 ELISA assay, with AUC values of 0.853 and 0.842 (Figure 3B and 4B). The optimal cutoff value for the anti-RPL30 ELISA assay was determined to be 0.0708, achieving 100% specificity and 75.0% sensitivity (75.0% specificity and 90.9% sensitivity for the AMA-2 PBC group). According to this cutoff value, all patients with autoantibody-negative PBC tested positive for anti-RPL30 compared with only 4 out of 20 HC, indicating a statistically significant difference between the two groups (P < 0.001, Figure 3C). A total of 33 patients with AMA-M2-negative PBC tested positive for anti-RPL30 compared with only 4 out of 20 HC, indicating a statistically significant difference between the two groups (P < 0.001, Figure 4C). In patients with AMA-M2-negative PBC, the AUC for anti-RPL30 was significantly higher than that for anti-gp210 and anti-Sp100 (Figure 4D).
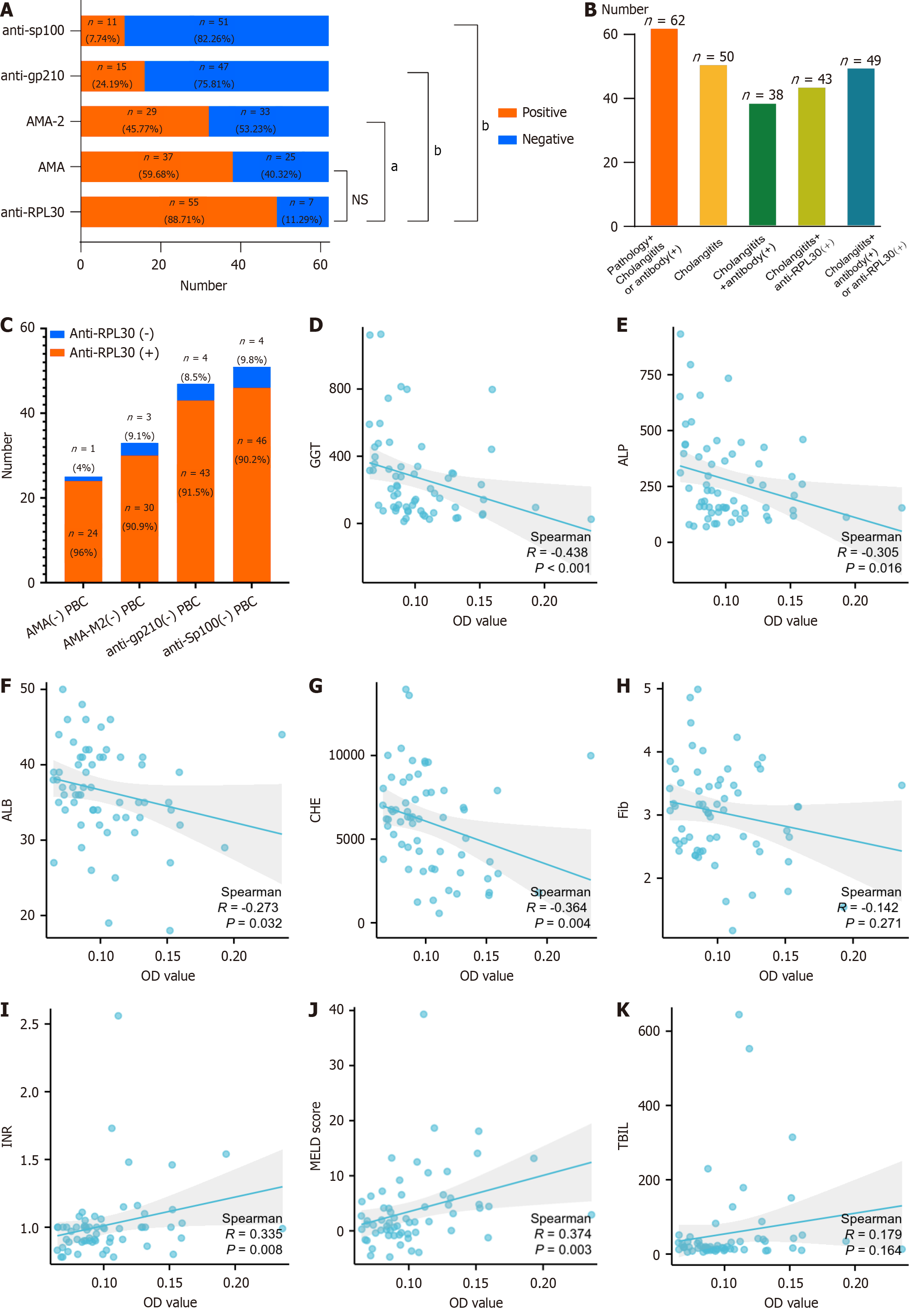
We then examined the relationship between RPL30 autoantibody levels and clinical features. Notably, the frequency of positive anti-RPL30 was significantly higher than that of AMA, AMA-M2, anti-gp210, and anti-Sp100, with statistical differences observed (88.71% vs 59.68%, P = 0.0004, 88.71% vs 46.77%, P < 0.0001, 88.71% vs 24.19%, P < 0.0001, and 88.71% vs 17.74%, P < 0.0001, Figure 5A) in the retrospective cohort. Among the 62 patients with PBC diagnosed based on pathology and cholangitis-related biochemical indicators [total bile acid, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT)], there was a notable improvement in diagnostic accuracy. Initially, the diagnostic yield was 61.3% (38 out of 62) for the group with cholangitis and positive autoantibodies. This increased to 69.4% (43 out of 62) when anti-RPL30 positivity was included.
Additionally, the diagnostic rate surged from 61.3% to 79.0% (P = 0.0489) for patients with cholangitis who tested positive for either anti-RPL30 or other autoantibodies (Figure 5B). In patients with AMA-negative PBC, the anti-RPL30 positivity rate reached 96.0% (24/25). Among patients with AMA-2-negative PBC, the anti-RPL30 positivity rate was 90.1% (30/33). In patients with PBC who were anti-gp210-negative and anti-Sp100-negative, the anti-RPL30 positivity rates were 91.5% (43/47) and 90.2% (46/51), respectively (Figure 5C).
Correlation analysis of anti-RPL30 optical density values with clinical data of patients with PBC revealed a negative association with GGT, ALP, albumin (ALB), cholinesterase (CHE), and fibrinogen (Fib; all P < 0.05, Figure 5D-H), while a positive association was observed with international normalized ratio (INR) and MELD scores (P = 0.008 and P = 0.003, respectively; Figure 5I and J). Based on the ELISA results, the 62 patients with PBC were further classified into 7 anti-RPL30-negative patients and 55 anti-RPL30-positive patients (Supplementary Table 3). The analysis showed no significant differences in total bile acid, TBIL, aspartate aminotransferase, and INR levels between the RPL30-negative and RPL30-positive groups. However, ALP, GGT, alanine aminotransferase, and prothrombin activity levels in the RPL30-positive group were significantly lower than those in the negative group, suggesting that RPL30 is inversely associated with impaired coagulation function.
In this study, we identified a specific RPL30 autoantigen present in patients with PBC using the Sengenics IMMU
The detection rate of PBC is increasing, driven by intensified research efforts, advancements in detection technologies, and improved evaluation methods[7]. According to the latest epidemiological survey in Japan, the annual prevalence of PBC is 33.1 per 100000 population[8]. However, many patients with PBC who develop jaundice often experience rapid progression to liver failure, leading to higher rates of liver transplantation and mortality[9]. Hence, early diagnosis and treatment of PBC are essential for improving prognosis and extending survival rates. The identification of anti-RPL30 in this study provides a promising new approach to PBC diagnosis.
Autoantibodies, particularly AMA, have been pivotal in PBC diagnosis, with positivity rates previously reported to exceed 90% of cases[10]. However, in our retrospective study, the AMA positivity rate was 59.68%, and the AMA-M2 positivity rate was 46.77%, both of which were notably lower than those reported in earlier studies. Like other auto
Furthermore, the current experimental methods for detecting PBC autoantibodies have certain limitations[12,13]. Anti-gp210 and anti-Sp100 antibodies are recognized as specific markers for PBC, especially in cases where AMA expression is negative. These antibodies may also be linked to disease progression[14]. However, these antibodies exhibit low sen
In the retrospective analysis (Figure 5A), anti-RPL30 showed a significantly higher positivity rate (88.71%) compared with AMA (59.68%), AMA-M2 (46.77%), anti-gp210 (24.19%), and anti-Sp100 (17.74%). This result is particularly meaningful for AMA-negative patients, as anti-RPL30 demonstrated strong diagnostic potential even in seronegative PBC cases. These comparisons underscore the enhanced diagnostic value of anti-RPL30, especially in early or atypical presentations of PBC. Liver histology is often required, leading to an underestimation of the actual number of cases.
RPL30 is a component of the large ribosomal subunit, a large ribonucleoprotein complex responsible for protein synthesis within the cell[16]. In the IMMUNOME™ protein assay, RPL30 exhibited the highest fold change differences between PBC cases and control groups, indicating a significantly higher frequency of antibody production compared with the HC group. Although the expression of anti-RPL30 has not yet been confirmed as disease-specific, it demonstrates potential as a diagnostic marker for autoantibody-negative PBC.
Furthermore, anti-RPL30 exhibits a high positivity rate (100%) in patients with PBC who are negative for anti-AMA, anti-gp210, and anti-Sp100. Anti-RPL30 functions as a sensitive autoimmune antibody biomarker, effectively distinguishing autoantibody-negative PBC from healthy groups. However, its ability to differentiate PBC from other autoimmune diseases requires further investigation. The association of anti-RPL30 with decreased GGT and ALP levels as well as increased INR and MELD scores presents contrasting findings. Notably, the relationship between anti-RPL30 and bile duct disappearance, which requires confirmation through pathology, is significant. RPL30 is not only a widely utilized stable housekeeping gene but also exhibits pleiotropic functions in cancer progression, development, immune regulation, and antimicrobial responses[17-19]. Its molecular mechanisms involve post-transcriptional regulation, ribosome assembly, and integration with cellular signaling networks, establishing it as a critical molecular nexus that bridges fundamental research and clinical translation[20,21]. Currently, no direct link between RPL30 and autoimmune diseases has been established. However, this study identified an association between RPL30 antibodies and PBC. Future research should aim to uncover the dynamic regulatory network of RPL30 in PBC pathogenesis and assess its viability as a novel therapeutic target.
This study had several limitations. The sample size was relatively small, and selection bias may have occurred due to the higher likelihood of liver biopsy in AMA-negative patients. Furthermore, the absence of an external validation cohort restricts the generalizability of our findings. Future research should incorporate independent, multicenter cohorts and longitudinal follow-ups to validate the diagnostic utility of anti-RPL30 and its association with disease progression.
The RPL30 autoantibody showed significant potential for accurately predicting PBC and may serve as an additional biomarker for patients with PBC who test negative for other autoantibodies. Our findings could improve diagnostic accuracy and subsequently enable more effective treatment strategies.
| 1. | Pratt DS. Primary Biliary Cholangitis--A New Name and a New Treatment. N Engl J Med. 2016;375:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A, Vierling JM, Poupon R. Changing nomenclature for PBC: From 'cirrhosis' to 'cholangitis'. Clin Res Hepatol Gastroenterol. 2015;39:e57-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1013] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 4. | Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) (federführend); Deutsche Gesellschaft für Innere Medizin (DGIM); Deutsche M. Crohn/Colitis ulcerosa Vereinigung (DCCV); Deutsche Leberhilfe e.V; Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM); Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (DGE-BV); Deutsche Gesellschaft für Kinder- und Jugendmedizin (DGKJ); Gesellschaft für Pädiatrische Gastroenterologie (GPGE); Deutsche Gesellschaft für Rheumatologie (DGRh); Deutsche Röntgengesellschaft (DRG); Deutsche Transplantationsgesellschaft (DTG); Deutsche Gesellschaft für Pathologie (DGP) und Bundesverband Deutscher Pathologen (BDP); Österreichische Gesellschaft für Gastroenterologie (ÖGG); Schweizer Gastroenterologische Gesellschaft (SGG); Authors; Collaborators:; Externe Begutachtung durch:. [Practice guideline autoimmune liver diseases - AWMF-Reg. No. 021-27]. Z Gastroenterol. 2017;55:1135-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Adeola HA, Smith M, Kaestner L, Blackburn JM, Zerbini LF. Novel potential serological prostate cancer biomarkers using CT100+ cancer antigen microarray platform in a multi-cultural South African cohort. Oncotarget. 2016;7:13945-13964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Duarte J, Serufuri J, Mulder N, Blackburn J. Protein Function Microarrays: Design, Use and Bioinformatic Analysis in Cancer Biomarker Discovery and Quantitation. In: Wang X, editor. Bioinformatics of Human Proteomics. Dordrecht: Springer, 2012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | French J, van der Mei I, Simpson S Jr, Ng J, Angus P, Lubel J, Nicoll A, Sood S, Roberts SK, Kemp W, Arachchi N, Dev A, Thompson A, Gow PJ. Increasing prevalence of primary biliary cholangitis in Victoria, Australia. J Gastroenterol Hepatol. 2020;35:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, Semela D, Zen Y, Mazzucchelli L, Voreck A, Kolbus N, Merlo E, Di Bartolomeo C, Messina P, Cerny A, Costantini S, Vergani D, Mieli-Vergani G; Swiss PBC Cohort Study Group. Primary biliary cholangitis with normal alkaline phosphatase: A neglected clinical entity challenging current guidelines. J Autoimmun. 2021;116:102578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Fan X, Wang T, Shen Y, Xi X, Yang L. Underestimated Male Prevalence of Primary Biliary Cholangitis in China: Results of a 16-yr cohort study involving 769 patients. Sci Rep. 2017;7:6560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Agmon-Levin N, Shapira Y, Selmi C, Barzilai O, Ram M, Szyper-Kravitz M, Sella S, Katz BS, Youinou P, Renaudineau Y, Larida B, Invernizzi P, Gershwin ME, Shoenfeld Y. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010;34:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Ma WT, Chang C, Gershwin ME, Lian ZX. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J Autoimmun. 2017;83:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Cançado GGL, Braga MH, Ferraz MLG, Villela-Nogueira CA, Terrabuio DRB, Cançado ELR, Nardelli MJ, Faria LC, de Faria Gomes NM, Oliveira EMG, Rotman V, Oliveira MB, da Cunha SMCF, Cunha-Silva M, Mendes LSC, Ivantes CAP, Codes L, de Almeida E Borges VF, de Lima Pace FH, Pessoa MG, Signorelli IV, Coral GP, Bittencourt PL, Levy C, Couto CA; Members of the Brazilian Cholestasis Study Group Consortium. Anti-mitochondrial Antibody-Negative Primary Biliary Cholangitis Is Part of the Same Spectrum of Classical Primary Biliary Cholangitis. Dig Dis Sci. 2022;67:3305-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Selmi C, Zuin M, Bowlus CL, Gershwin ME. Anti-mitochondrial antibody-negative primary biliary cirrhosis. Clin Liver Dis. 2008;12:173-185, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Barbouche MR, Chen Q, Carbone M, Ben-Mustapha I, Shums Z, Trifa M, Malinverno F, Bernuzzi F, Zhang H, Agrebi N, Norman GL, Chang C, Gershwin ME, Invernizzi P. Comprehensive review of autoantibodies in patients with hyper-IgM syndrome. Cell Mol Immunol. 2018;15:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Huang C, Han W, Wang C, Liu Y, Chen Y, Duan Z. Early Prognostic Utility of Gp210 Antibody-Positive Rate in Primary Biliary Cholangitis: A Meta-Analysis. Dis Markers. 2019;2019:9121207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Liang X, Zuo MQ, Zhang Y, Li N, Ma C, Dong MQ, Gao N. Structural snapshots of human pre-60S ribosomal particles before and after nuclear export. Nat Commun. 2020;11:3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Kim CW, Cha JM, Kwak MS. Identification of Potential Biomarkers and Biological Pathways for Poor Clinical Outcome in Mucinous Colorectal Adenocarcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Lin YL, Chung CL, Chen MH, Chen CH, Fang SC. SUMO Protease SMT7 Modulates Ribosomal Protein L30 and Regulates Cell-Size Checkpoint Function. Plant Cell. 2020;32:1285-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Doherty L, Sheen MR, Vlachos A, Choesmel V, O'Donohue MF, Clinton C, Schneider HE, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Glader B, Arceci RJ, Farrar JE, Atsidaftos E, Lipton JM, Gleizes PE, Gazda HT. Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet. 2010;86:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Thai A, Doescher C, Kamal N, Teramoto D, Fung C, Cha E, La V, Cheng P, Sedighim S, Keklikian A, Thankam FG. Single cell transcriptomics profiling of the stromal cells in the pathologic association of ribosomal proteins in the ischemic myocardium and epicardial fat. Cell Tissue Res. 2025;399:173-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Macías S, Bragulat M, Tardiff DF, Vilardell J. L30 binds the nascent RPL30 transcript to repress U2 snRNP recruitment. Mol Cell. 2008;30:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/