Published online May 28, 2025. doi: 10.3748/wjg.v31.i20.104038
Revised: March 27, 2025
Accepted: May 7, 2025
Published online: May 28, 2025
Processing time: 126 Days and 17.2 Hours
Pyoderma gangrenosum (PG) is one of the most severe extra-intestinal manifestations of ulcerative colitis (UC). The treatment of refractory UC combined with PG is challenging, particularly for patients with schizophrenia (SCZ) with a long-term history of risperidone use, and there have been no successfully treated patients reported in the literature.
A 36-year-old woman attended the gastroenterological clinic due to intermittent symptoms of diarrhea and mucous bloody stools. Prior to the emergence of these symptoms, the patient had a history of SCZ for 3 years. She had been receiving long-term risperidone treatment and had stable mental symptoms. In April 2023, she was diagnosed with UC E3 moderate and began taking mesalazine 3 g/day. In March 2024, her intestinal symptoms recurred and approximately 2 months later, PG developed in both lower limbs. Previous treatments with adalimumab and steroids were ineffective for PG and UC, and simultaneously, the patient experienced headache, confusion, and severe sleep disturbances. After switching to upadacitinib (UPA) 45 mg/day, PG lesions showed complete healing and fecal calprotectin was < 10 μg/g after 7 weeks of treatment. Following approximately 12 weeks of UPA therapy, colonoscopy indicated that the patient had achieved mucosal healing. No adverse events occurred during UPA induction and main
UPA treatment led to successful resolution of both intestinal and extra-intestinal manifestations in this patient with new-onset UC who had a history of SCZ. No adverse effects were observed with concurrent UPA and risperidone use.
Core Tip: Due to the potential anti-inflammatory effect of risperidone via the tumor necrosis factor pathway, patients with new-onset ulcerative colitis (UC) who have been receiving long-term risperidone cannot achieve a good therapeutic effect with anti-tumor necrosis factor therapy. We report the successful treatment of a patient with UC combined with pyoderma gangrenosum using upadacitinib, with no adverse events during the 6-month follow-up period.
- Citation: He SD, Tian Y. Upadacitinib for ulcerative colitis and pyoderma gangrenosum in a patient with schizophrenia on long-term risperidone: A case report. World J Gastroenterol 2025; 31(20): 104038
- URL: https://www.wjgnet.com/1007-9327/full/v31/i20/104038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i20.104038
Pyoderma gangrenosum (PG) is an uncommon inflammatory dermatological disorder and is one of the most severe extra-intestinal manifestations of ulcerative colitis (UC). The treatment of both PG and refractory UC is challenging[1]. Re
Psychiatric symptoms in inflammatory bowel disease (IBD) include depression, anxiety and other psychiatric symptoms, and are both common and affect the treatment effect of IBD. Previous studies have found that gut microbial dysbiosis identified in gastrointestinal diseases are associated with psychiatric disorders. These phenomena reflect the intricate gut-microbiota-brain axis[2].
Recently, a cohort study in Taiwan demonstrated a significant association between schizophrenia (SCZ) and subse
A 36-year-old female patient complained of intermittent symptoms of diarrhea and mucous bloody stools for more than one year.
The patient underwent colonoscopy due to intermittent symptoms of diarrhea and mucous bloody stools for at least 3 months in April 2023. Both colonoscopy and pathological examination suggested a typical manifestation of UC pancolitis; therefore, moderate UC E3 was diagnosed, and oral mesalazine (MEZ) 3 g/day was recommended. The patient's symptoms significantly improved after treatment, and the medication was discontinued after approximately 3 months.
In March 2024, the patient gradually experienced diarrhea and bloody mucous stools, with 4-6 bowel movements per day [stool frequency score (SFS) = 2] and obvious bloody mucous stools [rectal bleeding score (RBS) = 2], accompanied by lower abdominal pain and urgency in defecation. The patient was administered 3 g/day of MEZ for treatment, which slightly alleviated abdominal pain; however, there was no significant improvement in diarrhea and rectal bleeding symp
The patient had been suffering from SCZ for approximately three years (diagnosed at a psychiatric hospital in 2020) and had been receiving long-term treatment with risperidone, resulting in stable mental symptoms. She has no history of other illnesses.
The patient did not have a history of smoking or drinking alcohol. There were no IBD or genetic diseases in her family.
On March 21, 2024, the patient visited our IBD specialist clinic. She was conscious and had no abnormalities on cardio
The patient completed laboratory tests (Table 1), and according to the IBD specialist clinic, the dosage of oral MEZ for this moderate activity UC patient was increased to 4 g/day on March 24, 2024.
| Routine blood coagulation function | Liver function | Routine stool test, stool pathogen | Blood pathogen | Autoantibody1 | |||||
| WBC | 11.2 × 109/L | ALT | Normal | OBT | Positive | CMV-DNA | Negative | ANA | 1:320 |
| Hb | Normal | Tbil | Normal | BC | Negative | EBV-DNA | Negative | Sm | Negative |
| PLT | 431 × 109/L | GGT | Normal | TB | Negative | T-spot.TB | Negative | SSA/SSB | Negative |
| ESR | 21 mm/hour | TP | Normal | Fungi | Negative | HBsAg | Negative | RNP | Negative |
| D-dimer | Negative | ALB | 31.7 g/L | Clostridium difficile | Negative | Anti-HCV | Negative | pANCA | 1:80 |
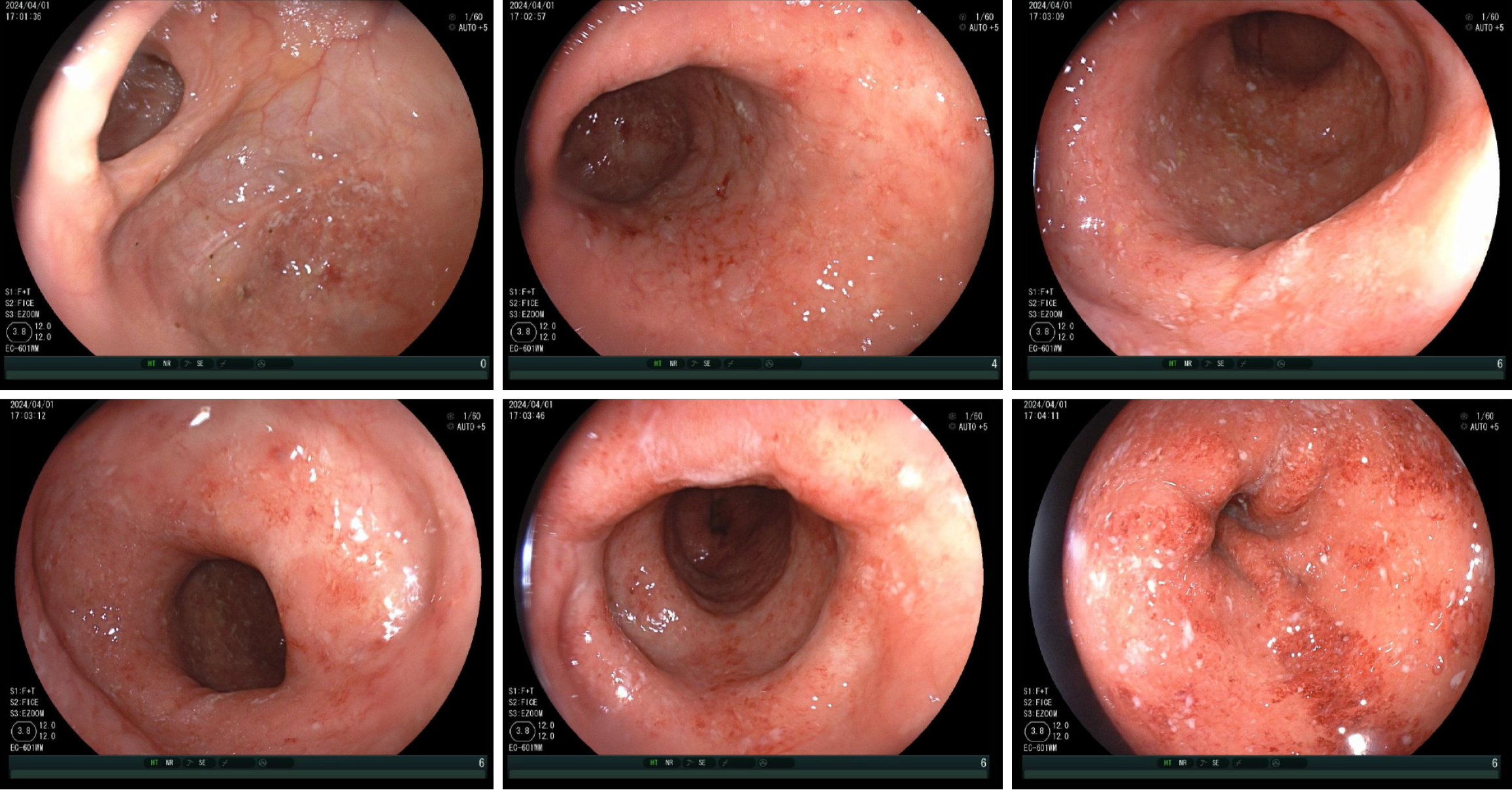
On April 1, 2024, she underwent colonoscopy (Figure 1) and the results were as follows: UC E3 Mayo endoscopic score (MES) = 2 and ulcerative colitis endoscopic index of severity (UCEIS) = 5. Pathological examination (transverse and sigmoid colon) revealed chronic inflammation of the colon mucosa with crypt inflammation and abscesses and a reduced number of glands with deformation.
On April 12, 2024, the patient visited an IBD specialist and reported improvement in symptoms after treatment. The frequency of bowel movements was 3-4 times per day (SFS = 1), and there were still unformed stools with mucus and blood (RBS = 1).
On April 20, the patient developed gangrene on the skin of both lower limbs. Local application of erythromycin ointment and oral antibiotics was ineffective, and the skin lesions rapidly expanded. Moreover, the symptoms of diarrhea, mucus, and bloody stool worsened, with more than six bowel movements per day. During this period, the patient continued to take 4 g MEZ per day.
On April 24, the patient visited the dermatology clinic and underwent a skin biopsy. On May 6, she was diagnosed with PG and received adalimumab (ADA) 80 mg injectio hypodermica treatment. On May 13, during follow-up at the dermatology clinic, the doctor found that the control of PG was unsatisfactory, and an additional 10 mg prednisone bid was added to her treatment.
The patient was diagnosed as UC (E3, severe) combined with PG (extra-intestinal manifestation of UC) and SCZ.
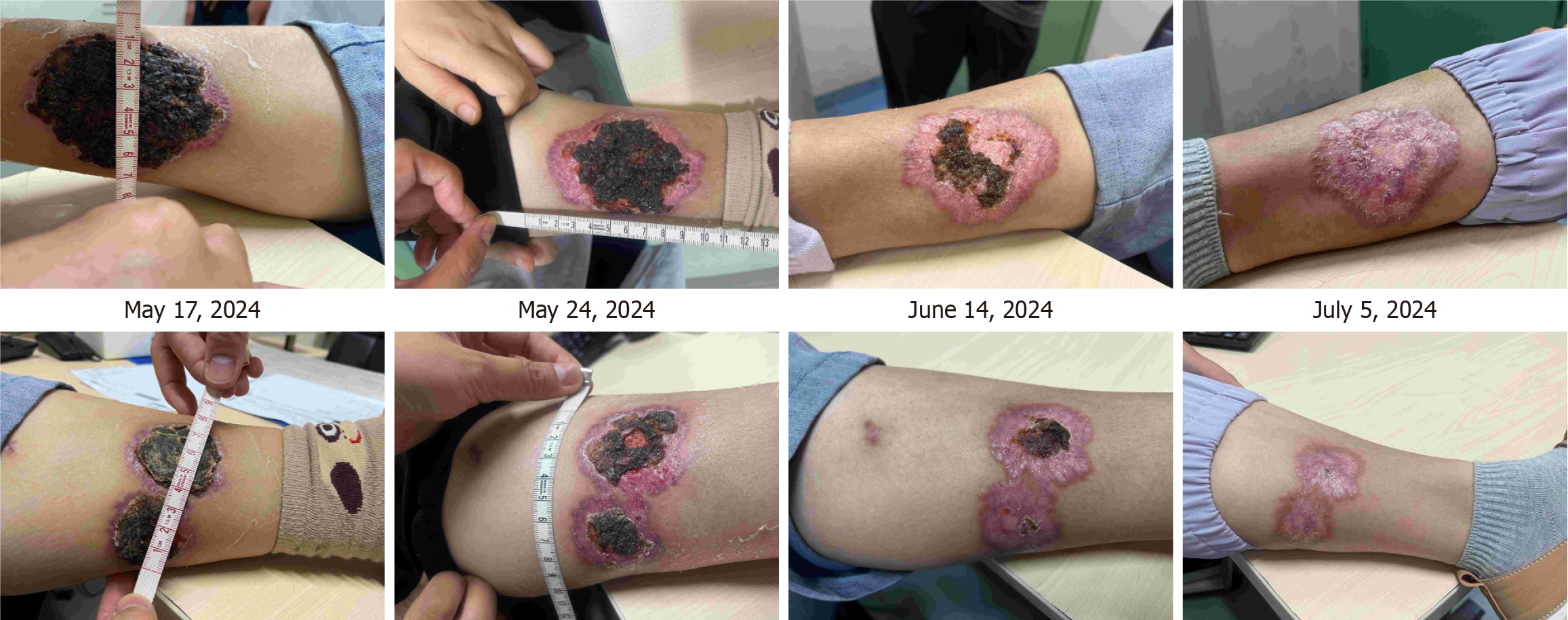
On May 17, 2024, the patient visited the IBD specialist accompanied by her husband and mother. The patient's husband reported that the patient had experienced headache, confusion, and severe sleep disturbances in the past week. Her psychiatric medication (risperidone) was administered consistently at the same dosage as suggested by the psychiatrist. Simultaneously, the patient exhibited large gangrenous changes on the skin of her lower limbs. The doctor inquired about changes in the patient's condition over the past month. Physical examinations were conducted at the IBD specialist clinic to assess the condition of the skin lesions, particularly in the right lower limb (10.5 cm × 7.0 cm and 3.5 cm × 2.5 cm) and left lower limb (7.5 cm × 5.0 cm and 7.5 cm × 4.0 cm; Figure 2). UC severity was defined as moderate activity, with a partial adapted Mayo score of 5 (SFS = 3, RBS = 2). The patient and her family believed that home care treatment was better than in-hospital due to her psychiatric symptoms, and they did not accept ADA optimized therapy. Due to incomplete response to ADA, and convenient home care with oral administration, after shared decision-making between the patient and doctor, the treatment was switched to UPA 45 mg qd, and the prednisone dosage was rapidly reduced to minimize the impact of steroids on psychiatric symptoms.
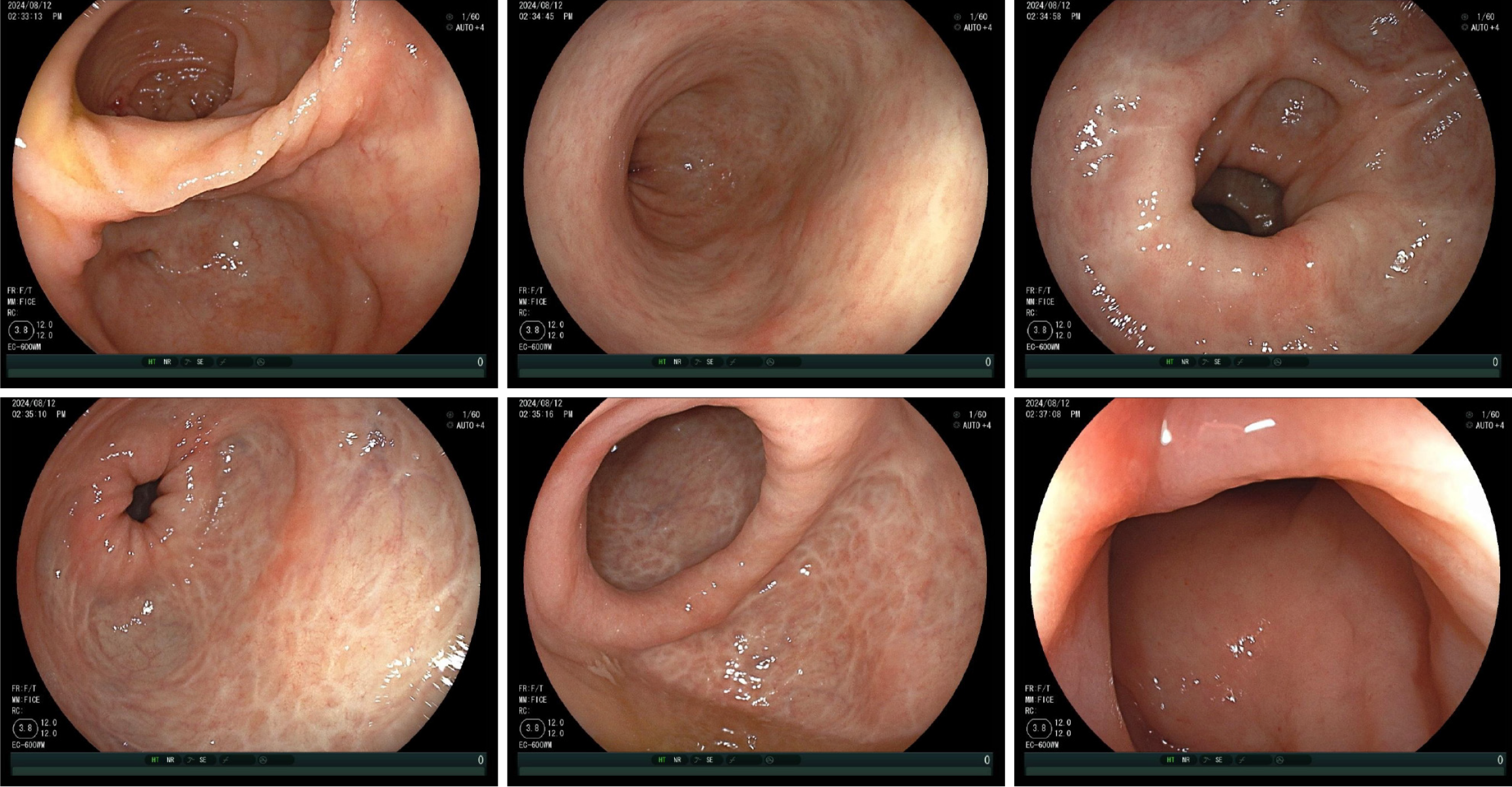
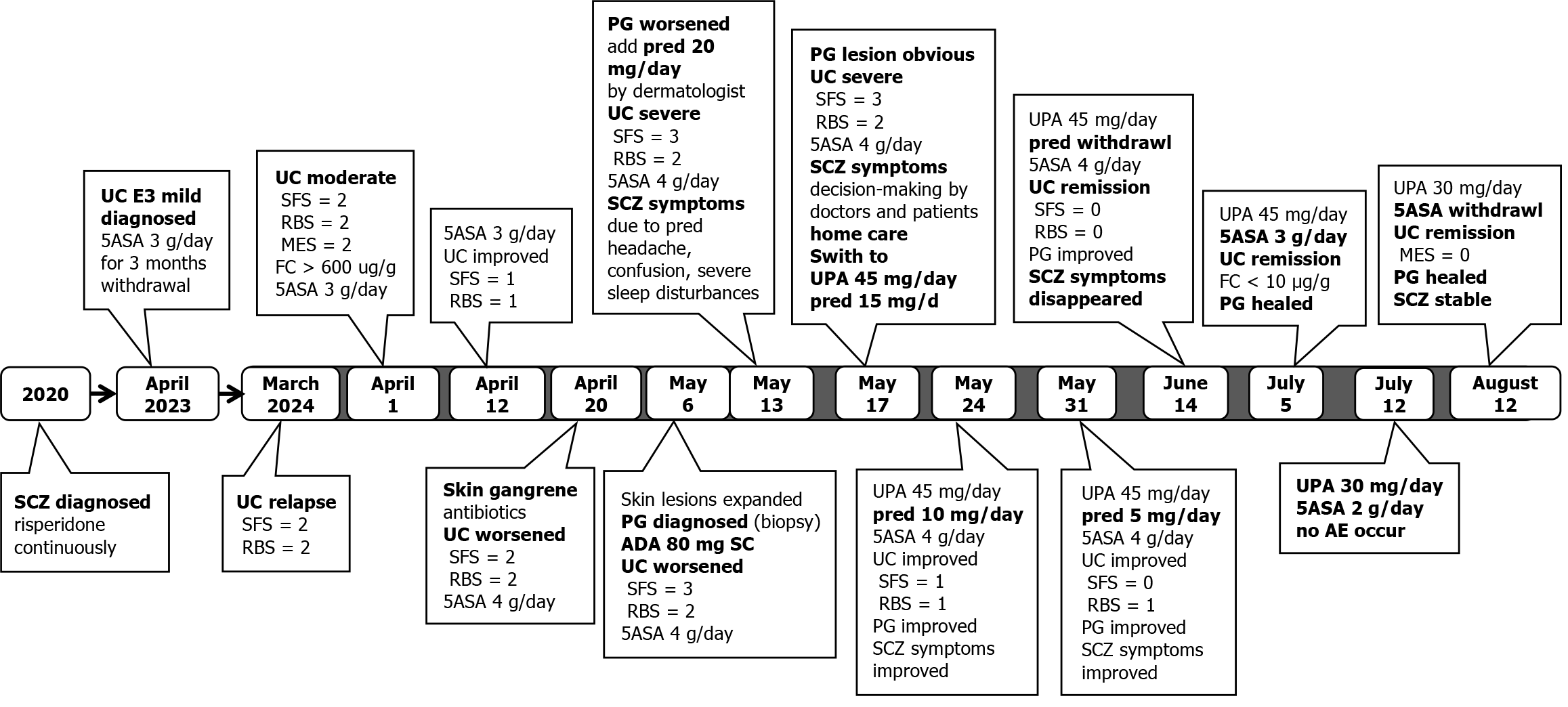
Subsequently, the patient received IBD specialist follow-up at 1, 4, and 7 weeks after switching to UPA treatment. After a one week of UPA treatment, the patient's intestinal symptoms significantly improved, with daily bowel movements and a small amount of blood visible in the soft stool. Skin lesions showed a clear healing trend: Right lower limb (8.5 cm × 6.0 cm and 3.0 cm × 2.0 cm) and left lower limb (5.5 cm × 4.0 cm and 4.5 cm × 3.0 cm). The prednisone dose was then reduced to 10 mg qd. After 4 weeks of UPA treatment, the patient achieved the goal of patient-reported outcomes. The skin damage healed further and prednisone was discontinued. After 7 weeks of UPA treatment, the patient achieved a laboratory test target (fecal calprotectin < 10 μg/g). The skin lesions healed completely (Figure 2). The patient's mental state was good, and she resumed a normal life. On July 12, after taking 45 mg qd of UPA for 8 weeks, the patient began maintenance treatment with 30 mg of UPA qd and underwent colonoscopy to evaluate the treatment effect. Colonoscopy re-examination results on August 12 showed UC E3 MES = 0 and UCEIS = 1 (Figure 3). The patient’s treatment is summarized in Figure 4.
On November 8, after 25 weeks of UPA treatment, the patient’s fecal occult blood test was negative and fecal calprotectin was normal (< 10 μg/g). The UPA dose was reduced to 15 mg qd maintenance treatment. The patient was consistently administered risperidone (regimen was not altered during the entire treatment process) to maintain treat
An important characteristic of this case is that the patient had a psychiatric disease and required long-term treatment with risperidone. Some studies have shown that increased tumor necrosis factor (TNF)-α levels in first-episode drug-naïve patients with SCZ are correlated with psychopathological symptoms[5]. Studies have concluded that serum TNF-α may contribute to the symptomatology of SCZ[6]. To date, the influence of risperidone on inflammatory factors such as TNF remains unclear. In vitro studies on anti-psychotics have not clearly shown the pro- and anti-inflammatory activity of risperidone[7]. A study demonstrated that TNF-α levels decrease after antipsychotic treatment[8], whereas another study reported no significant variation in TNF-α levels after treatment[9].
Several interesting studies have investigated the anti-inflammatory effects of risperidone. Zhang et al[10] found that risperidone affects the differentiation of osteoblasts by inhibiting autophagy and enhancing apoptosis via TNF-mediated downregulation of SATB2. In the field of intestinal inflammation related research, an extraordinary study showed that risperidone attenuates acetic acid-induced colitis in rats by inhibiting the TLR4/NF-kB signaling pathway[11]. These results suggest that risperidone exerts a regulatory effect on the inflammatory response via TNF-related molecular pathways. In this case, the patient developed new UC and PG during long-term treatment with risperidone, indicating that the TNF-mediated immune inflammatory pathway may not play an important role in its pathogenesis. Therefore, the clinical efficacy of ADA in UC and PG is unsatisfactory. Of course, an insufficient ADA dosage for initial treatment is another important factor. This highlights the importance of multi-disciplinary team diagnosis and treatment in UC patients with extra-intestinal manifestations. In the subsequent treatment process, the addition of prednisone led to deterioration of the patient’s psychiatric symptoms, which should be noted by physicians.
By switching to other inflammatory pathways, UPA treatment resulted in rapid symptom improvement and long-lasting therapeutic effects. UPA treatment for moderate-to-severe UC has the characteristics of fast onset and a high endoscopic mucosal healing rate, providing patients with rapid symptomatic relief from UC symptoms within 1-3 days and reducing intestinal inflammatory marker levels by week 2[12]. In a recent meta-analysis[13], UPA 45 mg qd was more efficacious than all other advanced therapies in achieving early symptomatic remission within 2 weeks. In a phase 3 clinical trial[14], a good therapeutic effect was observed in patients with extra-intestinal manifestations, with approximately 70% of extra-intestinal manifestations achieving regression after induction therapy.
PG is a rare and challenging neutrophilic dermatosis, with limited clinical studies and no guidelines on therapeutic options. It is often associated with various other immune-mediated diseases, most commonly IBD and rheumatoid arthritis[15]. Corticosteroids and/or cyclosporine remain the therapeutic options. However, cumulative data indicate that psychiatric complications of corticosteroid treatment are not rare and range from clinically significant anxiety and insomnia, to severe mood and psychotic disorders[16]. However, in recent years, there have been an increasing number of studies on the positive effects of biological therapies, such as anti-TNF agents. In a recent case report, two PG patients were successfully treated with tofacitinib and baricitinib. Both patients were cured with no relapses[17]. As more cases are reported[18,19], some scholars believe that Janus Kinase inhibitors may be the future for PG treatment[20].
The safety of UPA, particularly the severity of adverse events in patients with moderate-to-severe UC[21], has always been a concern. Recently, a study focusing on the safety of UPA that included 6991 patients who received at least one dose of UPA, representing 15425 patient-years of exposure across diseases, demonstrated that UPA was generally well tolerated[22]. In this case, the patient received long-term psychiatric medication and no adverse events occurred during UPA treatment, reflecting the safety of the combination of UPA and risperidone. There are no reports in the literature on the long-term use of UPA in combination with risperidone. The main pharmacokinetic pathways of risperidone and UPA are different[23], and there is only small metabolic overlap through CYP450 2D6 between UPA and risperidone. Theoretically a drug interaction is weak. The majority of clinical trials on IBD exclude patients with psychiatric disorders, making it difficult to obtain clinical data for this group of patients. Thus, we should pay more attention to those under-repre
Due to the potential anti-inflammatory effect of risperidone through the TNF pathway, the long-term use of risperidone in our patient with SCZ resulted in unsatisfactory anti-TNF treatment outcomes with de novo UC and concomitant PG. After switching to UPA, both UC and PG showed considerable improvement. The safety of long-term combination therapy with UPA and risperidone requires further investigation.
Sincere thanks to Professor Hua-Hong Wang who is the Former Director of the Department of Gastroenterology, Peking University First Hospital, for his high attention to those under-represented groups of patients in clinical trials and publication of relevant cases.
| 1. | Dissemond J, Marzano AV, Hampton PJ, Ortega-Loayza AG. Pyoderma Gangrenosum: Treatment Options. Drugs. 2023;83:1255-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Uellendahl-Werth F, Maj C, Borisov O, Juzenas S, Wacker EM, Jørgensen IF, Steiert TA, Bej S, Krawitz P, Hoffmann P, Schramm C, Wolkenhauer O, Banasik K, Brunak S, Schreiber S, Karlsen TH, Degenhardt F, Nöthen M, Franke A, Folseraas T, Ellinghaus D. Cross-tissue transcriptome-wide association studies identify susceptibility genes shared between schizophrenia and inflammatory bowel disease. Commun Biol. 2022;5:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Sung KY, Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, Hou MC, Lu CL, Wang YP, Chen MH. Schizophrenia and risk of new-onset inflammatory bowel disease: a nationwide longitudinal study. Aliment Pharmacol Ther. 2022;55:1192-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Wang J, Luo GY, Tian T, Zhao YQ, Meng SY, Wu JH, Han WX, Deng B, Ni J. Shared genetic basis and causality between schizophrenia and inflammatory bowel disease: evidence from a comprehensive genetic analysis. Psychol Med. 2024;54:2658-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Lin C, Chen K, Yu J, Feng W, Fu W, Yang F, Zhang X, Chen D. Relationship between TNF-α levels and psychiatric symptoms in first-episode drug-naïve patients with schizophrenia before and after risperidone treatment and in chronic patients. BMC Psychiatry. 2021;21:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Erbağci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1beta, sIL-2R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10:109-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl). 2016;233:1575-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Romeo B, Brunet-Lecomte M, Martelli C, Benyamina A. Kinetics of Cytokine Levels during Antipsychotic Treatment in Schizophrenia: A Meta-Analysis. Int J Neuropsychopharmacol. 2018;21:828-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neurosci Biobehav Rev. 2017;77:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Zhang S, He W, Li A, Zhao C, Chen Y, Xu C, Zhang Q, Zheng D, Chen M, Miao H, Huang Y. Involvement of the TNF-α/SATB2 axis in the induced apoptosis and inhibited autophagy of osteoblasts by the antipsychotic Risperidone. Mol Med. 2022;28:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 11. | Yousefi-Manesh H, Dejban P, Mumtaz F, Abdollahi A, Chamanara M, Dehpour A, Hasanvand A, Rashidian A. Risperidone attenuates acetic acid-induced colitis in rats through inhibition of TLR4/NF-kB signaling pathway. Immunopharmacol Immunotoxicol. 2020;42:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Loftus EV Jr, Colombel JF, Takeuchi K, Gao X, Panaccione R, Danese S, Dubinsky M, Schreiber S, Ilo D, Finney-Hayward T, Zhou W, Phillips C, Gonzalez YS, Shu L, Yao X, Zhou Q, Vermeire S. Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1 of Induction Treatment. Clin Gastroenterol Hepatol. 2023;21:2347-2358.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 13. | Ahuja D, Murad MH, Ma C, Jairath V, Singh S. Comparative Speed of Early Symptomatic Remission With Advanced Therapies for Moderate-to-Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Am J Gastroenterol. 2023;118:1618-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 409] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 15. | Maverakis E, Marzano AV, Le ST, Callen JP, Brüggen MC, Guenova E, Dissemond J, Shinkai K, Langan SM. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 16. | Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Castro LGM. JAK inhibitors: a novel, safe, and efficacious therapy for pyoderma gangrenosum. Int J Dermatol. 2023;62:1088-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 18. | Dos Santos MR, Ianhez M, Ribeiro BN, de Queiroz BB, Miot HA. Refractory pyoderma gangrenosum associated with rheumatoid arthritis successfully treated with upadacitinib. Comments on: "JAK inhibitors: a novel, safe, and efficacious therapy for pyoderma gangrenosum". Int J Dermatol. 2023;62:e595-e598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Mendolaro M, Morello E, Salacone P, Rocca R. A case of refractory severe pyoderma gangrenosum successfully treated with upadacitinib. Dig Liver Dis. 2024;56:1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Łyko M, Ryguła A, Kowalski M, Karska J, Jankowska-Konsur A. The Pathophysiology and Treatment of Pyoderma Gangrenosum-Current Options and New Perspectives. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 21. | Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 22. | Burmester GR, Cohen SB, Winthrop KL, Nash P, Irvine AD, Deodhar A, Mysler E, Tanaka Y, Liu J, Lacerda AP, Palac H, Shaw T, Mease PJ, Guttman-Yassky E. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 23. | Nader A, Stodtmann S, Friedel A, Mohamed MF, Othman AA. Pharmacokinetics of Upadacitinib in Healthy Subjects and Subjects With Rheumatoid Arthritis, Crohn's Disease, Ulcerative Colitis, or Atopic Dermatitis: Population Analyses of Phase 1 and 2 Clinical Trials. J Clin Pharmacol. 2020;60:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/