Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3367
Revised: June 19, 2024
Accepted: July 2, 2024
Published online: July 28, 2024
Processing time: 60 Days and 6.5 Hours
In this editorial, the roles of tata-box-binding protein-associated factor 15 (TAF15) in oncogenesis, tumor behavior, and as a therapeutic target in cancers in the context of gastrointestinal (GI) tumors are discussed concerning the publication by Guo et al. TAF15 is a member of the FET protein family with a comprehensive range of cellular processes. Besides, evidence has shown that TAF15 is involved in many diseases, including cancers. TAF15 contributes to carcinogenesis and tumor behavior in many tumors. Besides, its relationship with the mitogen-activated protein kinases (MAPK) signaling pathway makes TAF15 a new target for the
Core Tip: Recently, the role of tata-binding protein associated factor 15 (TAF15) in many diseases, including cancer, has been suggested. Current results support the hypothesis that TAF15 expression is related to aggressive behavior by contributing to many pathways that are involved in tumor progression. Although its role in prognosis has not been entirely determined in gastrointestinal cancers, the fact that increased TAF15 expression is associated with adverse clinicopathological parameters warrants further study with the aim of better understanding its role in predicting prognosis. Moreover, based on its association with the mitogen-activated protein kinases signal pathway, its significance as a therapeutic target, particularly in tumors resistant to treatment awaits investigation.
- Citation: Elpek GO. Tata-box-binding protein-associated factor 15 as a new potential marker in gastrointestinal tumors. World J Gastroenterol 2024; 30(28): 3367-3372
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3367
Tata-box-binding protein-associated factor 15 (TAF15) is a type of RNA-binding protein that belongs to FET protein family[1,2]. TAF15 is a crucial regulator of RNA metabolism and plays a significant role in the normal functions of RNA[3-5]. Prior research has shown that chromosomal translocation of FET genes, including TAF15 can result in the formation of fusion oncoproteins in several types of tumors, such as sarcomas and leukemias[6-8]. Moreover, the knockdown and inhibition of TAF15 has an impact on the expression of a substantial number of genes, a considerable proportion of which are implicated in the regulation of the cell cycle and programmed cell death[3,9-11]. Multiple studies have demonstrated that TAF15 plays a role in various types of human diseases including malignancies[12-14]. The involvement of TAF15 in the cellular interactions that promotes cell proliferation and migration has been demonstrated, suggesting a role in tumor progression[15-17]. Recent studies have demonstrated that TAF15 has the ability to activate the mitogen-activated protein kinases (MAPK) signaling pathway in malignant tumors[15-17]. Moreover, the contribution of TAF15 in the drug tolerance of cancer cells has been noted[18,19]. Despite the existence of this evidence regarding the relationship of TAF15 with oncogenesis and tumor behavior, the exact role of this protein in these events and whether it can be used as a therapeutic target has not been fully elucidated.
Therefore, in this article, the activity of TAF15, a protein with a multifaceted role, in cancer and its potential as a treatment target are discussed in the context of gastrointestinal (GI) tumors[20-32].
This protein is encoded by the TAF15 gene; this gene is located at chromosome 17q12 and was first reported in 2002 in a case of B-cell anaplastic large cell lymphoma[33]. As noted above, TAF15 is a member of the FET protein family (FUS-EWS), which contributes to cellular processes, including RNA splicing, transcription, mRNA transport, signaling, modification, translation, and preservation of genomic integrity[1,9,11]. Several evidence indicates that the suppression of the TAF15 gene with siRNA had a significant effect on the expression of a large number of genes, which are predominantly linked to cell proliferation and death[3,4]. On the other hand, the diverse localization of TAF15 at the cell surface and cytoplasm and its primary localization in the cell nucleus suggests that it has a function beyond DNA and RNA binding[34,35]. The contribution of TAF15 to the cellular stress response, cell adhesion, and migration via its regulation and interaction with numerous proteins has been demonstrated[36]. Accumulated data pointed out the contribution of TAF15 in different forms of diseases, including malignant tumors.
Recent experimental studies in lung carcinoma cell cultures have revealed a new role for TAF15 in carcinogenesis in squamous cell carcinomas and its association with long noncoding RNAs in this process[36]. In parallel to these findings, increased expression of TAF15 is associated with a decrease in survival rates. The contribution of TAF15 in carcinogenesis by stabilizing the MAPK signaling pathway has been also observed, a finding of significant clinical relevance[16]. Additionally, in adenocarcinomas, TAF15 has been shown to participate in interleukin-6-activated epithelial-to-mesenchymal transition and invasion to facilitate metastasis[37]. The experimental inhibition of TAF15 by transcriptional intermediary factor-1γ has been demonstrated to prevent this phenomenon, further highlighting the urgent need to elucidate TAF15-related mechanisms for potential therapeutic interventions[37]. Moreover, blockade of TAF15 with an antibody is a feasible approach for enhancing the cytotoxicity of radiation in lung cancer, and this approach may lead to improved outcomes in non-small cell lung cancer patients with TAF15 overexpression[10].
In breast cancer, recruitment of TAF15 by LINC00504 stabilizes CEPB2 mRNA, which is associated with radio resistance and contributes to its overexpression. In addition, LINC00504 silencing increased radiosensitivity by blocking TAF15[17].
In melanomas, the association of TAF15 with oncogenesis and the impact of its suppression on the inhibition of tumor cell proliferation have also been documented[35].
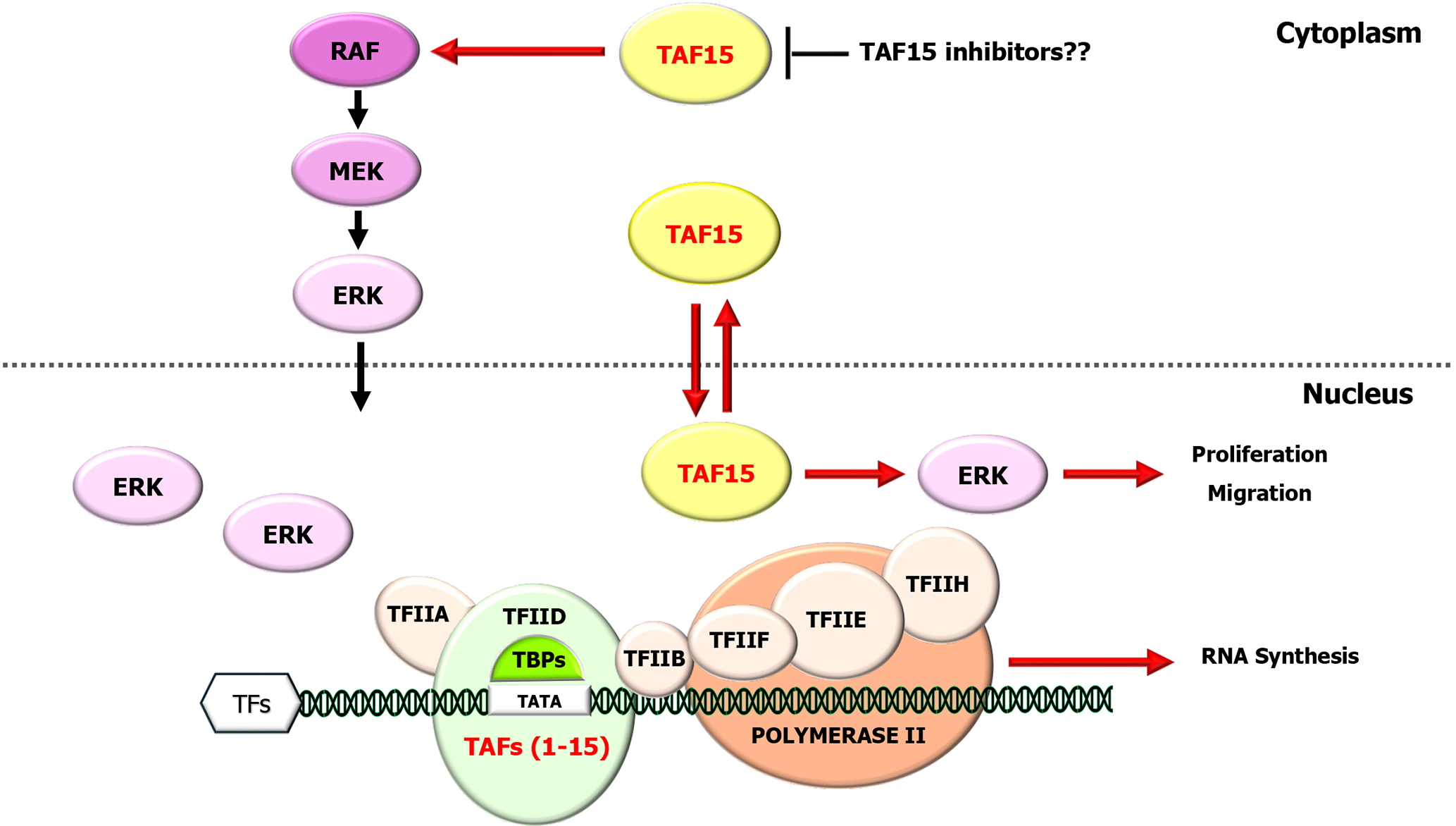
Regarding GI system, in colorectal cancer cell cultures, the interaction of long noncoding RNA blood vessel epicardial substance (BVES) antisense RNA 1 with miR-522-3p and TAF15 has been demonstrated to regulate BVES expression, which might offer a perspective for colorectal cancer treatment, but further study is needed[38]. In an elegant study, Tang et al[15] revealed significant upregulation of TAF15 in gastric cancer (GC) tumor tissues and cell lines. The overexpression of TAF15 was found to be correlated with increased tumor size, advanced pathological stage, and invasion. Importantly, the knockdown of TAF15 hindered the proliferation, migration, and invasion of tumor cells in cell culture and restrained tumor growth. Furthermore, this knockdown resulted in substantial decreases in the phosphorylation levels of RAF1, MEK, and ERK1/2, key components of the RAF1/MEK/ERK signaling, indicating the involvement of TAF15 in this pathway and suggesting that it could serve as a promising molecular diagnostic marker or therapeutic target for GC. A human antibody that recognizes a tumor-specific TAF15 antigen that inhibits tumor cell adhesion and spreading in stomach cancer, PAT-BA4, has been described[35].
In GI stromal tumors (GISTs), the influence of TAF15 expression on oncogenesis and prognosis has been analyzed in a recent report[39]. In this multidisciplinary study, the authors first discovered the significant upregulation of TAF15 in tumor tissues and cell lines. This upregulation was reflected by the overexpression of TAF15 in 31 patients with GIST and correlated with larger tumor size and high-risk stage, indicating its role in oncogenesis and tumor cell behavior. Although the number of patients was limited, these findings suggest that the TAF15 may be effective in determining the prognosis of GISTs and is worthy of further study. More interestingly, TAF15 knockdown, in addition to suppressing tumor cells and inhibiting tumor growth, also reduced the levels of phosphorylated RAF1, MEK and ERK1/2 in these cells. This observation suggested that TAF15 affects tumor cell behavior by regulating cell proliferation and migration via the RAF1/MEK/ERK signaling pathway.
These data support previous findings that TAF15 acts via the RAF1/MEK/ERK signaling in tumor progression across different tumor types and that TAF15 may be a potential therapeutic target in GI cancers, particularly in treatment-resistant tumors (Figure 1).
Furthermore, the discovery that α-AMA, an RNAPII inhibitor, is effective in reducing drug tolerance in cancer cells through TAF15 inhibition suggests that this protein may be a promising therapeutic target for preventing posttreatment relapses in solid tumors[40]. These findings indicate that a pharmacological strategy involving the use of novel chemicals that might also prevent aggressive behavior of tumor cells in patients with GI cancers by targeting TAF15.
Therefore, understanding the regulation of TAF15 is highly important considering the significant role of modifications in dysregulation of its expression, which affects malignant transformation and tumor progression in GI tumors. Moreover, new insight into its role as a distinct transcriptional regulator, together with its connection to signaling networks, especially the RAF1/MEK/ERK pathway, will facilitate the advancement of therapies for these tumors.
Beyond its involvement in gene fusions, TAF15 is a protein with a broad repertoire of influences on cancers, considering its roles in oncogenesis, tumor progression, and treatment. The expression of TAF15 in GI malignancies and its relationship with aggressive tumor cell behavior warrant further studies in larger cohorts. The association of TAF15 with progression-related clinicopathological factors and its value as an independent prognostic factor and indicator of aggressive tumor behavior in patients with GI tumors require further study. More importantly, the close relationship between TAF15 and the MAPK signaling pathway in GI cancers suggests that TAF15 may be a potential therapeutic target, especially in treatment-resistant cases.
| 1. | Kovar H. Dr. Jekyll and Mr. Hyde: The Two Faces of the FUS/EWS/TAF15 Protein Family. Sarcoma. 2011;2011:837474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Law WJ, Cann KL, Hicks GG. TLS, EWS and TAF15: a model for transcriptional integration of gene expression. Brief Funct Genomic Proteomic. 2006;5:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Ballarino M, Jobert L, Dembélé D, de la Grange P, Auboeuf D, Tora L. TAF15 is important for cellular proliferation and regulates the expression of a subset of cell cycle genes through miRNAs. Oncogene. 2013;32:4646-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 4. | Ibrahim F, Maragkakis M, Alexiou P, Maronski MA, Dichter MA, Mourelatos Z. Identification of in vivo, conserved, TAF15 RNA binding sites reveals the impact of TAF15 on the neuronal transcriptome. Cell Rep. 2013;3:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Luna-Arias JP, Castro-Muñozledo F. Participation of the TBP-associated factors (TAFs) in cell differentiation. J Cell Physiol. 2024;239:e31167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (4)] |
| 6. | Flucke U, van Noesel MM, Siozopoulou V, Creytens D, Tops BBJ, van Gorp JM, Hiemcke-Jiwa LS. EWSR1-The Most Common Rearranged Gene in Soft Tissue Lesions, Which Also Occurs in Different Bone Lesions: An Updated Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Zhu L, Bai W, Cheng Q, Fang J. ZNF384-Related Fusion Genes in Acute Lymphoblastic Leukemia. Cancer Control. 2023;30:10732748231182787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Georgakopoulos N, Diamantopoulos P, Micci F, Giannakopoulou N, Zervakis K, Dimitrakopoulou A, Viniou NA. An Adult Patient with Early Pre-B Acute Lymphoblastic Leukemia with t(12;17)(p13;q21)/ZNF384-TAF15. In Vivo. 2018;32:1241-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Chen L, Chen Q, Kuang S, Zhao C, Yang L, Zhang Y, Zhu H, Yang R. USF1-induced upregulation of LINC01048 promotes cell proliferation and apoptosis in cutaneous squamous cell carcinoma by binding to TAF15 to transcriptionally activate YAP1. Cell Death Dis. 2019;10:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 10. | Singh AK, Kapoor V, Thotala D, Hallahan DE. TAF15 contributes to the radiation-inducible stress response in cancer. Oncotarget. 2020;11:2647-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Wang D, Li Z, Yin H. Long Non-Coding RNA CCAT2 Activates RAB14 and Acts as an Oncogene in Colorectal Cancer. Front Oncol. 2021;11:751903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Tetter S, Arseni D, Murzin AG, Buhidma Y, Peak-Chew SY, Garringer HJ, Newell KL, Vidal R, Apostolova LG, Lashley T, Ghetti B, Ryskeldi-Falcon B. TAF15 amyloid filaments in frontotemporal lobar degeneration. Nature. 2024;625:345-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Ji J, Wu S, Bao X, Liu S, Ye Y, Liu J, Guo J, Liu J, Wang X, Xia Z, Wei L, Zhang Y, Hao D, Huang D. Mediating oxidative stress through the Palbociclib/miR-141-3p/STAT4 axis in osteoporosis: a bioinformatics and experimental validation study. Sci Rep. 2023;13:19560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Chen J, Li X, Liu H, Zhong D, Yin K, Li Y, Zhu L, Xu C, Li M, Wang C. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabet Med. 2023;40:e15031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 15. | Tang L, Guo C, Li X, Zhang B, Huang L. TAF15 promotes cell proliferation, migration and invasion of gastric cancer via activation of the RAF1/MEK/ERK signalling pathway. Sci Rep. 2023;13:5846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Zhu H, Liu Q, Yang X, Ding C, Wang Q, Xiong Y. LncRNA LINC00649 recruits TAF15 and enhances MAPK6 expression to promote the development of lung squamous cell carcinoma via activating MAPK signaling pathway. Cancer Gene Ther. 2022;29:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 17. | Feng J, Li Y, Zhu L, Zhao Q, Li D, Li Y, Wu T. STAT1 mediated long non-coding RNA LINC00504 influences radio-sensitivity of breast cancer via binding to TAF15 and stabilizing CPEB2 expression. Cancer Biol Ther. 2021;22:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ruan X, Zheng J, Liu X, Liu Y, Liu L, Ma J, He Q, Yang C, Wang D, Cai H, Li Z, Liu J, Xue Y. lncRNA LINC00665 Stabilized by TAF15 Impeded the Malignant Biological Behaviors of Glioma Cells via STAU1-Mediated mRNA Degradation. Mol Ther Nucleic Acids. 2020;20:823-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Zheng WH, Long ZQ, Zheng ZQ, Zhang LL, Liang YL, Li ZX, Lv JW, Kou J, Hong XH, He SW, Xu R, Zhou GQ, Liu N, Ma J, Sun Y, Lin L, Wei D. m6A-enriched lncRNA LINC00839 promotes tumor progression by enhancing TAF15-mediated transcription of amine oxidase AOC1 in nasopharyngeal carcinoma. J Biol Chem. 2023;299:104873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Butler JE, Kadonaga JT. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 2002;16:2583-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 434] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 21. | Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 633] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 22. | Akhtar W, Veenstra GJ. TBP-related factors: a paradigm of diversity in transcription initiation. Cell Biosci. 2011;1:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 23. | Feigerle JT, Weil PA. The C Terminus of the RNA Polymerase II Transcription Factor IID (TFIID) Subunit Taf2 Mediates Stable Association of Subunit Taf14 into the Yeast TFIID Complex. J Biol Chem. 2016;291:22721-22740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bernardini A, Hollinger C, Willgenss D, Müller F, Devys D, Tora L. Transcription factor IID parks and drives preinitiation complexes at sharp or broad promoters. Trends Biochem Sci. 2023;48:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Chen X, Xu Y. Structural insights into assembly of transcription preinitiation complex. Curr Opin Struct Biol. 2022;75:102404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Mishal R, Luna-Arias JP. Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation. Gene. 2022;833:146581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 27. | Gura MA, Mikedis MM, Seymour KA, de Rooij DG, Page DC, Freiman RN. Dynamic and regulated TAF gene expression during mouse embryonic germ cell development. PLoS Genet. 2020;16:e1008515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | He XD, Phillips S, Hioki K, Majhi PD, Babbitt C, Tremblay KD, Pobezinsky LA, Mager J. TATA-binding associated factors have distinct roles during early mammalian development. Dev Biol. 2024;511:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Säisä-Borreill S, Davidson G, Kleiber T, Thevenot A, Martin E, Mondot S, Blottière H, Helleux A, Mengus G, Plateroti M, Duluc I, Davidson I, Freund JN. General transcription factor TAF4 antagonizes epigenetic silencing by Polycomb to maintain intestine stem cell functions. Cell Death Differ. 2023;30:839-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Kalogeropoulou M, Voulgari A, Kostourou V, Sandaltzopoulos R, Dikstein R, Davidson I, Tora L, Pintzas A. TAF4b and Jun/activating protein-1 collaborate to regulate the expression of integrin alpha6 and cancer cell migration properties. Mol Cancer Res. 2010;8:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 31. | Li Q, Wu Q, Li Z, Hu Y, Zhou F, Zhai Z, Yue S, Tian H. LncRNA LINC00319 is associated with tumorigenesis and poor prognosis in glioma. Eur J Pharmacol. 2019;861:172556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Xu Y, Man N, Karl D, Martinez C, Liu F, Sun J, Martinez CJ, Martin GM, Beckedorff F, Lai F, Yue J, Roisman A, Greenblatt S, Duffort S, Wang L, Sun X, Figueroa M, Shiekhattar R, Nimer S. TAF1 plays a critical role in AML1-ETO driven leukemogenesis. Nat Commun. 2019;10:4925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Martini A, La Starza R, Janssen H, Bilhou-Nabera C, Corveleyn A, Somers R, Aventin A, Foà R, Hagemeijer A, Mecucci C, Marynen P. Recurrent rearrangement of the Ewing's sarcoma gene, EWSR1, or its homologue, TAF15, with the transcription factor CIZ/NMP4 in acute leukemia. Cancer Res. 2002;62:5408-5412. [PubMed] |
| 34. | Andersson MK, Ståhlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 35. | Schatz N, Brändlein S, Rückl K, Hensel F, Vollmers HP. Diagnostic and therapeutic potential of a human antibody cloned from a cancer patient that binds to a tumor-specific variant of transcription factor TAF15. Cancer Res. 2010;70:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Xiong Y, Yang C, Yang X, Ding C, Wang Q, Zhu H. LncRNA MIR9-3HG enhances LIMK1 mRNA and protein levels to contribute to the carcinogenesis of lung squamous cell carcinoma via sponging miR-138-5p and recruiting TAF15. Pathol Res Pract. 2022;237:153941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 37. | Su Z, Sun Z, Wang Z, Wang S, Wang Y, Jin E, Li C, Zhao J, Liu Z, Zhou Z, Wang Y, Chen X, Liu X, Lei Z, Zhang HT. TIF1γ inhibits lung adenocarcinoma EMT and metastasis by interacting with the TAF15/TBP complex. Cell Rep. 2022;41:111513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 38. | Wen W, Wang H, Xie S, Wu Z, Zhang C. BVES-AS1 inhibits the malignant behaviors of colon adenocarcinoma cells via regulating BVES. Cell Biol Int. 2021;45:1945-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 39. | Guo CM, Tang L, Li X, Huang LY. TATA-box-binding protein-associated factor 15 is a novel biomarker that promotes cell proliferation and migration in gastrointestinal stromal tumor. World J Gastroenterol. 2023;29:2932-2949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Kume K, Ikeda M, Miura S, Ito K, Sato KA, Ohmori Y, Endo F, Katagiri H, Ishida K, Ito C, Iwaya T, Nishizuka SS. α-Amanitin Restrains Cancer Relapse from Drug-Tolerant Cell Subpopulations via TAF15. Sci Rep. 2016;6:25895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/