Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1480
Peer-review started: December 29, 2023
First decision: January 19, 2024
Revised: January 30, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: March 21, 2024
Processing time: 82 Days and 21.7 Hours
During the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, particular interest rose regarding the interaction between metabolic dysfunction-associated fatty liver disease (MAFLD) and the COVID-19 infection. Several studies highlighted the fact that individuals with MAFLD had higher probability of severe acute respiratory syndrome coronavirus 2 infection and more severe adverse clinical outcomes. One of the proposed mechanisms is the inflammatory response pathway, especially the one involving cytokines, such as interleukin 6, which appeared particularly elevated in those patients and was deemed responsible for additional insult to the already damaged liver. This should increase our vigilance in terms of early detection, close follow up and early treatment for individuals with MAFLD and COVID-19 infection. In the direction of early diagnosis, biomarkers such as cytokeratin-18 and scoring systems such as Fibrosis-4 index score are proposed. COVID-19 is a newly described entity, expec
Core Tip: The intricate intertwining of metabolic dysfunction-associated fatty liver disease (MAFLD) and coronavirus disease 2019 (COVID-19) presents a critical nexus with severe clinical outcomes. The symbiotic impact of MAFLD increasing susceptibility to severe COVID-19, and the reciprocal exacerbation by the viral infection, mandate special attention. Early identification, vigilant monitoring and tailored evidence-based interventions, navigating both conditions, are pivotal in mitigating adverse effects. Investigating the molecular pathways underlying the synergistic effects of MAFLD and COVID-19, and the impact of specific COVID-19 treatment drugs on liver function and their potential exacerbation of MAFLD, stands as a promising research avenue that could unveil novel therapeutic targets.
- Citation: Brilakis L, Theofilogiannakou E, Lykoudis PM. Current remarks and future directions on the interactions between metabolic dysfunction-associated fatty liver disease and COVID-19. World J Gastroenterol 2024; 30(11): 1480-1487
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1480.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1480
As the world encounters the enduring impact of coronavirus disease 2019 (COVID-19), the interplay between this viral pandemic and metabolic dysfunction-associated fatty liver disease (MAFLD) emerges as a topic for comprehensive research. While MAFLD has a remarkable prevalence among COVID-19 patients and is expected to be increased even more by 2030 due to obesity and metabolic syndrome persistently growing, the topical question that rises is whether concurrent obesity and MAFLD epidemics could be contributing factors to the exacerbation and prevalence of the ongoing COVID-19 pandemic[1,2]. In addition to the metabolic syndrome, MAFLD might influence the severity of viral infections, potentially impacting conditions like COVID-19 or even representing an independent risk factor. Vice versa, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection exacerbates MAFLD and induces liver injury through various mechanisms.
In their consensus statement, Eslam et al[3] suggested that a positive diagnosis of MAFLD can be achieved through the confirmation of at least one criterion from each of the following two groups. The first group consists of the following: The presence of hepatic steatosis in adults, as it can be ascertained through imaging modalities such as ultrasound, magnetic resonance imaging or computerized tomography; blood biomarkers or blood test-based scoring systems; and liver histology. The second group consists of the following: Presence of overweight/obesity; type 2 diabetes mellitus; and evidence of metabolic dysregulation[3]. Metabolic dysregulation can be documented by the presence of at least two of the following metabolic risk abnormalities: (1) Waist circumference ≥ 102/88 cm in Caucasian men and women; (2) Blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) Plasma triglycerides ≥ 150 mg/dL or specific drug treatment; (4) Plasma High Density Lipoprotein–cholesterol < 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment; (5) Prediabetes; (6) Homeostasis model assessment of insulin resistance score ≥ 2.5; and (7) Plasma high-sensitivity C-reactive protein level > 2 mg/L[3]. Our ability to recognize individuals with MAFLD is very important as this condition is a favorable substrate for severe COVID-19 illness. In particular, Gao et al[4] reported that after adjustment for age and sex, patients with MAFLD had a 2.6-fold higher risk of severe COVID-19 illness over those without MAFLD. They also reported that MAFLD patients with elevated interleukin-6 (IL-6) Levels were at higher risk of severe COVID-19 illness over non-MAFLD patients[4].
Although there is an unmet need for further studies, it is a fact that COVID-19 affects liver function, which can be manifested straight from the onset of the disease, with mild and moderate elevations of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST)[2]. Tao et al[1] reported a negative impact of MAFLD on the course and clinical outcomes of COVID-19 illness but did not detect a statistically significant correlation[1].
The incidence of MAFLD in patients with COVID-19 has been noted as higher than in general population[1]. One possible explanation of the enhanced susceptibility to COVID-19 is the increased hepatic angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) expression in MAFLD patients, which are host cell proteins that contribute to SARS-CoV-2 infection[2,5,6]. In addition, COVID-19 patients with MAFLD under the age of 60 years have a higher risk of severe COVID-19 illness compared to those without MAFLD[5]. Due to liver's role in producing acute-phase reactants, coagulation factors and albumin, any dysfunction in the liver might affect the broader symptoms of COVID-19, potentially influencing conditions like acute respiratory distress syndrome (ARDS), coagulation disorders and multi-organ failure, aggravating the COVID-19 progression[2]. Therefore, MAFLD patients have higher risk of a poorer COVID-19 prognosis, higher prevalence of severe disease course, higher viral shedding time and more liver failure during hospitalization[7].
Notably, MAFLD is not a stand-alone disease. It can be characterized as the hepatic manifestation of metabolic syndrome and stands along with the obesity epidemic[5]. These comorbidities are associated with adverse clinical manifestation of type 2 diabetes and increased risk of severe infections, since obesity compromises the immune system due to chronic inflammation. It is essential to underline that increased liver steatosis is associated with a higher risk of symptomatic COVID-19 in obese patients[7]. A pro-inflammatory state in MAFLD patients that aggravates COVID-19 illness, is also a result of excess of free fatty acids that enter the liver, activating Kupffer cells[1]. The already burdened immune system and its effects on liver, lead to a shift of Kupffer cells from an anti-inflammatory M2 state to a proinflammatory M1 state[1]. Consequently, this transition enhances the production of cytokines such as tumor necrosis factor-α and IL-6. Studies suggest that IL-6 plays a pivotal role in the cytokine 'storm', which could be a primary factor for the aggravation of COVID-19[1,4,7].
Research is needed to ascertain the exact role of fibrosis in the spectrum of outcomes in patients with MAFLD and COVID-19. Angulo et al[8] after highlighting the difficulty and the risks of liver biopsy, turned to the examination of a non-invasive model for the identification of liver fibrosis among MAFLD patients. They proposed a score that includes age, body mass index, AST/ALT ratio, platelet count, hyperglycemia and albumin, comprehensively known as the non-alcoholic fatty liver disease fibrosis score (NFS)[8]. In their study, Campos-Murguía et al[9], in the process of diagnosing liver fibrosis, instead of using a single score, used a two-score system simultaneously in order to better stratify high-risk patients with liver fibrosis. In this system, only individuals with intermediate or high risk of fibrosis-as calculated by NFS- had AST to platelet ratio index calculated[9]. It is suggested that the existence of MAFLD, and especially the coexistence with significant or advanced fibrosis, stimulates the virus-triggered cytokine storm. This is mainly explained by the hepatic production of pro-inflammatory cytokines that significantly contribute to the aggravation of COVID-19 disease[10]. In the same context, Hegyi et al[7] underlined fibrosis as an independent factor for severe COVID-19 illness, in addition to the existence of fatty liver[7]. Data provided by Targher et al[11] corroborate a correlation between MAFLD with higher Fibrosis-4 (FIB-4) and NFS scores, and severe course of COVID-19 disease[11]. The severity of COVID-19 notably escalates with the progression of liver fibrosis. A FIB-4 score higher than 2.67 was associated with the highest risk of developing severe COVID-19. Moreover, evidence was provided that the coexistence of MAFLD alongside with a neutrophil-to-lymphocyte ratio (NLR) over 2.8 correlated with an elevated risk of severe COVID-19 disease, compared to non-MAFLD individuals with a normal NLR[7,11]. Therefore, there is an urgent need to evaluate the existence of fibrosis and its degree as it seems to significantly affect the course of the disease.
Reversely, SARS-CoV-2 infection can promote the progression of MAFLD. SARS-CoV-2 demonstrates wide organotropism, including the liver[12]. Hepatic dysfunction, characterized by abnormalities in liver enzymes, is frequently observed in individuals with COVID-19, notably in severe cases across multiple studies[2,5]. Patients experiencing severe COVID-19 infection tend to exhibit significantly higher incidence of abnormal liver function tests, which are correlated with poorer outcomes[12,13]. Underlying liver conditions can exacerbate liver damage resulting from COVID-19, and multiple mechanisms could explain the abnormal liver dysfunction indicated in COVID-19 patients. Direct liver injury induced by SARS-CoV-2, systemic inflammatory response syndrome (SIRS) and cytokine storms, hypoxia, drug-induced liver injury (DILI), dysregulation of hepatic lipid metabolism and liver steatosis, and disruption in gut microbiota balance are only some of the postulated underlying mechanisms[5,12,14].
A variety of studies point out that SARS-CoV-2 can directly damage liver cells. ACE2 and TMPRSS2 are involved in the process of SARS-CoV-2 endocytosis[6]. Therefore, the presence of these receptors in the liver and bile ducts can cause cytopathic damage[2,12,13]. SARS-CoV-2 found in the gut lumen may migrate to the liver through the portal circulation, causing direct damage by actively replicating in hepatic cells via ACE2 receptors[15]. Liver cell apoptosis studies further support the indication of direct viral cytopathic effects as reported by Chen H and Chen Q[2].Other factors that lead to liver damage and therefore can worsen the progression of MAFLD are bi-nucleated cells, swollen mitochondria, and changes in canalicular structures[16,17]. A retrospective cohort study by Wong et al[18] also demonstrated that patients with prolonged viral presence were more likely to develop elevation of ALT/AST[18].
As an infectious condition, COVID-19 can provoke systemic inflammation and there is evidence that patients with severe COVID-19 may have cytokine storm syndrome[19]. This can lead to multi-organ dysfunction following liver damage. As liver steatosis is associated with markers of inflammation such as IL-6, MAFLD can be exacerbated by the inflammatory response of COVID-19[20]. Alongside the hyper-inflammatory state due to the co-existence of MAFLD and COVID-19 illness, patients with severe COVID-19 develop multi-organ failure, ARDS, shock and hypoxia[12]. Liver ischemia may lead to increased hypoxia-induced factors (HIFs). Higher levels of HIFs in COVID-19 patients could aggravate obesity and insulin resistance, and both aforementioned conditions constitute significant risk factors associated with MAFLD, as suggested by Tian et al[13]. It is important to mention that studies have shown that HIF-1α could promote MAFLD[2]. Moreover, increased production of HIF-2α could induce lipogenesis in the liver[2,14,21]. Therefore, SIRS and hypoxia in severe COVID-19 may exacerbate the progression of MAFLD.
During an infection, the activated innate immune response not only directly triggers and amplifies liver inflammation but also disrupts the regulation of lipid metabolism[22]. This lipid derangement can promote the development of liver fibrosis in MAFLD/non-alcoholic steatohepatitis (NASH) patients, as mentioned by Chen H and Chen Q[2]. Analysis of proteins and metabolites in COVID-19 patients have revealed dyslipidemia, characterized by lipid accumulation and decreased levels of apolipoproteins[23,24]. Unsaturated fatty acids might be released as a defense mechanism during a cytokine storm, which can occur in reaction to COVID-19[24]. This condition can lead to changes where proinflammatory lipids and lipid mediators alter the immune response[5].
In turn, viruses modify lipid metabolism to facilitate their replication, impacting the expression and function of vital enzymes in lipid biosynthesis[25]. These alterations in lipid metabolism can also relate to the host's response to an infection[25]. SARS-CoV-2 follows this pattern, inducing substantial changes in lipid metabolism post-infection[5,25]. In particular, SARS-CoV-2 infection has been found to influence pathways involved in lipid synthesis and uptake, leading to increased accumulation of lipid droplets (LDs) within human cells. Interestingly, the virus utilizes these LDs to enhance its replication capacity[25]. Moreover, recent studies highlight the significance of ACE2 in maintaining metabolic balance[2]. However, SARS-CoV-2 infection impairs ACE2 expression, potentially inducing metabolic abnormalities[26]. This disruption in metabolic equilibrium due to ACE2 impairment may contribute to the progression of MAFLD in individuals affected by COVID-19[2].
Disruption of gut microbiome equilibrium might contribute to the severity of MAFLD during COVID-19. Growing evidence indicates that imbalances in the microbiota during COVID-19 correlate with disease severity and increased mortality[27]. The gastrointestinal tract serves as both the primary habitat for human microbiota and a target for SARS-CoV-2 infection due to its elevated expression of ACE2 and TMPRSS2[27]. Importantly, the intestine has a connection with the liver via the gut-liver axis, and intestinal microbiota significantly influences progression of MAFLD[28,29].
A variety of studies suggest an enrichment of opportunistic pathogens and a reduction of beneficial commensals in the intestinal microbiota of patients with severe COVID-19[30-33]. For example, a cohort study with 62 patients with COVID-19 found that anti-inflammatory bacteria such as Roseburia and Faecalibacterium decreased, while opportunistic pathogens such as Clostridium and Streptococcus increased in these patients[32]. These studies highlighted changes in gut microbiota linked to susceptibility to severe illness.
The relationship between gut microbiota and the liver appears to be strongly supported by evidence. Seventy percent of blood supply to the liver is provided by the portal vein which carries pathogens from the intestine, therefore liver acts as the primary defense against antigens originating from the gut. The balance and well-being of gut-liver axis heavily rely on the contribution of intestinal bacteria[28]. Therefore, disruptions in gut bacteria balance can lead to the passage of bacteria and endotoxins into the liver due to increased intestinal permeability, eventually triggering liver inflammation. This dysbiosis-induced hepatic inflammation, coupled with SIRS mentioned earlier in patients with severe COVID-19, can further aggravate MAFLD[2].
The liver, as the body's primary detoxifier, can be affected by numerous medications used to treat COVID-19[2]. Studies have demonstrated that treatment with antiviral drugs, such as lopinavir, ritonavir and remdesivir, and macrolides, used for superinfections, have the potential to cause DILI[2,34]. Lopinavir and ritonavir therapy in patients with COVID-19 is independently associated with elevated ALT/AST[2]. Hepatotoxicity has also been reported in association with antimalarial/antirheumatic drugs such as hydroxychloroquine, immunomodulatory drugs, corticosteroids and tocilizumab, as well as acetaminophen, commonly used as an antipyretic medication[2,35]. Importantly, corticosteroid therapy, currently recommended by the World Health Organization for patients with severe SARS-CoV-2 infection, is evidently linked to conditions such as steatosis or glycogenosis[36]. Therefore, amongst various drugs used to treat COVID-19, some can be harmful to the liver.
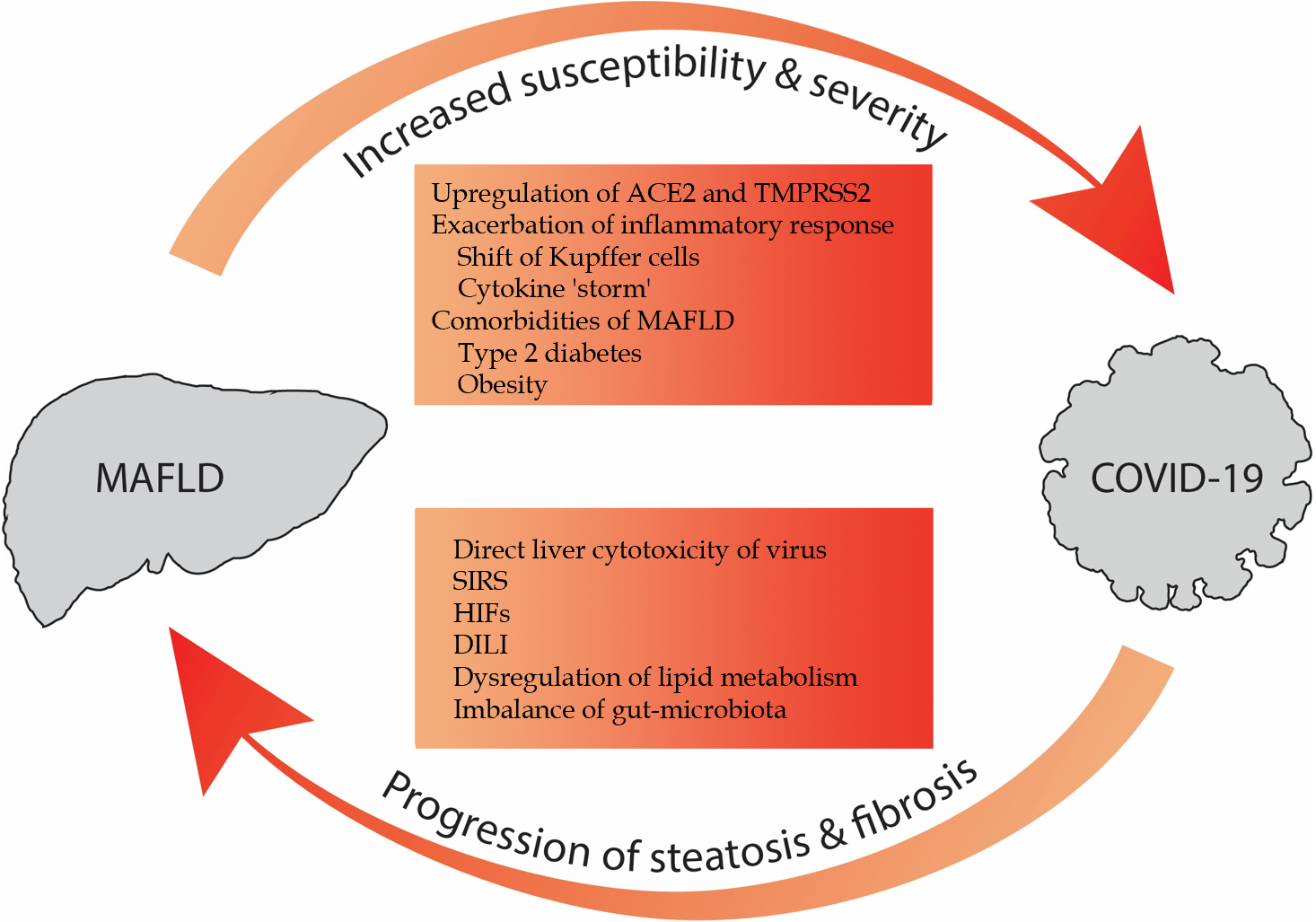
Underlying metabolic abnormalities and MAFLD have been identified as contributors to DILI[34]. MAFLD has the potential to increase the liver's sensitivity to hepatotoxicants like acetaminophen[34]. In addition, other compounds, such as corticoids, antiretroviral agents and methotrexate, appear to initiate the progression from simple fatty liver to NASH, or exacerbate pre-existing conditions like steatosis, necroinflammation and fibrosis[35]. Hence, in individuals with chronic liver disease, it's crucial to weigh the risk of potential liver injury when selecting medications for COVID-19 treatment and to carefully monitor these patients, as the use of drugs with elevated hepatotoxicity could potentially enhance the progression of MAFLD. Figure 1 provides an overview of the aforementioned interactions and highlights the bidirectional aggravating relationship.
Treatment of patients with COVID-19 and MAFLD is a very challenging task with significant implications. On the one hand, there is the need for appropriate treatment for COVID-19 and on the other, the need for the least possible burden on the already damaged liver. Given that many of the medications used to treat COVID-19 or its symptoms are harmful to or impair liver function, great care must be taken in their administration and close liver function monitoring is mandatory in order to deal with any adverse effects[37]. Hamid et al[38] highlighted the increased risk of drug-drug interactions and especially in liver transplant patients who are under immunosuppressive treatment. They also added that dexamethasone, which is effective in reducing mortality, does not appear to have an aggravating effect in patients with liver damage[38]. Elemam et al[12] emphasized the fact that the reduction in the drugs in COVID-19 patients with potential DILI, needs to be further evaluated[12]. Jeeyavudeen et al[5] reported medications such as dexamethasone, protease inhibitors, nucleoside analogue (remdesivir) and monoclonal antibodies to IL-6 as frequently used agents for COVID-19 treatment. Additionally, they proposed selection of medications according to disease severity, disease duration, ALT levels and cross-interactions with other treatments patients might be under. Τhey also recommended close monitoring of liver function[5]. It can be argued that once research provides evidence for a more precise treatment algorithm for COVID-19, areas of clear overlap with MAFLD treatment will emerge, and more specific questions will need to be addressed.
The triple association of chronic viral hepatitis B (CHB) and chronic viral hepatitis C (CHC), MAFLD, and COVID-19 introduces a complex interplay. Notably, there is a significant gap in the existing literature specifically addressing this triple association. The influence of pre-existing liver conditions on COVID-19-associated liver injury remains a topic of debate. Despite studies revealing the severity of COVID-19 in patients with chronic liver diseases (CLD), with MAFLD and alcoholic liver disease identified as independent risk factors for severe COVID-19, the relationship between COVID-19 and CLD caused by hepatitis C virus (HCV) and hepatitis B virus (HBV) has received less attention[5,18,39]. According to Elemam et al[12], there are conflicting results regarding the impact of viral hepatitis on COVID-19 outcomes[12]. Some studies suggest that individuals with viral hepatitis experienced more severe liver dysfunction due to pre-existing immune dysregulation, while other studies indicated that individuals with pre-existing liver disease or hepatitis B infection did not show more severe symptoms of COVID-19[12,18,39]. Postulated reasons for these discrepancies in literature, have been suggested by Lin et al[40] and include small sample size of patients with co-infection of HBV and COVID, heterogeneity of included patients and lack of thorough understanding of the complexity of enhanced liver injury caused by inflammatory response[40]. This conflicting evidence underscores the need for further research to elucidate the specific relationship between viral hepatitis and COVID-19-related liver injury. However, liver cirrhosis stands out as a critical factor contributing to severe outcomes in COVID-19, with CHB and CHC playing major roles in its development[5,12,38]. The World Gastroenterology Organisation underscores the need for more data to assess the risk of adverse outcomes in individuals with CHB or CHC without cirrhosis exposed to COVID-19[38]. While uncertainties persist about the susceptibility of patients with CHB or CHC to SARS-CoV-2-induced liver damage, those with advanced fibrosis or cirrhosis face a higher risk of severe outcomes, necessitating vigilant monitoring and tailored interventions[38].
The potential mechanisms underlying the association between viral hepatitis B and C with COVID-19 require thorough investigation and seem to be similar with those observed in the interplay between MAFLD and COVID-19. The enhanced liver injury induced by SARS-CoV-2 and HBV co-infection has been characterized as the hepatocyte type rather than the cholangiocyte type, emphasizing the primary involvement of hepatocytes in the pathogenesis[40]. One key aspect of this interaction is the inflammatory response and cytokine ‘storm’. Inflammatory factors, including abnormal lactate dehydrogenase, D-dimer, and IL-6 production, may contribute significantly to liver injury following SARS-CoV-2 coinfection[40]. Thrombocytopenia, more pronounced in COVID-19 cases with HBV co-infection, indicates a potential role of inflammatory factors in liver injury[40,41]. Furthermore, DILI poses a significant concern[42]. Corticosteroids, commonly administered in severe COVID-19 cases, introduce a dual risk by not only suppressing the immune response but also activating HBV replication[42]. This activation occurs through the suppression of cytotoxic T cell function and direct stimulation of HBV genomic sequences[42]. Similarly, tocilizumab raises concerns as it can cause liver injury and induce reactivation of hepatotropic viruses[42].
Notably, while some studies indicate that related inflammatory factors contribute to abnormal liver function, the exact mechanisms of enhanced liver injury caused by the inflammatory response need further elucidation[40]. Some mechanistic insights revealed stable expression levels of HBV-associated markers during SARS-CoV-2 infection, suggesting that chronic HBV infection alone may not significantly increase the severity of COVID-19[43]. Nevertheless, caution is warranted, as reactivation of hepatitis B has been observed, particularly when corticosteroids are employed, emphasizing the need for continuous antiviral therapy to manage and prevent such occurrences[42]. Similarly, for individuals co-infected with HCV and SARS-CoV-2, the continuation of antiviral therapy remains crucial[42]. Both the American Association for the Study of Liver Disease and the European Association for the Study of the Liver strongly recommend ongoing antiviral therapy in individuals diagnosed with COVID-19[42]. The parallel complexity seen in the MAFLD-COVID association prompts a closer examination of each potential mechanism to unveil common threads linking the diverse conditions of CHB/CHC and MAFLD to COVID-19 progression.
It is crucial to highlight that both CHB and CHC are frequently associated with hepatic steatosis, forming a connection with obesity, dyslipidemia and insulin resistance[44]. This shared link to metabolic factors is compounded by MAFLD as a common comorbidity, further emphasizing the interconnected nature of these conditions. This interplay highlights the need for comprehensive research to elucidate the complex dynamics of the triple association between CHB and CHC, MAFLD and COVID-19. While existing studies shed light on the impact of individual components, the triple association remains a relatively unexplored area in medical literature. Future investigations should aim to unravel the mechanisms underlying the interactions between these three entities. Understanding the synergistic effects could provide valuable insights into tailored interventions and preventive strategies.
In conclusion, the interaction of MAFLD and course of disease in patients with COVID-19 is particularly broad and there is a constant growing interest for research on this topic. Individuals with MAFLD have higher probability of SARS-COV-2 infection and more severe adverse clinical outcomes. Reversely, the disease from SARS-COV-2 can lead to triggering and progression of MAFLD. The multitude of already known mechanisms involved in this essential interaction need to be elucidated through focused research. Finally, it is imperative to quickly identify patients who combine the above two conditions and to apply tailored treatment, highly effective for COVID-19 and safe for the liver.
| 1. | Tao Z, Li Y, Cheng B, Zhou T, Gao Y. Risk of Severe COVID-19 Increased by Metabolic Dysfunction-associated Fatty Liver Disease: A Meta-analysis. J Clin Gastroenterol. 2021;55:830-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Chen H, Chen Q. COVID-19 Pandemic: Insights into Interactions between SARS-CoV-2 Infection and MAFLD. Int J Biol Sci. 2022;18:4756-4767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3162] [Article Influence: 527.0] [Reference Citation Analysis (2)] |
| 4. | Gao F, Zheng KI, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Association and Interaction Between Serum Interleukin-6 Levels and Metabolic Dysfunction-Associated Fatty Liver Disease in Patients With Severe Coronavirus Disease 2019. Front Endocrinol (Lausanne). 2021;12:604100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Jeeyavudeen MS, Chaudhari R, Pappachan JM, Fouda S. Clinical implications of COVID-19 in patients with metabolic-associated fatty liver disease. World J Gastroenterol. 2023;29:487-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 7. | Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne). 2021;8:626425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2370] [Article Influence: 124.7] [Reference Citation Analysis (2)] |
| 9. | Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, González-Regueiro JA, Solís-Ortega AA, Kúsulas-Delint D, Cruz-Contreras M, Cruz-Yedra N, Cubero FJ, Nevzorova YA, Martínez-Cabrera CF, Moreno-Guillén P, Lozano-Cruz OA, Chapa-Ibargüengoitia M, Gulías-Herrero A, Aguilar-Salinas CA, Ruiz-Margáin A, Macías-Rodríguez RU. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;53:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 12. | Elemam NM, Talaat IM, Maghazachi AA, Saber-Ayad M. Liver Injury Associated with COVID-19 Infection: Pathogenesis, Histopathology, Prognosis, and Treatment. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 663] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 14. | Chen J, Chen J, Fu H, Li Y, Wang L, Luo S, Lu H. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2α/PPARα pathway. Am J Physiol Endocrinol Metab. 2019;317:E710-E722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 18. | Wong GL, Yip TC, Wong VW, Tse YK, Hui DS, Lee SS, Yeoh EK, Chan HL, Lui GC. SARS-CoV-2 Viral Persistence Based on Cycle Threshold Value and Liver Injury in Patients With COVID-19. Open Forum Infect Dis. 2021;8:ofab205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6842] [Article Influence: 1140.3] [Reference Citation Analysis (1)] |
| 20. | Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ, Long MT. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol. 2019;17:1157-1164.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, Duan J, Sullards MC, Leiker TJ, Barkley RM, Quehenberger O, Armando AM, Milne SB, Mathews TP, Armstrong MD, Li C, Melvin WV, Clements RH, Washington MK, Mendonsa AM, Witztum JL, Guan Z, Glass CK, Murphy RC, Dennis EA, Merrill AH Jr, Russell DW, Subramaniam S, Brown HA. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Bai L, Li H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med (Berl). 2019;97:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, García de Vicuña A, Seco M, Bosch A, Palazón A, San Juan I, Laín A, Gil-Martínez J, Bernardo-Seisdedos G, Fernández-Ramos D, Lopitz-Otsoa F, Embade N, Lu S, Mato JM, Millet O. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience. 2020;23:101645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 24. | Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59-72.e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 1136] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 25. | Dias SSG, Soares VC, Ferreira AC, Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, Teixeira L, Nunes da Silva MA, Barreto E, Mattos M, de Freitas CS, Azevedo-Quintanilha IG, Manso PPA, Miranda MD, Siqueira MM, Hottz ED, Pão CRR, Bou-Habib DC, Barreto-Vieira DF, Bozza FA, Souza TML, Bozza PT. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16:e1009127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 26. | Li Z, Peng M, Chen P, Liu C, Hu A, Zhang Y, Peng J, Liu J, Li Y, Li W, Zhu W, Guan D, Chen H, Li J, Fan D, Huang K, Lin F, Zhang Z, Guo Z, Luo H, He X, Zhu Y, Li L, Huang B, Cai W, Gu L, Lu Y, Deng K, Yan L, Chen S. Imatinib and methazolamide ameliorate COVID-19-induced metabolic complications via elevating ACE2 enzymatic activity and inhibiting viral entry. Cell Metab. 2022;34:424-440.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 28. | Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 29. | Iruzubieta P, Medina JM, Fernández-López R, Crespo J, de la Cruz F. A Role for Gut Microbiome Fermentative Pathways in Fatty Liver Disease Progression. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1106] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 31. | Gu S, Chen Y, Wu Z, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71:2669-2678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 589] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 32. | Tao W, Zhang G, Wang X, Guo M, Zeng W, Xu Z, Cao D, Pan A, Wang Y, Zhang K, Ma X, Chen Z, Jin T, Liu L, Weng J, Zhu S. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol. 2020;5:100023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 995] [Cited by in RCA: 916] [Article Influence: 183.2] [Reference Citation Analysis (2)] |
| 34. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 35. | Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 36. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 37. | Hu X, Sun L, Guo Z, Wu C, Yu X, Li J. Management of COVID-19 patients with chronic liver diseases and liver transplants. Ann Hepatol. 2022;27:100653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Hamid S, Alvares da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, Gaspar R, LaBrecque D, Lee A, Macedo G, McMahon B, Ning Q, Reau N, Sonderup M, van Leeuwen DJ, Armstrong D, Yurdaydin C. WGO Guidance for the Care of Patients With COVID-19 and Liver Disease. J Clin Gastroenterol. 2021;55:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Aslam J, Qaisar F, Khaliq M, Khalid M, Sabeeh DE, Ali AY. Association between Chronic Liver Disease Caused by Viral Hepatitis, Hospitalisation for COVID-19 and Mortality. J Coll Physicians Surg Pak. 2023;33:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2021;8:484-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Liu R, Zhao L, Cheng X, Han H, Li C, Li D, Liu A, Gao G, Zhou F, Liu F, Jiang Y, Zhu C, Xia Y. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2021;41:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Li P, Liu Y, Cheng Z, Yu X, Li Y. COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother. 2022;154:113568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Yu R, Tan S, Dan Y, Lu Y, Zhang J, Tan Z, He X, Xiang X, Zhou Y, Guo Y, Deng G, Chen Y, Tan W. Effect of SARS-CoV-2 coinfection was not apparent on the dynamics of chronic hepatitis B infection. Virology. 2021;553:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 44. | Gordon A, McLean CA, Pedersen JS, Bailey MJ, Roberts SK. Hepatic steatosis in chronic hepatitis B and C: predictors, distribution and effect on fibrosis. J Hepatol. 2005;43:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tekin MS, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Cai YX