Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1368
Peer-review started: December 27, 2023
First decision: January 5, 2024
Revised: January 17, 2024
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 14, 2024
Processing time: 78 Days and 8.9 Hours
Colorectal cancer (CRC) is a global health concern, with advanced-stage diagnoses contributing to poor prognoses. The efficacy of CRC screening has been well-established; nevertheless, a significant proportion of patients remain unscreened, with > 70% of cases diagnosed outside screening. Although identifying specific subgroups for whom CRC screening should be particularly recommended is crucial owing to limited resources, the association between the diagnostic routes and identification of these subgroups has been less appreciated. In the Japanese cancer registry, the diagnostic routes for groups discovered outside of screening are primarily categorized into those with comorbidities found during hospital visits and those with CRC-related symptoms.
To clarify the stage at CRC diagnosis based on diagnostic routes.
We conducted a retrospective observational study using a cancer registry of patients with CRC between January 2016 and December 2019 at two hospitals. The diagnostic routes were primarily classified into three groups: Cancer screening, follow-up, and symptomatic. The early-stage was defined as Stages 0 or I. Multivariate and univariate logistic regressions were exploited to determine the odds of early-stage diagnosis in the symptomatic and cancer screening groups, referencing the follow-up group. The adjusted covariates were age, sex, and tumor location.
Of the 2083 patients, 715 (34.4%), 1064 (51.1%), and 304 (14.6%) belonged to the follow-up, symptomatic, and cancer screening groups, respectively. Among the 2083 patients, CRCs diagnosed at an early stage were 57.3% (410 of 715), 23.9% (254 of 1064), and 59.5% (181 of 304) in the follow-up, symptomatic, and cancer screening groups, respectively. The symptomatic group exhibited a lower likelihood of early-stage diagnosis than the follow-up group [P < 0.001, adjusted odds ratio (aOR), 0.23; 95% confidence interval (95%CI): 0.19-0.29]. The likelihood of diagnosis at an early stage was similar between the follow-up and cancer screening groups (P = 0.493, aOR for early-stage diagnosis in the cancer screening group vs follow-up group = 1.11; 95%CI = 0.82-1.49).
CRCs detected during hospital visits for comorbidities were diagnosed earlier, similar to cancer screening. CRC screening should be recommended, particularly for patients without periodical hospital visits for comorbidities.
Core Tip: Colorectal cancer (CRC) screening reduces CRC deaths, yet several patients remain unscreened. To encourage more individuals to participate in screening, identifying subgroups at high risk is crucial. This study used cancer registries from two Japanese facilities to clarify the stage at diagnosis in three groups: cancer screening, follow-up (patients detected during follow-up for other comorbidities), and symptomatic. The proportion of early-stage diagnoses was higher in the follow-up group than in the symptomatic group and was comparable to that in the cancer screening group. Therefore, CRC screening should be recommended, particularly for patients without periodical hospital visits for comorbidities.
- Citation: Agatsuma N, Utsumi T, Nishikawa Y, Horimatsu T, Seta T, Yamashita Y, Tanaka Y, Inoue T, Nakanishi Y, Shimizu T, Ohno M, Fukushima A, Nakayama T, Seno H. Stage at diagnosis of colorectal cancer through diagnostic route: Who should be screened? World J Gastroenterol 2024; 30(10): 1368-1376
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1368.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1368
Colorectal cancer (CRC) is a commonly diagnosed cancer and is a leading cause of cancer-related deaths worldwide[1]. CRC has a poor prognosis when diagnosed at an advanced stage, and screening can lead to more early-stage diagnoses, potentially improving patient survival[2-5]. Despite evidence showing the effectiveness of CRC screening, some (30%-50%) of the eligible population does not undergo the process, with over 70% of all CRC cases discovered through non-screening routes[6-8]. Although encouraging individuals to participate in CRC screening is essential, there remains insufficient guidance on effectively and efficiently recommending screening for the non-adherent population.
Understanding the individuals for whom screening should be particularly recommended is crucial for its efficient promotion. Screening should be recommended for all eligible individuals; however, personalized messages for all eligible individuals are impractical due to limited human and financial resources[9-13]. Targeted interventions to increase cancer screening are considered cost-effective[14]. Therefore, identifying individuals for whom CRC screening should be particularly recommended based on the subgroup to which the screening-eligible population belongs is beneficial. However, few reports have appreciated the value of the diagnostic route to clarify subgroups of patients that are more likely to be diagnosed at a later stage.
CRC is diagnosed through various routes besides screening, leading to different stages at diagnosis. In Western countries, diagnostic routes for CRC are primarily classified into emergency and non-emergency pathways, with emergency cases typically diagnosed at more advanced stages than those of non-emergency[8,15,16]. Conversely, the Japanese cancer registry categorizes CRC cases other than those in the screening pathway into two primary groups: Those discovered during hospital visits owing to comorbidities and those found through symptom-driven medical con
This study aimed to clarify the stages at diagnosis based on the diagnostic routes of CRC using hospital-based cancer registries from two Japanese facilities. We evaluated the diagnostic stage distribution and compared the proportion of early-stage cases among three groups: (1) Patients detected during hospital visits for other comorbidities; (2) patients detected following presentation with CRC-related symptoms; and (3) patients detected during cancer screening.
This retrospective observational study was conducted at Kyoto University Hospital (KU) in Kyoto Prefecture and Japanese Red Cross Wakayama Medical Center (WMC) in Wakayama Prefecture. Both facilities are among the hospitals that have treated the largest CRC cases in each prefecture and are designated cancer care hospitals. KU covers approximately 20% of patients with CRC in Kyoto Prefecture, and WMC covers approximately 40% of these patients in Wakayama Prefecture[18]. All patients with CRC registered in the hospital-based cancer registry at both institutions between January 2016 and December 2019 were included in this study.
Data from each registry were extracted from cancer registrars based on medical records according to the standard registry definition. The diagnostic routes were primarily classified into three groups: Cancer screening, follow-up (patients detected during the follow-up of comorbidities), and symptomatic[19]. The two other groups were CRC cases with unknown diagnostic routes and those discovered at autopsy. However, the total number is less than 5% nationwide[18]. The cancer screening group included patients who underwent a population-based or opportunistic screening. In the cancer screening group, almost all patients were estimated to undergo an immunochemical fecal occult blood test (iFOBT) because iFOBT is strongly recommended for population-based and opportunistic screening in the Japanese CRC screening guidelines[20]. The follow-up group comprised patients detected during examinations conducted as part of the follow-up for existing comorbidities (lifestyle-related diseases, such as hypertension and diabetes, heart disease, other organ cancers, and benign digestive disorders). Cases in which CRC was discovered due to CRC-related symptoms during a hospital visit for comorbidity follow-up were classified as the follow-up group. Patients with CRC who presented to the hospital primarily for CRC-related symptoms were classified as “other group” in the cancer registry, distinguished from the patients with CRC detected via screening or during hospital visits for comorbidities[17,19]. Stages were recorded according to the tumor-node-metastasis staging system of the Union for International Cancer Control (8th edition). If the patient had undergone surgery, the post-operative stage was used; if the patient had not, the preoperative stage was used. Data on age, sex, diagnostic routes, tumor location, clinical and pathological stages, and pathological findings were obtained from a hospital-based cancer registry. No data were missing. We excluded patients with non-epithelial or neuroendocrine tumors, those whose stage or route of discovery was unknown, and autopsy-detected cases. Patients with multiple CRCs were considered to have advanced-stage disease. Regarding tumor location, we defined right-sided CRC as tumors from the cecum to the splenic flexure, whereas left-sided CRC was defined as tumors from the descending colon to the rectum. For advanced lesions on both sides of the colorectum, left-sided lesions were excluded because previous reports indicated a poorer prognosis in right-sided primary CRC than in left-sided[21]. Localized disease, corresponding to Stages 0 and I, is associated with a 5-year survival rate of over 90%, making its prognosis more favorable than that in the regional or distant stages[2]. Hence, as in previous reports, we categorized Stages 0 and I as early stages in this study[22,23]. The stage distribution and proportion of patients with early-stage CRC were evaluated using the diagnostic routes. We compared the proportion of patients with early-stage CRC between the follow-up, symptomatic, and cancer screening groups.
Pearson’s Chi-square test was used to compare the proportion of early-stage CRCs in the follow-up group with those in the symptomatic and screening groups. Differences were considered statistically significant at P < 0.05. Logistic regression analysis was used to determine the odds of early-stage diagnosis in the symptomatic and cancer screening groups using the follow-up group as a reference. Univariate logistic regression analysis was used to calculate the crude odds ratios of early-stage detection, comparing the follow-up group with the symptomatic and cancer screening groups. Multivariate logistic regression analysis was also used to calculate adjusted odds ratios (aORs), adjusted for age, sex, and tumor location. Statistical results were calculated as point estimates with a 95% confidence interval (95%CI). All statistical analyses were performed using JMP Pro® 16.1.0 (SAS Institute Inc., Cary, NC, United States).
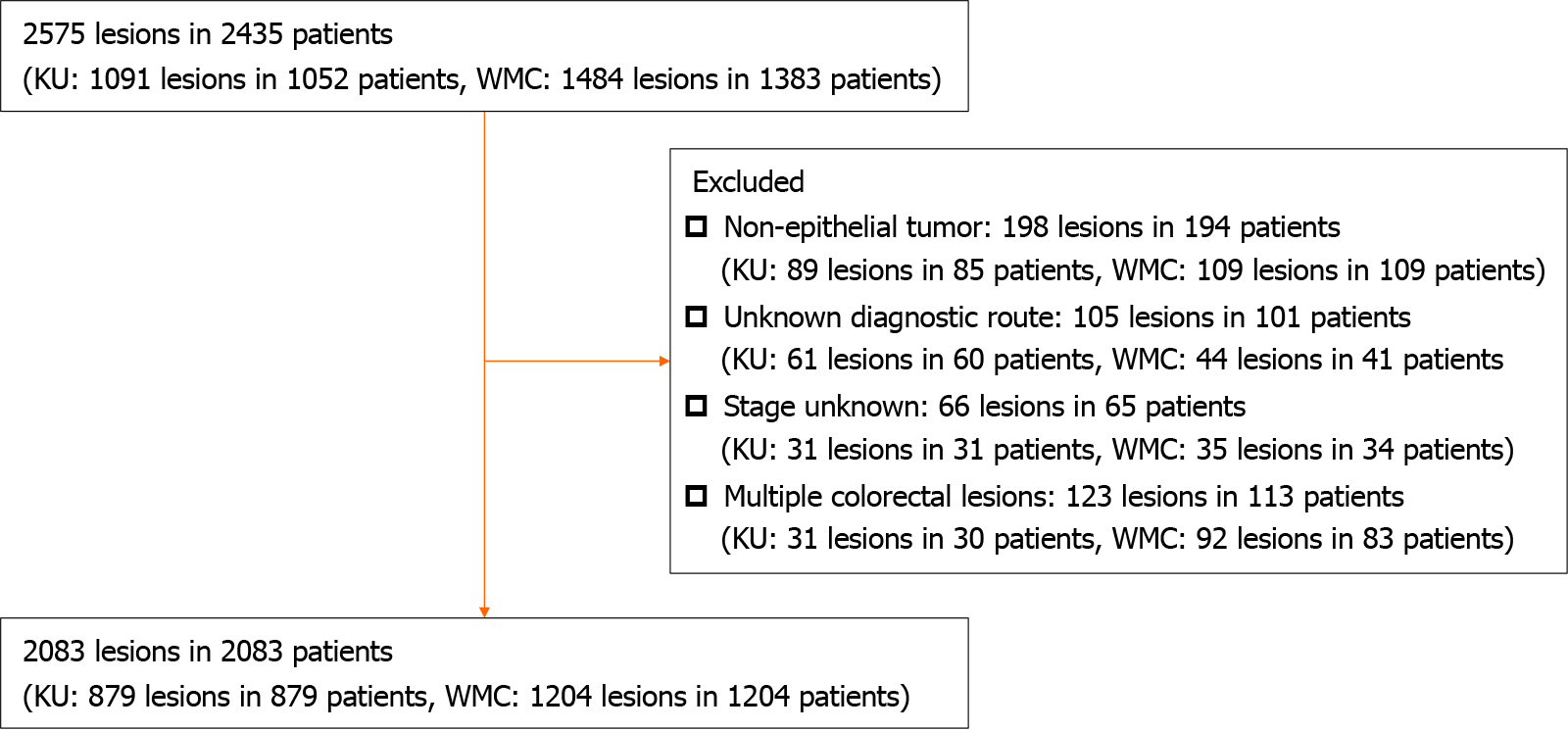
A flowchart of the participants is shown in Figure 1. Of the 2575 lesions and 2435 patients, the study included 2083 patients (879 at KU and 1204 at WMC). Table 1 presents patient characteristics for each discovery route. Among the 2083 patients included in the study, 715 (34.4%), 1064 (51.1%), and 304 (14.6%) belonged to the follow-up, symptomatic, and cancer screening groups, respectively. Patient characteristics at each hospital are presented in Table 2. The stage distribution and early-stage diagnosis proportions for each diagnostic route are presented in Table 3. In each hospital, as for the early-stage diagnosed proportion, the follow-up, symptomatic, and cancer screening groups had 55.7%, 21.7%, and 54.7%, respectively, in KU, and 58.3%, 25.7%, and 63.1%, respectively, in WMC, indicating similar trends at both institutions (Table 4). The proportion of patients with CRC diagnosed at an early stage was 57.3% (410 of 715), 23.9% (254 of 1064), and 59.5% (181 of 304) in the follow-up, symptomatic, and cancer screening groups, respectively. The symptomatic group exhibited a lower likelihood of early-stage diagnosis than the follow-up group (P < 0.001; aOR: 0.23; 95%CI: 0.19-0.29). The likelihood of being diagnosed at an early stage was similar between the follow-up and cancer screening groups (P = 0.493; aOR for early-stage diagnosis in the cancer screening group vs the follow-up group = 1.11, 95%CI = 0.82-1.49) (Table 5).
| Variable | Follow-up group (n = 715) | Symptomatic group (n = 1064) | Cancer screening group (n = 304) |
| Age (median, IQR) | 74 (68-79) | 71 (62-78) | 64 (56-71) |
| Sex, male, n (%) | 444 (62.1) | 559 (52.5) | 172 (56.6) |
| Right side, n (%) | 318 (44.5) | 302 (28.4) | 96 (31.6) |
| Variable | KU | WMC | ||||
| Follow-up group (n = 271) | Symptomatic group (n = 480) | Cancer screening group (n = 128) | Follow-up group (n = 444) | Symptomatic group (n = 584) | Cancer screening group (n = 176) | |
| Age (median, IQR) | 72 (66-79) | 69 (59-76) | 64 (54-70) | 74 (69-79) | 72.5 (64.3-80.0) | 64.0 (56.3-71.8) |
| Sex, male, n (%) | 168 (62.0) | 251 (52.3) | 71 (55.5) | 276 (62.2) | 308 (52.7) | 101 (57.4) |
| Right side, n (%) | 116 (42.8) | 125 (26.0) | 41 (32.0) | 202 (45.5) | 177 (30.3) | 55 (31.3) |
| Tumor stage | Follow-up group (n = 715) | Symptomatic group (n = 1064) | Cancer screening group (n = 304) |
| Stage 0 | 216 (30.2) | 92 (8.7) | 81 (26.6) |
| Stage I | 194 (27.1) | 162 (15.2) | 100 (32.9) |
| Stage II | 106 (14.8) | 240 (22.6) | 39 (12.8) |
| Stage III | 109 (15.2) | 297 (27.9) | 54 (17.8) |
| Stage IV | 90 (12.6) | 273 (25.7) | 30 (9.9) |
| Early-stage | 410 (57.3) | 254 (23.9) | 181 (59.5) |
| Tumor stage | KU | WMC | ||||
| Follow-up group (n = 271) | Symptomatic group (n = 480) | Cancer screening group (n = 128) | Follow-up group (n = 444) | Symptomatic group (n = 584) | Cancer screening group (n = 176) | |
| Stage 0 | 62 (22.9) | 22 (4.6) | 20 (15.6) | 154 (34.7) | 70 (12.0) | 61 (34.7) |
| Stage I | 89 (32.8) | 82 (17.1) | 50 (39.1) | 105 (23.7) | 80 (13.7) | 50 (28.4) |
| Stage II | 39 (14.4) | 113 (23.5) | 14 (10.9) | 67 (15.1) | 127 (21.8) | 25 (14.2) |
| Stage III | 43 (15.9) | 120 (25.0) | 23 (18.0) | 66 (14.9) | 177 (30.3) | 31 (17.6) |
| Stage IV | 38 (14.0) | 143 (29.8) | 21 (16.4) | 52 (11.7) | 130 (22.3) | 9 (5.1) |
| Early-stage | 151 (55.7) | 104 (21.7) | 70 (54.7) | 259 (58.3) | 150 (25.7) | 111 (63.1) |
| Variable | Early-stage detection rate (%) | Univariate | Multivariate | ||
| cOR (95%CI) | P value | aOR (95%CI) | P value | ||
| Follow-up group | 57.3 | Reference | Reference | ||
| Symptomatic group | 23.9 | 0.23 (0.19-0.29) | < 0.001 | 0.23 (0.19-0.29) | < 0.001 |
| Cancer screening group | 59.5 | 1.09 (0.83-1.44) | 0.516 | 1.11 (0.82-1.49) | 0.493 |
In this study, we conducted a comparative analysis of stages at diagnosis of CRC based on diagnostic routes. This study had two major findings. First, patients with CRC, which was detected during hospital visits for existing comorbidities, exhibited a higher proportion of early-stage diagnoses than those who presented with CRC-related symptoms. Second, the proportion of early-stage CRC diagnoses detected during hospital visits for comorbidities was comparable to that in the cancer screening group.
Patients with CRC detected at a comorbidity visit had a higher rate of early-stage diagnosis than those who presented with CRC-related symptoms. The first potential explanation for this derived from the fact that asymptomatic patients were included among the patients with CRC who were detected during hospital visits for other comorbidities. The frequency of symptomatic early-stage cancer is low, and the proportion of symptomatic cases increases with cancer progression[23]. Follow-up testing for comorbidities would incidentally detect asymptomatic CRC at an earlier stage compared to CRC detected in symptomatic patients. Second, the increased frequency of hospital visits owing to comorbidities likely results in early-stage cancer diagnosis due to incidental detection (surveillance hypothesis)[24]. Previous studies have shown that certain chronic diseases, such as end-stage kidney disease and high levels of comorbidities, are correlated with the detection of CRCs in earlier stages owing to frequent visits to healthcare providers[25,26]. In our study, patients with comorbidities were more likely to undergo imaging studies and colonoscopies because of abnormal tests and some symptoms, which could lead to an earlier diagnosis. Therefore, CRC detection during hospital visits for comorbidities may have been at an earlier stage than that in symptomatic patients.
We also found that the proportion of early-stage CRCs detected during hospital visits for comorbidities was comparable to that in the cancer screening group. The proportion of early-stage detection was 57.3% in the follow-up group compared with 59.5% in the screening group (P = 0.493). The odds of early detection were similar between groups, with an OR of 1.11 (95%CI: 0.82-1.49) (screening vs follow-up), and no significant differences were observed between the two groups. There have been no reports comparing the proportion of early-stage CRC diagnoses detected during hospital visits for comorbidities with those detected through regular screening. Our findings can be attributed to the unique medical context seen in Japan, characterized by unrestricted access to medical facilities facilitated by the universal insurance system[27]. The swift examinations during hospital visits potentially increase the chances of rapid diagnosis and early detection. Extended diagnosis periods are reportedly correlated with later detection stages[28]. Notably, Japan’s healthcare infrastructure facilitates easy access to advanced diagnostic technologies, such as colonoscopies and computed tomography, potentially contributing to early detection. Therefore, patients regularly monitored for comorbidities may have an increased early-stage diagnosis rate, which is comparable to that of patients who undergo cancer screening, even if they present with CRC-related symptoms.
Therefore, CRC screening should be recommended, particularly for patients without periodical hospital visits for comorbidities. Our study suggests that, in clinical practice, CRC is detected relatively early when it is found in patients presenting for follow-up for existing comorbidities. To date, no report has demonstrated that CRC detection during the follow-up of comorbidities is at an earlier stage than that after presenting with symptoms and that it occurs as early as that during cancer screening. Previous studies suggest that patients who visit hospitals for comorbidities can be recommended for cancer screening during consultation with their family physician[29,30]. Conversely, individuals without underlying conditions or those who do not undergo CRC screening may harbor undiagnosed CRC owing to a lack of hospital visits. CRC screening should be recommended for all screening-eligible individuals. Tailored message interventions for screening recommendations targeting segmented individuals reportedly increase screening attendance rates[9]. However, the resources required for screening outreach are limited, and colonoscopies for diagnostic testing require financial and human resources[10-13]. CRC detected at late stages contributes to higher medical costs[31]. Therefore, recommending screening would be essential, particularly for populations at a high risk of diagnosis with late-stage cancer, to take advantage of limited resources. Hence, it would be beneficial to develop policies that specifically encourage cancer screening for those who do not regularly visit the hospital due to comorbidities, considering the barriers to acceptance of screening and the causes of lack of access to healthcare facilities in this population. Encouraging populations other than those who regularly visit the hospital for any comorbidities to undergo screening could increase the earlier-stage detection, which would further contribute to an improved prognosis for patients with CRC.
Our study has some limitations in interpreting the results. First, the type and number of comorbidities and frequency of hospital visits in patients with CRC detected during follow-up for other comorbidities in this study have not been considered. Second, whether individuals presenting with CRC-related symptoms include those who undergo regular cancer screening is unclear. However, in Japan, approximately half of the eligible candidates do not undergo screening, and it is presumed that most individuals exist within the symptomatic group who do not undergo regular cancer screening[6]. Third, our study was conducted at two designated cancer care hospitals in Japan and may not apply to other regions or countries. Healthcare systems and classification of CRC routes for diagnosis may differ in other countries; however, there were no significant differences in the proportions and stage distributions of each population between our study population and the Japanese cohort[18]. While our study only had access to cancer registration data from two facilities, using cancer registration data from other facilities or nationwide cancer registration data in Japan would aid in investigating whether the trends observed in this study are universally applicable.
This study suggests that CRC detection during hospital visits for comorbidities is likely at earlier stages than those detected via the symptomatic route. Furthermore, CRCs detected during hospital visits for comorbidities may be detected as early as those detected through cancer screening. While CRC screening should be recommended for all eligible individuals, particular attention could be directed towards populations without periodical hospital visits for comorbidities as they may not derive early CRC detection benefits due to fewer opportunities for hospital visits.
Colorectal cancer (CRC) screening reduces CRC mortality, yet several patients remain unscreened.
Although identifying specific subgroups at high risk is crucial to encourage more individuals to participate in screening, the association between the diagnostic routes and identification of these subgroups has been less appreciated.
To determine the stage at diagnosis of CRC based on various diagnostic routes.
A retrospective observational study was conducted using data from the cancer registry of two hospitals to clarify the stage at diagnosis in three groups: Follow-up (patients detected during follow-up for other comorbidities), symptomatic (patients detected following presentation with CRC-related symptoms), and cancer screening.
In a study of 2083 patients, early-stage CRCs were diagnosed in 57.3% of the follow-up group, 23.9% of the symptomatic group, and 59.5% of the cancer screening group. The symptomatic group had a lower likelihood of early-stage diagnosis compared to the follow-up group, while the follow-up and cancer screening groups showed similar likelihoods of early-stage diagnosis.
CRCs detected during hospital visits for comorbidities were diagnosed earlier, similar to cancer screening.
Encouraging CRC screening in individuals who do not make regular hospital visits for comorbidities could enhance early detection and improve patient prognoses.
We would like to thank Ms. Hiroko Kimura and Ms. Naoko Nishioka at the Japanese Red Cross Wakayama Medical Center for registering the cancer registry.
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 10927] [Article Influence: 3642.3] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1847] [Reference Citation Analysis (5)] |
| 3. | Kubisch CH, Crispin A, Mansmann U, Göke B, Kolligs FT. Screening for Colorectal Cancer Is Associated With Lower Disease Stage: A Population-Based Study. Clin Gastroenterol Hepatol. 2016;14:1612-1618.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Friedrich K, Grüter L, Gotthardt D, Eisenbach C, Stremmel W, Scholl SG, Rex DK, Sieg A. Survival in patients with colorectal cancer diagnosed by screening colonoscopy. Gastrointest Endosc. 2015;82:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Araghi M, Arnold M, Rutherford MJ, Guren MG, Cabasag CJ, Bardot A, Ferlay J, Tervonen H, Shack L, Woods RR, Saint-Jacques N, De P, McClure C, Engholm G, Gavin AT, Morgan E, Walsh PM, Jackson C, Porter G, Møller B, Bucher O, Eden M, O'Connell DL, Bray F, Soerjomataram I. Colon and rectal cancer survival in seven high-income countries 2010-2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut. 2021;70:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Ministry of Health, Labour and Welfare. Comprehensive Survey of Living Conditions, 2022. [cited 1 September 2023]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa22/index.html. |
| 7. | Sabatino SA, Thompson TD, White MC, Shapiro JA, Clarke TC, Croswell JM, Richardson LC. Cancer Screening Test Use-U.S., 2019. Am J Prev Med. 2022;63:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Weller D, Menon U, Zalounina Falborg A, Jensen H, Barisic A, Knudsen AK, Bergin RJ, Brewster DH, Cairnduff V, Gavin AT, Grunfeld E, Harland E, Lambe M, Law RJ, Lin Y, Malmberg M, Turner D, Neal RD, White V, Harrison S, Reguilon I; ICBP Module 4 Working Group, Vedsted P. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP). BMJ Open. 2018;8:e023870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Hirai K, Ishikawa Y, Fukuyoshi J, Yonekura A, Harada K, Shibuya D, Yamamoto S, Mizota Y, Hamashima C, Saito H. Tailored message interventions versus typical messages for increasing participation in colorectal cancer screening among a non-adherent population: A randomized controlled trial. BMC Public Health. 2016;16:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kapinos KA, Halm EA, Murphy CC, Santini NO, Loewen AC, Skinner CS, Singal AG. Cost Effectiveness of Mailed Outreach Programs for Colorectal Cancer Screening: Analysis of a Pragmatic, Randomized Trial. Clin Gastroenterol Hepatol. 2022;20:2383-2392.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hamashima C, Sano H. Association between age factors and strategies for promoting participation in gastric and colorectal cancer screenings. BMC Cancer. 2018;18:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Sekiguchi M, Igarashi A, Matsuda T, Matsumoto M, Sakamoto T, Nakajima T, Kakugawa Y, Yamamoto S, Saito H, Saito Y. Optimal use of colonoscopy and fecal immunochemical test for population-based colorectal cancer screening: a cost-effectiveness analysis using Japanese data. Jpn J Clin Oncol. 2016;46:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, Keating NL, Kim P, Lave JR, Laveist TA, Ness RB, Optican RJ, Virnig BA. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Lairson DR, DiCarlo M, Myers RE, Wolf T, Cocroft J, Sifri R, Rosenthal M, Vernon SW, Wender R. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, Richards M. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 431] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 16. | Downing A, Aravani A, Macleod U, Oliver S, Finan PJ, Thomas JD, Quirke P, Wilkinson JR, Morris EJ. Early mortality from colorectal cancer in England: a retrospective observational study of the factors associated with death in the first year after diagnosis. Br J Cancer. 2013;108:681-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Center for Cancer Control and Information Services, National Cancer Center, Japan. Coding definitions of the hospital-based cancer registry in designated cancer care hospitals. 2023. [cited 1 September 2023]. Available from: https://ganjoho.jp/med_pro/cancer_control/can_reg/hospital/regulation.html. |
| 18. | Cancer Information Service, National Cancer Center, Japan. Annual report of hospital-based cancer registries. [cited 27 January 2023]. Available from: https://jhcr-cs.ganjoho.jp/hbcrtables/. |
| 19. | Kajiwara Saito M, Morishima T, Ma C, Koyama S, Miyashiro I. Diagnosis and treatment of digestive cancers during COVID-19 in Japan: A Cancer Registry-based Study on the Impact of COVID-19 on Cancer Care in Osaka (CanReCO). PLoS One. 2022;17:e0274918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Center for Cancer Control, National Cancer Center, Japan. Updated Version of Evidence-based Guidelines for Colorectal Cancer Screening. January 9, 2023. [cited 1 September 2023]. Available from: https://canscreen.ncc.go.jp/koukaiforum/2023/G_CRC_2023.pdf. |
| 21. | van de Veerdonk W, Hoeck S, Peeters M, Van Hal G, Francart J, De Brabander I. Occurrence and characteristics of faecal immunochemical screen-detected cancers vs non-screen-detected cancers: Results from a Flemish colorectal cancer screening programme. United European Gastroenterol J. 2020;8:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Gornick ME, Eggers PW, Riley GF. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv Res. 2004;39:1403-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Nakabayashi N, Hirose M, Suzuki R, Suzumiya J, Igawa M. How asymptomatic are early cancer patients of five organs based on registry data in Japan. Int J Clin Oncol. 2018;23:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Fleming ST, Pursley HG, Newman B, Pavlov D, Chen K. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Zafar SY, Abernethy AP, Abbott DH, Grambow SC, Marcello JE, Herndon JE 2nd, Rowe KL, Kolimaga JT, Zullig LL, Patwardhan MB, Provenzale DT. Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective, parallel analysis of two health systems. BMC Cancer. 2008;8:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Taneja S, Mandayam S, Kayani ZZ, Kuo YF, Shahinian VB. Comparison of stage at diagnosis of cancer in patients who are on dialysis versus the general population. Clin J Am Soc Nephrol. 2007;2:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Sugiyama K, Oshio T, Kuwahara S, Kimura H. Association between having a primary care physician and health behavioral intention in Japan: results from a nationwide survey. BMC Prim Care. 2023;24:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Tørring ML, Murchie P, Hamilton W, Vedsted P, Esteva M, Lautrup M, Winget M, Rubin G. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017;117:888-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Wang H, Roy S, Kim J, Farazi PA, Siahpush M, Su D. Barriers of colorectal cancer screening in rural USA: a systematic review. Rural Remote Health. 2019;19:5181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 569] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 31. | Utsumi T, Horimatsu T, Nishikawa Y, Hoshino N, Takahashi Y, Goto R, Kashihara S, Fukuyoshi J, Nakayama T, Seno H. Medical costs according to the stages of colorectal cancer: an analysis of health insurance claims in Hachioji, Japan. J Gastroenterol. 2021;56:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Verma V, United States S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY