Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5543
Peer-review started: July 20, 2023
First decision: August 25, 2023
Revised: September 5, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: October 28, 2023
Processing time: 99 Days and 6.5 Hours
Phosphatidylinositol-3-kinases (PI3K) is a well-known route in inflammation-related cancer. Recent discovery on PI3K-related genes revealed a potential variant that links ulcerative colitis (UC) and colorectal cancer (CRC) with colitis-associated cancer (CAC). PI3K/AKT pathway has been recommended as a potential additional therapeutic option for CRC due to its substantial role in modifying cellular processes. Buparlisib is a pan-class I PI3K inhibitor previously shown to reduce tumor growth.
To investigate the regulation of rs10889677 and the role of buparlisib in the PI3K signaling pathway in CAC pathogenesis.
Genomic DNA from 32 colonic samples, including CAC (n = 7), UC (n = 10) and CRC (n = 15), was sequenced for the rs10889677 mutation. The mutant and wildtype fragments were amplified and cloned in the pmirGLO vector. The luciferase activity of cloned vectors was assessed after transfection into the HT29 cell line. CAC mice were induced by a mixture of a single azoxymethane injection and three cycles of dextran sulphate sodium, then buparlisib was administered after 14 d. The excised colon was subjected to immunohistochemistry for Ki67 and Cleaved-caspase-3 markers and quantitative real-time polymerase chain reaction analysis for Pdk1 and Sgk2.
Luciferase activity decreased by 2.07-fold in the rs10889677 mutant, confirming the hypothesis that the variant disrupted miRNA binding sites, which led to an increase in IL23R expression and the activation of the PI3K signaling pathway. Furthermore, CAC-induced mice had a significantly higher disease activity index (P < 0.05). Buparlisib treatment significantly decreased mean weight loss in CAC-induced mice (P < 0.05), reduced the percentage of proliferating cells by 5%, and increased the number of apoptotic cells. The treatment also caused a downward trend of Pdk1 expression and significantly decreased Sgk2 expression.
Our findings suggested that the rs10889677 variant as a critical initiator of the PI3K signaling pathway, and buparlisib had the ability to prevent PI3K-non-AKT activation in the pathophysiology of CAC.
Core Tip: The role of phosphatidylinositol-3-kinases (PI3K) in promoting cancer progression has been widely acknowledged due to its crucial involvement in regulating the survival, differentiation, and proliferation of cancer cells. Here, we investigate the role of PI3K signaling in colitis-associated cancer (CAC) pathogenesis by studying the regulation of potential variant in PI3K-related gene in the colorectal cancer cell line and the utilization of PI3K inhibitor, buparlisib, in the CAC-induced mice model. We suggested that rs10889677 variant plays a crucial role in initiating the PI3K signaling pathway, and buparlisib has the capability to inhibit PI3K-non-AKT activation in the pathophysiology of CAC.
- Citation: Razali NN, Raja Ali RA, Muhammad Nawawi KN, Yahaya A, Mohd Rathi ND, Mokhtar NM. Roles of phosphatidylinositol-3-kinases signaling pathway in inflammation-related cancer: Impact of rs10889677 variant and buparlisib in colitis-associated cancer. World J Gastroenterol 2023; 29(40): 5543-5556
- URL: https://www.wjgnet.com/1007-9327/full/v29/i40/5543.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i40.5543
Inflammatory bowel disease (IBD), a chronic inflammatory disorder that goes into remission and then recurs, eventually leading to the development of colitis-associated cancer (CAC), is a kind of colorectal cancer (CRC)[1,2]. Crohn’s disease and ulcerative colitis (UC), the two main subtypes of IBD, had CAC risks of 1.4% and 0.8%, respectively[3,4]. The rising trend of IBD incidence and prevalence among Asians increased from 1.3% to 7.2% per 100000 population among Malaysian-Singaporeans[5]. Rapid urbanization, adoption of a Western diet, antibiotics use, personal hygiene standards, microbiological exposures, and pollution are all risk factors for developing IBD[6]. In Malaysia, the average annual incidence of IBD has risen to 1.46 per 100000 people over the last decade[7]. CAC accounted for 10%-15% of IBD fatality cases among Westerners, accounting for only 1% to 2% of CRC incidence in Malaysia[4,8].
CAC develops through the inflammation–dysplasia–carcinoma pathway[9]. Immune cells, including cytokines, chemokines, epithelial cells, and stromal cells, are among the cell types contributing to the inflammatory processes in the CAC development[10]. Moreover, inflammatory mediators had a substantial impact on the control of pre-neoplastic growth during CAC development, and their release is more likely to target multiple carcinogenesis-related signaling pathways involved, such as NFkB, PI3K, JAK/STAT and Wnt/B-catenin[11,12].
Phosphatidylinositol-3-kinases (PI3K) signaling pathway is an essential intracellular signaling mechanism in regulating the cell cycle[13]. Growth factors, cytokines, and other stimulatory chemicals activate PI3K via their receptors. The AKT-mTOR and SGK families receive signals from activated PI3K, which controls cell growth, proliferation, and apoptosis[14]. PI3K enzymatic activity has been linked to the aetiology of various diseases, including chronic inflammation and cancer[12,15]. Our earlier research identified PI3K as one of the critical pathways in long-duration UC associated with an increased risk of developing CAC[16]. A recent study on mutational analysis in CAC patients identified several potential variants, including rs10889677, in the IL23R, a cytokine-induced PI3K-related gene that may link inflammation to an increased risk of cancer[17].
PI3K/AKT pathway has been proposed as a viable therapeutic target for CRC due to its significant role in modulating cellular processes by targeting the downstream pathway[18]. Buparlisib is an oral pan-class I PI3K inhibitor that targets all four class I PI3K catalytic isoforms (p110α, p110β, p110δ and p110γ)[19,20]. This drug is the most clinically advanced medication to have passed a phase II clinical trial in the cancer[21,22]. Buparlisib treatment in in vivo study showed encouraging outcomes by enormously lowering the quantity of phosphorylated AKT and slowing tumor growth in tumor-bearing mouse models[19,23]. Therefore, the objective of this study was to study the regulation of the PI3K-related variant, rs10889677, in the CRC cell line and to investigate whether buparlisib may affect the non-AKT-independent branch of the PI3K signaling pathway, which was one of the keys signaling pathways implicated in cancer development and progression, using a mouse model.
The Universiti Kebangsaan Malaysia Research Ethics Committee (UKM/PPI/111/8/JEP-2019-572) approved this study. A total of 32 fresh frozen and archival samples (ranging from the year 2014 to 2019) from patients with long-standing UC, CAC, and CRC were collected from the Endoscopy Unit at Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia. Upon admission, all patients provided informed consent. All tissues were collected by December 2020, stored in RNAlater, and frozen at -80 oC until further processing. The hematoxylin and eosin-stained tissue sections were evaluated by an experienced pathologist for confirmation of diagnosis, inflammation, and metastatic status. The genomic DNA was extracted from fresh frozen tissues using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, United States) according to the manufacturer’s protocol. Meanwhile, GENEREAD DNA FFPE Kit (Qiagen, United States) was used to extract DNA from formalin-fixed paraffin-embedded blocks of archival samples. DNA concentration was measured using DeNovix DS11+ Spectrophotometer (DeNovix Inc., United States).
Primers were designed using the NCBI Primer Tool (National Center for Biotechnology Information, United States) and Primer3Plus[24] based on the location of the somatic variant. The predesigned primers were as follows: IL23R, forward 5’-TCT GTG CTC CTA CCA TCA CC-3’, and reverse 5’-TGT GCC TGT ATG TGT GAC CA-3’. SnapGene Viewer 5.3.2 was used to analyze the sequencing results.
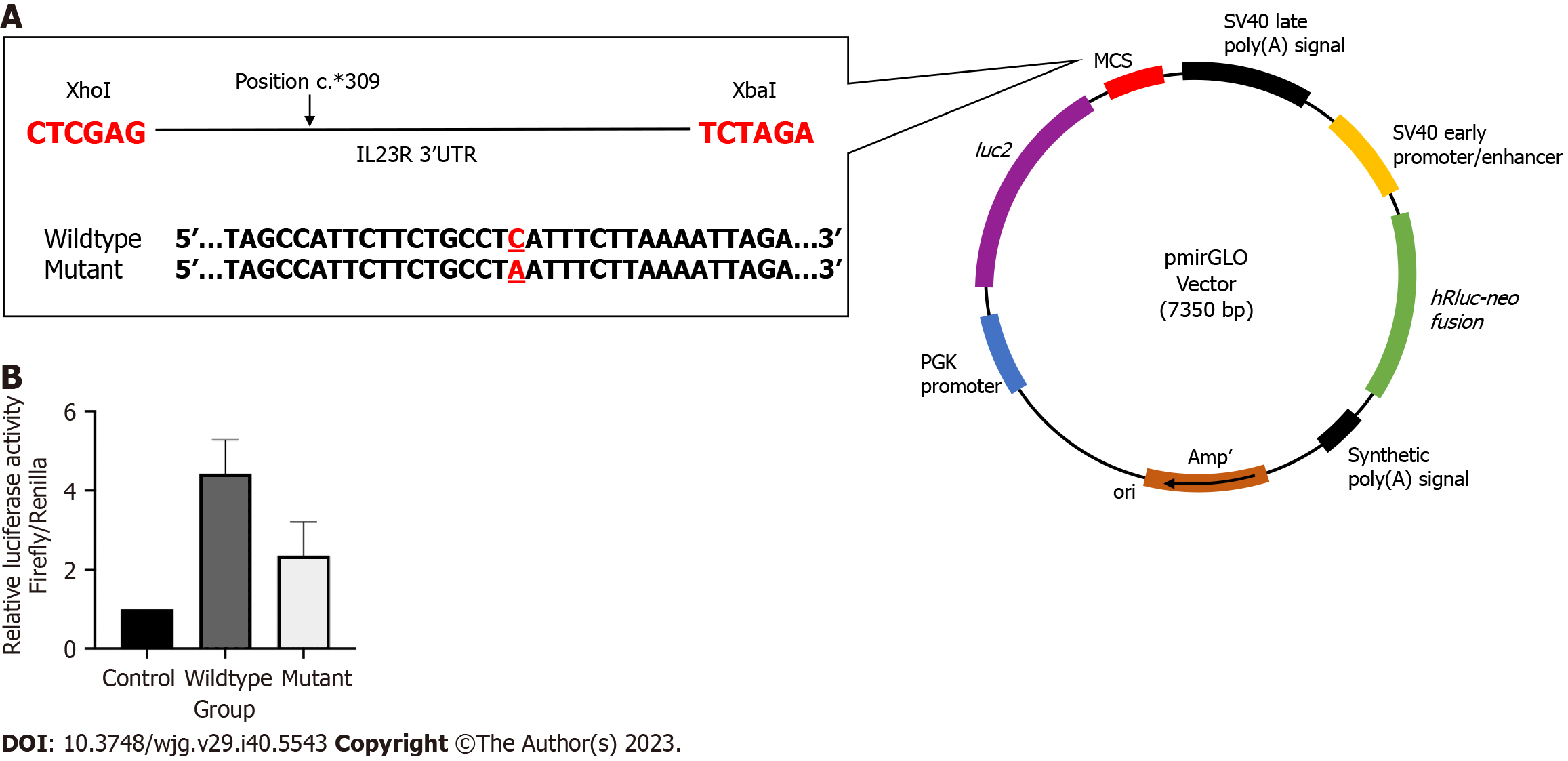
The fragments containing the wildtype and mutant rs10889677 variant on the 3’ untranslated region of IL23R were amplified from the genotyped DNA samples. The predesigned primers were added with the target sequences of the Xbal and XhoI restriction enzymes. The primers were as follows: Forward 5’-ATC GCT CGA GGC TGC CTT GCA ATC TGA ACT-3’ and reverse 5’-ATC GTC TAG ATC TGC CTT CCT GGT TCA AGT-3’. The polymerase chain reaction for fragment amplification was carried out using GoTaq® Green Master Mix (Promega, United States) with a pre-denaturation step at 95 oC for eight minutes, followed by 35-40 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, with an additional final elongation step at 72 °C for five minutes. Then, the polymerase chain reaction (PCR) products were run on gel electrophoresis to check for fragment bands, followed by the gel purification step using the QIAquick® Gel Extraction Kit (Qiagen, United States).
A total of 1 μg of pmirGLO (Promega, United States) vector was digested with the following enzymes: Xba1 (NEB, United Kingdom) and XhoI (NEB, United Kingdom) according to the manufacturer’s instructions. The ligation reactions were prepared using a T4 DNA Ligase kit (NEB, United Kingdom) with a molar ratio of 1:3 vector to insert. The ligated products were transformed utilizing the heat-shock technique with an E.coli competent cell (NEB, United Kingdom). Colonies from prior successful transformations were screened, and positive transformant plasmid was purified using PureYield™ Plasmid Miniprep System (Promega, United States).
The CRC cell line, HT29, was routinely cultured in a RPMI-1640 (Elabscience, United States) supplemented with 10% fetal bovine serum (Gibco, United States) and 1% of Penicillin/Streptomycin (Gibco, United States) and cultivated in 5% of CO2 level incubator at 37 oC. Before transfection, 5.0 × 104 HT29 cells were seeded into a 96-well white plate in 100 μL antibiotic-free growth media. Cultures were performed in triplicate. A total of 0.25 μg of wildtype, mutant and empty plasmids were transfected using Lipofectamine 2000 (Invitrogen, United States) transfection reagent according to the manufacturer’s protocol. Afterwards, the luciferase activity was measured 48 h after post-transfection using the Dual-Glo® Luciferase Assay system (Promega, United States) following the manufacturer’s protocol. Firefly and Renilla luciferase activity were measured using the GloMax® 20/20 Luminometer (Promega, United States). The assay was performed in triplicate, and all results were normalized with the internal control reading.
Forty male Balb/c mice (seven to eight weeks old, weighing 25-30 g) were purchased from the Animal Unit, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia. The mice were housed in individual cages with kenaf bedding and maintained on a 12 h light/dark cycle with controlled humidity at 25 oC kept in the animal laboratory unit, Faculty of Medicine, Universiti Kebangsaan Malaysia. Regular food and water were supplied ad libitum. All mice were acclimatized for ten days before the start of the treatment. The animal handling and protocol was approved by the Animal Ethics Committee of Universiti Kebangsaan Malaysia (PPUKM/2019/NORFILZA/25-SEPT./1035-SEPT.-2019-DEC.-2021).
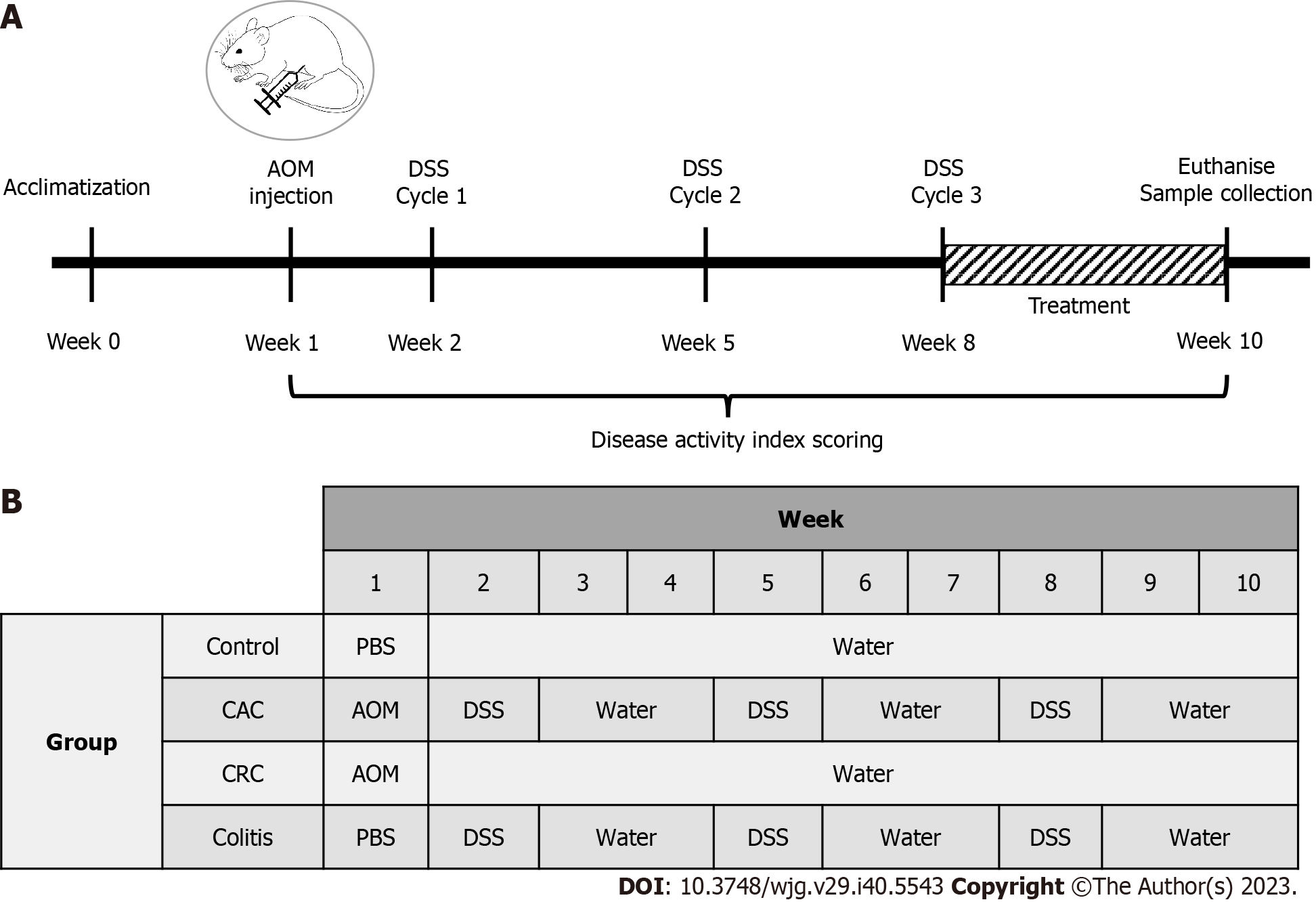
The mice were randomly divided into four groups (n = 10 each): Control, dextran sodium sulphate (DSS) induced, azoxymethane (AOM) induced, and AOM/DSS combination induced[25]. A single intraperitoneal injection of AOM (10 mg/kg body weight) (Sigma-Aldrich Chemicals, United States) was administered to the AOM and AOM/DSS-induced group. A week after injection, AOM/DSS and DSS-induced groups received 2.5% DSS (40kDa; Sigma-Aldrich Chemicals, United States) in their drinking water for seven days in three cycles (each cycle consisting of one-week DSS, followed by two weeks of sterile water). The control and AOM-induced groups were only provided with sterile water throughout the treatment. Beginning in week eight, mice from the AOM/DSS-induced group were treated daily for 14 d with a PI3K inhibitor buparlisib (NVP-BKM120, MedChemExpress, United States) at 30 mg/kg via oral administration (Figure 1).
Daily observations and records were made of body weight, bowel consistency, and rectal bleeding throughout ten weeks. Changes in these factors will be scored to determine the disease activity index (DAI), which is based on the rating of each component. Scores were assigned for weight loss (0: 0-1%, 1: 1%-5%, 2: 5%-10%, 3: 10%-20%, 4: > 20%), stool consistency (0: Normal, 2: Loose stool, 4: Diarrhea), and presence of blood in the stool (0: Negative, 2: Visual blood in stool, 4: Fresh rectal bleeding)[26]. A total score was determined, ranging from 0 to 12.
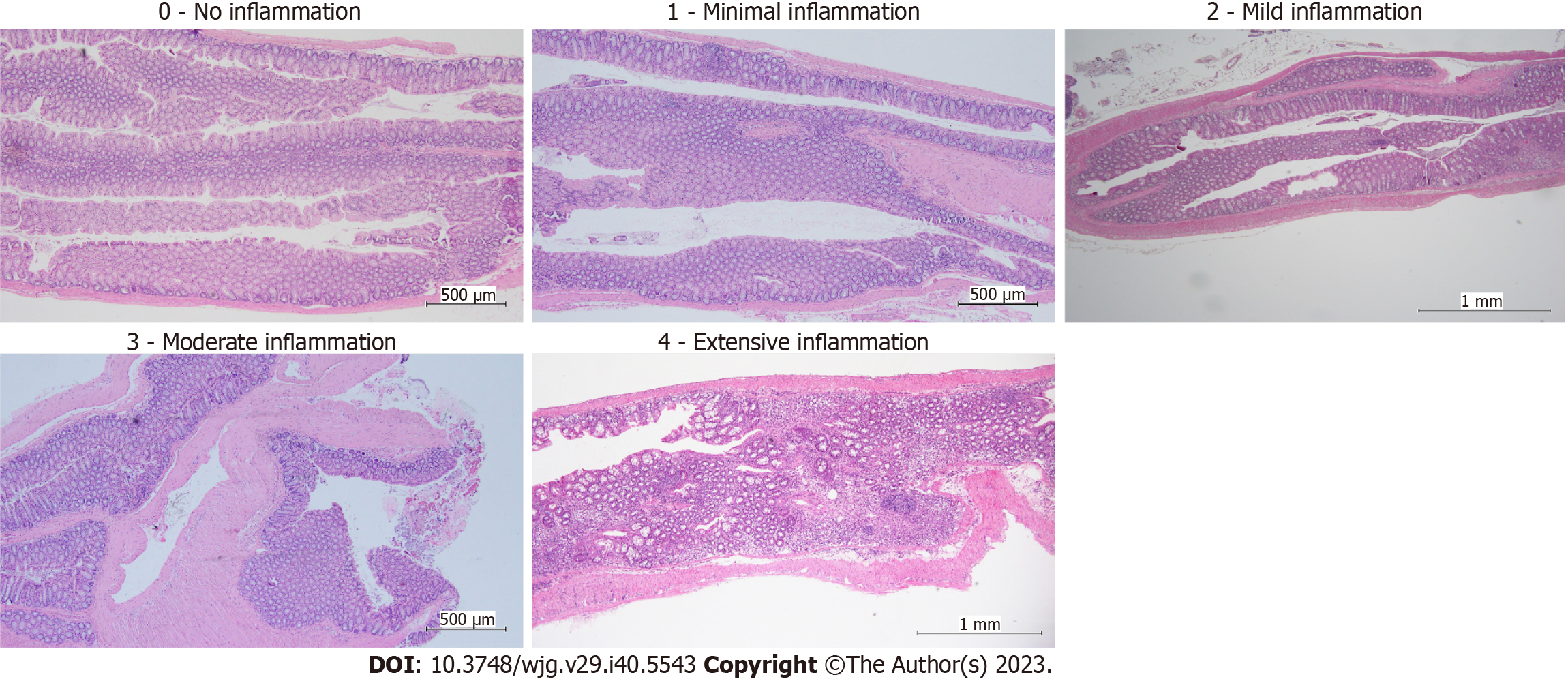
Colons were removed, cleaned, and flushed with sterile phosphate buffer saline and cut into two parts: Proximal and distal colon. The proximal colon was stored in RNAlater solution (ThermoFisher, United States), and the distal colons were rolled, placed in the tissue cassette, and soaked in 10% neutral-buffered formalin for at least one night. Then, the tissues were processed, embedded in paraffin, and sectioned for hematoxylin and eosin staining. Histological structures were assessed and scored (0: Healthy colon, 1: Minimal inflammation with minimal to no separation of crypts, 2: Mild inflammation with mild separation of crypts, 3: Moderate inflammation with separation of crypts, with or without focal effacement of crypts, 4: Extensive inflammation with marked separation and effacement of crypts, 5: Diffuse inflammation with marked separation and effacement of crypts)[27]. The pathologist used an inverted microscope to examine all slides.
Immunohistochemistry (IHC) staining was performed using the Rabbit-specific HRP/DAB Detection IHC Detection kit Micro-polymer, as instructed by the manufacturer (ab236469; Abcam, United States). Slides were incubated in primary antibodies: Rabbit monoclonal Cleaved caspase-3 (CC-3) (1:5000, ab214430; Abcam, United Kingdom) and rabbit monoclonal Ki67 (1:100, ab16667; Abcam, United Kingdom) for 30 minutes. The secondary antibody was goat anti-rabbit HRP Conjugate, included in the same kit. Images were obtained using an Olympus light microscope (Japan). Qualitative and quantitative scoring were scored blindly by the experienced pathologist.
The excised proximal colon was sectioned into smaller fragments and placed in different tubes at -80 oC. RNA was prepared using AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, United States) following the manufacturer’s instructions. The total RNA yield was quantified using a DS-11 spectrophotometer (DeNovix Inc., United States). cDNA conversion was conducted using QuantiNova Reverse Transcription Kit (Qiagen, United States) from 1 μg RNA following the manufacturer’s instructions. Quantitative PCR amplifications were prepared with approximately 100 ng cDNA using the QuantiNova SYBR Green PCR Kit (Qiagen, United States) and mouse primer sets (IDT, United States). The predesigned primers were as follows: Ppia, forward 5’-CAA ACA CAA ACG GTT AG-3’ and reverse 5’-TTC ACC TTC CCA AAG ACC AC-3’; Sgk2, forward, 5’-GCA TAG AGC CTA CCT GAT CAC-3’ and reverse 5’-CCC AGG TTG ATG TTC CCA TT-3’ and Pdk1, forward 5’-CAT GCA CAT CCA GAT CAC AGA-3’ and reverse 5’- CTT TTA CAC GCC GAC TTC TCT-3’. The gene expression was normalized to the reference gene, Ppia and all reactions were prepared in triplicate.
Normally distributed variables are presented as the mean ± standard error mean. For statistical significance, the Student T-test and ANOVA tests were performed. At P < 0.05, the differences between groups were considered significant. Statistical analyses were performed using the GraphPad Prism Version 9.3.1 (GraphPad Software Inc., United States).
Table 1 displays demographic data for all samples. The median age of all samples was 63 years old (IQR:11.5). Malays made up the majority of the samples (56%) and were followed by Chinese (28%) and Indians (15%). The gender distribution was equal between males and females. Most patients’ smoking status was non-smoker (91%) instead of smoker/ex-smoker (9%). Most UC patients were diagnosed with left-sided colitis or pancolitis, with a Mayo index score of 1 to 3 and a Geboes score of Grade 2A.1 to 2A.2. The majority of CRC and CAC patients were at stages 1 and 3, and the rectosigmoid and distal colon were the sites of the malignancies. Histologically, most CRCs were moderately differentiated, whereas tumors from CAC patients were categorized as poor, moderate and well differentiated. There was no reported familial history of CRC among any of the patients.
| CRC (n = 15) | CAC (n = 7) | UC (n = 10) | |
| Median age (range) | 63 (39-67) | 58 (20-71) | 65 (52-69) |
| Race | |||
| Malay | 11 | 2 | 5 |
| Chinese | 4 | 2 | 3 |
| Indian | - | 3 | 2 |
| Gender | |||
| Male | 9 | 3 | 4 |
| Female | 6 | 4 | 6 |
| Smoking status | |||
| Smoker/Ex-smoker | 2 | 1 | - |
| Non-smoker | 13 | 6 | 10 |
| Stage1 | |||
| I | 2 | 1 | Not applicable |
| II | - | - | |
| III | 7 | 4 | |
| Adenocarcinoma types1 | |||
| Poorly differentiated | - | 1 | Not applicable |
| Moderately differentiated | 7 | 1 | |
| Well differentiated | 1 | 3 | |
| Mayo score (range)1 | Not applicable | Data unavailable | 1-3 |
| Geboes score (range)1 | Not applicable | Data unavailable | 2A.1-2A.2 |
| Presentation | Rectal bleeding | Rectal bleeding, abdominal pain, severe diarrhea | Diarrhea, loss of appetite |
To understand the significance of the PI3K signaling pathway in the formation of CAC, the functional role of PI3K-related somatic variants that potentially correlate CAC with UC and CRC was investigated to gain insight into the role. Somatic variant rs10889677C>A has been successfully sequenced in CAC, UC and CRC samples. The proportion of homozygous CC (wildtype) was discovered to be the highest in CRC samples (60%), while the proportion of homozygous AA (mutant) was highest in CAC samples (85%). We discovered mutant rs10889677 had a 2.07-fold lower luciferase activity (2.35 ± 0.85) than the wildtype construct (4.42 ± 0.86). However, the difference was not statistically significant (Figure 2).
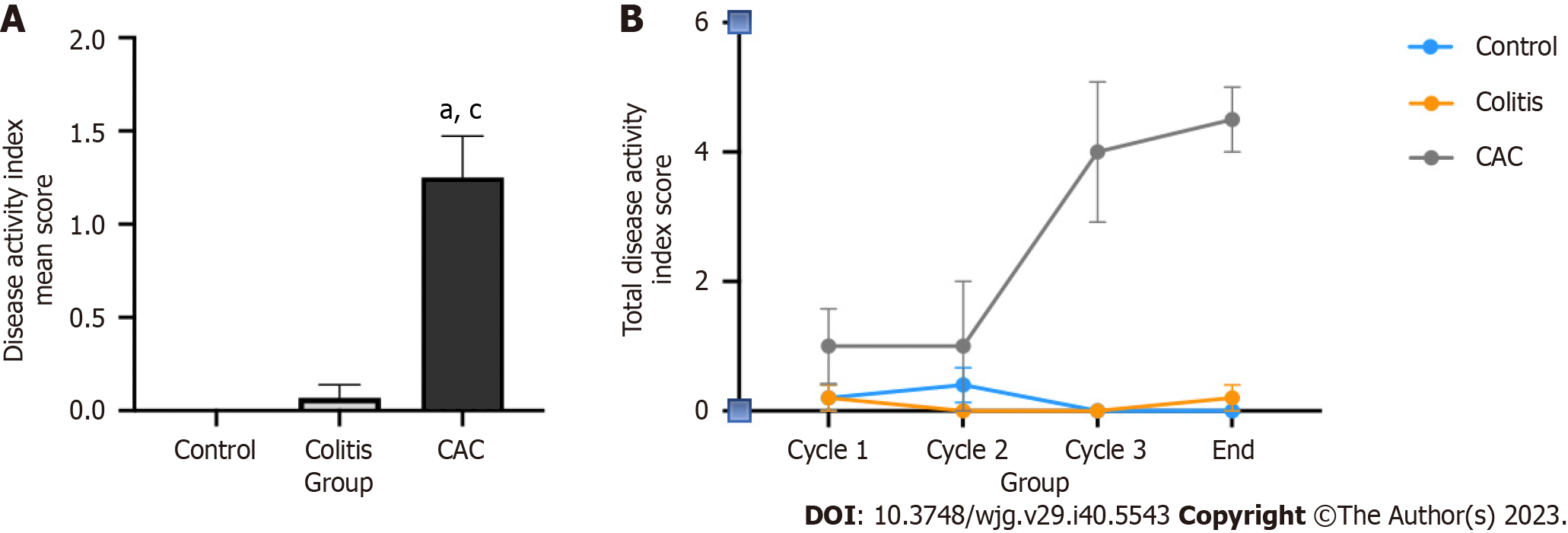
In a CAC mouse model, chemical induction of a single AOM injection with three cycles of 2.5% DSS was considered successful. The assessment of a successful CAC induction was based on observing body weight loss, the condition of the stool and the occurrence of any rectal bleeding. The CAC mice group endured reduced body weight, watery stools, and rectal bleeding for ten weeks. In light of this, the CAC mice group scored significantly higher on the DAI than the colitis and control groups, with a mean score of 1.25 ± 0.22 (P < 0.05) (Figure 3A). The DAI score of the CAC mice group began to rise dramatically after the second DSS induction (Figure 3B). On the other hand, the colitis group had a low DAI score, while the control group had none.
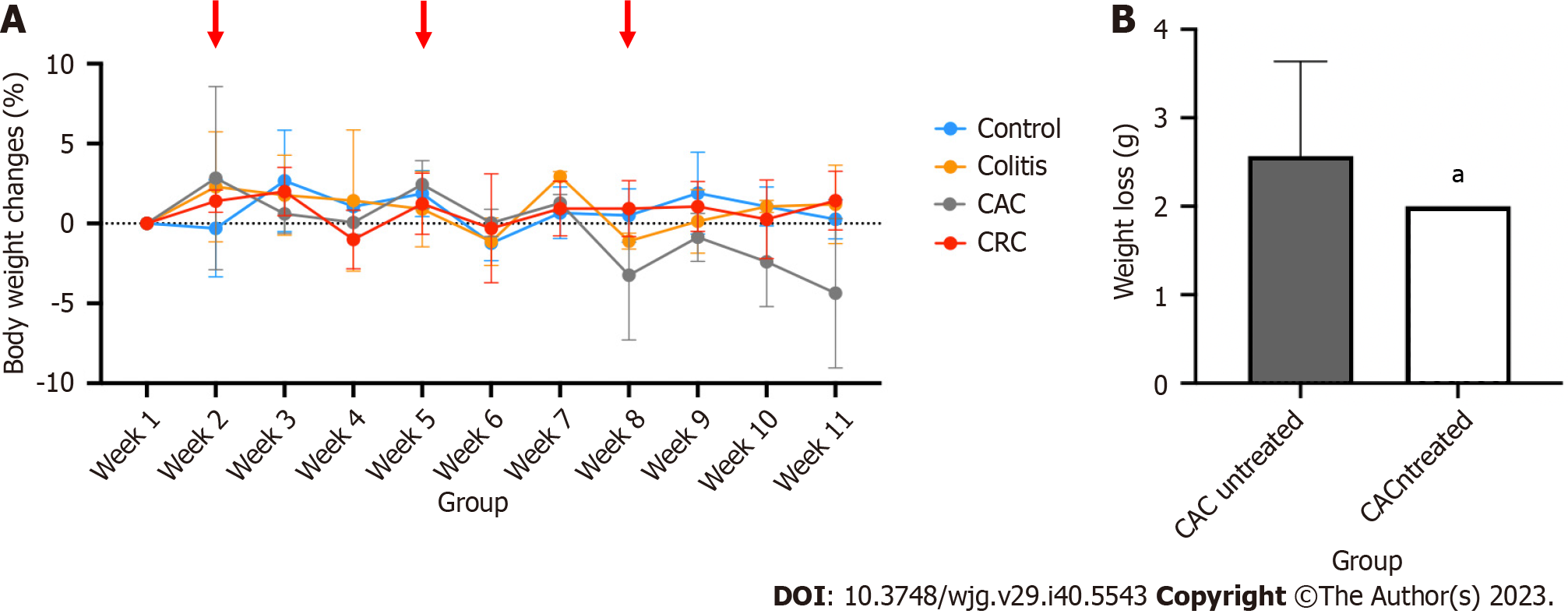
Due to ongoing DSS exposure, the colitis and CAC-induced mice group's body weights have fluctuated (Figure 4). At every DSS induction cycle, weight loss was predicted to occur. Following each cycle, mice had a two-weeks recovery period to help them regain any lost weight. However, starting with the third cycle of DSS induction, the CAC mice group had substantial weight loss, losing about 5% of their total body weight. This significant body weight reduction in the CAC group explained why they had a higher DAI score than other groups. Meanwhile, the mice in the other groups recovered and kept their body weight throughout the treatment (Figure 4A).
PI3K inhibitor (buparlisib) supplementation, started in the eight week of treatment, was used to perform additional research on the relationship between PI3K and the advancement of CAC. PI3K oral inhibitor dosage was calculated depending on the body weight of CAC mice. After receiving daily treatment for 14 d, the mean weight loss (g) in the CAC-induced mice was significantly lower (2.0 ± 0.0) than in the CAC group that had not received any treatment (2.6 ± 1.07) (P < 0.05) (Figure 4B).
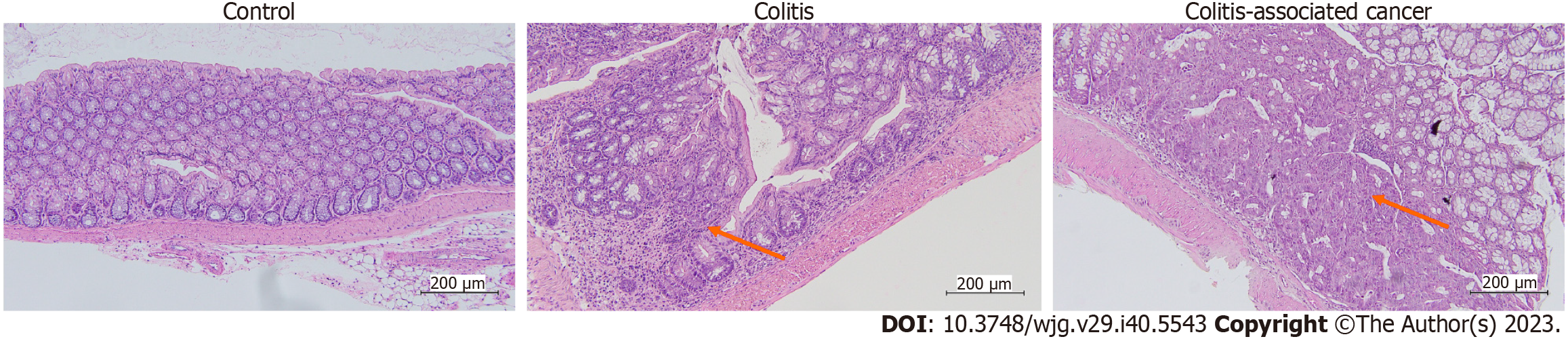
The distal colon underwent histological evaluation to check for any morphological alterations, and the results were reported in Figure 5. No diffuse inflammation was observed in any of the tissues in the present study, which ranged from 0 (no inflammation/healthy), 1 (minimal inflammation), 2 (mild inflammation), 3 (moderate inflammation), and 4 (extensive inflammation). Most control group mice had healthy colons with uniformly shaped glands and minimal inflammation. In the colon tissues of the colitis mice group, mild to moderate inflammation with separation of crypts, with or without focal effacement of crypts, and various gland shapes were seen. On the other hand, at least 50% of CAC-induced mice had tumors and varying degrees of mild to severe inflammation. These tumors had enlarged nuclei, reactive cellular changes, moderate inflammatory response, depleted glands, and an elevated neutrophil count. The scoring on colitis and CAC mice colon tissue were scored depending on the inflamed area (chronic) or specifically at the tumor location (Figure 6).
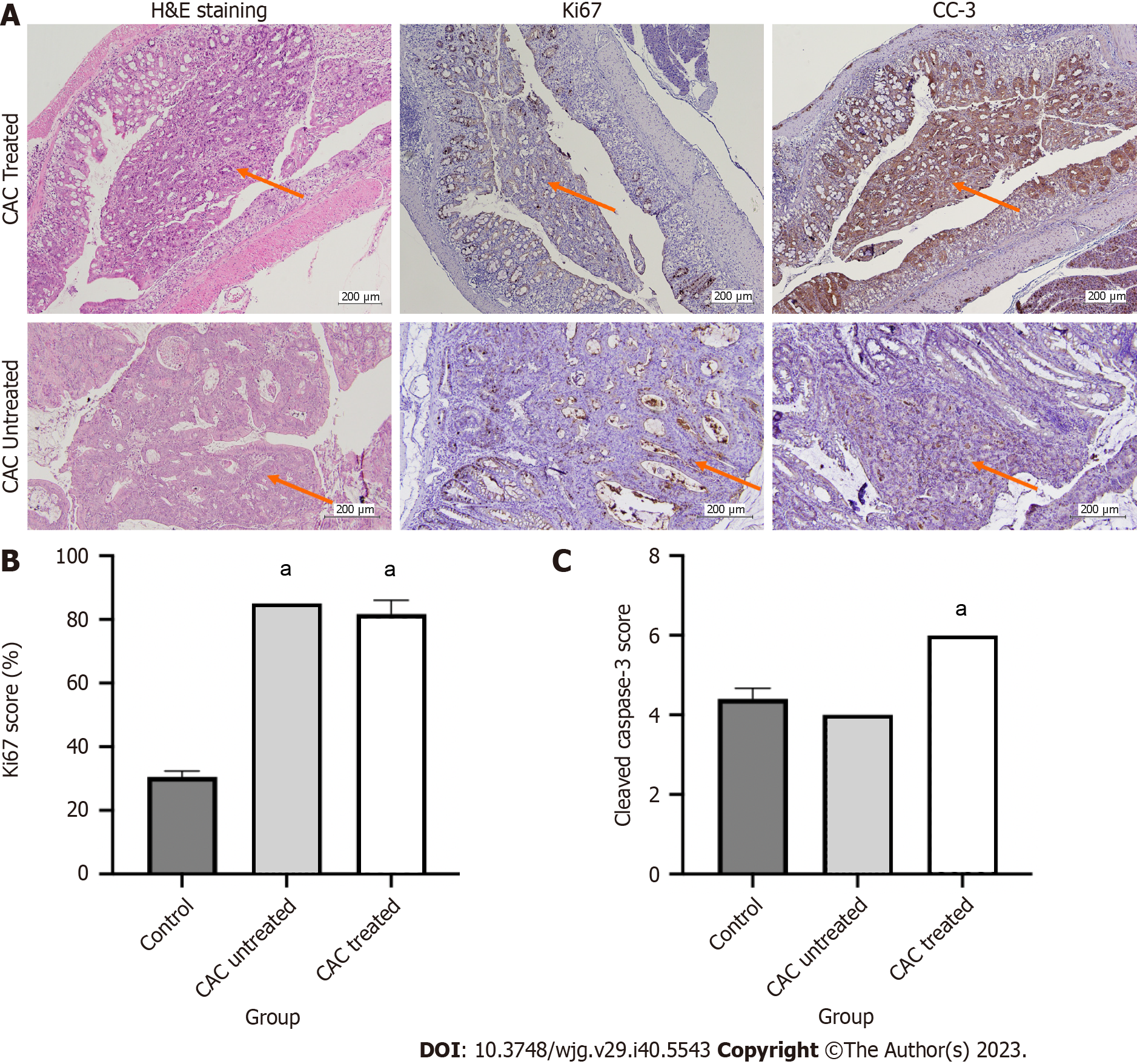
The apoptotic marker (CC-3) and the proliferative marker (Ki67) were both subjected (Figure 7). Every type of tissue, even the healthy tissue, had proliferating cells. The proliferative cells' distribution might vary depending on the different tissue types. In the control group, the healthy colon exhibited 30% of Ki67-positive cells, with the proliferating cells predominating in the basal regions of the glands. In contrast, more than 50% and 80% of Ki67-positive cells had irregular distribution in the colitis and CAC mice groups, respectively. The uneven distribution of proliferative cells, particularly in the upper section of the glands, suggested the cells were highly proliferating. Buparlisib administration to the CAC mice group reduced about 5% of the positive cells compared to the untreated CAC mice group (Figure 7).
CC-3 was assessed as an apoptotic marker based on its intensity and the number of positive cells. Most tissues from the control, colitis and CAC mice group have moderate intensity of CC-3. In contrast to the control group, which had fewer than 50% positive cells, most colitis and CAC mice had more than 75% CC-3 positive cells. Based on the PI3K inhibitor treatment evaluation, buparlisib-treated CAC mice achieved a high overall score of CC3-intensity and -positive cells (6/8) (Figure 7).
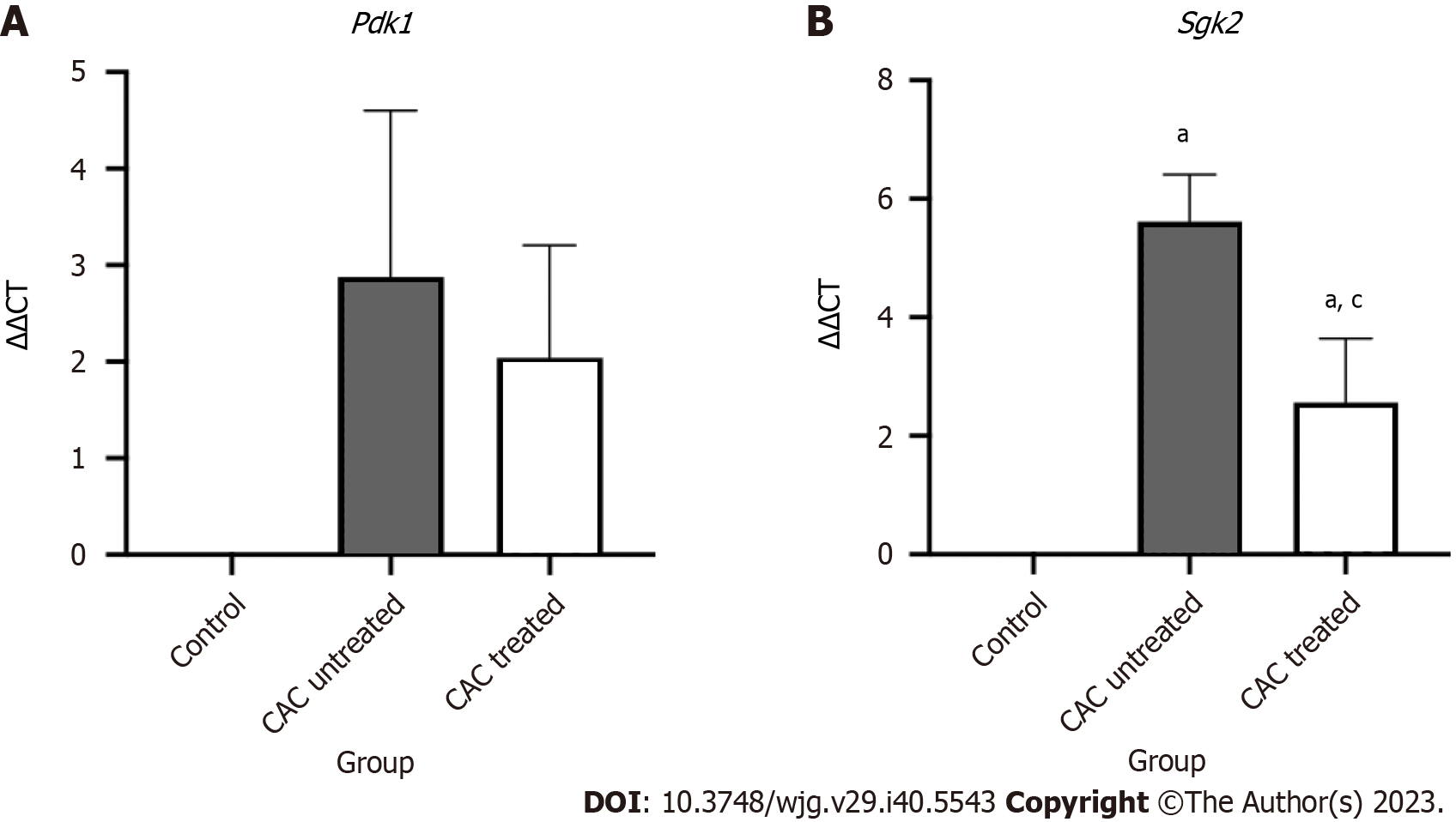
The relative gene expression of the downstream PI3K-non-AKT pathway genes Pdk1 and Sgk2, which are involved in CAC progression, was assessed to study further the impact of the PI3K inhibitor on CAC development. Buparlisib treatment showed a downward trend in the expression of Pdk1 (2.04 ± 1.17) in the CAC-induced mice group, however, it is not significant compared to the untreated group (Figure 8). Only Sgk2 expression showed a significant reduction in treated CAC-induced mice (2.56 ± 1.09) in comparison to the untreated CAC mice (5.61 ± 0.79) (P < 0.05).
A well-known pathway in the emergence of inflammation-related cancer is the PI3K pathway. Dysregulation of the PI3K signaling pathway may affect cellular development, metabolism, proliferation, and apoptosis[28]. Recent research on the function of cytokine-induced PI3K-related genes in CAC patients led to the discovery of possible mutations that might connect CAC with UC and CRC[17]. We are, therefore, interested in learning more about the pathophysiology of CAC and how the PI3K signaling pathway is involved. In this study, we utilized dual luciferase assay to investigate the functional significance of rs10889677, a somatic mutation related to PI3K, and we generated CAC in mice to assess the function and regulation of PI3K inhibitor in the modulation of PI3K-non-AKT signaling pathway.
The single nucleotide polymorphism (SNP) rs10889677 is a genetic variant identified in the IL23R, one of the cytokine-induced PI3K-related genes. This particular SNP is a variation in the DNA sequence of the IL23R, where a single nucleotide (cytosine, C) is replaced by another nucleotide (adenine, A). The rs10889677 SNP is situated precisely in the 3’ untranslated region of IL23R, which is also a miRNA binding site[29]. Any SNPs present in the miRNA binding site may affect how miRNA-mRNA interacts, changing the stability or translation efficiency of the mRNA[30]. This, in turn, may influence the expression of the IL23R protein, which is involved in immune response and inflammation, and may increase the susceptibility to certain diseases, such as CRC. The rs10889677 mutation has been linked strongly to IBD and several other cases, including breast, lung, bladder and colorectal[31-33]. In our recent publication, we have successfully predicted a notable decrease in the stability of mRNA secondary structure for the rs10889677 variant construct compared to the wildtype[17]. This finding supported the hypothesis that genetic mutations resulted in mRNA structural instability and disrupted the binding sites[17].
About 60%-70% of human CRC s were associated with activating the PI3K pathway, increased AKT signaling and PTEN inactivation[34]. Furthermore, PI3K inhibitors have been proposed as another effective targeted therapy for solid tumors, including CRC[35,36]. Nevertheless, further elucidation regarding the utilization of PI3K inhibitors in the context of CAC is warranted. Balb/c male mice were chosen because they are more susceptible and prone to grow tumors than other strains, and tumors were induced in them with a combination of AOM injection and repeated DSS cycles[37,38]. The application of combined AOM and DSS is a potent, reproducible, and reasonably priced initiation-promotion paradigm that has been widely used in CAC-related research[25,39]. A high DAI score indicates that the mouse CAC model is successful. The CAC-induced mice model manifested similar symptoms as CAC patients, where the mice were experiencing rectal bleeding, watering stool and body weight loss[40]. According to a previous study, symptoms including bloody stools, began to manifest between days 13 and 21 after the mice received DSS[41]. Similar results were shown in this study, where disease activity in CAC-induced mice began to increase dramatically following the second cycle of DSS, which occurred just 21 d after the first induction of DSS.
Histologically, three cycles of 2.5% DSS induced mild to moderate inflammatory response in the colitis group. No tumor formation was observed along the digestive system in the CRC group. Hence, no additional evaluation of those tissues was done. On the other hand, 50% of the CAC-induced mice group were found to have moderate inflammation. No diffuse inflammation was observed in the colonic mucosal of CAC-induced mice, even though it was reported that signs of diffuse inflammation can appear as early as third to fourth weeks after treatment[42]. Furthermore, compared to a prior study, where submucosal tumor infiltrations were sporadically seen in 20%–24% of AOM-DSS mice at week ten, the percentage of CAC-induced mice with tumors in this study was considered high[43]. The selection of the mouse strain may have affected the percentage of tumor occurrence because different strains of mice have varying susceptibilities.
Buparlisib was given daily for 14 d to the CAC-induced mice group to perform additional research on the function of PI3K inhibitors. Buparlisib is a potent oral pan-PI3K inhibitor that targets all four catalytic isoforms of class I PI3K (p110α, p110β, p110δ and p110γ)[44]. Buparlisib has been tested in clinical trials phase I and II in several solid tumors, including breast, brain, and lung cancer[20]. The preclinical studies showed buparlisib's inhibitory action against numerous PI3K isoforms and anti-proliferative effects in various in vitro cell lines[19]. Additionally, the authors provided the outcomes of in vivo studies with mouse xenograft models that demonstrate the effectiveness of buparlisib in reducing tumor growth[19]. Our findings showed that buparlisib treatment significantly decreased weight loss in CAC-induced mice. Another study supported this finding in which buparlisib therapy inhibited the growth of established patient-derived glioblastoma multiforme xenografts and increased survival in nude rats[45].
Additionally, buparlisib treatment in CAC-induced mice resulted in a slight rise in apoptotic cell activity and decreased cell proliferation. This result was consistent with several studies that found buparlisib to have the ability to induce apoptosis and reduce tumor growth in a variety of malignancies[44,46]. Buparlisib's effectiveness was also examined in CRC cell lines, where it was discovered that the drug had a considerable impact on the process of apoptosis induction, which progressed from AKT inhibition via FoxO3a-dependent p53 upregulated modulator of apoptosis induction[47].
Additionally, buparlisib treatment has been demonstrated to inhibit the PI3K/Akt signaling pathway, which causes apoptosis and slows tumor growth in medulloblastoma cells[44]. The most frequent downstream targets of buparlisib are AKT and mTOR. Studies have demonstrated that buparlisib effectively inhibited at serine 473 and threonine 308 and suppressed the phosphorylation of proteins downstream from mTORC1, including S6K and 4E-BP1 in bone and soft tissue sarcomas[48]. Despite that, there has been limited research conducted on the correlation between buparlisib and PI3K-non-AKT downstream pathways. Our results revealed that the non-AKT downstream of PI3K, Pdk1 and Sgk2 also showed a similar down-regulation tendency with buparlisib treatment. The expression of two PI3K-non-AKT downstream genes was previously found to be higher in malignant tissues than in healthy mucosa[49,50]. PDK1 is a gene that plays a critical role in regulating cell proliferation and survival through its ability to curb signal propagation to the downstream targets such as AKT and SGK2. PDK1 was discovered to interact with a specific protein known as HuR (human antigen R), which is known to attach to the mRNA of PI3K and stabilize it, enhancing the production of PI3K[51].
Meanwhile, SGK is activated by the same signaling cascade as AKT, and its inhibitor has been created and demonstrated encouraging results in preclinical tests as a possible therapeutic target in cancer[52]. The therapeutic potential of SGK1 inhibition in cancer treatment has been demonstrated in preclinical studies using a variety of small molecule inhibitors and RNAi-based strategies that target SGK1[53]. Buparlisib and wortmannin, both of which are PI3K inhibitors, decreased the stimulatory impact of insulin on hOAT4 transport activity, aided by SGK2[54]. This showed that PI3K inhibition, whether by buparlisib or wortmannin, can lessen the stimulatory effect of insulin on hOAT4 transport activity and that SGK2 may mediate this effect[54].
There are a few limitations in this study. There were only male mice used in the animal experiment. The AOM/DSS-induced mice model has been widely used in research, with a focus on male mice[42]. This preference for male mice is primarily caused by behavioural changes in female mice that can be altered by hormone cycles[55]. Additional studies using female balb/c mice will be required to establish that gender does not interfere with generalization of findings. Another limitation of this study is that the gene expression analysis was only performed on mouse tissues derived from inflammatory and malignant regions. It would be beneficial to include surrounding normal tissue in the analysis to observe and analyse any potential differences.
We investigated the functional relevance of the cytokine-induced PI3K-related gene variation, rs10889677, and it is hypothesized that this variant was a mediating factor in the beginning of the PI3K signaling pathway. Buparlisib, a PI3K inhibitor, was also administered, and it showed promise in preventing PI3K-non-AKT activation in the CAC-induced mice model. Overall, our research may offer a fresh perspective on how PI3K is used in the pathophysiology of CAC.
The phosphatidylinositol-3-kinases (PI3K)/AKT pathway has emerged as a potential new approach in the complicated landscape of inflammation-related cancer, providing new hope for patients with colitis-associated cancer (CAC). A recent discovery in the investigation of PI3K-related genes revealed a promising association between ulcerative colitis (UC), colorectal cancer (CRC), and the elusive rs10889677 mutation.
Understanding the involvement of the PI3K signalling pathway in the development and progression of CAC. Genetic Variants and CAC Susceptibility: Investigating the impact of genetic variants, such as the rs10889677 variant, on CAC susceptibility and their contribution to PI3K pathway activation. The potential of the pan-class I PI3K inhibitor buparlisib as a potential therapeutic option for the treatment of CAC was being examined.
To investigate the role of PI3K-related gene variation in CAC pathogenesis and to evaluate the therapeutic potential of buparlisib, a powerful pan-class I PI3K inhibitor with tumor-suppressive properties.
The study examined the genomic DNA from 32 colonic samples from cases of CAC, UC, and CRC. The rs10889677 mutation was highlighted, which was amplified and cloned for both mutant and wild-type fragments in the pmirGLO vector. Luciferase activity was measured using the HT29 cell line. CAC was induced using a precise protocol that included azoxymethane and sodium dextran sulphate. Buparlisib was administered after 14 d. Immunohistochemistry for Ki67 and Cleaved-caspase-3 markers as well as quantitative real-time polymerase chain reaction for Pdk1 and Sgk2 were performed on excised colonic tissues.
Buparlisib significantly reduced the mean weight loss in these mice, indicating an increase in general health and well-being. It has an effect in slowing cancer cell development and promoting cell death in CAC-induced mice. It also had an effect on the expression of particular genes involved in the PI3K pathway, with significantly decreased Sgk2 expression and a decreasing trend in Pdk1 expression.
Our study discovered that a specific genetic variant (rs10889677 variation) is critical in the activation of a cancer-promoting pathway known as PI3K. We also discovered that a drug called buparlisib can prevent this route from being triggered, which is great news for those fighting cancer (CAC).
Patient stratification: Future research could look into whether the rs10889677 variant can be used as a biomarker to predict CAC development in UC patients. This could help with early detection and personalised treatment initiatives. Mechanism Elucidation: The mechanisms by which the rs10889677 variation alters the PI3K pathway should be studied further in the future.
Special thanks to the Jameel Education Foundation for the student financial assistance. We also would like to thank Mrs Nazierah Abd Ghani from the Department of Physiology, Faculty of Medicine, UKM, for her assistance in animal handling; staff of Laboratory Animal Resource Unit (LARU), UKM, for providing the animal facilities; Mr Muaatamarulain Mustangin from Department of Pathology, Faculty of Medicine, UKM for his assistance in histology optimization and Dr Saiful Effendi Syafruddin from UMBI, UKM for his assistance in cloning works.
| 1. | Grivennikov SI, Cominelli F. Colitis-Associated and Sporadic Colon Cancers: Different Diseases, Different Mutations? Gastroenterology. 2016;150:808-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, Krieglstein CF, Senninger N, Anthoni C, Bruewer M. Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg. 2011;15:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Zhou Q, Shen ZF, Wu BS, Xu CB, He ZQ, Chen T, Shang HT, Xie CF, Huang SY, Chen YG, Chen HB, Han ST. Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019;2019:5363261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Zhiqin W, Palaniappan S, Raja Ali RA. Inflammatory Bowel Disease-related Colorectal Cancer in the Asia-Pacific Region: Past, Present, and Future. Intest Res. 2014;12:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 690] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 7. | Mokhtar NM, Nawawi KNM, Verasingam J, Zhiqin W, Sagap I, Azman ZAM, Mazlan L, Hamid HA, Yaacob NY, Rose IM, Den ELN, Wan MS, Raja Ali RA. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur, Malaysia. BMC Public Health. 2019;19:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Kameyama H, Nagahashi M, Shimada Y, Tajima Y, Ichikawa H, Nakano M, Sakata J, Kobayashi T, Narayanan S, Takabe K, Wakai T. Genomic characterization of colitis-associated colorectal cancer. World J Surg Oncol. 2018;16:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Hartnett L, Egan LJ. Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis. 2012;33:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 285] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (4)] |
| 11. | Zundler S, Neurath MF. Integrating Immunologic Signaling Networks: The JAK/STAT Pathway in Colitis and Colitis-Associated Cancer. Vaccines (Basel). 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Qu X, Tang Y, Hua S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front Immunol. 2018;9:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 13. | Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170:605-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1679] [Cited by in RCA: 2066] [Article Influence: 229.6] [Reference Citation Analysis (0)] |
| 14. | Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1507] [Article Influence: 251.2] [Reference Citation Analysis (0)] |
| 15. | Ciraolo E, Gulluni F, Hirsch E. Methods to measure the enzymatic activity of PI3Ks. Methods Enzymol. 2014;543:115-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Low END, Mokhtar NM, Wong Z, Raja Ali RA. Colonic Mucosal Transcriptomic Changes in Patients with Long-Duration Ulcerative Colitis Revealed Colitis-Associated Cancer Pathways. J Crohns Colitis. 2019;13:755-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Razali NN, Raja Ali RA, Muhammad Nawawi KN, Yahaya A, Mokhtar NM. Targeted Sequencing of Cytokine-Induced PI3K-Related Genes in Ulcerative Colitis, Colorectal Cancer and Colitis-Associated Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Pons-Tostivint E, Thibault B, Guillermet-Guibert J. Targeting PI3K Signaling in Combination Cancer Therapy. Trends Cancer. 2017;3:454-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chène P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, García-Echeverría C, Sellers WR, Voliva CF. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 444] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 20. | Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | Zhao W, Qiu Y, Kong D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm Sin B. 2017;7:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 806] [Article Influence: 100.8] [Reference Citation Analysis (1)] |
| 23. | Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, Luu V, Marco E, Ramkissoon LA, Kang YJ, Hayashi M, Nguyen QD, Ligon AH, Du R, Claus EB, Alexander BM, Yuan GC, Wang ZC, Iglehart JD, Krop IE, Roberts TM, Winer EP, Lin NU, Ligon KL, Zhao JJ. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22:723-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71-W74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1816] [Cited by in RCA: 1943] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 25. | Parang B, Barrett CW, Williams CS. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol Biol. 2016;1422:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 26. | Hidalgo-Cantabrana C, Algieri F, Rodriguez-Nogales A, Vezza T, Martínez-Camblor P, Margolles A, Ruas-Madiedo P, Gálvez J. Effect of a Ropy Exopolysaccharide-Producing Bifidobacterium animalis subsp. lactis Strain Orally Administered on DSS-Induced Colitis Mice Model. Front Microbiol. 2016;7:868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Rogers R, Eastham-Anderson J, DeVoss J, Lesch J, Yan D, Xu M, Solon M, Hotzel K, Diehl L, Webster JD. Image Analysis-Based Approaches for Scoring Mouse Models of Colitis. Vet Pathol. 2016;53:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Papadatos-Pastos D, Rabbie R, Ross P, Sarker D. The role of the PI3K pathway in colorectal cancer. Crit Rev Oncol Hematol. 2015;94:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 30. | Bhaumik P, Gopalakrishnan C, Kamaraj B, Purohit R. Single nucleotide polymorphisms in microRNA binding sites: implications in colorectal cancer. ScientificWorldJournal. 2014;2014:547154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2345] [Article Influence: 117.3] [Reference Citation Analysis (1)] |
| 32. | Zheng J, Jiang L, Zhang L, Yang L, Deng J, You Y, Li N, Wu H, Li W, Lu J, Zhou Y. Functional genetic variations in the IL-23 receptor gene are associated with risk of breast, lung and nasopharyngeal cancer in Chinese populations. Carcinogenesis. 2012;33:2409-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Peng LL, Wang Y, Zhu FL, Xu WD, Ji XL, Ni J. IL-23R mutation is associated with ulcerative colitis: A systemic review and meta-analysis. Oncotarget. 2017;8:4849-4863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013;14:2201-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat Rev. 2017;59:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Fernandes MS, Sanches JM, Seruca R. Targeting the PI3K Signalling as a Therapeutic Strategy in Colorectal Cancer. Adv Exp Med Biol. 2018;1110:35-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 39. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1983] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 40. | Moazzami B, Moazzami K, Rezaei N. Early onset inflammatory bowel disease: manifestations, genetics and diagnosis. Turk J Pediatr. 2019;61:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 569] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 42. | De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 43. | Tanimura Y, Fukui T, Horitani S, Matsumoto Y, Miyamoto S, Suzuki R, Tanaka T, Tomiyama T, Ikeura T, Ando Y, Nishio A, Okazaki K. Long-term model of colitis-associated colorectal cancer suggests tumor spread mechanism and nature of cancer stem cells. Oncol Lett. 2021;21:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Zhao P, Hall J, Durston M, Voydanoff A, VanSickle E, Kelly S, Nagulapally AB, Bond J, Saulnier Sholler G. BKM120 induces apoptosis and inhibits tumor growth in medulloblastoma. PLoS One. 2017;12:e0179948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Netland IA, Førde HE, Sleire L, Leiss L, Rahman MA, Skeie BS, Miletic H, Enger PØ, Goplen D. Treatment with the PI3K inhibitor buparlisib (NVP-BKM120) suppresses the growth of established patient-derived GBM xenografts and prolongs survival in nude rats. J Neurooncol. 2016;129:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PLoS One. 2018;13:e0192464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Yang S, Li X, Guan W, Qian M, Yao Z, Yin X, Zhao H. NVP-BKM120 inhibits colon cancer growth via FoxO3a-dependent PUMA induction. Oncotarget. 2017;8:83052-83062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Anderson JL, Park A, Akiyama R, Tap WD, Denny CT, Federman N. Evaluation of In Vitro Activity of the Class I PI3K Inhibitor Buparlisib (BKM120) in Pediatric Bone and Soft Tissue Sarcomas. PLoS One. 2015;10:e0133610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Liu T, Yin H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer. Oncol Rep. 2017;37:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Chen JB, Zhang M, Zhang XL, Cui Y, Liu PH, Hu J, Li HH, Jin H, Liu LF, Chen MF, Chen HQ, Liang CZ, Zu XB. Glucocorticoid-Inducible Kinase 2 Promotes Bladder Cancer Cell Proliferation, Migration and Invasion by Enhancing β-catenin/c-Myc Signaling Pathway. J Cancer. 2018;9:4774-4782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Dieterle AM, Böhler P, Keppeler H, Alers S, Berleth N, Drießen S, Hieke N, Pietkiewicz S, Löffler AS, Peter C, Gray A, Leslie NR, Shinohara H, Kurosaki T, Engelke M, Wienands J, Bonin M, Wesselborg S, Stork B. PDK1 controls upstream PI3K expression and PIP3 generation. Oncogene. 2014;33:3043-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Bruhn MA, Pearson RB, Hannan RD, Sheppard KE. Second AKT: the rise of SGK in cancer signalling. Growth Factors. 2010;28:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Sang Y, Kong P, Zhang S, Zhang L, Cao Y, Duan X, Sun T, Tao Z, Liu W. SGK1 in Human Cancer: Emerging Roles and Mechanisms. Front Oncol. 2020;10:608722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 54. | Wang H, Zhang J, You G. The mechanistic links between insulin and human organic anion transporter 4. Int J Pharm. 2019;555:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qin J, China; Sahin TT, Turkey; Sheykhhasan M, Iran S-Editor: Fan JR L-Editor: A P-Editor: Zhao S