Published online Aug 21, 2023. doi: 10.3748/wjg.v29.i31.4736
Peer-review started: June 26, 2023
First decision: July 5, 2023
Revised: July 17, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 21, 2023
Processing time: 53 Days and 8.5 Hours
Diabetes is a highly prevalent disease that was initially simplified into three major types: Type 1, type 2 and gestational diabetes. With the global rise in incidence of acute pancreatitis (AP), a lesser-known type of diabetes referred to as diabetes of the exocrine pancreas (DEP) is becoming more recognized. However, there is a poor understanding of the inherent relationship between diabetes and AP. There is established data about certain diseases affecting the exocrine function of the pancreas which can lead to diabetes. More specifically, there are well established guidelines for diagnosis and management of DEP caused be chronic pancreatitis. Conversely, the sequelae of AP leading to diabetes has limited recognition and data. The purpose of this review is to provide a comprehensive summary of the prevalence, epidemiology, pathophysiology and future research aims of AP-related diabetes. In addition, we propose a screening and diagnostic algorithm to aid clinicians in providing better care for their patients.
Core Tip: Acute pancreatitis (AP)-related diabetes has limited recognition and data. The disease occurs more often than previously recognized and patients are often misdiagnosed with type 2 diabetes. The purpose of this review is to provide a comprehensive summary of the prevalence, epidemiology, pathophysiology and future research aims of AP-related diabetes. In addition, we propose a screening and diagnostic algorithm to aid clinicians in providing better care for their patients.
- Citation: Charley E, Dinner B, Pham K, Vyas N. Diabetes as a consequence of acute pancreatitis. World J Gastroenterol 2023; 29(31): 4736-4743
- URL: https://www.wjgnet.com/1007-9327/full/v29/i31/4736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i31.4736
Acute pancreatitis (AP) is a condition defined by rapid onset pancreatic inflammation that is short-lived and may be associated with local and systemic complications[1]. Alcohol use and gallstones are the most common etiologies, which may explain its standing as one of the leading gastrointestinal diseases requiring hospitalization in the United States[2]. The severity of AP is graded based on the presence or absence of local complications, systemic complications, and persistent organ failure. Patients with mild disease recover with supportive care alone while those with severe disease may experience prolonged hospitalization and require procedural interventions to treat various complications. The severe form of the disease occurs in 20% of all patients with AP and carries a mortality rate as high as 30%-40%[1,3]. Following a single episode of AP, all patients are susceptible to the potential sequela of the injury sustained by the pancreas regardless of the severity of the episode[4]. The complication acquiring interest of late is new-onset diabetes mellitus (DM) after AP.
Diabetes is a term used to classify a group of diseases characterized by persistent hyperglycemia that afflicts 37 million adults in the United States alone. Type I diabetes (T1D) accounts for 5%-10% of the total prevalence of diabetes and is hypothesized to be a consequence of autoimmune destruction of beta cells, resulting in inadequate insulin levels[5]. The most common form of diabetes, type II DM (T2DM), is caused by insufficient insulin secretion by pancreatic beta cells and progressive development of insulin resistance (IR). Persistent impairment in glucose control may also be promoted by diseases of the exocrine pancreas, including AP[6]. This form of diabetes has been referred to as type 3c diabetes or diabetes of the exocrine pancreas (DEP)[6,7].
Hyperglycemia during AP was previously speculated to be a transient event that resolved as the inflammatory process improved[4]. Recent meta-analyses have questioned this notion by showing evidence of a high incidence of AP-related DM[4]. Two meta-analyses found 15%-23% of patients developed new-onset diabetes following a single episode of AP and the remaining patients had greater than a two-fold risk of diabetes over five years[8,9].
The various pancreatic diseases known to potentially cause DEP are often grouped together despite this collection encompassing multiple distinct conditions that should be further investigated on an individual basis. The unique mechanisms in which these conditions cause damage to the pancreas is important to take into consideration as they may allow for specific therapeutic options, change screening recommendations, and affect the length of time to diabetes. AP in particular, the most common disease of the exocrine pancreas, warrants further discussion. The goal of this review is to summarize the prevalence and pathophysiology of DEP after episode(s) of AP, elucidate diagnostic criteria for AP-related DM and identify risk factors of those at highest risk of developing the complication. We will also suggest management and screening recommendations, including the emergence of potential new ways to diagnose AP-related DM and identify areas for further study.
In the United States, AP is estimated to account for approximately 275000 hospital admissions and $2.5 billion in healthcare costs annually[10]. A meta-analysis of the global incidence of AP from 1961 to 2016 found the incidence of AP has been increasing over time. Select populations were noted to have disproportionate rises in incidence especially throughout the majority of countries within the Western world, including North America, Europe, and Oceania[3]. One study aimed at determining the global incidence of AP reported the countries with the greatest incidence of AP in 2019 were India, China, and the United States. Alcohol was found to be an important cause of increased morbidity and mortality. The study also uncovered the existence of significant differences in the burden of AP across geographic regions and differences in outcomes based on socioeconomic factors. For example, patients admitted to smaller hospitals with fewer resources were noted to have a higher risk of pancreatitis-related mortality[10].
The lesser-known sequelae of AP are the development of endocrine and exocrine insufficiency, which in turn can contribute to the development of diabetes. All patients are susceptible to these effects, even those with a mild case of AP and after a first-time episode[11]. In one cohort study, exocrine pancreatic dysfunction was associated with significantly increased risk for new-onset DM, including when the analysis was limited to mild AP[12]. A systematic review and meta-analysis from May 2019 found the incidence of AP-related DM was 23% and new onset insulin dependent DM (IDDM) was 15%. The incidence of DM was higher for severe AP (SAP) compared to mild AP, pancreatic necrosis compared to without, and alcoholic compared to biliary AP. The incidence of IDDM after SAP was 21% and 18% following an episode of alcohol-related AP. Within five years of the initial episode of AP, the rate of DM and IDDM was 20% and 14%, respectively. The rate of DM and IDDM after AP increased to 37% and 25%, respectively[9]. Another study demonstrated similar findings of the development of endocrine and exocrine insufficiency despite severity of AP, number of or time from episodes of AP, gender, and etiology. However, there was a statistically significant difference in the prevalence of DM when patients with SAP were compared with non-SAP[12].
Ongoing investigations have proposed multiple mechanisms believed to contribute to the development of AP-related DM. These mechanisms presumably occur in parallel and it is unknown which mechanism has the greatest impact. Prior to further discussing the theories it is important to understand the roles of the individual cell types of the pancreas and where they reside. This will aid in demonstrating the clinical implications of their malfunction or loss.
The pancreas consists of two tissue types which give rise to the distinct actions of the pancreas, the exocrine and endocrine functions. Exocrine tissue makes up an estimated 95% of the pancreas and produces enzymes necessary for digestion. The remaining tissue is made up of special endocrine cells embedded within the exocrine pancreas that are collectively known as the islets of Langerhans[13]. This collection consists of four cell types that play exclusive roles in regulating glucose levels and pancreatic secretions: Alpha cells, beta cells, pancreatic polypeptide (PP) cells and delta cells. Alpha cells release glucagon and make up 15% of the total islet cell volume in the anterior portions of the pancreas (head, body and tail). Glucagon is important in preventing hypoglycemia through several well-known mechanisms. Insulin-releasing beta cells make up 80% of the islet volume in the anterior pancreas and 20% of the volume in the posterior head. Insulin regulates glucose by promoting uptake and storage of glucose in the liver, muscle and adipose tissue. The hormone also regulates the action of alpha cells through paracrine inhibition. PP cells make up 80% of the islet volume in the posterior head and have a small presence (< 1%) in the anterior portions of the pancreas. They produce PP, which enhances the effect of insulin on the liver. Delta cells have a small overall existence, contributing to < 1% of islet volume in the posterior head and 5% in the anterior pancreas. Nevertheless, they are essential for their release of somatostatin which has multiple functions throughout the body. In the gastrointestinal system, somatostatin is important for regulating the release of glucagon and insulin through paracrine inhibition. The hormone also slows both gastric emptying and nutrient absorption from the intestinal tract[7].
Unlike T1D and T2DM, DEP is a consequence of damage to the pancreas that affects all of the highly specialized cells of the organ. Based on this observation and previous data showing a higher incidence of DEP in patients suffering from necrotizing pancreatitis compared to those without necrosis, a correlation between the extent of loss of islet cells and the risk of developing AP-related DM has been suggested[9]. This inference has been further substantiated by evidence that patients with T1D and T2DM have decreased pancreatic volume compared to non-diabetics[14]. Nevertheless, emerging evidence indicates the overall pathogenesis is likely more complicated and requires more investigation. One systematic review examining the risk of developing DM after partial pancreatectomy found the incidence of new-onset DM was significantly different between various types of resection. Those who underwent a distal pancreatectomy had the highest incidence of developing DEP followed by patients who underwent a Whipple. Patients who underwent a central pancreatectomy had the smallest incidence[15]. A study by Tu et al[16] reports that in comparison to necrosis in the head and body of the pancreas, necrosis in the tail was associated with a greater risk of developing diabetes. Others also report that surgical resection of up to 50% of the pancreas did not necessarily lead to diabetes[14]. These new findings may explain why the severity of AP does not necessarily positively correlate with the risk of developing AP-related DM.
Damage to the exocrine pancreas may contribute to endocrine dysfunction through mechanisms still undergoing analysis. When chronic pancreatic exocrine disease occurs, findings of decreased pancreatic endocrine function including islet function and insulin secretion can be seen. For example, one of the results of chronic pancreatitis is damage to nerve bundles, specifically vagal afferents, which are necessary for PP secretion. Volume loss within the posterior head of the pancreas, in which resides the majority of PP cells, further exacerbates the reduction in insulin secretion. Significant pancreatic endocrine dysfunction can thereby lead to DEP[14].
In a recent cohort study, Cho et al[12] found that patients with exocrine pancreatic dysfunction requiring pancreatic enzyme replacement therapy (PERT) after their initial attack of pancreatitis were at increased risk of new-onset DM. New-onset DM was defined as diagnostic coding of DM and/or receiving a new oral hypoglycemic medication. This association remained even when the analysis was limited to mild AP. Patients with exocrine dysfunction are prone to deficiencies in fat-soluble vitamins.
In the past, vitamin D was found to play a role in glucose control. Mirhosseini et al[17] describe the mechanism in a meta-analysis, reporting that the active form of vitamin D regulates the expression of the insulin receptor gene and facilitates the transport of glucose into muscle cells. The authors conclude that vitamin D supplementation may significantly reduce serum fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c)[17]. However, study results regarding the association of vitamin D and glucose control have been inconsistent. One randomized placebo-controlled trial found that daily vitamin D and calcium supplementation did not have a significant effect on insulin sensitivity, insulin secretion, or β-cell function in vitamin D deficient individuals at risk of T2DM[18]. An important point to note is that the authors classified patients “at risk of T2DM” based on a diabetes risk questionnaire. In a post hoc analysis restricted to patients with clinically diagnosed prediabetes, they found a significant beneficial effect of vitamin D and calcium supplementation on insulin sensitivity. There were no changes in insulin secretion or beta cell function.
In a more recent study, Norbitt et al[19] investigated potential connections between the intake of fat- and water-soluble vitamins with markers of glucose metabolism in patients after AP. They found a significant association between three fat-soluble vitamins (α-carotene, β-carotene, and total carotene) and homeostasis model assessment of β-cell function (HOMA-β) in patients with new-onset prediabetes/diabetes after AP[19]. Despite this association, there were no significant associations with FPG or HOMA-IR index. Although more studies are needed to help uncover the role of vitamin deficiencies in oxidative stress-driven disorders, it appears that optimizing the exocrine function of the pancreas plays an important role in glucose control. This may provide a key opportunity for intervention to prevent AP-related DM.
An important component of understanding the etiology of AP-related DM is identifying risk factors linked with heightened endocrine dysfunction after AP. One case-controlled study with multivariable analysis found AP-related DM occurred more often in patients with components of metabolic syndrome such as hypertension, obesity and hyperlipidemia[20]. The potential explanation is that this particular population has an underlying predisposition toward T2DM and the additional insult of AP accelerates the progression to sustained hyperglycemia[21,22]. In another study, Lv et al[23] found similar results but also discovered non-alcoholic fatty liver disease (NAFLD) was an additional independent risk factor. Until now, most studies have focused on the effect of the exocrine pancreas on endocrine function yet the liver also plays a critical role in regulating and maintaining glucose levels. Those with NAFLD have underlying IR due to chronic inflammation from the release of inflammatory mediators by adipose tissue. These mediators which include leucine-rich alpha-2-glycoprotein 1 and interleukin-6 have been found to inhibit insulin receptors and the action of insulin through different signaling pathways[23]. The authors suggest chronic inflammation combined with inflammation-induced lipolysis contribute to the process of IR. Investigations to further characterize risk factors for AP-related DM are needed to help identify patients who will benefit from closer follow-up and earlier interventions to control glucose levels following an episode of AP.
Diagnostic criteria for T1D and T2DM have been well established and have undergone very minor revision over the last decades. The diagnosis of DEP has been more challenging to standardize as it shares clinical features that overlap with T1D and T2DM. The development of standardized criteria is also complicated by the fact that DEP is the result of multiple clinically distinct diseases and/or mechanisms. These individual etiologies lead to variable levels of pancreatic dysfunction and have varying rates of progression to diabetes, making the timing of the initiation of screening difficult to determine[7,24]. Previously, Ewald and Bretzel[25] suggested the following to diagnose DEP: Major criteria (all must be fulfilled): Presence of exocrine pancreatic insufficiency (according to the monoclonal fecal elastase-1 test or direct function tests); pathological pancreatic imaging (endoscopic ultrasound, magnetic resonance imaging, computed tomography); absence of T1DM associated autoimmune markers. Minor criteria: Impaired beta cell function (e.g., HOMA-B, C-peptide/glucose-ratio); no excessive IR (e.g., HOMA-IR); impaired incretin secretion [e.g., glucagon-like peptide 1 (GLP-1), PP]; low serum levels of lipid-soluble vitamins (A, D, E, and K).
Although cited in numerous articles over the years, the criteria were never universally accepted. As we learn more about the underlying mechanisms of each disease process and how they may lead to DEP, most of the above criteria have become invalid. For example, pancreatic insufficiency is not a specific characteristic of any of the diseases of the exocrine pancreas. Pancreatic insufficiency is a risk factor for the development of DEP and therefore should not be considered a defining characteristic. Additionally, pathological pancreatic imaging varies among the different diseases and is not a reliable marker of pancreatic function. Not all patients with imaging findings consistent with necrotizing pancreatitis develop DEP. Patients with mild pancreatitis may lack significant radiographic findings yet are still at risk of developing DEP. Furthermore, patients with pancreatic adenocarcinoma are commonly diagnosed with diabetes prior to developing cancer visible on cross-sectional imaging[26].
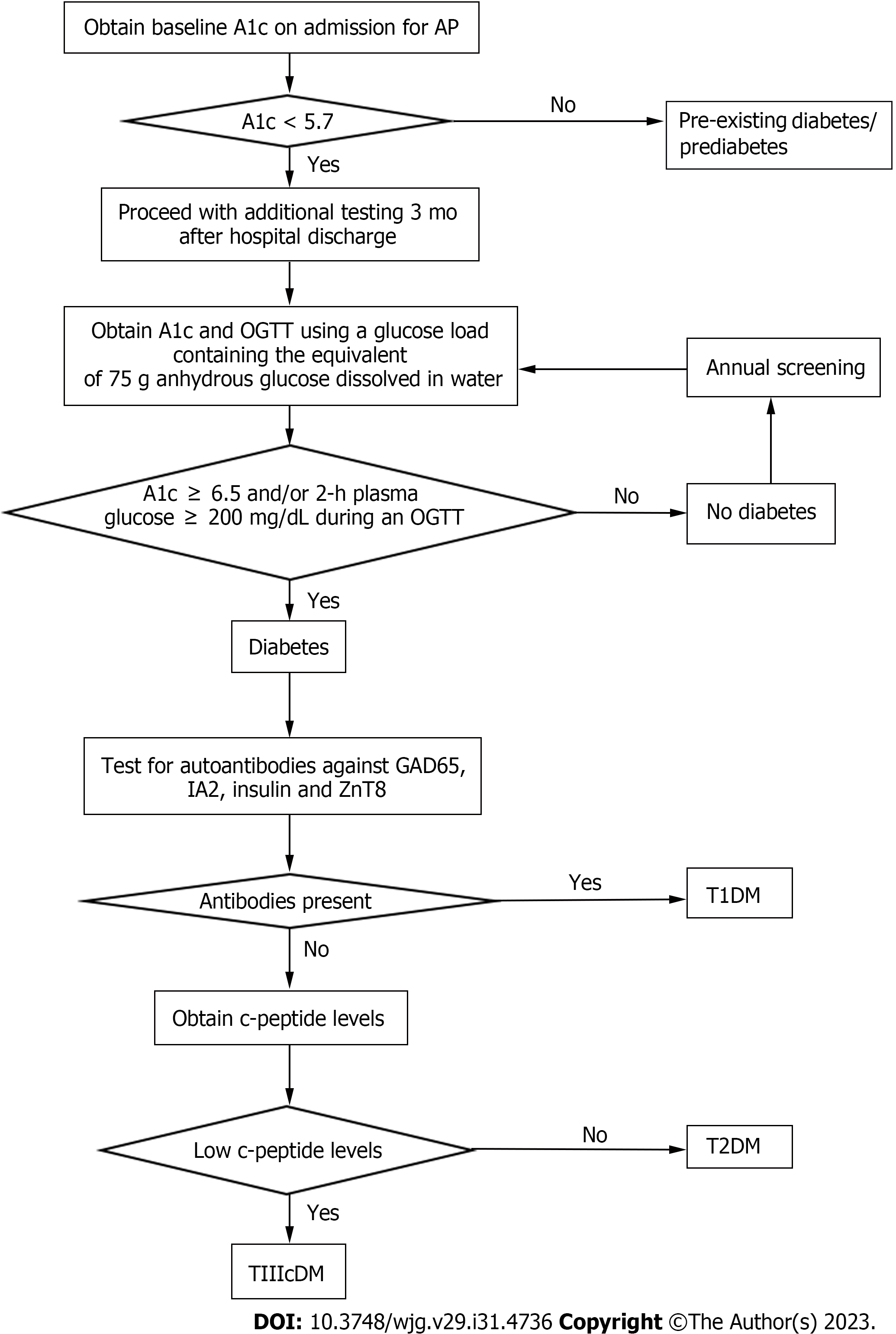
Here, we will focus on screening and diagnosis of AP-related diabetes (Figure 1). The first step should be to rule out undiagnosed diabetes/prediabetes by obtaining an HbA1c on admission for AP. We recommend screening for diabetes three months after hospital discharge by obtaining an HbA1c and an oral glucose tolerance test (OGTT). The timing of screening at three months post-hospitalization was selected to limit confounding by stress-related hyperglycemia and to help ensure that the HbA1c is an accurate reflection of changes in glucose levels after AP. Recommendations to use an OGTT are based on several observations. First, studies have shown poor test performance of HbA1c in diagnosing diabetes in patients with cystic fibrosis, another well-known disease affecting the exocrine pancreas. As a result, screening guidelines for cystic fibrosis-related diabetes recommend use of OGTT over HbA1c, and the committee concluded HbA1c was not sufficiently sensitive for diagnosis of diabetes in this patient population[27]. Similarly, Meier and Giese[28] advocate for the use of OGTT for earlier detection of diabetes in those with pancreatic disease. They found the OGTT diagnosed diabetes when the beta cell area was reduced by 64% compared to a more sizeable beta cell area loss of 89% when diabetes was diagnosed by HbA1c. A more recent study found that fasting glucose and HbA1c underestimate the prevalence of DEP and also recommend use of OGTT[29]. In this study, the diagnosis of over half of the new cases of diabetes after SAP were only possible by using the OGTT.
Once a diagnosis of diabetes is established, the next steps involve ruling out T1D and T2DM. The exclusion of T1D is more straightforward and can be performed by demonstrating the absence of autoantibodies. Duggan et al[29] suggest that c-peptide levels may be used to differentiate DEP and T2DM. Levels of c-peptide are typically used to distinguish T1D from T2DM, as levels are low in T1D and high in T2DM. They found that SAP patients with diabetes demonstrated a lower insulin response to glucose ingestion and had lower c-peptide secretion. This suggests the diagnosis of AP-related DM/DEP should be made in the setting of low c-peptide levels and the absence of autoantibodies[29]. More studies are needed to confirm the correlation, but it is a reasonable place to start. As studies showed risk of developing DEP up to five years after a single attack, it would be reasonable to consider continuing annual screening with HbA1c and OGTT vs more frequently depending on the presence of other risk factors.
While the overall pathophysiology of DEP is far more complex than the more common types of diabetes, the treatment algorithms for T2DM are often applied for management of DEP. This may be problematic on several fronts. Studies have shown that patients with CP have an increased risk of developing pancreatic adenocarcinoma through a mechanism potentiated by hyperinsulinemia[2,7,30]. As a result, interventions that reduce ambient insulin levels (i.e., exercise, weight loss, low-carb diet) coupled with the anti-neoplastic properties of Metformin has become the preferred first-line treatment option. If glucose levels remain elevated it is reasonable to consider the addition of oral agents from the thiazolidinedione and/or alpha-glucosidase inhibitor classes. Clinical trials evaluating the safety of sulfonylureas, GLP-1 analogs, and dipeptidyl peptidase-IV inhibitors are lacking and these medications should be avoided due to possible association with pancreatitis[7,30]. All patients should also be counseled on the importance of abstinence from alcohol and tobacco use.
Screening for malabsorption and fat-soluble vitamin deficiencies can help guide the initiation of PERT and vitamin supplementation. The response and release of incretins, such as GLP-1, to fat hydrolysis may even be impaired with subclinical fat-soluble vitamin deficiency and thereby promote post-prandial hyperglycemia. PERT may augment the post-prandial incretin response and has been demonstrated to stabilize glucose levels in patients with alcoholic pancreatitis and cystic fibrosis. Additionally, some believe that PERT may help improve pain associated with pancreatitis.
Every effort should be made to detect AP-related DM before the development of critical insulin deficiency. If insulin therapy is necessary, it is important to remember that DEP may cause enhanced peripheral sensitivity to insulin, increasing the risk of hypoglycemia[7,30]. Dosing calculations similar to those used for T1D should be used rather than those used for T2DM[7]. Additionally, patients with DEP may require more frequent dose adjustments compared to patients with T2DM.
Further investigation should focus on how to use IR and insufficient insulin production to distinguish T2DM from DEP. Although we have proposed a set of diagnostic criteria, further research is needed to confirm whether the absence of autoantibodies in conjunction with low c-peptide levels is enough to diagnose DEP. Additionally, exploration into the possible contribution of PP cell tracking and other biomarkers to characterize DEP is necessary[4].
Improved characterization of alpha cells, beta cells, PP cells and delta cells may also have implications on the diagnosis and treatment of DEP[4]. Methods to do so may include new radiological techniques as well as neural networks. Newer advanced magnetic resonance techniques, including diffusion-weighted imaging, T1 mapping, and T2 mapping, provide quantifiable imaging features to gain additional understanding of underlying irregularities in AP. This goes beyond the traditional magnetic resonance sequences’ qualitative imaging features to detect morphological changes in AP[31]. Further, these newer radiological techniques may be able to improve the detection of islet volume changes involved in DEP. Advanced analytic techniques, including radiomics and artificial neural networks, may be able to uncover imaging biomarkers to enable further risk stratification of DEP secondary to AP.
Artificial intelligence (AI) offers risk stratification and prognostication information for AP, including DEP secondary to AP. Machine learning through large data sets may be able to better determine an association between AP disease severity and DEP. An ongoing clinical trial is investigating whether AI machine learning can be used to estimate the disease severity in AP according to the revised Atlanta criteria, which may have implications on DEP[32]. Similarly, while parameters used in handcrafted AP severity prediction scores, such as APACHE II and Ranson, are typically unavailable until 24 to 72 h after hospitalization, the early achievable severity index prediction score which was developed using machine learning, can identify patients at high risk for SAP within 24 h of hospital admission[33]. Early identification of patients at risk of SAP allows for early interventions to decrease mortality, which may also be critical in preventing the development of DEP.
As deficiency of fat-soluble vitamins associated with exocrine pancreatic dysfunction may be linked to new-onset DM, early administration of fat-soluble vitamins needs to be further studied as a possible preventative measure against the development of DEP[12]. Additionally, serum levels of fat-soluble vitamins (vitamin D in particular) may have the potential to be used as markers of risk of AP-related DM, as mentioned by Norbitt et al[19]. The connection comes from previous findings that vitamin D levels negatively correlated with AP severity indexes[19,34].
Based on the data mentioned earlier, the incidence of AP continues to rise and we should anticipate an increasing incidence of AP-related DM. This escalates the importance of needing to screen for, monitor, and treat DEP[3]. The next major step in research should also include investigating the reasons for the increased incidence of AP to prevent the disease and DEP by extension.
AP is the most common disease of the exocrine pancreas and places a significant strain on healthcare costs as the incidence continues to rise. While many studies have focused on treatment during hospitalization, the sequela of the disease are becoming more important as more patients develop AP and recover from the disease. AP-related diabetes, a type of DEP, is likely more prevalent than initially thought as it is often misclassified as T2DM and treated as such. As new studies emerge, we have learned that the diagnostic criteria for AP-related diabetes needs to be better defined in order to improve management of this unique type of diabetes. Through common knowledge of the pathophysiology of T1D and T2DM and the review of newer studies attempting to find biomarkers to help characterize DEP, we were able to propose a set of diagnostic criteria for AP-related DM. Although studies are needed to test the utility, this may at least provide a starting point. Furthermore, we have identified specific areas in need of further research in hopes that it may help lead to the development of guidelines for the diagnosis and management of AP-related DM.
| 1. | Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (1)] |
| 2. | Gapp J, Tariq A, Chandra S. Acute Pancreatitis. 2023 Feb 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 3. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (1)] |
| 4. | Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, Bellin MD, Yadav D, Andersen DK, Serrano J, Papachristou GI. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Centers for Disease Control and Prevention. What Is Diabetes? [cited 26 November 2022]. Available from: https://www.cdc.gov/diabetes/basics/diabetes.html. |
| 6. | Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 7. | Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 9. | Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990-2019. BMC Gastroenterol. 2021;21:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 11. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 618] [Article Influence: 123.6] [Reference Citation Analysis (1)] |
| 12. | Cho J, Scragg R, Pandol SJ, Petrov MS. Exocrine Pancreatic Dysfunction Increases the Risk of New-Onset Diabetes Mellitus: Results of a Nationwide Cohort Study. Clin Transl Sci. 2021;14:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Columbia Surgery. The pancreas and its functions. [cited 15 November 2022]. Available from: https://columbiasurgery.org/pancreas/pancreas-and-its-functions. |
| 14. | Rickels MR, Norris AW, Hull RL. A tale of two pancreases: exocrine pathology and endocrine dysfunction. Diabetologia. 2020;63:2030-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Wu L, Nahm CB, Jamieson NB, Samra J, Clifton-Bligh R, Mittal A, Tsang V. Risk factors for development of diabetes mellitus (Type 3c) after partial pancreatectomy: A systematic review. Clin Endocrinol (Oxf). 2020;92:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Tu J, Zhang J, Ke L, Yang Y, Yang Q, Lu G, Li B, Tong Z, Li W, Li J. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J Clin Endocrinol Metab. 2017;102:3097-3110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, Jean S, Ebeling PR. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and β-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One. 2014;9:e109607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Norbitt CF, Kimita W, Bharmal SH, Ko J, Petrov MS. Relationship between Habitual Intake of Vitamins and New-Onset Prediabetes/Diabetes after Acute Pancreatitis. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Firkins SA, Hart PA, Papachristou GI, Lara LF, Cruz-Monserrate Z, Hinton A, Conwell DL, Bradley DP, Krishna SG. Identification of a Risk Profile for New-Onset Diabetes After Acute Pancreatitis. Pancreas. 2021;50:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Goodarzi MO, Petrov MS, Andersen DK, Hart PA. Diabetes in chronic pancreatitis: risk factors and natural history. Curr Opin Gastroenterol. 2021;37:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, Gatenby PAC, Jones SA, de Lusignan S. Incidence, Demographics, and Clinical Characteristics of Diabetes of the Exocrine Pancreas (Type 3c): A Retrospective Cohort Study. Diabetes Care. 2017;40:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Lv Y, Zhang J, Yang T, Sun J, Hou J, Chen Z, Yu X, Yuan X, Lu X, Xie T, Yu T, Su X, Liu G, Zhang C, Li L. Non-Alcoholic Fatty Liver Disease (NAFLD) Is an Independent Risk Factor for Developing New-Onset Diabetes After Acute Pancreatitis: A Multicenter Retrospective Cohort Study in Chinese Population. Front Endocrinol (Lausanne). 2022;13:903731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 24. | Richardson A, Park WG. Acute pancreatitis and diabetes mellitus: a review. Korean J Intern Med. 2021;36:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)--are we neglecting an important disease? Eur J Intern Med. 2013;24:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Petrov MS, Basina M. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur J Endocrinol. 2021;184:R151-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 503] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 28. | Meier JJ, Giese A. Diabetes associated with pancreatic diseases. Curr Opin Gastroenterol. 2015;31:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 29. | Duggan SN, O'Connor DB, Antanaitis A, Campion JR, Lawal O, Ahmed M, Tisdall AR, Sherlock M, Boran G, le Roux C, Gibney J, Conlon KC. Metabolic dysfunction and diabetes mellitus during long-term follow-up of severe acute pancreatitis: A case-matched study. Pancreatology. 2020;20:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11:279-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Ghandili S, Shayesteh S, Fouladi DF, Blanco A, Chu LC. Emerging imaging techniques for acute pancreatitis. Abdom Radiol (NY). 2020;45:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | İnce AT. Artificial Intelligence Prediction for the Severity of Acute Pancreatitis. [accessed 2023 Jan 24]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT04735055 ClinicalTrials.gov Identifier: NCT04735055. |
| 33. | Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, Vincze Á, Bajor J, Gódi S, Czimmer J, Szabó I, Illés A, Sarlós P, Hágendorn R, Pár G, Papp M, Vitális Z, Kovács G, Fehér E, Földi I, Izbéki F, Gajdán L, Fejes R, Németh BC, Török I, Farkas H, Mickevicius A, Sallinen V, Galeev S, Ramírez-Maldonado E, Párniczky A, Erőss B, Hegyi PJ, Márta K, Váncsa S, Sutton R, Szatmary P, Latawiec D, Halloran C, de-Madaria E, Pando E, Alberti P, Gómez-Jurado MJ, Tantau A, Szentesi A, Hegyi P; Hungarian Pancreatic Study Group. EASY-APP: An artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12:e842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 34. | Huh JH, Kim JW, Lee KJ. Vitamin D deficiency predicts severe acute pancreatitis. United European Gastroenterol J. 2019;7:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mao EQ, China; Xiao B, China S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX