Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.4072
Peer-review started: April 23, 2023
First decision: May 15, 2023
Revised: May 20, 2023
Accepted: June 2, 2023
Article in press: June 2, 2023
Published online: July 7, 2023
Processing time: 65 Days and 17.3 Hours
Acute bleeding due to esophageal varices (EVs) is a life-threatening complication in patients with cirrhosis. The diagnosis of EVs is mainly through upper gastrointestinal endoscopy, but the discomfort, contraindications and complications of gastrointestinal endoscopic screening reduce patient compliance. According to the bleeding risk of EVs, the Baveno VI consensus divides varices into high bleeding risk EVs (HEVs) and low bleeding risk EVs (LEVs). We sought to identify a non-invasive prediction model based on spleen stiffness measure
To develop a safe, simple and non-invasive model to predict HEVs in patients with viral cirrhosis and identify patients who can be exempted from upper gastrointestinal endoscopy.
Data from 200 patients with viral cirrhosis were included in this study, with 140 patients as the modelling group and 60 patients as the external validation group, and the EVs types of patients were determined by upper gastrointestinal endos
Univariate and multivariate analyses showed that SSM and LSM were associated with the occurrence of HEVs in patients with viral cirrhosis. On this basis, logistic regression analysis was used to construct a prediction model: Ln [P/(1-P)] = -8.184 -0.228 × SSM + 0.642 × LSM. The area under the curve of the new model was 0.965. When the cut-off value was 0.27, the sensitivity, specificity, positive predictive value and negative predictive value of the model for predicting HEVs were 100.00%, 82.43%, 83.52%, and 100%, respectively. Compared with the four prediction models of liver stiffness-spleen diameter to platelet ratio score, variceal risk index, aspartate aminotransferase to alanine aminotransferase ratio, and Baveno VI, the established model can better predict HEVs in patients with viral cirrhosis.
Based on the SSM and LSM measured by transient elastography, we established a non-invasive prediction model for HEVs. The new model is reliable in predicting HEVs and can be used as an alternative to routine upper gastrointestinal endoscopy screening, which is helpful for clinical decision making.
Core Tip: The non-invasive prediction model for predicting high risk esophageal varices (HEVs) in patients with viral cirrhosis was successfully established based on the spleen stiffness measurement and liver stiffness measurement. It is a novel model that has not been reported. The model was shown to be better than previous prediction models. The new model is reliable in predicting HEVs and can be used as an alternative to routine upper gastrointestinal endoscopy screening, which is helpful for clinical decision making.
- Citation: Yang LB, Gao X, Li H, Tantai XX, Chen FR, Dong L, Dang XS, Wei ZC, Liu CY, Wang Y. Non-invasive model for predicting high-risk esophageal varices based on liver and spleen stiffness. World J Gastroenterol 2023; 29(25): 4072-4084
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/4072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.4072
Liver cirrhosis is the end stage of chronic liver disease. When hepatic venous pressure gradient (HVPG) ≥ 10 mmHg is defined as clinically significant portal hypertension (CSPH), the patients who meet this criteria may suffer from complications such as esophageal variceal bleeding, ascites, hepatic encephalopathy, and jaundice due to portal hypertension and liver insufficiency[1,2]. Rupture of esophageal varices (EVs) is a common and life-threatening complication in patients with liver cirrhosis. The incidence of EVs bleeding is approximately 5%-15% per year, the re-bleeding rate within 6 wk after EVs rupture bleeding is 30% to 40%, and the mortality rate is 15%-25%[3-5]. The severity of liver cirrhosis, the size of EVs and the presence or absence of the red sign (RS) are related indicators of EVs bleeding[6]. For routine assessment of the above indicators, the Baveno VI consensus recommends that patients with cirrhosis need to undergo regular screening upper gastrointestinal endoscopy so that appropriate preventive treatment can be administered to prevent variceal bleeding events[7]. To date, HPVG and upper gastrointestinal endoscopy are considered the gold standards for the assessment of PH and EVs, respectively[6]. However, the prevalence of varicose veins requiring treatment (VNT), as defined by the Baveno VI guidelines, is very low in Compensated advanced chronic liver disease (cACLD) patients who are detected at an early stage[8]. HPVG and EGD are invasive procedures and expensive, and the patient compliance associated with them is poor[9,10]. Therefore, in clinical practice, it is necessary to develop a safe, non-invasive and patient-acceptable prediction model that can not only prevent frequent HPVG or gastrointestinal endoscopy examinations but also better predict HEVs in patients with viral cirrhosis. With the development of transient elastography (TE), studies have shown that liver stiffness (LS) and spleen stiffness (SS) detected by TE are associated with liver fibrosis, significant portal hypertension, and EVs. SS is increased in patients with viral hepatitis, and SS is positively correlated with HVPG, which has good predictive performance for CSPH and EVs in patients with cACLD[11-13].
At present, in addition to ultrasound, CT, MRI, and other imaging methods, there are also several common prediction models[14,15]. The LS-spleen diameter to platelet (PLT) ratio score (LSPS), variceal risk index (VRI), aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR) and Baveno VI model have all achieved good clinical effects in predicting HEVs[16-19]. In addition, the Baveno VI consensus states that LS measurement (LSM) combined with PLT helps to exclude HEVs[8,19,20]. To initially predict HEVs in patients with viral cirrhosis, the aim of this study is to establish a non-invasive prediction model that can predict HEVs based on SS measurement (SSM) and LSM and to evaluate the accuracy of the new model in identifying HEVs in patients with viral cirrhosis who can be exempted from upper gastrointestinal endoscopy.
The study was authorized by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, Shaanxi Province, China). As a retrospective study; therefore, the Ethics Committee waived the informed consent. This study retrospectively analyzed the data of patients with viral cirrhosis who were admitted to the Second Affiliated Hospital of Xi’an Jiaotong University and underwent upper abdominal computed tomography (CT) examination from March 2020 to November 2022. The inclusion criteria were: (1) Age > 18 years old; (2) patients with hepatitis B and hepatitis C cirrhosis; (3) patients who underwent endoscopy, upper abdominal CT, and laboratory examinations with complete results; and (4) the interval between two examinations was not more than 3 mo. Exclusion criteria: (1) Other liver injury factors, such as alcoholic liver disease, autoimmunity, metabolic liver disease, occult liver cirrhosis, etc; (2) suspicious liver tumor; (3) history of liver resection, liver transplantation or splenectomy; (4) frequent use of proton pump inhibitors; (5) other diseases that may impact the haemodynamics of the splenic vein or portal vein, such as cavernous degeneration, thrombosis, embolism; (6) cirrhotic patients with moderate or massive ascites; (7) previous treatment of portal hypertension, such as splenectomy, transjugular intrahepatic portosystemic shunt, endoscopic therapy, and nonselective β-blocker therapy; (8) diseases may affect the liver or spleen size, such as cysts, leukaemia, thrombocytopenic purpura, haemolytic anaemia, multiple myeloma, etc; (9) patients with a history of esophageal bleeding undergoing endoscopic or surgical treatment; (10) severe malnutrition or weight loss; (11) unreliable LSM: Quartile range/median > 0.3, success rate < 60%, or the number of effective measurements < 10; or (12) other conditions affecting LSM, such as body mass index > 35 kg/m2.
A total of 140 patients who met the inclusion criteria were selected as the modelling group. According to the results of upper gastrointestinal endoscopy and the Baveno VI criteria, the patients were divided into the HEVs group and the LEVs group; 66 patients were HEVs patients, and 74 patients were LEVs patients. In addition, 60 patients who met the inclusion criteria were used as the validation group. The patient’s data were collected when the model was established, and the data collection procedure and the model application did not interfere with each other.
Patients with liver cirrhosis were graded and scored using the Child-Pugh scoring system[21]. According to Baveno VI criteria, HEVs were defined as EVs with a diameter ≥ 5 mm, EVs with a diameter ≤ 5 mm and a positive RS, EVs in patients with Child grade C, and EVs that did not meet these criteria were LEVs with a low risk of bleeding[22,23].
The CT examinations were conducted by a multislice spiral CT scanner (GE 128-slice spiral CT scanner; Linux Medical System, United States) with a 5 mm reconstructed layer thickness, and the time interval was 5 s.
Actual liver volume measured by CT (CTLV), actual spleen volume measured by CT (CTSV), portal vein diameter (PVD) and spleen long diameter (SLD) were simultaneously measured by experienced radiologists who did not know the basic information of the patients. CTLV and CTSV were obtained by manually tracing the surface area of the liver and spleen at each level and multiplying by the layer thickness. The entire measurement process requires active avoidance of large blood vessels, gallbladder, and fissure. The SLD was defined as the length of the superior pole to the inferior line of the spleen at the plane of maximum surface area. The PVD needs to be measured at the midpoint between the portal vein bifurcation site and the vein confluence site[24].
For each patient, data on age, sex, height, weight, medical history, medication use, the presence or absence of ascites, and Child-Pugh score were collected. The white blood cell count, red blood cell count, PLT, ALT, AST, total bilirubin (TBil), alkaline phosphatase (ALP), glutamine transferase (GGT), albumin (ALB), total cholesterol (TCHO), prothrombin time (PT), international prothrombin ratio (INR), and prothrombin activity (PTA) of all patients included in the study were collected. The blood test was tested by an XN-9000 analyser (Xisen Meikang Medical Electronics Co. Ltd., Shanghai, China), and the coagulation function was tested by a Sysmex CO-CS-1500 system (SYSMEX Co., Ltd, Kobe, Japan) and the liver function test was performed using a Cobas 8000 analyser (Roche Diagnostics, Mannheim, Germany).
LSM and SSM were measured in all patients using FibroScan (Echosens, Paris, France) and FibroTouch (Hai’s Medical Technology Center, Beijing, China). The LSM was assessed by a trained and experienced operator after at least 4 h of fasting, and the SSM was performed on the same day as the LSM assessment. All measurements were obtained by experienced operators who had performed at least 300 tests in patients with chronic liver disease. The TE results of the patients were collected retrospectively, and the obtained results were expressed in kilopascals (kPa). The interquartile range (IQR) was defined as the intrinsic variation index between the 25th and 75th percentiles of the LS results containing 50% of the valid measurements. Therefore, LSM and SSM values were considered to be reliable when at least 10 valid measurements were obtained and the results were reliable, with an overall success rate of more than 60% and IQR/median ≤ 0.3[25,26].
Upper gastrointestinal endoscopy was performed by an endoscopic operator who was experienced in the assessment of patients with cirrhosis (with a minimum of 500 endoscopic procedures). Endoscopic examinations were performed to determine whether the patients had EVs, and if so, the EVs were graded according to the location (L), shape and size (F), colour (C), and presence or absence of RS of the lesion.
The non-invasive prediction models we choose to compare were as shown blow: LSPS = [LSM (KPa) × SLD (cm)]/PLT (× 109/L)[16]; VRI = -4.364 + 0.538 × SLD-0.049 × PLT-0.044 × LSM + 0.001 × (LSM × PLT)[17]; AAR = AST/ALT[18] and the Baveno VI criteria proposed by the consensus conference. The Baveno VI criteria were defined as LSM < 20 kPa and platelet count > 150 × 109/L. The extended Baveno VI criteria were defined as LSM < 25 kPa and platelet count > 110 × 109/L[8,27]. The results of upper gastrointestinal endoscopy were used as the gold standard. The receiver operating characteristic (ROC) curves of LSPS, VRI, AAR and Baveno VI were drawn, and the area under the ROC curve (AUC), sensitivity, specificity and Youden index were calculated to evaluate the performance of the new model and the previous four models in identifying HEVs. The point with the largest sum of sensitivity and specificity was selected as the best cut-off value for the diagnosis of HEVs.
The discrimination ability of the new model was assessed by the ROC curves in the modelling group and the external validation group. The Z test was used to evaluate differences in ROC curves. If there was no significant difference in the ROC between the two groups and AUC > 0.7, the model was considered to have good discrimination ability. The calibration ability of the prediction model was evaluated by the Hosmer-Lemeshow test and the two sets of calibration scatter plots. Decision curve analysis (DCA) was performed to evaluate the clinical efficacy of the new model.
SPSS 26.0 and R software (IBM SPSS, Chicago, IL, United States) were used for statistical analysis. Data are presented as the mean ± SD. The chi-square test was used to compare the measurement data between the HEVs group and the LEVs group. The Mann-Whitney U test was used for univariate analysis of continuous variable measurement data, and WALD backwards regression analysis was used for multivariate analysis. SPSS 26.0 software was used to draw the ROC curve and calculate the AUC to evaluate the diagnostic performance of the model. The maximum corresponding point of the Youden index was selected as the best cut-off value, and a positive prediction result was defined as equal to or greater than the best cut-off value. The best discrimination probability threshold, sensitivity, specificity and predictive value were calculated, and the diagnostic accuracy was compared. The higher the Youden index (1% or 100%), the more effective the correlation. Hosmer-Lemeshow test results, calibration charts and DCA were obtained using R software. All statistical tests were two-sided, with an alpha value of 0.05 and a statistical significance threshold of P < 0.05.
Tables 1 and 2 List the baseline characteristics of the modelling group and the external validation group, respectively. In the LEVs group, the mean age was 50.88 years ± 11.6 years, 43 (58.1%) were male, and 61 (82.4%) had hepatitis B. The age of the HEVs patients was 55.36 years ± 11.1 years old, 37 (56.1%) were male, and 53 (80.3%) had hepatitis B. In the modelling group, there was a significant difference in age between the HEVs group and the LEVs group (P < 0.05) but no significant difference in sex or hepatitis type (P > 0.05), and the two groups were comparable. In the external validation group, there was no significant difference in sex, age or hepatitis type (P > 0.05).
| Parameter | Patients with LEVs, n = 74 | Patients with HEVs, n = 66 | T value/χ2 value | P value |
| Age in yr | 50.88 ± 11.60 | 55.36 ± 11.10 | -2.333 | 0.021 |
| Male (%) | 43 (58.1%) | 37 (56.1%) | 0.060 | 0.807 |
| Etiology, HBV/HCV | 61/13 | 53/13 | 0.105 | 0.746 |
| Parameter | Patients with LEVs, n = 28 | Patients with HEVs, n = 32 | T value/χ2 value | P value |
| Age in yr | 52.54 ± 13.70 | 54.97 ± 10.40 | -0.780 | 0.438 |
| Male (%) | 15 (53.6%) | 14 (43.8%) | 0.577 | 0.448 |
| Etiology, HBV/HCV | 25/3 | 30/2 | 0.024 | 0.876 |
T tests and non-parametric rank sum tests were used for the univariate analysis. The summarized results are shown in Table 3. There were significant differences in SSM, PLT, LSM, ALT, AST, GGT, SLD, PT, INR, PTA, PVD, CTLV, and CTSV between the HEVs group and the LEVs group (P < 0.05). There were no significant differences in ALP, TBil, and TCHO between the two groups (P > 0.05).
| Parameter | Patients with LEVs, n = 74 | Patients with HEVs, n = 66 | t/Z | P value |
| SSM, KPa | 22.70 ± 6.00 | 19.06 ± 4.90 | 3.880 | < 0.001 |
| PLT, × 109/L | 108.55 ± 68.10 | 62.53 ± 29.00 | 5.096 | < 0.001 |
| LSM, KPa | 14.90 ± 5.10 | 24.83 ± 4.30 | -12.354 | < 0.001 |
| ALT, IU/L | 37.50 (26.00, 49.50) | 23.00 (16.00, 35.00) | -4.278 | < 0.001 |
| AST, IU/L | 43.00 (31.75, 69.00) | 34.50 (27.75, 45.25) | -2.796 | 0.005 |
| ALP, IU/L | 108.00 (81.25, 137.25) | 93.50 (78.00, 128.75) | -1.012 | 0.311 |
| GGT, IU/L | 60.50 (28.00, 114.00) | 33.00 (19.75, 58.75) | -3.609 | < 0.001 |
| SLD, mm | 13.58 ± 3.10 | 15.10 ± 3.30 | -2.806 | 0.006 |
| TBIL, μmol/L | 22.63 (16.01, 34.96) | 27.80 (18.05, 39.65) | -0.960 | 0.337 |
| ALB, g/dL | 37.16 ± 8.30 | 36.02 ± 5.80 | 0.935 | 0.351 |
| TCHO, mmol/L | 3.57 ± 1.40 | 3.18 ± 0.90 | 1.981 | 0.050 |
| PT, s | 12.45 ± 2.20 | 13.34 ± 2.50 | -2.284 | 0.024 |
| INR | 1.13 ± 0.20 | 1.21 ± 0.20 | -2.175 | 0.031 |
| PTA, % | 82.15 ± 20.80 | 75.09 ± 16.10 | 2.228 | 0.028 |
| PVD, mm | 12.20 ± 1.90 | 13.64 ± 2.30 | -4.024 | < 0.001 |
| CTLV, cm3 | 1031.88 ± 361.20 | 920.85 ± 241.50 | 2.111 | 0.037 |
| CTSV, cm3 | 558.11 ± 338.70 | 808.25 ± 409.90 | -3.951 | < 0.001 |
The parameters shown in Table 3 with statistically significant differences between the HEVs and LEVs groups were analyzed by multivariate analysis using backwards WALD regression analysis. As shown in Table 4, the SSM and LSM between the HEVs group and LEVs group were significantly different (P < 0.05). There were no significant differences in PLT, ALT, AST, GGT, SLD, PT, INR, PTA, PVD, CTLV, and CTSV (P > 0.05).
| Parameter | Patients with LEVs, n = 74 | Patients with HEVs, n = 66 | t/Z | P value |
| SSM, KPa | 22.70 ± 6.00 | 19.06 ± 4.90 | 3.880 | 0.009 |
| PLT, × 109/L | 108.55 ± 68.10 | 62.53 ± 29.00 | 5.096 | 0.606 |
| LSM, KPa | 14.90 ± 5.10 | 24.83 ± 4.30 | -12.354 | < 0.001 |
| ALT, IU/L | 37.50 (26.00, 49.50) | 23.00 (16.00, 35.00) | -4.278 | 0.669 |
| AST, IU/L | 43.00 (31.75, 69.00) | 34.50 (27.75, 45.25) | -2.796 | 0.125 |
| 60.50 (28.00, 114.00) | 33.00 (19.75, 58.75) | -3.609 | 0.790 | |
| SLD, mm | 13.58 ± 3.10 | 15.10 ± 3.30 | -2.806 | 0.952 |
| PT, s | 12.45 ± 2.20 | 13.34 ± 2.50 | -2.284 | 0.883 |
| INR | 1.13 ± 0.20 | 1.21 ± 0.20 | -2.175 | 0.777 |
| PTA, % | 82.15 ± 20.80 | 75.09 ± 16.10 | 2.228 | 0.920 |
| PVD, mm | 12.20 ± 1.90 | 13.64 ± 2.30 | -4.024 | 0.220 |
| CTLV, cm3 | 1031.88 ± 361.10 | 920.85 ± 241.50 | 2.111 | 0.892 |
| CTSV, cm3 | 558.11 ± 338.70 | 808.25 ± 409.90 | -3.951 | 0.713 |
Based on the results of the multivariate analysis, the parameters with no statistically significant difference between the two groups were excluded, and the parameters with statistically significant differences, including SSM and LSM, were used to establish a non-invasive prediction model. The logistic regression analysis showed that SSM and LSM were independent factors affecting the occurrence of HEVs and were statistically significant (P < 0.05). As shown in Table 5, the model was as follows: Ln [P/(1-P)] = -8.184 - 0.228 × SSM + 0.642 × LSM. HEVs in patients with viral cirrhosis were negatively correlated with SSM and positively correlated with LSM.
| Parameter | B | S.E. | Wald | P | Exp (B) | 95%CI of exp (B) |
| SSM | -0.228 | 0.074 | 9.647 | 0.002 | 0.796 | 0.689-0.919 |
| LSM | 0.642 | 0.123 | 27.245 | < 0.001 | 1.900 | 1.493-2.418 |
| Constant | -8.184 | 2.300 | 12.659 | < 0.001 | 0.000 | - |
The new model was compared with other models reported to predict EVs in patients with liver cirrhosis, namely, the LSPS, VRI, AAR, and Baveno VI models. The sensitivity, specificity and AUC of the new model based on the SSM and LSM and LSPS, VRI, AAR and Baveno VI models were calculated. The cut-off value of the model was defined as the maximum value of the sum of the specificity and sensitivity. Patients were considered to have HEVs when the P value calculated by the established formula was greater than the cut-off value. As shown in Figure 1A and Table 6, the AUC of this model was 0.965, while the AUCs of LSPS, VRI, AAR and Baveno VI were 0.835, 0.744, 0.641, and 0.675, respectively. The AUC > 0.7 indicated the good discrimination power of the model. The higher the AUC is, the better the discriminative power of the model, so the new model has good discriminative power.
| Area | SE | P | 95%CI of exp (B) | |
| LSPS | 0.835 | 0.033 | < 0.001 | 0.771-0.900 |
| VRI | 0.744 | 0.041 | < 0.001 | 0.663-0.824 |
| AAR | 0.641 | 0.046 | 0.004 | 0.550-0.732 |
| Baveno VI | 0.675 | 0.045 | < 0.001 | 0.586-0.764 |
| The new model | 0.965 | 0.015 | < 0.001 | 0.936-0.995 |
The accuracy, positive predictive value and negative predictive value of LSPS, VRI, ARR, Baveno VI, and the new model of 140 patients in the modelling group were calculated according to the calculation formula. As shown in Table 7, the non-invasive prediction model shown in this study had an accuracy of 89.30% and a positive predictive value of 83.52%. The accuracy and positive predictive values suggest the likelihood that the new model can correctly diagnose HEVs, with higher values indicating a more correct diagnosis.
| Sensitivity | Specificity | Accuracy, % | Positive predictive value, % | Negative predictive value, % | Cutoff value | |
| LSPS | 89.39 | 62.16 | 74.30 | 67.78 | 86.81 | 3.12 |
| VRI | 74.24 | 67.57 | 69.30 | 67.09 | 74.66 | 0.03 |
| AAR | 75.76 | 52.70 | 57.90 | 58.78 | 70.95 | 1.27 |
| Baveno VI1 | 98.48 | 36.49 | 65.70 | 57.99 | 96.42 | - |
| The new model | 100.00 | 82.43 | 89.30 | 83.52 | 100.00 | 0.27 |
Evaluation of the ability of the non-invasive model was performed by drawing the ROC curve of the established new model in the external validation group and using the Z test to compare the AUC curve between the modelling group and the external validation group and to evaluate the discriminative power of the new model. The AUC of the new model for predicting HEVs in the modelling group was 0.965, which was higher than that of the LSPS, VRI, AAR, and Baveno VI models. The AUC of the new model in the external validation group was 1. The Z test result was 0.896, and the P value was 0.37, indicating that there was no significant difference between the modelling group and the external validation group. The ROC curve of the external validation group is shown in Figure 1B.
The Hosmer-Lemeshow test was used to calculate the χ2 value of the modelling group and the external validation group to evaluate the calibration ability of the new model. The results showed that χ2 was -10.39 in the modelling group and 0.03 in the external validation group. The P values were 0.999 and 1.000, respectively. Both the groups’ P value were greater than 0.05, indicating that the model could predict HEVs accurately. The calibration scatter plots of the two groups were shown in Figure 2. As seen from the figure, all scatter points fluctuated around the baseline without significant deviation because the P values of both groups were greater than 0.05 and the difference between the groups was not statistically significant. The results showed that the HEVs patients who were predicted to have viral cirrhosis by using the new model were in good agreement with the actual HEVs patients.
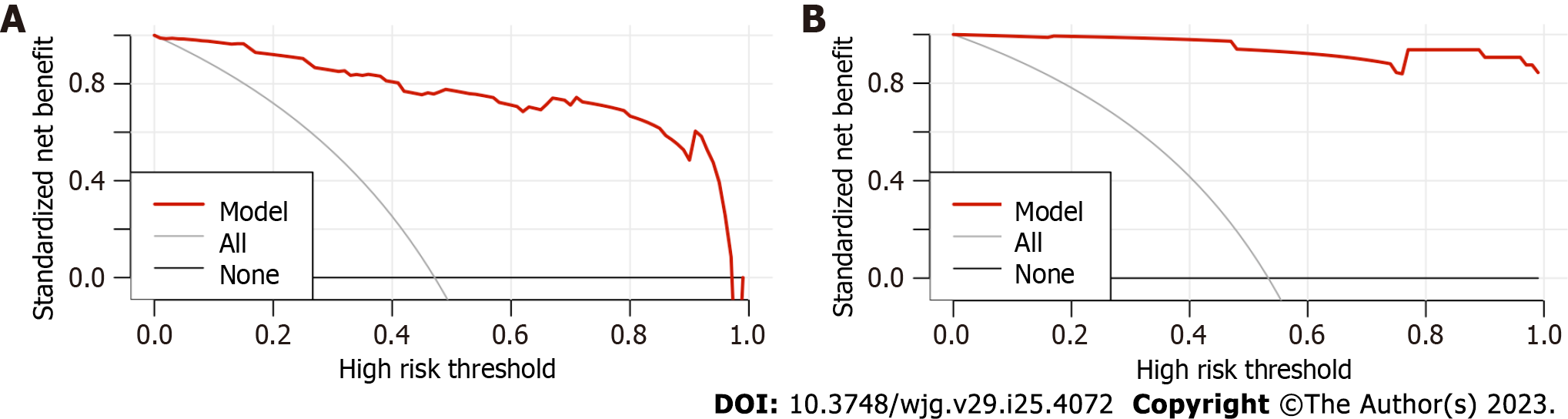
DCA was plotted against the probability of actual HEVs occurrence by predicting the probability of the modelling group and the external validation group by the new model. The DCA of the two groups is shown in Figure 3. In the DSA curve, the two dashed lines represent the two extreme cases, and the black line indicates that the new model predicts there was no HEVs, and a net clinical benefit of zero. The other grey line with a negative slope indicates that the new model predicts HEVs in all patients with viral cirrhosis, and the net clinical benefit is a back-slope with a negative slope[28]. The red line is the new model’s DCA. As the DCA curve shown, the red line was higher than the black and grey lines, indicating that when the new model was applied to the modelling group and the external validation group, both groups of patients could benefit, so the new model had certain clinical efficacy.
Long-term chronic viral hepatitis can lead to liver cirrhosis and is associated with high morbidity and mortality; therefore, it is a public health concern that deserves attention[29-31]. EVs rupture and bleeding are common causes of death in patients with liver cirrhosis. Clinical guidelines recommend the use of upper gastrointestinal endoscopy in screening and periodic reexamination of patients with cirrhosis regardless of the disease cause[9]. According to the results of upper gastrointestinal endoscopy and the Baveno VI criteria, EVs were divided by the low bleeding risk EVs (LEVs) and the high bleeding risk EVs (HEVs). For patients with HEVs, early precation measures can significantly reduce the esophageal variceal bleeding risk[22,32]. However, the invasiveness of upper gastrointestinal endoscopy, the high price, and the risk of anaesthesia make the compliance of patients to upper gastrointestinal endoscopy very low[9,10]. Considering that many patients do not have EVs in the early stage of liver cirrhosis, there is a need for non-invasive, simple, and safe means to identify liver cirrhosis patients with HEVs. In addition to upper gastrointestinal endoscopy, a variety of imaging methods, such as ultrasound, CT, and MRI, can be used to predict HEVs. However, these three methods cannot visually observe EVs, and the accuracy of identifying HEVs is poor[33]. A number of non-invasive models have been developed to predict HEVs, and several studies have shown that the LSPS, VRI, AAR, and Baveno VI models have achieved good results in predicting HEVs. Measurement of LS and SS by TE (using FibroScan) is a fast, non-invasive, easy to perform and reproducible procedure for predicting the presence of clinically significant EVs and PH, so LS and SS were measured by TE in this study[34]. In this study, to ensure the homogeneity of aetiology, we included patients with cirrhosis and HBV/HCV infection. Our study showed that LSM and SSM were two independent variables associated with the presence of HEVs. The results of our study are similar to those of several studies. LSM is associated with PH, and LSM combined with other indicators can predict HEVs[35,36]. Splenomegaly is common in patients with chronic viral cirrhosis, and splenic blood flow enters the portal vein system through the splenic vein. Therefore, SSM can simultaneously reflect static resistance fibrosis of the liver (LSM can also reflect) and dynamically capture PH-related visceral hypoperfusion, changes in spleen results, changes in blood flow, and PH-induced splenic fibrosis[37].
In this study, we constructed a non-invasive prediction model including LSM and SSM. The AUC of the new model was 0.965, the accuracy was 89.30%, which was better than that of the LSPS, VRI, AAR, and Baveno VI models, and the new model showed good diagnostic performance. When the optimal cut-off value was 0.27, the sensitivity and negative predictive value (NPV) of the new model in the modelling group were both 100%, and due to the Baveno VI criteria and other models, the new model could best identify non-HEVs patients so that these patients could be spared from undergoing upper gastrointestinal endoscopy. Morishita et al[38] used a similar approach in 135 patients with HCV-related cirrhosis to predict the presence of HEVs in patients with viral cirrhosis using a single indicator of LSM, and their results showed that the AUC of LSM for predicting the presence of HEVs was 0.868. The sensitivity, specificity, positive predictive value and negative predictive value were 81%, 82%, 69%, and 89%, respectively[38]. Similarly, in another study, Stefanescu et al[5] used a single-indicator SSM to assess the presence of HEVs, selecting an SSM@50 Hz with a 95% sensitivity for the best cut-off value[5]. Moreover, many studies have shown that when the HVPG value is ≥ 12 mmHg, LSM alone cannot reliably diagnose or exclude the risk grade of varices because of the poor correlation of extrahepatic factors. However, SSM can evaluate the severity of PH, the presence of EVs and the risk of bleeding but cannot predict the grade of EVs[39]. Therefore, this study developed and validated whether a non-invasive prediction model based on the combination of SSM and LSM indicators can be used as a useful tool to assess the severity of EVs and the risk of upper gastrointestinal bleeding (UGIB). These results of our study suggest that in the majority of patients with viral cirrhosis evaluated, the new model can accurately exclude patients with HEVs, thereby allowing these patients to avoid endoscopy or prophylactic therapy. Furthermore, the higher NPV and sensitivity, regardless of the cirrhosis severity, suggest that use of the new model may be more cost-effective, as endoscopic screening of patients with both compensated and decompensated cirrhosis proved to be cost-effective[40]. In addition, a second independent dataset was used to externally validate the clinical utility of the new model, and the results showed that the new model had high discrimination power. In addition, DCA was cited to illustrate the clinical benefit of the new model, and both the modelling group and the external validation group could benefit from the new model. In addition, the included indicators in the new model can be obtained by TE and B-ultrasound, which are non-invasive, inexpensive, do not require radiation, are highly feasible in clinical practice, and are easy to popularize in clinical work.
Generally speaking, we have successfully developed a non-invasive prediction model using LSM and SSM indicators to predict the presence of HEVs in patients with viral cirrhosis, which has not been reported in the literature. Compared with other models LSPS, VRI, AAR, and Baveno VI, the new model has a good diagnostic performance, a high discrimination ability, calibration ability and a clinical application value. In addition, we enrolled patients with viral cirrhosis, which provided good consistency while minimizing bias in the results. However, this retrospective study has limitations. First, the sample size of this study is relatively small, and more data need to be collected for evaluation. Second, the patients in this study were all Chinese, and it is unclear whether this model can be applied in other ethnic groups. Additionally, because the patients in this study were all patients with viral cirrhosis and because changes in liver and spleen volume can vary with cirrhosis from different causes, it is unclear whether the new model can be applied to cirrhosis from other causes.
In conclusion, the new model based on SSM and LSM indicators, ln [P/(1-P)] = -8.184 - 0.228 × SSM + 0.642 × LSM, can effectively rule out the presence of HEVs in patients with viral cirrhosis, and this model needs to be further verified in prospective trials. This model helps physicians recognize the presence of HEVs in patients with viral cirrhosis, make more informed decisions, and provide appropriate preventive treatment.
The new model can effectively rule out the presence of HEVs in patients with viral cirrhosis and can be used as an alternative to routine upper gastrointestinal endoscopy screening.
Acute bleeding due to esophageal varices (EVs) is a life-threatening complication in patients with cirrhosis. The diagnosis of EVs is mainly through upper gastrointestinal endoscopy, but the discomfort, contraindications and complications of gastrointestinal endoscopic screening reduce patient compliance.
To develop a safe, simple and non-invasive model to predict high risk EVs (HEVs) in patients with viral cirrhosis and identify patients who can be exempted from upper gastrointestinal endoscopy.
To establish a non-invasive prediction model based on spleen stiffness measurement (SSM) and live stiffness measurement (LSM) as an alternative to EVs screening.
Two hundred Chinese adults, from March 2020 to November 2022, were included at the Second Affiliated Hospital of Xi’an Jiaotong University. Required data were collected by the medical records, and the EVs types of patients were determined by upper gastrointestinal endoscopy and the Baveno VI consensus. The effect of each parameter on HEVs was analyzed by univariate and multivariate analyses, and a non-invasive prediction model was established, and then the effect of each parameter on HEVs was analyzed by univariate and multivariate analyses, and a non-invasive prediction model was established.
After univariate and multivariate analyses, SSM and LSM were used to established a prediction model. The new non-invasive model was better than other four models to predict HEVs in patients with viral cirrhosis.
The new model is reliable in predicting HEVs and can be used as an alternative to routine upper gastrointestinal endoscopy screening, which is helpful for clinical decision making.
In the future, we will try to apply the new model to predict HEVs in patients with viral cirrhosis.
We thank all the participants in this study.
| 1. | Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-37; quiz 38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 776] [Article Influence: 59.7] [Reference Citation Analysis (1)] |
| 2. | de Franchis R, Dell'Era A. Invasive and noninvasive methods to diagnose portal hypertension and esophageal varices. Clin Liver Dis. 2014;18:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Liang H, Si H, Liu M, Yuan L, Ma R, Zhang G, Yang J, Mo Z, Zhao Q. Non-Invasive Prediction Models for Esophageal Varices and Red Signs in Patients With Hepatitis B Virus-Related Liver Cirrhosis. Front Mol Biosci. 2022;9:930762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Yu S, Chen W, Jiang Z. Platelet count/spleen volume ratio has a good predictive value for esophageal varices in patients with hepatitis B liver cirrhosis. PLoS One. 2021;16:e0260774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Stefanescu H, Marasco G, Calès P, Fraquelli M, Rosselli M, Ganne-Carriè N, de Ledinghen V, Ravaioli F, Colecchia A, Rusu C, Andreone P, Mazzella G, Festi D. A novel spleen-dedicated stiffness measurement by FibroScan® improves the screening of high-risk oesophageal varices. Liver Int. 2020;40:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, Angeli P, Saggioro A, Alberti A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol. 2010;53:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Gaete MI, Díaz LA, Arenas A, González K, Cattaneo M, Fuster F, Henríquez R, Soza A, Arrese M, Barrera F, Arab JP, Benítez C. Baveno VI and Expanded Baveno VI criteria successfully predicts the absence of high-risk gastro-oesophageal varices in a Chilean cohort. Liver Int. 2020;40:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Wickremeratne T, Turner S, O'Beirne J. Systematic review with meta-analysis: ultra-thin gastroscopy compared to conventional gastroscopy for the diagnosis of oesophageal varices in people with cirrhosis. Aliment Pharmacol Ther. 2019;49:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | La Mura V, Garcia-Guix M, Berzigotti A, Abraldes JG, García-Pagán JC, Villanueva C, Bosch J. A Prognostic Strategy Based on Stage of Cirrhosis and HVPG to Improve Risk Stratification After Variceal Bleeding. Hepatology. 2020;72:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Singh S, Eaton JE, Murad MH, Tanaka H, Iijima H, Talwalkar JA. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:935-45.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Hu X, Huang X, Hou J, Ding L, Su C, Meng F. Diagnostic accuracy of spleen stiffness to evaluate portal hypertension and esophageal varices in chronic liver disease: a systematic review and meta-analysis. Eur Radiol. 2021;31:2392-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Manatsathit W, Samant H, Kapur S, Ingviya T, Esmadi M, Wijarnpreecha K, McCashland T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018;33:1696-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | de Alcantara RV, Yamada RM, Cardoso SR, de Fátima M, Servidoni CP, Hessel G. Ultrasonographic predictors of esophageal varices. J Pediatr Gastroenterol Nutr. 2013;57:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lipp MJ, Broder A, Hudesman D, Suwandhi P, Okon SA, Horowitz M, Clain DJ, Friedmann P, Min AD. Detection of esophageal varices using CT and MRI. Dig Dis Sci. 2011;56:2696-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim DY. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 18. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Marot A, Trépo E, Doerig C, Schoepfer A, Moreno C, Deltenre P. Liver stiffness and platelet count for identifying patients with compensated liver disease at low risk of variceal bleeding. Liver Int. 2017;37:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Bae J, Sinn DH, Kang W, Gwak GY, Choi MS, Paik YH, Lee JH, Koh KC, Paik SW. Validation of the Baveno VI and the expanded Baveno VI criteria to identify patients who could avoid screening endoscopy. Liver Int. 2018;38:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Edeline J, Blanc JF, Johnson P, Campillo-Gimenez B, Ross P, Ma YT, King J, Hubner RA, Sumpter K, Darby S, Evans J, Iwuji C, Swinson D, Collins P, Patel K, Muazzam I, Palmer DH, Meyer T. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int. 2016;36:1821-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A; Anticipate Investigators. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study. Hepatology. 2016;64:2173-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 23. | Lee E, Kim YJ, Goo DE, Yang SB, Kim HJ, Jang JY, Jeong SW. Comparison of hepatic venous pressure gradient and endoscopic grading of esophageal varices. World J Gastroenterol. 2016;22:3212-3219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Patel M, Tann M, Liangpunsakul S. CT-scan Based Liver and Spleen Volume Measurement as a Prognostic Indicator for Patients with Cirrhosis. Am J Med Sci. 2021;362:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 518] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 26. | Rigamonti C, Cittone MG, Manfredi GF, Sorge A, Moia R, Patriarca A, Donato MF, Gaidano G, Pirisi M, Fraquelli M. High reproducibility of spleen stiffness measurement by vibration-controlled transient elastography with a spleen-dedicated module. Hepatol Commun. 2022;6:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Zheng KI, Liu C, Li J, Zhao L, Zheng MH, Wang F, Qi X. Validation of Baveno VI and expanded Baveno VI criteria to identify high-risk varices in patients with MAFLD-related compensated cirrhosis. J Hepatol. 2020;73:1571-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Vickers AJ, Holland F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 2021;21:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 29. | Leoni S, Casabianca A, Biagioni B, Serio I. Viral hepatitis: Innovations and expectations. World J Gastroenterol. 2022;28:517-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Xiang Z, Li J, Lu D, Wei X, Xu X. Advances in multi-omics research on viral hepatitis. Front Microbiol. 2022;13:987324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Reiberger T, Püspök A, Schoder M, Baumann-Durchschein F, Bucsics T, Datz C, Dolak W, Ferlitsch A, Finkenstedt A, Graziadei I, Hametner S, Karnel F, Krones E, Maieron A, Mandorfer M, Peck-Radosavljevic M, Rainer F, Schwabl P, Stadlbauer V, Stauber R, Tilg H, Trauner M, Zoller H, Schöfl R, Fickert P. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (3)] |
| 33. | Zardi EM, Di Matteo FM, Pacella CM, Sanyal AJ. Invasive and non-invasive techniques for detecting portal hypertension and predicting variceal bleeding in cirrhosis: a review. Ann Med. 2014;46:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Roccarina D, Rosselli M, Genesca J, Tsochatzis EA. Elastography methods for the non-invasive assessment of portal hypertension. Expert Rev Gastroenterol Hepatol. 2018;12:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Kim BK, Kim DY, Han KH, Park JY, Kim JK, Paik YH, Lee KS, Chon CY, Ahn SH. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol. 2011;106:1654-1662, 1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Berger A, Ravaioli F, Farcau O, Festi D, Stefanescu H, Buisson F, Nahon P, Bureau C, Ganne-Carriè N, Berzigotti A, de Ledinghen V, Petta S, Calès P; multicenter groups Varices œsophagiennes - Vidéo-capsule œsophagienne, Agence Nationale de Recherches sur le Sida et les Hépatites Virales, Cohort 12 Cirrhoses Virales, M116, and Validation of Expanded Baveno VI Criteria. Including Ratio of Platelets to Liver Stiffness Improves Accuracy of Screening for Esophageal Varices That Require Treatment. Clin Gastroenterol Hepatol. 2021;19:777-787.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Reiberger T. The Value of Liver and Spleen Stiffness for Evaluation of Portal Hypertension in Compensated Cirrhosis. Hepatol Commun. 2022;6:950-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 38. | Morishita N, Hiramatsu N, Oze T, Harada N, Yamada R, Miyazaki M, Yakushijin T, Miyagi T, Yoshida Y, Tatsumi T, Kanto T, Takehara T. Liver stiffness measurement by acoustic radiation force impulse is useful in predicting the presence of esophageal varices or high-risk esophageal varices among patients with HCV-related cirrhosis. J Gastroenterol. 2014;49:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Buechter M, Kahraman A, Manka P, Gerken G, Jochum C, Canbay A, Dechêne A. Spleen and Liver Stiffness Is Positively Correlated with the Risk of Esophageal Variceal Bleeding. Digestion. 2016;94:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I, Matsueda K, Yamamoto H. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92-101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Ullah K, Pakistan S-Editor: Chen YL L-Editor: A P-Editor: Zhao S