Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.4009
Peer-review started: February 10, 2023
First decision: March 15, 2023
Revised: March 27, 2023
Accepted: June 5, 2023
Article in press: June 5, 2023
Published online: July 7, 2023
Processing time: 138 Days and 6.7 Hours
Endoscopic full-thickness resection (EFTR) has emerged as a viable technique in the management of mucosal and subepithelial lesions of the gastrointestinal tract (GIT) not amenable to conventional therapeutic approaches. While various devices and techniques have been described for EFTR, a single, combined full-thickness resection and closure device (full-thickness resection device, FTRD system, Ovesco Endoscopy AG, Tuebingen, Germany) has become commercially available in recent years. Initially, the FTRD system was limited to use in the colorectum only. Recently, a modified version of the FTRD has been released for EFTR in the upper GIT as well. This review provides a broad summary of the FTRD, highlighting recent advances and current challenges.
Core Tip: Endoscopic full-thickness resection (EFTR) is an emerging technique for tissue resection of lesions in the gastrointestinal tract (GIT) not amenable to conventional resection approaches. The novel full-thickness resection device (FTRD) is a combined full-thickness resection and closure device that allows for EFTR of lesions in the GIT. EFTR with FTRD is feasible, safe, and efficacious and should be considered as a viable option for resection of select lesions in the lower and upper GIT.
- Citation: Mun EJ, Wagh MS. Recent advances and current challenges in endoscopic resection with the full-thickness resection device. World J Gastroenterol 2023; 29(25): 4009-4020
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/4009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.4009
Traditionally, flexible endoscopic removal of gastrointestinal neoplasms has been accomplished by standard polypectomy, endoscopic mucosal resection (EMR), or endoscopic submucosal dissection (ESD). More recently, endoscopic full-thickness resection (EFTR) has emerged as a complementary resection technique for the removal of colorectal lesions, particularly for lesions that are scarred or non-lifting, early cancers with deeper invasion, and subepithelial lesions, where resection via standard endoscopic techniques is difficult or not possible[1,2]. EFTR also has the potential added benefit of decreasing procedural time and providing a minimally-invasive organ-sparing alternative approach, obviating need for surgery. As its name implies, EFTR refers to the technique whereby a lesion is resected endoscopically in its entirety including all layers of the gastrointestinal wall[1]. The two main techniques for EFTR include the “resect and close” method, where the full-thickness resection is performed first exposing the peritoneal cavity, followed by complete closure of the defect, and “close and resect” method, where approximation of the wall layers deep to the lesion is performed first followed by full-thickness resection without exposure and contamination of the peritoneum. Various strategies and devices have been developed to accomplish EFTR, including but not limited to needle-knives, ESD knives, nylon loop accompanied by standard through-the-scope clips, omental patches, endoscopic plicating and suturing devices, and endoscopic staplers[3-9]. These methods will not be covered in this review.
Recently, a single, combined full-thickness resection and closure device referred to as the full-thickness resection device (FTRD system, Ovesco Endoscopy AG, Tuebingen, Germany) has become available and is quickly being adopted across centers worldwide to accomplish EFTR. Its appeal over other strategies includes the ability to perform resection and closure with a single device integrating an over-the-scope clip (OTSC) and electrosurgical snare. It can offer a safe and nonsurgical alternative for removal of lesions that may be challenging to resect with conventional endoscopic methods and might otherwise require surgery. Main indications for the FTRD include non-lifting or scarred polyps, partially resected or residual polyps with submucosal fibrosis, early cancers, subepithelial lesions, and diagnostic EFTR for pathologic assessment of the gut wall. It is approved for use in the colorectum, and recently, received additional approval from the U.S. Food and Drug Administration for use in the upper gastrointestinal tract (GIT)[10].
This review article will provide a summary of the major studies on FTRD, highlighting recent advances and current challenges, in the hopes that practicing endoscopists will be better equipped to understand the role and limitations of, and potentially utilize, this novel and important resection technique.
The FTRD system, a combined full-thickness resection and closure device, was first made available in Europe in 2014[11]. It has since been approved for use in the United States for colorectal EFTR in 2017 and gastroduodenal EFTR in 2020[12].
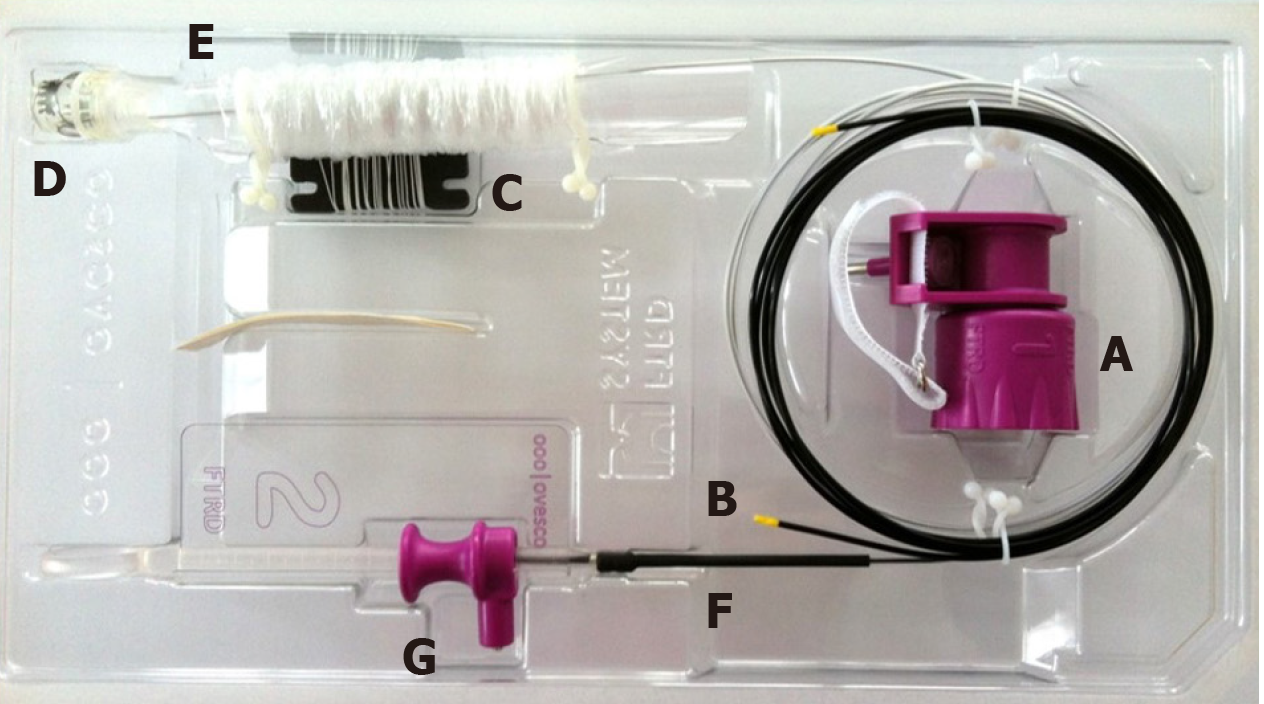
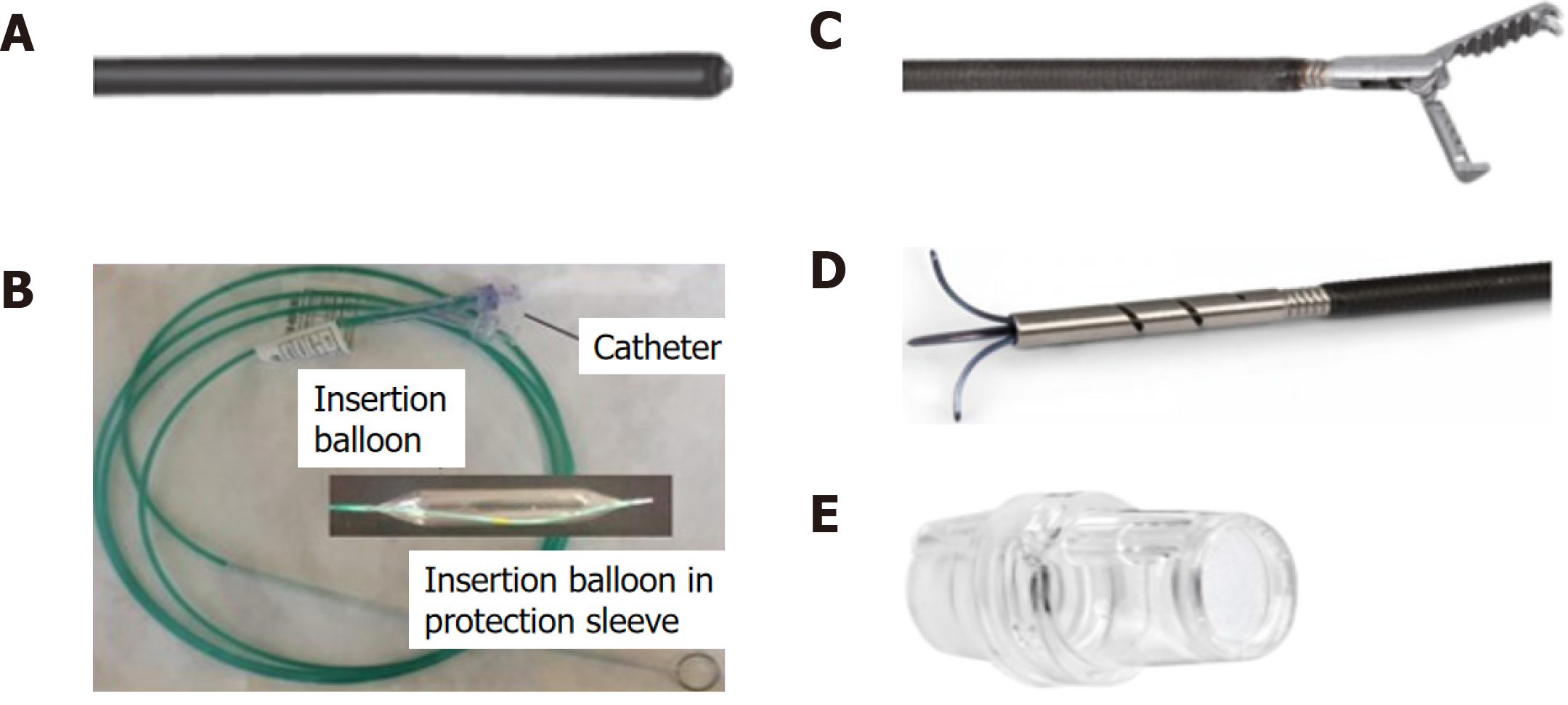
The FTRD system is based on the principle of “close and resect” for EFTR. It uses the well-established OTSC system (Ovesco Endoscopy AG, Tuebingen, Germany), which allows for full-thickness tissue apposition deeper to the lesion first, followed by full-thickness resection above the clip, thereby performing EFTR without exposure of the peritoneal cavity[13]. The FTRD system (Figure 1) consists of a clear, plastic distal attachment cap that has a mounted ready-for-use 14 mm nickel titanium alloy (Nitinol) clip, integrated 14 mm monofilament snare and thread, thread retriever, endoscope sleeve with fixation tapes, and a hand wheel. Separately, an FTRD Marking Probe (Figure 2A) and FTRD Grasper (Figure 2B) are provided as part of the FTRD set. These individual instruments can be advanced through the working channel for marking and mobilization of the target lesion, respectively. The FTRD system is suitable for use for endoscopes having a diameter of 11.5-13.2 mm and a working channel diameter of 3.2 mm. The cap diameter is 21 mm and the cap depth is 23 mm.
The initial FTRD system was fashioned for use in the colorectum and is now marketed as the colonic FTRD©. With recent advances demonstrating the role of the FTRD in the upper GIT, an additional FTRD system called the gastroduodenal FTRD© has now become available. The gastroduodenal FTRD system is smaller in caliber and additionally comes with an insertion balloon and guide wire to help facilitate passage of the FTRD through the upper esophageal sphincter and pylorus (Figure 2C). A separate modified Anchor (Figure 2D) is also available to allow for better tissue mobilization (i.e., for subepithelial lesions). The gastroduodenal FTRD is suitable for endoscopes having a diameter of 10.5-12.0 mm and a working channel diameter of 3.7 mm. The cap diameter is 19.5 mm and the cap depth is 23 mm.
Recently, a third FTRD system called the diagnostic FTRD© was made available for use in the colorectum for diagnostic resection, or full-thickness biopsy. This system is nearly identical to the colonic FTRD system but is smaller in size allowing for full-thickness resection of lesser tissue volume when desired for diagnostic purposes. The diagnostic FTRD system can also be applied to a pediatric colonoscope allowing for increased flexibility and mobility. The diagnostic FTRD system is suitable for endoscopes having a diameter of 10.5-12.0 mm and a working channel diameter of 3.2 mm. The cap diameter is 19.5 mm and the cap depth is 23 mm.
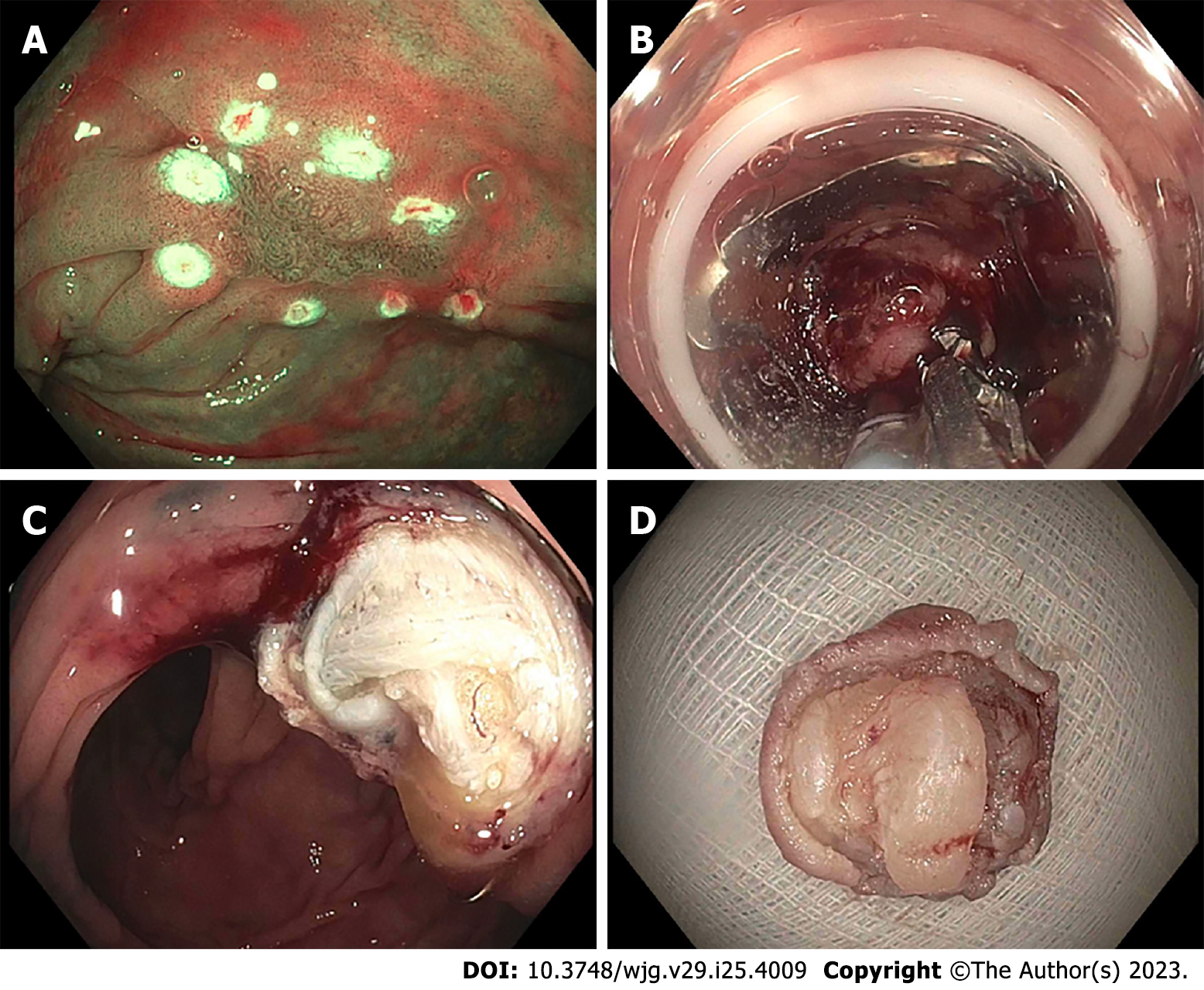
Once a target lesion in the GIT is identified, its margins are delineated with the FTRD Marking Probe ensuring a margin of normal surrounding tissue (Figure 3A). The endoscope is then withdrawn from the patient. It is often cumbersome to advance the endoscope (with the attached stiff FTRD cap) to the lesion (especially lesions located in the right colon), and sometimes difficult to grasp and bring a scarred lesion into the cap. Hence many endoscopists will first attach a test cap — the FTRD prOVE cap (a “blank” cap similar to the FTRD cap but without the mounted clip or snare; see Figure 2E) — to the endoscope to perform a “test run” without using the FTRD, which may otherwise be wasted if unable to be advanced to the lesion. Once it is confirmed that the endoscope with the attached prOVE cap can be advanced to the lesion and the lesion able to be brought inside the cap, the endoscope is withdrawn and fitted with the “real” FTRD system. The hand wheel (Figure 1A) is first inserted into the working channel of the endoscope. The thread retriever (Figure 1B) is then inserted into the working channel and used to retrieve the thread (Figure 1C) allowing for attachment of the plastic attachment cap (Figure 1D) onto the distal end of the endoscope. The distal attachment cap has a preloaded clip attached to the thread that runs inside the working channel of the endoscope and attaches to the hand wheel, as well as a preloaded snare that runs outside of the endoscope. The accompanying endoscope sleeve (Figure 1E) and adhesion tapes help affix the snare (Figure 1F) to the outside of the endoscope and prevent inadvertent twisting of the snare around the endoscope or entrapment of tissue between the snare and the endoscope. Turning of the hand wheel allows for adjusting tension on the thread, and eventually, deployment of the clip. A snare lock (Figure 1G) helps prevent inadvertent snare opening and can be removed to “unlock” the snare once ready for use. The endoscope with the attached FTRD system is then re-inserted into the GIT to the target lesion. Next, with the tip of the cap appropriately positioned over the lesion (Figure 3B), the FTRD Grasper is used to grasp (Figure 3C) and mobilize the lesion completely within the cap (Figure 3D). All markings made by the FTRD Marking Probe should be visible inside the cap. Suction should not be used to draw the lesion into the cap as this may bring in extraluminal structures (e.g., small bowel) into the cap causing significant injury to adjacent organs and structures. Finally, the clip is deployed (Figure 3E) by turning the hand wheel, and the lesion immediately resected with the snare using an electrosurgical current (Figure 3F) while being firmly grasped with the Grasper. It should be noted that once the lesion is brought into the cap, these steps should be performed quickly and sequentially in this specific order to prevent perforation and/or the lesion slipping out of the cap. The resected specimen is retrieved by withdrawing the endoscope with the lesion held by the Grasper. The endoscope without the attached FTRD is then reinserted to inspect the resection site for complete resection and confirm the absence of adverse events (AEs) (Figure 4).
Earlier studies established efficacy and safety of the OTSC system for endoscopic closure of gastrointestinal perforations and surgical anastomotic leaks and fistulas[14,15]. Following these data, several observational experiences and case series were described using an OTSC-assisted method for resection of gastrointestinal lesions, whereby the OTSC system was used to deploy the clip deep to a lesion prior to snare resection[16-18]. Based on these experiences, the basis for the FTRD system — a combined device containing an OTSC system plus a preloaded snare — was developed.
An early preclinical study using the FTRD in an animal (porcine) model demonstrated safety and efficacy[11]. The FTRD was used for resection of the colon wall in 11 pigs, and 7 and 28-d follow up study sessions were performed to evaluate the clip application site. Clips were still present in all but one case at 28 d and adherent stool and fibrin were found on the clips. There were mild inflammatory reactions seen histologically at day 7, but no acute inflammation was noted on day 28. The underlying tissue at the clip bed showed no signs of dehiscence or ischemia. Manometric pressure tests demonstrated no leak. Histology assessments revealed successful full-thickness resection (mucosa to serosa) in all cases.
A summary of the major studies investigating the FTRD are included below. The keywords "endoscopic full thickness resection", “full thickness resection device,” and “FTRD” were used for the initial query on PubMed. Studies published within a 10-year period from January 1, 2011-December 31, 2021, were considered for inclusion. To be considered for inclusion in this review, studies had to be original research studies using the Ovesco Endoscopy FTRD (other devices described for EFTR were not included) and be designed as prospective or retrospective studies. Observational studies, case reports, preclinical animal studies and proof of concept studies were not included. Studies that did not report the type of lesion, technical success rates, R0 resection rates, full-thickness resection rates, and rates of AEs were also not included.
The first clinical experience using the FTRD was published in 2015[19]. In this retrospective, two-center European study, 24 patients who underwent resection of colorectal lesions with the FTRD were identified. Indications included recurrent or incompletely resected adenomas with a non-lifting sign (n = 11), appendiceal adenomas (n = 5), untreated adenomas with a non-lifting sign (n = 2), incomplete resection of T1 carcinoma (n = 2), submucosal tumors (n = 2), adenoma within diverticulum (n = 1), flat adenoma in a coagulopathic patient (n = 1), and diagnostic resection in a patient with suspected Hirschsprung’s disease (n = 1). Technical success (complete en bloc resection) was achieved in 20/24 (83.3%) patients and R0 resection (histologically complete resection with tumor-free margins) in 18/24 (75.0%) patients. Two patients developed post-polypectomy syndrome and required antibiotics, but there were no other reported AEs including no perforations or major bleeding events.
This study has been followed by numerous retrospective and prospective studies demonstrating safety and efficacy of FTRD in the colon and rectum (see Table 1 for summary of major clinical studies with current applications of FTRD for EFTR)[12,20-27]. In the largest, prospective multicenter study to date, 181 patients were recruited to receive EFTR with the FTRD system[25]. Indications for EFTR included “difficult” adenomas (n = 143), early carcinomas (n = 15), and subepithelial tumors (n = 23). “Difficult” adenomas included adenomas with a non-lifting sign, adenomas at the appendiceal orifice, and adenomas at a diverticulum. Technical success was achieved in 89.5% of cases and R0 resection in 76.9% of cases. AEs were noted in 18/181 (9.9%) patients and included bleeding (4/18), post-polypectomy syndrome (3/18), perforation (6/18), acute appendicitis (3/18), abdominal pain without clear etiology on laboratory and radiographic work-up (1/18), and enterocolonic fistula formation (1/18). All four patients with bleeding were treated conservatively and did not require transfusion of blood products. Emergency surgery was required in 3/181 (2.2%) patients.
| Ref. | Study type | Total FTRD cases, n | Site (s) | Type of lesion | Technical success, % | R0 resection, % | Full-thickness resection, % | Adverse events, % | |
| 1 | Schmidt et al[19] | Retrospective | 24 | Colon; Rectum | Non-lifting polyps (n = 13); Appendiceal polyps (n = 5); Diagnostic (n = 3); Subepithelial tumor (n = 2); Polyp involving diverticulum (n = 1) | 83.3 | 75.0 | 87.5 | 8.3 |
| 2 | Schmidt et al[25] | Prospective | 181 | Colon; Rectum | Difficult adenomas (79.0%); Cancer (8.3%); Subepithelial tumor (12.7%) | 89.5 | 76.9 | 81.0 | 9.9 |
| 3 | Meier et al[12] | Retrospective | 1178 | Colon; Rectum | Difficult adenomas (67.1%); Cancer (18.4%); Subepithelial tumor (6.8%); Diagnostic (1.3%) | 88.2 | 80.0 | 89.9 | 12.1 |
| 4 | Boger et al[23] | Retrospective | 68 | Colon; Rectum | Non-lifting polyps (n = 29); Cancer (n = 13); Subepithelial tumor (n = 9); Polyps in appendix or diverticulum (n = 17) | 88.2 | 76.8 | NA | 5.9 |
| 5 | Hajifathalian et al[37] | Retrospective | 56 | Stomach; Duodenum | Mesenchymal neoplasm including GIST (n = 26); Diagnostic (n = 10), NET (n = 9); Adenomas (n = 6); Adenocarcinoma (n = 5) | 93.0 | 68.0 | NA | 21.0 |
| 6 | Meier et al[39] | Prospective | 29 | Stomach | Subepithelial tumor (n = 29) | 89.7 | 76.0 | 65.5 | 31.0 |
| 7 | Ichkhanian et al[20] | Retrospective | 66 | Colon/appendiceal orifice | Polyps involving appendiceal orifice | 89.0 | 93.0 | 91.0 | 17.0 |
| 8 | Zwager et al[28] | Prospective | 367 | Colon; Rectum | Difficult polyps (n = 133); T1 cancer (n = 71); Incomplete resection (n = 150); Subepithelial tumors (n = 13) | 83.9 | 82.4 | 83.2 | 9.3 |
| 9 | Zwager et al[29] | Retrospective | 330 | Colon; Rectum | Primary resection of T1 cancer (n = 132); Secondary resection of T1 cancer (n = 198) | 87.0 | 85.6 | NA | 2.2 |
The results of a recent large retrospective analysis using the German colonic FTRD registry (n = 1178) reported similar findings, corroborating the previously reported findings of smaller studies[12]. Indications included difficult adenomas, early carcinomas, subepithelial tumors, and diagnostic resection. The technical success rate was 88.2% and R0 resection rate 80.0. The AE rate was 12.1% with 3.1% being major AEs. A similar but smaller retrospective study from the UK FTRD registry (n = 68) reported similar outcomes23 with technical success rate 88.2%, R0 resection rate 76.8%, and AE rate 5.9%. This has since been further replicated in a larger Dutch FTRD registry (n = 367) with technical success rate 83.9%, R0 resection rate 82.4%, full-thickness resection rate 83.2%, and AE rate 9.3%[28].
Studies have reported slight variations (albeit with comparable success) in outcomes for FTRD depending on the indication for EFTR. Regarding FTRD use for early cancer, a recent study of 156 patients with a histologically proven diagnosis of adenocarcinoma showed FTRD to have a technical success rate of 92.3% and R0 resection rate of 71.8%. Serious AEs were reported in 3.9% of patients[24]. In a subgroup analysis from the previously mentioned retrospective study using the German colonic FTRD registry by Meier et al[12], 217 cases of FTRD in early cancers were identified. Technical success was reported to be 84.6% and R0 resection in 82.8% of cases. In a separate analysis by Schmidt et al[25], 29 cases of FTRD for early carcinomas were identified (14 harbored unsuspected cancer and 15 were known cancers). R0 resection was achieved in 72.4% of cases, but curative resection could only be achieved in 44.8% of cases. Similarly, a study from the Dutch FTRD registry showed overall technical success, R0 resection, and AE rates of 87.0%, 85.6%, and 2.2%. However, the curative resection rate was only 60.3% with a curative resection rate of 23.7% for primary resection of T1 cancers[29]. Hence, given these low curative resection rates, there is some uncertainty regarding the role of FTRD as primary use for early cancers.
For lesions at the appendiceal orifice, a recent study of 66 patients demonstrated a technical success rate of 89% and R0 resection rate of 79%[20] using FTRD. Of note, 17.2% of patients developed appendicitis and 10.3% of patients (60% of those who developed appendicitis) required surgical appendectomy. Factors associated with risk of appendicitis included male sex (odds ratio: 1.2, P = 0.03) and failure to achieve histologic full-thickness resection (odds ratio: 1.5, P = 0.04)[20]. The high rates of appendicitis for this indication have raised concerns regarding the use of FTRD for appendiceal lesions. Further highly powered studies are needed to clarify the safety of FTRD for these lesions.
There is a lack of highly powered studies investigating FTRD for subepithelial tumors. A recent study of 40 patients with rectal neuroendocrine tumors (NETs) did report a technical success rate of 100%, R0 resection rate of 100%, and no AEs[21]. While these outcomes should be interpreted cautiously in the setting of a small sample size, the reported success is nonetheless encouraging and in line with the success reported in other FTRD studies[12,22,23,25]. In a subgroup analysis using the German colonic FTRD registry by Meier et al[12], 80 cases of FTRD for subepithelial tumors were identified. The technical success rate was 97.3% and R0 resection rate was 97%. R0 resection was significantly higher for subepithelial tumors compared with that for other lesions in the German FTRD registry (77.2% for difficult adenomas, 82.8% for early cancers). Data regarding AE rates within the subgroup analysis were not available. In a separate subgroup analysis from Schmidt et al[25], 23 cases of FTRD for indication of subepithelial lesions were identified, with R0 resection rate of 87%. Overall, FTRD for subepithelial lesions seems to be very effective, with greater technical success and R0 resection than FTRD for other lesions.
Subgroup analyses have also been performed in studies to determine whether differences in outcomes may be related to other factors such as lesion location, lesion size, and prior treatment. Findings have varied[12,25], though by and large, there does not appear to be convincing evidence of any significant differences in technical success and other outcomes based on lesion location, size, or prior treatment[12]. Further studies may help better clarify what factors independently predict these outcomes of success.
Similar technical success and R0 resection rates have been reported for EMR and ESD[30,31]. ESD has been associated with greater R0 resection rates than EMR, but is also associated with greater rates of AEs like perforation and major bleeding. Direct comparisons of technical success and R0 resection between EMR/ESD and FTRD are difficult to make, as the majority of indications for FTRD currently are for lesions deemed difficult or challenging to resect via EMR or ESD. Few studies have reported on the efficacy of salvage ESD (ESD performed for residual or locally recurrent non-lifting lesions), which would likely be a better comparator group for FTRD, but these few small studies do appear to suggest comparable rates of success[32,33]. Similar AE rates have been reported for ESD and FRTD[31]. However, procedural time for ESD is considerably longer than FTRD[31].
Overall, EFTR with the FTRD system in the colorectum appears to be safe and efficacious. Common indications for FTRD in the lower GIT include select lesions difficult to resect by more conventional approaches (i.e., non-lifting adenomas, adenomas at appendiceal orifice, adenomas at diverticulum), early cancers, subepithelial lesions, and diagnostic resection. Very large lesions > 3 cm may not be appropriate for FTRD.
Recently, a newer and smaller caliber FTRD, the gastroduodenal FTRD©, has become available for use in the upper GIT.
Prior to the development of the newer gastroduodenal FTRD system, experiences using the traditional FTRD system (now known as the colonic FTRD©) in the upper GIT were reported[34-36]. These were primarily case reports and small case series dating as far back as 2014 (when the FTRD first became available in Europe) reporting safe and successful use of the FTRD in the stomach and duodenum in patients with malignant or large, nonmalignant subepithelial lesions who were deemed unsuitable for surgery[34-36]. Several retrospective analyses have followed demonstrating safety and efficacy of FTRD in the UGIT[37,38]. The largest of these was an international multicenter trial of 56 patients across 13 centers[37]. Common indications included gastrointestinal stromal tumors (GIST), adenomas, hamartomas, and adenocarcinomas. Results showed a technical success rate of 77% and R0 resection rate of 68%. The AE rate was 21%, though none were classified as severe AEs. Common AEs included intraprocedural and delayed bleeding. No perforations were reported.
Recently, a prospective multicenter pilot study (RESET trial) investigated the new gastroduodenal FTRD in the upper GIT[39]. 29 patients underwent EFTR with the gastroduodenal FTRD in this trial for gastric subepithelial tumors, which included leiomyomas, lipomas, schwannomas, ectopic pancreas, GISTs, and NETs. The technical success rate was 89% and the R0 resection rate was 76%. Minor bleeding occurred in 31% of cases and was able to be managed intraprocedurally in all cases. No transfusion of blood products was required nor was any further bleeding observed. No other AEs were reported. The success rates and AE rates reported here are similar to those reported with FTRD in the lower GIT.
To our knowledge, there is only one single report on the use of the FTRD in the esophagus[37]. However, this was reported as part of a larger multicenter study, and it is not evident whether this case was technically successful or associated with any AEs.
Similar to FTRD in the lower GIT, various subgroup analyses have been performed to ascertain whether outcomes of FTRD in the upper GIT are associated with other variables. Thus far, variables such as indication, lesion size, lesion location, prior treatment, participating center, or number of FTRD cases performed by the endoscopist have not been clearly associated with technical success and other success outcomes[37,39]. Further studies are needed to help delineate the impact of these individual factors on gastroduodenal FTRD outcomes.
Overall, early data suggests that FTRD in the upper GIT appears to be feasible, safe, and efficacious. Common indications include gastric and duodenal subepithelial lesions, adenomas, and early cancer. However, given the paucity of data of FTRD in the upper GIT compared to the lower GIT, further studies are needed to further clarify the indications, especially for the indication of early cancer, as well as clarify overall safety and efficacy.
In cases with lesions > 3 cm or where en bloc EFTR appears challenging for other reasons, various hybrid techniques have been proposed. These have been referred to by varying names including Hybrid FTRD, EMR + FTRD, and ESD + FTRD. In general, these hybrid techniques refer to using either piecemeal EMR or ESD followed by FTRD of scarred areas or residual abnormal appearing tissue. Hybrid techniques have shown some promise in small case series[40-44]. However, further evaluation in larger scale studies is needed to draw conclusions on their routine use.
Several limitations of the FTRD have been reported. One technical challenge is the difficulty in resecting larger lesions. With a cap diameter of 21 mm and cap depth of 23 mm for the standard colonic FTRD (and smaller for the gastroduodenal FTRD and diagnostic FTRD), it is often difficult to adequately grasp large lesions (> 3 cm) to accomplish en bloc resection. In addition, these lesions are often scarred and fixed to the colon wall, and therefore may be difficult to grasp and bring into the cap even if they are smaller than 3 cm. The length and stiffness of the plastic cap can also interfere with visualization and flexibility at the scope tip, thereby making it quite cumbersome to advance the colonoscope with the FTRD across a tortuous sigmoid and especially to the right colon. As described earlier, a proprietary test cap called the FTRD prOVE Cap© is available to help circumvent some of these issues and to determine whether a target lesion can be reached and might be suitable for resection with FTRD. The long plastic sheath that wraps around the scope shaft can increase friction of the device and also make advancement of the scope through tight or tortuous anatomic locations difficult. Some endoscopists leave a guidewire in the colon after the diagnostic colonoscopy is performed. The colonoscope with the attached FTRD is then advanced alongside the guidewire, following the guidewire to the lesion, overcoming the limited visualization and maneuverability with the FTRD cap. Device failure (failure of the clip to fully cinch down or complete malfunction of the clip or snare’s opening and closing functions) and premature clip deployment leading to immediate perforation or incomplete resection have been reported[12]. Risk factors for incomplete or partial resection include tissue fibrosis, right-sided colonic lesions, and size > 3 cm[25]. It should be emphasized that clip placement must be immediately followed by prompt snare resection, often requiring good communication and teamwork between the endoscopist and assistants handling the snare and grasper. It is important to have the endoscopist fire the clip and have two separate assistants operate the grasper and snare. Delay in snare resection after clip placement, slippage of the lesion from the grasper, or inadvertently performing snare resection before firing the clip can result in incomplete resection or immediate perforation. As with other endoscopic devices and techniques, EFTR with the FTRD requires adequate training and endoscopic skill. Completion of mandatory training is required prior to the purchase and use of FTRD.
Currently, there is a lack of direct comparison between FTRD and other standard resection approaches such as EMR and ESD. Direct comparisons between FTRD and more standard approaches are needed to further clarity the role of FTRD, whether it is merely adjunctive to standard approaches or may be a suitable primary or “standard” approach itself. Cost-effectiveness studies may help further clarify this role as well. Recently, laparoscopic and endoscopic cooperative surgery (LECS) has been described as a less invasive approach to resecting lesions in the GIT, such as GIST[45]. Prospective studies comparing FTRD with LECS and other similar novel minimally-invasive surgical techniques will help clarify the advantage of FTRD over these novel surgical approaches. Studies investigating longer term outcomes are also needed, especially regarding AEs and the ability to perform adequate endoscopic examination of the full-thickness resection site during follow-up and surveillance. This may be challenging if the FTRD clip is still in place and not fallen off. There are still concerns regarding AEs with EFTR with FTRD, especially for resection of lesions at the appendiceal orifice and with regards to delayed perforation. Long term data are needed on specific outcomes such as the development of an appendiceal mucocele or delayed appendicitis after using FTRD in this location. It is also tempting to consider whether there might be safe applications for the FTRD in the esophagus, particularly in the treatment of early carcinomas or subepithelial lesions. Future studies may help clarify this and other expanded roles of FTRD.
EFTR can be successfully and safely accomplished with the FTRD system. The role of FTRD has expanded in recent years and the FTRD is currently available for use in the lower and upper GIT. EFTR with FTRD is a crucial addition to the endoscopic armamentarium for resection of gastrointestinal lesions and reducing need for more invasive surgery. Common indications for its use include resection of lesions difficult to resect with conventional techniques like EMR and ESD, such as scarred or non-lifting polyps, early cancers, subepithelial lesions, and diagnostic resection for evaluation of various neuromuscular or entero-infiltrative conditions (e.g., Hirschsprung disease, gastrointestinal amyloidosis). EFTR with FTRD has a high technical success and R0 resection rate, and AE rates are acceptable, especially when used as a less invasive option for lesions not amenable for standard endoscopic resection. Common AEs include bleeding and perforation, and further long-term data on unique complications such as post-polypectomy syndrome, delayed perforation, acute appendicitis, and enterocolonic fistula are needed. Several technical challenges exist and endoscopists should be adequately trained on the FTRD system prior to use in clinical practice. FTRD, used in its proper context, has the ability to be a major advancement in endoscopic resection for populations felt to be too high risk for traditional resection techniques and surgery (e.g., elderly patients, patients at high risk of bleeding, high risk for anesthesia).
| 1. | Rajan E, Wong Kee Song LM. Endoscopic Full Thickness Resection. Gastroenterology. 2018;154:1925-1937.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: Current status. World J Gastroenterol. 2015;21:9273-9285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 3. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | ASGE Technology Committee; Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Konda V, Murad FM, Siddiqui UD, Banerjee S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Stavropoulos SN, Modayil R, Friedel D, Brathwaite CE. Endoscopic full-thickness resection for GI stromal tumors. Gastrointest Endosc. 2014;80:334-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Mönkemüller K, Sarker S, Baig KR. Endoscopic creation of an omental patch with an over-the-scope clip system after endoscopic excavation and resection of a large gastrointestinal stromal tumor of the stomach. Endoscopy. 2014;46 Suppl 1 UCTN:E451-E452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | ASGE Technology Committee; Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Wang A, Song LM, Rodriguez SA. Endoscopic closure devices. Gastrointest Endosc. 2012;76:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Kaehler G, Grobholz R, Langner C, Suchan K, Post S. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy. 2006;38:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | U. S. Food and Drug Administration. Center for Devices and Radiological Health. Gastroduodenal FTRD Set K200684 approval letter, June 2, 2020. [cited 7 December 2020]. In: U.S. Food and Drug Administration [Internet]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/K200684.pdf. |
| 11. | Schurr MO, Baur FE, Krautwald M, Fehlker M, Wehrmann M, Gottwald T, Prosst RL. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc. 2015;29:2434-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 12. | Meier B, Stritzke B, Kuellmer A, Zervoulakos P, Huebner GH, Repp M, Walter B, Meining A, Gutberlet K, Wiedbrauck T, Glitsch A, Lorenz A, Caca K, Schmidt A. Efficacy and Safety of Endoscopic Full-Thickness Resection in the Colorectum: Results From the German Colonic FTRD Registry. Am J Gastroenterol. 2020;115:1998-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 15. | Arezzo A, Verra M, Reddavid R, Cravero F, Bonino MA, Morino M. Efficacy of the over-the-scope clip (OTSC) for treatment of colorectal postsurgical leaks and fistulas. Surg Endosc. 2012;26:3330-3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Mönkemüller K. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014;46:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Mönkemüller K, Peter S, Toshniwal J, Popa D, Zabielski M, Stahl RD, Ramesh J, Wilcox CM. Multipurpose use of the 'bear claw' (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc. 2014;26:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Ichkhanian Y, Barawi M, Seoud T, Thakkar S, Kothari TH, Halabi ME, Ullah A, Edris W, Aepli P, Kowalski T, Shinn B, Shariaha RZ, Mahadev S, Mosko JD, Andrisani G, Di Matteo FM, Albrecht H, Giap AQ, Tang SJ, Naga YM, van Geenen E, Friedland S, Tharian B, Irani S, Ross AS, Jamil LH, Lew D, Nett AS, Farha J, Runge TM, Jovani M, Khashab MA. Endoscopic full-thickness resection of polyps involving the appendiceal orifice: a multicenter international experience. Endoscopy. 2022;54:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Meier B, Albrecht H, Wiedbrauck T, Schmidt A, Caca K. Full-thickness resection of neuroendocrine tumors in the rectum. Endoscopy. 2020;52:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Ichkhanian Y, Vosoughi K, Diehl DL, Grimm IS, James TW, Templeton AW, Hajifathalian K, Tokar JL, Samarasena JB, Chehade NEH, Lee J, Chang K, Mizrahi M, Barawi M, Irani S, Friedland S, Korc P, Aadam AA, Al-Haddad MA, Kowalski TE, Novikov A, Smallfield G, Ginsberg GG, Oza VM, Panuu D, Fukami N, Pohl H, Lajin M, Kumta NA, Tang SJ, Naga YM, Amateau SK, Brewer GOI, Kumbhari V, Sharaiha R, Khashab MA. A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions. Surg Endosc. 2021;35:1296-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Boger P, Rahman I, Hu M, Ayaru L, Bhandari P, Chedgy F, Green S, Hayat M, Hopper AD, Ishaq S, Martin J, McCallum I, Phull P, Pugh S, Russo E, Suzuki N, Thomas-Gibson S, Zeino Z, Patel P. Endoscopic full thickness resection in the colo-rectum: outcomes from the UK Registry. Eur J Gastroenterol Hepatol. 2021;33:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89:1180-1189.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H, Neuhaus H, Albers D, Birk M, Thimme R, Probst A, Faehndrich M, Frieling T, Goetz M, Riecken B, Caca K. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (2)] |
| 26. | Andrisani G, Pizzicannella M, Martino M, Rea R, Pandolfi M, Taffon C, Caricato M, Coppola R, Crescenzi A, Costamagna G, Di Matteo FM. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Dig Liver Dis. 2017;49:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Backes Y, Kappelle WFW, Berk L, Koch AD, Groen JN, de Vos Tot Nederveen Cappel WH, Schwartz MP, Kerkhof M, Siersema PD, Schröder R, Tan TG, Lacle MM, Vleggaar FP, Moons LMG; T1 CRC Working Group. Colorectal endoscopic full-thickness resection using a novel, flat-base over-the-scope clip: a prospective study. Endoscopy. 2017;49:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Zwager LW, Bastiaansen BAJ, Bronzwaer MES, van der Spek BW, Heine GDN, Haasnoot KJC, van der Sluis H, Perk LE, Boonstra JJ, Rietdijk ST, Wolters HJ, Weusten BLAM, Gilissen LPL, Ten Hove WR, Nagengast WB, Bekkering FC, Schwartz MP, Terhaar Sive Droste JS, Vlug MS, Houben MHMG, Rando Munoz FJ, Seerden TCJ, Beaumont H, de Ridder R, Dekker E, Fockens P; Dutch eFTR Group. Endoscopic full-thickness resection (eFTR) of colorectal lesions: results from the Dutch colorectal eFTR registry. Endoscopy. 2020;52:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Zwager LW, Bastiaansen BAJ, van der Spek BW, Heine DN, Schreuder RM, Perk LE, Weusten BLAM, Boonstra JJ, van der Sluis H, Wolters HJ, Bekkering FC, Rietdijk ST, Schwartz MP, Nagengast WB, Ten Hove WR, Terhaar Sive Droste JS, Rando Munoz FJ, Vlug MS, Beaumont H, Houben MHMG, Seerden TCJ, de Wijkerslooth TR, Gielisse EAR, Hazewinkel Y, de Ridder R, Straathof JA, van der Vlugt M, Koens L, Fockens P, Dekker E; Dutch eFTR Group. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy. 2022;54:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 31. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 32. | Azzolini F, Camellini L, Sassatelli R, Sereni G, Biolchini F, Decembrino F, De Marco L, Iori V, Tioli C, Cavina M, Bedogni G. Endoscopic submucosal dissection of scar-embedded rectal polyps: a prospective study (Esd in scar-embedded rectal polyps). Clin Res Hepatol Gastroenterol. 2011;35:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Hurlstone DP, Shorthouse AJ, Brown SR, Tiffin N, Cross SS. Salvage endoscopic submucosal dissection for residual or local recurrent intraepithelial neoplasia in the colorectum: a prospective analysis. Colorectal Dis. 2008;10:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Roberts JR, Koro K, Yeh MM, Saunders MD, Templeton AW. Endoscopic resection of gastric adenocarcinoma by use of a full-thickness resection device. VideoGIE. 2018;3:244-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Schmidt A, Meier B, Cahyadi O, Caca K. Duodenal endoscopic full-thickness resection (with video). Gastrointest Endosc. 2015;82:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Andrisani G, Di Matteo FM. Endoscopic full-thickness resection of duodenal lesions (with video). Surg Endosc. 2020;34:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Hajifathalian K, Ichkhanian Y, Dawod Q, Meining A, Schmidt A, Glaser N, Vosoughi K, Diehl DL, Grimm IS, James T, Templeton AW, Samarasena JB, Chehade NEH, Lee JG, Chang KJ, Mizrahi M, Barawi M, Irani S, Friedland S, Korc P, Aadam AA, Al-Haddad M, Kowalski TE, Smallfield G, Ginsberg GG, Fukami N, Lajin M, Kumta NA, Tang SJ, Naga Y, Amateau SK, Kasmin F, Goetz M, Seewald S, Kumbhari V, Ngamruengphong S, Mahdev S, Mukewar S, Sampath K, Carr-Locke DL, Khashab MA, Sharaiha RZ. Full-thickness resection device (FTRD) for treatment of upper gastrointestinal tract lesions: the first international experience. Endosc Int Open. 2020;8:E1291-E1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J. 2018;6:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Meier B, Schmidt A, Glaser N, Meining A, Walter B, Wannhoff A, Riecken B, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surg Endosc. 2020;34:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Zimmer V. Hybrid precutting EMR-EFTR resection for a tricky rectal adenoma recurrence with high-grade fibrosis. Dig Liver Dis. 2020;52:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Meier B, Caca K, Schmidt A. Hybrid endoscopic mucosal resection and full-thickness resection: a new approach for resection of large non-lifting colorectal adenomas (with video). Surg Endosc. 2017;31:4268-4274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Lupu A, Jacques J, Rivory J, Saurin JC, Rostain F, Ponchon T, Pioche M. Hybrid endoscopic submucosal dissection using a full-thickness resection device allows en bloc resection of a large adenoma deeply invading the appendix. Endoscopy. 2018;50:E296-E298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Andrisani G, Di Matteo FM. Hybrid resection with endoscopic submucosal dissection and full-thickness resection device of a large cecal laterally spreading tumor involving the appendix. VideoGIE. 2020;5:372-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Andrisani G, Di Matteo FM. Hybrid resection with ESD and FTRD: Could this be a rescue treatment in the presence of severe submucosal fibrosis? Dig Liver Dis. 2019;51:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 101273.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin L, China; Nagata J, Japan S-Editor: Gao CC L-Editor: A P-Editor: Xu ZH