Published online Jun 21, 2023. doi: 10.3748/wjg.v29.i23.3668
Peer-review started: March 2, 2023
First decision: April 8, 2023
Revised: April 21, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 21, 2023
Processing time: 105 Days and 23.6 Hours
Endoscopic resection (ER) with bipolar snare, in which the electric current only passes through the tissue between the device’s two electrodes, is a prominent method used to prevent perforation due to electricity potentially. ER using bipolar snare with or without submucosal injection enabled safe resection of colorectal lesions measuring 10–15 mm in an ex vivo porcine model. ER with bipolar snare is expected to have good treatment outcomes in 10–15 mm colorectal lesions, with high safety even without submucosal injection. However, no clinical reports have compared treatment outcomes with and without submucosal injection.
To compare the treatment outcomes of bipolar polypectomy with hot snare polypectomy (HSP) to those with endoscopic mucosal resection (EMR).
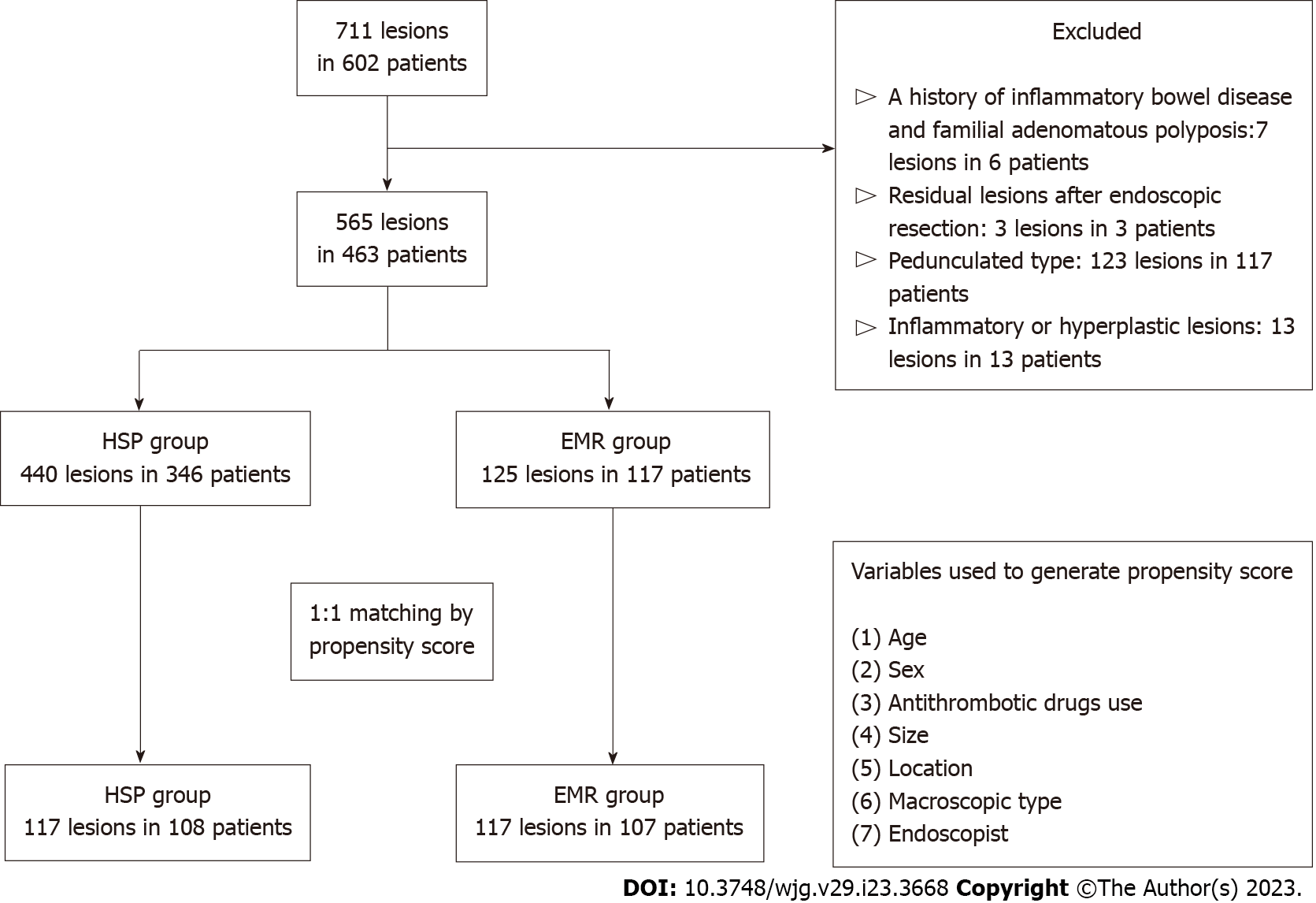
In this single-centre retrospective study, we enrolled 10–15 mm nonpedunculated colorectal lesions (565 Lesions in 463 patients) diagnosed as type 2A based on the Japan Narrow-band Imaging Expert Team classification, resected by either HSP or EMR between January 2018 and June 2021 at the National Cancer Center Hospital East. Lesions were divided into HSP and EMR groups, and propensity score matching was performed. In the matched cohort, en bloc and R0 resection rates and adverse events were compared between the two groups.
Of the 565 lesions in 463 patients, 117 lesions each in the HSP and EMR groups were selected after propensity score matching. In the original cohort, there was a significant difference in antithrombotic drug use (P < 0.05), lesion size (P < 0.01), location (P < 0.01), and macroscopic type (P < 0.05) between the HSP and EMR groups. In the matched cohort, the en bloc resection rates were comparable between both groups [93.2% (109/117) vs 92.3% (108/117), P = 0.81], and there was no significant difference in the R0 resection rate [77.8% (91/117) vs 80.3% (94/117), P = 0.64]. The incidence of delayed bleeding was similar in both groups [1.7% (2/117)]. Perforation occurred in the EMR group [0.9% (1/117)] but not in the HSP group.
Using bipolar snare, ER of nonpedunculated 10–15 mm colorectal lesions may be performed safely and effectively, even without submucosal injection.
Core Tip: This study is the first to compare treatment outcomes between hot snare polypectomy (HSP) and endoscopic mucosal resection (EMR) using a bipolar snare for nonpedunculated colorectal lesions measuring 10–15 mm. First, there was no significant difference in en bloc and R0 resection rates between the HSP and EMR groups. Second, the incidence of adverse events was similar in both groups, but perforation occurred only in the EMR group. These results suggest that comparable treatment efficiency and safety may be obtained even without submucosal injection when resecting nonpedunculated colorectal lesions measuring 10–15 mm using a bipolar snare.
- Citation: Minakata N, Murano T, Wakabayashi M, Sasabe M, Watanabe T, Mitsui T, Yamashita H, Inaba A, Sunakawa H, Nakajo K, Kadota T, Shinmura K, Ikematsu H, Yano T. Hot snare polypectomy vs endoscopic mucosal resection using bipolar snare for intermediate size colorectal lesions: Propensity score matching. World J Gastroenterol 2023; 29(23): 3668-3677
- URL: https://www.wjgnet.com/1007-9327/full/v29/i23/3668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i23.3668
Colorectal cancer (CRC) is the leading cause of morbidity and mortality worldwide[1]. Detection and resection of colorectal lesions via colonoscopy reduce CRC-related mortality[2,3]. However, complications associated with endoscopic resection (ER), such as post-procedural bleeding and perforation, are concerning[4,5]. Perforation, although infrequent, is the most serious complication that may result in hospitalization, stoma formation, and mortality[6]. Various devices and techniques related to ER have been developed to reduce the risk of complications.
ER using a bipolar snare, wherein the electric current only passes through the tissue between the two electrodes of the device, may potentially prevent perforation due to electricity. Apart from reducing electric damage to tissues, using a bipolar snare does not require a counter electrode, carries a lower risk of burns, and can be used even when a metal, such as a pacemaker, is present inside the patient’s body[7]. In an ex vivo porcine model, using a bipolar snare for intramucosal lesions measuring 10–15 mm did not cause thermal damage to the muscularis propria. However, perforation occurred with a monopolar snare. Furthermore, thermal damage to the muscularis propria was not observed in bipolar polypectomy, regardless of whether a submucosal injection was performed before resection[8].
An ER with a bipolar snare for 10–15 mm colorectal lesions could result in favourable outcomes even without submucosal injection. However, no reports have compared bipolar polypectomy with and without submucosal injection in clinical practice. Hence, using a bipolar snare for colorectal lesions measuring 10–15 mm, this study aimed to compare the safety and efficiency of hot snare polypectomy (HSP), which involves resection without submucosal injection, to those of endoscopic mucosal resection (EMR), which involves resection with submucosal injection.
We retrospectively enrolled 10–15-mm nonpedunculated colorectal lesions diagnosed as type 2A based on the Japan Narrow-band Imaging Expert Team (JNET) classification, resected by either HSP or EMR using a bipolar snare between January 2018 and June 2021 at the National Cancer Center Hospital East. The exclusion criteria were: (1) History of inflammatory bowel disease and familial adenomatous polyposis; (2) Pedunculated type lesions; (3) Residual lesions after previous ER; and (4) Lesions pathologically diagnosed as inflammatory or hyperplastic polyps.
Before the colonoscopy, a polyethylene glycol electrolyte solution with ascorbic acid (MobiPrep, EA Pharma, Tokyo, Japan) or magnesium citrate (Magcorol P, Horii Pharmaceutical Industries, Osaka, Japan) was administered to all patients according to the manufacturer’s instructions. The examinations were performed using a magnifying endoscope (PCF-H290ZI, CF-HQ290, CF-H290ECI, CF-EZ1500DI, CF-XZ1200I colonoscope, Olympus, Tokyo, Japan; EC-L590ZP, EC-L600ZP, Fujifilm Co., Tokyo, Japan), light source, and video processor (EVIS LUCERAELITE, EVIS X1, Olympus; LASEREO, Fujifilm Co.).
All procedures were performed by either four experts (≥ 2000 colonoscopies performed) or 18 nonexperts (< 2000 colonoscopies performed) endoscopists. The treatment choice (HSP or EMR) was at the endoscopist’s discretion. For EMR, a submucosal injection was performed using saline solution alone or combined with sodium hyaluronate acid. A bipolar snare (Dragonare® Xemex Co. Ltd., Tokyo, Japan) was used in both HSP and EMR. An electrosurgery generator unit (ICC200, VIO300D, VIO3; ERBE Elektromedizin GmbH Co. Ltd., Tubingen, Germany; ESG100, Olympus) was used for all ERs. The cutting mode in the forced coagulation mode was used for resection. Subsequently, resection margins were evaluated endoscopically to confirm the absence of remnants. Prophylactic clipping after resection was performed at the endoscopist’s discretion. Resected lesions were retrieved by suctioning through the endoscope into a trap, using pentapod-type grasping forceps or a retrieval net. The endocopists recorded the size, location, and macroscopic type of the lesions, diagnosis according to the JNET classification, and en bloc or piecemeal resection. The location was recorded as right colon if the lesion was in the caecum, ascending, or transverse colon, and as left colon if it was in the descending or sigmoid colon. Once removed, the lesions were fixed in formalin, embedded in paraffin, sectioned into 2–3 mm slices, stained with hematoxylin-eosin, and evaluated by two experienced pathologists blinded to the patient’s clinical information. Pathological results were described according to World Health Organization criteria[9].
Study outcomes were en bloc and R0 resection rates, and adverse events, including delayed bleeding and perforation. En bloc resection was defined as ER with the entire lesion resected in a single piece. Upon histological evaluation of the horizontal and vertical margins of specimens, R0 resection was defined as negative margins both horizontally and vertically; RX resection, as unclear resection margins either horizontally or vertically; and R1 resection, as positive resection margins either horizontally or vertically[10]. Delayed bleeding was defined as haemorrhage requiring endoscopic intervention within 2 wk after polypectomy. Perforation was defined as any organ or fat outside the muscularis layer visualized on endoscopy during the procedure or free air observed on computed tomography after the procedure. For subgroup analysis, the R0 resection rate according to each clinical characteristic related to lesions was assessed.
This was a single-centre retrospective study, and the protocol was approved by the Institutional Review Board of the National Cancer Center (2017-434). All data were collected from the medical records. All procedures were performed after written informed consent was obtained.
Propensity score matching was applied at a 1:1 HSP-to-EMR ratio using greedy matching with a calliper width of 0.20 of the standard deviation of the logit transformation for the estimated propensity score[11,12]. The propensity score was estimated using the multivariate logistic regression model, which included the following: age (continuous), sex (male/female), antithrombotic drug use (no/yes), size (continuous), location (right-sided colon/Left-sided colon/rectum), macroscopic type (0-Is/0-Isp/0-IIa), and endoscopist experience (expert/nonexpert) as explanatory variables without considering outcome variables. To evaluate the balance of patient characteristics between the HSP and EMR groups, we calculated standardized differences and created histograms and box plots. The chi-square and Mann–Whitney U tests were used to compare patient characteristics between groups. For treatment outcomes, univariable analyses were performed using the χ2 test in all enrolled lesions and the McNemar test in pair-matched lesions. All P values were two-sided with a significance level of 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) and SAS (version 9.4) graphical user interface for R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R commander (version 2.7-0), designed to add statistical functions frequently used in biostatistics[13]. The statistical methods of this study were reviewed by Masashi Wakabayashi from the Biostatistics Division of Center for Research Administration and Support at National Cancer Center.
Of the 711 consecutive lesions in 602 patients, 463 patients were enrolled, and 565 Lesions in them were analysed in the present study. Of the 565 Lesions, 440 in 346 patients were resected via HSP; 125 in 117 patients were resected via EMR. A flowchart of patient enrolment is shown in Figure 1. Patient characteristics before and after propensity score matching are shown in Table 1. There was a significant difference in antithrombotic drug use (P < 0.05), lesion size (P < 0.01), location (P < 0.01), and macroscopic type (P < 0.05) between the HSP and EMR groups in the original cohort. After propensity score matching, 117/440 Lesions in the HSP group and 117/125 in the EMR group were selected. Nearly all baseline characteristics were balanced (Table 1; standardized differences < 0.1 between HSP and EMR).
| Original cohort | Propensity score-matched cohort | |||||||
| HSP | EMR | P value | Standardized difference | HSP | EMR | P value | Standardized difference | |
| No. of lesions | 440 | 125 | 117 | 117 | ||||
| Age (yr), mean ± SD (range) | 69.7 ± 9.47 (36–90) | 69.8 ± 8.36 (46–89) | 0.53 | 0.013 | 69.5 ± 8.34 (43–85) | 70.1 ± 8.78 (46–89) | 0.67 | 0.072 |
| Sex, n (%) | 0.83 | 0.022 | 1.00 | 0 | ||||
| Male | 328 (74.6) | 92 (73.6) | 87 (74.4) | 87 (74.4) | ||||
| Female | 112 (25.5) | 33 (26.4) | 30 (25.6) | 30 (25.6) | ||||
| Antithrombotic drugs use, n (%) | 0.031 | 0.21 | 0.87 | 0.021 | ||||
| Yes | 66 (15.0) | 29 (23.2) | 25 (21.4) | 26 (22.2) | ||||
| No | 374 (85.0) | 96 (76.8) | 92 (78.6) | 91 (77.8) | ||||
| Size (mm), mean ± SD (range) | 11.2 ± 1.76 (10–15) | 12.7 ± 2.02 (10–15) | < 0.01 | 0.77 | 12.4 ± 2.08 (10–15) | 12.5 ± 2.01 (10–15) | 0.72 | 0.054 |
| Location, n (%) | < 0.01 | 0.38 | 0.94 | 0.054 | ||||
| Right-sided colon | 240 (54.6) | 63 (50.4) | 61 (52.1) | 63 (53.9) | ||||
| Left-sided colon | 155 (35.2) | 36 (28.8) | 32 (27.4) | 32 (27.4) | ||||
| Rectum | 45 (10.2) | 26 (20.8) | 24 (20.5) | 22 (18.8) | ||||
| Histological findings, n (%) | 0.087 | 0.26 | 0.081 | 0.23 | ||||
| SSL | 5 (1.1) | 0 (0) | 3 (2.6) | 0 (0) | ||||
| LGD/HGD | 434 (98.6) | 123 (98.4) | 114 (97.4) | 117 (100) | ||||
| T1a/T1b | 1 (0.2) | 2 (1.6) | 0 (0) | 0 (0) | ||||
| Macroscopic type, n (%) | 0.011 | 0.3 | 0.79 | 0.034 | ||||
| Type 0–Is, 0–Isp | 176 (40.0) | 66 (52.8) | 62 (53.0) | 60 (51.3) | ||||
| Type 0–IIa | 264 (60.0) | 59 (47.2) | 55 (47.0) | 57 (48.7) | ||||
| Endoscopist, n (%) | 0.053 | 0.2 | 0.51 | 0.086 | ||||
| Expert | 186 (42.3) | 65 (52.0) | 63 (53.9) | 58 (49.6) | ||||
| Non-expert | 254 (57.7) | 60 (48.0) | 54 (46.2) | 59 (50.4) | ||||
In the original cohort, the en bloc and R0 resection rates between the HSP and EMR groups were similar [94.8% (417/440) vs 92.8% (116/125), P = 0.40; 81.6% (359/440) vs 80.8% (101/125), P = 0.84, respectively] (Table 2). In the propensity score-matched cohort, the en bloc resection rate remained similar between the HSP and EMR groups [93.2% (109/117) vs 92.3% (108/117), P = 0.81]. Furthermore, there was no significant difference between the HSP and EMR groups in the R0 resection rate [77.8% (91/117) vs 80.3% (94/117), P = 0.64] (Table 2). All patients who underwent RX/R1 resection were classified as HMX/HM1, and there were no patients with VMX/VM1. There was no significant difference in the R0 resection rate between the HSP and EMR groups according to size, macroscopic type, and endoscopist experience. However, in terms of rectal location, the R0 resection rate was significantly higher in the EMR group than in the HSP group [75.0% (18/24) vs 100% (22/22), P = 0.022] (Table 3).
| Original cohort | Propensity score-matched cohort | |||||
| HSP | EMR | P value | HSP | EMR | P value | |
| No. of lesions | 440 | 125 | 117 | 117 | ||
| En bloc resection, n, % (95%CI) | 417, 94.8 (92.3-96.7) | 116, 92.8 (86.8-96.7) | 0.4 | 109, 93.2 (87.0-97.0) | 108, 92.3 (85.9-96.4) | 0.81 |
| Piecemeal resection, n, % (95%CI) | 23, 5.2 (3.3-7.7) | 9, 7.2 (3.3-13.2) | 8, 6.8 (3.0-13.0) | 9, 7.7 (3.6-14.1) | ||
| R0 resection, n, % (95%CI) | 359, 81.6 (77.6-85.1) | 101, 80.8 (72.8-87.3) | 0.84 | 91, 77.8 (69.2-84.9) | 94, 80.3 (72.0-87.1) | 0.64 |
| RX/R1 resection, n, % (95%CI) | 81, 18.4 (14.9-22.4) | 24, 19.2 (12.7-27.2) | 26, 22.2 (15.1-30.8) | 23, 19.7 (12.9-28.0) | ||
| Delayed bleeding, n, % (95%CI) | 5, 1.1 (0.4-2.6) | 2, 1.6 (0.2-5.7) | 0.68 | 2, 1.7 (0.2-6.0) | 2, 1.7 (0.2-6.0) | 1.00 |
| Perforation, n, % (95%CI) | 0, 0 (0-0.8) | 1, 0.8 (0.0-4.4) | 0.06 | 0, 0 (0-3.1) | 1, 0.9 (0-4.7) | N/A |
| R0 resection rate, %, n/n (95%CI) | |||
| HSP | EMR | P value | |
| Total | 77.8, 91/117 (69.2-84.9) | 80.3, 94/117 (72.0-87.1) | |
| Size | |||
| 10–12 mm | 77.9, 53/68 (66.2-87.1) | 82.9, 58/70 (72.0-90.8) | 0.52 |
| 13–15 mm | 77.6, 38/49 (63.4-88.2) | 76.6, 36/47 (62.0-87.7) | 1.00 |
| Macroscopic type | |||
| Type 0-Is, 0-Isp | 77.4, 48/62 (65.0-87.1) | 86.7, 52/60 (75.4-94.1) | 0.24 |
| Type 0-IIa | 78.2, 43/55 (65.0-88.2) | 73.7, 42/57 (60.3-84.5) | 0.66 |
| Location | |||
| Right-sided colon | 75.4, 46/61 (62.7-85.5) | 74.6, 47/63 (62.1-84.7) | 1.00 |
| Left-sided colon | 84.4, 27/32 (67.2-94.7) | 78.1, 25/32 (60.0-90.7) | 0.75 |
| Rectum | 75.0, 18/24 (53.3-90.2) | 100, 22/22 (84.6-100) | 0.022 |
| Endoscopist | |||
| Expert | 81.0, 51/63 (69.1-89.8) | 82.8, 48/58 (70.6-91.4) | 0.82 |
| Non-expert | 74.1, 40/54 (60.3-85.0) | 78.0, 46/59 (65.3-87.7) | 0.66 |
In the original cohort, there was no significant difference in the incidence of delayed bleeding between the HSP and EMR groups [1.1% (5/440) vs 1.7% (2/125), P = 0.68]. Perforation occurred in the EMR group [0.9% (1/125); P = 0.060] but not in the HSP group (Table 2). In the propensity score-matched cohort, the incidence of delayed bleeding was similar in both groups [1.7% (2/117)]. Perforation occurred in the EMR group [0.9% (1/117)] but not in the HSP group. One case of perforation in the EMR group occurred intraoperatively.
This study is the first to compare treatment outcomes between HSP and EMR using a bipolar snare for nonpedunculated colorectal lesions measuring 10–15 mm in clinical practice. Two important results were obtained in this study. First, there was no significant difference in en bloc and R0 resection rates between the HSP and EMR groups. Second, the incidence of adverse events was similar in both groups; however, perforation occurred only in the EMR group. These results suggest that comparable treatment efficiency and safety may be obtained when resecting nonpedunculated colorectal lesions measuring 10–15 mm using a bipolar snare and even without submucosal injection.
Several ER methods have been widely adopted for colorectal lesions, including cold snare polypectomy (CSP), HSP, and EMR. The European Society of Gastrointestinal Endoscopy clinical guidelines proposed selecting ER methods according to the size and macroscopic type of lesions. For nonpedunculated lesions, CSP is recommended for lesions ≤ 9 mm, HSP or EMR for lesions 10–19 mm, and EMR or piecemeal EMR for lesions ≥ 20 mm[14,15]. For HSP and EMR, electrosurgical resection with a monopolar snare is generally performed. However, there is a risk of thermal damage because high-frequency current derived from the monopolar snare flows to the patient’s plate through the deep part of the patient’s living tissue[7]. In fact, in treatment outcomes of ER using a monopolar snare, perforation occurs in approximately 1.3%–2.8% of cases[16,17]. Therefore, when using a monopolar snare during ER for 10–19 mm colorectal lesions, EMR is recommended instead of HSP as it can reduce the risk of deep thermal damage[14].
We recently reported on endoscopic procedures using either a monopolar or bipolar snare in an ex vivo porcine model[8]. When 10–15-mm lesions were resected by HSP, the muscularis propria was thermally damaged when a monopolar snare was used but not when a bipolar snare was used. Therefore, greater safety can be expected even without submucosal injection when a bipolar snare is used. However, no studies compared the safety and effectivity between HSP and EMR using a bipolar snare in clinical practice. This study revealed that HSP had a similar complication rate as EMR when a bipolar snare was used for 10–15-mm nonpedunculated colorectal lesions. Notably, perforation did not occur following HSP, suggesting a better safety profile when using a bipolar snare over a monopolar snare. The delayed bleeding rate was also reported to be 1.4%–3.1% for EMR[16,17] and 5.3% for HSP[18] when a monopolar snare was used. However, in this study, the delayed bleeding rate for both HSP and EMR when a bipolar snare was used was 1.7%, which is relatively lower than previously reported values. Saraya et al[19] also reported that HSP and EMR using a bipolar snare had a similar or lower risk of delayed bleeding and perforation than EMR using a monopolar snare[19]. Although the present study had a small number of cases, HSP did not cause perforation and resulted in less delayed bleeding, suggesting that HSP using a bipolar snare might be an option that results in fewer complications than EMR using a monopolar or bipolar snare.
For ER of medium-sized lesions ≥ 10 mm, determining whether en bloc or R0 resection is possible is important for ER method selection. Piecemeal ER and RX/R1 resection are known risk factors for local recurrence after ER[20,21]. As the lesion grows, piecemeal endoscopic and RX/R1 resection rates increase, leading to an increased risk of local recurrence. However, the R0 resection rate is equivalent between HSP and EMR using a monopolar snare for colorectal lesions measuring 10–14 mm[22]. Therefore, the en bloc and R0 resection rates are equivalent between HSP and EMR with a bipolar snare, although no studies have been conducted to confirm this. In this study, for nonpedunculated colorectal lesions measuring 10–15 mm, HSP resulted in en bloc and R0 resection rates equivalent to those of EMR with a bipolar snare. Meanwhile, the en bloc resection rate was as high as ≥ 90% in both groups, and the R0 resection rate was approximately 80%, lower than previously reported values[22]. This could be because resection at our hospital is performed under the coagulation mode. Also, given the characteristics of ER using a bipolar snare, it is difficult to horizontally evaluate the pathological specimen due to crushing by cauterization in lesions where the resection margin is close to the lesion edge. However, resection in coagulation mode may have contributed to a lower rate of delayed bleeding than previously reported because of the ability to coagulate the vessel[16-18].
The following points contributed to this innovative study design. First, patient and lesion backgrounds were matched using property score matching. The size, location, and macroscopic type of the lesions and endoscopic experience are known factors related to en bloc and R0 resection, and oral intake of antithrombotic agents is related to delayed bleeding[23-26]. This is the only study to balance the many confounding factors that may affect the estimation of results by property score matching and to compare treatment outcomes between HSP and EMR with a bipolar snare.
Second, only lesions diagnosed as JNET type 2A using magnifying observation combined with narrow-band imaging were included. In the qualitative diagnosis of colorectal lesions, those diagnosed as JNET type 2A can be diagnosed as adenomas or intramucosal cancers with high accuracy[27]. On the other hand, lesions diagnosed as JNET type 2B or 3 are generally known to develop into T1 cancer more frequently, resulting in bias wherein endoscopists who perform therapeutic endoscopy more carefully select ER methods that secure lesion margins. In this study, only JNET type 2A lesions potentially avoided bias due to differences in ER methods based on the preoperative diagnosis. Therefore, although the results of this study can be applied to adenomas and intramucosal cancers, it is unclear whether ER for T1 cancers will provide similar results. Third, we analysed the R0 resection rate as a factor between HSP and EMR. There was no difference in the R0 resection rate between HSP and EMR for most of the factors. However, the R0 resection rate for lesions in the rectum was better for EMR. No study has reported a change in the R0 resection rate with or without submucosal injection for rectal lesions. However, the R0 resection rate was reportedly significantly better in EMR than in HSP for the left colon[22]. Additionally, it can be difficult to diagnose a range of lesions due to factors such as ‘skirt’ in the rectum compared to the colon[28]. In rectal lesions of a difficult-to-identify extent with ‘skirt’, EMR provides wider margins, which may lead to fewer leftovers and improved R0 resection rates.
This study had some limitations. First, this was a single-centre retrospective study. In particular, the sample size was not large enough to prove the non-inferiority of HSP to EMR for any of the outcomes. Furthermore, since the treatment choices for HSP and EMR were left to the discretion of each endoscopist, the present results may be strongly influenced by the skill of a specific endoscopist. They may have been affected by imbalanced confounding factors that were not included in the analysis between the two groups. Second, there was no clear standard for measuring lesion size. Each endoscopist judged the size of the lesion by comparing it with the size of the snare, suggesting inaccurate lesion measurements. Third, the size of the bipolar snare used for resection was not specified, and the effects on the results cannot be denied. To eliminate these biases, conducting a large-cohort, multicentre, prospective, randomized controlled trial after clarifying ER methods, size of the snare, and lesion measurement methods is desirable.
When using a bipolar snare, HSP has comparable treatment outcomes to EMR for nonpedunculated colorectal lesions measuring 10–15 mm. This suggests that the use of a bipolar snare may replace submucosal injection and may enable a more accessible ER while maintaining efficiency and safety.
In endoscopic resection (ER) of colorectal lesions, it is important to develop resection methods that enable efficient and safe resection. Most recently, we have reported in ex vivo porcine model that endoscopic resection using bipolar snare for intermediate size lesions didn’t lead to thermal injury for the intrinsic muscle layer even without submucosal injection. Therefore, the bipolar ER for intermediate size colorectal lesions of 10-15 mm has the potential to provide prominent outcomes in an efficient and highly safe manner even without submucosal injection.
We would like to assess the treatment outcomes of the bipolar resection with and without submucosal injection.
The present study aims to compare the resection results of endoscopic mucosal resection (EMR), which refers to the resection following submucosal injection, and hot snare polypectomy (HSP), which refers to the resection with no submucosal injection, to evaluate the efficacy and safety of HSP with bipolar snare for 10-15 mm lesions.
We conducted the single-centre retrospective analysis of all 10-15 mm size colorectal lesions with a diagnosis of JNET Type 2A and resected by either EMR or HSP from January 2018 to June 2021. The target lesions were divided into two groups, HSP group and EMR group, and treatment outcomes and the adverse events were compared by conducting propensity score matching analysis.
Of the 565 lesions in 463 patients, 117 lesions each in the HSP and EMR groups were selected after propensity score matching. In the original cohort, there was a significant difference in antithrombotic drug use (P < 0.05), lesion size (P < 0.01), location (P < 0.01), and macroscopic type (P < 0.05) between the HSP and EMR groups. In the matched cohort, the en bloc resection rates were 93.2% (109/117) in the HSP group and 92.3% (108/117) in the EMR group, in which there was no significant difference (P = 0.81). Moreover, no significant difference was observed in the R0 resection rate [77.8% (91/117) vs 80.3% (94/117), P = 0.64]. The rates of delayed bleeding were comparable between the groups [1.7% (2/117)]. Perforation occurred in the EMR group [0.9% (1/117)] but not in the HSP group.
Using bipolar snare, ER of nonpedunculated 10–15 mm colorectal lesions may be performed safely and effectively, even without submucosal injection.
A large-cohort, multicentre, prospective, randomized controlled trial is warranted to prove the non-inferiority of bipolar HSP to bipolar EMR in treatment outcomes with ER of nonpedunculated 10–15 mm colorectal lesions.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 13046] [Article Influence: 1304.6] [Reference Citation Analysis (3)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3173] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 3. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2386] [Article Influence: 170.4] [Reference Citation Analysis (2)] |
| 4. | Kim SY, Kim HS, Park HJ. Adverse events related to colonoscopy: Global trends and future challenges. World J Gastroenterol. 2019;25:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (9)] |
| 5. | Paszat LF, Sutradhar R, Luo J, Rabeneck L, Tinmouth J. Perforation and post-polypectomy bleeding complicating colonoscopy in a population-based screening program. Endosc Int Open. 2021;9:E637-E645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Derbyshire E, Hungin P, Nickerson C, Rutter MD. Colonoscopic perforations in the English National Health Service Bowel Cancer Screening Programme. Endoscopy. 2018;50:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Rey JF, Beilenhoff U, Neumann CS, Dumonceau JM; European Society of Gastrointestinal Endoscopy (ESGE). European Society of Gastrointestinal Endoscopy (ESGE) guideline: the use of electrosurgical units. Endoscopy. 2010;42:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Shinmura K, Ikematsu H, Kojima M, Nakamura H, Osera S, Yoda Y, Hori K, Oono Y, Ochiai A, Yano T. Safety of endoscopic procedures with monopolar vs bipolar instruments in an ex vivo porcine model. BMC Gastroenterol. 2020;20:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Digestive System Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2019. |
| 10. | Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019;3:175-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 499] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 11. | Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 12. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 8045] [Article Influence: 536.3] [Reference Citation Analysis (0)] |
| 13. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14478] [Article Influence: 1113.7] [Reference Citation Analysis (0)] |
| 14. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 797] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 15. | Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M, Akamatsu T, Tada K, Komeda Y, Iwatate M, Kawakami K, Nishikawa M, Watanabe D, Yamauchi A, Fukata N, Shimatani M, Ooi M, Fujita K, Sano Y, Kashida H, Hirose S, Iwagami H, Uedo N, Teramukai S, Tanaka K. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 16. | van Hattem WA, Shahidi N, Vosko S, Hartley I, Britto K, Sidhu M, Bar-Yishay I, Schoeman S, Tate DJ, Byth K, Hewett DG, Pellisé M, Hourigan LF, Moss A, Tutticci N, Bourke MJ. Piecemeal cold snare polypectomy vs conventional endoscopic mucosal resection for large sessile serrated lesions: a retrospective comparison across two successive periods. Gut. 2021;70:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 17. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection vs endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 18. | Ket SN, Mangira D, Ng A, Tjandra D, Koo JH, La Nauze R, Metz A, Moss A, Brown G. Complications of cold vs hot snare polypectomy of 10-20 mm polyps: A retrospective cohort study. JGH Open. 2020;4:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Saraya T, Ikematsu H, Fu KI, Tsunoda C, Yoda Y, Oono Y, Kojima T, Yano T, Horimatsu T, Sano Y, Kaneko K. Evaluation of complications related to therapeutic colonoscopy using the bipolar snare. Surg Endosc. 2012;26:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Yoshida N, Fukumoto K, Hasegawa D, Inagaki Y, Inoue K, Hirose R, Dohi O, Ogiso K, Murakami T, Tomie A, Okuda K, Inada Y, Okuda T, Rani RA, Morinaga Y, Kishimoto M, Itoh Y. Recurrence rate and lesions characteristics after cold snare polypectomy of high-grade dysplasia and T1 Lesions: A multicenter analysis. J Gastroenterol Hepatol. 2021;36:3337-3344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Horiuchi A, Makino T, Kajiyama M, Tanaka N, Sano K, Graham DY. Comparison between endoscopic mucosal resection and hot snare resection of large nonpedunculated colorectal polyps: a randomized trial. Endoscopy. 2016;48:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Ito A, Suga T, Ota H, Tateiwa N, Matsumoto A, Tanaka E. Resection depth and layer of cold snare polypectomy vs endoscopic mucosal resection. J Gastroenterol. 2018;53:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 25. | Tavakkoli A, Law RJ, Bedi AO, Prabhu A, Hiatt T, Anderson MA, Wamsteker EJ, Elmunzer BJ, Piraka CR, Scheiman JM, Elta GH, Kwon RS. Specialist Endoscopists Are Associated with a Decreased Risk of Incomplete Polyp Resection During Endoscopic Mucosal Resection in the Colon. Dig Dis Sci. 2017;62:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kishida Y, Hotta K, Imai K, Ito S, Yoshida M, Kawata N, Tanaka M, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Ono H. Risk Analysis of Colorectal Post-Polypectomy Bleeding Due to Antithrombotic Agent. Digestion. 2019;99:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Hirata D, Kashida H, Iwatate M, Tochio T, Teramoto A, Sano Y, Kudo M. Effective use of the Japan Narrow Band Imaging Expert Team classification based on diagnostic performance and confidence level. World J Clin Cases. 2019;7:2658-2665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Miyamoto H, Ikematsu H, Fujii S, Osera S, Odagaki T, Oono Y, Yano T, Ochiai A, Sasaki Y, Kaneko K. Clinicopathological differences of laterally spreading tumors arising in the colon and rectum. Int J Colorectal Dis. 2014;29:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martino A, Italy; Nagami Y, Japan S-Editor: Li L L-Editor: A P-Editor: Li L