Published online Jun 21, 2023. doi: 10.3748/wjg.v29.i23.3678
Peer-review started: March 22, 2023
First decision: April 14, 2023
Revised: April 28, 2023
Accepted: May 22, 2023
Article in press: June 21, 2023
Published online: June 21, 2023
Processing time: 85 Days and 20.5 Hours
The lymphocyte-to-white blood cell ratio (LWR) is a blood marker of the systemic inflammatory response. The prognostic value of LWR in patients with hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) remains unclear.
To explore whether LWR could stratify the risk of poor outcomes in HBV-ACLF patients.
This study was conducted by recruiting 330 patients with HBV-ACLF at the Department of Gastroenterology in a large tertiary hospital. Patients were divided into survivor and non-survivor groups according to their 28-d prognosis. The independent risk factors for 28-d mortality were calculated by univariate and multivariate Cox regression analyses. Patients were divided into low- and high-LWR groups according to the cutoff values. Kaplan-Meier analysis was performed according to the level of LWR.
During the 28-d follow-up time, 135 patients died, and the mortality rate was 40.90%. The LWR level in non-surviving patients was significantly decreased compared to that in surviving patients. A lower LWR level was an independent risk factor for poor 28-d outcomes (hazard ratio = 0.052, 95% confidence interval: 0.005-0.535). The LWR level was significantly negatively correlated with the Child-Turcotte-Pugh, model for end-stage liver disease, and Chinese Group on the Study of Severe Hepatitis B-ACLF II scores. In addition, the 28-d mortality was higher for patients with LWR < 0.11 than for those with LWR ≥ 0.11.
LWR may serve as a simple and useful tool for stratifying the risk of poor 28-d outcomes in HBV-ACLF patients.
Core Tip: This manuscript introduced a simple and effective inflammatory marker, the lymphocyte-to-white blood cell ratio (LWR). Our study found that a lower LWR level was associated with poor 28-d outcomes in hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) patients. It may serve as a simple and useful tool for stratifying the risk of poor 28-d outcomes in HBV-ACLF patients, and it may be helpful in guiding a clinician to treatment allocation and assist in the prediction of prognosis.
- Citation: Zhang Y, Chen P, Zhu X. Lymphocyte-to-white blood cell ratio is associated with outcome in patients with hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol 2023; 29(23): 3678-3687
- URL: https://www.wjgnet.com/1007-9327/full/v29/i23/3678.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i23.3678
Acute-on-chronic liver failure (ACLF) is a life-threatening clinically complex syndrome characterized by high short-term mortality due to different combinations of multiorgan failures[1-3]. The main etiology of ACLF is hepatitis B virus (HBV) infection, with HBV-associated ACLF (HBV-ACLF) accounting for more than 70% of ACLF cases in most Asian countries[4]. The clinical characteristics of HBV-ACLF patients differ from those of alcoholic-related ACLF patients in Western countries, wherein coagulation and liver failure are the most common types of organ failure[5]. Early prediction of the prognosis of HBV-ACLF is important for clinical management and diminishing mortality. However, current score models are based on complicated assessments of organ failure. Therefore, it is necessary to identify an accurate and simple indicator to detect high-risk patients.
A growing body of research evidence suggests that HBV-ACLF is associated with systemic inflammation and immune paralysis[6,7]. In many recent studies, inflammation-related markers such as the platelet (PLT) to white blood cell ratio, neutrophil-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio have received increasing attention in clinical settings and are used in predicting the prognosis of HBV-ACLF[8-10]. The lymphocyte-to-white blood cell ratio (LWR) is a blood marker of the systemic inflammatory response. Studies have suggested that LWR has good prognostic value for patients with cancer, infective endocarditis and COVID-19[11-13]. However, the prognostic role of LWR in HBV-ACLF patients remains unclear. Therefore, our study aims to reveal whether LWR can risk-stratify poor prognosis in HBV-ACLF patients.
A total of 330 patients diagnosed with HBV-ACLF were retrospectively included from May 2014 to February 2021 at the Department of Gastroenterology, the First Affiliated Hospital of Nanchang University. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Chronic liver disease due to HBV infection; and (3) HBV-ACLF diagnosed based on the diagnostic guidelines for liver failure established in 2019[14]. The exclusion criteria were as follows: (1) Coinfection with hepatitis A/C/D/E virus; (2) Other etiologies such as drugs, autoimmunity, alcohol, or toxins that may contribute to HBV-ACLF; (3) Complicated with hepatocellular carcinoma; (4) Human immunodeficiency virus infection; and (5) Loss to follow-up. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Nanchang University.
Demographic information and clinical data were comprehensively collected by searching medical records. Laboratory blood tests were measured in the first 24-h period on admission. LWR was computed as the lymphocyte count (× 109/L) divided by the white blood cell count (× 109/L)[12]. The Child-Turcotte-Pugh (CTP), model for end-stage liver disease (MELD) and Chinese Group on the Study of Severe Hepatitis B-ACLF II (COSSHACLFII) scores were calculated as previously described[15-17]. All patients were followed from their diagnosis until either their death or the end of the 28-d follow-up period. The survival rates at 28 d were obtained from patients’ medical records or by telephone calls with the patients or their kinsfolks.
Chronic HBV infection was defined by the presence of hepatitis B surface antigen for > 6 mo[18]. HBV-ACLF was defined according to the Asian Pacific Association for the Study of the Liver criteria in 2019: (1) Serum bilirubin ≥ 5 mg/dL; (2) International normalized ratio (INR) ≥ 1.5 or prothrombin activity < 40%; (3) Complicated within 4 wk by clinical ascites and/or encephalopathy in patients with pre-existing chronic liver diseases (diagnosed or undiagnosed); and (4) High 28-d mortality[14].
Statistical analysis was performed using the SPSS 24.0 statistical package (SPSS Inc., Chicago, IL), R software version 4.1.0 (http://www.r-project.org/), and X-tile software (Version 3.6.1, Yale University, New Haven, CT, United States). Continuous variables were compared using the t test or the Mann-Whitney U test, whereas categorical variables were compared using the chi-square test or Fisher’s exact test. Univariate analysis and multivariate Cox proportional hazards models were performed to identify whether LWR was related to poor outcomes. The optimal cutoff value of LWR was determined by using X-tile. The Kaplan-Meier survival curve was generated by the “survival” and “survminer” packages in R software. All statistical tests were two-sided with a statistical significance level set at P values < 0.05.
The baseline characteristics of the patients are summarized in Table 1. A total of 330 patients with HBV-ACLF were recruited. In the cohort, the average age of patients was 49.68 ± 12.39 years, and approximately 83.9% of patients were male. The HBV-ACLF patients were divided into survivor and non-survivor groups according to the prognosis at 28 d. At follow-up, the age, prothrombin time (PT), INR, bilirubin, CTP score (CTPs), MELD score (MELDs), and COSSHACLFII score (COSSHACLFIIs) of the non-survivors were significantly higher than those of the survivors (P < 0.05). However, the PLT count and LWR level of the non-survivors were significantly lower than those of the survivors (P < 0.05). In addition, there were no significant differences in sex, costs, hemoglobin, albumin, creatinine, blood urea nitrogen (BUN), or serum Na between the non-survivor group and survivor group (P > 0.05).
| All patients (n = 330) | Survivor patients (n = 195) | Non-survivor patients (n = 135) | P value | |
| Age (yr) | 49.68 ± 12.39 | 47.91 ± 12.12 | 52.23 ± 12.39 | 0.002 |
| Male, n (%) | 277 (83.9) | 162 (83.1) | 115 (85.2) | 0.068 |
| Costs (dollars) | 10133.88 (5886.57-15955.07) | 10734.72 (5958.59-16913.77) | 9107.89 (5727.32-14945.14) | 0.065 |
| Ascites, n (%) | 0.016 | |||
| Mild | 158 (47.9) | 104 (53.3) | 54 (40.0) | |
| Medium | 102 (30.9) | 59 (30.3) | 43 (31.9) | |
| Severe | 70 (21.2) | 32 (16.4) | 38 (28.1) | |
| PLT (109/L) | 108.00 (72.75-144.25) | 115.00 (82.00-148.00) | 89.00 (57.00-138.00) | 0.004 |
| PT (s) | 22.70 (19.30-29.25) | 21.10 (18.90-25.20) | 25.40 (21.80-32.80) | < 0.001 |
| INR | 2.01 (1.74-2.64) | 1.88 (1.69-2.30) | 2.4 (1.94-3.07) | < 0.001 |
| Hemoglobin (g/L) | 122.00 (102.00-136.00) | 123.00 (107.00-137.00) | 121.00 (94.00-135.00) | 0.166 |
| Bilirubin (μmol/L) | 312.91 ± 135.85 | 299.15 ± 126.15 | 332.79 ± 146.96 | 0.027 |
| Albumin (g/L) | 31.40 (28.20-34.25) | 31.50 (28.20-34.60) | 31.10 (28.20-33.30) | 0.151 |
| Creatinine (μmol/L) | 66.60 (57.08-84.73) | 65.60 (57.00-81.00) | 68.30 (57.50-91.90) | 0.294 |
| BUN (mmol/L) | 4.00 (2.80-6.10) | 4.00 (2.80-5.50) | 4.10 (3.00-7.60) | 0.074 |
| Serum Na (mmol/L) | 137.00 (133.30-139.10) | 137.00 (133.20-139.00) | 136.90 (133.30-139.10) | 0.882 |
| LWR | 0.17 (0.11-0.23) | 0.19 (0.12-0.25) | 0.13 (0.08-0.20) | < 0.001 |
| CTPs | 11.00 (10.00-12.00) | 11.00 (10.00-12.00) | 12.00 (11.00-13.00) | < 0.001 |
| MELDs | 23.17 (20.03-27.27) | 21.59 (18.86-25.46) | 25.04 (21.78-29.10) | < 0.001 |
| COSSHACLFIIs | 7.18 (6.54-8.12) | 6.80 (6.30-7.37) | 7.95 (7.20-8.70) | < 0.001 |
The association between the LWR level and 28-d mortality is shown in Table 2. In univariate analysis, age, PLT, PT, hemoglobin, bilirubin, BUN, and LWR were significant factors for 28-d mortality (all P < 0.05). In multivariable analysis, the results showed that age, PT, bilirubin, and LWR were associated with short-term mortality [hazard ratio (HR) = 1.015, 95% confidence interval (CI): 1.001-1.030; HR = 1.028, 95%CI: 1.015-1.042; HR = 1.001, 95%CI: 1.000-1.003; HR = 0.052, 95%CI: 0.005-0.535, respectively].
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.021 (1.007-1.035) | 0.002 | 1.015 (1.001-1.030) | 0.037 |
| Male sex | 1.202 (0.747-1.932) | 0.448 | ||
| PLT (109/L) | 0.997 (0.994-0.999) | 0.046 | ||
| PT (s) | 1.020 (1.011-1.030) | < 0.001 | 1.028 (1.015-1.042) | < 0.001 |
| INR | 1.042 (0.993-1.094) | 0.093 | ||
| Hemoglobin (g/L) | 0.993 (0.987-0.999) | 0.027 | ||
| Bilirubin (μmol/L) | 1.001 (1.000-1.003) | 0.025 | 1.001 (1.000-1.003) | 0.041 |
| Albumin (g/L) | 0.968 (0.933-1.005) | 0.091 | ||
| Creatinine (μmol/L) | 1.001 (1.000-1.001) | 0.179 | ||
| BUN (mmol/L) | 1.036 (1.014-1.058) | 0.001 | ||
| Serum Na (mmol/L) | 1.001 (0.999-1.003) | 0.249 | ||
| LWR | 0.011 (0.001-0.088) | < 0.001 | 0.052 (0.005-0.535) | 0.013 |
Next, we investigated the correlation between LWR levels and other score models, including CTPs, MELDs and COSSHACLFIIs. As shown in Figures 1A-C, LWR levels were significantly correlated with the CTPs, MELDs and COSSHACLFIIs (r = -0.29, P < 0.001; r = -0.31, P < 0.001; r = -0.49, P < 0.001, respectively).
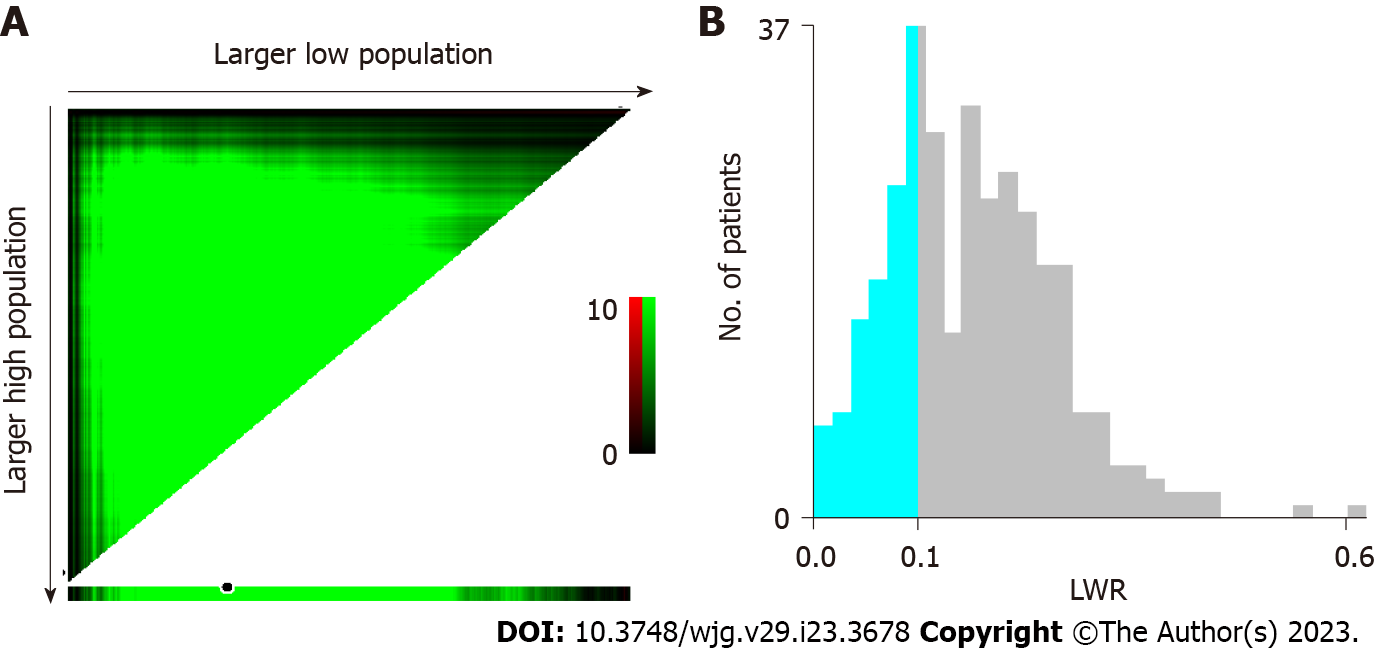
The median LMR of HBV-ACLF patients was 0.17 (0.11-0.23), and X-tile software was used to determine the optimal cutoff values for LWR for 28-d mortality. Consequently, the threshold of 0.11 enabled us to distinguish favorable and poor outcomes that were most significant in HBV-ACLF patients (Figure 2). HBV-ACLF patients were stratified into low LWR (LWR < 0.11) and high LWR (LWR ≥ 0.11) groups according to the cutoff values. As shown in Table 3, the patients in the group with LWR < 0.11 had an older age, lower PLT count, higher PT, higher INR, lower hemoglobin, lower albumin, higher creatinine, higher BUN, higher CTPs, higher MELDs, higher COSSHACLFIIs, and significantly shorter survival rate than those in the group with LWR ≥ 0.11.
| Low LWR level (n = 77) | High LWR level (n = 253) | P value | |
| Age (yr) | 52.99 ± 12.57 | 48.67 ± 12.19 | 0.007 |
| Male, n (%) | 59 (76.6) | 218 (86.2) | 0.046 |
| Costs (dollars) | 7625.18 (3899.37-12070.55) | 10984.59 (6639.92-16693.10) | < 0.001 |
| Ascites, n (%) | 0.007 | ||
| Mild | 25 (32.5) | 133 (52.6) | |
| Medium | 29 (37.7) | 73 (28.9) | |
| Severe | 23 (29.9) | 47 (18.6) | |
| PLT (109/L) | 86.00 (55.50-138.50) | 110.00 (79.50-145.50) | 0.039 |
| PT (s) | 23.20 (19.70-33.50) | 22.40 (19.20-28.00) | 0.016 |
| INR | 2.13 (1.76-3.11) | 1.99 (1.73-2.54) | 0.018 |
| Hemoglobin (g/L) | 109.00 (89.50-125.50) | 125.00 (108.00-139.00) | < 0.001 |
| Bilirubin (μmol/L) | 331.52 ± 153.46 | 307.25 ± 129.82 | 0.170 |
| Albumin (g/L) | 30.10 (26.30-32.50) | 31.80 (28.85-34.55) | < 0.001 |
| Creatinine (μmol/L) | 82.40 (58.40-126.10) | 64.80 (56.85-77.95) | < 0.001 |
| BUN (mmol/L) | 7.00 (4.00-11.10) | 3.70 (2.70-5.20) | < 0.001 |
| Serum Na (mmol/L) | 135.30 (131.50-139.05) | 137.20 (133.90-139.10) | 0.086 |
| CTPs | 12.00 (11.00-13.00) | 11.00 (10.00-12.00) | < 0.001 |
| MELDs | 25.79 (22.52-30.91) | 22.44 (19.55-26.13) | < 0.001 |
| COSSHACLFIIs | 8.11 (7.26-9.06) | 6.94 (6.40-7.79) | < 0.001 |
| 28-d mortality, n (%) | 46 (59.7) | 89 (35.2) | < 0.001 |
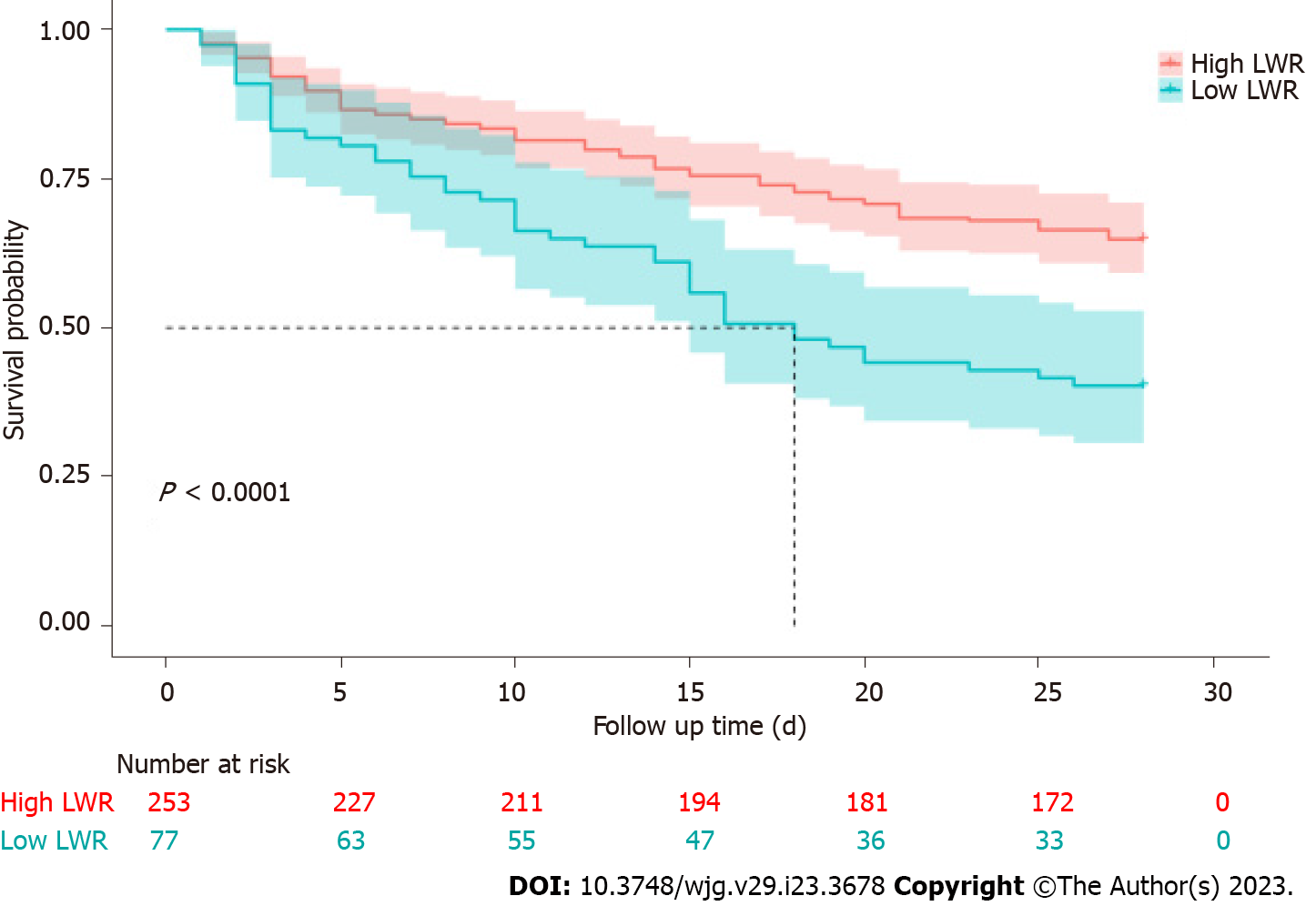
As shown above, the patients with LWR < 0.11 had higher 28-d mortality than the high LWR group (P < 0.05). To confirm the association of the LWR level and 28-d outcomes in detail, Kaplan-Meier analysis was performed to assess LWR in HBV-ACLF patients, and patients with low LWR levels had a worse outcome than those with high LWR levels (Figure 3).
In our study, we found that a low LWR level was an independent prognostic factor related to poor 28-d outcomes in patients with HBV-ACLF. Patients with LWR < 0.11 had higher 28-d mortality than those with high LWR levels.
Systemic inflammation plays an important role in the development of HBV-ACLF[19]. The activation of inflammatory cytokines causes organ hypoperfusion and systemic circulatory dysfunction, which increase the activation of coagulation, tissue microthrombosis, and the development of organ failure[20]. Many studies have indicated that the inflammatory response can be reflected by inflammatory markers such as lymphocytes, white blood cells, PLTs, and neutrophils[21,22]. The combination of these inflammatory markers, such as the neutrophil-lymphocyte ratio (NLR), platelet-to-white blood cell ratio (PWR), and LWR, has been confirmed as a prognostic marker in a variety of liver diseases. Bernsmeier et al[23] reported that the NLR was an independent risk factor in patients with acute decompensation (AD) cirrhosis. Kim et al[22] included 1670 AD patients from a prospective cohort and found that patients with a PWR ≤ 12.1 had a higher 28-d mortality than those with a PWR > 12.1, and a lower PWR level was a prognostic factor for 28-d adverse outcomes. Overall, these inflammation-based markers could be useful for stratifying the severity of liver disease.
Our study found that LWR levels were significantly decreased in non-survivor HBV-ACLF patients, and low LWR levels were an independent risk factor for 28-d mortality in HBV-ACLF patients. The decreased LWR levels may reflect an enhanced inflammatory response and/or impaired immune response, which may explain the results. A previous study confirmed that lymphocytes play a critical role in the body’s immune defense functions, immune response, and immune surveillance[12]. The elevated white blood cell count showed severe systemic inflammation, which was related to the prognosis of HBV-ACLF patients[24]. In addition, a recent study indicated that a low LWR level was an independent factor for poor outcomes in patients with decompensated liver cirrhosis, and the cut off value of LWR for 1 mo was 0.163. Patients with LWR < 0.163 had higher mortality than patients with LWR > 0.163[25]. Similar to this study, our research found that the cutoff value of LWR was 0.11, and patients with LWR < 0.11 had higher mortality than patients with LWR ≥ 0.11, and the results showed a significant negative correlation between LWR and CTPs, MELDs and COSSHACLFIIs. There are several limitations in our study. First, this is a single-center and retrospective study, which may cause selection biases. Second, lymphocytes and white blood cells were not tested dynamically during follow-up.
In conclusion, LWR is easily accessible and conveniently calculated, and it might be a good marker for identifying the risk of poor outcomes in HBV-ACLF patients. Therefore, our findings can help clinicians intervene in high-risk patients as early as possible.
The lymphocyte-to-white blood cell ratio (LWR) is a blood marker that reflects the systemic inflammatory response. The prognostic value of the LWR remains unclear in hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) patients.
It is necessary to find an easy and effective marker that can reflect the prognosis in HBV-ACLF patients, so we explored whether LWR can risk-stratify poor prognosis in HBV-ACLF patients.
This study aimed to investigate whether LWR could be an easy and useful marker that can identify the risk of poor outcomes in HBV-ACLF patients.
A total of 330 HBV-ACLF patients were included in this study, and patients were divided into survivor and non-survivor groups according to 28-d outcome. Univariate and multivariate Cox regression analyses were performed to select independent risk factors for 28-d mortality. The correlation test was performed to evaluate the correlation between LWR and Child-Turcotte-Pugh score (CTPs), model for end-stage liver disease score (MELDs), and Chinese Group on the Study of Severe Hepatitis B-ACLF II score (COSSHACLFIIs). The cutoff value of LWR was calculated by X-tile software, and Kaplan-Meier analysis was performed to assess the association of the LWR level and 28-d outcomes in HBV-ACLF patients.
Low LWR was an independent risk factor for 28-d mortality in patients with HBV-ACLF (hazard ratio = 0.052, 95% confidence interval: 0.005-0.535), and LWR levels were significantly negatively correlated with CTPs, MELDs and COSSHACLFIIs. Moreover, the patients with low LWR levels had a higher 28-d mortality than those with high LWR levels.
LWR is a simple, useful, and effective marker that could stratify the risk of 28-d adverse outcomes in HBV-ACLF patients.
Further large-sample and multicenter prospective studies should be conducted to verify and confirm the prognostic value of the LWR.
| 1. | Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3:100176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2269] [Article Influence: 174.5] [Reference Citation Analysis (6)] |
| 3. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Janicko M, Steib C, Reiberger T, Acevedo J, Gatti P, Bernal W, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Bendtsen F, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solè C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Özdogan OC, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Curto A, Pitarch C, Putignano A, Moreno E, Shawcross D, Aguilar F, Clària J, Ponzo P, Jansen C, Vitalis Z, Zaccherini G, Balogh B, Vargas V, Montagnese S, Alessandria C, Bernardi M, Ginès P, Jalan R, Moreau R, Angeli P, Arroyo V; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 4. | Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, Chen YP. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol. 2011;9:351-356.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (2)] |
| 6. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 583] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 7. | Bernsmeier C, van der Merwe S, Périanin A. Innate immune cells in cirrhosis. J Hepatol. 2020;73:186-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Jie Y, Gong J, Xiao C, Zhu S, Zhou W, Luo J, Chong Y, Hu B. Low Platelet to White Blood Cell Ratio Indicates Poor Prognosis for Acute-On-Chronic Liver Failure. Biomed Res Int. 2018;2018:7394904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Sun J, Guo H, Yu X, Zhu H, Zhang X, Yang J, Wang J, Qian Z, Shen Z, Mao R, Zhang J. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol. 2021;21:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Liu XY, He X, Cai M, Peng SQ. Prognostic Value of Complete Blood Cell Count-Derived Inflammatory Markers in Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Zhao W, Wang P, Jia H, Chen M, Gu X, Liu M, Zhang Z, Cheng W, Wu Z. Lymphocyte count or percentage: which can better predict the prognosis of advanced cancer patients following palliative care? BMC Cancer. 2017;17:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Pitre T, Jones A, Su J, Helmeczi W, Xu G, Lee C, Shamsuddin A, Mir A, MacGregor S, Duong M, Ho T, Beauchamp MK, Costa AP, Kruisselbrink R; COREG Investigators. Inflammatory biomarkers as independent prognosticators of 28-day mortality for COVID-19 patients admitted to general medicine or ICU wards: a retrospective cohort study. Intern Emerg Med. 2021;16:1573-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Zhang M, Ge Q, Qiao T, Wang Y, Xia X, Zhang X, Zhou J. Prognostic Value of Lymphocyte-to-White Blood Cell Ratio for In-Hospital Mortality in Infective Endocarditis Patients. Int J Clin Pract. 2022;2022:8667054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 642] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 15. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5821] [Article Influence: 109.8] [Reference Citation Analysis (2)] |
| 16. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 17. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3776] [Article Influence: 151.0] [Reference Citation Analysis (2)] |
| 18. | Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, Amarapurkar D, Cooksley G, Jafri W, Mohamed R, Hou JL, Chuang WL, Lesmana LA, Sollano JD, Suh DJ, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 19. | Premkumar M, Saxena P, Rangegowda D, Baweja S, Mirza R, Jain P, Bhatia P, Kumar G, Bihari C, Kalal C, Vyas T, Choudhury A, Sarin SK. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: An observational cohort study. Liver Int. 2019;39:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Lisman T, Luyendyk JP. Systemic inflammation and disorders of hemostasis in the AD-ACLF syndrome. J Hepatol. 2021;74:1264-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Cai YJ, Dong JJ, Dong JZ, Chen Y, Lin Z, Song M, Wang YQ, Chen YP, Shi KQ, Zhou MT. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45:1413-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Kim JH, Kim SE, Song DS, Kim HY, Yoon EL, Kim TH, Jung YK, Suk KT, Jun BG, Yim HJ, Kwon JH, Lee SW, Kang SH, Kim MY, Jeong SW, Jang JY, Yoo JJ, Kim SG, Jin YJ, Cheon GJ, Kim BS, Seo YS, Kim HS, Sinn DH, Chung WJ, Lee HA, Nam SW, Kim IH, Suh JI, Kim JH, Chae HB, Sohn JH, Cho JY, Kim YJ, Yang JM, Park JG, Kim W, Cho HC, Kim DJ. Platelet-to-White Blood Cell Ratio Is Associated with Adverse Outcomes in Cirrhotic Patients with Acute Deterioration. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Bernsmeier C, Cavazza A, Fatourou EM, Theocharidou E, Akintimehin A, Baumgartner B, Dhar A, Auzinger G, Thursz M, Bernal W, Wendon JA, Karvellas CJ, Antoniades CG, McPhail MJW. Leucocyte ratios are biomarkers of mortality in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. Aliment Pharmacol Ther. 2020;52:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Chen L, Zhang J, Lu T, Cai J, Zheng J, Yao J, Yi S, Li H, Chen G, Zhao H, Zhang Y, Yang Y. A nomogram to predict survival in patients with acute-on-chronic hepatitis B liver failure after liver transplantation. Ann Transl Med. 2021;9:555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Xie Y, He C, Wang W. A potential novel inflammation biomarker for predicting the prognosis of decompensated liver cirrhosis. Ann Med. 2022;54:3201-3210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Desai GS, India; Kao JT, Taiwan; Papazafiropoulou A, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ