Published online Jun 7, 2023. doi: 10.3748/wjg.v29.i21.3318
Peer-review started: February 27, 2023
First decision: March 14, 2023
Revised: March 23, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 7, 2023
Processing time: 94 Days and 5.3 Hours
Artifacts are common when using two-dimensional shear wave elastography (2-D SWE) to measure liver stiffness (LS), but they are poorly recognized.
To investigate the presence and influence of artifacts in 2-D SWE of liver.
We included 158 patients with chronic liver disease, who underwent 2-D SWE examination by a novice and an expert. A cross line at the center of the elastogram was drawn and was divided it into four locations: top-left, top-right, bottom-left, and bottom-right. The occurrence frequency of artifacts in different locations was compared. The influence of artifacts on the LS measurements was evaluated by comparing the elastogram with the most artifacts (EMA) and the elastogram with the least artifacts (ELA).
The percentage of elastograms with artifacts in the novice (51.7%) was signi
Artifacts are common when using 2-D SWE to measure LS, especially for the novice. Artifacts may lead to the overestimation of LS and reduce the repeatability and reliability of LS measurements.
Core Tip: Artifacts are common when using two-dimensional shear wave elastography (2-D SWE) to measure liver stiffness (LS), especially for the novice. We investigated the presence and influence of artifacts in 2-D SWE of liver. Our results showed artifacts were more likely to occur in the bottom-left corner of the elastogram. Artifacts may lead to the overestimation of LS and reduce the repeatability and reliability of LS measurements. For the elastograms with artifacts, we should place the Q-Box away from the artifacts.
- Citation: Wang HP, Zheng PC, Wang XM, Sang L. Artifacts in two-dimensional shear wave elastography of liver. World J Gastroenterol 2023; 29(21): 3318-3327
- URL: https://www.wjgnet.com/1007-9327/full/v29/i21/3318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i21.3318
Chronic liver disease is a growing problem worldwide. The main causes of chronic liver disease include hepatitis virus infection, alcoholic liver disease, and non-alcoholic fatty liver disease[1]. It mainly causes diffuse liver fibrosis, which in turn leads to liver cirrhosis. Some of them eventually develop hepatocellular carcinoma, portal hypertension, and hepatic encephalopathy[2,3]. Accurate assessment of liver fibrosis is important for treatment prioritization, surveillance, and determination of prognosis[4]. Moreover, liver biopsy allows the assessment of the degree of fibrosis[5]. However, liver biopsy is an expensive and invasive diagnostic tool. Its main complications are bleeding and pain[6,7], which limit its clinical application.
Recently, the application of ultrasound elastography in the diagnosis of non-invasive assessment of liver fibrosis has developed rapidly[8]. US elastography is mainly classified into two major types: Strain elastography and shear wave elastography[9,10]. Two-dimensional shear wave elastography (2-D SWE) is a type of shear wave elastography that uses acoustic radiation force to create shear waves. The velocity of the shear wave can be used to calculate the tissue stiffness by the formula E = 3ρc2, where E is tissue elasticity (Young’s modulus, kPa), ρ is tissue density (kg/m3), and c is shear wave velocity (m/s). The 2-D SWE is based on the quantification of the propagation speed of shear waves in the liver to create an elastogram. The elastogram is displayed using a color-coded map superimposed on a conventional B-mode image, where different colors represent different stiffness, allowing an assessment of homogeneity[10].
It has been reported that 2-D SWE has shown sufficient accuracy in evaluating the degree of liver fibrosis[11-13]. However, there was significant heterogeneity in the results of these studies. This heterogeneity may be caused by different patient populations, research designs and equipment used[14]. Another important reason may be that the presence of artifacts leads to inaccurate liver stiffness (LS) measurements. Bruce et al[15] reported that 2-D SWE artifacts resulted in a significant variability in the assessed LS.
Although 2-D SWE artifacts of the liver are common in clinical practice, they are poorly recognized, and there is even no clear definition. To the best of our knowledge, only a few review articles have been published[15,16]. Therefore, the purpose of this study was to investigate the presence and effects of artifacts in 2-D SWE of the liver. This is important to avoid artifacts and improve diagnostic perfor
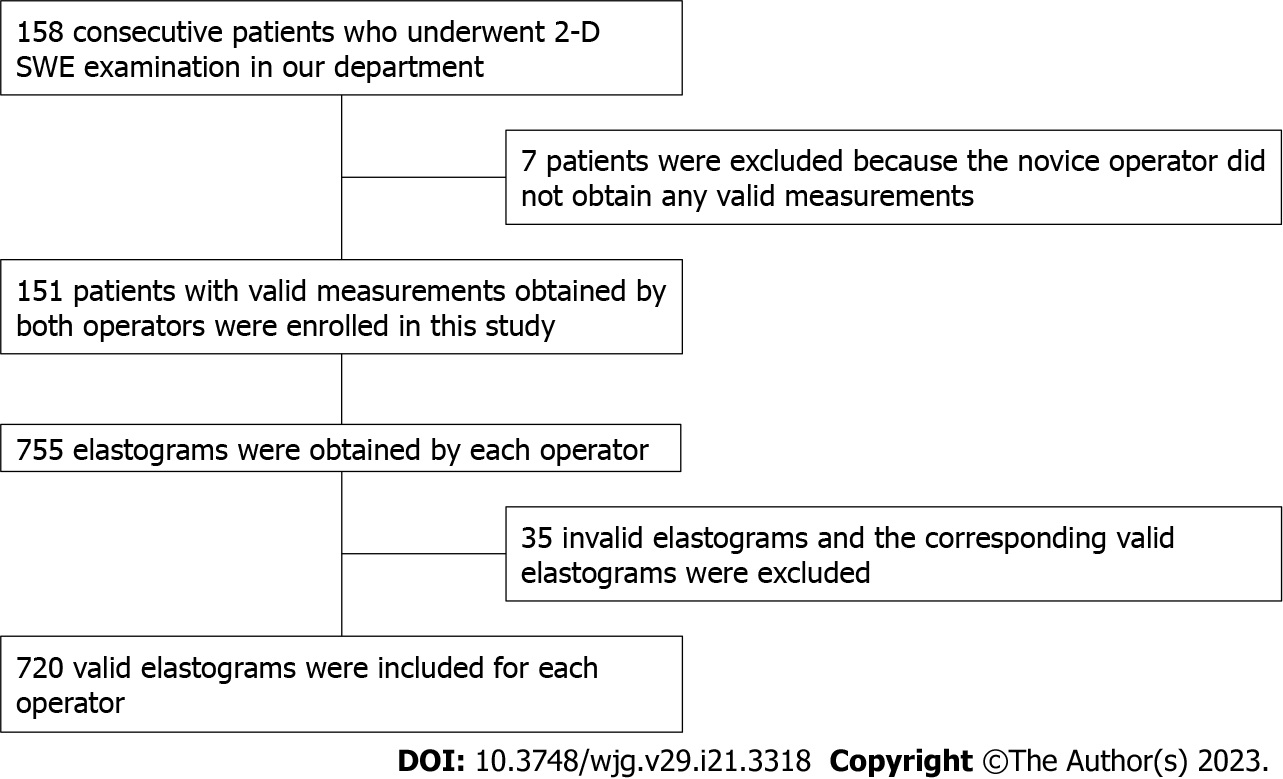
This prospective study was approved by the institutional ethical review board of our hospital. All patients signed a written informed consent document to participate in the study. We included 158 consecutive patients with chronic liver disease, who underwent 2-D SWE examination in our department. The study was conducted according to the principles reported in the Declaration of Helsinki and approved by the authors’ institutional review board. The exclusion criterion was that no valid measurement was obtained by either operator. Seven patients were excluded because the novice operator did not obtain any valid measurements after five consecutive measurements. The baseline characteristics of the patients were presented in Table 1.
| Characteristic | Value |
| Age (yr) | 46.2 ± 13.1 (19-75) |
| Liver stiffness value (kPa) | 9.7 ± 7.8 (3.8-34.9) |
| Liver cirrhosis, n (%) | 17 (11.3) |
| Subcutaneous fat thickness (cm) | 0.4 ± 0.3 (0.1-2.4) |
| Sex, n (%) | |
| Male | 72 (47.7) |
| Female | 79 (52.3) |
| Body mass index (kg/m2) | 23.3 ± 3.4 (17.2-36.3) |
| Normal (< 25 kg/m2), n (%) | 101 (66.9) |
| Overweight (25-30 kg/m2), n (%) | 41 (27.2) |
| Obese (> 30 kg/m2), n (%) | 9 (5.9) |
| Etiology of chronic liver disease, n (%) | |
| Hepatitis B virus | 122 (80.8) |
| Hepatitis C virus | 8 (5.3) |
| Alcoholic liver disease | 10 (6.6) |
| Nonalcoholic fatty liver disease | 6 (4) |
| Autoimmune disease | 5 (3.3) |
LS measurements were performed with an Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France) with a convex probe (SC6-1, 1-6 MHz). Patients fasted for more than 6 h and were examined in the supine position with the right arm in maximal abduction. The right anterior lobe of the liver was examined by intercostal scanning, and the SWE mode was started with neutral breathing during breath-holding. The upper limit of the color-coding scale was set to 70 kPa. The sampling frame was approximately 2.5 cm × 3.5 cm, placed at least 1 cm below the liver capsule, avoiding the large vascular structures. Image acquisition was performed after the elastography image was stable for 3-5 s. The quantitative analysis system (Q-Box) was then activated and placed at the center of the sampling frame. The Q-Box was 2 cm in diameter and the measurement depth was 3-5 cm. The LS measurement was considered invalid if there was no color-coding or the coded area was smaller than the Q-Box size[17]. When the area of color-coding is larger than the Q-Box size, the LS measurement was considered valid even if there are artifacts within it.
Each patient was continuously measured five times by an expert and a novice, respectively. The operators performed consecutive LS measurements in a randomized blinded manner. The median value of all valid measurements performed by the two operators represents the LS value (LSV) of the subject and was used for the correlation analysis with artifacts. The expert operator had 9 years of experience in the 2-D SWE examinations and had successfully performed approximately 15000 2D SWE examinations. The novice operator was trained by an expert operator and successfully performed 50 2-D SWE examinations.
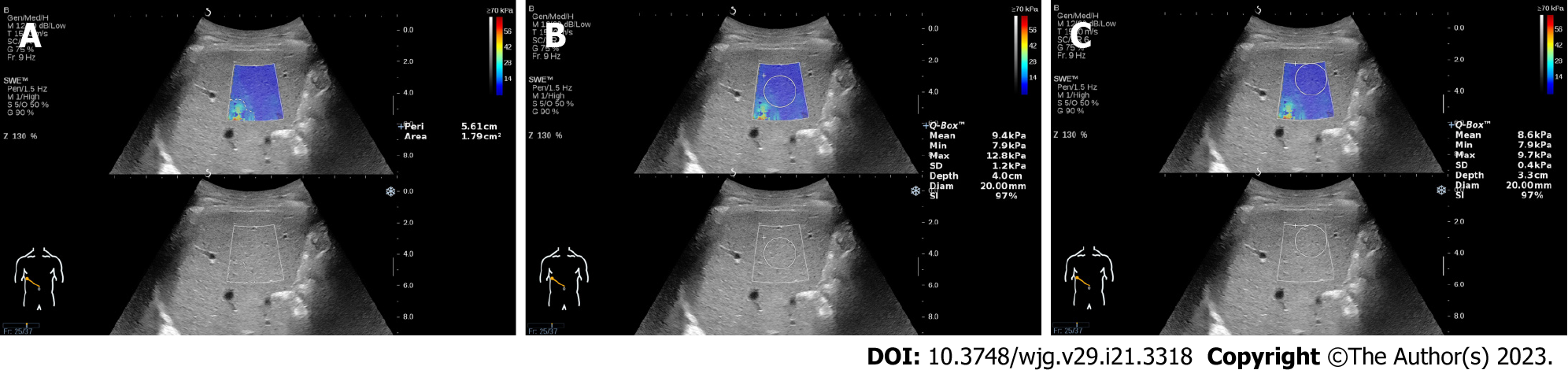
Artifacts were defined as the mottled area in the elastograms, and the area of the artifacts was measured using a tracing instrument attached to the device. We can manually trace the edge of the artifacts and automatically display the area and perimeter of the artifacts (Figure 1A). We drew a cross line at the center of the elastogram and divided it into four locations: top-left, top-right, bottom-left, and bottom-right. The location of the artifacts in each elastogram was recorded. The elastogram with the most artifacts (EMA) and the elastogram with the least artifacts (ELA) in each patient measured by the two operators were found by all authors. For the elastograms with artifacts, the Q-Box was placed in the center of the sampling frame (Figure 1B) and away from the artifacts for measurements (Figure 1C). The influence of artifacts on LS measurement was evaluated by comparing the differences in LSVs, standard deviation (SD) values and stability index (SI) values.
All quantitative data are expressed as mean ± SD (range), and qualitative variables are expressed as numbers (percentages). The Shapiro-Wilk test was used to test whether the numeric variables were normally distributed. Non-parametric tests with the Kruskal-Wallis method were used to compare the difference in numeric variables with a non-normal distribution. Differences between numeric variables with a normal distribution were assessed using a parametric test (t-test). The χ2-test was used to compare the proportions expressed as percentages. Interobserver repeatability was evaluated using intraclass correlation coefficient (ICC). Relationships between various parameters were examined using Pearson’s correlation test. Statistical significance was set at P < 0.05, and all P values were two-sided. Statistical analysis was performed using MedCalc software (MedCalc Software, version 17.4, Ostend, Belgium).
Among the 158 patients, 151 patients with valid measurements obtained by both operators were enrolled in this study. In theory, each operator should obtain 755 (151 × 5 = 755) elastography images. However, in the examination of 12 patients by the two operators, 35 elastography images were invalid and excluded. To ensure that the two operators had the same number of valid elastograms for each patient, valid measurements corresponding to the 35 invalid measurements were also excluded. Therefore, 720 elastography images from each operator were included (Figure 2).
For the expert operator, the percentage of elastograms with artifacts was 19.6% (141/720), and the area of artifacts was 0.92 ± 0.68 cm2. For the novice operator, the percentage of elastograms with artifacts was 51.7% (372/720), and the area of artifacts was 1.36 ± 0.87 cm2. The percentage of elastograms with artifacts and the area of artifacts in the novice were significantly higher than those in the expert, and the difference were both statistically significant (both P < 0.001). We counted all the artifacts according to their locations, and the results are shown in Table 2. There were no significant differences in the frequency of the occurrence of artifacts between the two operators at the same location (all P > 0.05). Comparing the occurrence frequency of artifacts in all locations of the two operators, it was found that both operators had the highest frequency of bottom-left, followed by top-left and bottom-right, and top-right had the lowest frequency. No statistical difference was found between the frequency of top-left and bottom-right (P > 0.05), but the frequency among other locations was statistically different (all P < 0.001) (Table 3).
| Locations | Expert, n (%) | Novice, n (%) | P value |
| Top-left | 40 (21.5) | 120 (20.5) | 0.769 |
| Bottom-left | 102 (54.8) | 309 (52.8) | 0.634 |
| Top-right | 7 (3.8) | 42 (7.2) | 0.098 |
| Bottom-right | 37 (19.9) | 114 (19.5) | 0.904 |
The LSVs of EMAs were higher than those of ELAs for both operators, and the differences were statistically significant (both P < 0.001). There was a significant difference in the LSVs of the EMAs between the two operators (P = 0.006). However, there was no statistically significant difference in the LSVs of the ELAs between the two operators (P = 0.051) (Table 4).
The ICC values and 95%CIs were calculated by comparing the LSVs of the EMAs and ELAs of the two operators. An ICC value of 0.96 (95%CI: 0.94-0.98) was found in the LSVs of EMAs, and it increased to 0.98 (95%CI: 0.97-0.99) when the LSVs of the ELAs were used. The SD values of EMAs were higher than those of ELAs for both operators, and the differences were statistically significant (both P < 0.001). The SI values of the EMAs were lower than those of the ELAs for both operators. The difference was only statistically significant for the novice (P = 0.002), but not for the expert (P = 0.135) (Table 5).
For the elastograms with artifacts, the LSVs and SD values of the Q-Box placed in the center of the sampling frame were higher than those of the Q-Box placed away from the artifacts. The SI values of the Q-Box placed in the center of the sampling frame were lower than those of the Q-Box placed away from the artifacts. There were significant differences in LSVs, SD values and SI values between the Q-Box placed in the center of the sampling frame and away from the artifacts for both operators (all P < 0.05) (Table 6).
| Number | Q-Box in the center of the sampling frame | Q-Box away from the artifacts | |||||
| LSV | SD | SI | LSV | SD | SI | ||
| Expert | 141 | 14.6 ± 9.5 | 1.7 ± 1.1 | 93% ± 6% | 14.1 ± 9.3 | 0.8 ± 0.6 | 94% ± 7% |
| Novice | 372 | 12.1 ± 9.5 | 1.9 ± 1.3 | 90% ± 7% | 11.6 ± 9.4 | 0.9 ± 0.7 | 93% ± 6% |
The total number of elastograms with artifacts measured by the two operators was 513 (141 by the expert, 372 by the novice). The number of elastograms with artifacts in male subjects was 238 (46.4%), and that in female subjects was 275 (53.6%). There was no significant difference between the male and female subjects (P = 0.378). Pearson's correlation test showed that there was no significant linear correlation between age and the number of elastograms with artifacts (r = 0.21, P = 0.126). In the entire cohort, Pearson’s correlation test showed that there was a positive correlation between LSV, body mass index (BMI), subcutaneous fat thickness and the number of elastograms with artifacts (r = 0.47, P = 0.001; r = 0.41, P = 0.002; and r = 0.42, P = 0.002, respectively).
When using 2-D SWE to measure LS in clinical practice, artifacts are commonly observed in elastograms[18]. It is difficult for some subjects to obtain satisfactory elastograms, such as obesity, poor acoustic window and inability of the subjects to hold their breath. Despite our best efforts to avoid artifacts, even operators with 9 years of operating experience still have a certain percentage of artifacts. In this study, we compared the difference in the frequency of occurrence artifacts between two different experienced operators. The results showed that the percentage of elastograms with artifacts and the area of artifacts in the novice were significantly higher than that of the expert. This may be because the expert operator can obtain high-quality B-mode imaging, which is required for accurately tracking shear waves[18]. Previous studies have shown that experts have better repeatability and reliability in measuring LS, which may have an important relationship with the fact that there were few artifacts in their elastograms[19,20]. Therefore, some studies have suggested that novices should perform at least 300 abdominal US scans or more than 50 supervised 2-D SWE examinations; however, this may not be sufficient[19,21]. A learning curve has been observed for 2-D SWE, a proportion of operator error would decrease over time[22].
We divided the elastogram into four locations and calculated the frequency of occurrence of artifacts at each location. The occurrence frequency of artifacts is arranged in descending order: bottom-left, top-left, bottom-right, and top-right. The two operators in this study had the same results, indicating that this difference may have certain regularity. The reason for this result may be that the aerated lung leads to a shadowing artifact on the left side of the B-mode image, which makes it impossible to form a well-defined push beam in this area[15,23]. On the other hand, to avoid liver capsule reverberation artifacts, the depth of the sampling frame has increased, especially in obese or overweight patients. When the depth exceeds the penetration limit, attenuation artifacts and larger vessels may have more pulsatile artifacts at the bottom of the sampling frame[16,23]. We found the same phenomenon on another 2-D SWE ultrasound system (Aplio500, Canon, Tochigi, Japan). We found that artifacts were more likely to occur in the bottom-left corner of the elastogram, where distortion waves were noted in the propagation map of the corresponding site. The distribution of artifacts may also be applicable to other devices of 2-D SWE technology, because they have the same imaging principles.
Usually a color-coding scale of up to 30 kPa is sufficient, but in this study the upper limit of the color-coding scale was set to 70 kPa. The reason is that some patients have an LSV greater than 30 kPa, and a lower color-coding scale setting will make the elastogram appear only in red. At this time, it is impossible to distinguish whether there is an artifact or not. Although the color-coding scale was set to 70 kPa may ignore tiny artifacts, it is easier to show obvious artifacts.
The presence of artifacts affects the assessment of LS, but there is no detailed research report yet. This study showed that the LSVs of the EMAs were higher than those of the ELAs. This indicates that artifacts may lead to the overestimation of LS. This study compared the differences between the two operators in the LSVs of EMAs and ELAs. The results showed that in either the EMAs or ELAs, the LSVs of the novice were higher than that of the expert, which may be due to the higher proportion of artifacts in the elastograms measured by the novice. The ICC value between the two operators calculated with the LSVs of the EMAs was lower than that calculated with the LSVs of ELAs. This shows that artifacts can reduce inter-observer repeatability.
Although the degree of liver fibrosis in chronic liver disease will be slightly different, the color-coded LSmapping image will hardly show obvious mottled area. These mottled areas are considered as artifacts and belong to noise. Some studies use signal-to-noise ratio as the standard to evaluate image quality[24,25]. The new software version of the device provides SD and SI as indicators to evaluate the reliability of LS measurement[26-28]. The SD reflects the homogeneity of LSVs in the measurement area of the Q-Box. The higher the SD values, the greater heterogeneity of the LSVs in the measurement area. Thiele et al[29] reported that the diagnostic accuracy for cirrhosis by 2D SWE increased at SD < 1.75 kPa. The SI is an indicator of temporal stability of the measurement area, and the manufacturer recommends that a reliable LS measurement should have a SI greater than 90%. Our study showed that the SD values of the EMAs were much higher than those of the ELAs, which indicated that artifacts made the elastograms heterogeneous. The SI values of the EMAs were lower than those of the ELAs, which showed that artifacts may reduce the temporal stability of the elastograms. In short, artifacts can reduce the reliability of the LS measurements. For the elastograms with artifacts, we found that placing the Q-Box away from the artifacts can obtain more reliable LS measurements than placing it in the center of the sampling frame (generally the default measurement position of the equipment).
Furthermore, we investigated the relationship between patient characteristics and the occurrence of artifacts. We found that the occurrence of artifacts had no significant relationship with sex or age. However, we found that patients’ BMI, subcutaneous fat thickness and LSVs were positively correlated with the occurrence of artifacts. Higher BMI and subcutaneous fat thickness usually indicate overweight or obesity with a thicker abdominal wall. Artifacts are prone to occur when measuring LS in overweight or obese subjects due to the combined effects of attenuation artifacts, reverberation artifacts, and vessels[16,30]. Previous studies have also shown that a high BMI is the main reason for measurement failure and unreliable assessment[17,31,32]. Patients with liver cirrhosis usually have higher LSVs, and they often have artifacts because of their shrunken liver volumes and poor sonic window. Other studies have demonstrated that severe liver fibrosis is a risk factor for unreliable LS measurements[17,33].
To the best of our knowledge, this is the first prospective study to analyze artifacts in 2-D SWE of the liver. This study analyzed the predilection sites and people for artifacts, and explored the effects of artifacts on LS measurements. Knowledge of the artifacts is essential to improve operation technology to obtain high-quality images. It is very important to obtain accurate measurements in an attempt to optimize its performance and application value. In addition, knowledge from this and other studies on artifacts can be used to investigate how training and education could reduce the occurrence of artifacts. Hopefully, engineers and researchers can improve the product design, provide quality indicators and other ways to avoid the acquisition of improper data due to artifacts.
Our study had several limitations. First, artifacts may be ignored when the color changes are inconspicuous. Second, only one device was tested in this study. Third, this study did not analyze the causes of artifacts, because it is sometimes difficult to accurately determine. Finally, we analyzed only a small sample of data from two operators. Therefore, a larger sample study involving more operators and devices needs to be conducted in future.
In conclusion, artifacts are common when using 2-D SWE to measure LS, especially for the novice. Artifacts may lead to the overestimation of LS and reduce the repeatability and reliability of LS measurements. For the elastograms with artifacts, we should place the Q-Box away from the artifacts.
Chronic liver disease is a growing problem worldwide. Accurate assessment of liver fibrosis is important for treatment prioritization, surveillance, and determination of prognosis. Liver biopsy is still considered as the gold standard for staging liver fibrosis. However, liver biopsy is an expensive and invasive diagnostic tool. Its main complications are bleeding and pain, which limit its clinical application. Recently, the application of two-dimensional shear wave elastography (2-D SWE) in the diagnosis of non-invasive assessment of liver fibrosis has developed rapidly. However, the presence of artifacts leads to inaccurate liver stiffness (LS) measurements.
Although 2-D SWE artifacts of the liver are common in clinical practice, they are poorly recognized, and there is even no clear definition. To the best of our knowledge, only a few review articles have been published. Knowledge of the artifacts is essential to improve operation technology to obtain high-quality images. It is very important to obtain accurate measurements in an attempt to optimize its performance and application value.
We aim to investigate the presence and influence of artifacts in 2-D SWE of liver.
In this study, we performed 2-D SWE examination in patients with chronic liver disease by a novice and an expert. The elastogram was divided into four locations: top-left, top-right, bottom-left, and bottom-right. The occurrence frequency of artifacts in different locations was compared. The effect of artifacts on the LS measurements was evaluated by comparing the elastogram with the most artifacts (EMA) and the elastogram with the least artifacts (ELA).
Each operator had 720 elastography images were included for analysis. The percentage of elastograms with artifacts and the area of artifacts in the novice were significantly higher than those in the expert (both P < 0.001). Comparing the occurrence frequency of artifacts in all locations of the two operators, it was found that both operators had the highest frequency of bottom-left, followed by top-left and bottom-right, and top-right had the lowest frequency. This study showed that the LS values and standard deviation values of the EMAs were higher than those of the ELAs. Both operators had lower stability index values and intraclass correlation coefficient values for EMAs than ELAs.
Artifacts are common when using 2-D SWE to measure LS, especially for the novice. Our results showed artifacts were more likely to occur in the bottom-left corner of the elastogram. Artifacts may lead to the overestimation of LS and reduce the repeatability and reliability of LS measurements.
In this study, we only analyzed a small sample of data from two operators of one device. Therefore, a larger sample study involving more operators and devices needs to be conducted in future studies.
| 1. | Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 564] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 2. | Aydın MM, Akçalı KC. Liver fibrosis. Turk J Gastroenterol. 2018;29:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 3. | Pryke R, Guha IN. Time to focus on chronic liver diseases in the community: A review of primary care hepatology tools, pathways of care and reimbursement mechanisms. J Hepatol. 2023;78:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Soon G, Wee A. Updates in the quantitative assessment of liver fibrosis for nonalcoholic fatty liver disease: Histological perspective. Clin Mol Hepatol. 2021;27:44-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 2013] [Article Influence: 671.0] [Reference Citation Analysis (3)] |
| 6. | Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20:16820-16830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 7. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, Dienstag JL; HALT-C Trial Group. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 8. | Ferraioli G, Roccarina D. Update on the role of elastography in liver disease. Therap Adv Gastroenterol. 2022;15:17562848221140657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 609] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 10. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 11. | Abe T, Kuroda H, Fujiwara Y, Yoshida Y, Miyasaka A, Kamiyama N, Takikawa Y. Accuracy of 2D shear wave elastography in the diagnosis of liver fibrosis in patients with chronic hepatitis C. J Clin Ultrasound. 2018;46:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Luo QT, Zhu Q, Zong XD, Li MK, Yu HS, Jiang CY, Liao X. Diagnostic Performance of Transient Elastography Versus Two-Dimensional Shear Wave Elastography for Liver Fibrosis in Chronic Viral Hepatitis: Direct Comparison and a Meta-Analysis. Biomed Res Int. 2022;2022:1960244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Villani R, Cavallone F, Romano AD, Bellanti F, Serviddio G. Two-Dimensional Shear Wave Elastography versus Transient Elastography: A Non-Invasive Comparison for the Assessment of Liver Fibrosis in Patients with Chronic Hepatitis C. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Xie LT, Yan CH, Zhao QY, He MN, Jiang TA. Quantitative and noninvasive assessment of chronic liver diseases using two-dimensional shear wave elastography. World J Gastroenterol. 2018;24:957-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Bruce M, Kolokythas O, Ferraioli G, Filice C, O'Donnell M. Limitations and artifacts in shear-wave elastography of the liver. Biomed Eng Lett. 2017;7:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Naganuma H, Ishida H, Uno A, Nagai H, Kuroda H, Ogawa M. Diagnostic problems in two-dimensional shear wave elastography of the liver. World J Radiol. 2020;12:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Yoon JH, Lee JM, Han JK, Choi BI. Shear wave elastography for liver stiffness measurement in clinical sonographic examinations: evaluation of intraobserver reproducibility, technical failure, and unreliable stiffness measurements. J Ultrasound Med. 2014;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (1)] |
| 19. | Moga TV, Stepan AM, Pienar C, Bende F, Popescu A, Șirli R, Dănilă M, Sporea I. Intra- and Inter-Observer Reproducibility of a 2-D Shear Wave Elastography Technique and the Impact of Ultrasound Experience in Achieving Reliable Data. Ultrasound Med Biol. 2018;44:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Wang H, Zheng P, Sang L, Wang X. Does Operator Experience and the Q-Box Diameter Affect the Repeatability of Liver Stiffness Measurements Obtained by 2-Dimensional Shear Wave Elastography? J Ultrasound Med. 2020;39:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Fang C, Konstantatou E, Romanos O, Yusuf GT, Quinlan DJ, Sidhu PS. Reproducibility of 2-Dimensional Shear Wave Elastography Assessment of the Liver: A Direct Comparison With Point Shear Wave Elastography in Healthy Volunteers. J Ultrasound Med. 2017;36:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Woo H, Lee JY, Yoon JH, Kim W, Cho B, Choi BI. Comparison of the Reliability of Acoustic Radiation Force Impulse Imaging and Supersonic Shear Imaging in Measurement of Liver Stiffness. Radiology. 2015;277:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Dubinsky TJ, Shah HU, Erpelding TN, Sannananja B, Sonneborn R, Zhang M. Propagation Imaging in the Demonstration of Common Shear Wave Artifacts. J Ultrasound Med. 2019;38:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Dighe M, Hippe DS, Thiel J. Artifacts in Shear Wave Elastography Images of Thyroid Nodules. Ultrasound Med Biol. 2018;44:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Wang CZ, Zheng J, Huang ZP, Xiao Y, Song D, Zeng J, Zheng HR, Zheng RQ. Influence of measurement depth on the stiffness assessment of healthy liver with real-time shear wave elastography. Ultrasound Med Biol. 2014;40:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | O'Hara S, Zelesco M, Rocke K, Stevenson G, Sun Z. Reliability Indicators for 2-Dimensional Shear Wave Elastography. J Ultrasound Med. 2019;38:3065-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Gatos I, Tsantis S, Spiliopoulos S, Karnabatidis D, Theotokas I, Zoumpoulis P, Loupas T, Hazle JD, Kagadis GC. Temporal stability assessment in shear wave elasticity images validated by deep learning neural network for chronic liver disease fibrosis stage assessment. Med Phys. 2019;46:2298-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Hong EK, Choi YH, Cheon JE, Kim WS, Kim IO, Kang SY. Accurate measurements of liver stiffness using shear wave elastography in children and young adults and the role of the stability index. Ultrasonography. 2018;37:226-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Thiele M, Madsen BS, Procopet B, Hansen JF, Møller LMS, Detlefsen S, Berzigotti A, Krag A. Reliability Criteria for Liver Stiffness Measurements with Real-Time 2D Shear Wave Elastography in Different Clinical Scenarios of Chronic Liver Disease. Ultraschall Med. 2017;38:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Bouchet P, Gennisson JL, Podda A, Alilet M, Carrié M, Aubry S. Artifacts and Technical Restrictions in 2D Shear Wave Elastography. Ultraschall Med. 2020;41:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 32. | Guo HY, Liao M, Zheng J, Huang ZP, Xie SD. Two-dimensional shear wave elastography utilized in patients with ascites: a more reliable method than transient elastography for noninvasively detecting the liver stiffness-an original study with 170 patients. Ann Transl Med. 2023;11:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lattanzi S, Italy; Macias-Rodriguez RU, Mexico; Singh D, India S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY