Published online Nov 7, 2022. doi: 10.3748/wjg.v28.i41.5957
Peer-review started: July 26, 2022
First decision: August 31, 2022
Revised: September 15, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 7, 2022
Processing time: 101 Days and 0.6 Hours
Endoscopic submucosal dissection (ESD) is an established technique for the treatment of early gastrointestinal neoplasia. Generally, multi-day (M-D) admission is required for patients undergoing ESD due to potential complications.
To evaluate the feasibility of a same-day (S-D) discharge strategy for ESD of the esophagus or stomach.
The data of patients who underwent esophageal or gastric ESD were retro
Among the 479 patients reviewed, 470 patients, including 91 in the S-D group and 379 in the M-D group, fulfilled the inclusion and exclusion criteria. Following PSM, 78 patients in each group were paired using the 1:1 nearest available score match algorithm. No significant difference was found between groups with respect to intraoperative and postprocedural major adverse events (AEs). Tumor size, complete resection rate, and procedural duration were comparable between the groups. The S-D group demonstrated a significantly shorter length of hospital stay (P < 0.001) and lower overall medical expenses (P < 0.001) compared with the M-D group.
The S-D discharge strategy may be feasible and effective for esophagogastric ESD, and the procedural-related AEs can be managed successfully.
Core Tip: Generally, multi-day (M-D) admission is required for patients with early gastrointestinal neoplasia undergoing endoscopic submucosal dissection (ESD) due to potential complications. We evaluated the feasibility of a same-day (S-D) discharge strategy for ESD of the esophagus or stomach. No significant difference was found between the S-D and M-D groups with respect to intraoperative and postprocedural major adverse events. However, the S-D group demonstrated a significantly shorter length of hospital stay (P < 0.001) and lower overall medical expenses (P < 0.001) compared to the M-D group. The S-D discharge strategy may be feasible and effective for esophagogastric ESD.
- Citation: Wang J, Li SJ, Yan Y, Yuan P, Li WF, Cao CQ, Chen WG, Chen KN, Wu Q. Feasibility of same-day discharge following endoscopic submucosal dissection for esophageal or gastric early cancer. World J Gastroenterol 2022; 28(41): 5957-5967
- URL: https://www.wjgnet.com/1007-9327/full/v28/i41/5957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i41.5957
Endoscopic submucosal dissection (ESD) has been advocated as an effective treatment approach for early esophageal cancer and early gastric cancer[1-3]. ESD is safer, more cost-effective, has greater efficacy, and has a positive impact on health-related quality of life compared with surgery[4,5]. As ESD is associated with complications, including intraprocedural perforation rates between 2.2% and 4.5%[6-8] and postprocedural bleeding rates between 1% and 5.1%[6-9], a multi-day (M-D) hospital admission of 5 d to 7 d is generally required in daily practice[10]. Reducing the length of hospital stay can decrease medical expenses, and some studies have attempted to shorten the duration of postprocedural hospitalization after esophageal[11], gastric[12], and colorectal[13] ESD. However, data on the feasibility of same-day (S-D) discharge after esophagogastric ESD remain limited. Based on our previous studies with relatively low complications in ESD[14-16], our department has applied the S-D strategy to selected patients since 2020. In this study, we describe our preliminary experience with the S-D discharge strategy following ESD of the esophagus or stomach compared with conventional M-D hospital admission.
We retrospectively reviewed clinical data from a prospectively maintained database of ESD for consecutive patients at Peking University Cancer Hospital between January 2018 and December 2021. The inclusion criteria were receipt of esophageal or gastric ESD and malignant final diagnosis. The exclusion criteria were receipt of laparoscopic endoscopic collaborative surgery, recurrent lesions, multiple lesions, or a history of esophagectomy or gastrectomy. The present study was approved by the Ethics Committee of Peking University Cancer Hospital (2022KT13) in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients or their families.
According to the length of hospitalization, patients were divided into S-D and M-D groups. Patients in the S-D group were admitted to the ambulatory care unit on the morning of the ESD procedure day, whereas patients in the M-D group were admitted to the hospital ward the day before ESD. After the ESD procedure, patients in the S-D group were discharged from the ambulatory care unit on the S-D, while the M-D group patients returned to the hospital ward for at least one night before discharge. Patients in the S-D group were informed that they might be transferred to a hospital ward for hospital stay after the procedure if there was an intraprocedural perforation, unsatisfactory postanesthesia recovery, or other serious unexpected adverse events (AEs). Concerning patients who received antithrombotic therapy, after consultation with a cardiologist, agents were discontinued 5-7 d before ESD and resumed on day 7 after the procedure.
The following demographic and clinical information were collected: Age, sex, American Society of Anesthesiologists (ASA) physical status classification, comorbidities, history of antithrombic agent use, duration of ESD procedure, length of hospital stay, cost of hospitalization, pathological evaluation of specimen, and AEs during or after the procedure.
In this study, the primary endpoint was the presence of ESD-related major AEs (MAEs) within 30 d of the procedure. MAEs included bleeding and perforation. Bleeding was defined as active or oozing bleeding of the ESD wound requiring hemostasis during scheduled second-look endoscopy (SSLE), with or without a decrease in hemoglobin level of ≥ 2 g/dL. Perforation was defined as a muscle layer defect, allowing the observation of mesenteric fat or intraabdominal space during the procedure or free air found on a radiograph in symptomatic patients after the ESD procedure. AEs were categorized as intraprocedural and postprocedural according to the time point in which they emerged.
The secondary endpoints were the rates of en bloc resection and complete resection, length of hospital stay, and medical expenses. The tumor location was divided into the esophagus, and the upper, middle, and lower stomach. The upper stomach consists of the cardia and upper part of the gastric body, the middle stomach consists of the angle and middle body, and the lower stomach consists of the pylorus, antrum, and lower body. The macroscopic classification was divided into elevated (0-I), flat (0-II), and depressed (0-III) types according to the Paris classification of superficial neoplastic lesions in the digestive tract[17]. En bloc resection was defined as resection of the lesion in a single piece, and complete resection was defined as resection of a tumor without histological evidence of tumor cell involvement on the lateral and vertical resection margins[18].
All ESD patients in our department followed the M-D strategy before 2020. Patients with an estimated specimen size < 4 cm or who lived nearby were selected as S-D strategy candidates since 2020; they were assigned to the S-D or M-D group based on the anesthesiologist‘s recommendation and the patient’s intention after full consultation. All ESD procedures were performed under general anesthesia with tracheal intubation and propofol administration. A single-channel upper gastrointestinal endoscope (GIF Q260J; Olympus Co., Tokyo, Japan) was used in all ESD procedures. A premixed sterilized solution of glycerol (10% glycerol and 5% fructose; Cisen Pharmaceutical, Co., Ltd., Shandong, China) with indigo carmine was injected into the submucosal layer. A single-use electrosurgical knife with water injection function (Micro-Tech Co., Nanjing, China) was used for lesion marking, incision, and dissection with an electrosurgical unit (VIO 200S; ERBE Elektromedizin GmbH, Tübingen, Germany). The ENDO CUT Q mode (parameter setting effect 3, cutting duration 2, and cutting interval 4) was applied for both mucosal incision and submucosal dissection. Hemostasis was achieved with the FORCED COAG E2 mode, and the power was set to 40 W in the esophagus and 50 W in the stomach. If perforation occurred during the procedure, suturing was performed using hemoclips (Micro-Tech Co.). All ESD wounds were sprayed with porcine fibrin sealant (5 mL kit; Guangzhou Bioseal Biotechnology Co., Ltd., Guangzhou, China) after the lesions were resected.
All ESD procedures were performed by the same endoscopist. For patients without intraprocedural perforation, nasogastric tube was not placed. For all patients, water drinking was initiated 2 h after anesthesia recovery. All patients also underwent an SSLE the next day to identify possible bleeding, even if they had been discharged on the same day of ESD. If no bleeding was discovered in the SSLE, oral enteral nutritional suspension was prescribed for 1 wk, followed by soft diet for 1 wk before the full diet resumption. For patients with intraprocedural perforation or postprocedural bleeding, the oral diet was postponed depending on recovery. For all patients, proton pump inhibitor therapy (standard dosing) was administered intravenously until the patient was discharged, followed by oral administration for 4 wk. Follow-up consisted of telephonic contact, and AEs reported after discharge were recorded by a physician associate.
To minimize the effect of selection bias, the propensity score matching (PSM) method was applied to balance the unevenly distributed patient baseline characteristics in this non-randomized trial. Individual propensity scores were generated through a logistic regression model that included the following covariates: Age, sex, ASA physical status, comorbidities, use of antithrombotic agent, tumor location, macroscopic appearance, tumor differentiation, depth of invasion, and specimen size. Subsequently, patients in the S-D and M-D groups were paired using a 1:1 nearest available score match algorithm with a match tolerance of 0.02.
Further statistical analyses were conducted to compare the differences between the two groups based on the matched data. Quantitative data with normal distribution are presented as the means ± SD, and categorical data are presented as frequencies. Differences between groups were examined using the student’s t-test, χ2 test, or Fisher’s exact test where appropriate. Logistic regression was used to identify the risk factors for AEs. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS statistical software (version 22.0; IBM Corp., Armonk, NY, United States).
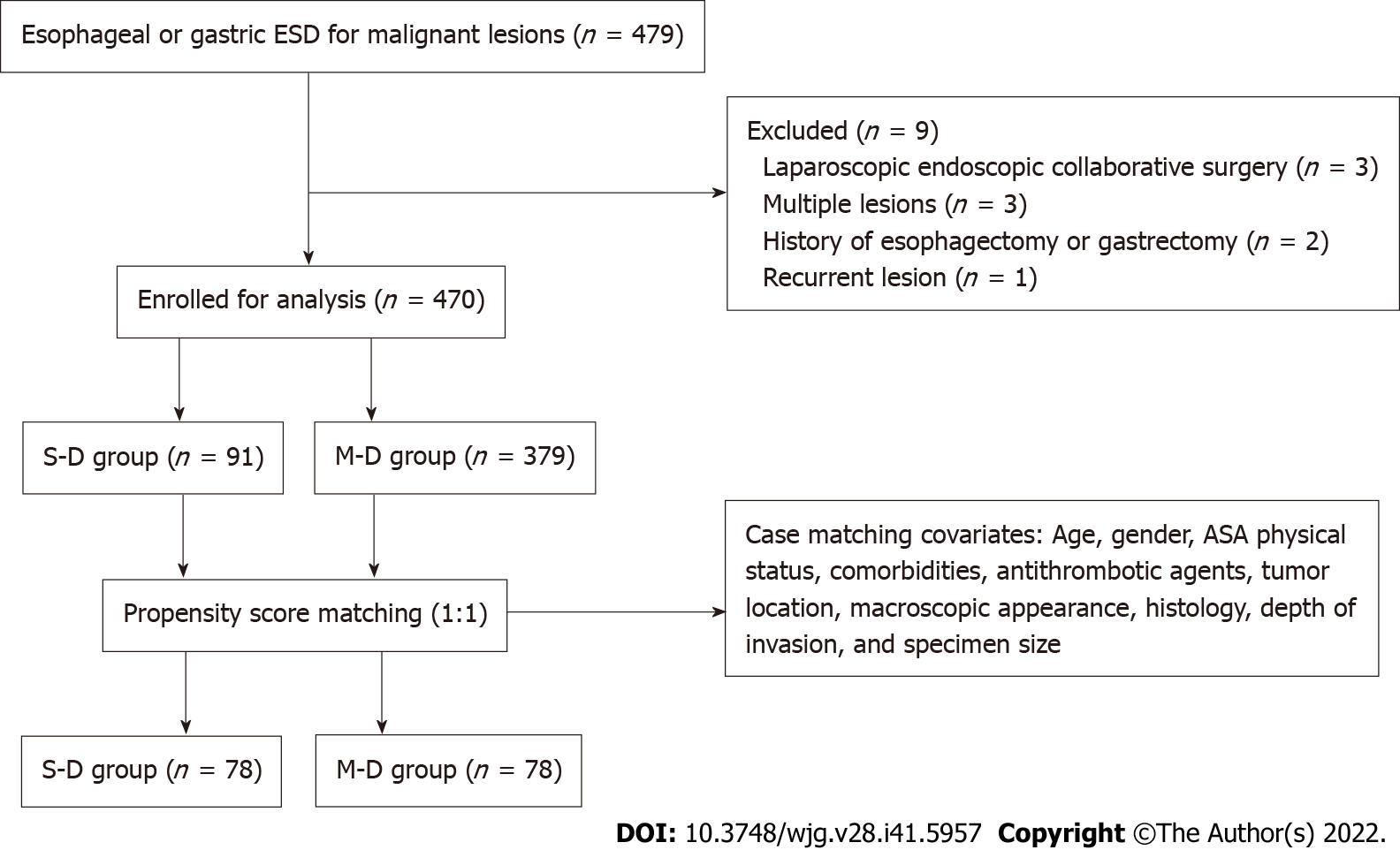
A total of 479 patients who underwent 482 esophageal or gastric ESD procedures were reviewed retrospectively (Figure 1). Among these, 3 patients who underwent laparoscopic endoscopic collaborative surgery, 3 patients with multiple lesions, 2 patients with history of esophagectomy or gastrectomy, and 1 patient with a recurrent lesion were excluded. Therefore, 470 patients were enrolled in the study. The clinicopathological characteristics of the patients are presented in Table 1. Before PSM, there were 91 patients in the S-D group and 379 patients in the M-D group. There were significant differences in ASA score (P = 0.039), tumor differentiation (P = 0.004), depth of invasion (P = 0.022), and specimen size (P < 0.001) between the two groups. After PSM, there were 78 patients in each group, and all baseline parameters were balanced between the two groups.
| Characteristics | Overall sample, n = 470 | SMD value | P value | Matched sample, n = 156 | SMD value | P value | ||
| S-D, n = 91 | M-D, n = 379 | S-D, n = 78 | M-D, n = 78 | |||||
| Age in yr | -0.052 | 0.657 | 0.025 | 0.402 | ||||
| ≤ 60 | 33 (36.3) | 147 (38.8) | 30 (38.5) | 25 (32.1) | ||||
| > 60 | 58 (63.7) | 232 (61.2) | 48 (61.5) | 53 (67.9) | ||||
| Sex | 0.069 | 0.550 | -0.057 | 1.000 | ||||
| Female | 21 (23.1) | 99 (26.1) | 16 (20.5) | 16 (20.5) | ||||
| Male | 70 (76.9) | 280 (73.9) | 62 (79.5) | 62 (79.5) | ||||
| ASA physical status | 0.224 | 0.039 | 0.000 | 1.000 | ||||
| ≤ 2 | 89 (97.8) | 347 (91.6) | 76 (97.4) | 75 (96.2) | ||||
| ≥ 2 | 2 (2.2) | 32 (8.4) | 2 (2.6) | 3 (3.8) | ||||
| Comorbidities | -0.087 | 0.457 | -0.050 | 0.423 | ||||
| No | 45 (49.5) | 171 (45.1) | 37 (47.4) | 42 (53.8) | ||||
| Yes | 46 (50.5) | 208 (54.9) | 41 (52.6) | 36 (46.2) | ||||
| Antithrombotic agents use | -0.164 | 0.141 | -0.041 | 1.000 | ||||
| Yes | 5 (5.5) | 40 (10.6) | 4 (5.1) | 5 (6.4) | ||||
| No | 86 (94.5) | 339 (89.4) | 74 (94.9) | 73 (93.6) | ||||
| Location of lesion | -0.197 | 0.168 | 0.176 | 0.357 | ||||
| Esophagus | 23 (25.3) | 115 (30.3) | 22 (28.2) | 27 (34.6) | ||||
| Upper 1/3 of the stomach | 18 (19.8) | 97 (25.6) | 14 (17.9) | 20 (25.6) | ||||
| Middle 1/3 of the stomach | 22 (24.2) | 89 (23.5) | 20 (25.6) | 15 (19.2) | ||||
| Lower 1/3 of the stomach | 28 (30.7) | 78 (20.6) | 22 (28.3) | 16 (20.6) | ||||
| Macroscopic appearance, type | 0.184 | 0.092 | 0.000 | 1.000 | ||||
| 0-II | 89 (97.8) | 383 (93.1) | 76 (97.4) | 75 (96.2) | ||||
| 0-I and 0-III | 2 (2.2) | 26 (6.9) | 2 (2.6) | 3 (3.8) | ||||
| Tumor differentiation | 0.316 | 0.004 | -0.064 | 0.442 | ||||
| Differentiated | 85 (93.4) | 307 (81.0) | 73 (93.6) | 76 (97.4) | ||||
| Undifferentiated | 6 (6.6) | 72 (19.0) | 5 (6.4) | 2 (2.6) | ||||
| Depth of invasion | 0.258 | 0.022 | 0.030 | 1.000 | ||||
| Intramucosal | 81 (89.0) | 297 (78.4) | 69 (88.5) | 69 (88.5) | ||||
| Submucosal | 10 (11.0) | 82 (21.6) | 9 (11.5) | 9 (11.5) | ||||
| Specimen size in mm, mean ± SD | 31.2 ± 12.3 | 43.9 ± 17.0 | 0.749 | 0.000 | 33.3 ± 11.9 | 33.6 ± 15.5 | 0.031 | 0.913 |
As shown in Table 2, after PSM, no significant difference was found between the groups across pathological parameters including tumor size, rate of free vertical margin, and complete resection. As shown in Table 3, the ESD procedural time was comparable between the two groups after PSM (60.5 ± 34.9 min in the S-D group vs 65.8 ± 43.0 min in the M-D group; P = 0.397). In addition, the duration of hospitalization was significantly shorter in the S-D group than in the M-D group (1 d vs 4.9 ± 2.5 d, respectively; P < 0.001). In this study, the total medical expense was determined by categorizing the costs of the procedure, medical devices, medication, diagnostic tests, and administration. The overall medical expenses and the subitem costs were lower in the S-D group.
| Characteristics | Matched sample, n = 156 | P value | |
| S-D, n = 78 | M-D, n = 78 | ||
| Tumor size in mm | 17.8 ± 11.6 | 17.3 ± 10.4 | 0.778 |
| En bloc resection | 77 (98.7) | 76 (94.7) | 1.000 |
| Free horizontal margin | 78 (100.0) | 77 (98.7) | 1.000 |
| Free vertical margin | 76 (97.4) | 76 (97.4) | 1.000 |
| Complete resection | 75 (96.2) | 73 (93.6) | 0.719 |
| Characteristics | Matched sample, n = 156 | P value | |
| S-D, n = 78 | M-D, n = 78 | ||
| Procedure time in min | 60.5 ± 34.9 | 65.8 ± 43.0 | 0.397 |
| Hospitalization in d | |||
| Total | 1 | 4.6 ± 2.0 | 0.000 |
| Postprocedural | 0 | 3.0 ± 1.8 | 0.000 |
| Medical expenses in CNY | 25749.0 ± 4389.3 | 37000.8 ± 8510.7 | 0.000 |
| Procedure: ESD, anesthesia, other procedures | 3616.1 ± 942.8 | 6079.3 ± 1646.5 | 0.000 |
| Medical devices | 13112.0 ± 1884.5 | 17956.7 ± 4977.2 | 0.000 |
| Medication | 6390.7 ± 3866.4 | 7759.9 ± 2241.8 | 0.008 |
| Diagnostic test: Endoscopy, laboratory, radiology, pathology | 3625.5 ± 1133.9 | 4025.9 ± 1561.5 | 0.069 |
| Administration: Hospitalization, nursing | 260.0 ± 232.7 | 1178.9 ± 1506.7 | 0.000 |
Thirty-five MAEs occurred in 35 (7.4%) patients, including 14 intraprocedural perforations (1 in the esophagus), 18 cases of oozing bleeding (1 in the esophagus) without hemoglobin decreased, and 3 cases of active bleeding (all in the stomach) with hemoglobin decreased 2 g/dL to 2.5 g/dL during SSLE. All MAEs were managed endoscopically. There was no recurrent bleeding that occurred after SSLE, and no rehospitalization was needed within 7 d of discharge in either group. Both before and after PSM, no significant differences were found between the groups with respect to intraprocedural and postprocedural MAEs (Table 4). Factors associated with postprocedural bleeding and intraprocedural perforation were also investigated. Following multivariate analysis, lesions located in the middle and lower thirds of the stomach were significantly associated with postprocedural bleeding (odds ratio: 5.3, 95% confidence interval: 1.3-22.2; P = 0.023) (Table 5), whereas no risk factor was identified for intraoperative perforation.
| Characteristics | Overall sample, n = 470 | P value | Matched sample, n = 156 | P value | ||
| S-D, n = 91 | M-D, n = 379 | S-D, n = 78 | M-D, n = 78 | |||
| Total major adverse events | 3 (3.3) | 32 (8.4) | 0.093 | 3 (3.8) | 6 (7.7) | 0.495 |
| Intraprocedural perforation | 0 | 14 (3.7) | 0.083 | 0 | 3 (3.8) | 0.245 |
| Postprocedural bleeding during SSLE | 3 (3.3) | 18 (4.7) | 0.778 | 3 (3.8) | 3 (3.8) | 1.000 |
| Oozing bleeding | 2 (2.2) | 16 (4.2) | 0.546 | 2 (2.6) | 3 (3.8) | 1.000 |
| Active bleeding | 1 (1.1) | 2 (0.5) | 0.476 | 1 (1.3) | 0 | 1.000 |
| Variable | Total, n | PB, n (%) | OR (95%CI) | P value |
| Male sex | 350 | 17 (4.9) | 3.0 (0.8-11.1) | 0.105 |
| Age ≤ 60 yr | 180 | 10 (5.6) | 1.5 (0.6-4.2) | 0.392 |
| ASA score ≤ 2 | 436 | 19 (4.4) | 2.7 (0.2-38.3) | 0.457 |
| ATA usage | 45 | 2 (4.4) | 1.7 (0.2-12.7) | 0.610 |
| Multi-day discharge group | 379 | 17 (4.5) | 1.9 (0.5-7.4) | 0.361 |
| Non-flat appearance | 28 | 2 (7.1) | 1.4 (0.3-7.6) | 0.710 |
| Located in the lower 1/3 of the stomach | 106 | 11 (10.4) | 2.1 (0.7-6.2) | 0.163 |
| Located in the lower 2/3 of the stomach | 211 | 17 (7.7) | 5.3 (1.3-22.2) | 0.023 |
| Differentiated type | 392 | 17 (4.3) | 1.2 (0.3-5.2) | 0.799 |
| Submucosal invasion | 92 | 4 (4.3) | 1.3 (0.3-4.7) | 0.723 |
| Lesion ≥ 2 cm | 236 | 11 (4.7) | 1.2 (0.4-3.8) | 0.818 |
| Specimen ≥ 4 cm | 234 | 10 (4.3) | 1.1 (0.3-3.7) | 0.879 |
In China, ESD has developed rapidly over the recent years due to the popularization of digestive endoscopic screening and the improved detection rate of early neoplastic lesions. Generally, an M-D admission is required for patients undergoing ESD because of the known potential complications[10]. Based on our previous experience, the risk of AEs is relatively low and generally can be managed conservatively or endoscopically in esophagogastric ESD, with reported intraprocedural perforation rates being between 1.9% and 2.6% and postprocedural bleeding rates between 1.4% and 8.7%[14-16]. Our department has performed the S-D discharge strategy since 2020, and this study demonstrates the feasibility and efficacy of S-D discharge procedures in selected esophagogastric ESD patients.
It was gratifying that we did not find any significant differences in the incidence of MAEs between the groups both before and after PSM in this study. Postprocedural bleeding is the most common complication in upper gastrointestinal ESD, with a reported incidence of 1% in the esophagus and 5.1% in the stomach[6-9]. Tumor in the lower third of stomach is an independent risk factor for post-ESD bleeding[19], and active antral peristalsis as well as bile reflux might lead to a higher incidence of post-ESD bleeding[8]. In our series, a slightly higher incidence of postprocedural bleeding (6.0%) was noted in the stomach, whereas mid- to lower location in the stomach was identified as the only risk factor for postprocedural bleeding, suggesting that we should not only pay attention to the lesions in the antrum but also those in the angle and gastric body to minimize the risk of postprocedural bleeding. In addition, male sex, antithrombotic drugs, tumor size > 20 mm, resected specimen size ≥ 40 mm, and flat/depressed lesion types are also risk factors for postprocedural bleeding[9,20], but none were identified in our study, possibly because we expanded the definition of postprocedural bleeding. We not only included patients with massive bleeding but also patients with active or oozing bleeding that necessitated hemostasis during SSLE without an overt hemoglobin decrease, which might maximize the safety of the S-D strategy in patients.
Although routine use of SSLE is not advocated as it does not reduce the risk of delayed bleeding[21,22], this technique has been carried out in many studies[23-25]. The purpose of SSLE in our study was to detect oozing and active bleeding and perform hemostasis. We did not perform prophylactic coagulation on nonbleeding visible vessels smaller than 0.3 mm in the post-ESD ulcer. Our previous study showed that a wound microvessel-protective hemostatic technique followed by porcine fibrin sealant can promote ESD-induced ulcer healing without increasing delayed bleeding events[15]. Prophylactic hemostasis-induced tissue damage or necrosis may lead to the exposure of arteries on the base of the ulcer, which in turn would contribute to delayed episodes of bleeding[21]. Although the inconvenience of SSLE might limit benefit of the S-D discharge strategy, it does provide help in the early detection of postprocedural bleeding, especially as a nasogastric tube is not routinely deployed in our department. Meanwhile, a fairly short distance to the hospital would allow for the patients to obtain timely treatments in the case of MAE development[11].
As a relatively rare complication, intraprocedural perforation can be treated endoscopically in most cases, with a reported incidence of 2.2% in the esophagus and 4.5% in the stomach[6-8]. Larger tumor size (> 2 cm) and longer procedure time (> 2 h) are risk factors for perforation[26,27]. In this study, the rate of intraoperative perforation was 0.7% in the esophagus and 3.9% in the stomach. All perforations were sutured by hemoclips successfully, with no delayed perforation occurring. To avoid intraoperative perforation, it is important to obtain a good intraoperative field of view and to reliably discern the muscularis propria. The traction method is useful in many such cases[28], but we did not perform it routinely in our procedure. Greater experience and more delicate operation techniques might also reduce the risk of intraoperative perforation.
Achieving tumor-free margins is essential for the efficacy of ESD in early gastrointestinal malignancies. In this study, we obtained a similar complete resection rate of 96.2% and 93.6% in the S-D and M-D groups, respectively, which are comparable with previous studies[7,29,30]. Larger specimen sizes correlate with longer procedural duration[11], which is an independent risk factor for pulmonary risk during anesthesia[31], and specimen size ≥ 4 cm is associated with delayed bleeding[20]. So when we started the S-D strategy in 2020, patients with estimated specimens smaller than 4 cm were selected as the S-D discharge candidates to minimize the associated risk above. Tumor differentiation should be noticed in specimen size estimation. In undifferentiated lesions, it is difficult to delineate the cancerous areas and easily obtain a positive lateral margin. Therefore, a further distance from the estimated border is usually needed to establish complete resection[32,33].
ASA physical status classification can reflect the severity of a patient’s comorbidities, and those with an ASA score of 1 or 2 could be considered suitable for S-D discharge or outpatient ESD[11,34]. The results of the present study supported this data, as the proportion of patients with ASA score of 1 or 2 in the S-D group was more than that in the M-D group, but those patients experienced a similar profile of MAEs before and after PSM. Although the Charlson Comorbidity Index can provide a more detailed risk evaluation for patients with multiple comorbidities[35,36], the ASA score system is considered easier to apply in clinical settings.
ESD can greatly reduce the medical care costs associated with gastric cancer[37]. In Japan, ESD patients are usually admitted for 5-7 d, and in Europe for 2-4 d following ESD[10]. A reduction of hospitalization stay length or practice in an outpatient setting would minimize the medical expenses further[34]. A benchmark cost estimate for ESD treatment including 4 d of postoperative hospitalization in China is reportedly approximately 5400 United States Dollars[38], which is similar to our M-D group. Labor costs for doctors and nurses remain low in many East Asian countries, whereas medication and medical devices account for most of the total cost of ESD. A significant reduction in total cost could be established if ESD was performed with S-D discharge, as applied in our study. This is very important for Western countries, as their medical expenses increase with length of hospitalization. Using proper selection criteria, S-D discharge ESD could be a cost-effective strategy for esophagogastric early malignancies.
Our study had several limitations. First, all of the procedures were performed by a single skilled endoscopist with 14 years’ experience in gastrointestinal ESD, and our experience reflected that of a high-volume center with a specialized endoscopist to perform ESD. Thus, our results might not be applicable to other centers. A further investigation involving more endoscopists, with varying degree of experience, from more centers, with diverse structure, is being designed and planned, and we hope to provide more conclusive findings in the future. Second, as a retrospective study, selection bias could not be ignored, although the PSM method was used to balance the characteristics of the patients in both groups. As an oncology-specific territory center, we lack specific experience in handling complex comorbidities. Most of the included patients had an ASA score of 1 or 2. Thus, we cannot generalize these results to patients with ASA scores of 3 or more. Third, we had implemented a S-D discharge policy for only 2 years. Due to a relatively small patient number, we were unable to identify a more detailed selection criterion other than an estimated specimen size of less than 4 cm for S-D discharge ESD to avoid potential complications during and after the procedure, and further investigation is needed.
In conclusion, this study, the first retrospective propensity score-matched study evaluating S-D discharge procedures for esophagogastric ESD in China, demonstrates that this strategy may be feasible and effective, and that the AEs related to ESD could be managed successfully. Additional prospective studies are warranted to establish more detailed standards to select patients for S-D discharge ESD.
Endoscopic submucosal dissection (ESD) is an established technique for the treatment of early gastrointestinal neoplasia. Generally, a multi-day (M-D) admission is required for patients undergoing ESD due to potential complications. This retrospective study demonstrates that the same-day (S-D) discharge procedures for esophagogastric ESD may be feasible and effective.
ESD is safer, more cost-effective, has greater efficacy, and exhibits a positive impact on health-related quality of life in comparison with surgery. Reducing the length of hospital stay can decrease medical expenses, and some studies have attempted to shorten the duration of postprocedural hospitalization after esophageal, gastric, and colorectal ESD. However, data on the feasibility of S-D discharge after esophagogastric ESD remain limited.
In this study, we describe our preliminary experience with the S-D discharge strategy following ESD of the esophagus or stomach compared with conventional M-D hospital admission.
To minimize the effect of selection bias, the propensity score matching method was applied to balance the unevenly distributed patient baseline characteristics in this non-randomized trial. Subsequently, patients in the S-D and M-D groups were paired using the 1:1 nearest available score match algorithm with a match tolerance of 0.02. Further statistical analyses were conducted to compare the differences between the two groups based on the matched data.
No significant difference was found between the groups with respect to intraoperative and postprocedural major adverse events (AEs). The tumor size, complete resection rate, and procedural duration were comparable between the groups. The S-D group demonstrated a significantly shorter length of hospital stay (P < 0.001) and lower overall medical expenses (P < 0.001) compared to the M-D group.
This is the first retrospective study evaluating S-D discharge procedures for esophagogastric ESD in China. The result demonstrated the S-D discharge strategy may be feasible and effective for esophagogastric ESD, and the procedural-related AEs can be managed successfully.
This first retrospective study evaluating S-D discharge procedures for esophagogastric ESD in China demonstrates that this strategy may be feasible and effective, and that the AEs related to ESD could be managed successfully. Additional prospective studies are warranted to establish more detailed standards to select patients for S-D discharge ESD.
We thank Zhong-Hu He for his generous help in the data analysis.
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (2)] |
| 2. | Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M, Goda K, Goto O, Tanaka K, Yano T, Yoshinaga S, Muto M, Kawakubo H, Fujishiro M, Yoshida M, Fujimoto K, Tajiri H, Inoue H; Japan Gastroenterological Endoscopy Society Guidelines Committee of ESD/EMR for Esophageal Cancer. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 3. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (1)] |
| 4. | Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic Submucosal Dissection: Indications and Application in Western Endoscopy Practice. Gastroenterology. 2018;154:1887-1900.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 5. | Libânio D, Braga V, Ferraz S, Castro R, Lage J, Pita I, Ribeiro C, Abreu De Sousa J, Dinis-Ribeiro M, Pimentel-Nunes P. Prospective comparative study of endoscopic submucosal dissection and gastrectomy for early neoplastic lesions including patients' perspectives. Endoscopy. 2019;51:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Ahmed Y, Othman M. EMR/ESD: Techniques, Complications, and Evidence. Curr Gastroenterol Rep. 2020;22:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Aadam AA, Abe S. Endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Kim GH, Jung HY. Endoscopic Resection of Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:563-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016;84:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Probst A, Ebigbo A, Märkl B, Schaller T, Anthuber M, Fleischmann C, Messmann H. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy. 2017;49:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hanada Y, Wang KK. Safety and feasibility of same-day discharge after esophageal endoscopic submucosal dissection. Gastrointest Endosc. 2021;93:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Choi JY, Park YS, Na G, Park SJ, Yoon H, Shin CM, Kim N, Lee DH. Safety and effectiveness of endoscopic mucosal resection or endoscopic submucosal dissection for gastric neoplasia within 2 days' hospital stay. Medicine (Baltimore). 2019;98:e16578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Tomiki Y, Kawai M, Takehara K, Tashiro Y, Munakata S, Kure K, Ishiyama S, Sugimoto K, Kamiyama H, Takahashi M, Sakamoto K. Clinical pathway to discharge 3 days after colorectal endoscopic submucosal dissection. Dig Endosc. 2015;27:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Li SJ, Wang J, Li ZY, Bu ZD, Su XQ, Li ZW, Wu Q. [Application of endoscopic submucosal dissection in treatment of early gastric cancer]. Beijing Da Xue Xue Bao Yi Xue Ban. 2015;47:945-951. [PubMed] |
| 15. | Wang J, Li SL, Wu N, Wu Q. Effectiveness of fibrin sealant as a hemostatic technique in accelerating endoscopic submucosal dissection-induced ulcer healing and preventing stricture in the esophagus: A retrospective study. Oncol Lett. 2020;20:2322-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Wang J, Shan F, Li S, Li Z, Wu Q. Effect of administration of a proton pump inhibitor for ulcerative differentiated early gastric cancer prior to endoscopic submucosal dissection. Dig Endosc. 2021;33:939-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (2)] |
| 18. | Park CH, Yang DH, Kim JW, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intest Res. 2021;19:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, Nakajima A, Hoshino E, Igarashi M. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Mochizuki S, Uedo N, Oda I, Kaneko K, Yamamoto Y, Yamashina T, Suzuki H, Kodashima S, Yano T, Yamamichi N, Goto O, Shimamoto T, Fujishiro M, Koike K; SAFE Trial Study Group. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut. 2015;64:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Kim EH, Park SW, Nam E, Eun CS, Han DS, Park CH. Role of second-look endoscopy and prophylactic hemostasis after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Ono S, Ono M, Nakagawa M, Shimizu Y, Kato M, Sakamoto N. Delayed bleeding and hemorrhage of mucosal defects after gastric endoscopic submucosal dissection on second-look endoscopy. Gastric Cancer. 2016;19:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Izumikawa K, Iwamuro M, Inaba T, Ishikawa S, Kuwaki K, Sakakihara I, Yamamoto K, Takahashi S, Tanaka S, Wato M, Okada H. Bleeding in patients who underwent scheduled second-look endoscopy 5 days after endoscopic submucosal dissection for gastric lesions. BMC Gastroenterol. 2018;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Baldaque-Silva F, Marques M, Andrade AP, Sousa N, Lopes J, Carneiro F, Macedo G. Endoscopic submucosal dissection of gastrointestinal lesions on an outpatient basis. United European Gastroenterol J. 2019;7:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Nonaka S, Oda I, Makazu M, Haruyama S, Abe S, Suzuki H, Yoshinaga S, Nakajima T, Kushima R, Saito Y. Endoscopic submucosal dissection for early gastric cancer in the remnant stomach after gastrectomy. Gastrointest Endosc. 2013;78:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Ikehara H, Oshima T, Fukui H, Miwa H. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: A prospective pilot study. World J Gastrointest Endosc. 2013;5:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Misumi Y, Nonaka K. Prevention and Management of Complications and Education in Endoscopic Submucosal Dissection. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Kim SG, Park CM, Lee NR, Kim J, Lyu DH, Park SH, Choi IJ, Lee WS, Park SJ, Kim JJ, Kim JH, Lim CH, Cho JY, Kim GH, Lee YC, Jung HY, Lee JH, Chun HJ, Seol SY. Long-Term Clinical Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver. 2018;12:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Shichijo S, Uedo N, Kanesaka T, Ohta T, Nakagawa K, Shimamoto Y, Ohmori M, Arao M, Iwatsubo T, Suzuki S, Matsuno K, Iwagami H, Inoue S, Matsuura N, Maekawa A, Nakahira H, Yamamoto S, Takeuchi Y, Higashino K, Ishihara R, Fukui K, Ito Y, Narahara H, Ishiguro S, Iishi H. Long-term outcomes after endoscopic submucosal dissection for differentiated-type early gastric cancer that fulfilled expanded indication criteria: A prospective cohort study. J Gastroenterol Hepatol. 2021;36:664-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Urdaneta F, Wardhan R, Wells G, White JD. Prevention of pulmonary complications in sedated patients undergoing interventional procedures in the nonoperating room anesthesia setting. Curr Opin Anaesthesiol. 2022;35:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Yoshimizu S, Yamamoto Y, Horiuchi Y, Yoshio T, Ishiyama A, Hirasawa T, Tsuchida T, Fujisaki J. A suitable marking method to achieve lateral margin negative in endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endosc Int Open. 2019;7:E274-E281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Maselli R, Galtieri PA, Di Leo M, Ferrara EC, Anderloni A, Carrara S, Vanni E, Mangiavillano B, Genco A, Al Awadhi S, Fuccio L, Hassan C, Repici A. Cost analysis and outcome of endoscopic submucosal dissection for colorectal lesions in an outpatient setting. Dig Liver Dis. 2019;51:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Kim GH, Choi KD, Ko Y, Park T, Kim KW, Park SY, Na HK, Ahn JY, Lee JH, Jung KW, Kim DH, Song HJ, Lee GH, Jung HY. Impact of Comorbidities, Sarcopenia, and Nutritional Status on the Long-Term Outcomes after Endoscopic Submucosal Dissection for Early Gastric Cancer in Elderly Patients Aged ≥ 80 Years. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Othman MO, Bahdi F, Ahmed Y, Gagneja H, Andrawes S, Groth S, Dhingra S. Short-term clinical outcomes of non-curative endoscopic submucosal dissection for early esophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2021;33:e700-e708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Kim JH, Kim SS, Lee JH, Jung DH, Cheung DY, Chung WC, Park SH. Early Detection is Important to Reduce the Economic Burden of Gastric Cancer. J Gastric Cancer. 2018;18:82-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Cui N, Zhao Y, Yu H. Cost Analysis of Endoscopic Submucosal Dissection for the Treatment of Colorectal Lesions in China. Biomed Res Int. 2019;2019:6983896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hisada H, Japan; Noh CK, South Korea S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ