Published online Nov 7, 2022. doi: 10.3748/wjg.v28.i41.5968
Peer-review started: August 19, 2022
First decision: September 12, 2022
Revised: September 24, 2022
Accepted: October 13, 2022
Article in press: October 13, 2022
Published online: November 7, 2022
Processing time: 76 Days and 19.4 Hours
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a form of rare primary liver cancer that combines intrahepatic cholangiocarcinoma (ICC) and hepatocellular carcinoma.
To investigate overall survival (OS) and recurrence-free survival (RFS) after radical resection in patients with cHCC-CCA, and the clinicopathological factors affecting prognosis in two center hospitals of China.
We reviewed consecutive patients with cHCC-CCA who received radical rese
Our study included 95 patients who received radical resection. The majority of these patients were male and 82.7% of these patients were infected with HBV. The mean tumor size was 4.5 cm, and approximately 40% of patients had more than one lesion. The median OS was 26.8 (95%CI: 18.5-43.0) mo, and the median RFS was 7.27 (95%CI: 5.83-10.3) mo. Independent predictors of OS were CA19-9 ≥ 37 U/mL (HR = 8.68, P = 0.002), Child-Pugh score > 5 (HR = 5.52, P = 0.027), tumor number > 1 (HR = 30.85, P = 0.002), tumor size and transarterial chemoembolization (TACE) after surgery (HR = 0.2, P = 0.005).
The overall postoperative survival of cHCC-CCA patients is poor, and most patients experience relapse within a short period of time after surgery. Preoperative tumor biomarker (CA19-9, alpha-fetoprotein) levels, tumor size, and Child-Pugh score can significantly affect OS. Adjuvant TACE after surgery prolongs RFS, suggesting that TACE is a possible option for postoperative adjuvant therapy in patients with cHCC-CCA.
Core Tip: Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a relatively rare type of primary liver cancer. Hepatectomy combined with lymph node dissection is the only possible cure. In our study, we found that the prognosis for this group of patients is poor, with a 2-year survival rate of approximately 50% after radical resection. Preoperative CA19-9 Level, tumor number, tumor size and whether or not to receive tumor size and transarterial chemoembolization (TACE) after surgery were independent factors affecting overall survival. Therefore, we recommend that patients with cHCC-CCA actively receive adjuvant TACE therapy after surgery.
- Citation: Zhang G, Chen BW, Yang XB, Wang HY, Yang X, Xie FC, Chen XQ, Yu LX, Shi J, Lu YY, Zhao HT. Prognostic analysis of patients with combined hepatocellular-cholangiocarcinoma after radical resection: A retrospective multicenter cohort study. World J Gastroenterol 2022; 28(41): 5968-5981
- URL: https://www.wjgnet.com/1007-9327/full/v28/i41/5968.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i41.5968
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a relatively rare primary liver cancer (PLC) and accounts for 0.4% to 14.2% of the incidence of PLC[1-4]. The definition of cHCC-CCA has been updated because of unclear understanding. In 2019, the WHO updated the cHCC-CCA classification[5], and in conventional histopathology of hematoxylin and eosin (H&E) staining, cHCC-CCA shows two different degrees of differentiation, hepatocellular and cholangiocarcinoma, within the same lesion. In contrast to the well-established management pathways for hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), treatment remains a gray area for cHCC-CCA currently. The overall prognosis of patients with cHCC-CCA is worse than that of patients with HCC, and the prognosis is similar to that of patients with ICC. Vascular invasion actually seems to occur more frequently in cHCC-CCA than in HCC. In addition, lymph node metastases exhibit similar characteristics[6]. The treatment of cHCC-CCA has not been standardized in comparison to HCC and ICC, and a number of therapy strategies have been suggested. Radical tumor resection and lymph node dissection are the only curative options for patients with cHCC-CCA[7,8]. Nonetheless, the 5-year survival rate does not reach 30%, and the tumor recurrence rate is considerable (up to 80% after 5 years) in most studies[9-11].
In our research, we retrospectively analyzed cHCC-CCA patients who received surgical resection at two institutions to explore clinical case information for this rare tumor on prognosis, looking for factors affecting recurrence and long-term survival. All patients underwent rigorous organizational path
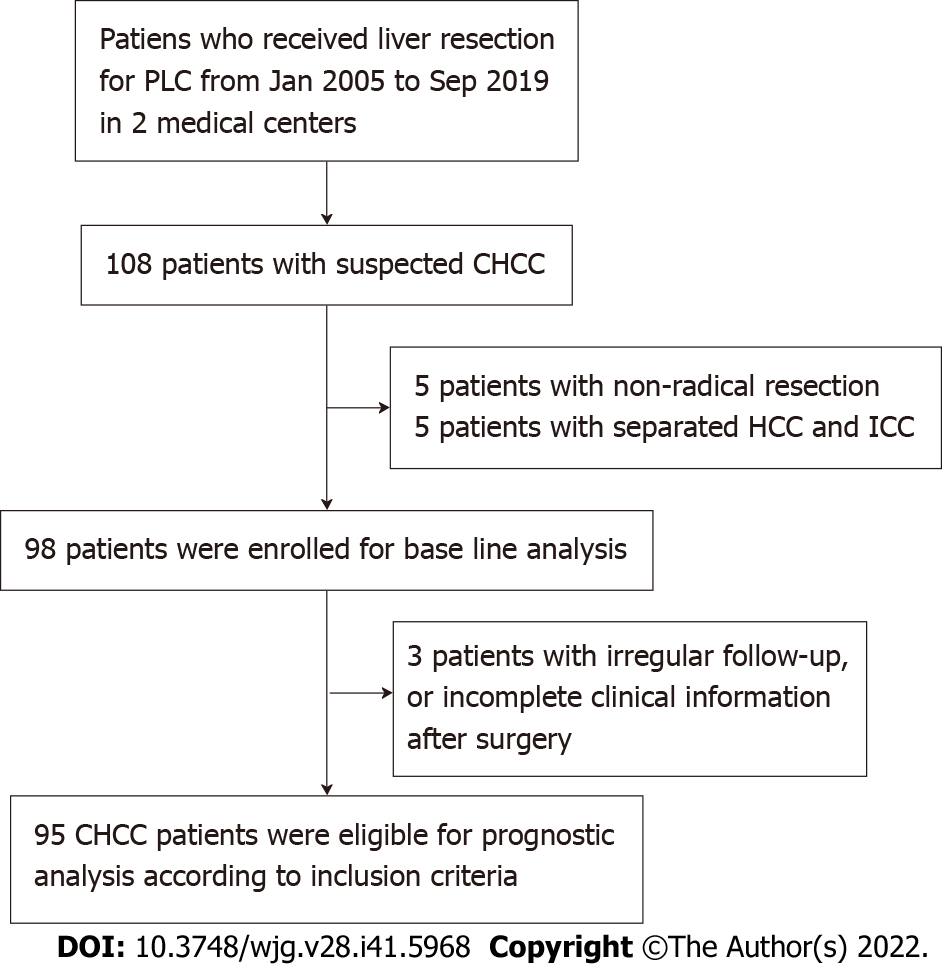
Among the patients who received hepatectomy for PLC in Peking Union Medical College Hospital and The 5th Medical Center of the PLA General Hospital from January 2005 to September 2021, 95 patients were pathologically diagnosed with cHCC-CCA based on the latest WHO criteria in 2019. Among these patients, 61 were treated in Peking Union Medical College Hospital, and 34 were treated in The 5th Medical Center of the PLA General Hospital. The inclusion criteria for these patients are described below: (1) Patients who received radical liver resection; (2) patients were pathologically diagnosed with cHCC-CCA; and (3) patients with complete clinical information and at least 2 follow-up visits after surgery. The exclusion criteria are described below: (1) Non-radical resection; (2) separated HCC and ICC; (3) incomplete clinical information, or irregular follow-up after surgery; and (4) history of other malignancies.
Based on regular medical records and telephone follow-up records, we determined how these patients were treated after surgery, whether they survived, and whether they experienced recurrence. Two patients had HCC and ICC at the same time, but the growth was dissociative, so they were excluded. Due to lost follow-up or too short follow-up time, another three patients were only used for baseline information statistics and not for prognosis analysis (Figure 1).
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (Reg. numbers JS-3390) and The 5th Medical Center of the PLA General Hospital (Reg. number KY-2022-4-23-1), and the study protocol conforms to the ethical guidelines of the Declaration of Helsinki. All participants signed written informed consent.
Through a search of the patients’ medical records, we collected the following clinical information: Age, sex, background of liver disease, Eastern Cooperative Oncology Group (ECOG) score, gallstones, CA19-9 Level, alpha-fetoprotein (AFP) level, carcinoembryonic antigen (CEA) level, total bilirubin (TBil) level, direct bilirubin (DBil) level, albumin, ascites, and cirrhosis before surgery. The preserved liver functional was evaluated using the Child-Pugh (C-P) scoring system[12].
By reviewing the radiological reports, pathology reports and pathology sections of patients, we collected the following pathological information: tumor size, tumor number, macrovascular invasion (Macro VI), microvascular invasion (Micro VI), lymph node metastasis, distance to section, Ki-67, cytokeratin 7 (CK7), cytokeratin 19 (CK19), Hepatocyte paraffin 1 (HepPar-1), Glypican-3 (GPC-3), HCC differentiation, HCC percent, ICC differentiation, and ICC percent. HepPar-1 and GPC-3 were used as HCC markers, and CK7 and CK19 were used as biliary epithelial markers. Due to the absence of an optimal staging system for cHCC-CCA, we applied the American Joint Committee on Cancer (AJCC) staging manual (8th edition) to cHCC-CCA[13].
Overall survival (OS, defined as the time interval from the date of surgery to death or the last follow-up, depend on which came first) and recurrence-free survival (RFS, defined as the time interval from the date of surgery to recurrence, death, or the last follow-up, depend on which came first) were the primary measures for this study.
Normality tests for continuous variables were performed by the Shapiro-Wilk test[14]. Normal continuous variables were compared between patients in the two centers by analysis of variance. To compare nonnormal continuous variables, the Kruskal-Wallis test was utilized[15]. Categorical variable data were compared by Fisher’s exact test[16]. Normal continuous variables were shown as the mean ± SD. Nonnormal continuous variables are shown as the median and IQR. Categorical variable data were displayed as numbers and percentages. The survival rate was determined using the Kaplan-Meier method. Univariate and multivariate analysis were performed using the log-rank test and Cox proportional hazards regression model, respectively. To identify independent prognostic factors, variables with P values < 0.15 in univariate analysis were incorporated into the Cox proportional hazards model. A P value with two tails < 0.05 was regarded as statistically significant. All analysis were performed using R 4.1.0.
In our research, we analyzed the preoperative clinical data of 98 (95 plus 3) patients (Table 1). Of the 98 patients, 86 (87.8%) were male. The mean age was 55.3 ± 10.4 years. The majority of patients had well-preserved liver function (Child-Pugh class A or B), the vast majority had an ECOG score of 0-1 (96.9%), and the majority had HBV infection (82.7%).
| Overall | The 5th Medical Center of the PLA General Hospital | Peking Union Medical College Hospital | P value | |
| Number | 98 | 34 | 64 | |
| Age, mean ± SD | 55.3 (10.4) | 53.5 (10.4) | 56.3 (10.3) | 0.219 |
| Sex | ||||
| Male | 86 (87.8) | 32 (88.2) | 56 (87.5) | 1 (Fisher) |
| Female (%) | 12 (12.2) | 4 (11.8) | 8 (12.5) | |
| ECOG (%) | 0.009 (Fisher) | |||
| 0 | 84 (85.7) | 26 (76.5) | 58 (90.6) | |
| 1 | 11 (11.2) | 8 (23.5) | 3 (4.7) | |
| NA | 3 (3.1) | 0 (0) | 3 (4.7) | |
| Child-Pugh class | 0.435 (Fisher) | |||
| A | 86 (87.8) | 32 (94.1) | 54 (84.4) | |
| B | 6 (6.1) | 1 (2.9) | 5 (7.8) | |
| NA | 6 (6.1) | 1 (2.9) | 5 (7.8) | |
| Liver disease (%) | 0.823 (Fisher) | |||
| NA | 4 (4.1) | 1 (2.9) | 3 (4.7) | |
| HBV | 81 (82.7) | 28 (82.4) | 53 (82.8) | |
| HCV | 4 (4.1) | 2 (5.9) | 2 (3.1) | |
| Fatty liver | 2 (2.0) | 0 (0.0) | 2 (3.1) | |
| Alcohol | 7 (7.1) | 3 (8.8) | 4 (6.2) | |
| Gallstones (%) | 13 (13.3) | 3 (8.8) | 10 (15.6) | 0.533 (Fisher) |
| CA19-9 (U/mL) | 26.5 [13.1, 56.2] | 29.7 [15.1, 46.5] | 23.6 [12.4, 56.4] | 0.775 (non-norm) |
| < 37 | 58 (59.2) | 21 (61.8) | 37 (57.8) | 0.813 (Fisher) |
| ≥ 37 | 31 (31.6) | 11 (32.4) | 20 (31.2) | |
| NA | 9 (9.2) | 2 (5.9) | 7 (10.9) | |
| AFP (ng/mL) | 44.1 [7.0, 338.4] | 43.4 [5.8, 294.7] | 44.1 [7.8, 724.3] | 0.389 (non-norm) |
| < 200 | 61 (62.2) | 24 (70.6) | 37 (57.8) | 0.122 (Fisher) |
| ≥ 200 | 30 (30.6) | 10 (29.4) | 20 (31.2) | |
| NA | 7 (7.1) | 0 (0.0) | 7 (10.9) | |
| CEA (ng/mL) | 2.7 [1.6, 4.4] | 2.5 [1.5, 3.5] | 2.7 [1.7, 4.8] | 0.173 (non-norm) |
| < 6 | 80 (81.6) | 32 (94.1) | 48 (75.0) | 0.038 (Fisher) |
| ≥ 6 | 9 (9.2) | 0 (0.0) | 9 (14.1) | |
| NA | 9 (9.2) | 2 (5.9) | 7 (10.9) | |
| TBil (μmol/L) | 12.6 [10.4, 16.4] | 12.2 [10.4, 14.0] | 12.9 [10.7, 17.8] | 0.260 (non-norm) |
| DBil (μmol/L) | 4.3 [3.8, 5.7] | 4.2 [3.8, 5.0] | 4.5 [3.8, 5.8] | 0.334 (non-norm) |
| Albumin (g/L) | 41.0 [39.0, 43.5] | 40.0 [38.0, 42.0] | 41.0 [39.0, 44.0] | 0.055 (non-norm) |
| Ascites (%) | 0.094 (Fisher) | |||
| No | 75 (76.5) | 30 (88.2) | 45 (70.3) | |
| Yes | 18 (18.4) | 4 (11.8) | 14 (21.9) | |
| NA | 5 (5.1) | 0 (0.0) | 5 (7.8) | |
| Liver cirrhosis (%) | 82 (83.7) | 32 (94.1) | 50 (78.1) | 0.143 (Fisher) |
Most patients had well-preserved liver function (C-P class A or B), and most (96.9%) had an ECOG score of 0-1. HBV infection was present in 82.7% of the patients. Preoperative level of CA19-9 was higher than normal in 31 patients (31.6%) (≥ 37 U/mL), preoperative level of AFP was higher than normal in 51 patients (52.0%) (20 ng/mL, not listed), of which 30 patients (31.6%) had levels higher than 200 ng/mL, and preoperative CEA levels were higher than normal in 9 patients (9.2%) (≥ 6 ng/mL). Ascites and liver fibrosis were present in 18 patients (18.4%) and 82 patients (83.7%), respectively.
Table 2 demonstrated the pathological features of our two-center cohorts. In more than half (56.1%) of the patients, the number of lesions was more than one. The mean tumor size was 4.5 cm [range (2.9, 6.5)], and 62 patients (63.2%) had tumors smaller than 5 cm. Surgical margin did not exceed 1 cm in more than half (55.1%) of the cases. The proportions of macrovascular and microvascular invasion were 24.5% and 63.3%, respectively. Lymph node metastases were found in 12.2% of these patients. Using the AJCC staging system, we evaluated the TNM stage in 98 patients. 18 (18.3%) patients were stage I (17 IA, 1 IB), 59 (60.2%) patients were stage II, 19 (19.4%) patients were stage III (3 IIIA, 16 IIIB), and 2 patients could not be evaluated.
| Item | Patients (n = 98) |
| Tumor number | |
| Solitary | 55 (56.1) |
| Multiple | 39 (39.8) |
| NA | 4 (4.1) |
| Tumor size, median [IQR] | 4.5 [2.9, 6.5] |
| ≤ 3cm (%) | 26 (26.5) |
| 3-5 cm (%) | 36 (36.7) |
| > 5 cm (%) | 34 (34.7) |
| NA | 2 (2.0) |
| Resection margin (%) | |
| ≤ 1cm (%) | 54 (55.1) |
| > 1cm (%) | 21 (21.4) |
| NA | 23 (23.5) |
| Macro VI (%) | 24 (24.5) |
| Micro VI (%) | 62 (63.3) |
| Lymph node metastasis (%) | 12 (12.2) |
| TNM Stage (AJCC 8th) (%) | |
| I | 18 (18.4) |
| II | 59 (60.2) |
| III | 19 (19.4) |
| NA | 2 (2.0) |
| Ki-67 (%) | |
| ≤ 50% | 36 (55.4) |
| > 50% | 29 (44.6) |
| CK7 (%) | |
| Negative | 9 (11.1) |
| Weak positive | 29 (35.8) |
| Strong Positive | 43 (53.1) |
| CK19 (%) | |
| Negative | 9 (10.8) |
| Weak positive | 27 (32.5) |
| Strong Positive | 47 (56.6) |
| HepPar-1 (%) | |
| Negative | 29 (34.1) |
| Weak positive | 23 (27.1) |
| Strong Positive | 33 (38.8) |
| GPC-3 (%) | |
| Negative | 16 (28.6) |
| Weak positive | 13 (23.2) |
| Strong Positive | 27 (48.2) |
| HCC differentiation (%) | |
| Poorly differentiated | 19 (41.3) |
| Well or moderate differentiated | 27 (58.7) |
| ICC differentiation (%) | |
| Poorly differentiated | 30 (65.2) |
| Well or moderate differentiated | 16 (34.8) |
| ICC percent (%) | |
| ≤ 50% | 11 (30.7) |
| > 50% | 16 (59.3) |
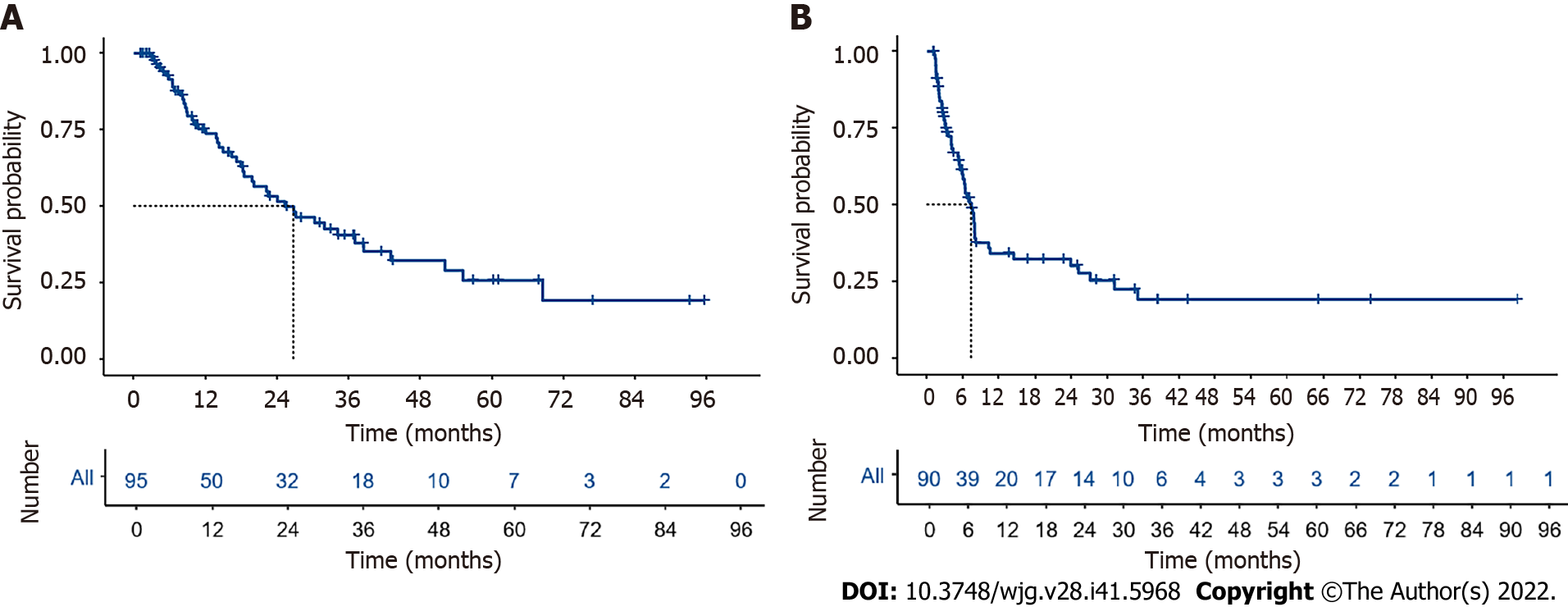
Ninety-five patients with follow-up longer than 1 mo were used in survival and recurrence analysis. The median follow-up time was 34.2 mo (95%CI: 28.0-43.3), and the median OS was 26.8 mo (95%CI: 18.5-43.0) (Figure 2A). The estimated cumulative survival rates at 1, 2, 3, and 5 years were 73.9%, 51.7%, 38.2%, and 23.6%, respectively. The median RFS was 7.27 mo (95%CI: 5.83-10.3) (Figure 2B), and the estimated cumulative RFS rates at 6 mo, 1 year, and 2 years were 58.4%, 33.6%, and 30.4%, respectively. Most patients experienced relapse within 1 year after surgery. In addition, we further staged the patients using the AJCC Staging Manual (8th edition), and the results the results revealed a substantial difference in the median OS between stage I/II patients and stage III patients.
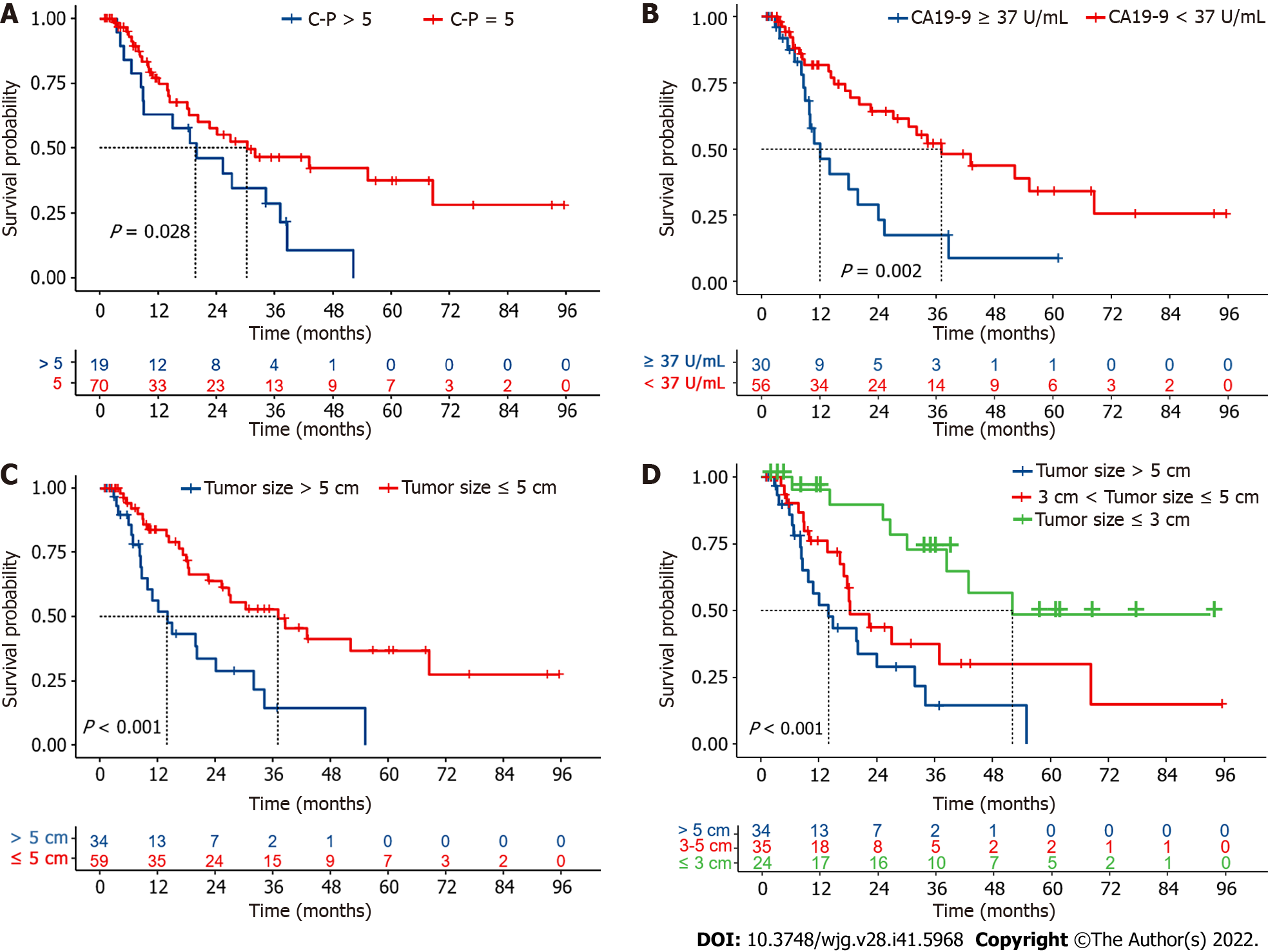
Subgroup analysis showed that preoperative liver function grading (C-P score 5 vs > 5) remarkably affected prognosis, and patients with a preoperative C-P score of 5 had a significantly better survive than those with a preoperative C-P score greater than 5 (Figure 3A). The median OS was considerably lower for patients with baseline CA19-9 Levels over 37 U/mL than it was for those with levels below 37 U/mL (Figure 3B); however, subgrouping for AFP levels did not yield similar results (Supplemen
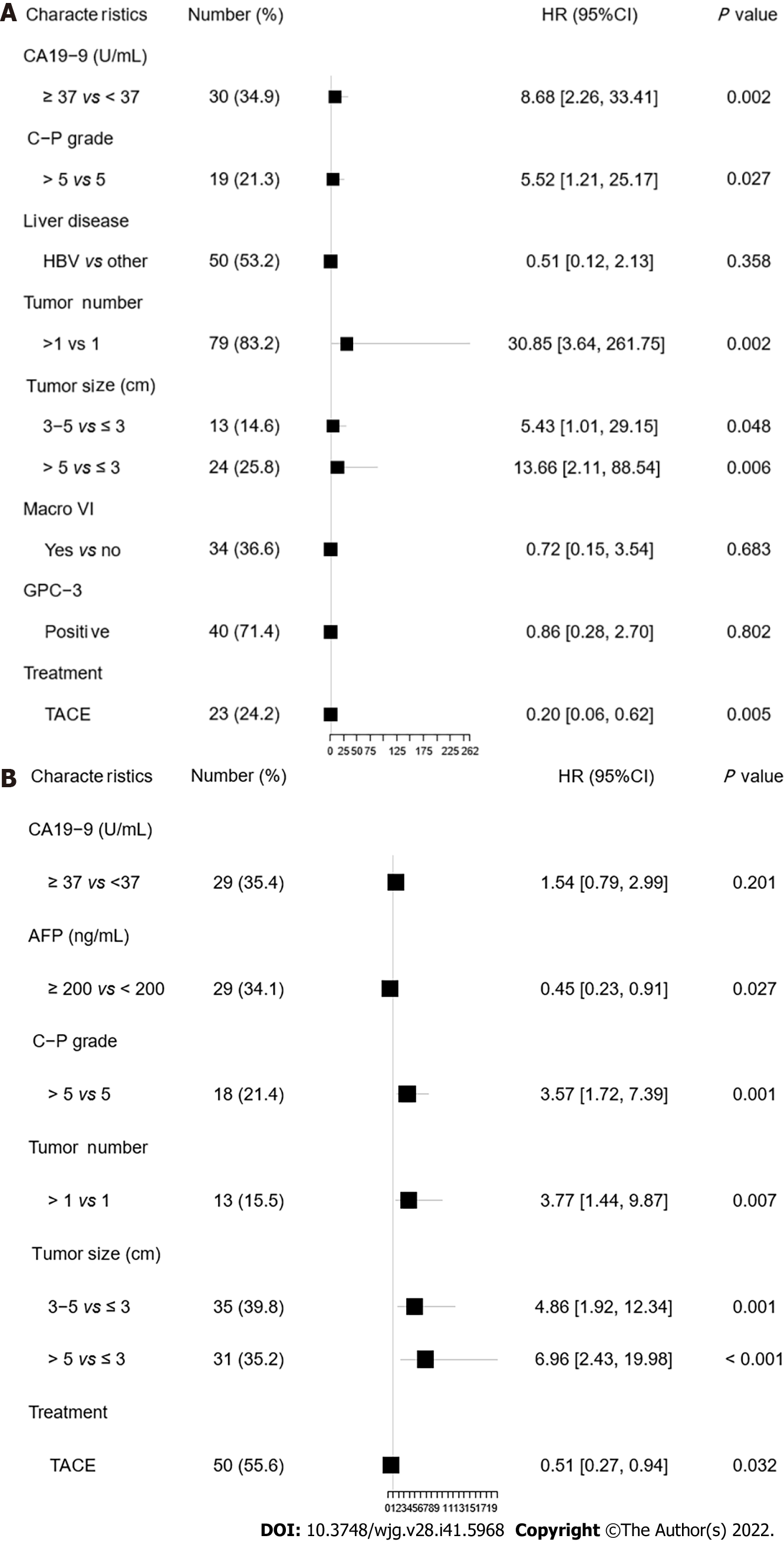
The results of univariate analysis indicated that the factors that prominently influenced OS were CA19-9 Level (≥ 37 U/mL vs < 37 U/mL), C-P score (> 5 vs 5), tumor size, and postoperative transarterial chemoembolization (TACE) intervention. The background of liver disease, macrovascular invasion, GPC-3 expression, and HCC differentiation showed similar effects (0.05 < P < 0.10). In contrast, age, gender, AFP level (≥ 200 ng/mL vs < 200 ng/mL), number of lesions, cut margins, and Micro VI were not associated with OS (Supplementary Figure 2). Further multivariate analysis revealed CA19-9 ≥ 37 U/mL (HR = 8.68, P = 0.002), C-P score > 5 (HR = 5.52, P = 0.027), tumor number > 1 (HR = 30.85, P = 0.002), tumor size, and postoperative TACE intervention (HR = 0.2, P = 0.005) as independent prognostic factors affecting OS (Figure 4A).
The similar subgroup analysis was carried out to further evaluate the variables impacting patient recurrence as patients with cHCC-CCA typically suffered recurrence within a short period of time. The results showed that patients with a preoperative C-P score of 5 had an actually longer RFS than patients with a C-P score greater than 5 (Supplementary Figure 3A). In addition, RFS was also significantly shorter in patients with multiple lesions (Supplementary Figure 3B), with patients with a tumor size ≤ 3 cm having a significantly longer RFS than those with tumors larger than 3 cm (Supplementary Figure 3).
The univariate analysis results were consistent with the subgroup analysis. Factors that significantly affected RFS were the C-P score, tumor number, tumor size and ICC differentiation (P < 0.05). In addition, postoperative TACE intervention was effective in prolonging patients’ RFS (Supplementary Figure 4). Further multivariate analysis showed that the C-P score > 5 (HR = 3.57, P = 0.001), AFP ≥ 200 ng/mL (HR = 0.45, P = 0.027), tumor number (HR = 3.77, P = 0.007), tumor size, and TACE intervention before recurrence (HR = 0.51, P = 0.032) were independent prognostic factors affecting RFS. AFP ≥ 200 ng/mL and postoperative TACE treatment were protective factors for RFS (Figure 4B).
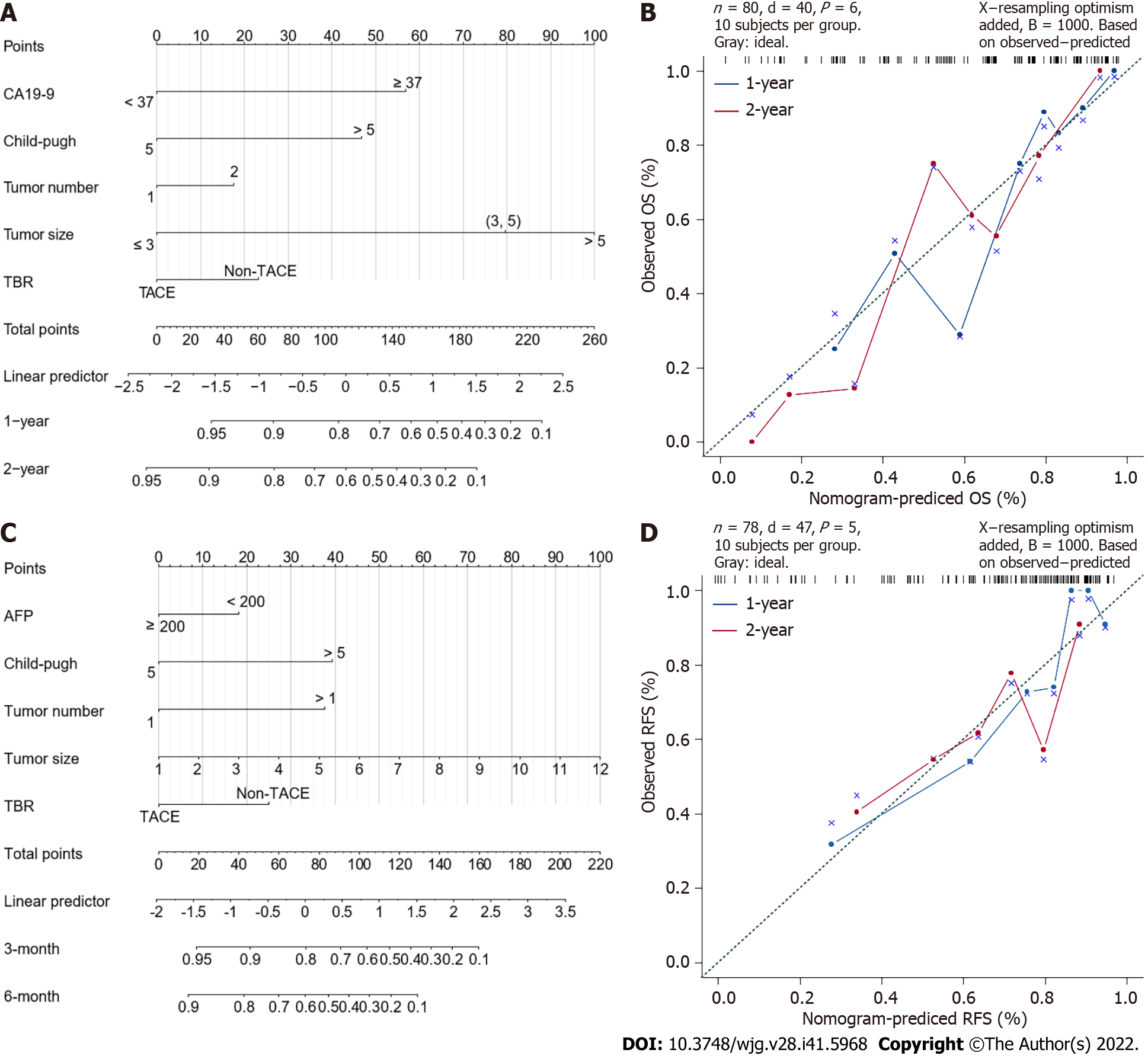
According to the results of the multivariate analysis, we constructed a nomogram which integrated the important factors for predicting OS and RFS in patients with cHCC-CCA. For predicting OS, Harrell’s concordance index (C-index) was 0.767 (Figure 5A), and this value was 0.737 when predicting RFS (Figure 5B).
As a rare kind of PLC, the percentage of cHCC-CCA varies in different studies, with the vast majority of studies concluding that its incidence is less than 15%[3,17-19]. Previous definitions of cHCC-CCA have also been changing, from the Allen and Lisa class proposed in 1949[18]; to the Goodman type proposed in 1985[19], the 2010 WHO classification (4th edition) and the 2019 WHO classification (5th edition)[1]. Currently, the pathological definition of cHCC-CCA has been refined; however, its clinical features, treatment and prognosis are still controversial, with some studies suggesting that cHCC-CCA is more comparable to HCC, and some suggesting that it is analogous to ICC[20-22], and the latest AJCC Staging Manual also suggests applying the ICC staging system to cHCC-CCA[13].
The comparison of prognosis between cHCC-CCA, HCC, and ICC has long been contentious. In present research, the median OS of cHCC-CCA patients was 26.8 mo. In previous studies, most studies concluded that the long-term survival of cHCC-CCA was worse than HCC and better than ICC[23-25], and some researchers concluded that the prognosis of cHCC-CCA was comparable to ICC[26]. However, many recent studies using propensity score matching have found no significant differences between the prognosis of cHCC-CCA and HCC or ICC when appropriate matching conditions were used[25,27], suggesting that the poorer prognosis of cHCC-CCA may be related to the behavior of the tumor.
In terms of predictive factors of cHCC-CCA in our cohort, multivariate analysis showed that CA19-9 was an important factor influencing the survive after radical surgery, and patients with high CA19-9 had a significantly worse prognosis. This is consistent with previous studies[7,28], suggesting that the ICC component may be a key factor affecting the prognosis of cHCC-CCA. Notably, AFP ≥ 200 ng/mL was a protective factor for prognosis, although in another study, there was no significant correlation between AFP and prognosis[6]. Overall, few researches have stated the connection between AFP and cHCC-CCA prognosis, and more studies are needed to investigate it.
In addition to tumor biomarkers, tumor size was an important factor affecting prognosis in our study. The median OS for patients with tumors > 5 cm was only 14 mo, and the prognosis was significantly worse in this subgroup patients (P < 0.001). And this result is in line with the findings of several prior investigations[28-30]. Based on the latest AJCC Staging Manual, ICC staging system is also applicable to cHCC-CCA, and in this TNM staging system, 5 cm is also used as a basis for differentiating between stages IA and IB. However, considering that a variable proportion of cHCC-CCA also has an HCC component, a further stratified analysis was performed for these patients. This analysis showed that patients with tumors up to 3 cm in size had a significantly better prognosis than those with tumors 3-5 cm in size (median OS: 52.1 mo vs 18.5 mo, P < 0.001), whereas patients in the 3-5 cm subgroup did not have a significantly better prognosis than those in the > 5 cm subgroup (median OS: 18.5 mo vs 14.0 mo), a phenomenon that suggests the need for more precise differentiation of cHCC-CCA patients with a tumor size ≤ 5 cm. However, in a previously conducted study of small HCC[31], the three-year OS rate after surgical resection was 91.4%, and in another similar study enrolling small HCC patients (≤ 3 cm) without vascular invasion, the 3-year survival rate after surgical resection was 96%[32]. In addition, in a recent retrospective study of ICC, the 5-year OS rate was 52.6% in 53 patients with small ICC (≤ 3 cm)[33]. In contrast, in another study, the 5-year OS rate was 40% in 44 patients with ICC, although the mean tumor size in that study was 5.5 cm[34]. These results imply that patients with cHCC-CCA have a considerably poorer prognosis than those with HCC of the same size, and their prognosis is even inferior to that of patients with ICC of the same size, suggesting that cHCC-CCA is a distinct entity of PLC that should be treated separately.
Due to the lack of accepted treatment protocols for cHCC-CCA, there are many discussions on postoperative adjuvant treatment choices for patients after resectable cHCC-CCA[22]. In our study, the univariate and multivariate results showed that postoperative TACE therapy significantly prolonged OS and RFS. TACE is a common adjuvant therapy after HCC, and previous studies have shown that TACE prolongs OS and RFS in HCC patients[35], which is based on the rationale of hindering the rich blood supply of HCC, thus promoting tumor necrosis[36]. TACE treatment has also been linked to improved survival in patients with cHCC-CCA following radical surgery, according to recent researches[24,25]. Studies including patients with unresectable cHCC-CCA have also shown that cHCC-CCA lesions with a rich blood supply have a higher response rate and better treatment outcomes for TACE[37]. These phenomena suggest that TACE might be an efficient postoperative adjuvant therapy modality for some patients with cHCC-CCA, and more studies are needed to further identify appropriate postoperative adjuvant treatment options.
Our study has some limitations. First, although our data were derived from multiple centers, selective bias in some of the data as a retrospective study and irregularities in postoperative follow-up are unavoidable. Second, our cohort was predominantly HBV-infected cHCC-CCA patients, and the applicability of these findings to non-HBV-infected cHCC-CCA patients remains to be further validated. Third, among patients with tumors ≤ 5 cm, our study found that the prognosis was significantly better for patients with tumors ≤ 3 cm, but further investigation with bigger samples is still required for this subgroup of patients. Fourth, there is still a large gap in postoperative adjuvant therapy for cHCC-CCA. In addition to TACE therapy, the role of targeted therapy and immunotherapy in preventing recurrence needs more research.
Herein, we discuss the clinical situation and prognostic features of resectable cHCC-CCA, using data from two centers. Overall, the prognosis of these patients is poor, with most patients recurring rapidly. TACE is an effective postoperative adjuvant therapy that may prolong RFS and improve patient prognosis.
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a relatively rare type of primary liver cancer. For patients who undergo radical resection, despite being able to undergo surgery, the overall postoperative prognosis is poor and the factors affecting postoperative recurrence and survival are unknown.
The motivation for this study was the poor prognosis of patients with cHCC-CCA who underwent radical surgery. Factors affecting postoperative survival remain controversial. There is a lack of clear guidelines for the choice of postoperative adjuvant therapy.
To explore the factors affecting postoperative recurrence and survival in patients with cHCC-CCA who underwent radical resection, leading to better risk stratification of patients and to investigate the impact of postoperative adjuvant therapy on prognosis.
This study is a multicenter retrospective study focusing on rare cancer types. Ninety-five patients who underwent radical resection and had surgical pathology confirmed cHCC-CCA were included. Clinical information was collected and follow-up was performed for these patients. The number of patients enrolled in this study was large and the follow-up was adequate.
For patients with cHCC-CCA undergoing radical resection, most patients recur within 1 year after surgery, with a median survival of approximately 2 years. The 5-year survival rate does not exceed 30%. In addition to the biological characteristics of the tumor, postoperative transarterial chemoembolization (TACE) can significantly affect the prognosis. This finding helps to assist physicians and patients in the selection of postoperative adjuvant therapy.
Most patients with cHCC-CCA experience recurrence within a short period of time after surgery. Postoperative adjuvant TACE prolongs RFS and is a possible option for postoperative adjuvant therapy.
The main direction of future research is to explore appropriate preoperative diagnostic methods as well as postoperative adjuvant treatment options.
| 1. | Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol. 2021;74:1212-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Ramai D, Ofosu A, Lai JK, Reddy M, Adler DG. Combined Hepatocellular Cholangiocarcinoma: A Population-Based Retrospective Study. Am J Gastroenterol. 2019;114:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 4. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2761] [Article Influence: 460.2] [Reference Citation Analysis (3)] |
| 6. | Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol. 2019;34:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Kim KH, Lee SG, Park EH, Hwang S, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009;16:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Yang Z, Shi G. Survival outcomes of combined hepatocellular-cholangiocarcinoma compared with intrahepatic cholangiocarcinoma: A SEER population-based cohort study. Cancer Med. 2022;11:692-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Kim M, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Hong SM. Postresection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 World Health Organization classification: single-center experience of 168 patients. Ann Surg Treat Res. 2021;100:260-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, Zhou Y, Fan J. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (3)] |
| 11. | Yamashita YI, Aishima S, Nakao Y, Yoshizumi T, Nagano H, Kuroki T, Takami Y, Ide T, Ohta M, Takatsuki M, Nanashima A, Ishii F, Kitahara K, Iino S, Beppu T, Baba H, Eguchi S. Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: High rate of early recurrence and its predictors. Hepatol Res. 2020;50:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 13. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4704] [Article Influence: 522.7] [Reference Citation Analysis (4)] |
| 14. | Shapiro SS. Citation Classic - an Analysis of Variance Test for Normality (Complete Samples). Curr Contents/Soci & Behav Sci. 1985;26:14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1885] [Cited by in RCA: 1928] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 15. | Kruskal WH, Wallis WA. Citation-Classic - Use of Ranks in One-Criterion Variance Analysis. Curr Contents/Soci & Behav Sci. 1987;40:20. |
| 16. | Fisher RA. The logic of inductive inference. J of the Roy Stat Soc. 1935;98:39-82. |
| 17. | Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | ALLEN RA, LISA JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 19. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanché H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, Degott C, Zucman-Rossi J. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Aoki K, Takayasu K, Kawano T, Muramatsu Y, Moriyama N, Wakao F, Yamamoto J, Shimada K, Takayama T, Kosuge T. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology. 1993;18:1090-1095. [PubMed] |
| 22. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 23. | Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Chen PD, Chen LJ, Chang YJ. Long-Term Survival of Combined Hepatocellular-Cholangiocarcinoma: A Nationwide Study. Oncologist. 2021;26:e1774-e1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Tang Y, Wang L, Teng F, Zhang T, Zhao Y, Chen Z. The clinical characteristics and prognostic factors of combined Hepatocellular Carcinoma and Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma after Surgical Resection: A propensity score matching analysis. Int J Med Sci. 2021;18:187-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Zhou YW, Li QF, Chen YY, Wang K, Pu D, Chen XR, Li CH, Jiang L, Wang Y, Li Q, Yang Y, Gou HF, Bi F, Liu JY, Chen Y, Qiu M. Clinicopathologic features, treatment, survival, and prognostic factors of combined hepatocellular and cholangiocarcinoma: A nomogram development based on SEER database and validation in multicenter study. Eur J Surg Oncol. 2022;48:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Lin CW, Wu TC, Lin HY, Hung CM, Hsieh PM, Yeh JH, Hsiao P, Huang YL, Li YC, Wang YC, Shu CW, Chen YS. Clinical features and outcomes of combined hepatocellular carcinoma and cholangiocarcinoma versus hepatocellular carcinoma versus cholangiocarcinoma after surgical resection: a propensity score matching analysis. BMC Gastroenterol. 2021;21:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 29. | Wang T, Yang X, Tang H, Kong J, Shen S, Qiu H, Wang W. Integrated nomograms to predict overall survival and recurrence-free survival in patients with combined hepatocellular cholangiocarcinoma (cHCC) after liver resection. Aging (Albany NY). 2020;12:15334-15358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Bagante F, Merath K, Squires MH, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Pawlik TM. The Limitations of Standard Clinicopathologic Features to Accurately Risk-Stratify Prognosis after Resection of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2018;22:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Mohkam K, Dumont PN, Manichon AF, Jouvet JC, Boussel L, Merle P, Ducerf C, Lesurtel M, Rode A, Mabrut JY. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Yang HJ, Lee JH, Lee DH, Yu SJ, Kim YJ, Yoon JH, Kim HC, Lee JM, Chung JW, Yi NJ, Lee KW, Suh KS, Lee HS. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Ruzzenente A, Conci S, Viganò L, Ercolani G, Manfreda S, Bagante F, Ciangherotti A, Pedrazzani C, Pinna AD, Iacono C, Torzilli G, Guglielmi A. Role of Lymph Node Dissection in Small (≤ 3 cm) Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2019;23:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1040] [Article Influence: 54.7] [Reference Citation Analysis (1)] |
| 35. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 369] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 36. | Ebeling Barbier C, Heindryckx F, Lennernäs H. Limitations and Possibilities of Transarterial Chemotherapeutic Treatment of Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, Shin JH, Sung KB. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hietanen S, Finland; Mahmud N, United States; Thacoor A, United Kingdom S-Editor: Chen YL L-Editor: A P-Editor: Yu HG