Published online Sep 21, 2022. doi: 10.3748/wjg.v28.i35.5093
Peer-review started: May 6, 2022
First decision: June 8, 2022
Revised: June 21, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: September 21, 2022
Processing time: 132 Days and 3.4 Hours
Robotic colonoscopes could potentially provide a comfortable, less painful and safer alternative to standard colonoscopy. Recent exciting developments in this field are pushing the boundaries to what is possible in the future. This article provides a comprehensive review of the current work in robotic colonoscopes including self-propelled, steerable and disposable endoscopes that could be alternatives to standard colonoscopy. We discuss the advantages and disad
Core Tip: Colorectal cancer is a common cause of cancer related mortality. Detection and removal of precancerous polyps reduces the risk of colorectal cancer. Colonoscopy is the gold standard investigation for colorectal polyps and cancer but can be uncomfortable due to the mechanics of the procedure. Robotics has the potential to reduce the discomfort and improve the procedure for patients, while improving key performance indicators. Robotics can offer a more intuitive endoscopic appearance, using various methods to negotiate the colon to reduce discomfort. Robotics could lead to a more effective, better tolerated, safer, autonomous colonoscopy with minimal operator interaction.
- Citation: Winters C, Subramanian V, Valdastri P. Robotic, self-propelled, self-steerable, and disposable colonoscopes: Reality or pipe dream? A state of the art review. World J Gastroenterol 2022; 28(35): 5093-5110
- URL: https://www.wjgnet.com/1007-9327/full/v28/i35/5093.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i35.5093
Colorectal cancer (CRC) is the third most diagnosed cancer and the second most common cause of cancer related mortality worldwide[1]. Many countries, particularly those with a high human development index (HDI), continue to see an increasing incidence[2]. Rapid increases in the incidence of CRC can be seen in countries transitioning from medium to high HDI[2]. Although CRC aetiology is heterogeneous, the majority are sporadic, and the increasing incidence has been attributed to the western lifestyle and increasing levels of obesity[3].
The majority of CRCs have a detectable precursor lesion, a colonic polyp, which can be present for many years before cancer develops[4]. Endoscopic resection of colonic polyps has been shown to reduce cancer related mortality by up to 53%[5]. More and more countries are utilising screening programmes to control the increasing incidence of CRC. Screening programmes vary in nature and often utilise faecal testing such as faecal immunochemical testing (FIT). However, colonoscopy remains the gold standard for diagnosing and removing colonic polyps. Colonoscopy is also utilised in many polyp, colitis, and hereditary CRC surveillance programmes[6-9].
Despite the recommendations for colonoscopy as a diagnostic, screening, or surveillance tool, it is often underutilised. Delays in colonoscopy following a positive FIT has been shown to increase the likelihood of finding advanced adenomas, CRC, and advanced CRC[10]. A meta-analysis looking at second evaluation after a positive faecal occult blood test found that only 72.5% underwent a second evaluation in the form of any lower gastrointestinal investigation[11]. Additionally, a recent international survey of FIT screening programmes found that only 79% of those with a positive FIT underwent a colonoscopy[12]. Studies from Lee et al[13] and Zorzi et al[14] suggest the risk of CRC related mortality is double in those with a positive FIT who refuse colonoscopy than those who undergo a colonoscopy[13,14].
The reason for a lack of follow-up investigation may be the decision of a healthcare worker, for example due to co-morbidity. However, approximately 10% of those with a positive faecal blood test that are not followed up are due to patient refusal to have a colonoscopy[15]. Alternative tests such as computed tomography colonography (CTC) and colon capsule are available, and their merits will be discussed in future sections. Several qualitative studies have been done exploring the barriers and facilitators to colonoscopy. Although the barriers are numerous, complex, and interrelated, previous bad experiences of colonoscopy and fear of pain or discomfort are commonly cited[15-17].
The first descriptions of an endoscope came from Bozzini in 1805 which he called the Lichtleiter (light conductor)[18,19]. Several rigid endoscopes were subsequently invented, but it was over a century before Wolfe and Schindler developed the semi-rigid gastroscope which paved the way for our endoscopes of today[20]. The evolution of the modern endoscope gained speed in the 1950s when Hirschowitz and Curtiss developed the fibreoptic endoscope[21]. A flexible endoscope was developed in Japan to assess of the lower GI tract, the first colonoscope, and by the late 1960s Shinya introduced therapeutics by performing the first polypectomy[21,22]. The charged couple device (CCD) was developed and in the 1980s the videoendoscope was born. Since then, the optics have been the mainstay of research focus and endoscope advancements. We have ever evolving improvements in the optics such as high definition, magnification, and an increasing range of image enhancement virtual chromoendoscopy options. And although the technique by which we perform colonoscopy has been refined, reducing risk and discomfort, we still push and torque a semirigid tube from the distal end to advance around the colon. The introduction of electromagnetic guidance, such as ScopeGuide (ScopeGuide Endoscope Insertion Tube System, Olympus America, Inc., Allentown, PA), allowed further refinement of the technique. However, it was not until the 1990s, with advances in robotics, that the potential for a new way to perform colonoscopy was explored.
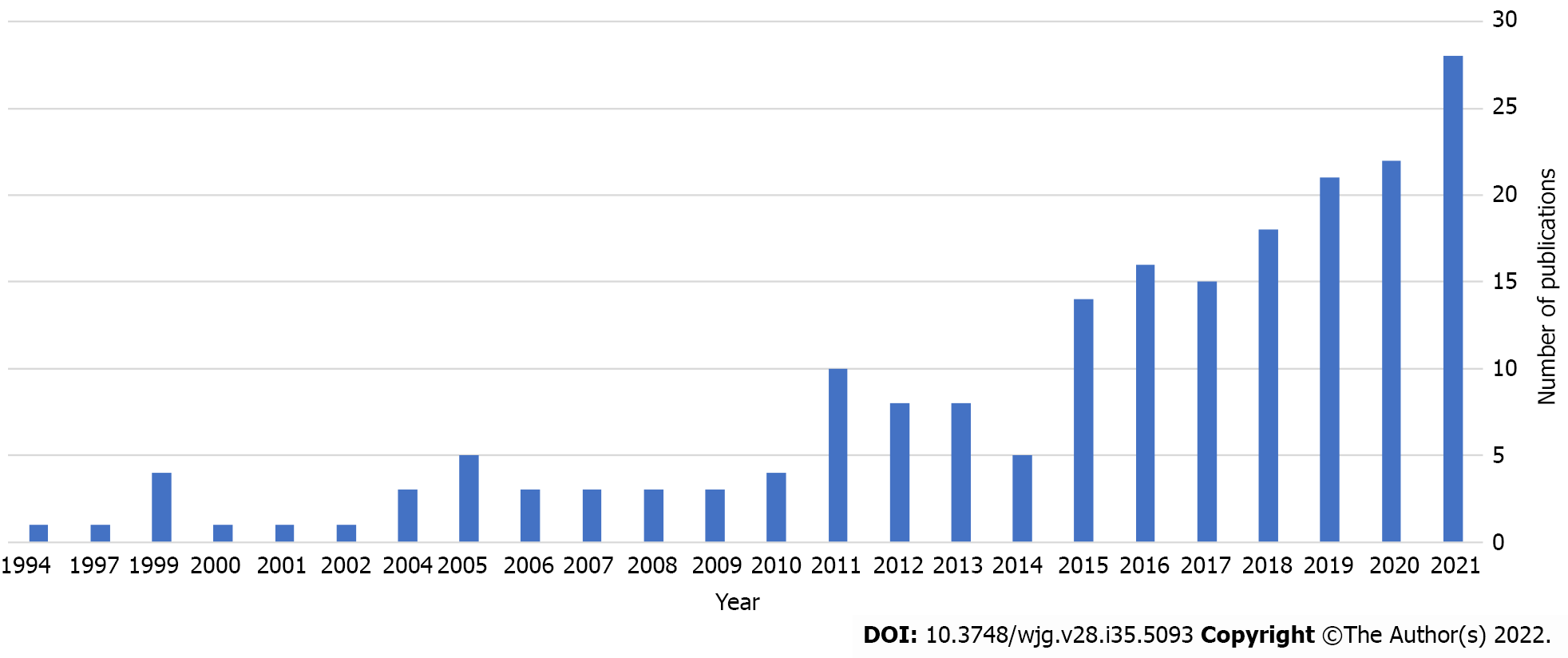
Following Food and Drug Administration (FDA) approval in 2000 of robotic surgical master-slave platforms such as da Vinci and Zeus, there has been an upsurge in interest in robotic endoscopic devices. Figure 1 shows the increasing number of robotic colonoscopy articles on PubMed[23]. Robotic colonoscopy has potential benefits over conventional colonoscopy (CC) for the patient as well as the endoscopist and service provider. Robotics in colonoscopy is not only limited to diagnostic procedures, the additional dexterity and triangulation offered by many robotic platforms has great potential in therapeutic procedures such as endoscopic submucosal dissection (ESD).
Recent advances in robotics and miniaturisation, as well as the evolving nature of artificial intel
For robotics to be accepted in endoscopy they must not only offer the same role as CC, but more. The ideal robotic colonoscope should fulfil several criteria as listed in Table 1.
| No. | Ideal robotic colonoscope features |
| 1 | Affordable |
| 2 | Acceptable to patients and endoscopists |
| 3 | More comfortable than conventional colonoscopy |
| 4 | Lower risk than conventional colonoscopy |
| 5 | Improved caecal intubation rate compared to conventional colonoscopy |
| 6 | Offer at least comparable mucosal visibility with the option of image enhancement (virtual chromoendoscopy) |
| 7 | Capable of taking biopsies and therapeutics such as polypectomy |
| 8 | Offer integration with artificial intelligence for polyp detection and characterisation |
| 9 | Ideally have autonomous features, such as self-navigation |
| 10 | Reduce the training time to achieve competence compared to conventional colonoscopy |
| 11 | Procedure times should be less than, but must not be significantly longer than, conventional colonoscopy |
| 12 | Have sustainability in mind in the manufacturing, reprocessing or disposal of the device |
Much work in robotic colonoscopy is aimed at delivering a less painful and lower risk endoscopic procedure by reducing the forces on the colonic mucosa and tethering structures. The principal method of reducing this discomfort is through altering the actuation method. Many devices have taken inspiration from nature through biomimicry, for example inchworm or snake like movements. Others use external actuation, such as magnets to pull the device through the colon. A lower discomfort procedure also offers the potential for less anaesthetic/sedative use, further lowering the risk of colonoscopy[24]. Miniaturisation, and particularly capsule endoscopy, have the potential to offer a less invasive, less embarrassing, and more convenient option for patients. Some devices may even be provided in a community setting, negating the need to travel to hospital. Although incomplete colonoscopy rates have improved over the last few decades, there remain a number of patients who require a second procedure. Robotics has the potential to reduce the number of failed procedures by improving caecal intubation rates (CIRs).
Training in both the diagnostic and therapeutic aspects of colonoscopy take considerable time[25,26]. Robotics has the potential to make colonoscopy training easier through more intuitive controls, autonomous features and the increased dexterity offered by many platforms. Less loop formation and lower discomfort rates could translate into improved completion rates. The improvement in manoeuvrability could reduce polyp miss rates and potential post-colonoscopy CRCs. Robotics has the potential to ‘democratise’ colonoscopy, reducing the variability in polyp detection rates by improving the detection rates of less well performing endoscopists. Many platforms are aiming to improve the ergonomics of colonoscopy in an attempt to reduce fatigue and endoscopy related musculoskeletal injuries[27,28].
Our forebearers in surgical robotics have yet to provide any clear answers as to the cost effectiveness of robotic procedures. The initial purchase costs are often high, but the cost benefit thereafter is unclear and variable between procedures[29-32]. The Endotics robotic colonoscope, discussed in detail later, claims to offer a cost neutral alternative to standard colonoscopy[33]. Although few robotic colonoscopes are at a technology readiness level (TRL) to perform health economics studies, we will aim to discuss several potential benefits to service providers.
Unsedated procedures offer cost and efficiency saving benefits through utilisation of less anaesthetic and recovery staff, as well as a quicker turnaround within the department. There is an increasing demand on endoscopy services caused by an aging population, increasing environmental and behavioural risk factors, and ever increasing screening and surveillance populations[34]. The addition of the recent pandemic associated service provision issues and enhanced infection control and prevention measures are stretching endoscopy departments to their limit[35]. The ideal robotic colonoscope to ease these pressures would require less staff and be quicker, pain-free, teleoperated, mobile and have therapeutic capabilities. A single use device would negate the cost associated with reprocessing, as well as potentially reducing cross contamination[36]. Improvements in sustainability may also be achieved using single use devices which reduce the need for carbon- and water-heavy reprocessing facilities.
Alternative procedures to assess the colon are required if a patient refuses or is unfit for a colonoscopy, or in the event of an incomplete colonoscopy. There are a number of alternatives to CC, most commonly CTC and colon capsule endoscopy (CCE). The advantages and limitations of these commonly used alternatives are shown in Table 2.
| Procedure | Advantages | Limitations |
| Conventional colonoscopy | Extensive knowledge base and expertise already available, diagnostic and therapeutic capabilities. Gold standard | Bowel cleansing required, painful for some (sedative and analgesics often required), prolonged training period required, risk of perforation due to forces required |
| CT colonography[39,41,101] | Lower intensity bowel cleansing, shorter procedure, less discomfort (no sedation or analgesia needed), other intraabdominal pathology can be detected, lower risk of perforation, better patient tolerance | Low dose radiation used, lower sensitivity for small and flat polyps, no therapeutic capability, no direct mucosal visualisation, limited evidence of a benefit in CRC incidence or mortality |
| Wireless capsule colonoscopy | Minimally invasive, painless, better patient tolerability, low perforation risk | Aggressive bowel cleansing required, lower sensitivity than CC for polyps, no control of the capsule, no therapeutic capability, risk of capsule retention, limited battery life can cut out before complete colon visualisation |
CTC, until the recent adoption of CCE, was the most commonly used alternative to colonoscopy. CTC has a similar sensitivity to colonoscopy for the detection of large (> 10 mm) polyps and CRC[37,38]. CTC is better tolerated than CC and over half of patients have an additional pathology detected on the scan[37,39]. However, approximately 30% of CTC patients require further investigation, often in the form of a colonoscopy to remove polyps[38]. CTC is significantly less sensitive than colonoscopy for detection of small polyps and flat lesions such as sessile serrated lesions[40].
CCE is better tolerated than CC[41]. A recent meta-analysis showed that when compared with CC, CCE had a sensitivity and specificity of 87% and 88% respectively, for detection of polyps ≥ 6 mm[42]. However, a large retrospective study by Benech et al[43], showed that 19% of CCEs had a missed advanced adenoma when they underwent a further procedure[43]. When comparing CTC to CCE, Spada et al[44] compared 100 patients with an incomplete colonoscopy and found a relative sensitivity of 2.0 in favour of CCE for identifying polyps ≥ 6 mm[44]. The recently published TOPAZ study also suggested CCE was superior to CTC for the detection of polyps ≥ 6 mm and non-inferior for polyps ≥ 10 mm[45]. However, some inherent biases and flaws in methodology of the TOPAZ study have been pointed out by Burr et al[46] which cast some doubt on the validity of these results[46]. A meta-analysis of CTC vs CCE in incomplete colonoscopies showed that CCE had superior diagnostic yields for all polyp sizes (37% vs 10%), but poorer completion rates (76% vs 98%)[47].
Magnetic resonance imaging techniques, magnetic resonance colonography (MRC), have also been evaluated as an alternative to CC. MRC has the benefits of being non-invasive and not carry the radiation risks associated with CTC. However, magnetic resonance imaging scanners can be claustrophobic for some and are very time consuming procedures. A meta-analysis in 2009 found the sensitivity of MRC for the detection of CRC was 100%, but was only 84% for polyps ≥ 10 mm[48]. A study in 30 lynch syndrome patients who underwent tandem MRC then colonoscopy, found that MRC was unable to detect any lesions under 10 mm, including one missed cancer[49]. Another study of MRC in asymptomatic individuals found MRC and colonoscopy had sensitivities of 78.4% and 97.3%, respectively, for polyps ≥ 6 mm[50]. Although some meta-analysis suggest MRC has a good sensitivity for the detection of CRC, other meta-analysis suggest the sensitivity for detection of all lesions could be as low as 75%[51].
As technologies advance, many of the technical challenges to robotic colonoscopy are being resolved. The deformable, slick surface of the colon and it’s many and varying orientations and bends make for a challenging environment and a multitude of solutions have been proposed by various research teams.
The first challenge for any robotic device is locomotion. Passive locomotion is adopted by most capsule endoscopes, in which the device passes through the gastrointestinal tract by natural peristalsis. However, the lack of control has the consequence of potentially missing lesions and rules out any therapeutic application.
Actively controlled robotic colonoscopes can be divided into internally and externally actuated devices. Internally actuated devices control propulsion by interacting directly with the surrounding environment by means of wheels, propellers, belts or bio-mimicked animal-like movements. Much inspiration for the locomotion of these devices has been taken from nature with many attempting to copy the movements of animals such as snakes, earthworms, or caterpillars, and some even mimicking the movement of micro cilia. These actuation principles are achieved using electromechanical or pneumatic mechanisms, or a combination of both. External actuation involved controlling the internal device by means of an external mechanism such as magnetic fields and field gradients. With external magnetic actuation comes the challenge of localisation of the endoscope within its environment.
Localisation refers to the position and orientation (i.e., pose) of the device. Localisation can be relative or absolute. Relative localisation is a well-developed technology and is implemented in a number of wireless capsule endoscopes (WCE) giving an estimated position with respect to the anatomy. Absolute localisation gives an accurate position and orientation. Several proposed solutions have been investigated in endoscopes including magnetic, ultrasound, radiofrequency and computer-vision technology. Many WCEs use radiofrequency localisation, current capsules have an error in position on average 38 mm and up to 100 mm[52]. Magnetic localisation is gaining increasing academic interest, and can be combined with magnetic actuation. Magnetic localisation can perform a 6 degrees of freedom (DOF) localisation with an average error in position and orientation under 5 mm and 6 mm, respectively[53].
Miniaturisation is a challenge for all robotic colonoscopes, but is of particular importance in capsule endoscopy. A capsule endoscope contains several components: External case, optical window, light emitting diode (LED), lens, image sensor, radio frequency transmitter, antenna, and a power source. Powering a capsule endoscope to allow for the variable colonic transit times requires a battery sufficiently large and often means adjusting the frames per seconds, and ultimately sensitivity, to preserve battery life. All this must then fit within a safe size for transit through the GI tract. Most capsule endoscopes are 11 mm in diameter and up to 32 mm in length with a battery life now exceeding 10 h and often containing two cameras[34]. One proposed method to overcome the miniaturisation challenge is external actuation via magnetic coupling, reducing the size of the battery in capsules and the need for intricate mechanisms of locomotion. One such commercially available capsule endoscope which already takes advantage of magnetic coupling is the NaviCam (Ankon Technologies, Wuhan, Shanghai, China) for inspection of the upper gastrointestinal tract[54].
The goal for most robotic colonoscopes is to improve the patient experience. For most devices this takes the form of reducing discomfort and risk. Capsule endoscopes achieve this by causing no distortion to the GI tract during their passive transit. Actively controlled devices aim to traverse the colon retrograde while reducing distortion and pressure exerted, resulting in less pain. Potentially also reducing the risk of perforation associated with the forces often required to torque and push around the colon in CC. Various actuation methods aim to tackle this problem by rolling, walking, inching, slithering, or pulling with magnets along the colon.
Not many robotic colonoscopy devices have health economic studies assessing their financial impact. Even the colon capsule, which is now available as an alternative to CC in some health services such as the National Health Service in the United Kingdom, has yet to show any cost effectiveness benefit over CC[55].
Reprocessing presents a massive challenge in robotic colonoscopy due to the intricacy of some devices. Many researchers are pursuing the potential of single use devices. With over 18 million endoscopic procedure per year in the United States, endoscopy is the third highest generator of waste in healthcare. Endoscope reprocessing uses up to 100 L of water as well as the electricity, heat, disinfectants and detergents[56]. Single use endoscopes have the potential to avoid this carbon heavy process. Provided the disposal of the device does not incur a higher carbon footprint, single use robotic devices have the potential to present a greener option for colonoscopy.
Perhaps the most notable evidence of robotic autonomy in day-to-day life is in automotive vehicles which can park and even navigate with minimal external input. However, medical robotics is also seeing an evolution in autonomy. Standard colonoscopy is completely under the control of the endoscopist with no autonomy. The introduction of AI for polyp detection has moved endoscopy into the first level of autonomy by visually assisting the endoscopist. Robotic colonoscopes have the potential to push the levels of autonomy much further. Devices capable of autonomously navigating the colon are being researched. However, with such levels of autonomy come challenges such as ethical and legal considerations.
TRL assessment was introduced by NASA in the 1980 to replace the traditional research and development categories: Basic research, feasibility, development, and demonstration[57]. TRL provides an objective 9 stage assessment of how advanced a technology is towards adoption[58]. When discussing each device, we will attempt to give our objective assessment of the TRL of each device. The score assigned will range from 1 (basic principles of the technology observed and report) to 9 (proof of real-world successful use of the technology) as described in Table 3, and a score will be assigned to each device described alongside the device characteristics in Table 4.
| TRL | Definition | Supporting information relevant to robotic colonoscopy |
| 1 | Basic principles observed and reported | Published research on the core principals of the technology |
| 2 | Technology concept and/or application formulated | Moving from principals to applied research with potential applications speculated |
| 3 | Analytical and experimental proof of concept | Active research and development proving the concept within a laboratory setting. Benchtop testing |
| 4 | Component validation in laboratory environment | Proof of concept and safety in an ex-vivo animal colon |
| 5 | Component/system validation in a relevant environment | In-vivo animal testing with an aim at providing relevant evidence for human testing or FDA approval |
| 6 | High fidelity alpha protype demonstration in a relevant environment | Clinical trials assessing feasibility and safety in small number of humans |
| 7 | Beta prototype demonstrated in a relevant environment | Clinical safety and effectiveness trials. Determination of risks and adverse events. Final design validation |
| 8 | Completed system and qualified to relevant requirement/standards through testing and demonstration | FDA or equivalent approval |
| 9 | Actual system proven through successful operation | Device being marked with post-market studies proving real world operational capability |
| Device name (manufacturer) | Latest study | Outcomes | TRL |
| Aer-O-Scope-GI View Ltd., Ramat Gan, Israel[21,59-61,63,64] | 2016: Human tandem study, 58 CRC screening patients. CIR: 98.2%. CIT: 11 min. 87.5% of polyps detected. No PREMs | CE marked and FDA approved. Balloon propulsion model no longer manufactured | 8 |
| ColonoSight-Stryker GI Ltd., Haifa, Israel[65,66] | 2008: Human study on 178 participants. CIR 90%. CIT: 11.2 min. No PREMs | FDA approved. No longer manufactured | 8 |
| Consis medical-Beer’Sheva, Israel[21,67] | None available | No regulatory approvals | 3 |
| Endoculus-Department of Mechanical Engineering & Division of Gastroenterology, University of Colorado, United States[20] | 2020: In-vivo and ex-vivo porcine colon in one. Unable to traverse an in-vivo colon, but capable of negotiating an ex-vivo porcine colon | No regulatory approvals | 4 |
| ENDOO robotic colonoscope-Endoo Project, Pisa, Italy[87-89] | 2020: Ex-vivo porcine colon human simulator study | No regulatory approvals | 4 |
| Endotics-ERA Endoscopy SRL, Peccioli, Italy[69-74] | 2020: Learning curve study of 57 participants. CIR and CIT improved to 100% and 22 min following a learning block. PREMs: Mild or no discomfort in most | CE marked and FDA approved. Commercially available in Europe and Japan | 8 |
| Invendoscope-Invendo Medical GmbH, Weinheim, Germany (acquired by Ambu A/S, Copenhagen, Denmark in 2017)[21,77-79] | 2018: Human study on 40 participants using the SC210 model. CIR 95%. CIT 14.2 min. No PREMs on this study, but previous studies reported lower pain scores than CC | CE marked and FDA approved. No longer manufactured | 8 |
| Magnetic Flexible Endoscope-STORM lab, Leeds, United Kingdom & Nashville, TN, United States[4,53,85,86,104] | 2020: In-vivo porcine study. Clinical trial due 2022 | No regulatory approvals | 5 |
For the purpose of this review article a selection of the most advanced platforms will be discussed, dividing them into active flexible platforms, passive devices, robot assisted platforms and robotic platforms with therapeutic applications. A table offering additional detail on the devices, as well as several more devices, is available in Supplementary Table 1.
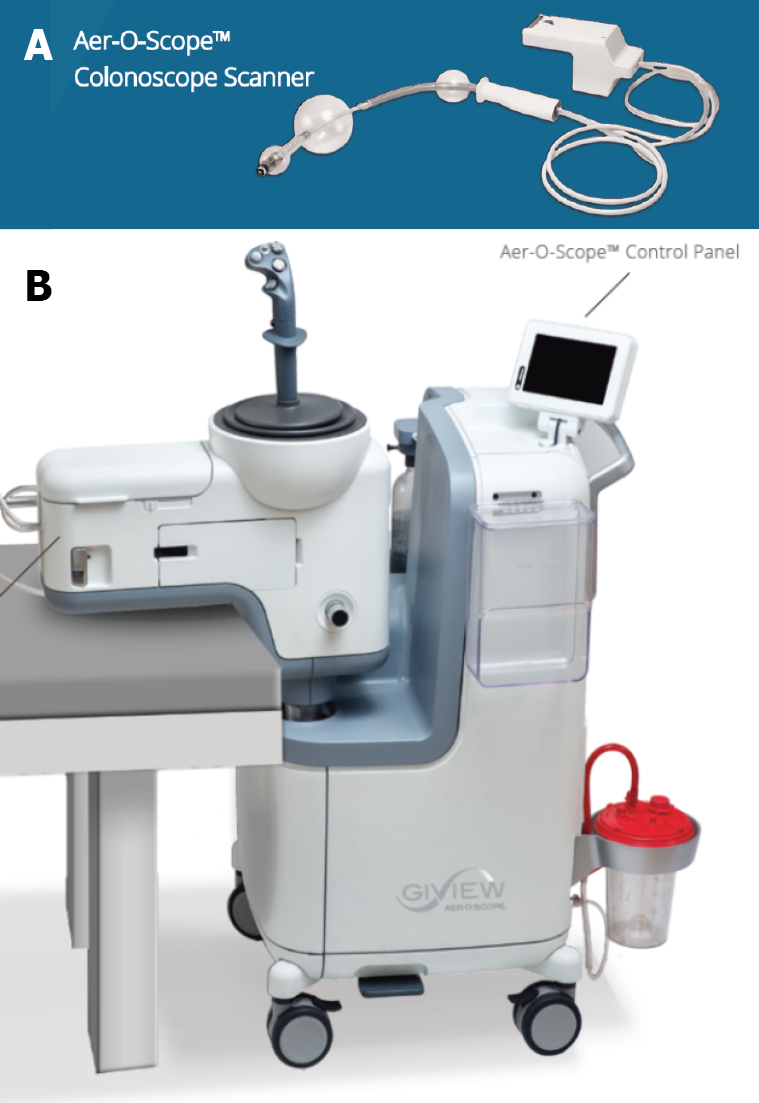
Aer-O-Scope: The Aer-O-Scope (GI View Ltd., Ramat Gan, Israel) is a single use flexible colonoscope that uses a CO2 propulsion system to self-advance through the colon. With a 200o field of view offered by two cameras it aims to offer better visibility behind folds while reducing discomfort. The device works by inflating two polyurethane balloons (Figure 2), the first balloon is inflated at the rectum to create a seal, a second balloon which pulsates and has a hydrophilic coating to reduce friction is inflated proximal to the first. Using the seal between the balloons, CO2 is inflated, and the pneumatic force propels the proximal balloon forward. To protect the colon, the system controls the pressure so it does not exceed 60 mbar[59]. The most recent version of the device is equipped with two working channels to allow therapeutics[21]. Tip control is achieved using a hand-held controller. Once in the caecum, the pressures are reversed to propel the proximal balloon back towards the rectum while maintaining colonic distension for visibility.
Safety of this novel propulsion method was first demonstrated in 20 pigs in 2006 were only minor petechiae and no adverse events were noted[60]. That study was quickly followed by a clinical trial in the same year on 12 healthy young adults[61]. In this study 10 out of 12 procedures were completed to the caecum with the other two stopping at the hepatic flexure. In both these cases CC was also unable to progress beyond the hepatic flexure due to redundant colon in one and pain in the other. Caecal intubation occurred in an average of 14 min for the 10 completed. Two subjects requested analgesia, while the other 10 had a non-sedated procedure. Mild localised submucosal petechial lesions, thought to be related to balloon friction, were noted on 4 of the follow-up conventional colonoscopies. No other clinically significant adverse events were reported.
A further prospective, non-randomised clinic trial was published in 2016 on 58 CRC screening patients who underwent tandem colonoscopies with the Aer-O-Scope followed by CC[59]. Caecal intubation was achieved in 98.2% (94.4% in the training cohort and 100% in the study cohort). The Aer-O-Scope detected 87.5% of the polyps found by CC, and 100% of those ≥ 5 mm. Caecal intubation was achieved in a mean of 11 min. No mucosal damage or adverse events were reported. Patients were sedated and no patient related experience measures have been reported on.
A prospective clinical trial using the Aer-O-Scope completed recruitment on clinicaltrial.gov in November 2021 but the results have not yet been published at the time of writing and are likely to be for a newer model of the colonoscope[62]. The Aer-O-Scope is CE marked and received FDA approval in 2016[63]. GI View Ltd no longer produce the self-propelled version of the Aer-O-Scope, instead opting to use the technology on a new version which is a single use robotic colonoscope without the balloon self-propulsion[64].
Following correspondence with GI View Ltd, they felt the advances in colonoscopy technique, with improved CIRs and within shorter times, limited the need for a self-propelled device. Instead, they are choosing to concentrate on a single use colonoscope with a larger lens aimed at improved polyp detection, while maintaining similar controls to reduce the need for new training.
ColonoSight: ColonoSight (Stryker GI Ltd., Haifa, Israel) is a self-advancing system composed of a reusable colonoscope (EndoSight) covered by a single use plastic sleeve (ColonoSleeve). The ColonoSleeve is a multi-lumen sheath that is inflated with air and progressively unfolds to propel the device through the colonic lumen. The IntraPull force that insufflates the air for propulsion is controlled using a foot pedal. The other controls resemble those of a standard colonoscope using a wheel and pully angulation control system. Push, pull and torque can also be used, similar to standard colonoscopy. On reaching the caecum, air insufflation is directed toward the tip, insufflating and reversing the pressure. A CCD camera with an LED is used to capture image, eliminating the need for fibre optics. The device contains a working channel for therapeutic tools. The outer surface, tip and channels of the endoscope are single use, therefore negating the need for reprocessing of the reusable EndoSight component[65].
Shike et al[65] describe that the operation and safety of the instrument was first tested on animals (12 pigs and 7 sheep) with a mean progression into the colon of 80 cm. In-vitro dye and culture studies as well as in-vivo culture testing confirmed the integrity of the disposable sleeve to protect against bacterial transfer. A clinical trial published in 2008 included 178 participants achieved a 90% CIR in a mean caecal intubation time (CIT) of 11.2 min. The CIR in the final 50 participants was 94%, suggesting a possible learning curve effect. 40 participants underwent polypectomy, with biopsies and argon plasma coagulation was also performed. No immediate complications were noted, and no complications were reported at a two week follow-up telephone call. Participants received intravenous sedation and no patient experience measures are reported on. Physicians reported the IntraPull helped progress the device[65]. The ColonoSight Model 510B received FDA approval in 2004[66]. Stryker GI Ltd acquired Sightline Technologies Ltd, the original manufacturer, in 2006 and the device is no longer manufactured. The reason for discontinuation is not clear, but Stryker GI Ltd have focused on laparoscopic endoscopic equipment rather than colonoscopes, so they may not have had the infrastructure to market a new colonoscope or they may have chosen to integrate the technology into laparoscopic equipment.
Consis medical: Consis medical (Beer’Sheva, Israel) have developed a semi-disposable, single use colonoscope that self-propels along the colon by means of an inverted sleeve pressurised with water. The head of the device can be removed and sterilised at the end of the procedure, with the sleeve cartridge being disposable[67]. The reusable head contains a camera, light source, steering system and water/air nozzle. Human colon simulation and animal tests were due to take place in 2018 but no published results are yet available[21].
Endoculus: Endoculus (University of Colorado, United States) is a multi-DOF fixed tether single use robotic capsule endoscope. Locomotion is via two independently controlled motor drives with micro-pillared treads, offering 2-DOF skid steering. Via a fixed tether the Endoculus contains channels for insufflation and irrigation, and a working channel for introduction of endoscopic instruments. The tip of the capsule contains a complementary metal-oxide-semiconductor (CMOS) camera and an adjustable LED. The fixed tether is narrow in diameter and very flexible, however the motorised tip is larger than a standard colonoscope at 6.0 cm × 3.0 cm × 2.3 cm. The research team feel the device could be made smaller with advanced manufacturing techniques[68].
In-vivo testing was carried out in a porcine colon. The device was inserted manually to 10 cm but was then unable to negotiate the sigmoid colon. The research team postulated that this was due to friction from the narrow sigmoid colon in a pig on the non-tread sides of the device. Ex-vivo testing on a porcine colon was subsequently conducted on a 40 cm mid colon section. The Endoculus was capable of negotiating the ex-vivo colon at speeds up to 40 mm/s both in an insufflated 6 cm diameter colon and also in a collapsed lumen[68].
Endotics: Endotics (ERA Endoscopy SRL, Peccioli, Italy) is an FDA and CE marked biomimicking colonoscope on sale in Europe and Japan[69]. The electropneumatic system uses proximal and distal clamps to attach to the colon wall and in a semi-autonomous process of extension and retraction of the shaft between the clamps it progresses along the colon in an inchworm fashion. Image is captured via an integrated CMOS camera lit by an LED light source. The device has a 3 mm working channel, insufflation and suction, and is single use. A 180o steerable head is controlled using a handheld control unit[70].
In-vitro studies reported in 2009 showed the Endotics E-worm system reduced the pressure on sensors around a pig colon by 90% compared to CC. An in-vivo animal study showed no complication of the clamping system at follow-up colonoscopy 7 d later[71]. In a pilot tandem study of 40 patients in 2009 the Endotics system only managed to achieve a CIR of 27.5% with mean CIT of 57 minutes, compared to a CIR of 82.5% for CC. The patient experience for the Endotics system was reported to be much better with average pain and discomfort scores of 0.9 and 1.1 out of 10, compared to 6.9 and 6.8 for CC[71].
A second tandem human study on 71 participants was published by Tumino et al[72] in 2010 aimed at assessing the Endotics ability to detect polyps. The CIR was 81.6% for the Endotics system and 94.3% for CC, with mean CIT 45.1 (± 18.5) min compared to 23.7 (± 7.2) min, respectively. None of the participants required sedation for the Endotics procedure, with 14/71 asking for sedation during the subsequent CC procedure. The Endotics system showed a sensitivity of 93.3% for the detection of polyps, detecting 14/15 polyps seen on CC. 6/71 (8.4%) of participants reported mild adverse events (nausea, headache, abdominal pain and discomfort) which the authors say, due to the tandem nature of the study, couldn’t be distinguished from bowel cleansing or CC related adverse events[72].
A retrospective analysis of 5 years of Endotics examination in a single centre in Italy reported on 102 procedures performed due to incomplete CC. The Endotics system was able to complete a colonoscopy in 95/102 (93.1%) of these patients. Mean CIT was 51 (± 22.5) min[73]. A learning curve study from Trecca et al[74] in 2020 presents two blocks of 27 and 28 participants. The CIR improved from 92.7% to 100% and the CIT from 55 to 22 min between the first and second block. The polyp detection rate and adenoma detection rate was 40% and 26.7%, respectively. The procedure was reported as mild or no distress in most cases with 92.7% willing to have a repeat Endotics procedure[74].
Endotics have performed a health economics study comparing Endotic robotic colonoscopy to standard colonoscopy in the Italian healthcare system and found the robotic colonoscopy to be comparable in price to standard colonoscopy, €441.25 vs €426.25, respectively[33].
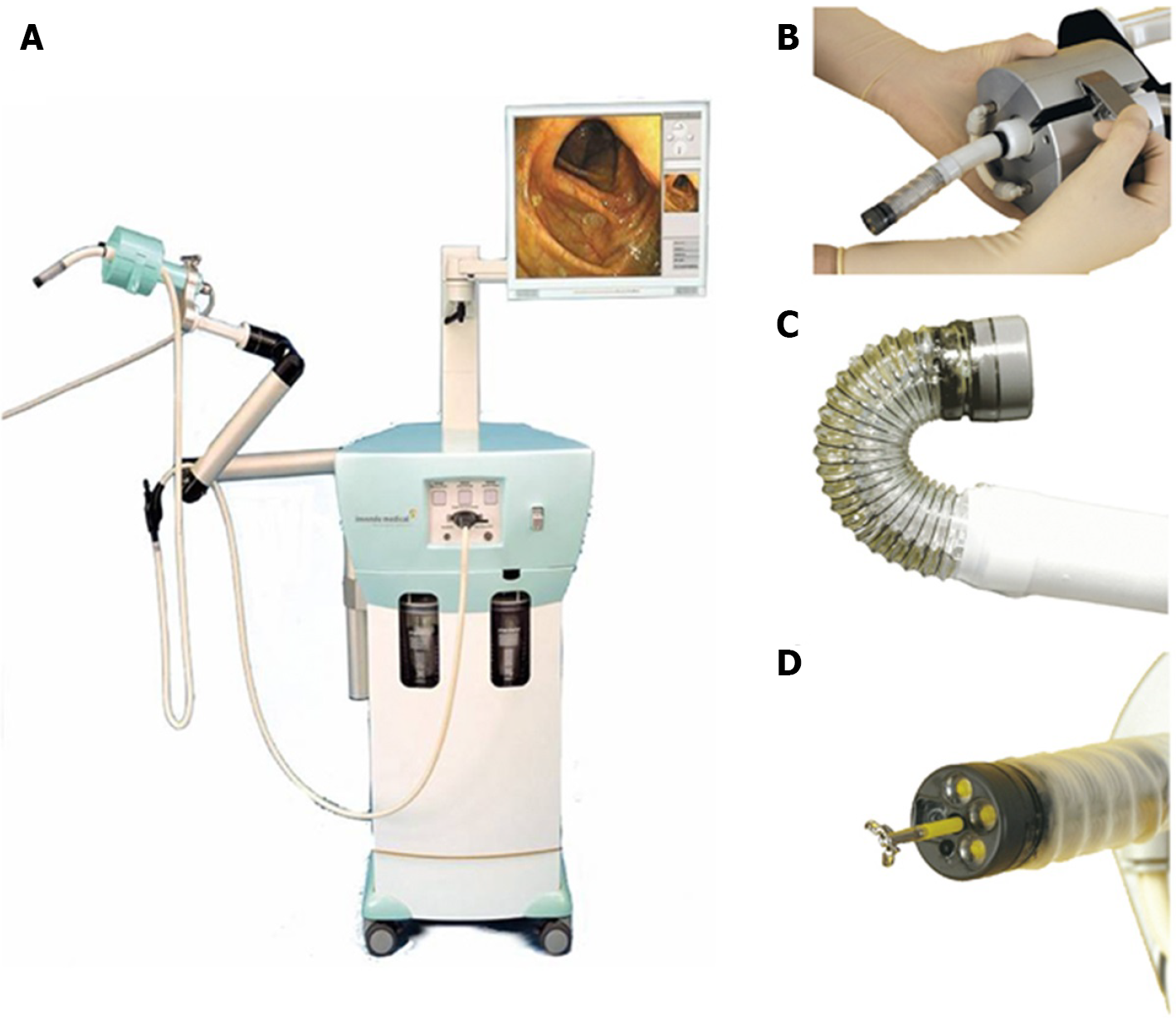
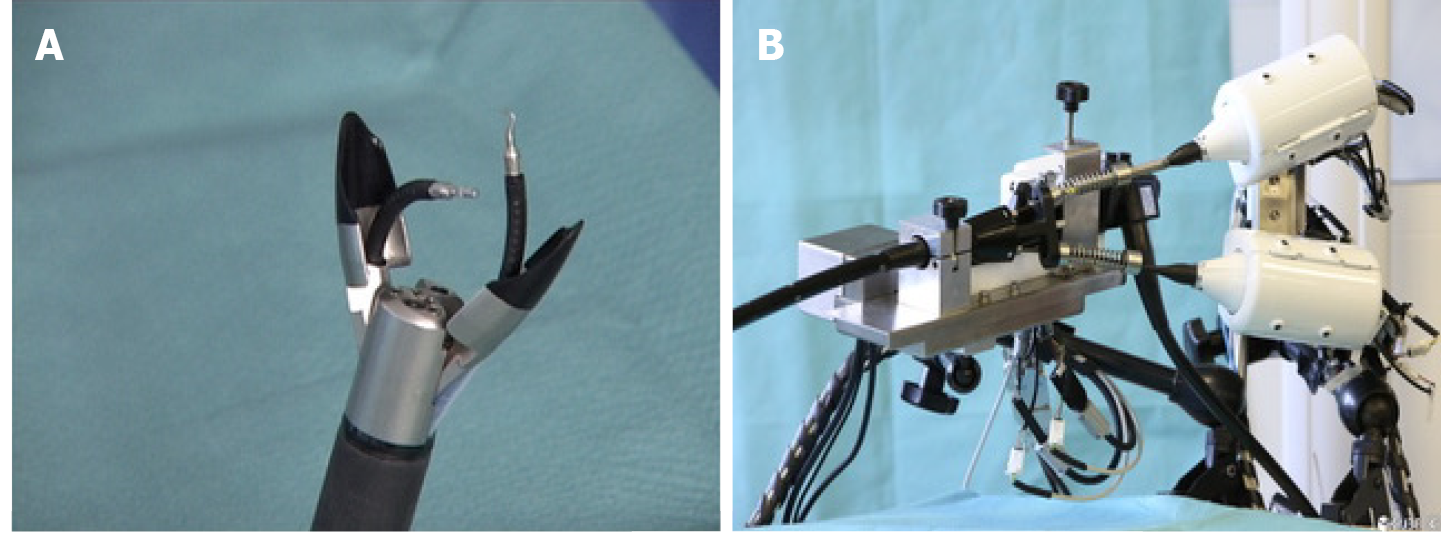
The Invendoscope (Invendo Medical GmbH, Weinheim, Germany) has a number of models with several CE and FDA approvals[75,76]. The single use computer-assisted device is propelled through the colon by an inverted sleeve driven by 8 wheels (Figure 3). The wheels grip the inside of the inverted sleeve to move forward and backwards. Control is via a handheld joystick. The tip of the colonoscope is robotically assisted and can be defected up to 180o, is equipped with three LEDs and a CMOS vision chip with an 114o field of view. Standard colonoscope functions such as insufflation, suction and irrigation are available, as well as a 3.2 mm working channel.
Animal testing using the SC20 model on the in-vivo small bowel of 5 pigs showed the device could be inserted through 3 small bowel loops and there was no mucosal damage found on microscopic pathological examination[77]. In a clinical trial of the SC40 model published in 2008, using two varying length prototypes in 34 participants, CIRs of 79% and 90% were achieved. CITs were 26 min and 20 min. The shorter of the two prototypes had a lower CIR, longer procedure time and more pain and bloating. Five cases had to be excluded due to instrument defects. Two of the failures to achieve caecal intubation were due to pain. Overall, the participants gave a mean acceptance rating of 1.96 (range 1-6). No immediate or delayed complications were reported[77].
A clinical trial on the Invendo SC20 model published by Groth et al[78] in 2011 found a CIR of 98.4% in 61 volunteers. Median CIT was 15 min and only 3/61 (4.9%) requested sedation. There were 32 polypectomies and no device-related complications reported. Water instillation was used in half of the cases to aid progression. Endoscope malfunctions were experienced in 2 cases. Post-procedure pain and discomfort scores were 1.6 and 2.3 out of 6, respectively[78].
Invendo Medical GmbH was acquired by Ambu in 2017 and the SC40 inverted sleeve wheel driven prototype has been replaced by the SC200 and then the SC210 which are manually inserted single use devices utilising robotic tip controls[21].
Only a conference abstract could be found on the Invendo SC210 model. In this clinical trial 40 participants underwent colonoscopy with the SC210, CIR was 95% with a median CIT of 14.2 min. Most patients (35/40) received propofol sedation. Twelve patients had polypectomies performed. There were 3 complaints of abdominal pain post-procedure and one self-limiting haemorrhage from the sigmoid colon, but no major complications[79].
The SC210 model no longer has any robotic locomotion, with the only robotics left being tip deflection, making it predominantly a single use colonoscope. Since the acquisition by Ambu, the Invendoscope is no longer on the market. Following correspondence with Ambu, the long scope length (220 cm) required for the self-propelled version made it difficult to control looping. Physicians also reportedly found it difficult to transition to the robotic tip control of the SC210 so Ambu have decided to focus on conventional control mechanisms.
A European FP6 project called “Versatile Endoscopic Capsule for GI TumOr Recognition and therapy” (VECTOR project) was tasked to explore magnetic capsule endoscopy from 2006 to 2011. One of the outputs of the project was a magnetically controlled capsule for colonoscopy. A 13.5 mm × 29.5 mm endoscopic capsule containing 6 LEDs and a CCD camera capable of 550 × 582 pixel resolution and a 120o field of view was designed. The capsule also contained 3 permanent magnets and a triaxial magnetic sensor to monitor the magnetic link. Power and transmission to and from the capsule was achieved via a 2 mm cable. The capsule was controlled using an external permanent magnet fixed to a robotic arm end effector which was found to offer more precision than manual control[80]. The robotic arm has 6 DOF and an additional 7th DOF was added at the end effector-magnet connection. The capsule trajectory is controlled by means of controlling the robotic arm and the magnetic attraction between the external and internal magnets pulls the capsule along the colon. The initial version of the device was not able to insufflate the colon, so a Foley catheter had to be passed rectally to insufflate[81].
A benchtop study of the magnetic capsule colonoscope was undertaken, including 11 endoscopist and 11 trainees, who performed the robotic capsule colonoscopy and CC on an ex-vivo porcine colon used to simulate a human colon. All 22 participants were able to complete the procedure using both the robotic capsule and conventional colonoscope. Pins inserted in the phantom colon were found with a mean accuracy of 80.9% (± 11.0%) in the robotic capsule procedures and 85.8% (± 9.9%) in the CC procedures[81].
The next prototype from the same team of researchers, named the Magnetic Air Capsule, included a soft multi-lumen tether to allow insufflation, washing, suctioning, lens cleaning and a working channel for passing therapeutic tools. Ex-vivo testing on a porcine colon within a human phantom model was undertaken using 12 participants and all participants were able to complete an 85 cm phantom colonoscopy, with 85% (± 11%) of the beads found. In-vivo porcine testing proved the device was capable of traversing an average distance of 800 (± 40) mm in an average of 900 (± 195) seconds[82].
The VECTOR project team were also able to explore several other aspects of robotic colonoscope design. Experiments exploring the human robot interface were conducted and teleoperated (remote control) was found to be more reliable than a human/robot cooperative haptic control using a torque/force sensor[83].
Following on from the VECTOR project, some of the research team have gone on to develop the technologies further in the ENDOO magnetic colonoscope and the magnetic flexible endoscope (MFE).
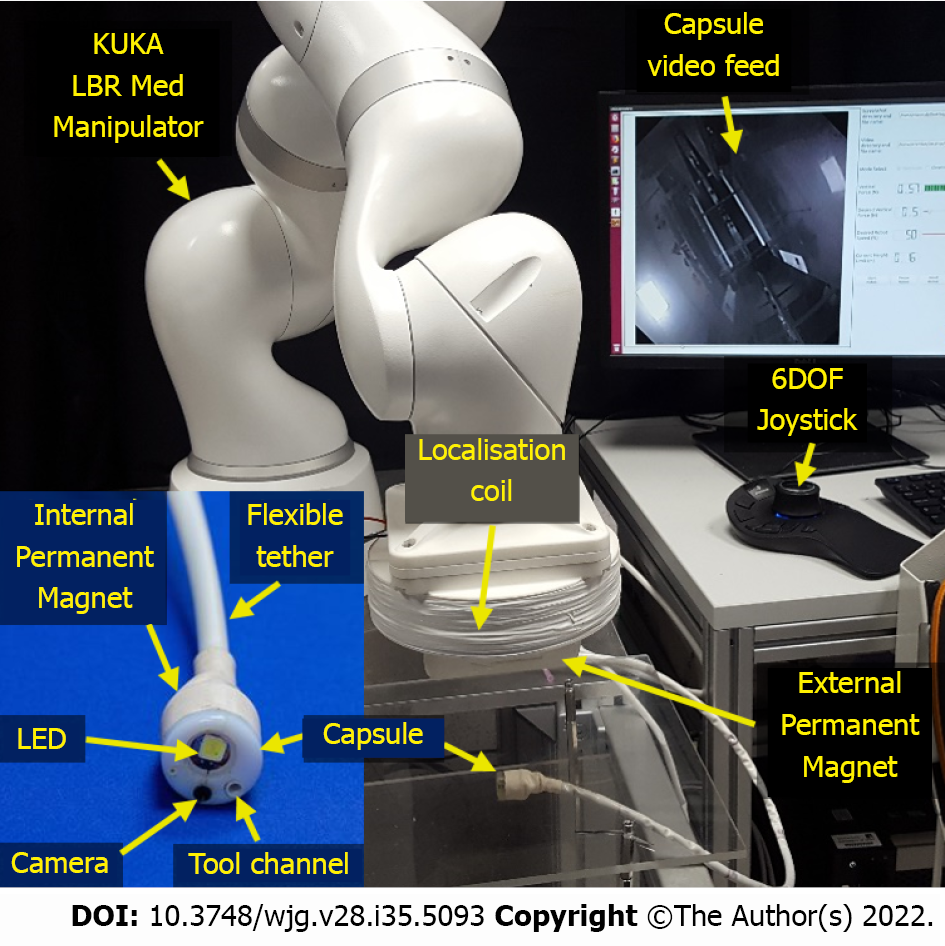
The MFE research team (STORM lab, Leeds, United Kingdom and Nashville, TN, United States) has developed the soft tether capsule colonoscope technology further (Figure 4). Advancements in the localisation and real time pose estimation allow closed loop control of the endoscope. Closed loop control allows the user to steer the endoscope based on what they see from the video feed rather than controlling movement of the robotic arm and attempting to translate that into the movement desired of the endoscope tip[53,84]. Further developments have allowed the capsule to levitate in the colon lumen rather than skimming along the mucosal surface. Autonomous navigation and autonomous retroflexion have been demonstrated in porcine studies[4,85]. Laboratory based learning curve studies and task load assessments suggest a quicker learning curve than with CC and a lower perceived workload[86]. In-vivo porcine studies have not demonstrated any safety concerns and even novice user were able to navigate the device 85 cm into a pig colon[84]. The platform is currently undergoing final pre-clinical steps in preparation for first-in-human trials.
The ENDOO project (Pisa, Italy) was a European H2020 project between 2015 and 2019 that also designed a magnetically actuated soft tether robot. A permanent magnet in the capsular tip of the endoscope is pulled through the colon using closed loop interaction with an external permanent magnet on an industrial anthropomorphic robotic arm[87,88]. The capsular tip has two 1080p CMOS cameras to allow stereoscopic, 3-dimensional, views. Four LEDs offer white light illumination, and 4 ultraviolet LEDs allow narrow band imaging. The 160 cm soft tether carries 4 channels, including a 3.7 mm working channel, providing suction, irrigation, lens cleaning and insufflation. The soft tether also integrates nylon cables to allow variable stiffness of the shaft.
Ex-vivo testing using a porcine colon in a human abdomen simulator compared key functionalities, forces generated, simulated polyp detection and usability when compared to CC in 10 expert endoscopists and 5 trainees. The forces generated by the robotic colonoscope were significantly less than those generated by CC, with cumulative interaction forces of 16.5N for CC and 1.67N for the robotic colonoscope. The robotic colonoscope was able to complete the procedure 67% of the time with a comparable polyp detection rate to CC[89].
Passive colonoscopes are capsule devices designed to assess the colon with movement through the gastrointestinal tract passively via natural peristalsis. There is now a commercially available colon capsule endoscope, the PillcamTM COLON2 (Given Imaging, Yokneam Illit, Israel). Researchers have also been exploring the addition of further robotics within capsular devices in an attempt to overcome some of their disadvantages. Advances in miniaturisation, image capture and batteries have allowed researchers the space within the small confines of a safe capsule size to add further robotics. A full review of these devices is beyond the scope of this review but have been done in detail by Manfredi[90] and Slawinski et al[91].
The previously described robotic platforms aim to provide an alternative to CC. However, some researchers have been developing platforms to assist with some of the challenges of performing CC by adapting the current colonoscope. Adding robotic controls to existing endoscopes could improve the ergonomics and assist with the difficult learning curve associated with colonoscopy.
The Endodrive (ECE Medical Products, Erlangen, Germany) is a platform using a rotating engine and foot pedal to control shaft insertion. Rotation and tip control is still required[92]. Rozeboom et al[93] present a robotic-assisted flexible colonoscope that uses an add-on to the handle of the colonoscope that convert the wheel control mechanism into a robotic joystick control. CIR using Rozeboom’s device was 68%[93]. Robotic steering and automated lumen centralisation (RS-ALC, Enschede, Netherlands) is another platform which uses joystick control of an add-on to the endoscope handle with the addition of lumen centralisation software. A study of novices and experts on a colon simulation model found that novices had a faster CIT and polyp detection rate with the RS-ALC than CC. The opposite was found of experts, who found CC better[94]. The master–slave endoscopic operation robot (EOR; Kyushu Institute of Technology, Kitakyushu, Japan) which is in its third iteration, added on therapeutic tools. The EOR version 3 uses a rotary motor, rotating handle, torque sensor and mini joystick which allows haptic feedback and 4 axis movement. An ex-vivo colon phantom study using 8 endoscopists performed 48 colonoscopies with a CIR of 100% and CIT of 118.5 (± 89.4) seconds[69].
Advances in the therapeutic capabilities of gastrointestinal endoscopy and the evolution of natural orifice transluminal endoscopy (NOTES) have led researchers to look to robotics to enhance the dexterity offered by our endoscopes. A number of systems have been developed that claim to offer greater triangulation, dexterity and DOF to improve our ability to perform procedures such as ESD, third space endoscopy and NOTES. Examples include Endoluminal Assistant for Surgical Endoscopy (EASE; KARL STORZ/IRCAD, Strasbourg, France) (Figure 5), K-FLEX (EasyEndo Surgical, Daejeon, Korea) and the MASTER (EndoMASTER Pte, Singapore)[95-97]. A detailed review of these devices is beyond the scope of this review article but has been done by Lim[98].
Endoscopy is an expanding speciality with colonoscopy demand rising by at least 5% per year[99,100]. Yet a significant number of people decline to undergo colonoscopy due to several factors including pain and previous bad experiences[16,17]. Robotics has the potential to provide an alternative to CC. However, the ideal robotic platform must be affordable; versatile and capable of performing precise movements while maintaining patient comfort. The potential for robotics to improve the dexterity of an endoscope in the ever-expanding field of therapeutic endoscopy is also worth consideration and likely to be something we will see in the future. Researchers developing new medical devices must keep sustainability in mind. And with a global pandemic in the forefronts of everyone’s minds, the potential for robotics to provide a ‘medically distanced’ procedure will be appealing to many.
AI has not been covered in this review article but is likely to be integrated in robotic colonoscopes. AI in endoscopy has many facets, including image assistance for lesion detection or characterisation, but also integrates closely with robotics in the form of autonomous movement/navigation. Although for many this seems like science fiction and not yet requiring discussion, the technology is available. The MFE device can autonomously navigate a pig colon.
However, advances in AI and increasingly autonomous tasks introduce a number a challenges. Regulatory, ethical and legal uncertainty is likely to take time to overcome, hindering commercialisation. Integration is therefore likely to be slow, with a gradual increase in the autonomy of devices despite the technology being available for further autonomy.
The technology to allow a completely autonomous colonoscopy, including diagnostics, with an endoscopist only required on a supervisory capacity is not so far away. Autonomous robotic colonoscopy has the potential to increased capacity with fewer operators required to perform more procedures. Endoscopists could potentially be trained much quicker. Robotics has the potential to raise the standard of procedures, democratising colonoscopy, thus reducing post-colonoscopy CRCs, reducing complications and improving the colonoscopy experience for patients.
Some of the devices discussed have reached TRLs of 8 and gained FDA or CE marking, however few have yet been adopted enough to perform post-marketing studies or health economics studies. Although, the Endotics health economics study has shown promising result, health economics studies in other areas of robotic healthcare, such as surgery, have been fraught with conflicting results.
Several of the discussed robotic colonoscopes have been modified, removing some of the robotics and opting to pursue the single use colonoscope market. It is probable that the improvements in endoscopy technique have put off commercialisation for the purpose of improved caecal intubation. However, much of the need for these devices is to improve patient experience, which now seems to be the focus of many research teams. What may seem like more intuitive controls will still be different to the standard endoscope controls experienced endoscopists have grown used to. Resistance to change is going to be a challenge for all new devices.
These technologies are often expensive initially, but the cost should not be confused with the potential cost effectiveness of a test which is more acceptable, with the subsequent reduction in morbidity and mortality. Technological advances are happening at an exponential rate and although none of the device to date have challenged the dominance of CC, it is probable that one will in the very near future.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68715] [Article Influence: 13743.0] [Reference Citation Analysis (201)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3523] [Article Influence: 391.4] [Reference Citation Analysis (4)] |
| 3. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1781] [Article Influence: 254.4] [Reference Citation Analysis (3)] |
| 4. | Slawinski PR, Taddese AZ, Musto KB, Sarker S, Valdastri P, Obstein KL. Autonomously Controlled Magnetic Flexible Endoscope for Colon Exploration. Gastroenterology. 2018;154:1577-1579.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Medical Technologies Innovation and Knowledge Centre. The Medical Technologies Innovation and Knowledge Centre. [cited 10 April 2022]. Available from: https://medical-technologies.co.uk/. |
| 6. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1723] [Article Influence: 246.1] [Reference Citation Analysis (0)] |
| 7. | Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, Ilyas M, Kaur A, Lalloo F, Latchford A, Rutter MD, Tomlinson I, Thomas HJW, Hill J; Hereditary CRC guidelines eDelphi consensus group. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020;69:411-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 8. | Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, Kaye PV, Monahan KJ, Novelli MR, Plumb A, Saunders BP, Thomas-Gibson S, Tolan DJM, Whyte S, Bonnington S, Scope A, Wong R, Hibbert B, Marsh J, Moores B, Cross A, Sharp L. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 9. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 361] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 10. | Mutneja HR, Bhurwal A, Arora S, Vohra I, Attar BM. A delay in colonoscopy after positive fecal tests leads to higher incidence of colorectal cancer: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Gingold-Belfer R, Leibovitzh H, Boltin D, Issa N, Tsadok Perets T, Dickman R, Niv Y. The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:424-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Selby K, Senore C, Wong M, May FP, Gupta S, Liang PS. Interventions to ensure follow-up of positive fecal immunochemical tests: An international survey of screening programs. J Med Screen. 2021;28:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, Yueh-Hsia Chiu S, Ching-Yuan Fann J, Chuang SL, Chiang TH, Chou CK, Chiu HM, Wu MS, Wu CY, Chia SL, Chiou ST, Chen HH. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Zorzi M, Battagello J, Selby K, Capodaglio G, Baracco S, Rizzato S, Chinellato E, Guzzinati S, Rugge M. Non-compliance with colonoscopy after a positive faecal immunochemical test doubles the risk of dying from colorectal cancer. Gut. 2022;71:561-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Dalton ARH. Incomplete diagnostic follow-up after a positive colorectal cancer screening test: a systematic review. J Public Health (Oxf). 2018;40:e46-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Kerrison RS, Sheik-Mohamud D, McBride E, Whitaker KL, Rees C, Duffy S, von Wagner C. Patient barriers and facilitators of colonoscopy use: A rapid systematic review and thematic synthesis of the qualitative literature. Prev Med. 2021;145:106413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Kerrison RS, Travis E, Dobson C, Whitaker KL, Rees CJ, Duffy SW, von Wagner C. Barriers and facilitators to colonoscopy following fecal immunochemical test screening for colorectal cancer: A key informant interview study. Patient Educ Couns. 2022;105:1652-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Meester RG, Doubeni CA, Zauber AG, Goede SL, Levin TR, Corley DA, Jemal A, Lansdorp-Vogelaar I. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281-2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Ramai D, Zakhia K, Etienne D, Reddy M. Philipp Bozzini (1773-1809): The earliest description of endoscopy. J Med Biogr. 2018;26:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Visconti TAC, Otoch JP, Artifon ELA. Robotic endoscopy. A review of the literature. Acta Cir Bras. 2020;35:e202000206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ciuti G, Skonieczna-Żydecka K, Marlicz W, Iacovacci V, Liu H, Stoyanov D, Arezzo A, Chiurazzi M, Toth E, Thorlacius H, Dario P, Koulaouzidis A. Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Lekven J, Schneede J, Tveit KM, Vatn M, Ursin G, Hoff G; NORCCAP Study Group†. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med. 2018;168:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | PubMed. PubMed search "Robotic Colonoscopy". National Library of Medicine; 2021. [cited 10 January 2022]. Available from: https://pubmed.ncbi.nlm.nih.gov/. |
| 24. | Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology. 2016;150:888-94; quiz e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 25. | Maida M, Alrubaiy L, Bokun T, Bruns T, Castro V, China L, Conroy G, Trabulo D, Van Steenkiste C, Voermans RP, Burisch J, Ianiro G. Current challenges and future needs of clinical and endoscopic training in gastroenterology: a European survey. Endosc Int Open. 2020;8:E525-E533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Siau K, Anderson JT, Valori R, Feeney M, Hawkes ND, Johnson G, McKaig BC, Pullan RD, Hodson J, Wells C, Thomas-Gibson S, Haycock AV, Beales ILP, Broughton R, Dunckley P; Joint Advisory Group on Gastrointestinal Endoscopy (JAG). Certification of UK gastrointestinal endoscopists and variations between trainee specialties: results from the JETS e-portfolio. Endosc Int Open. 2019;7:E551-E560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Shergill AK, Harris Adamson C. Failure of an engineered system: The gastrointestinal endoscope. Tech Gastrointest Endosc. 2019;21:116-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Yung DE, Banfi T, Ciuti G, Arezzo A, Dario P, Koulaouzidis A. Musculoskeletal injuries in gastrointestinal endoscopists: a systematic review. Expert Rev Gastroenterol Hepatol. 2017;11:939-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Merola G, Sciuto A, Pirozzi F, Andreuccetti J, Pignata G, Corcione F, Milone M, De Palma GD, Castaldo R, Pecchia L, Ceccarelli G, Bracale U. Is robotic right colectomy economically sustainable? Surg Endosc. 2020;34:4041-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Gomolin A, Gotlieb W, Lau S, Salvador S, Racovitan F, Abitbol J. Mandate to evaluate robotic surgery implementation: a 12-year retrospective analysis of impact and future implications. J Robot Surg. 2022;16:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Partelli S, Ricci C, Cinelli L, Montorsi RM, Ingaldi C, Andreasi V, Crippa S, Alberici L, Casadei R, Falconi M. Evaluation of cost-effectiveness among open, laparoscopic and robotic distal pancreatectomy: A systematic review and meta-analysis. Am J Surg. 2021;222:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Lundin ES, Carlsson P, Wodlin NB, Nilsson L, Kjölhede P. Cost-effectiveness of robotic hysterectomy vs abdominal hysterectomy in early endometrial cancer. Int J Gynecol Cancer. 2020;30:1719-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Sicuro R, Tumino E, Lambiase C, Mamone D. Robotic Colonoscopy: Comparative Analysis of Costs Compared to Painless Conventional Colonoscopy. 2020 Preprint. Available from: Research Square. [DOI] [Full Text] |
| 34. | Shenbagaraj L, Thomas-Gibson S, Stebbing J, Broughton R, Dron M, Johnston D, Shaw T, Haboubi HN, Green JT. Endoscopy in 2017: a national survey of practice in the UK. Frontline Gastroenterol. 2019;10:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 35. | Calderwood AH, Calderwood MS, Williams JL, Dominitz JA. Impact of the COVID-19 Pandemic on Utilization of EGD and Colonoscopy in the United States: An Analysis of the GIQuIC Registry. Tech Innov Gastrointest Endosc. 2021;23:313-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Larsen S, Kalloo A, Hutfless S. The hidden cost of colonoscopy including cost of reprocessing and infection rate: the implications for disposable colonoscopes. Gut. 2020;69:197-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Halligan S, Dadswell E, Wooldrage K, Wardle J, von Wagner C, Lilford R, Yao GL, Zhu S, Atkin W. Computed tomographic colonography compared with colonoscopy or barium enema for diagnosis of colorectal cancer in older symptomatic patients: two multicentre randomised trials with economic evaluation (the SIGGAR trials). Health Technol Assess. 2015;19:1-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O, Teare J, Lilford RJ, Morton D, Wardle J, Halligan S; SIGGAR investigators. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Halligan S, Wooldrage K, Dadswell E, Shah U, Kralj-Hans I, von Wagner C, Faiz O, Teare J, Edwards R, Kay C, Yao G, Lilford RJ, Morton D, Wardle J, Atkin W; SIGGAR Investigators. Identification of Extracolonic Pathologies by Computed Tomographic Colonography in Colorectal Cancer Symptomatic Patients. Gastroenterology. 2015;149:89-101.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | IJspeert JE, Tutein Nolthenius CJ, Kuipers EJ, van Leerdam ME, Nio CY, Thomeer MG, Biermann K, van de Vijver MJ, Dekker E, Stoker J. CT-Colonography vs. Colonoscopy for Detection of High-Risk Sessile Serrated Polyps. Am J Gastroenterol. 2016;111:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Ghanouni A, Halligan S, Plumb A, Boone D, Wardle J, von Wagner C. Non- or full-laxative CT colonography vs. endoscopic tests for colorectal cancer screening: a randomised survey comparing public perceptions and intentions to undergo testing. Eur Radiol. 2014;24:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Kjølhede T, Ølholm AM, Kaalby L, Kidholm K, Qvist N, Baatrup G. Diagnostic accuracy of capsule endoscopy compared with colonoscopy for polyp detection: systematic review and meta-analyses. Endoscopy. 2021;53:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 43. | Benech N, Vinet O, Gaudin JL, Benamouzig R, Dray X, Ponchon T, Galmiche JP, Sacher-Huvelin S, Samaha E, Saurin JC; ONECC Study Group. Colon capsule endoscopy in clinical practice: lessons from a national 5-year observational prospective cohort. Endosc Int Open. 2021;9:E1542-E1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Minelli Grazioli L, Senore C, Brizi G, Costamagna I, Alvaro G, Iannitti M, Salsano M, Ciolina M, Laghi A, Bonomo L, Costamagna G. Colon capsule vs CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015;64:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Cash BD, Fleisher MR, Fern S, Rajan E, Haithcock R, Kastenberg DM, Pound D, Papageorgiou NP, Fernández-Urién I, Schmelkin IJ, Rex DK. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy vs CT colonography in a screening population (the TOPAZ study). Gut. 2021;70:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Burr NE, Plumb A, Sood R, Rembacken B, Tolan DJM. CT colonography remains an important test for colorectal cancer. Gut. 2022;71:217-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Deding U, Kaalby L, Bøggild H, Plantener E, Wollesen MK, Kobaek-Larsen M, Hansen SJ, Baatrup G. Colon Capsule Endoscopy vs. CT Colonography Following Incomplete Colonoscopy: A Systematic Review with Meta-Analysis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Zijta FM, Bipat S, Stoker J. Magnetic resonance (MR) colonography in the detection of colorectal lesions: a systematic review of prospective studies. Eur Radiol. 2010;20:1031-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Lim EJ, Leung C, Pitman A, Stella DL, Brown G, Slattery M, Marion K, Macrae F. Magnetic resonance colonography for colorectal cancer screening in patients with Lynch syndrome gene mutation. Fam Cancer. 2010;9:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Graser A, Melzer A, Lindner E, Nagel D, Herrmann K, Stieber P, Schirra J, Mansmann U, Reiser MF, Göke B, Kolligs FT. Magnetic resonance colonography for the detection of colorectal neoplasia in asymptomatic adults. Gastroenterology. 2013;144:743-750.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Gao Y, Wang J, Lv H, Xue Y, Jia R, Liu G, Bai W, Wu Y, Zhang L, Yang J. Diagnostic value of magnetic resonance and computed tomography colonography for the diagnosis of colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e17187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 52. | Bianchi F, Masaracchia A, Shojaei Barjuei E, Menciassi A, Arezzo A, Koulaouzidis A, Stoyanov D, Dario P, Ciuti G. Localization strategies for robotic endoscopic capsules: a review. Expert Rev Med Devices. 2019;16:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Taddese AZ, Slawinski PR, Pirotta M, De Momi E, Obstein KL, Valdastri P. Enhanced Real-Time Pose Estimation for Closed-Loop Robotic Manipulation of Magnetically Actuated Capsule Endoscopes. Int J Rob Res. 2018;37:890-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Dupont PE, Nelson BJ, Goldfarb M, Hannaford B, Menciassi A, O'Malley MK, Simaan N, Valdastri P, Yang GZ. A decade retrospective of medical robotics research from 2010 to 2020. Sci Robot. 2021;6:eabi8017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 55. | England PH. Bowel Cancer Screening: Programme Overview. 2019. [cited 10 April 2022]. Available from: https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview. |
| 56. | Siau K, Hayee BH, Gayam S. Endoscopy's Current Carbon Footprint. Tech Innov Gastrointest Endosc. 2021;23:344-352. [DOI] [Full Text] |
| 57. | Tapia-Siles SC, Coleman S, Cuschieri A. Current state of micro-robots/devices as substitutes for screening colonoscopy: assessment based on technology readiness levels. Surg Endosc. 2016;30:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Taherdoost H. A review of technology acceptance and adoption models and theories. Procedia Manuf. 2018;22:960-967. |
| 59. | Gluck N, Melhem A, Halpern Z, Mergener K, Santo E. A novel self-propelled disposable colonoscope is effective for colonoscopy in humans (with video). Gastrointest Endosc. 2016;83:998-1004.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Pfeffer J, Grinshpon R, Rex D, Levin B, Rösch T, Arber N, Halpern Z. The Aer-O-Scope: proof of the concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope in a pig model. Endoscopy. 2006;38:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Vucelic B, Rex D, Pulanic R, Pfefer J, Hrstic I, Levin B, Halpern Z, Arber N. The aer-o-scope: proof of concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope. Gastroenterology. 2006;130:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | View G. Validation of Aer-O-Scope Colonoscope System Cecal Intubation. ClinicalTrials.gov: U.S. National Library of Medicine; 2019. [cited 8 April 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03949777?cond=aer-o-scope&draw=2&rank=1. |
| 63. | Federal Drug Administration. Aer-O-Scope FDA approval. Department of Health & Human Services 30th June 2016. [cited 8 April 2022]. Available from: https://www.gao.gov/products/gao-16-729r. |
| 64. | View G. Aer-O-Scope. GI View. [cited 8 April 2022]. Available from: https://www.giview.com/. |
| 65. | Shike M, Fireman Z, Eliakim R, Segol O, Sloyer A, Cohen LB, Goldfarb-Albak S, Repici A. Sightline ColonoSight system for a disposable, power-assisted, non-fiber-optic colonoscopy (with video). Gastrointest Endosc. 2008;68:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Federal Drug Administration. Colonosight FDA approval. Department of Health & Human Services 24th November 2003. [cited 8 April 2022]. Available from: https://www.fda.gov/. |
| 67. | Medical C. Consis Medical. Consis-medical; 2018 [updated 2018]. [cited 8 April 2022]. Available from: http://consis-medical.com/. |
| 68. | Formosa GA, Prendergast JM, Edmundowicz SA, Rentschler ME. Novel Optimization-Based Design and Surgical Evaluation of a Treaded Robotic Capsule Colonoscope. IEEE Transact Robot. 2020;36:545-552. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Kume K, Sakai N, Goto T. Development of a novel endoscopic manipulation system: the Endoscopic Operation Robot ver.3. Endoscopy. 2015;47:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Boškoski I, Orlandini B, Papparella LG, Matteo MV, De Siena M, Pontecorvi V, Costamagna G. Robotics and Artificial Intelligence in Gastrointestinal Endoscopy: Updated Review of the Literature and State of the Art. Curr Robot Rep. 2021;2:43-54. [DOI] [Full Text] |
| 71. | Cosentino F, Tumino E, Passoni GR, Morandi E, Capria A. Functional evaluation of the endotics system, a new disposable self-propelled robotic colonoscope: in vitro tests and clinical trial. Int J Artif Organs. 2009;32:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Tumino E, Sacco R, Bertini M, Bertoni M, Parisi G, Capria A. Endotics system vs colonoscopy for the detection of polyps. World J Gastroenterol. 2010;16:5452-5456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Tumino E, Parisi G, Bertoni M, Bertini M, Metrangolo S, Ierardi E, Cervelli R, Bresci G, Sacco R. Use of robotic colonoscopy in patients with previous incomplete colonoscopy. Eur Rev Med Pharmacol Sci. 2017;21:819-826. [PubMed] |
| 74. | Trecca A, Catalano F, Bella A, Borghini R. Robotic colonoscopy: efficacy, tolerability and safety. Preliminary clinical results from a pilot study. Surg Endosc. 2020;34:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Federal Drug Administration. Invendoscope E210 FDA approval. Public Health Service 27 September 2017. [cited 8 April 2022]. Available from: https://www.fda.gov/. |
| 76. | Federal Drug Administration. Invendoscope E200 FDA approval. Public Health Service: Department of Health & Human Services 15 July 2016. [cited 8 April 2022]. Available from: https://www.docin.com/p-1892232862.html. |
| 77. | Rösch T, Adler A, Pohl H, Wettschureck E, Koch M, Wiedenmann B, Hoepffner N. A motor-driven single-use colonoscope controlled with a hand-held device: a feasibility study in volunteers. Gastrointest Endosc. 2008;67:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Groth S, Rex DK, Rösch T, Hoepffner N. High cecal intubation rates with a new computer-assisted colonoscope: a feasibility study. Am J Gastroenterol. 2011;106:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Straulino F, Genthner A, Kiesslich R, Eickhoff A. Sa1938 Colonoscopy with the sterile single use endoscope INVENDOSCOPE SC210. Gastrointest Endosc. 2018;87. [DOI] [Full Text] |
| 80. | Ciuti G, Donlin R, Valdastri P, Arezzo A, Menciassi A, Morino M, Dario P. Robotic vs manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010;42:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Arezzo A, Menciassi A, Valdastri P, Ciuti G, Lucarini G, Salerno M, Di Natali C, Verra M, Dario P, Morino M. Experimental assessment of a novel robotically-driven endoscopic capsule compared to traditional colonoscopy. Dig Liver Dis. 2013;45:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Valdastri P, Ciuti G, Verbeni A, Menciassi A, Dario P, Arezzo A, Morino M. Magnetic air capsule robotic system: proof of concept of a novel approach for painless colonoscopy. Surg Endosc. 2012;26:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Salerno GCM, Lucarini G, Valdastri P, Arezzo A, Menciassi A, Morino M, Dario P. A Comparative Evaluation of Control Interfaces for a Robotic-Aided Endoscopic Capsule Platform. IEEE Trans Robot. 2012;28:534-538. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Martin JW, Scaglioni B, Norton JC, Subramanian V, Arezzo A, Obstein KL, Valdastri P. Enabling the future of colonoscopy with intelligent and autonomous magnetic manipulation. Nat Mach Intell. 2020;2:595-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 85. | Pittiglio G, Barducci L, Martin JW, Norton JC, Avizzano CA, Obstein KL, Valdastri P. Magnetic Levitation for Soft-Tethered Capsule Colonoscopy Actuated With a Single Permanent Magnet: A Dynamic Control Approach. IEEE Robot Autom Lett. 2019;4:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Mamunes AP, Campisano F, Martin J, Scaglioni B, Mazomenos E, Valdastri P, Obstein KL. Magnetic flexible endoscope for colonoscopy: an initial learning curve analysis. Endosc Int Open. 2021;9:E171-E180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Bianchi F, Ciuti G, Koulaouzidis A, Arezzo A, Stoyanov D, Schostek S, Oddo CM, Menciassi A, Dario P. An innovative robotic platform for magnetically-driven painless colonoscopy. Ann Transl Med. 2017;5:421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Endoo Project. Endoo EU Project; 2020. [cited 8 April 2022]. Available from: http://www.endoo-project.eu/. |
| 89. | Verra M, Firrincieli A, Chiurazzi M, Mariani A, Lo Secco G, Forcignanò E, Koulaouzidis A, Menciassi A, Dario P, Ciuti G, Arezzo A. Robotic-Assisted Colonoscopy Platform with a Magnetically-Actuated Soft-Tethered Capsule. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Manfredi L. Endorobots for Colonoscopy: Design Challenges and Available Technologies. Front Robot AI. 2021;8:705454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Slawinski PR, Obstein KL, Valdastri P. Capsule endoscopy of the future: What's on the horizon? World J Gastroenterol. 2015;21:10528-10541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 92. | Endoskopie EC. Endodrive systems workstation. www.ece-medical-systems.com: Ece medical systems. [cited 8 April 2022]. Available from: http://www.endodrive.de/. |
| 93. | Rozeboom ED, Bastiaansen BA, de Vries ES, Dekker E, Fockens PA, Broeders IA. Robotic-assisted flexible colonoscopy: preliminary safety and efficiency in humans. Gastrointest Endosc. 2016;83:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Pullens HJ, van der Stap N, Rozeboom ED, Schwartz MP, van der Heijden F, van Oijen MG, Siersema PD, Broeders IA. Colonoscopy with robotic steering and automated lumen centralization: a feasibility study in a colon model. Endoscopy. 2016;48:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Mascagni P, Lim SG, Fiorillo C, Zanne P, Nageotte F, Zorn L, Perretta S, de Mathelin M, Marescaux J, Dallemagne B. Democratizing Endoscopic Submucosal Dissection: Single-Operator Fully Robotic Colorectal Endoscopic Submucosal Dissection in a Pig Model. Gastroenterology. 2019;156:1569-1571.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Hwang M, Kwon DS. K-FLEX: A flexible robotic platform for scar-free endoscopic surgery. Int J Med Robot. 2020;16:e2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Lomanto D, Wijerathne S, Ho LK, Phee LS. Flexible endoscopic robot. Minim Invasive Ther Allied Technol. 2015;24:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Lim SG. The development of robotic flexible endoscopic platforms. Inter J Gastrointest Interven. 2020;9:9-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Comas M, Mendivil J, Andreu M, Hernández C, Castells X. Long-Term Prediction of the Demand of Colonoscopies Generated by a Population-Based Colorectal Cancer Screening Program. PLoS One. 2016;11:e0164666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Ravindran S, Bassett P, Shaw T, Dron M, Broughton R, Johnston D, Healey CJ, Green J, Ashrafian H, Darzi A, Coleman M, Thomas-Gibson S. National census of UK endoscopy services in 2019. Frontline Gastroenterol. 2021;12:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K; International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med. 2018;378:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 102. | Director RD. Technology Readiness Assessment (TRA) Deskbook. US Department of Defense, 2009. |
| 103. | Engineering ASoDfRa. Technology Readiness Assessment (TRA) Guidance. US Department of Defense, 2011. |
| 104. | Slawinski PR, Taddese AZ, Musto KB, Obstein KL, Valdastri P. Autonomous Retroflexion of a Magnetic Flexible Endoscope. IEEE Robot Autom Lett. 2017;2:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: British Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng KC, China; Pattarajierapan S, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Fan JR