Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.4993
Peer-review started: March 14, 2022
First decision: April 11, 2022
Revised: May 15, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 14, 2022
Processing time: 176 Days and 21 Hours
Oxidized low-density lipoprotein (ox-LDL), which is abnormally increased in the serum of colorectal cancer (CRC) patients consuming a high-fat diet (HFD), may be one of the risk factors for the development of CRC. Ox-LDL exerts a regulatory effect on macrophages and may influence CRC through the tumor microenvironment. The role of ox-LDL in CRC remains unclear.
To investigate the role of ox-LDL through macrophages in HFD associated CRC.
The expression of ox-LDL and CD206 was detected in colorectal tissues of CRC patients with hyperlipidemia and HFD-fed mice by immunofluorescence. We stimulated the macrophages with 20 μg/mL ox-LDL and assessed the expression levels of CD206 and the cytokines by cell fluorescence and quantitative poly
The expression of ox-LDL and the CD206 was significantly increased in the stroma of colorectal tissues of CRC patients with hyperlipidemia, and also upregulated in the HFD-fed mice. Moreover, an increased level of CD206 and decreased level of inducible nitric oxide synthase were observed in macrophages after ox-LDL continuous stimulation. Such effects were inhibited when the surface receptor LOX-1 was knocked down in macrophages. Ox-LDL could induce CD206+ macrophages, which resulted in high expression of CD44 and CD133 in co-cultured LoVo cells.
Ox-LDL stimulates CD206+ macrophages to upregulate CD44 and CD133 expression in HFD related CRC.
Core Tip: Obesity increases the risk of colorectal cancer (CRC), but the mechanism remains unknown. CD206+ macrophages promote CRC. It has been established that the prevalence of CRC was higher in people consuming a high-fat diet (HFD) and HFD fed mice with up-regulated CD206+ macrophages levels in colorectal tissue. Oxidized low-density lipoprotein (ox-LDL) is a lipid peroxide which has been found to be increased in serum of CRC patients. Importantly, ox-LDL exerts a regulatory effect on macrophages and may regulate CRC through the tumor microenvironment. Our study showed that ox-LDL stimulates CD206+ macrophages to up-regulate CD44 and CD133 expression in HFD associated CRC.
- Citation: Zheng SM, Chen H, Sha WH, Chen XF, Yin JB, Zhu XB, Zheng ZW, Ma J. Oxidized low-density lipoprotein stimulates CD206 positive macrophages upregulating CD44 and CD133 expression in colorectal cancer with high-fat diet. World J Gastroenterol 2022; 28(34): 4993-5006
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/4993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.4993
Obesity is a widespread health condition. There is ample evidence to suggest that obesity increases the risk of colorectal cancer (CRC)[1]. A prospective cohort study of 85256 women found that obese individuals had a 1.93 times higher risk of CRC by age 50 than normal-weight individuals[2]. Excessive intake of dietary fat, e.g., a high-fat diet (HFD), is the main cause of obesity and one of the important reasons for the increased incidence of CRC[3]. However, the specific mechanism of CRC remains unknown. It is currently believed that HFD promotes intestinal cancer by increasing the number and malignant potential of intestinal stem cells[4,5].
Interestingly, it has been shown that HFD could activate systemic inflammation and increase the malignant potential of intestinal tumors by upregulating the expression of macrophages[6,7]. It has been established that macrophages exhibit multiple phenotypes, like those positive for CD206, CD163, or inducible nitric oxide synthase (iNOS)[8]. Importantly, some types of macrophages promote cell repair and proliferation, like CD206+ macrophages[9]. An increasing body of evidence suggests that CD206+ macrophages in the tumor microenvironment promote CRC development, and a positive correlation has been documented between the level of CD206+ macrophages and the degree of tumor malignancy[10]. Moreover, metastasis can be promoted through the interactions of CD206+ macrophages with CRC cells[11]. Liu et al[7] found that the prevalence of CRC was higher in people on a HFD with upregulated CD206+ macrophage levels in colon tissue. Moreover, when mice with intestinal microflora disorder were fed an HFD, it was found that CD206+ macrophages in colon tissues correlated with the number of colon tumors and the degree of malignancy, suggesting that the increased CD206+ macrophage level induced by an HFD could exert a significant promoting effect on CRC.
HFD can cause low-grade inflammation and oxidative stress in the whole body, leading to increased lipid levels such as cholesterol and low-density lipoprotein[6]. Oxidized low-density lipoprotein (ox-LDL) is a lipid peroxide produced under oxidative stress that can be used to assess oxidative stress and lipid metabolism in the body. Several studies on obese people have found that the serum ox-LDL level in CRC patients was higher than that in the control group with a healthy intestinal tract[12-14]. Importantly, ox-LDL exerts a regulatory effect on macrophages and may regulate CRC through the tumor microenvironment[15]. However, the mechanisms underlying macrophage regulation in CRC by ox-LDL remain unclear. In addition, the regulatory role of macrophages on tumors may be related to tumor stem cells. In this regard, Yang et al[16] found that CD44 levels were gradually increased in lung cancer tissue with an increase in the level of CD206+ macrophages. Lv et al[17] also found that CD133 levels were upregulated in thyroid cancer with the increasing CD206+ macrophages. CD206+ macrophages share a similar relationship with CD44 and CD133 in CRC. In this study, the expression and corresponding effects of ox-LDL in colorectal tissue from hyperlipidemia patients were studied to explore the regulatory effects of ox-LDL on macrophages and the tumor stem cell markers CD44 and CD133 in the colorectal stroma. Our findings will provide a new mechanism of increased CRC susceptibility with HFD.
Colonoscopy was performed on hyperlipidemia patients with CRC, with no prior radiotherapy, chemotherapy, or surgery. Healthy colorectal tissues were collected from volunteers who underwent colonoscopy in the physical examination. CRC (cancer, n = 16, male:female = 10:6) and normal colorectal tissues (normal, n = 20, male:female = 11:9) were collected. The average age of all patients was 57 ± 6 years old. All tissue samples were examined by experienced pathologists. All the CRC tissues that we collected were adenocarcinoma. After sampling, tissue samples were fixed in 4% paraformaldehyde. All patients were treated at the Guangdong Provincial People’s Hospital, and the tissue samples were collected by the same endoscopist. The human study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital. Informed consent was obtained from all patients before the beginning of the study.
Six C57/BL6 mice aged 4 wk assigned to an HFD group were fed an HFD (#H10141, China) for a total of 12 wk. Another six C57/BL6 mice aged 4 wk were assigned to a control group and fed a normal diet (normal grade, #02, China) for the same duration. After 12 wk, the mice were sacrificed for colorectal tissue harvesting. All tissues were immediately transferred to 4% paraformaldehyde. Animal experiments were also approved by the Ethics Committee of Guangdong Provincial People’s Hospital.
After the above clinical and animal specimens were fixed at room temperature for 24 h, tissue sections were prepared as follows. The tissues were first dehydrated and then embedded with paraffin. The paraffin-embedded tissues were cut into 3 μm-thick sections and cross-sections of the intestinal tissue were observed.
Three- micron-thick mice tissue slices were cut and dried in a 65 °C oven for 120 min. The slices were stained with hematoxylin and eosin, dehydrated, and cleared. Finally, the slices were dried and sealed with neutral gum. All samples were examined by experienced pathologists.
The specimens were dried in a 65 °C oven for 2 h for dewaxing and dehydration, and then soaked in deionized water for 5 min. The tissue specimens were soaked in sodium citrate solution overnight in a 60 °C water bath to expose the antigen. The sections were immersed in phosphate buffer solution (PBS) and then incubated with 10% goat serum at 37 °C for 1 h for antigen blocking. The slices were incubated at 4 °C for 12 h with the corresponding primary antibody, rewarmed at room temperature, and washed with PBS. The cells were incubated with 100 μL/well working solution containing Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody at room temperature for 1 h in the dark. 4,6-diamino-2-phenylindole (DAPI; Thermo Fisher Science, United States) was used for nuclear counterstaining. The stained slides were imaged using an inverted fluorescence microscope (magnification, × 400; Olympus Corporation).
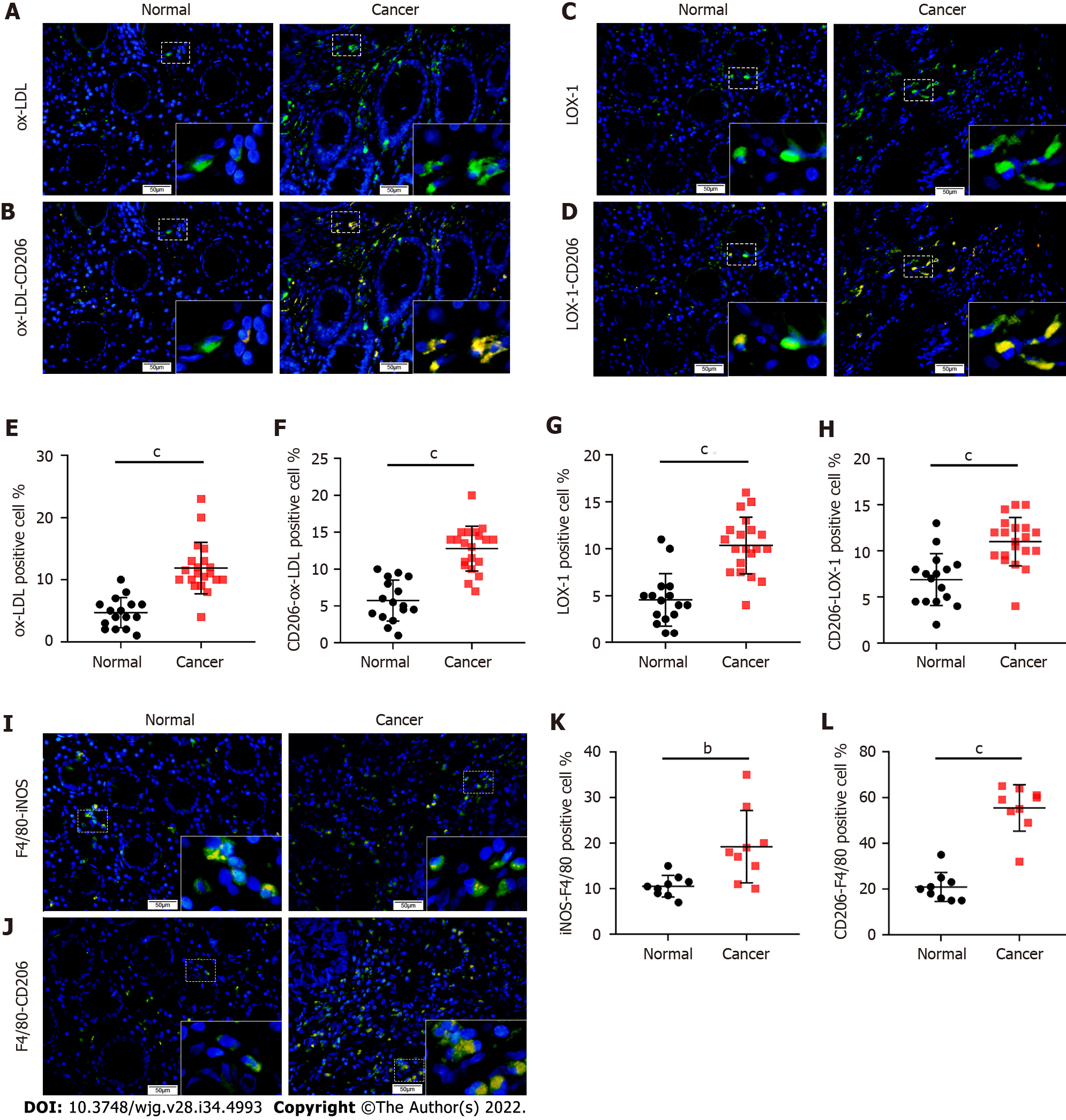
For calculation of the rate of ox-LDL positive cells in immunofluorescence (IF) staining, three fields were randomly selected from each section to observe the stroma of colorectal tissue under a 400 × microscope. For iNOS-F4/80 or CD206-F4/80 double staining, the number of iNOS, CD206, and F4/80 positive cells and total cells was counted, and the rate of iNOS and F/480, or CD206 and F4/80 positive cells was calculated. For ox-LDL-CD206 or LOX-1-CD206 double staining, the number of positive cells and total cells was counted, and their rate was calculated. The mean value of the rates from three fields was the positive cell rate of each section. All the analyses were double-blind and graded by two or more observers.
Cell lines and culture: The human colorectal adenocarcinoma cell line (LoVo) and mouse monocyte-macrophage leukemia cell line (RAW 264.7) were purchased from the American Center for Typical Culture Preservation (ATCC, United States). The human monocytic leukemia cell line was purchased from Wuhan Penoside Company (THP-1, #CL-0233, China). The maintenance medium for cell culture was Dulbecco modified Eagle medium (DMEM, Gibco) containing glucose (4.5 g/L), 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. All cells were cultured under standard cell culture conditions of 37 °C, 5% CO2, and 95% humidity.
Lipoprotein induction and RNA extraction: THP-1 cells were inoculated in 6-well plates, and 50 μg/mL ox-LDL was added 24 h and 72 h after sample collection. The confluence of cells in both groups was maintained at 60%-70% at the beginning of treatment, while the control group did not receive any treatment. The samples were centrifuged (800 rpm, 3 min) and washed with sterile PBS, and re-centrifuged (800 rpm, 3 min). Then, total RNA was extracted from tissues or cells using TRIzol reagent, according to the manufacturer’s instructions. The RNA samples were stored at -80 °C.
Cell fluorescence: RAW264.7 cells were inoculated in 12-well plates and treated with 50 μg/mL ox-LDL for 72 h when the cell confluence reached 60%-70%, while the control group did not receive any treatment. After 72 h, the culture medium was discarded, and the cells were treated with 4% paraformaldehyde for 15 min. The cells were washed with PBS, blocked with 10% sheep serum at 37 °C for 1 h, and incubated with PBS solution dissolved in 1% BSA and 0.1% TritonX-100 followed by primary antibody [rabbit anti-CD206 antibody (1:100, #18704-1-AP, United States)] at 4 °C for 12 h. The cells were then washed with PBS, and incubated with the AlexaFluor594 conjugated secondary antibody (1:500) at room temperature in the dark for 1 h. The cells were washed with PBS, one drop of DAPI was added to each well, and images were obtained under the corresponding fluorescence channel using an inverted fluorescence microscope. The method used to calculate the IF-positive cell rate was the same as above.
Co-culture and protein extraction: LoVo cells and THP-1 cells were inoculated in the lower and upper chambers of 12-well transwell plates, respectively. The cell density was 60%-70%, and cells were incubated with ox-LDL (#S24879, China) for 72 h. There were no THP-1 cells in the upper compartment of the culture plate in the control group, and the other conditions were the same as those for the treatment group. The culture medium was discarded 72 h later, and cells were gently moistened with PBS. Total protein of LoVo cells in each group was extracted with RIPA lysis buffer on ice. Protein concentration was detected by the BCA method, and protein samples were stored at -80 °C.
RNA concentration was determined first, and the samples were diluted to ensure that the concentrations were consistent. The RNA was reverse-transcribed to cDNA in a 20 μL system, and the total RNA content was kept below 1000 ng. Then, the cDNA was used as the template for quantitative polymerase chain reaction (qPCR) amplification. ABI 7300 real-time PCR software was used to analyze the PCR results and detect the Ct value of the sample. GAPDH was used as the internal reference gene, and the relative quantitative analysis was carried out by the 2-ΔΔCt method. The primer sequences used are shown in Table 1.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| GAPDH | CTGTTCGACAGTCAGCCGCATC | GCGCCCAATACGACCAAATCCG |
| INOS | TTCAGTATCACAACCTCAGCAAG | TGGACCTGCAAGTTAAAATCCC |
| CD206 | TGGAGAGGGAAGAGAGTGAACA | GCCCATAAGTGTGCTCTGAA |
| LOX-1 | GTGGCATGGAGAAAACTGTTAC | CATCCAAAGACAAGCACTTCTC |
Small interfering RNA (siRNA) was used to inhibit the expression of LOX-1 in macrophages. LOX-1 siRNA or siRNA negative control (GenePharma, GenePharma, Suzhou, China) was introduced into THP-1 cells via Liposome 3000 (3 μL/mL) for 24 h. The sequence of LOX-1 siRNA used is shown in Table 2.
| si-LOX-1 | Forward sequence (5’-3’) | Reverse sequence (5’-3’) |
| 1 | UUUGCUACUCUCUUCAGUGTT | CACUGAAGAGAGUAGCAAATT |
| 2 | UUGCUUGCUGGAUGAAGUCTT | GACUUCAUCCAGCAAGCAATT |
| 3 | UUUCUGACUCCUGUGAAGCTT | GCUUCACAGGAGUCAGAAATT |
Protein samples (10 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The membrane was then blocked with 5% skim milk. One hour later, anti-CD44 antibody (1:5000, # AB189524, United Kingdom) and anti-GAPDH antibody (1:10000, # AB9458, United Kingdom) were added. After incubating with the primary antibody, the membrane was washed with TBST. The samples were then incubated with horseradish peroxidase-labeled secondary antibody (1:3000) for 1 h at room temperature. After washing with TBST, immune reactive bands were analyzed after protein band visualization with enhanced chemiluminescence reagents.
Microsoft Excel 2019 worksheet was used to summarize the experimental results and generate tables. Adobe Photoshop 2020 software was used to process the images, and GraphPad Prism 8.0.2 software was used for statistical analyses. The Chi-square test or unpaired t-test was performed to compare the difference between two groups. One-way ANOVA test was performed to compare the difference among more than two groups, and the Dunnett post-hoc test was used for multiple comparisons. The data were assessed for normality before conducting the unpaired t-test and one-way ANOVA test. A P value < 0.05 was considered statistically significant. All tests were carried out more than three times.
To explore the distribution and expression of ox-LDL in CRC, we collected tissue samples from CRC patients with hyperlipidemia (n = 16) and normal subjects (n = 20) and performed IF staining for ox-LDL. The results showed that compared with normal colorectal tissues, the expression of ox-LDL was significantly increased in the stroma of CRC tissues (Figures 1A and 1E). It has been reported that LOX-1 is the surface receptor of ox-LDL in macrophages[12]. LOX-1-positive cells were also abundantly detected in CRC tissues (Figures 1C and 1G).
Double IF for CD206-F4/80 or iNOS-F4/80 showed that the number of CD206+ macrophages increased significantly in the stroma of CRC tissue (Figures 1I-L). Besides, double IF also showed the number of CD206+/ox-LDL+ cells and CD206+/LOX-1+ cells increased abundantly in CRC tissue (Figures 1B, 1D, 1F, and 1H).
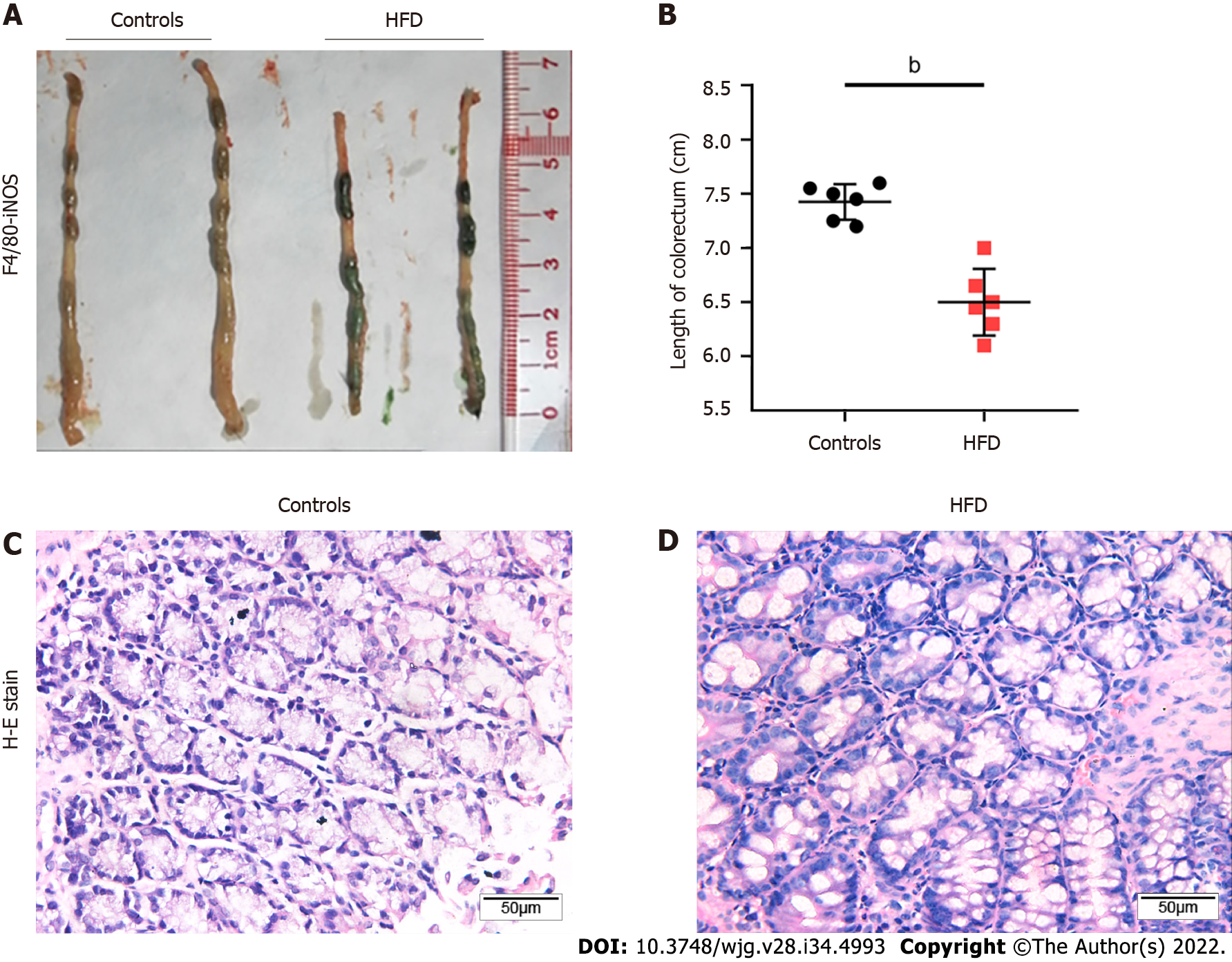
Four-week-old C57 mice were fed an HFD, and mice of the same age were fed a normal diet as controls. After 20 wk, the mice were sacrificed, and colorectal tissue specimens were harvested (n = 6). The colorectal length of control mice and HFD-fed mice was measured, respectively. We found that the colorectal length of HFD mice was shorter (Figures 2A and 2B). Subsequently, the tissue samples were sectioned and stained with hematoxylin and eosin. The staining results showed that glandular nuclei and interstitial cells in the colorectal tissues of HFD mice were slightly enlarged but to a milder degree than those usually observed with intraepithelial neoplasia (Figure 2C).
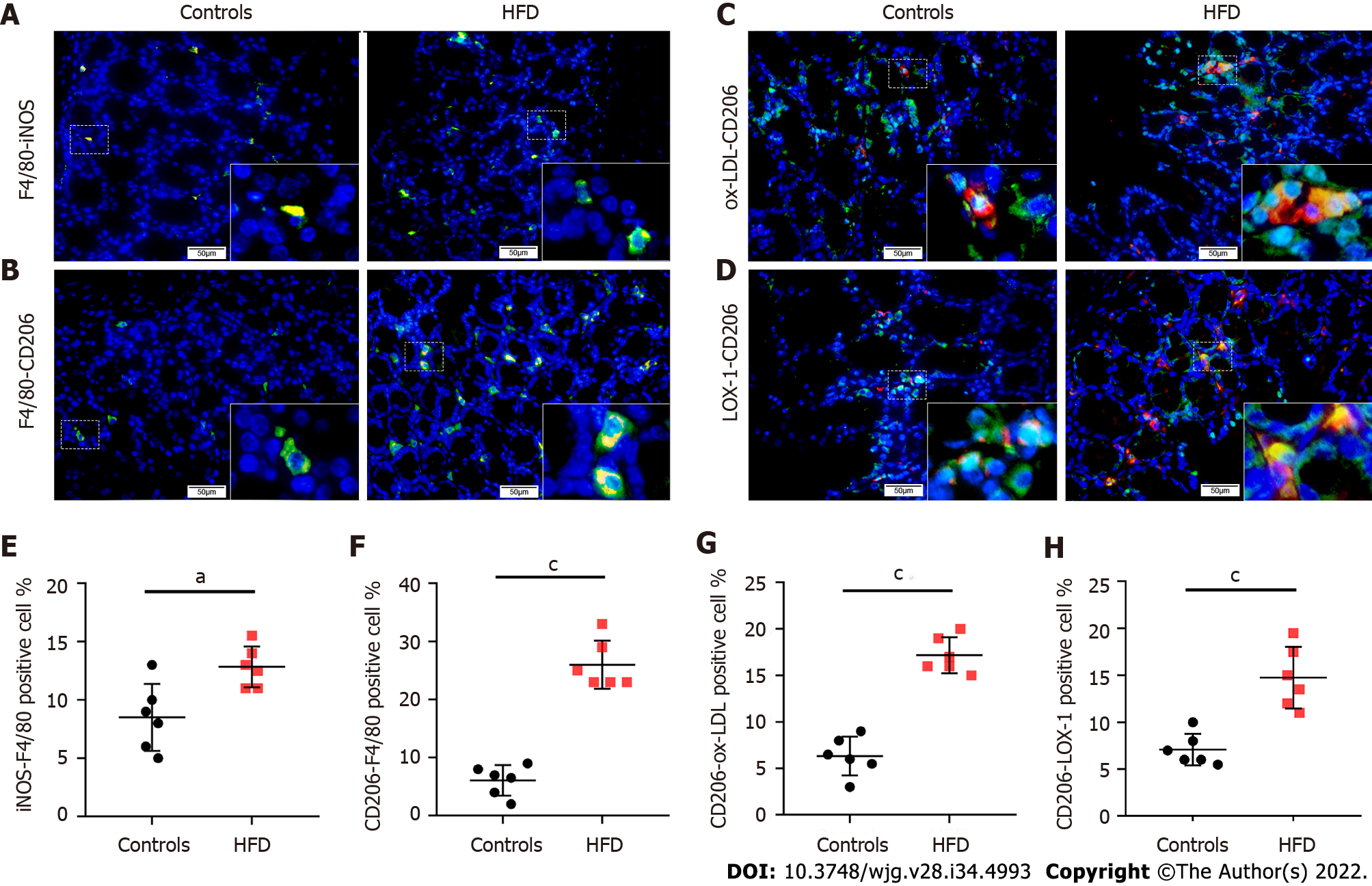
After establishing the HFD-fed mouse model, we performed double IF staining for CD206-F4/80 or iNOS-F4/80 in the colorectal tissue sections of the control and HFD-fed mice. We found that the number of CD206 positive macrophages was significantly increased in the colorectal stroma of HDF-fed mice (Figures 3A, 3B, 3E, and 3F). The number of CD206+/ox-LDL+ cells and CD206+/LOX-1+ cells also increased significantly in the colorectal stroma of HDF-fed mice (Figures 3C, 3D, 3G, and 3H).
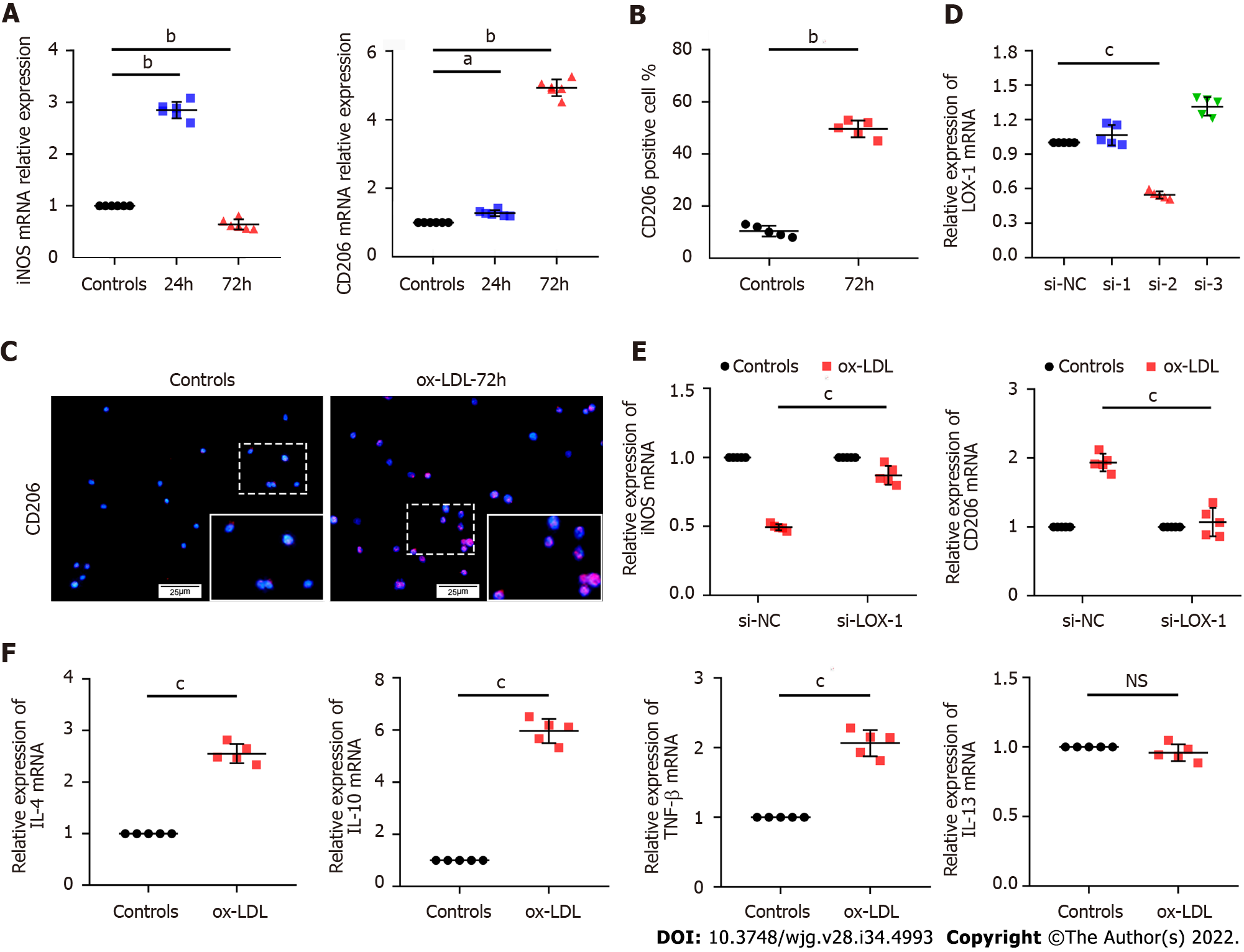
To explore whether ox-LDL is related to macrophage polarization, we stimulated human monocytic leukemia cells (THP-1) with ox-LDL for 24 h and 72 h in vitro. qPCR results showed that the expression level of CD206 gradually increased with increased stimulation time. However, the expression level of iNOS increased in a short period and then decreased significantly below the initial level (Figure 4A). In addition, ox-LDL was used to stimulate mouse leukemic monocyte/macrophage cell line (RAW 264.7) for 72 h, and IF detection for CD206 was conducted. The results showed that CD206+ macrophages significantly increased in RAW 264.7 cells 3 d after ox-LDL stimulation (Figures 4B and 4C). Overall, we found that CD206+ macrophages gradually increased with continuous stimulation with ox-LDL, while iNOS+ macrophages initially increased then decreased in the later stages.
In order to confirm the relationship between ox-LDL and CD206+ cells, we transfected LOX-1 siRNA into THP-1 cells to inhibit the expression of LOX-1, the specific receptor of ox-LDL (Figure 4D). After 72 h of ox-LDL stimulation, the inhibition of iNOS expression and the promotion of CD206 expression were significantly weakened in THP-1 cells transfected with LOX-1 siRNA (Figure 4E). Further examination of the function of CD206+ macrophages showed that after 72 h of ox-LDL stimulation, the levels of the CD206+ macrophage-related cytokines interleukin IL-4, IL-10, and tumor necrosis factor TNF-β increased significantly in THP-1 cells except IL-13 (Figure 4F).
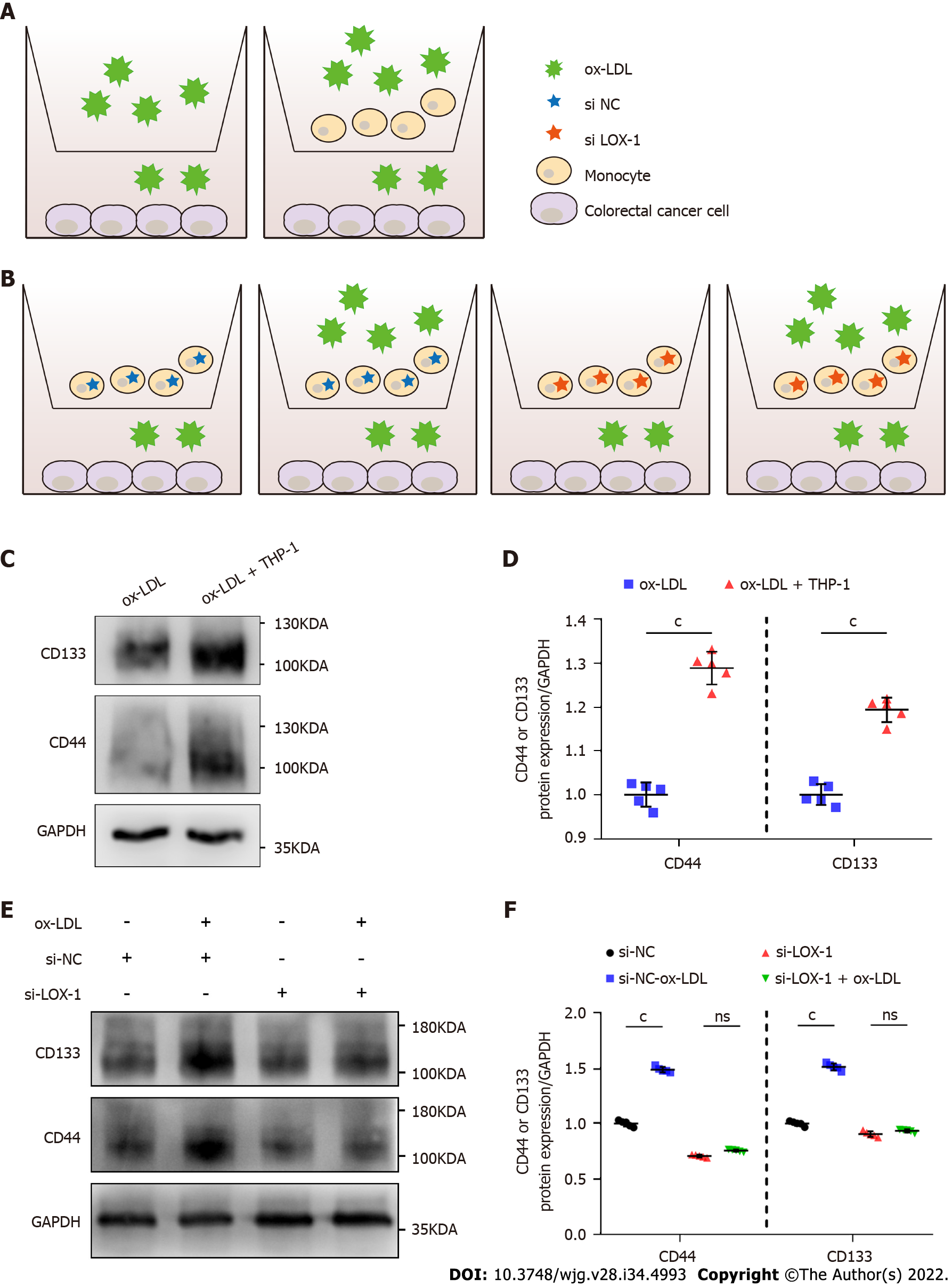
To investigate whether the occurrence of CRC is related to macrophages in the colorectal stroma and a high-fat microenvironment, we simultaneously inoculated human colorectal adenocarcinoma cells (LoVo) and THP-1 cells into transwell culture plates supplemented with 20 μg/mL ox-LDL for 72 h (Figure 5A). Thus, a high-lipid microenvironment was established to stimulate macrophages in vitro (n = 5). Western blot analysis showed that CD44 and CD133 expression was significantly increased in LoVo cells co-cultured with THP-1+ ox-LDL compared with ox-LDL alone (Figures 5C and 5D). We further transfected LOX-1 siRNA or siRNA negative control into THP-1 cells, and provided ox-LDL stimulation and co-cultured with LoVo cells again (Figure 5B). After ox-LDL stimulation, the levels of CD44 and CD133 in LoVo cells were inhibited when we knocked down LOX-1 in THP-1 cells (Figures 5E and 5F).
An increasing body of evidence suggests that HFD increases the risk of CRC. Importantly, studies have demonstrated the relationship between HFD and cancer by establishing animal models. HFD is often associated with abnormal oxidative stress and elevated lipid levels. Ox-LDL is a metabolite that reflects oxidative stress and lipid metabolism and is associated with various tumors. Ma et al[18] hypothesized that ox-LDL could promote gastric cancer metastasis and demonstrated that ox-LDL could promote vascular proliferation and lymphatic metastasis in gastric cancer by activating the nuclear factor-kappa B signaling pathway in animal and cell experiments. Ox-LDL may play a potential role in promoting HFD associated CRC. It has been reported that ox-LDL is correlated with CRC in patients with dyslipidemia, and the serum ox-LDL level of CRC patients is higher than that in subjects without tumors[19]. A study from Egypt found that the serum ox-LDL level of obese colon cancer patients was higher than that in obese patients with a healthy intestine; a positive correlation was found between the serum ox-LDL level and the degree of tumor malignancy[13]. Furthermore, it was found that the serum ox-LDL level of patients after surgery was significantly lower than that before surgery[14]. These findings suggest that ox-LDL is a potential predictor and prognostic biomarker of CRC. However, the above experiments were conducted using blood serum tests of CRC patients. To the best of our knowledge, no study has documented ox-LDL expression in colorectal tissue. Herein, we provided compelling evidence that ox-LDL was abnormally expressed in CRC patients with hyperlipidemia at the tissue level. In this regard, the ox-LDL level was upregulated in the cancer tissue of CRC patients, especially in the stroma. Besides, we demonstrated that LOX-1, the surface receptor of ox-LDL, was also upregulated in CRC tissues. Consistently, increased ox-LDL and LOX-1 levels were documented in the colorectal tissues of HFD-fed mice, suggesting the regulatory role of ox-LDL in the tumor microenvironment in CRC.
Macrophages, the most important immune cells in the tumor microenvironment, exhibit multiple phenotypes and exert various function[8]. It has been shown that iNOS+ macrophages inhibit tumor progression mainly by playing a pro-inflammatory role[20], while CD206+ macrophages promote cell repair and cell proliferation and growth, thus promoting tumor progression[9]. Different macrophages exhibit dynamic changes in the tumor microenvironment, and CD206+ macrophages are closely related to CRC development[21]. Existing clinical studies have reported that the CD206+ macrophage level is positively correlated with the TNM stage, the number of metastasized lymph nodes, and the degree of vascular invasion[22], and high CD206+ macrophage expression suggests a poor prognosis. Han et al[11] found that CD206+ macrophages in the tumor microenvironment could promote CRC metastasis through interaction with CRC cells. CD206+ macrophages have been reported to be elevated in colorectal tissues in patients with hyperlipidemia. A higher prevalence of CRC was detected in subjects with an HFD in a retrospective cohort study conducted by Liu et al[7]. In the present study, CD206+ macrophages were upregulated in colorectal tissues in patients with hyperlipidemia. In addition, animal experiments also confirmed that an increase in the number of intestinal tumors in HFD-fed mice was accompanied by a higher level of CD206+ macrophages, which correlated with the degree of mali
In addition, ox-LDL is also associated with tumor stem cells. Yang et al[23] found that ox-LDL could increase the malignancy of tumor stem cells in bladder cancer, thus promoting the development of bladder cancer. Active cell proliferation has been documented in gastrointestinal tissue from HFD-fed mice, with increased malignancy of tumor stem cells, which has been attributed to the inflammatory environment in colorectal tissue[4,5,24]. The high level of CD206+ macrophages in HFD-fed mice may play a certain role in this process. After THP-1 cells were stimulated with ox-LDL for 72 h and co-cultured with LoVo cells, the levels of the tumor stem cell markers CD44 and CD133 significantly increased in LoVo cells. Further, when we knocked down LOX-1 in macrophages, the increase in the levels of CD44 and CD133 was not that obvious in CRC cells, confirming that ox-LDL mediated the CD206+ macrophages to upregulate CD44 and CD133 expression in colon cancer cells.
In this study, we hypothesized that HFD could induce ox-LDL and its surface receptor LOX-1 accumulation in CRC tissue, suggesting the regulatory role of ox-LDL in the microenvironment of CRC. Furthermore, continuous stimulation of ox-LDL on macrophages induced CD206+ macrophages, which could further promote the increase of CD44 and CD133 levels in CRC cells. However, there are many limitations to our study. First of all, this study was a single-center study, and the sample size of included clinical specimens was relatively small. Larger sample size and multi-center prospective studies are needed to confirm our findings. Moreover, the mechanism underlying the effects of ox-LDL and the relevant signaling pathways were not explored, warranting further studies. In conclusion, we demonstrated that HFD causes ox-LDL accumulation in the colorectal tissue and upregulates CD44 and CD133 expression in colorectal cells by inducing CD206+ macrophages. These findings provide evidence for a new mechanism of increased CRC susceptibility with HFD.
Oxidized low-density lipoprotein (ox-LDL), abnormally increased in the serum of patients with colorectal cancer (CRC) associated with a high-fat diet (HFD), may be one of the risk factors for CRC. Ox-LDL exerts a regulatory effect on macrophages, is associated with cancer stem cells, and may regulate CRC through the tumor microenvironment. The role of ox-LDL in CRC remains unclear. It is essential to explore the function of ox-LDL to explore the pathogenesis of HFD associated CRC.
The expression of ox-LDL in human colorectal cancerous tissues and colorectal tissues of hyperlipidemic mice was detected, and the function of ox-LDL in the macrophages in the tumor microenvironment was explored. Our key point is that ox-LDL up-regulates CD44 and CD133 in HFD associated CRC, which is mediated by macrophages. Our study will provide a new idea for the mechanism of HFD associated CRC.
The study aimed to investigate the role of ox-LDL through macrophage in HFD associated CRC.
The expression of ox-LDL and CD206 was detected in colorectal tissues of CRC patients with hyperlipidemia and HFD-fed mice by immunofluorescence. We stimulated macrophages with 20 ug/mL ox-LDL and assessed the expression levels of CD206 and cytokines by cell fluorescence and quantitative polymerase chain reaction. We further knocked down LOX-1, the surface receptor of ox-LDL, to confirm the function of ox-LDL in macrophages. Then, LoVo cells were co-cultured with the stimulated macrophages to analyze the CD44 and CD133 expression by western blot.
The expression of ox-LDL and CD206 was significantly increased in the stroma of colorectal tissues of CRC patients with hyperlipidemia, and also upregulated in the HFD-fed mice. Moreover, an increased level of CD206 and decreased level of inducible nitric oxide synthase were observed in macrophages after the continuous stimulation of ox-LDL. Such effects were inhibited when the surface receptor LOX-1 was knocked down in macrophages. Ox-LDL could induce CD206+ macrophages, which resulted in high expression of CD44 and CD133 in co-cultured LoVo cells.
Our study found that HFD could induce ox-LDL accumulation in CRC tissue, suggesting the regulatory role of ox-LDL in the microenvironment of CRC. Continuous stimulation of macrophages with ox-LDL induced CD206+ macrophages, which could further promote the increase of CD44 and CD133 levels in CRC cells.
Our future study will collect more samples. We look forward to make a convincing analysis to identify the correlation between ox-LDL and progression and survival of the enrolled patients in the near future. We will explore the potential signal pathways related to ox-LDL promoting M2-type macrophages by using the technology of single cell sequencing and/or RNA-Seq assay. We are confident that it will provide exciting data in the near future.
We thank Ms. Lin-Lin Huang (Department of Gastroenterology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China) for kindly providing LoVo cells. We really appreciate Mr. Ren-Gui Lin and Ms. Yu-Liang Huang (The Third School of Clinical Medicine, Southern Medical University, Guangzhou, China) for kindly assisting in the completion of the experiments.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68603] [Article Influence: 13720.6] [Reference Citation Analysis (201)] |
| 2. | Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, Chan AT, Giovannucci EL, Cao Y. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019;5:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 386] [Article Influence: 55.1] [Reference Citation Analysis (2)] |
| 3. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1773] [Article Influence: 253.3] [Reference Citation Analysis (2)] |
| 4. | Mana MD, Hussey AM, Tzouanas CN, Imada S, Barrera Millan Y, Bahceci D, Saiz DR, Webb AT, Lewis CA, Carmeliet P, Mihaylova MM, Shalek AK, Yilmaz ÖH. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 2021;35:109212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 5. | van Driel MS, van Neerven SM, Vermeulen L. High-Fat Diet Impacts on Tumor Development in the Gut. Trends Cancer. 2021;7:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Lin HY, Weng SW, Shen FC, Chang YH, Lian WS, Hsieh CH, Chuang JH, Lin TK, Liou CW, Chang CS, Lin CY, Su YJ, Wang PW. Abrogation of Toll-Like Receptor 4 Mitigates Obesity-Induced Oxidative Stress, Proinflammation, and Insulin Resistance Through Metabolic Reprogramming of Mitochondria in Adipose Tissue. Antioxid Redox Signal. 2020;33:66-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Liu T, Guo Z, Song X, Liu L, Dong W, Wang S, Xu M, Yang C, Wang B, Cao H. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence. J Cell Mol Med. 2020;24:2648-2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4787] [Article Influence: 398.9] [Reference Citation Analysis (1)] |
| 9. | Li R, Zhou R, Wang H, Li W, Pan M, Yao X, Zhan W, Yang S, Xu L, Ding Y, Zhao L. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26:2447-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 10. | Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2889] [Cited by in RCA: 3201] [Article Influence: 266.8] [Reference Citation Analysis (0)] |
| 11. | Han L, Wang S, Wei C, Fang Y, Huang S, Yin T, Xiong B, Yang C. Tumour microenvironment: a non-negligible driver for epithelial-mesenchymal transition in colorectal cancer. Expert Rev Mol Med. 2021;23:e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Khaidakov M, Mehta JL. Do atherosclerosis and obesity-associated susceptibility to cancer share causative link to oxLDL and LOX-1? Cardiovasc Drugs Ther. 2011;25:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Keshk WA, Zineldeen DH, Wasfy RE, El-Khadrawy OH. Fatty acid synthase/oxidized low-density lipoprotein as metabolic oncogenes linking obesity to colon cancer via NF-kappa B in Egyptians. Med Oncol. 2014;31:192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Salehi SS, Mirmiranpour H, Rabizadeh S, Esteghamati A, Tomasello G, Alibakhshi A, Najafi N, Rajab A, Nakhjavani M. Improvement in Redox Homeostasis after Cytoreductive Surgery in Colorectal Adenocarcinoma. Oxid Med Cell Longev. 2021;2021:8864905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Pei C, Zhang Y, Wang P, Zhang B, Fang L, Liu B, Meng S. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways. Phytother Res. 2019;33:294-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Yang P, Rhea PR, Conway T, Nookala S, Hegde V, Gagea M, Ajami NJ, Harribance SL, Ochoa J, Sastry JK, Cohen L. Human Biofield Therapy Modulates Tumor Microenvironment and Cancer Stemness in Mouse Lung Carcinoma. Integr Cancer Ther. 2020;19:1534735420940398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Lv J, Liu C, Chen FK, Feng ZP, Jia L, Liu PJ, Yang ZX, Hou F, Deng ZY. M2like tumourassociated macrophagesecreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Ma C, Xie J, Luo C, Yin H, Li R, Wang X, Xiong W, Zhang T, Jiang P, Qi W, Zhou T, Yang Z, Wang W, Ma J, Gao G, Yang X. OxLDL promotes lymphangiogenesis and lymphatic metastasis in gastric cancer by upregulating VEGFC expression and secretion. Int J Oncol. 2019;54:572-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Toyoshima H, Kojima M, Tokudome S, Hayakawa N, Watanabe Y, Tamakoshi K, Suzuki S, Ozasa K, Tamakoshi A; Japan Collaborative Cohort Study Group. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev. 2004;13:1781-1787. [PubMed] |
| 20. | Lee JW, Lee SM, Chun J, Im JP, Seo SK, Ha N, Il Choi Y, Kim JS. Novel Histone Deacetylase 6 Inhibitor CKD-506 Inhibits NF-κB Signaling in Intestinal Epithelial Cells and Macrophages and Ameliorates Acute and Chronic Murine Colitis. Inflamm Bowel Dis. 2020;26:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Inagaki K, Kunisho S, Takigawa H, Yuge R, Oka S, Tanaka S, Shimamoto F, Chayama K, Kitadai Y. Role of tumor-associated macrophages at the invasive front in human colorectal cancer progression. Cancer Sci. 2021;112:2692-2704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Ma X, Gao Y, Chen Y, Liu J, Yang C, Bao C, Wang Y, Feng Y, Song X, Qiao S. M2-Type Macrophages Induce Tregs Generation by Activating the TGF-β/Smad Signalling Pathway to Promote Colorectal Cancer Development. Onco Targets Ther. 2021;14:5391-5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Yang L, Sun J, Li M, Long Y, Zhang D, Guo H, Huang R, Yan J. Oxidized Low-Density Lipoprotein Links Hypercholesterolemia and Bladder Cancer Aggressiveness by Promoting Cancer Stemness. Cancer Res. 2021;81:5720-5732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 647] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen XX, China; Elchaninov AV, Russia; Ortiz-Masia D, Spain S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wu RR