Published online Jun 14, 2022. doi: 10.3748/wjg.v28.i22.2482
Peer-review started: November 2, 2021
First decision: December 3, 2021
Revised: December 9, 2021
Accepted: April 28, 2022
Article in press: April 28, 2022
Published online: June 14, 2022
Processing time: 219 Days and 15.7 Hours
The appearance of the intestinal mucosa during endoscopy varies among patients with primary intestinal lymphangiectasia (PIL).
To classify the endoscopic features of the intestinal mucosa in PIL under endoscopy, combine the patients’ imaging and pathological characteristics of the patients, and explain their causes.
We retrospectively analyzed the endoscopic images of 123 patients with PIL who were treated at the hospital between January 1, 2007 and December 31, 2018. We compared and analyzed all endoscopic images, classified them into four types according to the endoscopic features of the intestinal mucosa, and analyzed the post-lymphographic computed tomography (PLCT) and pathological characteristics of each type.
According to the endoscopic features of PIL in 123 patients observed during endoscopy, they were classified into four types: nodular-type, granular-type, vesicular-type, and edematous-type. PLCT showed diffuse thickening of the small intestinal wall, and no contrast agent was seen in the small intestinal wall and mesentery in the patients with nodular and granular types. Contrast agent was scattered in the small intestinal wall and mesentery in the patients with vesicular and edematous types. Analysis of the small intestinal mucosal pathology revealed that nodular-type and granular-type lymphangiectasia involved the small intestine mucosa in four layers, whereas ectasia of the vesicular- and edematous-type lymphatic vessels largely involved the lamina propria mucosae, submucosae, and muscular layers.
Endoscopic classification, combined with the patients’ clinical manifestations and pathological examination results, is significant and very useful to clinicians when scoping patients with suspected PIL.
Core Tip: Primary intestinal lymphangiectasia (PIL) is a rare disorder that typically presents as protein-losing enteropathy, diarrhea, and limb edema. Here we analyzed the endoscopic presentation, post-lymphographic computed tomography findings, and pathological features of 123 patients with PIL. We classified PIL into four categories - nodular, granular, vesicle, and edema types, proposed the theory of nodular and vesicle types domestically through observation, and made breakthroughs in overcoming the issue of inaccurate diagnosis based on a few independent early-stage case reports.
- Citation: Meng MM, Liu KL, Xue XY, Hao K, Dong J, Yu CK, Liu H, Wang CH, Su H, Lin W, Jiang GJ, Wei N, Wang RG, Shen WB, Wu J. Endoscopic classification and pathological features of primary intestinal lymphangiectasia. World J Gastroenterol 2022; 28(22): 2482-2493
- URL: https://www.wjgnet.com/1007-9327/full/v28/i22/2482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i22.2482
Primary intestinal lymphangiectasia (PIL), also known as Waldmann disease, is a type of abnormal intestinal lymphatic drainage that may be caused by congenital dysplasia of the lymphatic system[1]. Infants, children, and adolescents account for a large proportion of PIL patients, with occasional reports of cases in middle-aged and elderly patients[2]. PIL can cause leakage of lymphatic fluid into the gastrointestinal tract, eventually leading to protein-losing enteropathy (PLE). The main symptoms include diarrhea with limb edema, hypoalbuminemia, lymphopenia, and hypogammaglobulinemia[3,4]. Prasad et al[5] concluded that the interval between symptom onset and a definitive diagnosis of PIL was as long as 54.8 mo due to the lack of knowledge of PIL by most clinicians, and their diagnosis relied on specialized examinations, such as endoscopy and histological diagnosis. At present, the diagnostic basis of such diseases includes visible typical endoscopic features, such as swelling and whitening of the intestinal villi, visible lymphangiectasis seen in the mucosa and submucosa through histopathological intestinal mucosal biopsy, and the characteristics of immunohistochemistry D2-40+[6].
As a rare disease, PIL is rarely reported domestically or internationally, and most reports have involved individual cases. Endoscopic images of individual cases revealed that patients had different mucosal manifestations, such as pinpoint white dots, diffuse villi thickening and whitening, and white spots[7-9]. A retrospective analysis of endoscopic images of patients diagnosed with PIL during a 12-year period at our research center summarized and classified them into four distinct endoscopic features and further explored the differences in radiologic and pathologic features of the different endoscopic features.
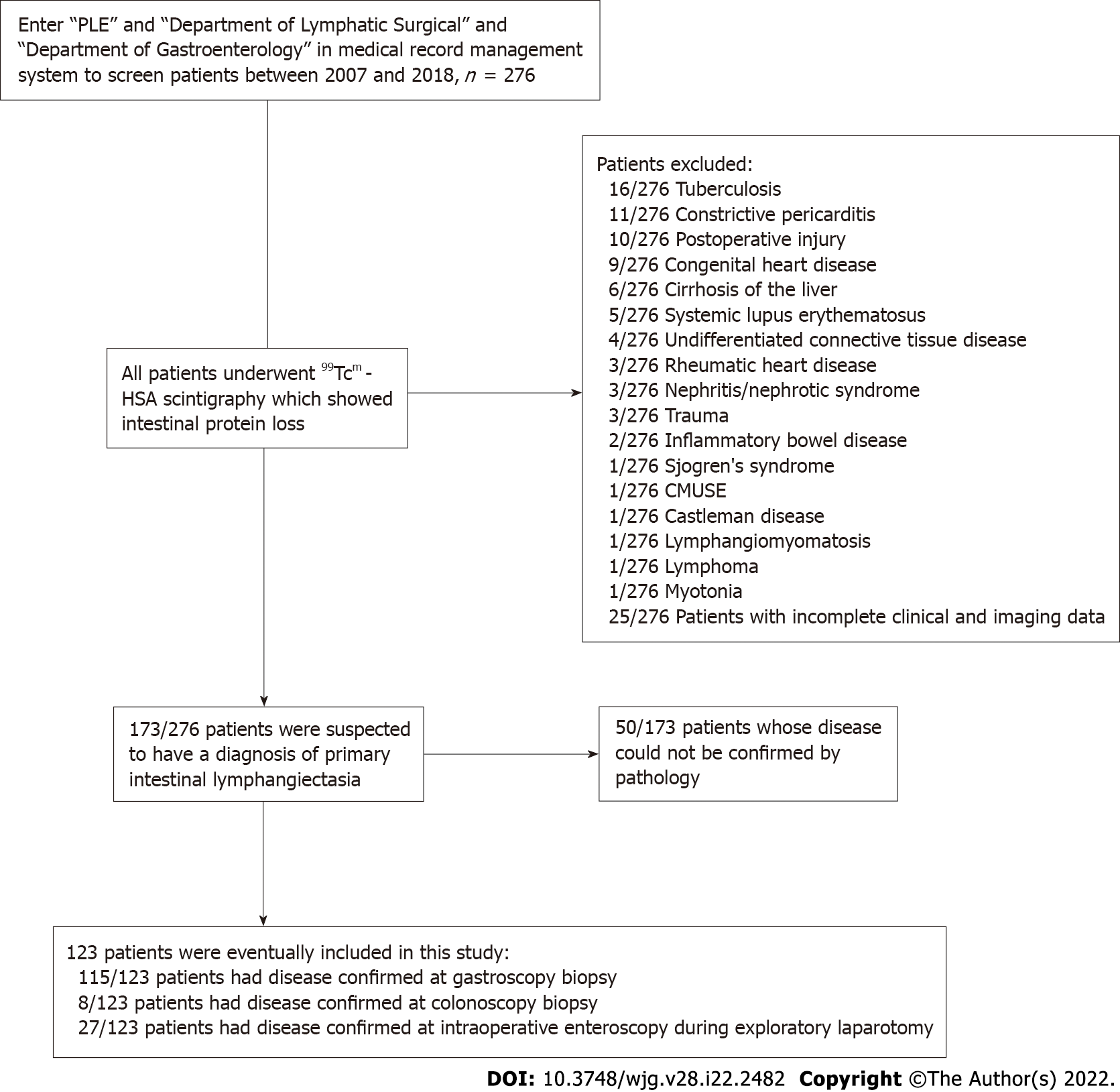
We retrospectively analyzed 685 cases of PLE at Beijing Shijitan Hospital, Capital Medical University between January 1, 2007, and December 31, 2018. After strict screening, 123 patients were included in this study. The direct indicators of diagnosis were manifestations of intestinal lymphangiectasia obtained through endoscopy, visible lymphangiectasis found in the mucosa and submucosa through histopathological intestinal mucosal biopsy or surgical specimen pathology, and a positive D2-40 on immunohistochemistry. In contrast, indirect indicators were typical clinical manifestations (e.g., diarrhea, edema, and abdominal pain), hypoproteinemia (albumin concentration < 30 g/L) with positive imaging of technetium-99m human serum albumin, and secondary factors[4,10]. We summarized the patients’ clinical data, including age, sex, family history, clinical symptoms, laboratory inspection results, endoscopic images, imaging examination results, and pathological examination results. The imaging data were obtained from the host computer imaging system of Beijing Shijitan Hospital. This study was reviewed and approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, and all patients signed a consent form.
All patients included in the study underwent electronic gastroscopy (GIF-H260; Olympus, Tokyo, Japan); of them, those with no significant abnormal manifestations were examined using electronic colonoscopy (CF-H260AI; Olympus, Tokyo, Japan). In addition, patients who underwent surgery underwent colonoscopy during the operation. All patients underwent endoscopic biopsy, the specimens from which were preserved after surgery. We obtained the first endoscopic images of the patients at admission for observation and invited three experienced endoscopists (deputy chief doctors or chief doctors with at least 10 years of experience in gastroscopy and enteroscopy) to interpret the endoscopic images. In cases of disagreement regarding the classification, it was determined by the endoscopists through discussion.
All the patients underwent direct lymphangiography. The contrast agent injection spot was located between the 1st and 2nd toes on certain sides. The lymphatic vessel was penetrated and the contrast agent iodinated oil (10 mL/ampule; Guerbet, France) was injected at a flow rate of 4-6 mL/h. Digital subtraction angiography images were dynamically collected at different intervals to observe the development of the lymphatic system. Two hours after surgery, computed tomography (CT) examination of the chest, abdomen, and pelvis was performed using the following scanning parameters: tube voltage, 80-120 kVp; tube current, 100-120 mAs; thickness of recombination layer, 2.0 mm; and interval, 1.8 mm. Post-lymphographic CT (PLCT) images were analyzed using the following evaluation items: (1) Intestinal wall thickening, when small intestinal wall thickness was ≥ 3 mm[10]; (2) edema manifestations: hydrops in the abdominal cavity, retina, and mesentery; and (3) lymphangiectasis of the small intestinal wall and mesentery, with manifestations of irregular distribution of contrast agent in multiple dots, lines, and clusters in the small intestinal wall and/or mesentery. All CT images were evaluated by two radiologists with > 10 years of experience in abdominal imaging diagnosis. In case of any differences in the evaluation results, consensus was reached through discussion and the results were recorded.
We collected the pathological data of 123 specimens from patients with PIL, of which 27 specimens were obtained through surgical resection. Hematoxylin and eosin (H&E) and immunohistochemical staining (D2-40 and CD34) were conducted on the specimens postoperatively. Visible dilated lymphatic vessels were distributed in the lamina propria of the intestinal mucosa, submucosa, muscular layer, and the serosal layer. All pathological sections were evaluated by two physicians with 5 and 10 years of experience in pathological diagnosis.
The statistical analysis was performed using the SPSS software for Windows (version 20.0; IBM Statistics, Armonk, NY, United States). Data are presented as medians with ranges and proportions. To determine the differences between the four groups, the chi-square test (with Fisher’s exact test whenever applicable) was used to compare categorical variables, while the t-test was used to compare continuous variables. Statistical significance was set at P < 0.05.
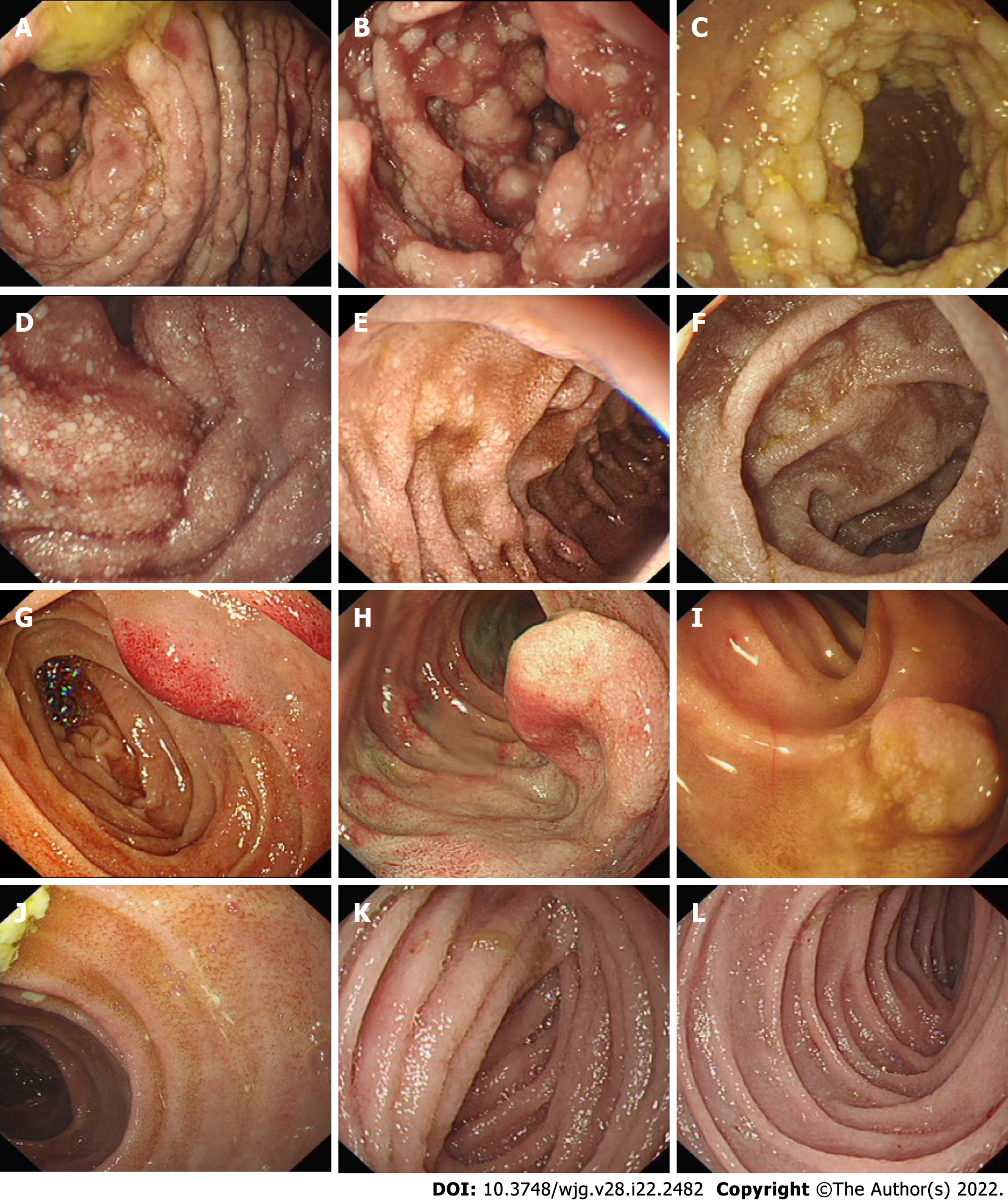
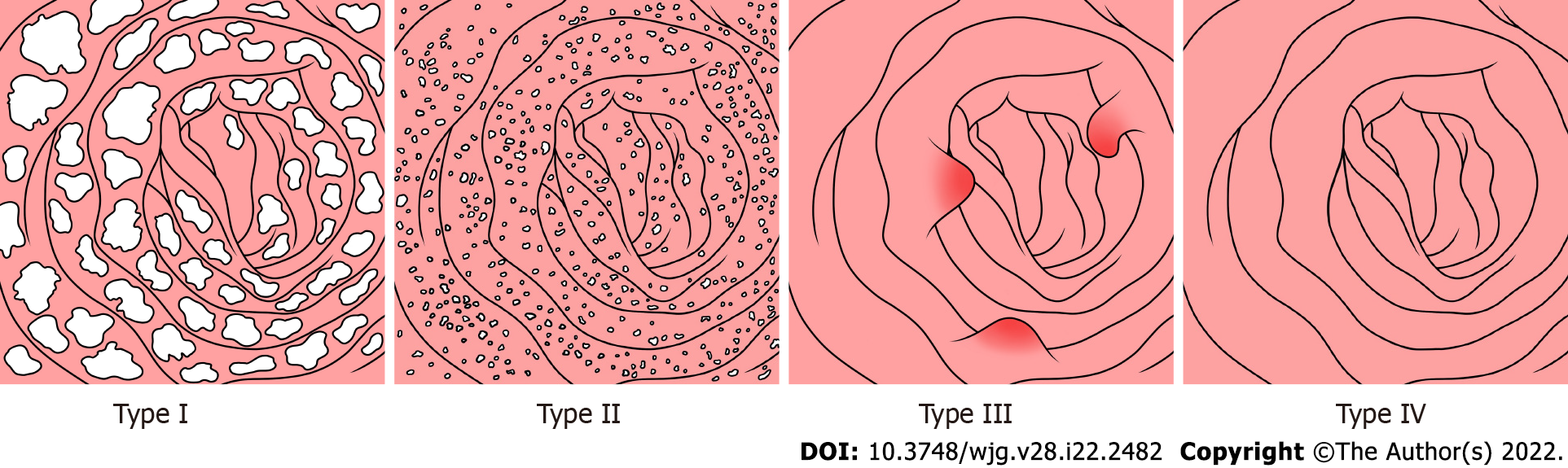
Figure 1 shows a flowchart of the patient selection process. A total of 123 patients were included in this study. Gastroscopy and biopsy results were used for 115 of 123 patients with PIL, while colonoscopy and biopsy results were used for the other 8 patients with PIL. Additionally, small intestinal endoscopy was used for 27 of 123 patients with PIL, and further confirmation was made when the pathological specimens were obtained postoperatively (Figure 1). Through unanimous judgment, PIL was classified into four types according to the different morphologies of the intestinal mucosa under endoscopy: (1) Nodular type [n = 17 (13.8%); Figure 2A-C], which had a snowflake or cream yellow nodular-shaped protrusion at the surface of the mucosa, and for patients with serious symptoms, the white flakes were ring-coelom shaped, the mucosa was swollen and hypertrophic, and intestinal stenosis was present; (2) granular type [n = 74 (60.2%); Figure 2D-F], which showed white granules with disseminated changes at the surface of the mucosa, swollen villi, and lesions that were distributed regionally or in a whole section; (3) vesicular type [n = 5 (4.1%); Figure 2G-I], which showed a vesicle-shaped structure protruding from the mucosa, that part of the surface of the mucosa was hyperemic, and the lesion was isolated; and (4) edematous type [n = 27 (21.9%); Figure 2J-L], in which the villi of the small intestine were swollen and blisters were small and round, villus color was normal, and no change in the characteristic white villus was noted (Figure 2).
Among 123 patients, 61 (49.6%) were men and 62 (50.4%) were women. The average age at onset was 17.0 ± 17.4 years (range, 0-68 years), while that at diagnosis was 22.8 ± 19.2 years. Based on the clinical manifestations, 58.5% (72/123) of the patients, including four with celiac stools, had five or more episodes of diarrhea per day. Additionally, 78.0% (96/123) of the patients had edema of the lower limbs, eyelids, and face, 17.7% (17/96) had anasarca, 17.7% (17/96) had unilateral limb edema, and the rest had bilateral edema. Furthermore, 1.6% (2/123) of patients had concurrent cryptococcal or streptococcal infections. Other symptoms included abdominal distention [28 patients (22.8%)], abdominal pain [10 patients (8.1%)], nausea and vomiting [8 patients (6.5%)], and alimentary tract hemorrhage [4 patients (3.3%)]. There were no obvious differences in clinical symptoms among the four patient types. However, the laboratory results of the four types of PIL patients showed a significant decline in peripheral blood lymphocyte counts and IgG concentrations (Table 1).
| Clinical features | Total (n = 123) | Type | P value | |||
| Nodular type (n = 17) | Granular type (n = 74) | Vesicular type (n = 5) | Edematous type (n = 27) | |||
| Demographic characteristics, mean ± SD (age range)/n (%) | ||||||
| Age of diagnosis (yr) | 22.8 ± 19.2 | 10.2 ± 7.6 | 23.7 ± 20.6 | 25.2 ± 15.7 | 27.7 ± 18.3 | < 0.001 |
| Age of onset (yr) | 17.0 ± 17.4 (0, 68) | 3.7 ± 6.9 (0, 22) | 17.9 ± 18.0 (0, 68) | 22.2 ± 15.1 (3, 44) | 21.7 ± 17.4 (0, 66) | < 0.001 |
| Male | 61 (49.6) | 7 (41.2) | 35 (47.3) | 2 (40.0) | 17 (63.0) | 0.408 |
| Clinical characteristics, n (%) | ||||||
| Diarrhea | 72 (58.5) | 12 (70.6) | 47 (63.5) | 3 (60.0) | 10 (37.0) | 0.074 |
| Edema | 96 (78.0) | 13 (76.5) | 59 (79.7) | 2 (40.0) | 22 (81.5) | 0.238 |
| Abdominal distension | 28 (22.8) | 6 (35.3) | 15 (20.3) | 1 (20.0) | 6 (22.2) | 0.567 |
| Abdominal pain | 10 (8.1) | 0 (0) | 7 (9.5) | 0 (0) | 3 (11.1) | 0.603 |
| Nausea and vomiting | 8 (6.5) | 0 (0) | 8 (10.8) | 0 (0) | 0 (0) | 0.145 |
| Gastrointestinal bleeding | 4 (3.3) | 0 (0) | 2 (2.7) | 0 (0) | 2 (7.4) | 0.423 |
| Fever | 2 (1.6) | 0 (0) | 1 (1.4) | 0 (0) | 1 (3.7) | 0.640 |
| Laboratory examination, mean ± SD | ||||||
| LY | 1.2 ± 0.9 | 1.0 ± 0.5 | 1.2 ± 1.1 | 0.3 ± 0.1 | 1.4 ± 0.9 | < 0.001 |
| HGB (g/L) (110-150) | 129.9 ± 23.5 | 137.4±17.9 | 127.9 ± 24.5 | 116.4 ± 13.3 | 133.1 ± 24.3 | 0.221 |
| TP (g/L) (60-80) | 41.2 ± 9.0 | 39.9 ± 6.7 | 40.4 ± 8.7 | 39.9 ± 7.1 | 44.4 ± 10.8 | 0.218 |
| Alb (g/L) (35-55) | 25.4 ± 7.2 | 24.3 ± 5.9 | 25.5 ± 6.7 | 26.5 ± 5.7 | 25.6 ± 9.4 | 0.911 |
| Glb (g/L) (20-30) | 15.8 ± 4.3 | 15.3 ± 4.4 | 15.0 ± 3.2 | 13.4 ± 3.0 | 18.8 ± 5.5 | 0.024 |
| IgG (g/L) (7-16) | 3.91 ± 2.30 | 3.35 ± 1.48 | 3.73 ± 2.12 | 2.26 ± 0.43 | 5.01 ± 2.90 | < 0.001 |
| Ca (mmol/l) (2.1-2.75) | 1.93 ± 0.28 | 1.90 ± 0.18 | 1.91 ± 0.31 | 1.86 ± 0.09 | 1.99 ± 0.26 | 0.556 |
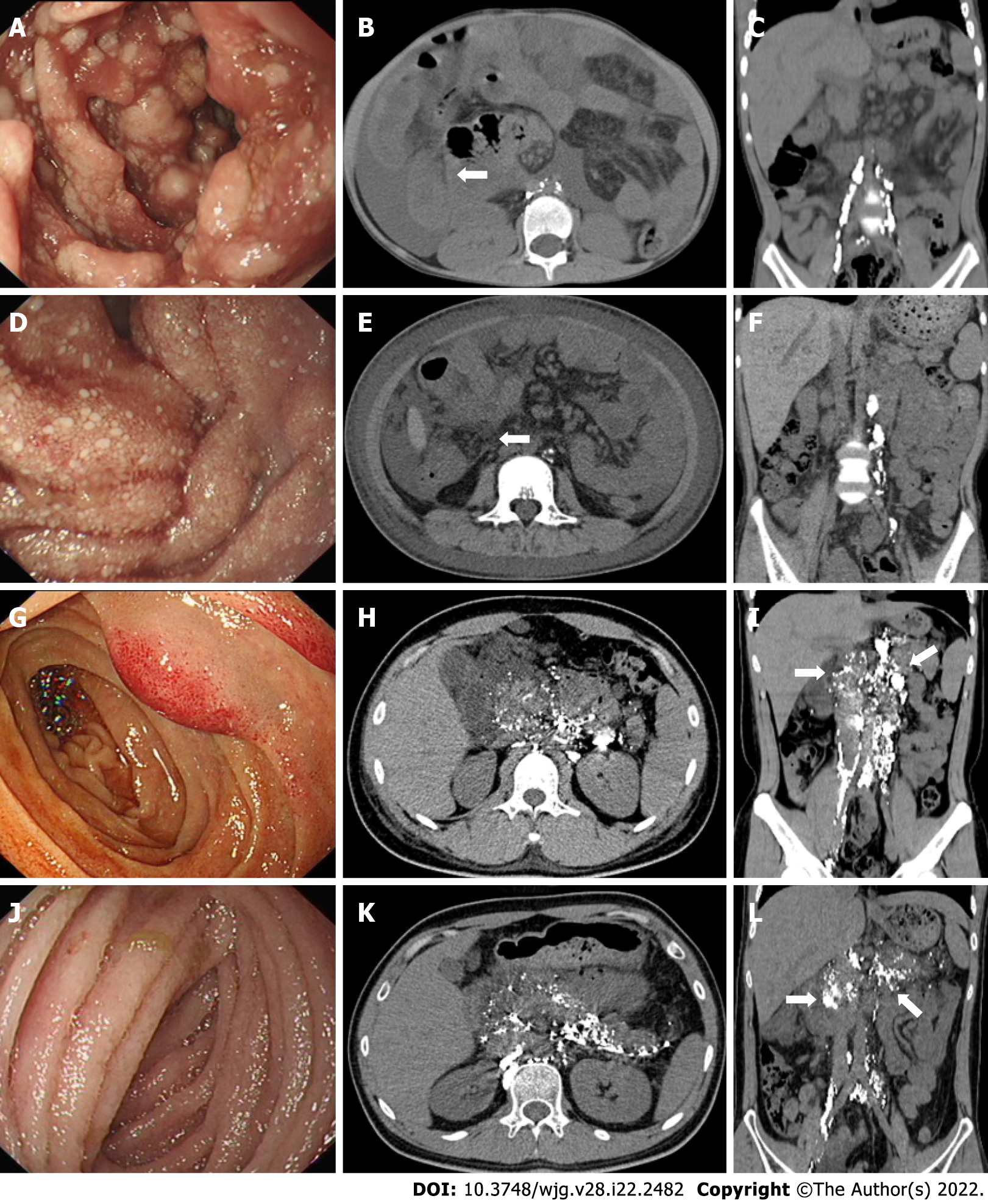
We summarized and analyzed the corresponding PLCT images of the four types of patients with PIL observed during endoscopy. The specific characteristics were as follows: (1) Nodular type: the images showed signs of intestinal wall thickening, increased mesenteric density, and no contrast distribution in the mesentery or intestinal wall (Figure 3A-C); (2) granular type: the images showed annular thickening of the small intestinal wall, increased mesenteric density, and no contrast distribution in the mesentery and intestinal wall (Figure 3D-F); (3) vesicular type: the images revealed an increased mesenteric density and distribution of contrast agents in the intestinal wall, mesentery, peripancreatic area, gallbladder fossa, hepatic hilum, and retroperitoneum (Figure 3G-I); and (4) edematous type: the images showed distribution of contrast agents in the intestinal wall, mesentery, peripancreatic area, hepatic hilum, and retroperitoneum (Figure 3J-L).
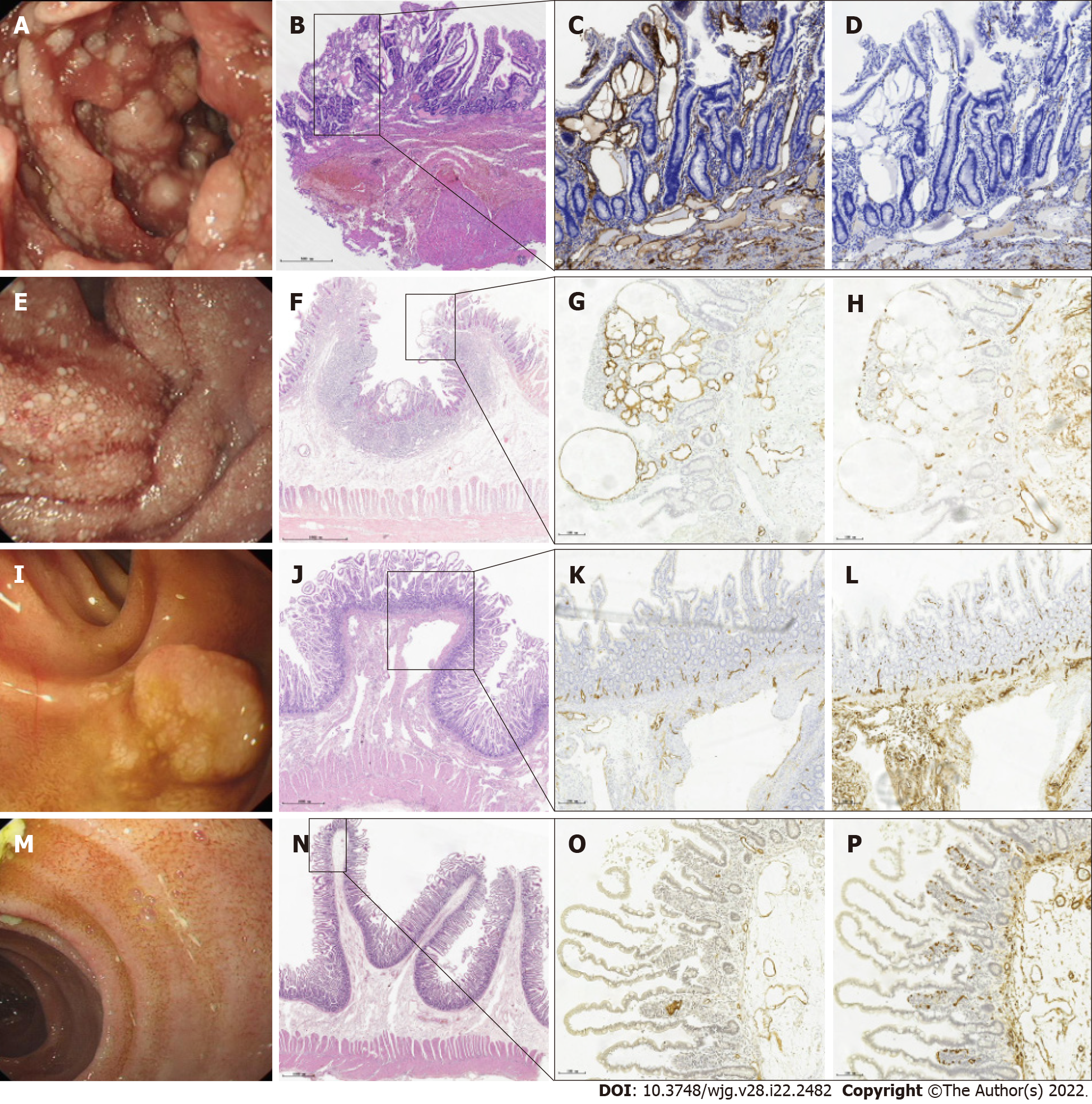
Pathological data of 123 specimens from PIL patients were collected, and lymphatic dilation was observed in different layers of the intestinal mucosa. The specific characteristics were as follows: (1) Nodular type: Enteroscopic findings showed a white-flake nodular-type protrusion (Figure 4A). H&E staining revealed that the lamina propria and submucosa lymphatic vessels were clearly dilated. The pink liquid in the dilated lumen was lymphatic fluid, which was consistent with the manifestation observed on endoscopy (Figure 4B). Additionally, lymphangiectasia was observed in the muscular and serosal layers, and immunohistochemical staining with D2-40 (+) more clearly showed the lymphatic vessels (Figure 4C and D); (2) granular type: Enteroscopic findings showed scattered white granules (Figure 4E), and the pathological features showed that the lymphatic vessels in the mucosal lamina propria and submucosa were significantly dilated (Figure 4F). Scattered lymphangiectasia was also seen in the muscular layer and serosal layers, and the distribution of tiny blood vessels was observed around the lymphatic vessels (Figure 4G and H); (3) vesicular type: Enteroscopic findings revealed raised submucosal lesions (Figure 4I). Pathological features showed thick lymphatic vessels in the raised lesions and no obvious lymphatic dilatation in the mucosa (Figure 4J and K). A distribution of tiny blood vessels was observed around the lymphatic vessels (Figure 4L); and (4) edematous type: Enteroscopic findings revealed swollen villi and small round blisters (Figure 4M). Pathological features showed lymphatic dilatation in the submucosa and no obvious lymphatic dilatation in the lamina propria (Figure 4N and O). Tiny blood vessels were observed around the lymphatic vessels (Figure 4P).
To date, there have been few studies on PIL in the English literature, most of which were reports of single cases and reviews of small cases. At present, the diagnosis of PIL is based on small intestinal endoscopy or capsule endoscopy to identify the typical characteristics of the intestinal mucosa and is confirmed through histopathological examination of the biopsy specimen of the intestinal mucosa; intestinal lymphangiectasia due to secondary causes is excluded[9,11]. The classification of intestinal lymphangiectasis using endoscopy is limited. Ohmiya et al[12] divided 14 patients with intestinal lymphangiectasis into white and non-white villi. However, these few cases included patients with secondary lymphangiectasias. We evaluated the endoscopic characteristics of 123 patients with PIL and found significant differences in the intestinal mucosal findings under endoscopy in different patients. Based on their endoscopic appearance, they were divided into four types: nodular, granular, vesicular, and edematous. The corresponding imaging and histopathological findings of each type were comprehensively analyzed. In this study, the endoscopic mucosal signs of patients with PIL were mainly white granule-like changes, a finding that was consistent with the endoscopic images reported in most studies. The nodular and vesicular types proposed in this study have not been reported previously.
PIL often develops in childhood or youth, and most cases are diagnosed before the age of 3 years. In recent years, an increasing number of cases have been reported in adults[2,13-17]. In this study, we found that the age at onset differed among the four types of endoscopy. Among them, the age at onset in patients with nodular-type PIL was the youngest (3.7 ± 6.9 years), and the earlier the age at onset, the more severe the intestinal mucosal findings. We also found clinical differences in peripheral blood lymphocyte counts and IgG levels among the four types in the laboratory examinations. Among the four types, the values of these two indicators were the lowest in patients with vesicular-type PIL, while the corresponding hemoglobin values were the lowest. By analyzing the clinical features of five patients with vesicular-type PIL, we found that four were diagnosed with gastrointestinal bleeding. The simultaneous loss of lymphatic fluid and blood from the intestinal tract can lead to a decrease in hemoglobin, lymphocyte, and immunoglobulin levels.
In recent years, PLCT has been of great value in the diagnosis of PIL. Our previous studies suggested that the specific signs of PIL include diffuse nodular thickening and edema of the intestinal wall as well as the presence of a "halo sign" on the intestinal wall[14]. The abnormal distribution of contrast agents in the small intestine and mesentery of the affected segment suggests the local presence of abnormally dilated lymphatic vessels and abnormal lymphatic circulation. In this study, the PLCT signs in nodular- and granular-type patients showed intestinal wall thickening and increased mesenteric density, consistent with the mucosal findings on endoscopy. In contrast, no significant intestinal wall thickening was found in vesicular- and edematous-type patients. By observing the contrast agent distribution, we found that the PLCT images of vesicular- and edematous-type patients showed contrast agent distribution in the intestinal wall, mesentery, peripancreatic, gallbladder fossa, hepatic hilum, and retroperitoneum, indicating lymphatic dilation and an abnormal distribution in these areas. However, there was no contrast agent distribution in the small intestine or mesentery in the PLCT images of nodular- and granular-type patients, which was inconsistent with the segmental and/or diffuse lymphatic dilation of the intestinal mucosa shown by the endoscopy results. Combined with the pathological results, we speculated that the lymphatic fluid in the intestinal mucosa and submucosal lymphatic vessels of nodular- and granular-type patients are subject to retention, which increases the pressure in the lymphatic vessels. In addition, there were many submucosal muscle fiber tissues that were not conducive to the counterflow of the contrast agent into the small intestine. In the nodular type, among the five children with contrast agent negativity, the lymphatic contrast agent was sparsely distributed throughout the body, which may be related to lymphatic dysplasia.
Previous reports[10,12,15] and histopathology of PIL biopsies showed that the mucosal and submucosal lymphatic vessels were significantly dilated and the lymphatic vessels were filled with protein-rich fluid and scattered lymphocytes. There are no reports on whether lymphatic dilation exists in the muscular and serosal layers. Macdonald et al[16] reported a strong correlation between small intestinal vascular hypoplasia and lymphatic dilation, but there was a lack of pathological examination supporting their argument. In this study, pathological examination of nodular- and granular-type PIL showed that the villi were enlarged and clubbed or cystic, with some tips ruptured. The lamina propria, submucosa, muscular layer, and serosa of the diffuse or localized mucosa showed significant dilation of the lymphatic vessels, with the most obvious lymphatic vessel dilation in the lamina propria of the intestinal mucosa, consistent with the endoscopy findings. Among them, nodular pathology has shown a large amount of muscle fiber tissue in the submucosa, which may be related to the long course of disease and chronic inflammation. Pathological examination of vesicular-type patients showed a large number of thin-walled and irregularly dilated lymphatic vessels in the submucosa; lymphatic fluid, lymphocytes, and red blood cells in the lumen; and the accumulation of lymphocytes in the stroma, which can form lymphoid follicles. This was consistent with the prominent polyp-like morphology observed on endoscopy, and a large number of small blood vessels were observed around the lymphatic vessels, further verifying that the third type of lymphatic dilation reported by Macdonald et al[16] was consistent with the vesicular type in our study. Therefore, our study findings complement the histopathological findings. Pathological examination of edematous-type patients showed that the lymphatic vessels in the submucosa were significantly dilated, and the lymphatic vessels in the lamina propria were not significantly dilated, which was consistent with the endoscopy findings. The histopathological results showed that the distribution of lymphatic dilation in the intestinal mucosa and the degree of lumen dilation differed; therefore, the signs of intestinal mucosa on endoscopy varied in patients with PIL.
This study analyzed the endoscopic images of 123 patients with PIL and found that the intestinal mucosa had different endoscopic features such as diffuse white nodules, white granules, polypoid protrusion, and mucosal edema, the basis of the division into four types of endoscopic features (Figure 5). However, the four distinctive phenotypic appearances may simply be endoscopic variations at different stages of the same disease, similar to the different endoscopic findings of eosinophilic esophagitis. The classification of PIL also requires support in animal models and molecular or genotypic bases to confirm the presence of distinct phenotypes. This study is preliminary because of its small sample size and retrospective design, and requires further validation in a multicenter prospective study.
In conclusion, PIL is a rare disorder that typically presents as PLE, diarrhea, and limb edema. Unspecific symptoms and a wide range of clinical manifestations can significantly hamper the establishment of a definitive diagnosis. PIL demonstrates four characteristics of digestive endoscopy. The identification of intestinal mucosal chorionic abnormalities in this rare disorder might be helpful for ensuring an early diagnosis and optimal treatment.
Primary intestinal lymphangiectasia (PIL) is a rare disorder that typically presents as PLE, diarrhea, and limb edema.
Unspecific symptoms and a wide range of clinical manifestations can significantly hamper the establishment of a definitive diagnosis.
This study aimed to classify the endoscopic features of the intestinal mucosa in PIL under endoscopy, combine post-lymphographic computed tomography findings and the patients’ pathological characteristics of the patients, and explain their causes.
This retrospective analysis of endoscopic images of patients diagnosed with PIL during a 12-year period at our research center summarized and classified types into four distinct endoscopic features and further explored the differences in radiologic and pathologic features of the different endoscopic features.
The endoscopic symptoms of 123 patients with PIL can be divided into four types: Nodular (13.8%), granular (60.2%), vesicular (4.1%), and edematous (21.9%).
We proposed the theory of nodular and vesicle types domestically through observation, and made breakthroughs in overcoming the issue of inaccurate diagnosis based on a few independent early-stage case reports.
According to the different partings under endoscopy, the clinical symptoms, therapeutic efficacy, and prognosis of patients with PIL were observed.
| 1. | Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS Jr. The role of the gastrointestinal system in "idiopathic hypoproteinemia". Gastroenterology. 1961;41:197-207. [PubMed] |
| 2. | Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (39)] |
| 3. | Takenaka H, Ohmiya N, Hirooka Y, Nakamura M, Ohno E, Miyahara R, Kawashima H, Itoh A, Watanabe O, Ando T, Goto H. Endoscopic and imaging findings in protein-losing enteropathy. J Clin Gastroenterol. 2012;46:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ingle SB, Hinge Ingle CR. Primary intestinal lymphangiectasia: Minireview. World J Clin Cases. 2014;2:528-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (3)] |
| 5. | Prasad D, Srivastava A, Tambe A, Yachha SK, Sarma MS, Poddar U. Clinical Profile, Response to Therapy, and Outcome of Children with Primary Intestinal Lymphangiectasia. Dig Dis. 2019;37:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Asakura H, Miura S, Morishita T, Aiso S, Tanaka T, Kitahora T, Tsuchiya M, Enomoto Y, Watanabe Y. Endoscopic and histopathological study on primary and secondary intestinal lymphangiectasia. Dig Dis Sci. 1981;26:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Cammarota G, Cianci R, Gasbarrini G. High-resolution magnifying video endoscopy in primary intestinal lymphangiectasia: a new role for endoscopy? Endoscopy. 2005;37:607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 8. | Hirano A, Matsumoto T, Esaki M, Fujita K, Iida M. Intestinal lymphangiectasia presenting with duodeno-jejunal polyposis: enteroscopic findings. Endoscopy. 2010;42 Suppl 2:E281-E282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Oh TG, Chung JW, Kim HM, Han SJ, Lee JS, Park JY, Song SY. Primary intestinal lymphangiectasia diagnosed by capsule endoscopy and double balloon enteroscopy. World J Gastrointest Endosc. 2011;3:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (37)] |
| 11. | Lai Y, Yu T, Qiao XY, Zhao LN, Chen QK. Primary intestinal lymphangiectasia diagnosed by double-balloon enteroscopy and treated by medium-chain triglycerides: a case report. J Med Case Rep. 2013;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Ohmiya N, Nakamura M, Yamamura T, Yamada K, Nagura A, Yoshimura T, Hirooka Y, Hirata I, Goto H. Classification of intestinal lymphangiectasia with protein-losing enteropathy: white villi type and non-white villi type. Digestion. 2014;90:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wen J, Tang Q, Wu J, Wang Y, Cai W. Primary intestinal lymphangiectasia: four case reports and a review of the literature. Dig Dis Sci. 2010;55:3466-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Dong J, Xin J, Shen W, Wen T, Chen X, Sun Y, Wang R. CT Lymphangiography (CTL) in Primary Intestinal Lymphangiectasia (PIL): A Comparative Study with Intraoperative Enteroscopy (IOE). Acad Radiol. 2019;26:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Huber T, Paschold M, Eckardt AJ, Lang H, Kneist W. Surgical therapy of primary intestinal lymphangiectasia in adults. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Macdonald J, Porter V, Scott NW, McNamara D. Small bowel lymphangiectasia and angiodysplasia: a positive association; novel clinical marker or shared pathophysiology? J Clin Gastroenterol. 2010;44:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Huber R, Semmler G, Mayr A, Offner F, Datz C. Primary intestinal lymphangiectasia in an adult patient: A case report and review of literature. World J Gastroenterol. 2020;26:7707-7718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (7)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balakrishnan DS, India; Khayat AA, Saudi Arabia; Kotelevets SM, Russia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP