Published online Feb 21, 2021. doi: 10.3748/wjg.v27.i7.576
Peer-review started: October 27, 2020
First decision: December 13, 2020
Revised: December 24, 2020
Accepted: January 13, 2021
Article in press: January 13, 2021
Published online: February 21, 2021
Processing time: 116 Days and 1.1 Hours
Interdigestive migrating motor complexes (MMC) produce periodic contractions in the gastrointestinal tract, but the exact mechanism of action still remains unclear. Intramuscular interstitial cells of Cajal (ICC-IM) participate in gastrointestinal hormone and neuromodulation, but the correlation between ICC-IM and MMC is also unclear. We found that xiangbinfang granules (XBF) mediated the phase III contraction of MMC. Here, the effects of XBF on gastric antrum motility in W/Wv mice and the effects of ICC-IM on gastric antrum MMC are reported.
To observe the effects of ICC-IM on gastric antrum motility and to establish the mechanism of XBF in promoting gastric antrum motility.
The density of c-kit-positive ICC myenteric plexus (ICC-MP) and ICC-IM in the antral muscularis of W/Wv and wild-type (WT) mice was examined by confocal microscopy. The effects of XBF on gastric antrum slow waves in W/Wv and WT mice were recorded by intracellular amplification recording. Micro-strain-gauge force transducers were implanted into the gastric antrum to monitor the MMC and the effect of XBF on gastric antrum motility in conscious W/Wv and WT mice.
In the gastric antrum of W/Wv mice, c-kit immunoreactivity was significantly reduced, and no ICC-IM network was observed. Spontaneous rhythmic slow waves also appeared in the antrum of W/Wv mice, but the amplitude of the antrum slow wave decreased significantly in W/Wv mice (22.62 ± 2.23 mV vs 2.92 ± 0.52 mV, P < 0.0001). MMCs were found in 7 of the 8 WT mice but no complete MMC cycle was found in W/Wv mice. The contractile frequency and amplitude index of the gastric antrum were significantly increased in conscious WT compared to W/Wv mice (frequency, 3.53 ± 0.18 cpm vs 1.28 ± 0.12 cpm; amplitude index, 23014.26 ± 1798.65 mV·20 min vs 3782.16 ± 407.13 mV·20 min; P < 0.0001). XBF depolarized smooth muscle cells of the gastric antrum in WT and W/Wv mice in a dose-dependent manner. Similarly, the gastric antrum motility in WT mice was significantly increased after treatment with XBF 5 mg (P < 0.05). Atropine (0.1 mg/kg) blocked the enhancement of XBF in WT and W/Wv mice completely, while tetrodotoxin (0.05 mg/kg) partially inhibited the enhancement by XBF.
ICC-IM participates in the regulation of gastric antrum MMC in mice. XBF induces MMC III-like contractions that enhance gastric antrum motility via ICC-IM in mice.
Core Tip: This study shows that intramuscular interstitial cells of Cajal (ICC-IM) play crucial roles in regulating the activity of gastric antral migrating motor complexes (MMC). This may be an important bridge between the vagus, enteric nervous system and motilin to regulate smooth muscle contraction. Through the muscarinic receptor pathway on ICC-IM, the Chinese medicine Xiangbinfang granules depolarize smooth muscle cells and initiates an action potential, changing the periodic motion of MMC into a phase III-like contraction pattern in the gastric antrum of mice.
- Citation: Chen QC, Jiang Z, Zhang JH, Cao LX, Chen ZQ. Xiangbinfang granules enhance gastric antrum motility via intramuscular interstitial cells of Cajal in mice. World J Gastroenterol 2021; 27(7): 576-591
- URL: https://www.wjgnet.com/1007-9327/full/v27/i7/576.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i7.576
Interdigestive migrating motor complexes (MMC) induce periodic contractions of the gastric, small intestine, or colon smooth muscle, propagating along the gastrointestinal (GI) tract. Although the exact mechanism of MMC action still remains unclear, MMC have been classified into four stages. The MMC phase III consists of a high-frequency, high-amplitude rhythmic contraction, which has important physiological significance in normal GI motility and digestion[1]. Interstitial cells of Cajal (ICC), which are electrically coupled to smooth muscle cells (SMCs), produce slow waves that function as pacemaker activity[2]. There are several ICC subtypes based on their anatomical locations, morphology, and function in the GI tract. ICC associated with the myenteric plexus (ICC-MP) are responsible for producing slow waves. Intramuscular ICC (ICC-IM), which are located in muscle bundles between muscle cells, mediate information transmission from autonomic nerves to SMCs[3]. In the small intestine of W/Wv mice lacking ICC-MP, there are no rhythmic slow waves and there are irregular contractions of longitudinal and circular muscles[4]. However, the MMC cycle still exists in the isolated intestine of W/Wv mice, suggesting that MMC do not require ICC-MP[5]. Other studies have shown that with the depletion of gastric ICC, disorders of the MMC cycle are common in diabetic gastroparesis[6,7]. This seems to indicate that ICC, especially ICC-IM, may also be involved in regulating the movement of MMC.
The Chinese herbal medicine xiangbinfang granules (XBF) is an effective treatment for promoting the post-surgical recovery of GI function[8,9]. Previous studies have shown that XBF significantly enhanced MMC activity in the antrum, pylorus, duodenum, jejunum, and the colon in beagles[10]. XBF was also found to promote contraction of the stomach, duodenum, and jejunum in healthy volunteers[11]. However, the mechanism of XBF in enhancing MMC activity in the GI tract is still unclear.
In this study, we observed the MMC activity of the gastric antrum in W/Wv mice that lacked ICC-IM and analyzed the effects of the traditional Chinese medicine XBF in promoting gastric antrum motility.
This study included 36 W/Wv and 36 wild-type (WT) mice. W/Wv mice were obtained from Jackson Laboratory (Bar Harbor, ME, United States) and bred in Guangdong Provincial Experimental Animal Center. The W/Wv and WT mice (weighing 18-25 g) were allowed free access to a standard laboratory chow diet and water and housed at a temperature of 22-24°C under a 12:12 h light/dark cycle. The study protocol was approved by the Institutional Animal Care and Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine (No. 2018003). All animal procedures were conducted according to the Regulations for the Care and Use of Laboratory Animals in Guangzhou University of Chinese Medicine.
The mice were initially anesthetized with isoflurane before being killed by cervical dislocation. The whole stomach was placed in Krebs-Ringer buffer that was constantly perfused with oxygen. A cut was made along the lesser curvature of the stomach, the stomach was washed with ice-cold Krebs with the mucosa facing upward, and then the mucosa and submucosa were carefully removed.
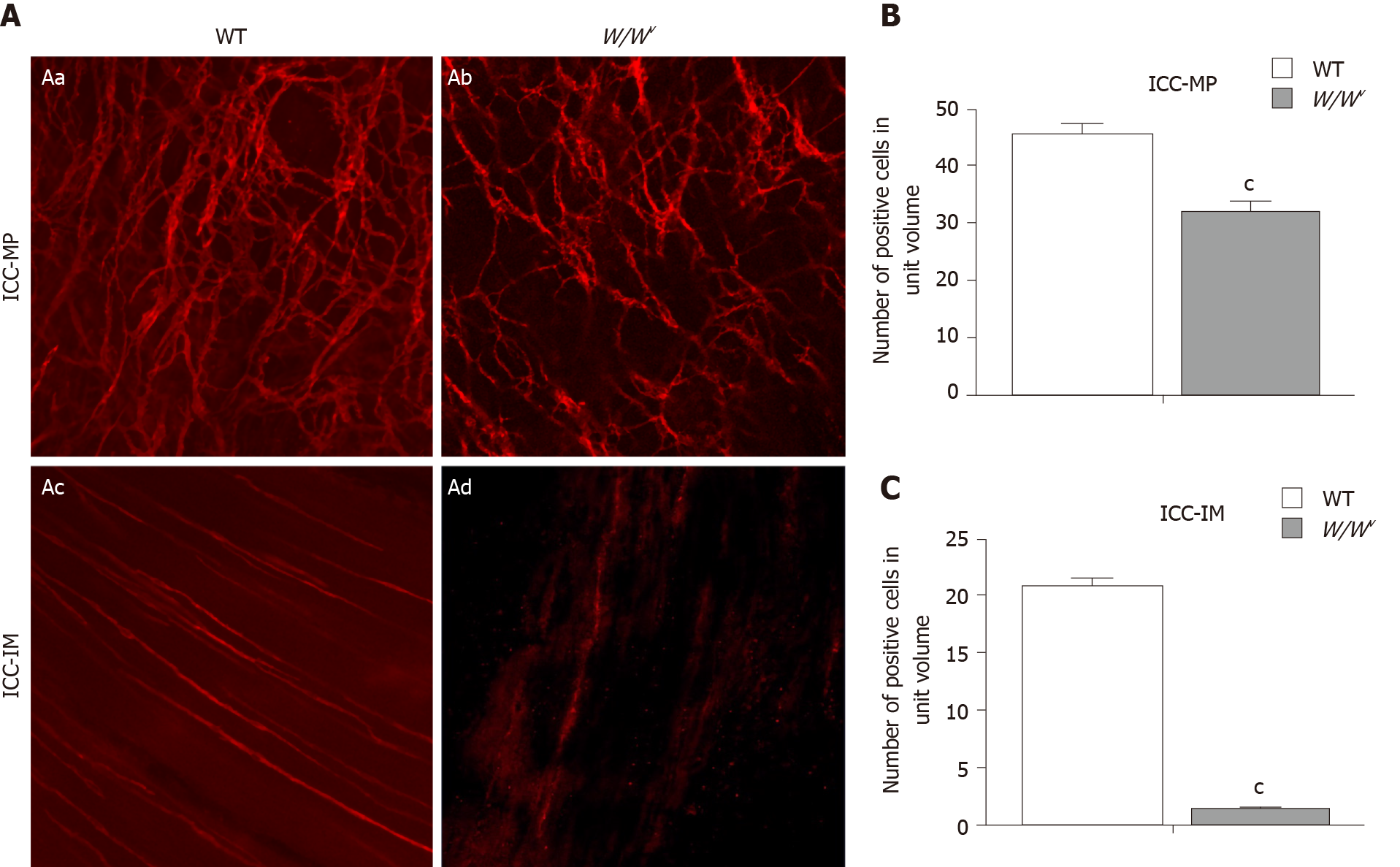
Ten W/Wv and 10 WT mice were used for immunofluorescence staining of the gastric antrum. The immunolabeling of mouse tissues was carried out on whole tissue mounts devoid of mucosa and submucosa. The whole mounts were stained with the primary antibodies for 1:100 c-kit (SC-168; Santa Cruz, CA, United States) and incubated for 1 h with 1:400 donkey anti-Rb IgG and Alexa Fluor 594. All immunostaining was imaged with a confocal microscope (Zeiss LSM 710; Göttingen, Germany) at an excitation wavelength of 594 nm. The c-kit-positive cells were observed under a 20 × objective lens. The confocal micrographs shown are digital composites of the Z-series with a depth of 6-10 µm for ICC-MP and 15-30 µm for ICC-IM. The density of ICC-MP and ICC-IM was estimated by scanning through a 6-µm thickness of tissue area, counting the number of positive cells. The area of each micrograph was 424.3 µm × 424.3 µm = 0.18 mm2. The unit volume was 424.3 µm × 424.3 µm × 6 µm = 0.0011 mm3. The immunofluorescence staining of the gastric antrum was performed as previously described[12].
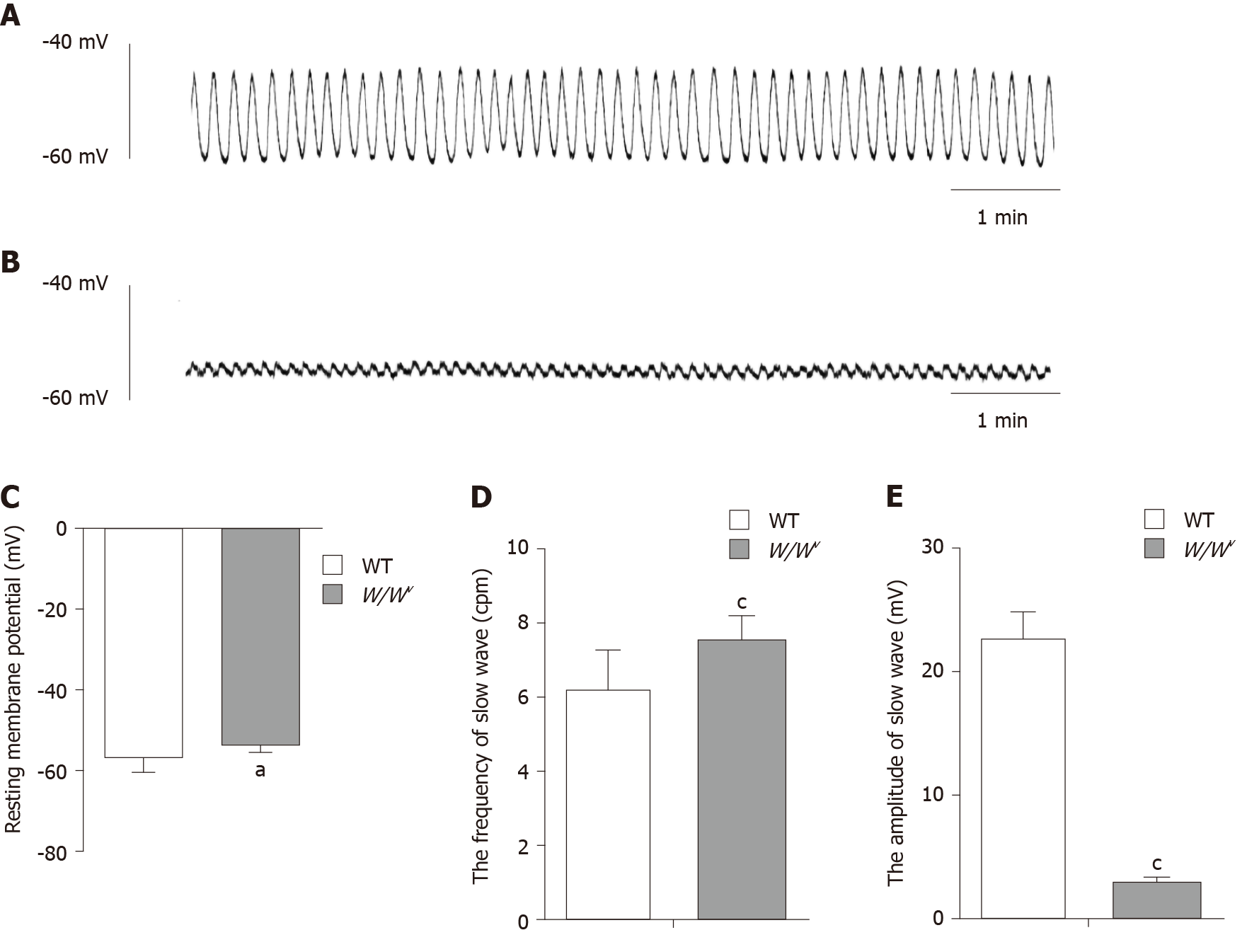
Eighteen W/Wv and 18 WT mice were used for intracellular microelectrode recording. Full-thickness muscle strips (8 mm × 4 mm) were cut from the gastric antrum and pinned onto the base of a silica layer and continuously perfused with oxygenated Krebs-Ringer buffer at 37°C. Before recording, the strips were incubated for 2 h. The glass microelectrodes with resistance ranging between 50 and 80 MΩ were filled with 1 mol/L KCl for penetrating cells. The electrical response was recorded and amplified using a high input impedance amplifier (AXON 210B; Molecular Devices, San Jose, CA, United States). The data were recorded on a computer using Clampfit 10.4 software.
When the 8 W/Wv and 8 WT mice were anesthetized by isoflurane inhalation, the limbs were fixed on the Baoding frame, and the abdominal hair was shaved with a razor and disinfected with iodophor. The abdominal cavity was carefully opened and the gastric antrum fully exposed. Then the micro-strain-gauge force transducers (J2A-06-S108N-10C; Micro-Measurements, Raleigh, NC, United States) were sutured to the gastric serosa with a needle and nylon thread. The electrode leads were drawn from the back of the neck through the subcutaneous layer before closing the abdomen. After monitoring the animal’s wakefulness for more than 15 min, the micro-strain-gauge force transducers were connected to the Porti physiological recorder (TMSI, Netherlands) to measure the gastric contractile motion. The first 30 min were recorded for baseline control and the next 30 min for treatment. The gastric contractile frequency and amplitude index (sum of amplitudes within 20 min) were analyzed.
XBF was produced by Hunan Academy of Chinese Medicine and contains Areca catechu L. (Binlang), Ginseng (Renshen), Fructus amomi (Sharen), Radix linderae (Wuyao) and Prunus persica Batsch (Taoren). Atropine (Lot A0132), tetrodotoxin (TTX, Lot T8024) and other chemicals were all acquired from Sigma (Sigma-Aldrich, St. Louis, MO, United States) unless indicated otherwise. The XBF, atropine, and TTX were dissolved in dH2O and further diluted in Krebs-Ringer buffer to the final concentration. Krebs buffer consisted of 121.9 mmol/L NaCl, 15.5 mmol/L NaHCO3, 5.9 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 11.5 mmol/L glucose, and 2.5 mmol/L CaCl2. The pH of the Krebs buffer was 7.3-7.4 when bubbled with 95% O2-5% CO2 at 37 ± 0.5 °C.
The data are shown as mean ± SD. Welch’s t-test was used to compare the difference between WT and W/Wv mice. The paired t-test was used to analyze the data before and after administration. The number of MMC was compared by Fisher’s exact test. A P value < 0.05 was considered significant. Data statistics were calculated with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, United States).
Immunofluorescence showed that there were many c-kit-positive ICC in the myenteric plexus of the gastric antrum in WT mice. The c-kit-positive cells were connected to form a rich and dense ICC-MP network (Figure 1Aa). In W/Wv mice, the c-kit immunogenicity of ICC-MP decreased (Figure 1Ab). Compared with WT mice, the density of ICC-MP in the gastric antrum of W/Wv mice was significantly decreased (Figure 1B; P < 0.001). A large number of bipolar ICC were found in the longitudinal and circular muscles of the gastric antrum in WT mice (Figure 1Ac). Although sparse c-kit immunoreactivity was visible occasionally, no ICC-IM network was found in the antrum of W/Wv mice (Figure 1Ad). There was a significant difference in ICC-IM density between WT and W/Wv mice (Figure 1C; P < 0.001).
The spontaneous periodic depolarization slow wave of the gastric antrum was observed in WT mice (Figure 2A). In WT mice, the resting membrane potential (RMP) was –(56.49 ± 3.58) mV, the amplitude was 22.62 ± 2.23 mV, and the frequency was 6.16 ± 1.12 cpm. Spontaneous periodic depolarization slow waves with low amplitude were also observed in the antrum of W/Wv mice (Figure 2B). In W/Wv mice, the RMP was –(52.95 ± 2.34) mV, the amplitude was 2.92 ± 0.52 mV, and the frequency was 7.48 ± 0.66 cpm. The difference determined was statistically significant between WT and W/Wv mice (Figure 2C-E;aP < 0.05, cP < 0.0001).
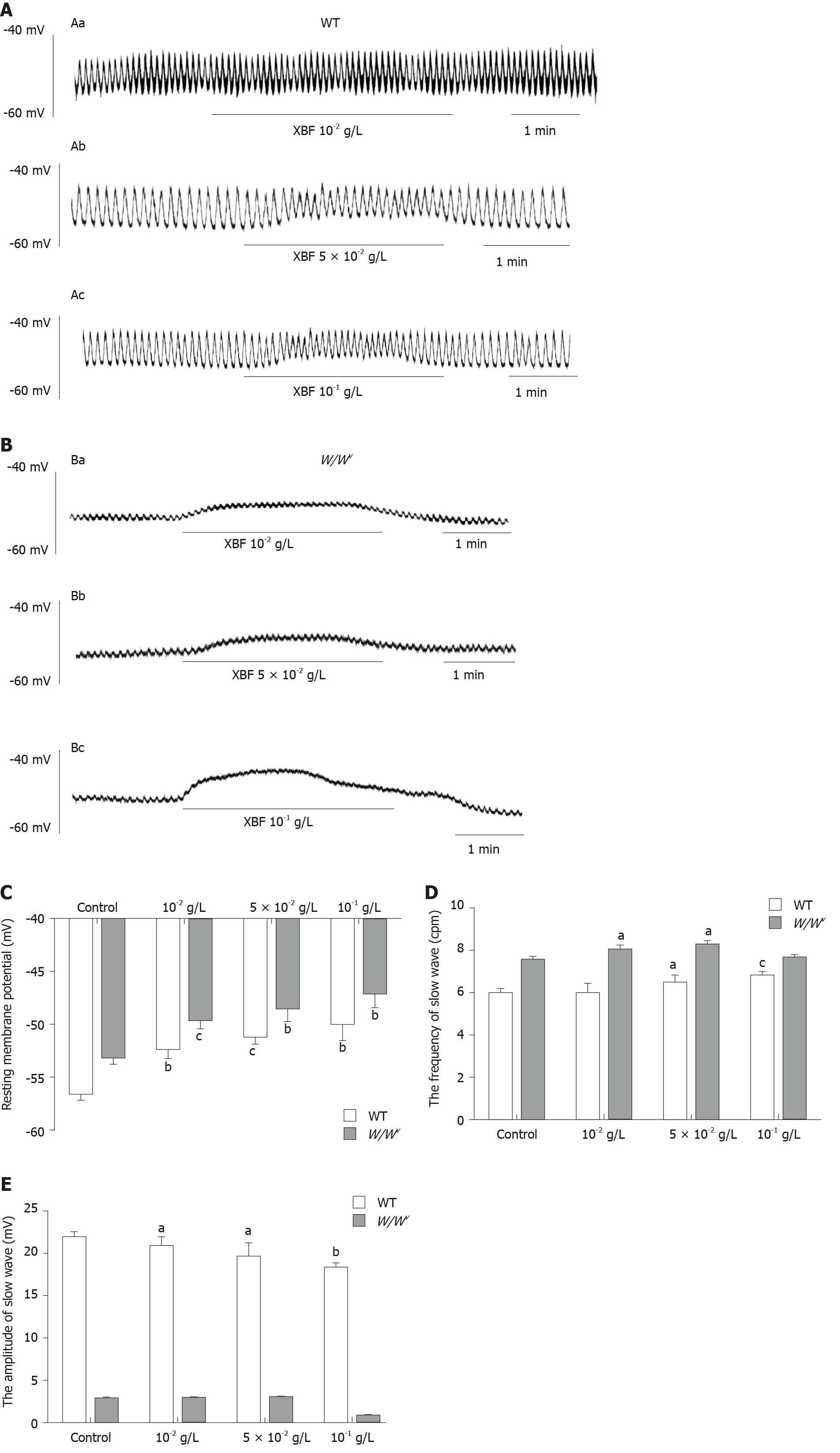
With perfused XBF at the concentration of 10-2 g/L, the slow waves of the gastric antrum began to depolarize in WT and W/Wv mice (Figure 3A and B). After treatment with concentrations of 10-2 g/L, 5 × 10-2 g/L, and 10-1 g/L XBF, RMP decreased from −(56.49 ± 0.53) mV to −(52.31 ± 0.82) mV, −(51.08 ± 0.61) mV, and −(49.87 ± 1.58) mV, respectively. The frequency changed from 5.99 ± 0.18 cpm to 5.95 ± 0.44 cpm, 6.47 ± 0.37 cpm, and 6.79 ± 0.14 cpm, respectively, and the amplitude decreased from 21.78 ± 0.65 mV to 20.85 ± 0.90 mV, 19.56 ± 1.60 mV, and 18.15 ± 0.65 mV, respectively in WT mice. The differences in RMP and amplitude were significantly different at all XBF concentrations between the control and treatment groups (P < 0.05 or 0.01), and the frequency increased significantly except at the concentration of 10-2 g/L (Figure 3C-E). In W/Wv mice, the RMP decreased from −(53.13 ± 0.53) mV to −(49.47 ± 0.79) mV, −(48.41 ± 1.24) mV, and −(46.98 ± 1.40) mV, respectively. The frequency changed from 7.55 ± 0.12 cpm to 8.02 ± 0.19 cpm, 8.25 ± 0.18 cpm, and 7.65 ± 0.15 cpm, respectively, and the amplitude decreased from 2.84 ± 0.08 mV to 2.71 ± 0.11 mV, 2.87 ± 0.10 mV, and 2.66 ± 0.21 mV, respectively, at concentrations of 10-2 g/L, 5 × 10-2 g/L, and 10-1 g/L. Compared to the WT controls, the differences in RMP were statistically significant at all concentrations (P < 0.01). At concentrations of 10-2 g/L and 5 × 10-2 g/L, the slow-wave frequency increased significantly (P < 0.05). However, the amplitude was not significantly altered at any XBF concentration in W/Wv mice.
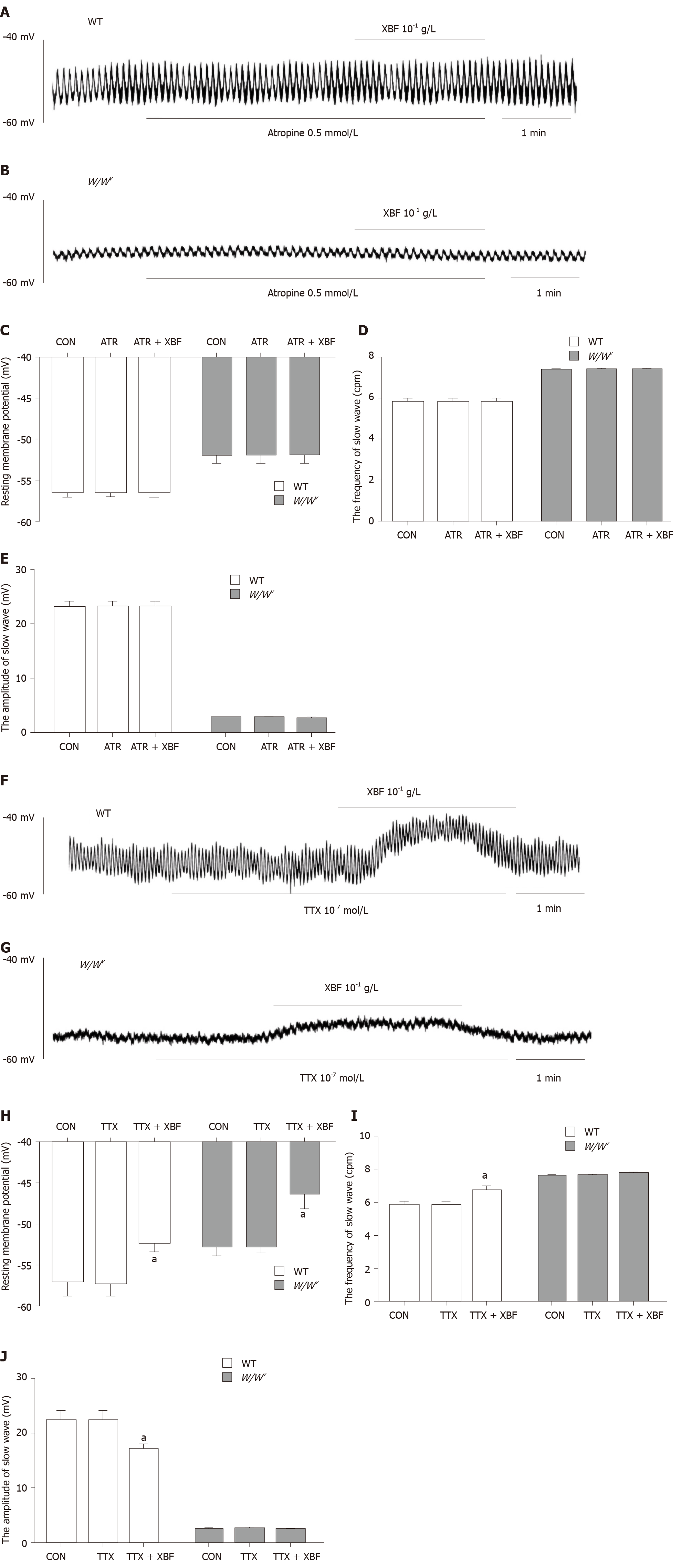
The results of intracellular recording showed that atropine could completely abolish the effect of XBF on gastric antrum slow waves in WT and W/Wv mice (Figure 4A and B). After pretreatment with 0.5 mmol/L atropine, perfusion of XBF at a concentration of 10-1 g/L did not alter the RMP, frequency, or amplitude of the gastric antrum slow waves in WT and W/Wv mice (Figure 4C-E). In the presence of TTX 10-7 M, perfusion of XBF at a concentration of 10-1 g/L was able to decrease the RMP of gastric antrum slow waves in WT and W/Wv mice (Figure 4F and G). TTX did not block the enhancement of gastric antrum slow waves in WT and W/Wv mice (Figure 4H-J).
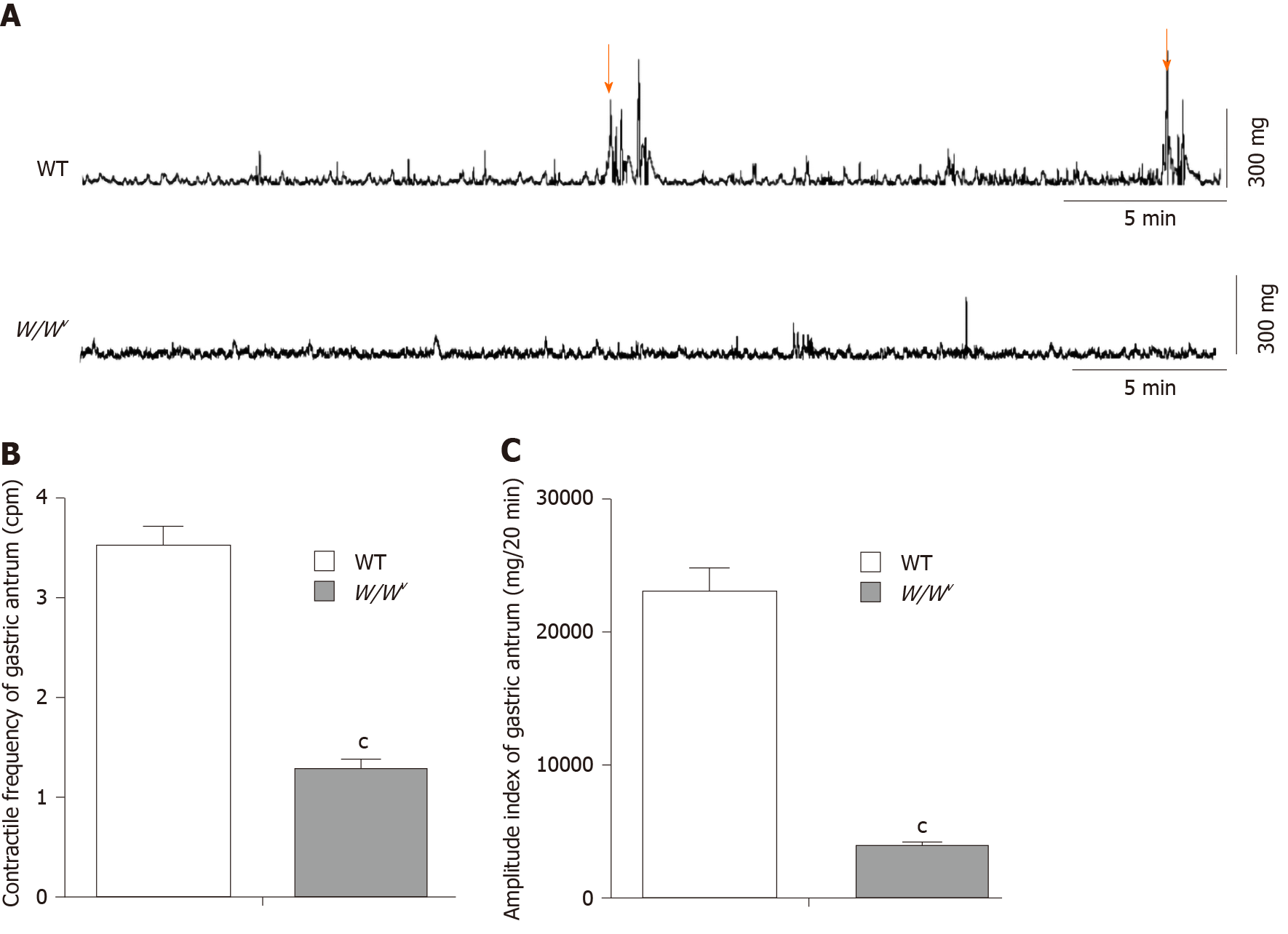
The MMCs in the gastric antrum were recorded by a micro-strain-gauge force transducer for 30 min. As shown in Figure 5, it was very difficult to distinguish between MMC phase I and phase II in WT. With the increase of irregular contraction frequency, regular contractions with the frequency 3.53 ± 0.18 cpm and amplitude index 23014.26 ± 1798.65 mV·20 min occurred in the antrum of WT mice. In the 30 min recording, there were only sporadic contractions in the gastric antrum of W/Wv. Occasionally, there was a strong paroxysmal contraction with a frequency of 1.28 ± 0.12 cpm and an amplitude index of 3782.16 ± 407.13 mV·20 min. The differences in the frequency and amplitude index were all statistically significant (P < 0.0001). We observed that 7 out of 8 WT mice developed a total of 13 MMC III phase contractions, while in the 8 W/Wv mice, only 2 developed 3 MMC phase III-like contractions of shorter duration, lower amplitude, and lower frequency. There was a significant difference in the number of MMCs between both groups (Fisher’s exact test, P = 0.0406). For the gastric antrum of WT mice, the duration of the MMC phase III was 151.08 ± 8.87 s, the amplitude was 315.45 ± 5.55 mV, and the interval between MMCs was 10.75 ± 0.61 min. In the gastric antrum of W/Wv mice, the duration of the MMC phase III-like contractions was 123.67 ± 2.96 s, the amplitude was 194.12 ± 4.76 mV, and during the 30 min observation, no phase III-like contractions were found twice in the same W/Wv mice. Compared to WT mice, the duration and amplitude of the MMC III phase in the gastric antrum was significantly reduced in W/Wv mice (P = 0.0117 and 0.0020, respectively), suggesting there was no typical MMC cycle in the gastric antrum of W/Wv mice.
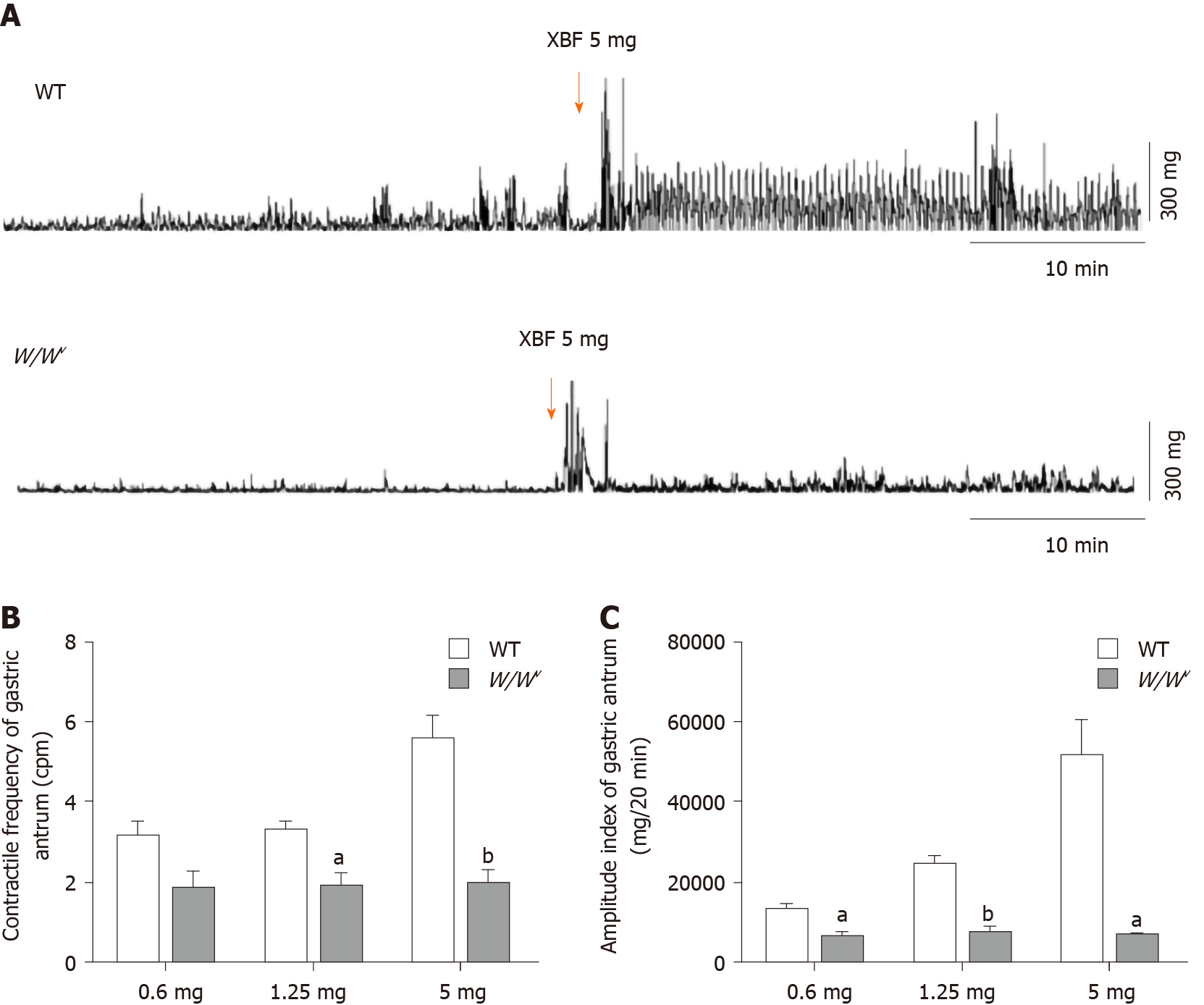
After the administration of XBF (5 mg), strong contractions in the antrum of WT mice were observed instantaneously (Figure 6). The periodic MMC movement turned into a high frequency and high amplitude MMC III phase contraction. However, XBF did not significantly enhance the contraction of the gastric antrum in W/Wv mice (Figure 6). After treatment in turn with 0.6 mg, 1.25 mg, and 5 mg of XBF, the frequency index of the gastric antrum in WT mice was 3.16 ± 0.39 cpm, 3.30 ± 0.26 cpm, and 5.58 ± 0.62 cpm and the amplitude index was 13415.25 ± 1694.38 mV·20 min, 24537.89 ± 2406.33 mV·20 min, and 51807.48 ± 9255.04 mV·20 min, respectively, while the frequency index of the gastric antrum in W/Wv mice was 1.85 ± 0.48 cpm, 1.93 ± 0.37 cpm, and 1.99 ± 0.37 cpm and the amplitude index was 6488.43 ± 1490.04 mV·20 min, 7733.07 ± 1469.27 mV·20 min, and 6901.26 ± 807.34 mV·20 min, respectively. These results demonstrated that with high-dose XBF treatment, there was a significant difference in the frequency and amplitude indices of the gastric antrum between WT and W/Wv mice (all P < 0.05 or 0.01).
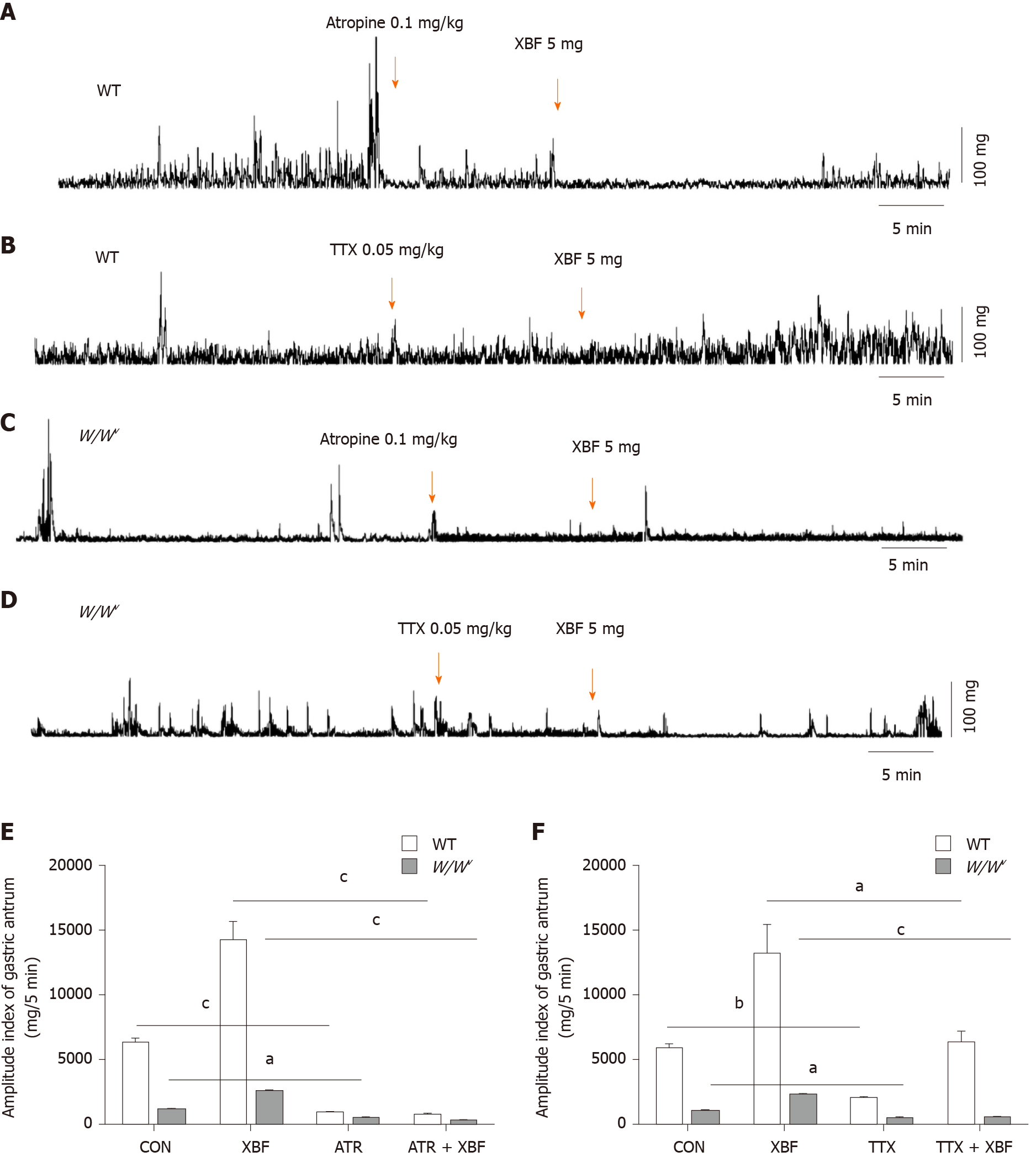
To clarify the mechanism of XBF in promoting antral motility, atropine and TTX were injected intraperitoneally, and then XBF was administered by gavage. The results indicated that contractions were inhibited in the antrum of WT and W/Wv mice after intraperitoneal injection of 0.1 mg/kg of atropine, and subsequent treatment with 5 mg of XBF did not increase the contractile activity of the gastric antrum (Figure 7A and C). These results suggest that atropine could completely eliminate the motility-promoting effect of XBF in both WT and W/Wv mice (Figure 7E). The frequency and amplitude of gastric antral contractions were abolished by 0.05 mg/kg of TTX. Subsequent treatment with 5 mg of XBF induced a contraction enhancement in WT and W/Wv mice (Figure 7B and D). In comparison with XBF without TTX, TTX pretreatment could partially reduce the gastric antrum motility-promoting effect mediated by XBF (Figure 7F).
C-kit is a necessary condition for the development of a GI pacemaker system[13]. Previous studies have shown that the W-mutation causes loss of the ICC network in the GI tract of W/Wv mice, such as the disappearance of ICC-MP and decreased ICC-MP in the jejunum and ileum[4]. In the W/Wv colon, the density of c-kit positive ICC-MP was reduced by 50%-60% compared to that of WT mice[12]. While a normal ICC-MP network was observed, the ICC-IM disappeared completely in the gastric antrum of W/Wv mice[14]. We scanned whole muscle specimens to obtain a more accurate number of ICC and compared them to WT mice; about 60% of ICC-MP was retained in the antrum of W/Wv mice, and no ICC-IM network was observed. GI slow waves are mainly produced by ICC-MP[2], and ICC-IM is responsible for the regenerative component of the slow wave[15]. Our immunofluorescent staining showed that the ICC-MP network density decreased but did not disappear, and the slow-wave activity in W/Wv mice was preserved. However, due to the lack of an ICC-IM network in the antrum of W/Wv mice, there is no regenerative component in the W/Wv antrum slow wave. Therefore, W/Wv mice showed a low-amplitude slow wave and disturbed movement in the antrum. ICC-IM were also innervated and provided mechanisms for neural signal transduction to the gastric musculature[16]. ICC-IM were densely innervated by excitatory and inhibitory enteric motor neurons and in close contact with nerve terminals. ICC-IM played a role in both nitrergic inhibitor and cholinergic excitatory motor neurotransmission in the gastric antrum[17,18]. Excitatory junctional potential and inhibitory junctional potential after intrinsic nerve stimulation were greatly attenuated in the antrum of W/Wv mice, and the reduced density of ICC-IM leads to reduced neural regulation in the W/Wv antrum[19]. Hirst et al[19] have shown that excitatory vagal stimulation response resembles the regenerative response which was initiated in this tissue by ICC-IM. Regenerative responses were the dominant responses produced by neural stimulation. This suggested that ICC-IM is regulated by vagus nerve.
The MMC has been found to be a complex system that may be regulated by motilin[20], the enteric nervous system, and the vagus nerve. Mondal et al[21] found that treatment with motilin induced phase III contractions in vivo and in vitro, while motilin antagonists can abolish the occurrence of spontaneous gastric phase III contractions. Their other experiment showed that motilin-induced gastric contractions were mediated through the myenteric plexus in a vagus-independent manner[22]. ICC-IM may play an important role in this process.
The MMC have a temporally coordinated cyclic motor pattern during the interdigestive state of the stomach and small intestine in many animals. In the human and dog gastrointestinal tracts, the occurrence of MMC is regulated at 90-120-min intervals[23,24]. Because of motilin and motilin receptor pseudogenes[25], mice and rats have shown different MMC patterns. Strain-gauge force transducer implantation is a crucial technique for recording GI MMC movement in conscious animals[23,24,26,27]. Takayama et al[26] examined the gastrointestinal motility of W-mutant rats by an extraluminal strain-gauge force transducer method. They found the duration of MMC was 2.5 ± 2.3 min and the interval of MMC was 5.4 ± 2.9 min. Similar results were observed by Taniguchi et al[27] in their study, spontaneous phase III contractions were observed every 13-16 min. It was difficult to record the gastrointestinal motor pattern of the mice in situ. Spencer et al[5] tried to record MMC in the isolated small intestine of mice. They found the interval between MMCs in the mouse small intestine was 5 ± 1 min, and the durations of MMC contractions were about 30 s. The MMC was regulated by motilin, the enteric nervous system, and the vagus nerve. Motilin-induced contractions are much less potent than those of MMC in vivo[28]. The complex regulatory system of MMC was not complete in vitro. Therefore, we miniaturized the strain-gauge force transducer to facilitate MMC recording in the gastric antrum of conscious mice. In this study, we found that the duration of the MMC phase III was 151.08 ± 8.87 s, the amplitude was 315.45 ± 5.55 mV, and the interval between MMCs was 10.75 ± 0.61 min in WT mice. There was no complete MMC cycle in the gastric antrum of W/Wv mice. The gastric antrum of W/Wv mice lacked ICC-IM and the corresponding motor nerve conduction was inhibited[29]. The typical MMC disappeared in W/Wv mice, suggesting that ICC-IM is an important factor in regulating the MMC activity.
In this study, we found that XBF enhanced the contractions of the gastric antrum in WT mice through slow-wave depolarization of SMCs. The effect of XBF on enhancing the antral motility was greatly reduced in W/Wv mice. ICC specifically express calcium-activated chloride channels (CACC), which are regulated by anoctamine 1 (Ano1). Exogenous nerve stimulation or GI hormones could act on ICC to produce slow waves by CACC[30]. SMCs respond to slow waves to generate action potentials by activating voltage-dependent L-type calcium channels[31]. Under physiological conditions, cell-membrane potential depolarization increased the probability of the L-type calcium channels opening, allowing calcium influx to induce SMCs to generate action potentials and contractions[32-34]. In Ano1-knockout mice, the excitability of smooth muscle contraction induced by carbachol was decreased. This is caused by cholinergic nerve stimulation that first activates muscarinic receptors on ICC-IM and then stimulates the CACC channel, thereby causing the SMCs to depolarize and generate action potentials through gap junctions[35]. Thus, the response of the distal stomach to cholinergic stimulation was weakened and blocked the neural activation of regenerative potentials in W/Wv mice deficient in ICC-IM, and the low level of potential depolarization during the slow-wave plateau period reduced the probability of L-type calcium channels opening. Therefore, XBF depolarized the SMCs slow waves (Figure 3) but did not significantly enhance the mechanical contraction (Figure 6) of the gastric antrum in W/Wv mice. Our study showed that atropine completely blocked the gastric antral motility induced by XBF, but TTX just partially reduced the effect of XBF in both WT and W/Wv mice (Figure 7). This suggested that XBF enhances the smooth muscle contraction of the gastric antrum through the cholinergic pathway of ICC-IM and the enteric nervous system.
The Chinese medicine XBF, composed of Areca catechu L., Ginseng, Fructus amomi, Radix linderae, and Prunus persica Batsch, promotes entire GI motility in the treatment of postoperative ileus[8-11]. Previous studies have shown that Areca catechu L. enhances gastric motility in healthy people[36]. Arecoline, the main active component of Areca, with an XBF content of 0.112 mg/g[37], promoted GI motility through the M3 receptor[38,39]. Ginsenoside RF, an extract of Ginseng, induced membrane depolarization of ICC[40] and Fructus amomi promoted the contraction of the antrum and duodenum in beagle dogs[41]. The promotion of GI motility by XBF is the result of the interaction of these Chinese herbs, and the most important active components still need to be further characterized.
Our study shows that ICC-IM play a crucial role in regulating gastric antral MMC activity. They may be an important bridge between the vagus nerve, the enteric nervous system, and motilin in regulating smooth muscle contraction. Through the muscarinic receptor pathway on ICC-IM, XBF depolarizes SMCs and initiates an action potential, changing the periodic motion of MMC into a phase III-like contraction pattern in the gastric antrum of mice.
The Chinese medicine Xiangbinfang granule (XBF) is an effective prescribed treatment for promoting the recovery of gastrointestinal (GI) function post-surgery. In previous studies, we found that XBF mediated the phase III contraction of migrating motor complexes (MMC). However, the mechanism of XBF in enhancing MMC activity in the GI tract is still unclear.
In this study, we observed the MMC activity of gastric antrum in W/Wv mice that lack intramuscular interstitial cells of Cajal (ICC-IM) and analyzed the effect of the traditional Chinese medicine XBF in promoting gastric antrum movement. From this study, we will further understand the role of ICC-IM in MMC activities. Meanwhile, the mechanism of XBF promoting gastrointestinal motility through ICC-IM was discussed, so as to provide the basis for the development and application of XBF.
W/Wv mice were used to observe the effects of ICC-IM on gastric antrum motility and to establish the mechanism of XBF in promoting gastric antrum motility. We further investigated the correlation between ICC-IM and MMC in mouse gastric antrum.
The density of c-kit positive ICC myenteric plexus and ICC-IM in the antral muscularis of W/Wv and wild-type (WT) mice was examined by confocal microscopy. The effects of XBF on the gastric antrum slow waves in W/Wv and WT mice were recorded by intracellular amplification recording. The micro-strain-gauge force transducers were implanted into the gastric antrum to monitor the MMC and the effect of XBF on gastric antrum motility in conscious W/Wv and WT mice.
In the gastric antrum of W/Wv mice, no ICC-IM network was observed. Spontaneous rhythmic slow waves with the low amplitude also appeared in the antrum of W/Wv mice. In this study, we found that the duration of MMC phase III was 151.08 ± 8.87 s, the amplitude was 315.45 ± 5.55 mg, and the interval between MMCs was 10.75 ± 0.61 min in the gastric antrum of WT mice. There was no complete MMC cycle in W/Wv gastric antrum lacking ICC-IM. The gastric antrum motility in WT and W/Wv antrum was significantly increased after treatment with XBF. Atropine blocked the enhancement of XBF completely, while tetrodotoxin partially inhibited the enhancement of XBF.
In this study, we first examined the gastrointestinal motility of W-mutant mice by an extraluminal strain-gauge force transducer method. It showed that ICC-IM plays an important role in the regulation of gastric antrum MMC.
In this study, MMC were recorded only at a single site in the gastric antrum. It was impossible to describe the propulsion of gastrointestinal movement. Therefore, recording at multiple gastrointestinal sites is important to further clarify the motility of gastrointestinal MMC in mice.
| 1. | Singaram K, Gold-Smith FD, Petrov MS. Motilin: a panoply of communications between the gut, brain, and pancreas. Expert Rev Gastroenterol Hepatol. 2020;14:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Parsons SP, Huizinga JD. Slow wave contraction frequency plateaux in the small intestine are composed of discrete waves of interval increase associated with dislocations. Exp Physiol. 2018;103:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Yadak R, Breur M, Bugiani M. Gastrointestinal Dysmotility in MNGIE: from thymidine phosphorylase enzyme deficiency to altered interstitial cells of Cajal. Orphanet J Rare Dis. 2019;14:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 192] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;553:881-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hayashi Y, Toyomasu Y, Saravanaperumal SA, Bardsley MR, Smestad JA, Lorincz A, Eisenman ST, Cipriani G, Nelson Holte MH, Al Khazal FJ, Syed SA, Gajdos GB, Choi KM, Stoltz GJ, Miller KE, Kendrick ML, Rubin BP, Gibbons SJ, Bharucha AE, Linden DR, Maher LJ 3rd, Farrugia G, Ordog T. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017; 153: 521-535. e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Park KS, Cho KB, Hwang IS, Park JH, Jang BI, Kim KO, Jeon SW, Kim ES, Park CS, Kwon JG. Characterization of smooth muscle, enteric nerve, interstitial cells of Cajal, and fibroblast-like cells in the gastric musculature of patients with diabetes mellitus. World J Gastroenterol. 2016;22:10131-10139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Gan H, Lin J, Jiang Z, Chen Q, Cao L, Chen Z. Xiangbin prescription for the recovery of gastrointestinal function after abdominal surgery (the XBPRS trial): study protocol for a randomized controlled trial. Trials. 2018;19:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wen SL, Feng X, Chen ZQ, Xiao J, Zhang WX. Effect of XiangBin granules on post-operative gastrointestinal function and brain-gut peptides after transabdominal gynecological surgery. Eur J Obstet Gynecol Reprod Biol. 2016;205:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Chen ZQ, Cao LX, Shang WF, Yang RX, Ye F, Chen QC, Pang FS, Jiang Z, Liu WP, Zhou L. The effect of xiangbin fang on gastrointestinal motility of dogs after abdominal operation. Zhongyi Zazhi. 2015;56:1953-1957. [DOI] [Full Text] |
| 11. | Jiang Z, Cao LX, Liu B, Chen QC, Shang WF, Zhou L, Li DY, Guo DA, Chen ZQ. Effects of Chinese herbal medicine Xiangbin prescription on gastrointestinal motility. World J Gastroenterol. 2017;23:2987-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Wang XY, Chen JH, Li K, Zhu YF, Wright GW, Huizinga JD. Discrepancies between c-Kit positive and Ano1 positive ICC-SMP in the W/Wv and wild-type mouse colon; relationships with motor patterns and calcium transients. Neurogastroenterol Motil. 2014;26:1298-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Klüppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998;211:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Dickens EJ, Edwards FR, Hirst GD. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Edwards FR, Hirst GD. An electrical description of the generation of slow waves in the antrum of the guinea-pig. J Physiol. 2005;564:213-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wright GW, Parsons SP, Loera-Valencia R, Wang XY, Barajas-López C, Huizinga JD. Cholinergic signalling-regulated KV7.5 currents are expressed in colonic ICC-IM but not ICC-MP. Pflugers Arch. 2014;466:1805-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jiménez M, Friebe A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2014;307:G98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Blair PJ, Bayguinov Y, Sanders KM, Ward SM. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil. 2012;24:e437-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Hirst GD, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol. 2002;541:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Deloose E, Vos R, Corsetti M, Depoortere I, Tack J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol Motil. 2015;27:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Mondal A, Xie Z, Miyano Y, Tsutsui C, Sakata I, Kawamoto Y, Aizawa S, Tanaka T, Oda S, Sakai T. Coordination of motilin and ghrelin regulates the migrating motor complex of gastrointestinal motility in Suncus murinus. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1207-G1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Mondal A, Kawamoto Y, Yanaka T, Tsutsui C, Sakata I, Oda SI, Tanaka T, Sakai T. Myenteric neural network activated by motilin in the stomach of Suncus murinus (house musk shrew). Neurogastroenterol Motil. 2011;23:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Itoh Z, Honda R, Hiwatashi K, Takeuchi S, Aizawa I, Takayanagi R, Couch EF. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl. 1976;39:93-110. [PubMed] |
| 24. | Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci. 1979;24:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 269] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | He J, Irwin DM, Chen R, Zhang YP. Stepwise loss of motilin and its specific receptor genes in rodents. J Mol Endocrinol. 2010;44:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Takayama I, Seto E, Zai H, Ohno S, Tezuka H, Daigo Y, Fujino MA. Changes of in vivo gastrointestinal motor pattern in pacemaker-deficient (WsRC-Ws/Ws) rats. Dig Dis Sci. 2000;45:1901-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol. 2008;14:6299-6302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Mizumoto A, Sano I, Matsunaga Y, Yamamoto O, Itoh Z, Ohshima K. Mechanism of motilin-induced contractions in isolated perfused canine stomach. Gastroenterology. 1993;105:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Kito Y. The functional role of intramuscular interstitial cells of Cajal in the stomach. J Smooth Muscle Res. 2011;47:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370-G1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 31. | Kim YC, Suzuki H, Xu WX, Hashitani H, Choi W, Yun HY, Park SM, Youn SJ, Lee SJ, Lee SJ. Voltage-dependent Ca Current Identified in Freshly Isolated Interstitial Cells of Cajal (ICC) of Guinea-pig Stomach. Korean J Physiol Pharmacol. 2008;12:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Thornbury KD, Hollywood MA, McHale NG, Sergeant GP. Cajal beyond the gut: interstitial cells in the urinary system--towards general regulatory mechanisms of smooth muscle contractility? Acta Gastroenterol Belg. 2011;74:536-542. [PubMed] |
| 33. | McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol. 2015;1:631-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 35. | Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, Webb TI, Pardo DM, Rock JR, Sanders KM, Ward SM. The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol. 2018;596:1549-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Sun J, Cao LX, Chen QC, Jiang Z, Chen ZQ, Zhou L. Effect of semen arecae on multichannel electrogastrogram and motilin and corticotropin-releasing hormone levels of healthy people. Zhongyao Xinyao Yu Linchuang Yaoli. 2016;27:281-285. [DOI] [Full Text] |
| 37. | Xu FF, Qi R, Jiang SW, Wang W, Chen ZQ, Guo DA, Liu B. Simultaneous determination of ten compounds in xiangbin fang by LC-MRM-MS. Zhonghua Zhongyiyao Zazhi. 2017;32:2226-2229. |
| 38. | Xie DP, Chen LB, Liu CY, Zhang CL, Liu KJ, Wang PS. Arecoline excites the colonic smooth muscle motility via M3 receptor in rabbits. Chin J Physiol. 2004;47:89-94. [PubMed] |
| 39. | Zhang JH, Cao LX, Deng SG, Chen QC, Jiang Z, Chen ZQ, Zhou L. Effects of arecoline hydrobromide on the motility of isolated gastric smooth muscle strips in rats. Guangdong Yixue. 2016;37:2881-2885. [DOI] [Full Text] |
| 40. | Han S, Kim JS, Jung BK, Han SE, Nam JH, Kwon YK, Nah SY, Kim BJ. Effects of ginsenoside on pacemaker potentials of cultured interstitial cells of Cajal clusters from the small intestine of mice. Mol Cells. 2012;33:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Chen QC, Pang FS, Cao LX, Jiang Z, Zhou L, Chen ZQ. Effect of Chinese herbs on gastrointestinal motility of chronic experimental beagle model. Guangzhou Zhongyiyao Daxue Xuebao. 2016;33:674-678. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baker SA, Huizinga JD S-Editor: Chen XF L-Editor: Webster JR P-Editor: Ma YJ