Published online Feb 14, 2021. doi: 10.3748/wjg.v27.i6.534
Peer-review started: December 4, 2020
First decision: December 24, 2020
Revised: January 6, 2021
Accepted: January 26, 2021
Article in press: January 26, 2021
Published online: February 14, 2021
Processing time: 63 Days and 5.8 Hours
Pancreaticoduodenectomy (PD) for advanced gastric cancer is rarely performed because of the high morbidity and mortality rates and low survival rate. However, neoadjuvant chemotherapy for advanced gastric cancer has improved, and chemotherapy combined with trastuzumab may have a preoperative tumor-reducing effect, especially for human epidermal growth factor receptor 2 (HER2)-positive cases.
We report a case of successful radical resection with PD after neoadjuvant S-1 plus oxaliplatin (SOX) and trastuzumab in a patient (66-year-old male) with advanced gastric cancer invading the pancreatic head. Initial esophagogastroduodenoscopy detected a type 3 advanced lesion located on the lower part of the stomach obstructing the pyloric ring. Computed tomography detected lymph node metastasis and tumor invasion to the pancreatic head without distant metastasis. Pathological findings revealed adenocarcinoma and HER2 positivity (immunohistochemical score of 3 +). We performed staging laparoscopy and confirmed no liver metastasis, no dissemination, negative lavage cytological findings, and immobility of the distal side of the stomach due to invasion to the pancreas. Laparoscopic gastrojejunostomy was performed at that time. One course of SOX and three courses of SOX plus trastuzumab were administered. Preoperative computed tomography showed partial response; therefore, PD was performed after neoadjuvant chemotherapy, and pathological radical resection was achieved.
We suggest that radical resection with PD after neoadjuvant chemotherapy plus trastuzumab is an option for locally advanced HER2-positive gastric cancer invading the pancreatic head in the absence of non-curative factors.
Core Tip: Because of the high surgical risk and poor prognosis, pancreaticoduodenectomy (PD) is rarely performed for gastric cancer. However, due to advances in surgery and improvements in perioperative management, PD may be considered for gastric cancer to improve long-term survival. We present the successful case of radical resection with PD after neoadjuvant chemotherapy combined with trastuzumab for human epidermal growth factor receptor 2-positive locally advanced gastric cancer invading the pancreatic head without postoperative severe complication. This case suggests that radical resection with PD after neoadjuvant chemotherapy combined with trastuzumab is an option for locally advanced human epidermal growth factor receptor 2-positive gastric cancer invading the pancreatic head in the absence of non-curative factors.
- Citation: Yura M, Takano K, Adachi K, Hara A, Hayashi K, Tajima Y, Kaneko Y, Ikoma Y, Fujisaki H, Hirata A, Hongo K, Yo K, Yoneyama K, Dehari R, Koyanagi K, Nakagawa M. Pancreaticoduodenectomy after neoadjuvant chemotherapy for gastric cancer invading the pancreatic head: A case report. World J Gastroenterol 2021; 27(6): 534-544
- URL: https://www.wjgnet.com/1007-9327/full/v27/i6/534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i6.534
Gastric cancer is the fifth most frequent cancer and the third most frequent cause of cancer death worldwide, according to the global cancer statistics presented in 2018[1]. Curative resection of gastric cancer is essential to achieve long-term survival. In patients with locally advanced gastric cancer invading adjacent organs, extended multivisceral resection is required to achieve an R0 resection. Pancreaticoduodenectomy (PD) is theoretically needed to achieve an R0 resection for locally advanced gastric cancer with invasion to the head of the pancreas and duodenum. This procedure has rarely been performed for gastric cancer because of the significant morbidity and mortality and poor prognosis[2,3]; however, some recent studies have shown that resection combined with PD is associated with improved survival in patients with locally advanced gastric cancer[4-7]. Li et al[8] described in their recent systematic review that PD is a feasible option for locally advanced gastric cancer invading the duodenum and/or pancreas, with acceptable surgical risk, and offers survival benefits for selected patients. In addition, several randomized controlled studies showed that postoperative adjuvant chemotherapy for gastric cancer has improved the prognosis of advanced cases[9-11], and preoperative chemotherapy is also expected to be effective, with several clinical trials currently underway[12,13]. Therefore, multidisciplinary treatment is considered important especially for advanced cases, and the indications for PD to achieve an R0 resection for locally advanced gastric cancer invading the pancreas should be reconsidered.
However, there are no reports showing the therapeutic effect of PD on advanced gastric cancer after neoadjuvant chemotherapy especially in combination with trastuzumab. Our case is the successful report of radical resection with PD after neoadjuvant S-1 plus oxaliplatin (SOX) combined with trastuzumab in a patient with locally advanced gastric cancer invading the pancreatic head.
A 66-year-old man had symptoms of abdominal pain, distension, and weight loss (from 62 to 47 kg within 6 mo).
The patient complained of abdominal distension and weight loss and had visited the hospital previously. Esophagogastroduodenoscopy (EGD) was performed, and an advanced type 3 lesion was detected on the lower part of the gastric body with stenosis, causing resistance to passage of the scope. He was then admitted to our hospital and underwent a detailed medical examination and treatment.
He had no specific past illness but had a current active smoking status [Brinkman Index: 920 (20 × 46 years)].
No family history to note.
Mild tenderness was noted in the upper abdomen.
Initial laboratory data revealed a hemoglobin level of 11.0 g/dL, white blood cell count of 9700 cells/µL, and platelet count of 3.17 × 105/μL. The creatinine level was 0.81 mg/dL, total bilirubin level was 0.3 mg/dL, direct bilirubin level was 0.1 mg/dL, aspartate aminotransferase level was 43 IU/L, alanine aminotransferase level was 72 IU/L, and albumin level was 3.5 g/dL. Tumor marker level of the carcinoembryonic antigen was 23.00 ng/mL, and carbohydrate antigen 19-9 level was 53.20 U/mL.
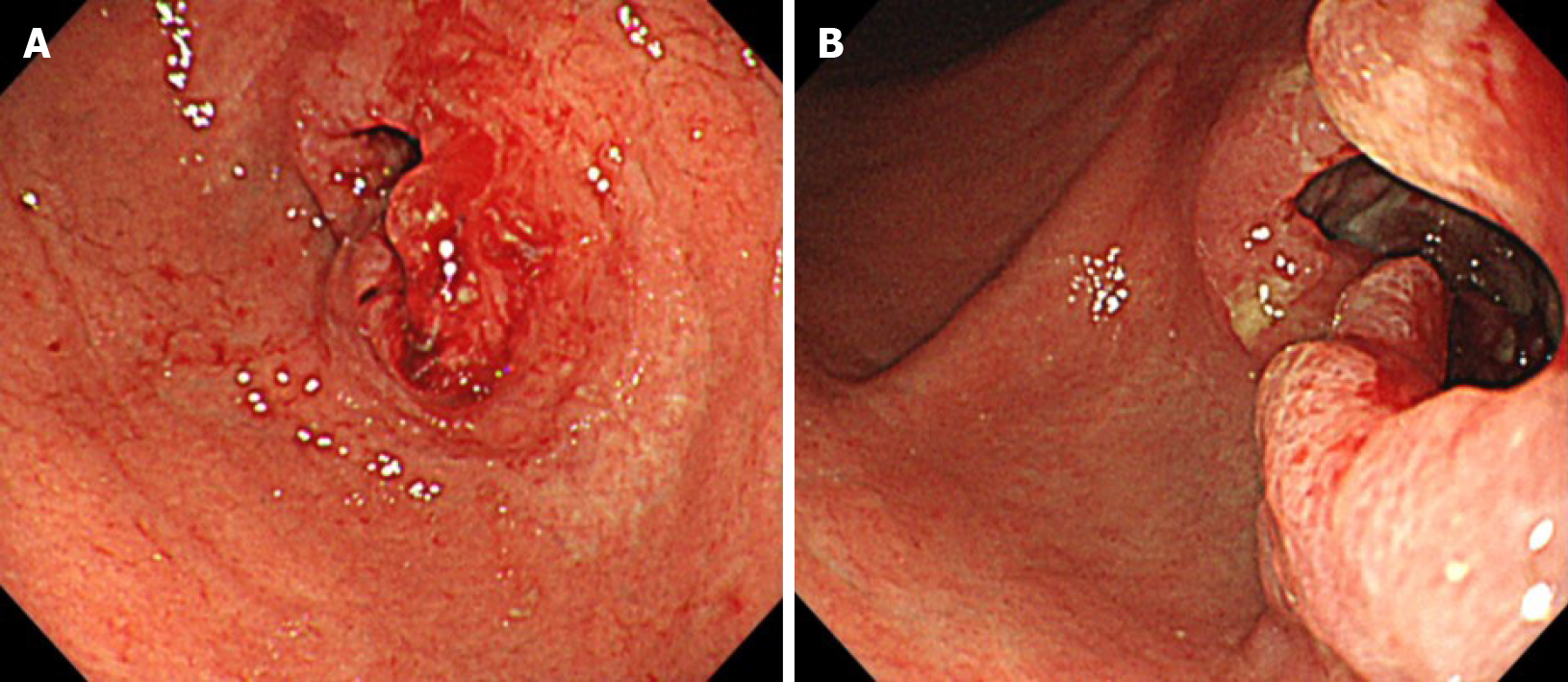
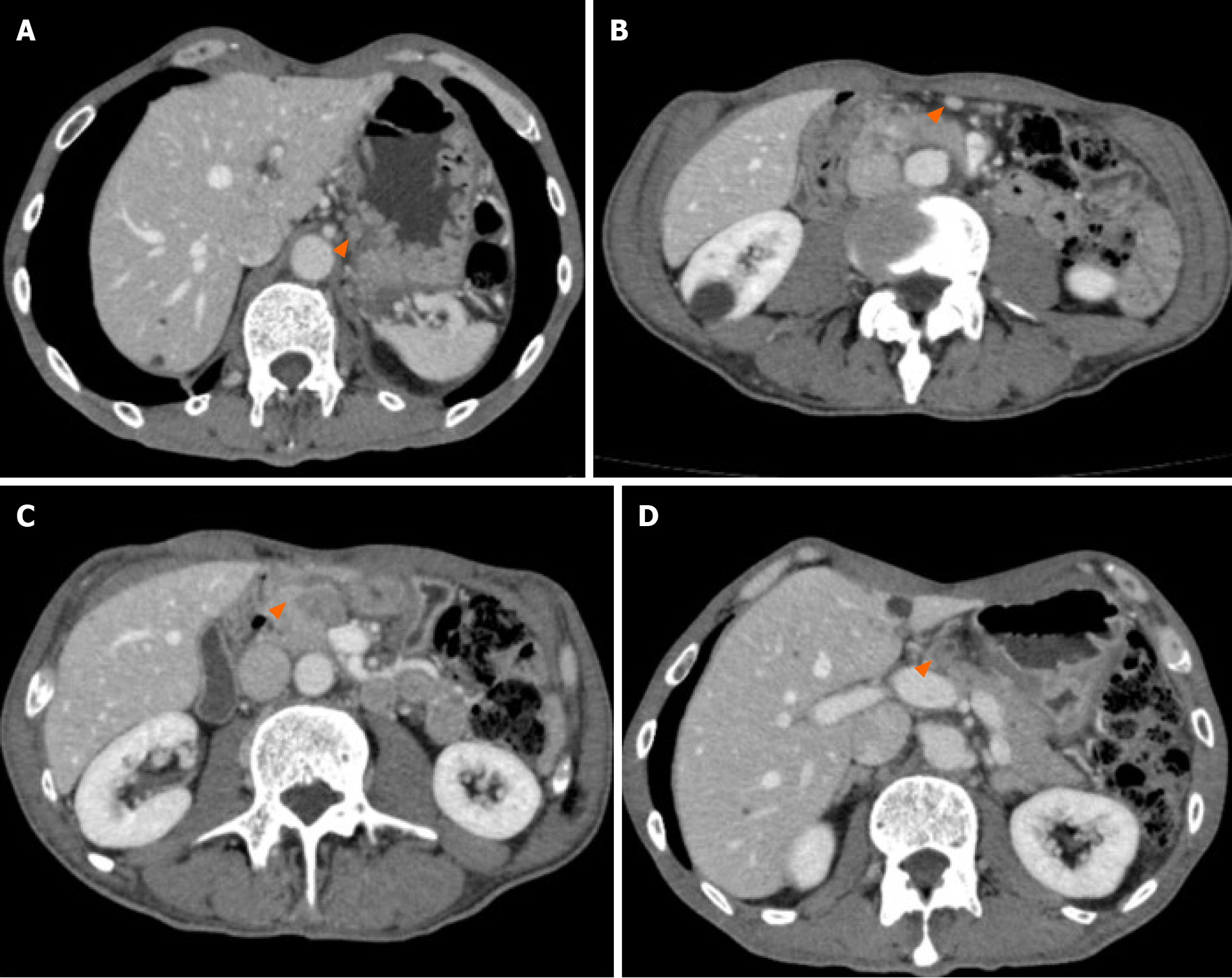
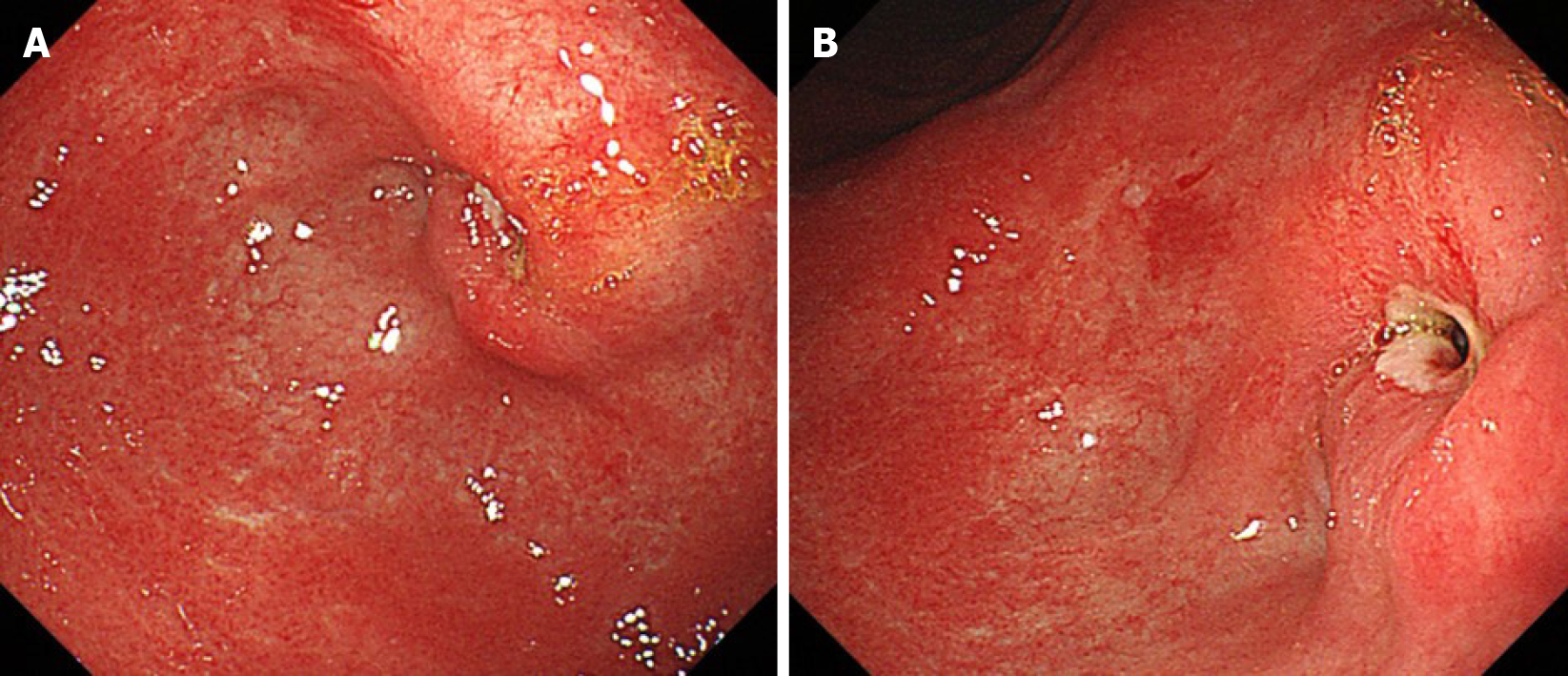
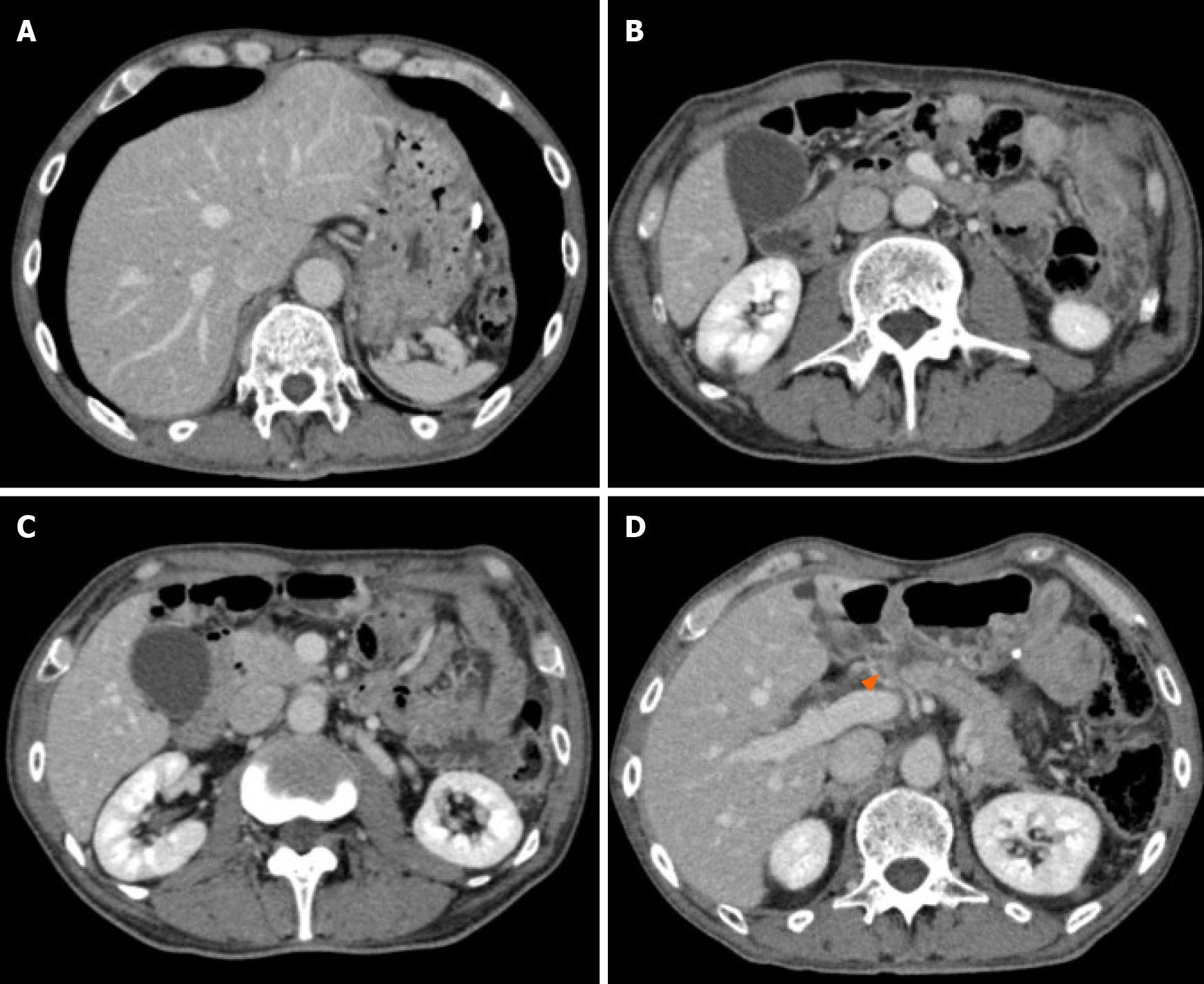
EGD identified stenosis caused by a large tumor (Figure 1). Computed tomography (CT) showed lymph node (LN) metastases at the station of the lesser curvature (#3 LN; 11.8 mm × 8.5 mm, Figure 2A), right greater curvature nodes along the right gastroepiploic artery (#4d LN; 10.3 mm × 8.4 mm, Figure 2B), infrapyloric nodes (#6 LN; 21.6 mm × 14.7 mm, Figure 2C), anterosuperior LNs along the common hepatic artery (#8a; 14.0 mm × 13.4 mm, Figure 2D), and suspicion of metastatic #6 LN invasion to the pancreatic head (the names of the LN station are provided in Table 1). There were no findings of distant metastasis.
| Station No. | Definition |
| 1 | Right paracardial nodes |
| 3 | Lesser curvature nodes |
| 4sb | Left greater curvature nodes along the left gastroepiploic artery |
| 4d | Right greater curvature nodes along the right gastroepiploic artery |
| 5 | Suprapyloric nodes |
| 6 | Infrapyloric nodes |
| 7 | Nodes at the root of the left gastric artery |
| 8a | Anterosuperior LNs along the common hepatic artery |
| 8p | Posterior LNs along the common hepatic artery |
| 9 | Nodes at the celiac artery |
| 11p | Nodes along the proximal splenic artery |
| 12a | Hepatoduodenal ligament LNs along the proper hepatic artery |
| 12b | Hepatoduodenal ligament LNs along the bile duct |
| 12p | Hepatoduodenal ligament LNs along the portal vein |
| 14v | Nodes along the superior mesenteric vein |
| 13a | Superior posterior pancreatoduodenal lymph nodes |
| 13b | Inferior posterior pancreatoduodenal lymph nodes |
| 17a | Superior anterior pancreatoduodenal lymph nodes |
| 17b | Inferior anterior pancreatoduodenal lymph nodes |
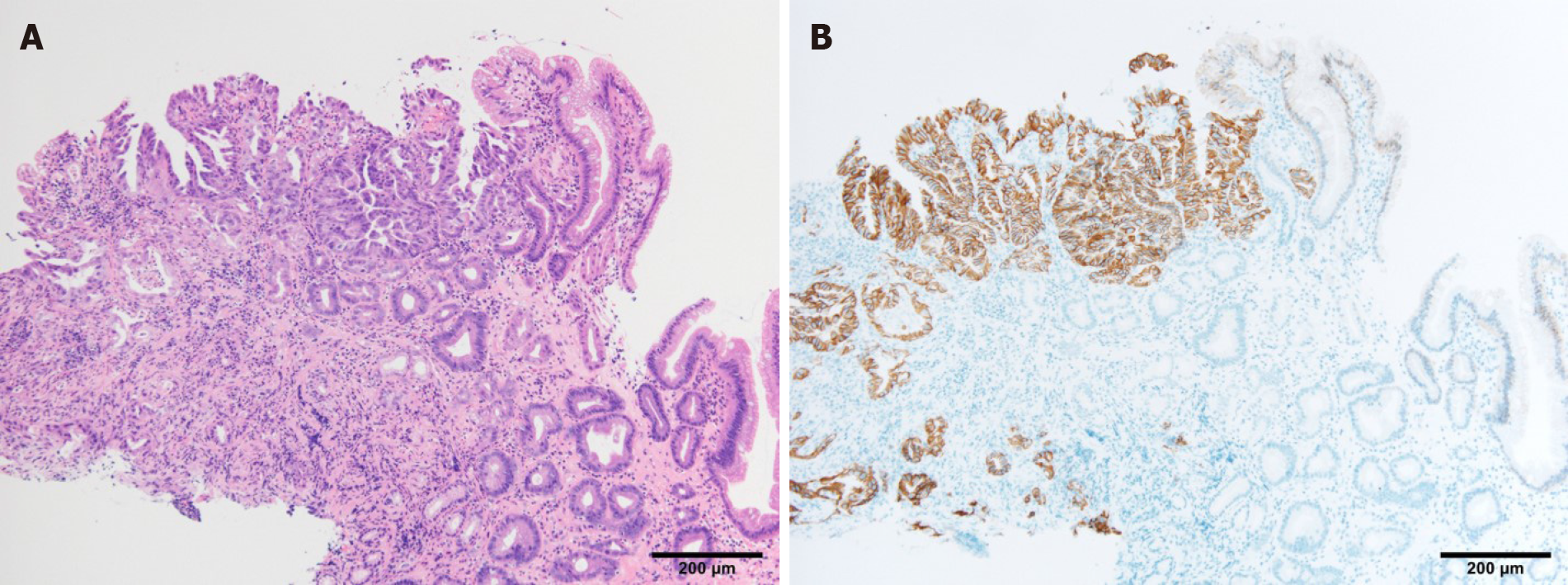
Biopsies were taken, and the histological examination led to a diagnosis of adenocarcinoma (papillary and well-differentiated adenocarcinoma; Figure 3A). Additional pathological examination revealed human epidermal growth factor receptor 2 (HER2) positivity based on an immunohistochemical score of 3 + (Figure 3B).
The clinical diagnosis was gastric cancer LD circ cType3 cT4b (panc) N2M0 cStageIVA according to the Union for International Cancer Control Tumor, Node Metastasis Classification of Malignant Tumors, Eighth Edition[14]. The lymph node station was defined according to the Japanese Classification of Gastric Cancer, 15th Edition[15].
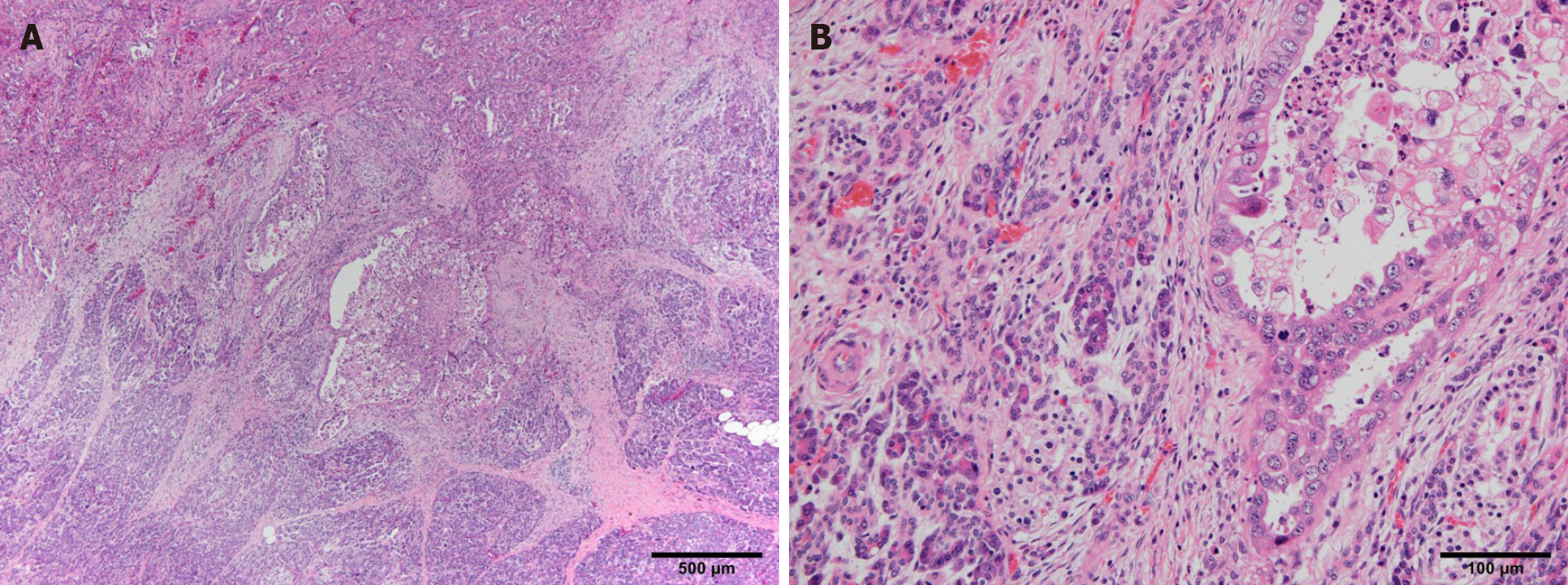
Pathological examination of the resected specimen revealed that the primary tumor was mixed-type adenocarcinoma (papillary adenocarcinoma, moderately differentiated adenocarcinoma > poorly differentiated adenocarcinoma non-solid type, mucinous adenocarcinoma) and subserosal invasion (35 mm × 30 mm) with lymphatic and venous infiltration (Ly1a, V1a). The horizontal and vertical surgical margins were negative, and all dissected LNs were negative for metastasis (0/54). Pathological findings also suggested pancreatic infiltration. The cancer cells infiltrating the pancreas may have invaded along the lymph nodes or nerves on the surface of the pancreas. (Figure 4). The pathological diagnosis was gastric cancer LD Circ ypType3, ypT4b (panc) N0M0, ypStage IIIA. Degeneration of cancer cells was limited, and the pathological therapeutic effect was classified as grade 1a according to the Japanese Classification of Gastric Cancer, 15th Edition[15].
As the patient was undernourished, we inserted a double elemental diet tube to administer enteral nutrition and drain food residue from the stomach. After 10 d of continuous enteral nutrition, we performed staging laparoscopy and confirmed no liver metastasis, no dissemination, and negative lavage cytological results. In addition, immobility of the lower part of the stomach body due to cancerous invasion to the pancreatic head was confirmed. Thus, laparoscopic ante-colic gastrojejunostomy (GJ) and half-cut of the gastric body at the distal side of the anastomosis were performed.
Considering the clinical and intraoperative findings, it was a locally advanced gastric cancer with pancreatic head invasion without non-curative factors such as dissemination and distant metastasis. As a treatment strategy, preoperative chemotherapy was given first, and in the case of stable disease or partial response, R0 resection with PD could be adopted.
After laparoscopic GJ, one course of SOX (S-1 120 mg/d on days 1-14, oxaliplatin 100 mg/m2 on day 1) was performed during one continuous hospitalization stay after surgery, and three courses of SOX with trastuzumab (S-1 120 mg/d on days 1-14, oxaliplatin 100 mg/m2 on day 1, trastuzumab 8 mg/kg during the first cycle and 6 mg/kg during the second and third cycles) was performed in an outpatient setting. For this regimen, only grade 2 elevations in aspartate aminotransferase and alanine aminotransferase levels (84 and 96 IU/L, respectively) were observed, and no grade 3 or more severe adverse events were detected according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0[16].
After neoadjuvant chemotherapy, EGD showed shrinkage of the primary tumor, but the scope could not pass through (Figure 5A and B), while CT showed marked reduction of the primary tumor and metastatic LNs. The #3 (Figure 6A) and #4d (Figure 6B) LNs could not be detected, and #6 LN, which had been suspected of invading the pancreatic head before chemotherapy, was scarred and could not be measured (Figure 6C). The #8a LN had shrunk (from 14.0 mm × 13.4 mm to 9.2 mm × 7.0 mm; Figure 6D). The clinical therapeutic effect was classified as a partial response on radiological examination according to the Response Evaluation Criteria in Solid Tumors criteria version 1.0.
Although the invasion of the pancreas was not clear on CT, magnetic resonance imaging showed that most of the boundary between the stomach or lymph nodes around the stomach and pancreas had fatty tissue, but some of the boundaries were unclear; thus, the invasion could not be denied. Thus, the preoperative diagnosis was ycT4b (panc) N1M0 ycStage IVA.
The surgical risks for this patient included his age (66 years), obstructive pulmonary dysfunction [vital capacity (%), 101.5; forced expiratory volume in 1 s (%), 69.5; forced expiratory volume in 1 s (L), 2.48], current heavy smoker status [Brinkman Index: 920 (20 × 46 years)], undernutrition (total protein level 5.8 g/dL, albumin level 3.2 g/dL), a history of GJ, and the patient’s clinical state after four courses of neoadjuvant chemotherapy. Cardiac function was normal (normal wall motion, ejection fraction of 72.8%) after neoadjuvant chemotherapy with trastuzumab, and the brain natriuretic peptide level was 33.3 pg/mL. Tumor marker levels were decreased compared with initial blood tests, and the carcinoembryonic antigen level improved from 23.00 to 11.00 ng/mL and the carbohydrate antigen 19-9 level from 53.20 to 26.80 U/mL. The therapeutic strategy was explained to the patient, who decided to undergo surgical treatment.
After detachment of adhesions between the omentum and abdominal wall, we confirmed no liver metastasis or dissemination. Rapid lavage cytology revealed no cancer cells in the ascites (Class I). We first performed omentectomy to expose the layer of the colic vein and superior mesenteric vein and performed Kocher mobilization until the inferior vena cava could be identified; the layers were continued up to the layer of the omentectomy. Near the right gastroepiploic artery and vein, the metastatic LN and/or tissues with desmoplastic and inflammatory reactions near the primary lesion invaded the pancreatic head and could not be separated. Therefore, PD was required to achieve R0 resection. We made a jejunum incision at the proximal and distal sides of the anastomosis, which was bypassed in a previous surgery, and performed D2 lymphadenectomy (#1, #3a, #3b, #4sb, #4d, #5, #6, #7, #8a, #9, #11p, #12a) with #8p and #14v LN dissection. The gastric body was cut using an Endo GIA 60 mm stapler (Covidien, Mansfield, MA, United States). After regional LN dissection of the gastric cancer, the gastroduodenal artery was ligated. The dorsal side of the pancreas was tunneled in front of the portal vein. We decided that resection of the tumor could be performed if the pancreas was cut at the line just on the portal vein.
The vein branches of the superior mesenteric vein (first jejunal vein, inferior pancreaticoduodenal vein, gastrocolic trunk, posterior superior pancreatoduodenal vein) were processed, and the artery branches (first jejunal artery, inferior pancreaticoduodenal artery, dorsal pancreatic artery) were processed outside the plexus of the superior mesenteric artery. After excision of the gallbladder, the common bile duct was dissected, and PD was completed (#13a, #13b, #17a, #17b, #12b, and #12p were taken together with the specimen).
Reconstruction of modified Child’s method with Braun enteroenterostomy was performed. First, pancreaticojejunostomy and cholangiojejunostomy were performed, followed by antecolic reverse peristaltic GJ. Jejunum of the afferent loop was sutured to the gastric body to prevent reflux to the afferent loop. Finally, jejunostomy was performed distal to the GJ, and Braun enteroenterostomy was made. The total operation time was 11 h and 16 minutes, and total blood loss was 490 mL.
On the day after the operation, the patient started drinking water and using a jejunostomy tube for enteral nutrition, and he resumed eating on the second day. Surgical complications included aspiration pneumonia, pancreatic fistula, and intraabdominal abscess, each of which was cured with antibiotics and defined as Clavien-Dindo grade 2[17]. The patient was discharged on postoperative day 16 without the need for enteral nutrition. Adjuvant S-1 (100 mg/body) was started 2 mo after the surgery and SOX (S-1; 120 mg/d on days 1-14, oxaliplatin; 100 mg/m2 on day 1) was started 3 mo after the surgery. Postoperative SOX chemotherapy was performed for three courses. There are no recurrences 8 mo after surgery. The timeline was showed in Table 2.
| Time series | Symptoms and treatment details |
| 5 mo ago | He had symptoms of abdominal pain, distension and weight loss (from 62 to 47 kg within 6 mo) and visited hospital |
| Double elemental diet tube was inserted to administer enteral nutrition and drain food residue from the stomach | |
| 4 mo ago | Staging laparoscopy confirmed no liver metastasis, no dissemination, and negative lavage cytological results |
| Laparoscopic ante-colic gastrojejunostomy and half-cut of the gastric body at the distal side of the anastomosis were performed | |
| 1-4 mo ago | SOX 1 |
| SOX + Trastuzumab 2-4 | |
| The date of surgery | Pancreaticoduodenectomy with D2 lymph node dissection for distal gastric cancer was done |
| Adjuvant S1 1 | |
| 2-8 mo after surgery | Adjuvant SOX 1-3 |
| CT shows no recurrence (7 mo after surgery) |
We present the successful case of radical resection with PD after neoadjuvant chemotherapy combined with trastuzumab for HER2-positive locally advanced gastric cancer invading the pancreatic head.
The principle of surgical oncology with curative intent is en bloc resection of organs potentially invaded by the cancer. In cases of pancreatic head invasion, PD with gastrectomy is required. Because of the high surgical risk and poor prognosis, PD is rarely performed for gastric cancer. However, due to advances in surgery and improvements in perioperative management, PD may be considered for gastric cancer to improve long-term survival. Recent reports from two Japanese high-volume cancer centers reported morbidity rates of 45.1% (14/31) and 73.9% (17/23) and mortality rates of 0% and 12.9% (4/31), respectively, after PD for gastric cancer[5,7]. Pancreatic fistula was the most common complication in these studies (12.9% and 43.5%). Saka et al[7] reported that early detection of pancreatic fistulas and drain discharge management are important to prevent fatal complications, such as an intraabdominal abscess leading to rupture of pseudoaneurysms, and emphasized that medical staff (including the surgeon and nursing staff) should be trained in drain management. Therefore, those authors recommended that PD should be performed only at institutions comfortable with PD management.
In our institution, PD is typically performed for pancreatic cancer by hepatobiliary-pancreatic surgeons and appropriate drainage treatment can be performed by radiologists if necessary. Thus, in this case, upper gastrointestinal surgeon and hepatobiliary-pancreatic surgeon performed the surgery in collaboration, allowing us to perform adequate regional LN dissection of the stomach and provide safe surgery and postoperative management for PD. In the present case, although severe inflammatory reactions around the tumor and degeneration and desmoplastic changes due to neoadjuvant chemotherapy were observed, the intraoperative blood loss was 490 mL, blood transfusion was not required, the intensive care unit stay was 1 d, postoperative complications did not exceed grade 2, and the patient was discharged 16 d after surgery. In addition, from an oncological perspective, R0 resection was achieved, and the number of LNs dissected was 54.
Li et al[8] reported a 5-year overall survival (OS) rate of gastric cancer patients after PD of 39.3%, which is comparable with that of patients with stage IIIB (34.8%) gastric cancer, as is registered nationally by the Japanese Gastric Cancer Society[18]. Katai et al[18] also reported, in a nationwide study, a rate of PD performed for gastric cancer of 0.1% (120/12202) and a 5-year OS rate of 41.4%. These findings suggest that PD is a beneficial technique under the correct indications. Saka et al[7] reported that the 5-year OS rate was 0% in patients with non-curative factors [such as para-aortic LN (PAN) metastasis, positive lavage cytological results, and peritoneal dissemination] and 47.4% in patients those without these factors. Nunobe et al[5] reported that the 5-year OS rate after R0 resection with PD was significantly better in the ≤ pN2 group (50.0%) compared with the ≥ pN3 group (7.7%). In our case, we confirmed negative lavage cytological results, no PAN, no dissemination during surgery, and no detected LN metastasis pathologically after surgery. All of these factors may affect survival after PD. According to these studies, PD is considered to be required in gastric cancer surgery in about 1 in 500 to 1000 cases[7,18], of which about one-third may have incurable factors[7]. Combining neoadjuvant chemotherapy may reduce the proportion of incurable cases and increase the rate of radical resection with PD.
Several randomized studies have shown a survival benefit of postoperative adjuvant chemotherapy for advanced gastric cancer[9-11]. However, no reports have described the efficacy or completion rate of adjuvant chemotherapy after PD. The completion rate of adjuvant chemotherapy after routine gastrectomy was reported to be 65.8% for S-1[9], 67% for capecitabine plus oxaliplatin[10], and 49% for docetaxel plus S-1[11]. This indicates that 33%-51% of patients do not benefit from chemotherapy. Adjuvant chemotherapy may be inadequate if the procedure becomes more invasive because of major surgical complications combined with organ resection and loss of appetite or physical fitness. Thus, preoperative chemotherapy is considered to have benefits in these patients compared with postoperative chemotherapy. Clinical trials of neoadjuvant chemotherapy for gastric cancer with extensive nodal metastasis (bulky and/or PANs) reported high completion rates of 65%-81%[19-21]. Despite the inclusion of double or triple chemotherapy regimens in these trials, compliance was greater compared with single-agent postoperative adjuvant chemotherapy. Considering the effects of preoperative chemotherapy and the highly invasive postoperative situation, patients with locally advanced gastric cancer who require PD for radical resection are thought to be good candidates for neoadjuvant chemotherapy. We used SOX plus trastuzumab as preoperative chemotherapy because, at our facility, we typically use the SOX regimen as preoperative chemotherapy for advanced gastric cancer with multiple LN metastases, according to a recent ongoing randomized clinical trial (JCOG1509)[13]. Furthermore, preoperative and perioperative chemotherapies combined with trastuzumab for HER2-positive gastric cancer are currently underway[12,22]. Thus, we think that SOX plus trastuzumab combination is expected as effective preoperative chemotherapy for HER-2 positive advanced gastric cancer.
Another problem is that invasion of adjacent organs cannot always be confirmed pathologically[7]. Desmoplastic and inflammatory reactions surrounding the tumor cannot be differentiated from tumor invasion during surgery; thus, PD cannot be avoided in such cases. However, neoadjuvant chemotherapy has a possibility to differentiate between cases of true tumor invasion and inflammatory reactions and may even avoid PD in some cases.
There were some limitations to this case report. First, the long-term survival benefit of neoadjuvant chemotherapy combined with trastuzumab in this patient was unknown, and a longer observation time is required. However, we achieved R0 resection with PD after this regimen and confirmed no LN metastasis pathologically. Previous reports have reported a survival benefit in such cases. Second, we use SOX combined with trastuzumab, for which no clear evidence has been established. However, the therapeutic effects of neoadjuvant SOX (JCOG1509; UMIN000024065) and neoadjuvant chemotherapy combined with trastuzumab for advanced gastric cancer (JCOG1301; UMIN000016920[12]) are currently being verified in clinical trials in Japan.
We suggest that radical resection with PD after neoadjuvant chemotherapy combined with trastuzumab is one option for locally advanced HER2-positive gastric cancer invading the pancreatic head in the absence of non-curative factors.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56643] [Article Influence: 7080.4] [Reference Citation Analysis (134)] |
| 2. | Ohashi I. Combined resection of adjacent organs for advanced cancer of the stomach: pancreatoduodenectomy and left upper abdominal evisceration (in Japanese). Surg Ther. 1985;52:173-180. |
| 3. | Shchepotin IB, Chorny VA, Nauta RJ, Shabahang M, Buras RR, Evans SR. Extended surgical resection in T4 gastric cancer. Am J Surg. 1998;175:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Wang XB, Yang LT, Zhang ZW, Guo JM, Cheng XD. Pancreaticoduodenectomy for advanced gastric cancer with pancreaticoduodenal region involvement. World J Gastroenterol. 2008;14:3425-3429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Nunobe S, Hiki N, Ohyama S, Fukunaga T, Seto Y, Yamaguchi T. Survival benefits of pancreatoduodenectomy for gastric cancer: relationship to the number of lymph node metastases. Langenbecks Arch Surg. 2008;393:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Chan WH, Cheow PC, Chung AY, Ong HS, Koong HN, Wong WK. Pancreaticoduodenectomy for locally advanced stomach cancer: preliminary results. ANZ J Surg. 2008;78:767-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Saka M, Mudan SS, Katai H, Sano T, Sasako M, Maruyama K. Pancreaticoduodenectomy for advanced gastric cancer. Gastric Cancer. 2005;8:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Li DB, You J, Wang SJ, Zhou YM. Pancreaticoduodenectomy for locally advanced gastric cancer: Results from a pooled analysis. Asian J Surg. 2019;42:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1978] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 10. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1331] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 11. | Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 12. | Kataoka K, Tokunaga M, Mizusawa J, Machida N, Katayama H, Shitara K, Tomita T, Nakamura K, Boku N, Sano T, Terashima M, Sasako M; Stomach Cancer Study Group/Japan Clinical Oncology Group. A randomized Phase II trial of systemic chemotherapy with and without trastuzumab followed by surgery in HER2-positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301 (Trigger Study). Jpn J Clin Oncol. 2015;45:1082-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Terashima M, Yoshikawa T, Boku N, Ito S, Tsuburaya A, Iwasaki Y, Fukagawa T, Tokunaga M, Sano T, Sasako M; Stomach Cancer Study Group; Japan Clinical Oncology Group. Current status of perioperative chemotherapy for locally advanced gastric cancer and JCOG perspectives. Jpn J Clin Oncol. 2020;50:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Brierley, James D, Gospodarowicz, MK, Wittekind, C. TNM classification of malignant tumors: International union against cancer. 8th ed. Oxford: Wiley; 2017. |
| 15. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2945] [Article Influence: 196.3] [Reference Citation Analysis (1)] |
| 16. | Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available from: https://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm#ctc_50. |
| 17. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9186] [Article Influence: 540.4] [Reference Citation Analysis (1)] |
| 18. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 377] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 19. | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 21. | Takahari D, Ito S, Mizusawa J, Katayama H, Terashima M, Sasako M, Morita S, Nomura T, Yamada M, Fujiwara Y, Kimura Y, Ikeda A, Kadokawa Y, Sano T; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Long-term outcomes of preoperative docetaxel with cisplatin plus S-1 therapy for gastric cancer with extensive nodal metastasis (JCOG1002). Gastric Cancer. 2020;23:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Wagner AD, Grabsch HI, Mauer M, Marreaud S, Caballero C, Thuss-Patience P, Mueller L, Elme A, Moehler MH, Martens U, Kang YK, Rha SY, Cats A, Tokunaga M, Lordick F. EORTC-1203-GITCG - the "INNOVATION"-trial: Effect of chemotherapy alone vs chemotherapy plus trastuzumab, vs chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu J, Zhou ZH S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH