Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6951
Peer-review started: February 4, 2021
First decision: March 6, 2021
Revised: March 17, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: October 28, 2021
Processing time: 265 Days and 6.3 Hours
Various liver and gastrointestinal involvements occur in patients with coronavirus disease 2019 (COVID-19) at variable prevalence. Most studies report mild liver function disturbances correlated with COVID-19 severity, though liver failure is unusual.
To study liver and gastrointestinal dysfunctions in Egyptian patients with COVID-19 and their relation to disease outcomes
This multicentre cohort study was conducted on 547 Egyptian patients from April 15, 2020 to July 29, 2020. Consecutive polymerase chain reaction-confirmed COVID-19 cases were included from four quarantine hospitals affiliated to the Egyptian ministry of health. Demographic information, laboratory characteristics, treatments, fibrosis-4 (FIB-4) index, COVID-19 severity, and outcomes were recorded and compared according to the degree of liver enzyme elevation and the presence of gastrointestinal symptoms. Follow-ups were conducted until discharge or death. Regression analyses were performed to determine the independent factors affecting mortality.
This study included 547 patients, of whom 53 (9.68%) died during hospitalization and 1 was discharged upon his request. Patients’ mean age was 45.04 ± 17.61 years, and 21.98% had severe or critical COVID-19. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were available for 430 and 428 patients, respectively. In total, 26% and 32% of patients had elevated ALT and AST, respectively. Significant liver injury with ALT or AST elevation exceeding 3-fold was recorded in 21 (4.91%) and 16 (3.73%) patients, respectively. Male gender, smoking, hypertension, chronic hepatitis C, and lung involvement were associated with elevated AST or ALT. AST was elevated in 50% of patients over 60-years-old. FIB-4 was significantly higher in patients admitted to the intensive care unit (ICU), those with more severe COVID-19, and non-survivors. The independent variables affecting outcome were supplementary vitamin C intake (1 g daily capsules) [odds ratio (OR): 0.05, 95% confidence interval (CI): 0.008–0.337]; lung consolidation (OR: 4.540, 95%CI: 1.155–17.840); ICU admission (OR: 25.032, 95%CI: 7.110–88.128); and FIB-4 score > 3.25 (OR: 10.393, 95%CI: 2.459-43.925). Among 60 (13.98%) patients with gastrointestinal symptoms, 52 (86.67%) had diarrhoea. Patients with gastrointestinal symptoms were predominantly females with higher body mass index, and 50 (83.40%) patients had non-severe COVID-19.
Few Egyptian patients with COVID-19 developed a significant liver injury. The independent variables affecting mortality were supplementary vitamin C intake, lung consolidation, ICU admission, and FIB-4 score.
Core Tip: The prevalence and severity of liver and gastrointestinal dysfunction in patients with coronavirus disease 2019 (COVID-19) vary among populations with different underlying characteristics and disease outcomes. This is the first report from Egypt specifically exploring hepatic and gastrointestinal involvement in Egyptian patients with COVID-19. In this study, we analyzed multicentre data of patients with polymerase chain reaction-confirmed COVID-19 from April 15, 2020 to July 29, 2020. Based on these data, we assessed the degree of liver injury and presence of gastrointestinal symptoms concerning COVID-19 disease severity, intensive care unit admission, and outcome.
- Citation: Shousha HI, Afify S, Maher R, Asem N, Fouad E, Mostafa EF, Medhat MA, Abdalazeem A, Elmorsy H, Aziz MM, Mohammed RS, Ibrahem M, Elgarem H, Omran D, Hassany M, Elsayed B, Abdelaziz AY, El Kassas M. Hepatic and gastrointestinal disturbances in Egyptian patients infected with coronavirus disease 2019: A multicentre cohort study. World J Gastroenterol 2021; 27(40): 6951-6966
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6951.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6951
Hepatic and gastrointestinal (GI) involvement occurs in 16% to 78% of patients infected with the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1-3]. In some of these populations, liver enzyme elevation exceeds five times the upper limit of normal (ULN)[4]. Most studies report mild liver function disturbances correlated with coronavirus disease 2019 (COVID-19) severity, though liver failure is unusual[5]. Hepatic and GI involvement can occur with and without pulmonary manifestations of COVID-19[1]. Different underlying mechanisms may contribute to liver injury related to COVID-19, including a direct viral effect, as the virus enters through the angiotensin-converting enzyme-2 receptor on cholangiocytes. Other indirect pathways include sepsis, drug-related hepatic injury, uncontrolled immune reactions, and cytokine storm[6].
Diarrhoea is the most frequently recorded GI symptom reported with COVID-19, ranging from 3% to 30%, followed by anorexia, nausea, vomiting, and abdominal pain[7]. SARS-CoV-2 RNA has been detected in stool specimens from patients with polymerase chain reaction-confirmed COVID-19 in respiratory samples. Those patients showed extended viral shedding in stool up to 11.2 d after viral eradication from respiratory specimens, suggesting faecal-oral transmission and warranting subsequent precautions[8]. The possible underlying mechanisms for GI involvement are the direct viral invasion of cells in the GI tract via the angiotensin-converting enzyme-2 receptor on gastric and duodenal glandular cells and proximal and distal enterocytes[8]. This viral invasion disrupts absorption and intestinal secretions, which activates the enteric nervous system and causes diarrhoea[9]. Other indirect mechanisms include antibiotic-associated diarrhoea, indirect inflammatory damage, and the ‘‘gut-lung axis” theory of immune-mediated effects on the respiratory tract by disturbed digestive tract flora[1,10]. This study aimed to determine the prevalence and extent of liver and GI derangements in Egyptian patients with COVID-19 infection and their relation to COVID-19 disease outcomes.
This multicentre, prospective cohort study recruited 547 consecutive patients from April 15, 2020 to July 29, 2020 who were hospitalized in four quarantine centres affiliated to the Egyptian Ministry of Health and Population in three Egyptian governorates: 15 Mayo Smart Hospital in Cairo governorate, National Hepatology and Tropical Medicine Research Institute in Cairo governorate, Students Hospital in Giza governorate, and Alraghy Hospital in Assuit Governorate. This study included patients with a confirmed diagnosis of SARS-CoV-2, defined as a positive real-time reverse-transcriptase polymerase chain reaction assay of nasal and pharyngeal swab specimens. Patients were followed until discharge or death.
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects or patients were approved by the research ethics committee of the Faculty of Medicine at Cairo University (number N-37-2020, May 14, 2020) and the research ethics committee of the Ministry of Health and Population (number 17-2020/8, June 21, 2020). Written informed consent was obtained from all patients before they participated in the study.
Collected baseline data included demographic information (age, gender, cigarette smoking, comorbidities), presenting symptoms (general, respiratory, GI), and laboratory features [complete blood count, liver and renal function, coagulation test, D-dimer, ferritin, C-reactive protein (CRP)]. Liver injury was defined as transaminase elevation exceeding three times the ULN[5]. The fibrosis-4 index (FIB-4) was calculated on admission using Sterling’s formula: Age (years) × aspartate aminotransferase (AST) (IU/L) / platelet count (109/L) × [alanine aminotransferase (ALT) (IU/L) × 1/2][11]. Patients were classified according to the respective FIB-4 cut-off values (≤ 1.45 and ≥ 3.25) for predicting advanced liver fibrosis. Other baseline data included chest computed tomography (CT) findings, treatments administered, and COVID-19 disease classification and outcome. Laboratory results before discharge were collected.
COVID-19 severity was categorized as mild, moderate, or severe according to the management protocol of the Egyptian Ministry of Health and Population. Mild cases included asymptomatic and symptomatic cases with lymphopenia (defined as an absolute lymphocyte count < 1.0 × 103/L)[12] or leukopenia[12] (defined as a total leucocyte count < 4.0 × 103/L) and no radiological evidence of pneumonia. Moderate cases included symptomatic patients with radiological features of pneumonia with or without leukopenia and lymphopenia. Severe and critical cases were defined by the presence of any of the following: Respiratory rate > 30 per min, SaO2 < 92 without oxygen therapy; PaO2/FiO2 ratio < 300 without oxygen or < 200 with oxygen, chest radiology showing more than 50% lung involvement, or progressive lung involvement within 24 h to 48 h. Severe and critical cases were indicated for intensive care unit (ICU) admission. Treatments were applied according to the protocol[13].
Analysis of data was performed using SPSS 25 for Windows (Armonk, NY, United States). Numerical variables are presented as mean, standard deviation, median, and 25th and 75th percentiles. Categorical variables are presented as numbers (n) and percentages (%). According to the distribution of numerical data, suitable tests for inferential statistics were used. The Kruskal-Wallis and Wilcoxon or the Mann–Whitney U test was used for comparing two groups of independent variables[14]. Comparisons between categorical variables were carried out by Chi-square test (
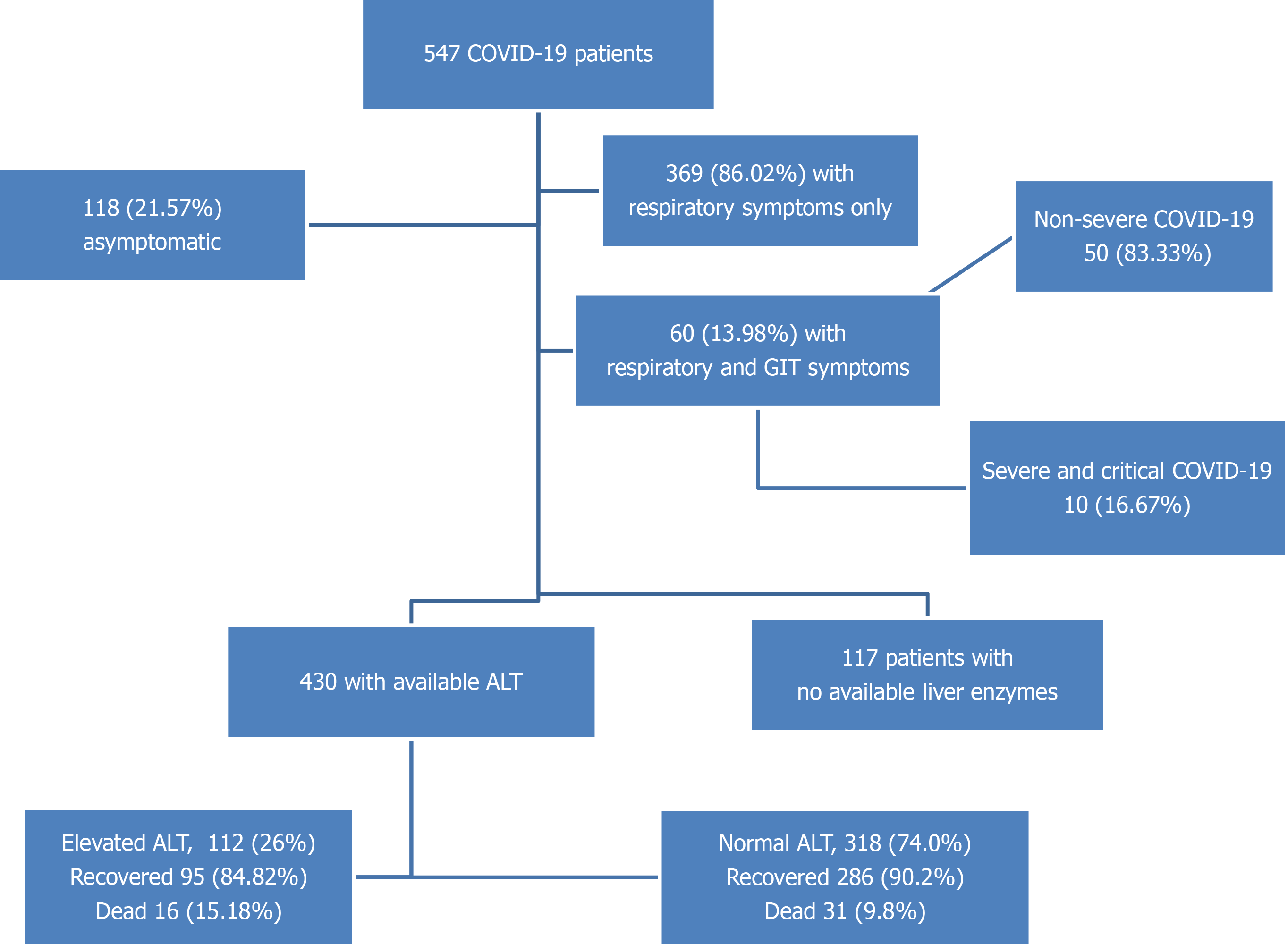
This study included 547 COVID-19 patients hospitalized in four quarantine hospitals affiliated to the Egyptian Ministry of Health and Population (see SupplementaryTables 1 and 2 for baseline clinical, laboratory, imaging, treatment, and outcome features of the studied cohort). Of the 547 patients, 53 (9.68%) died during hospitalization, 493 (90.46%) recovered and were discharged, and 1 was discharged upon his request. The most common symptoms were fever (54.66%), cough (52.47%), dyspnoea (32.54%), and fatigue (30.71%), and 118 (21.57%) patients were asymptomatic. The mean age of the studied patients was 45.04 ± 17.61 years (the median age interquartile range) was 45.00 (30.00:60.00) years and 300 (54.84%) were male. Diabetes was the most common comorbidity (24.86%), followed by hypertension (23.77%) and coronary artery disease (4.20%). Chronic liver disease was reported in 18 (3.29%) patients: 14 had chronic hepatitis C (of whom 7 had liver cirrhosis), 2 had chronic hepatitis B, and 2 had fatty liver disease. We confirmed their liver condition using their most recent abdominal ultrasound study before hospitalization for COVID-19. Regarding COVID-19, 427 (78.02%) had mild and moderate disease, 120 (21.98%) had severe or critical disease, and 122 (22.34%) were admitted to the ICU during their hospitalization. Flow chart of the study cohort is illustrated in Figure 1.
In our study, ALT and AST were available for 430 and 428 patients, respectively, and their other liver function tests and inflammatory markers were also available. Most patients had normal AST (291; 67.99%) and normal ALT levels (318; 74%). We divided patients as follows: Those with liver enzyme levels within the normal range, 1–2 fold over the ULN, 2–3 fold over the ULN, or >3 fold over the ULN (Tables 1-4). Among patients who required ICU admission, 48.50% had elevated AST and 35.60% had elevated ALT. On admission, FIB-4 was significantly higher in patients admitted to the ICU, those with more severe COVID-19 disease, and non-survivors (SupplementaryTable 3).
| Variable | AST level | P value | |||||
| Normal291 (67.99) | 1-2 UNL97 (22.66) | 2-3 UNL19 (4.44) | > 3UNL21 (4.91) | Total | |||
| Age | < 18 | 7 (87.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 8 (100.0) | < 0.001 |
| 18:60 | 237 (72.7) | 65 (19.9) | 9 (2.8) | 15 (4.6) | 326 (100.0) | ||
| > 60 | 47 (50.0) | 32 (34.0) | 9 (9.6) | 6 (6.4) | 94 (100.0) | ||
| Gender | Male | 142 (61.2) | 66 (28.4) | 10 (4.3) | 14 (6.0) | 232 (100) | 0.009 |
| Female | 149 (76.0) | 32 (16.3) | 8 (4.1) | 7 (3.6) | 196 (100) | ||
| Cigarette smoking | 10 (47.6) | 10 (47.6) | 0 (0.0) | 1 (4.8) | 21 (100) | 0.04 | |
| Diabetes mellitus | 68 (61.8) | 32 (29.1) | 6 (5.5) | 4 (3.6) | 110 (100) | 0.23 | |
| Hypertension | 60 (55.6) | 34 (31.5) | 10 (9.3) | 4 (3.7) | 108 (100) | 0.001 | |
| Chronic hepatitis C | 3 (21.4) | 7 (50.0) | 0 (0.0) | 4 (28.6) | 14 (100) | < 0.001 | |
| CT chest | Normal | 105 (78.9) | 21 (15.8) | 5 (3.8) | 2 (1.5) | 133 (100) | 0.008 |
| Abnormal | 181 (63.1) | 76 (26.5) | 13 (4.5) | 17 (5.9) | 287 (100) | ||
| Lung consolidation | 37 (56.1) | 20 (30.3) | 6 (9.1) | 3 (4.5) | 66 (100) | 0.05 | |
| Variable | AST level | P value | ||||
| Normal291 (67.99) | 1-2 UNL97 (22.66) | 2-3 UNL19 (4.44) | > 3UNL21 (4.91) | Total | ||
| Hemoglobin (gm/L) median (IQR) | 12.80 (11.80:14.00) | 13.00 (12.00:14.23) | 12.85 (11.23:14.05) | 13.00 (10.75:15.00) | 12.60 (12.00:13.90) | 0.49 |
| white blood cell count (× 109) | 5.00 (3.80:7.00) | 6.20 (4.88:10.68) | 6.30 (4.73:9.48) | 7.40 (4.35:9.85) | 5.00 (4.40:7.60) | < 0.001 |
| Neutrophils absolute count | 2.58 (1.58:4.40) | 3.90 (2.20:7.87) | 4.60 (3.01:8.13) | 6.00 (2.74:8.33) | 2.95 (1.70:5.60) | < 0.001 |
| Neutrophils percentage | 53.00 (42.00:69.00) | 61.50 (43.13:80.85) | 55.50 (42.75:68.25) | 73.00 (55.00:89.88) | 56.50 (42.63:70.75) | 0.25 |
| Lymphocytes absolute count | 1.63 (1.30:2.30) | 1.40 (1.01:2.35) | 1.50 (0.89:1.92) | 1.07 (0.80:1.48) | 1.58 (1.10:2.20) | < 0.001 |
| Lymphocytes percentages | 38.00 (26.00:45.90) | 23.30 (14.50:37.60) | 23.40 (16.85:28.90) | 14.00 (8.00:28.00) | 32.70 (20.15:45.00) | < 0.001 |
| Neutrophils/lymphocytes ratio | 2.16 (1.30:3.73) | 6.11 (2.12:11.26) | 3.66 (3.26) | 5.31 (3.83:6.84) | 3.11 (1.56:6.16) | 0.002 |
| Platelet Count | 225.00 (173.00:283.00) | 221.00 (174.50:286.50) | 251.00 (180.75:319.75) | 210.00 (146.50:293.50) | 208.00 (184.00:272.00) | 0.62 |
| AST | 22.00 (17.00:28.00) | 46.00 (41.00:55.50) | 85.00 (72.75:95.25) | 156.00 (117.00:252.00) | 28.00 (19.00:43.00) | < 0.001 |
| ALT | 22.00 (16.00:30.00) | 48.00 (36.00:68.00) | 68.00 (44.00:92.75) | 142.00 (93.50:217.50) | 27.00 (18.00:46.00) | < 0.001 |
| Alkaline phosphatase | 89.00 (68.50:112.00) | 82.50 (76.50:88.50) | 155.00 (125.25:349.00) | 111.00 (84.00:144.00) | 89.00 (71.00:114.50) | 0.01 |
| GGT | 31.00 (20.00:34.00) | 39.50 (31.25:62.25) | 67.50 (65.00) | 90.00 (65.00) | 34.00 (22.00:50.00) | 0.005 |
| Total bilirubin | 0.80 (0.50:1.10) | 0.90 (0.70:1.13) | 1.15 (0.88:1.65) | 1.80 (0.75:2.15) | 0.80 (0.60 :1.13) | 0.09 |
| Serum albumin | 4.20 (3.80:4.50) | 4.10 (3.90:4.45) | 3.80 (3.80) | 3.80 (2.90:4.10) | 4.20 (3.80:4.50) | 0.13 |
| D-dimer | 0.54 (0.35:1.02) | 0.57 (0.40:1.21) | 1.08 (0.55:1.50) | 0.90 (0.40:2.24) | 0.58 (0.40:1.10) | 0.005 |
| Ferritin | 230.00 (110.00:486.00) | 406.85 (200.00:773.25) | 804.00 (317.50:960.50) | 540.00 (231.45:1219.10) | 300.00 (132.80:590.00) | < 0.001 |
| C-reactive protein | 32.00 (5.00:64.00) | 43.22 (14.40:64.00) | 64.00 (31.50:109.85) | 39.23 (32.00:64.00) | 45.00 (8.25:64.00) | 0.002 |
| Recovery | 273 (93.8) | 78 (81.3) | 11 (61.1) | 17 (81.0) | 379 (89.0) | < 0.001 |
| Death | 18 (6.2) | 18 (18.8) | 7 (38.9) | 4 (19.0) | 47 (11.0) | |
| Variable | ALT levels | P value | |||||
| Normal318 (74.0) | 1-2 UNL73 (17.0) | 2-3 UNL23 (5.3) | > 3UNL16 (3.7) | Total | |||
| Age | < 18 | 9 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (100.0) | 0.53 |
| 18:60 | 246 (75.0) | 53 (16.2) | 18 (5.5) | 11 (3.4) | 328 (100.0) | ||
| > 60 | 63 (67.7) | 20 (21.5) | 5 (5.4) | 5 (5.4) | 93 (100.0) | ||
| Gender | Male | 157 (67.4) | 50 (21.5) | 16 (6.9) | 10 (4.3) | 233 (100.0) | 0.02 |
| Female | 160 (81.6) | 23 (11.7) | 7 (3.6) | 6 (3.1) | 196 (100.0) | ||
| Cigarette smoking | 10 (47.6) | 5 (23.8) | 4 (19.0) | 2 (9.5) | 21 (100.0) | 0.02 | |
| Diabetes mellitus | 79 (71.2) | 27 (24.3) | 3 (2.7) | 2 (1.8) | 111 (100.0) | 0.04 | |
| Hypertension | 79 (73.1) | 25 (23.1) | 2 (1.9) | 2 (1.9) | 108 (100.0) | 0.05 | |
| Chronic hepatitis C | 5 (35.7) | 6 (42.9) | 1 (7.1) | 2 (14.3) | 14 (100.0) | 0.005 | |
| CT chest | Normal | 110 (82.1) | 16 (11.9) | 7 (5.2) | 1 (.7) | 134 (100.0) | 0.04 |
| Abnormal | 203 (70.5) | 56 (19.4) | 16 (5.6) | 13 (4.5) | 288 (100.0) | ||
| Consolidation | 43 (65.2) | 17 (25.8) | 3 (4.5) | 3 (4.5) | 66 (100.0) | 0.06 | |
| Variable | ALT levels | P value | ||||
| Normal318 (74.0) | 1-2 UNL73 (17.0) | 2-3 UNL23 (5.3) | > 3UNL16 (3.7) | Total | ||
| Hemoglobin (gm/L) median (IQR) | 12.75 (11.78:14.00) | 13.00 (12.10:14.45) | 13.30 (12.30:14.80) | 13.35 (10.63:14.98) | 12.60 (12.00:13.90) | 0.09 |
| white blood cell count (× 109) | 5.00 (4.00:7.40) | 6.30 (4.70:10.55) | 6.30 (4.20:9.80) | 7.55 (3.88:9.33) | 5.00 (4.40:7.60) | 0.003 |
| Neutrophils absolute count | 2.70 (1.60:4.69) | 4.00 (2.40:7.73) | 2.70 (1.80:7.10) | 5.00 (2.60:6.97) | 2.95 (1.70:5.60) | 0.02 |
| Neutrophils percentage | 53.00 (41.50:66.50) | 57.00 (46.00:77.50) | 82.60 (44.93:92.08) | 66.50 (53.25:74.50) | 56.50 (42.63:70.75) | 0.17 |
| Lymphocytes absolute count | 1.60 (1.20:2.30) | 1.60 (1.02:2.00) | 1.26 (1.06:1.80) | 1.07 (0.83:1.50) | 1.58 (1.10:2.20) | 0.04 |
| Lymphocytes percentages | 36.00 (24.23:45.00) | 22.00 (15.70:43.40) | 25.55 (15.05:42.38) | 22.60 (13.75:33.75) | 32.70 (20.15:45.00) | 0.01 |
| Neutrophils/lymphocytes ratio | 2.19 (1.40:4.19) | 6.07 (2.96:9.15) | 6.24 (2.83:7.96) | 4.14 (2.70:15.85) | 3.11 (1.56:6.16) | 0.002 |
| Platelet count | 220.00 (168.00:273.25) | 238.00 (168.00:309.00) | 219.00 (202.00:297.00) | 271.00 (160.00:343.75) | 208.00 (184.00:272.00) | 0.11 |
| AST | 23.00 (17.00:31.00) | 46.00 (34.00:60.00) | 61.00 (52.00:84.00) | 154.50 (109.25:236.00) | 28.00 (19.00:43.00) | < 0.001 |
| ALT | 22.00 (16.00:29.25) | 56.00 (48.00:63.60) | 96.00 (91.00:110.00) | 183.50 (144.50:228.50) | 27.00 (18.00:46.00) | < 0.001 |
| Alkaline phosphatase | 88.00 (65.50:108.50) | 88.00 (77.50:134.00) | 86.00 (86.00:86.00) | 120.00 (90.00:151.00) | 89.00 (71.00:114.50) | 0.19 |
| GGT | 23.00 (20.00:34.00) | 39.50 (34.00:67.75) | 34.00 (22.00:50.00) | 77.50 (65.00:101.25) | 34.00 (22.00:50.00) | 0.001 |
| Total bilirubin | 0.80 (0.50:0.90) | 1.10 (0.75:1.40) | 0.80 (0.35: 0.88) | 1.80 (1.25:2.15) | 0.80 (0.60 :1.13) | 0.01 |
| Serum albumin | 4.20 (3.70:4.50) | 4.00 (3.83:4.45) | 3.10 (2.60) | 3.80 (3.50:4.10) | 4.20 (3.80:4.50) | 0.29 |
| D-dimer | 0.55 (0.36:1.02) | 0.58 (0.40:1.27) | 0.63 (0.36:1.50) | 0.58 (0.40:1.48) | 0.58 (0.40:1.10) | 0.23 |
| Ferritin | 245.20M (122.93:540.00) | 427.80 (200.00:847.00) | 438.00 (86.50:922.00) | 430.50 (215.73:1076.00) | 300.00 (132.80:590.00) | 0.009 |
| C-reactive protein | 32.00 (5.00:64.00) | 32.00 (20.00:64.00) | 32.00 (9.40:64.00) | 55.44 (32.00:78.23) | 45.00 (8.25:64.00) | 0.09 |
| Recovery | 286 (90.2) | 60 (83.3) | 22 (95.7) | 13 (81.3) | 381 (89.0) | 0.18 |
| Death | 31 (9.8) | 12 (16.7) | 1 (4.3) | 3 (18.8) | 47 (11.0) | |
We found that male gender, cigarette smoking, systemic hypertension, chronic hepatitis C, and abnormal chest CT findings were associated with significant elevation of baseline AST and ALT. Also, AST levels were elevated in patients older than 60 years, and diabetes mellitus was associated with elevation of baseline ALT. Elevated AST was also associated with elevated white blood cell count, absolute neutrophils count, neutrophils/lymphocytes ratio, ALT, gamma-glutamyl transferase (GGT), D-dimer, ferritin, and CRP and negatively correlated with absolute lymphocyte count and serum albumin. Elevated ALT was associated with elevated white blood cell count, Absolute Neutrophils count, neutrophils/lymphocytes ratio, AST, alkaline phosphatase (ALP), GGT, D-dimer, ferritin, and CRP and negatively correlated with absolute lymphocyte count and serum albumin (Tables 1-4).
No patient under 18-years-old showed elevated ALT levels, and only 1 had less than a 2-fold elevation of AST. All patients under 18 recovered and were discharged. Elevated AST levels were observed in 32 (18.4%) patients with mild disease and 23 (44.20%) with the critical disease, whereas elevated ALT levels were observed in 33 (19%) patients with mild disease and 32 (61.5%) with the critical disease (SupplementaryTable 3).
Univariate analysis revealed several factors associated with survival (age < 60 years; no fever; no dyspnoea; normal findings on chest CT) and several associated with increased mortality (age > 60 years; leucocytosis; increased neutrophil/lymphocyte ratio; elevated AST, CRP, D-dimer or ferritin; baseline renal function impairment with elevations 1–2 above ULN) (Table 5).
| Variable | Outcome | P value | ||||
| Death | Recovery | Total | ||||
| Age | < 18 yr | 0 (0.0) | 20 (100.0) | 20 (100.0) | < 0.001 | |
| 18-60 yr | 25 (6.1) | 384 (93.9) | 409 (100.0) | |||
| > 60 yr | 27 (23.5) | 88 (76.5) | 115 (100.0) | |||
| Gender | Male | 29 (9.7) | 270 (90.3) | 299 (100.0) | 0.94 | |
| Female | 23 (9.4) | 221 (90.6) | 244 (100.0) | |||
| BMI (mean ± SD) | 24.90 ± 2.08 | 28.11 ± 4.9 | 0.36 | |||
| Cigarette smoking | 2 (9.5) | 19 (90.5) | 21 (100.0) | 0.64 | ||
| Diabetes mellitus | 17 (12.6) | 118 (87.4) | 135 (100.0) | 0.17 | ||
| Hypertension | 17 (13.2) | 112 (86.8) | 129 (100.0) | 0.11 | ||
| Pulmonary diseases | 2 (40.0) | 3 (60.0) | 5 (100.0) | 0.07 | ||
| Symptoms | Asymptomatic | 1 (0.9) | 116 (99.1) | 117 (100.0) | < 0.001 | |
| Respiratory | 47 (12.8) | 320 (87.2) | 367 (100.0) | 0.17 | ||
| GIT | 4 (6.7) | 56 (93.3) | 60 (100.0) | |||
| Fever | 43 (14.5) | 254 (85.5) | 297 (100.0) | < 0.001 | ||
| Headache | 16 (10.3) | 140 (89.7) | 156 (100.0) | 0.73 | ||
| Dry cough | 40 (14.0) | 245 (86.0) | 285 (100.0) | < 0.001 | ||
| Dyspnea | 38 (21.5) | 139 (78.5) | 177 (100.0) | < 0.001 | ||
| Sore throat | 2 (2.3) | 86 (97.7) | 88 (100.0) | 0.01 | ||
| Diarrhoea | 2 (3.8) | 50 (96.2) | 52 (100.0) | 0.21 | ||
| Steroids | 18 (14.2) | 109 (85.8) | 127 (100.0) | 0.04 | ||
| Lactoferrin | 1 (2.2) | 45 (97.8) | 46 (100.0) | 0.11 | ||
| Hydroxy-chloroquine | 12 (8.8) | 124 (91.2) | 136 (100.0) | 0.74 | ||
| Chloroquine sulphate | 26 (8.3) | 287 (91.7) | 313 (100.0) | 0.25 | ||
| Vitamin C | 22 (6.5) | 318 (93.5) | 340 (100.0) | 0.002 | ||
| Azithromycin | 5 (4.1) | 117 (95.9) | 122 (100.0) | 0.02 | ||
| Other antibiotic | 21 (10.9) | 171 (89.1) | 192 (100.0) | 0.42 | ||
| Subcutaneous heparin | 43 (19.4) | 179 (80.6) | 222 (100.0) | < 0.001 | ||
| Oral anticoagulants | 0 (0.0) | 33 (100.0) | 33 (100.0) | 0.06 | ||
| COVID-19 disease classification | Mild | 1 (0.4) | 247 (99.6) | 248 (100.0) | < 0.001 | |
| Moderate | 7 (4.0) | 169 (96.0) | 176 (100.0) | |||
| Severe | 10 (17.2) | 48 (82.8) | 58 (100.0) | |||
| Critical | 34 (55.7) | 27 (44.3) | 61 (100.0) | |||
| CT chest | Normal | 4 (2.0) | 194 (98.0) | 198 (100.0) | < 0.001 | |
| Abnormal | 48 (14.2) | 290 (85.8) | 338 (100.0) | |||
| Lug consolidation | 29 (42.6) | 39 (57.4) | 68 (100.0) | < 0.001 | ||
| ICU admission | 45 (37.2) | 76 (62.8) | 121 (100.0) | < 0.001 | ||
| Fibrosis 4-score | < 1.45 | 17 (6.1) | 263 (93.9) | 280 (100.0) | < 0.001 | |
| 1.45:3.25 | 16 (15.1) | 90 (84.9) | 106 (100.0) | |||
| > 3.25 | 14 (38.9) | 22 (61.1) | 36 (100.0) | |||
| Creatinine | Normal | 35 (7.3) | 445 (92.7) | 480 (100.0) | < 0.001 | |
| 1-2 UNL | 14 (25.9) | 40 (74.1) | 54 (100.0) | |||
| 2-3 UNL | 1 (50.0) | 1 (50.0) | 2 (100.0) | |||
| > 3UNL | 2 (28.6) | 5 (71.4) | 7 (100.0) | |||
| Urea | Normal | 24 (7.5) | 295 (92.5) | 319 (100.0) | < 0.001 | |
| 1-2 UNL | 18 (26.5) | 50 (73.5) | 68 (100.0) | |||
| 2-3 UNL | 3 (37.5) | 5 (62.5) | 8 (100.0) | |||
| > 3UNL | 4 (44.4) | 5 (55.6) | 9 (100.0) | |||
| Estimated-glomerular filtration rate | < 15 | 2 (28.6) | 5 (71.4) | 7 (100.0) | < 0.001 | |
| 15:29 | 4 (50.0) | 4 (50.0) | 8 (100.0) | |||
| 30:44 | 4 (22.2) | 14 (77.8) | 18 (100.0) | |||
| 45:59 | 10 (18.2) | 45 (81.8) | 55 (100.0) | |||
| 60:89 | 17 (8.6) | 181 (91.4) | 198 (100.0) | |||
| ≥ 90 | 15 (5.8) | 243 (94.2) | 258 (100.0) | |||
| Hemoglobin (gm/L)1 | 12.35 (11.28:13.70) | 12.60 (12.00: 13.90) | 12.60 (12.00:13.90) | 0.23 | ||
| white blood cell count (× 109)1 | 8.95 (5.08:13.68) | 5.00 (4.30:6.90) | 5.00 (4.40:7.60) | < 0.001 | ||
| Neutrophils/lymphocytes ratio | 9.85 (5.14:14.85) | 2.71 (1.56:5.14) | 3.11 (1.56:6.16) | 0.003 | ||
| Platelet count1 | 201.50 (182.25:278.50) | 210.00 (187.00:271.50) | 208.00 (184.00:272.00) | 0.84 | ||
| AST1 | 43.00 (28.00:67.00) | 26.00 (18.00:40.00) | 28.00 (19.00:43.00) | < 0.001 | ||
| ALT1 | 31.00 (18.00:51.00) | 26.70 (18.00:45.00) | 27.00 (18.00:46.00) | 0.35 | ||
| Alkaline phosphatase1 | 152.00 (49.00) | 89.00 (71.50:114.00) | 89.00 (71.00:114.50) | 0.37 | ||
| Total bilirubin1 | 0.75 (0.70:0.80) | 0.90 (0.60:1.20) | 0.80 (0.60 :1.13) | 0.38 | ||
| Serum albumin1 | 4.00 (2.60) | 4.20 (3.80:4.50) | 4.20 (3.80:4.50) | 0.36 | ||
| D-dimer1 | 0.89 (0.40:1.96) | 0.56 (0.40:1.05) | 0.58 (0.40:1.10) | 0.01 | ||
| Ferritin1 | 429.40 (200.00:957.00) | 269.00 (125.80:550.00) | 300.00 (132.80:590.00) | 0.001 | ||
| C-reactive protein1 | 60.60 (32.00:64.00) | 44.90 (7.00:64.00) | 45.00 (8.25:64.00) | 0.02 | ||
Patients were followed up till discharge or death. On discharge, patients showed significant improvement in haemoglobin, gamma-glutamyl transferase, serum albumin, ferritin, CRP, and lactate dehydrogenase levels with mild elevations in ALT and AST levels (Supplementary Table 4).
Multiple stepwise logistic regression analyses were conducted to identify significant predictors for outcomes of death and recovery. Independent variables included age, asymptomatic status, fever, dry cough, dyspnoea, sore throat, steroid use, supplementary vitamin C intake, azithromycin intake, subcutaneous heparin, chest CT findings, ICU admission, FIB-4 score, creatinine level, and urea level. The model was significant [χ2 (144)], and the P-value was < 0.001, meaning the independent variable could explain the change in the dependent variable by up to 90.8%. Significant predictors in our model included treatment with supplementary vitamin C (1 g daily capsules according to the Egyptian Ministry of Health protocol[13]) [odds ratio (OR): 0.05, 95% confidence interval (CI): 0.008–0.337]; lung consolidation (OR: 4.540, 95%CI: 1.155–17.840); ICU admission (OR: 25.032, 95%CI: 7.110–88.128), and FIB-4 score > 3.25 (OR: 10.393, 95%CI: 2.459-43.925). The most significant predictor was ICU admission (Table 6).
| Variable | B | P value | adjusted OR | 95% confidence interval for OR | |
| Lower | Upper | ||||
| Vitamin C intake | -2.960 | 0.002 | 0.05 | 0.008 | 0.337 |
| Lung consolidation | 1.513 | 0.030 | 4.540 | 1.155 | 17.840 |
| ICU admission | 3.220 | < 0.001 | 25.032 | 7.110 | 88.128 |
| FIB-4 score levels | 0.001 | ||||
| FIB-4 index levels (1.45:3.25) | 0.054 | 0.921 | 1.055 | 0.368 | 3.025 |
| Fib-4 index levels (above 3.25) | 2.341 | 0.001 | 10.393 | 2.459 | 43.925 |
| Constant | -4.821 | 0.008 | |||
Within our cohort, 60 (13.98%) patients presented with GI symptoms in addition to respiratory symptoms. They were predominantly females with significantly higher body mass index. Diarrhoea was the most common symptom, affecting 52 (86.67%) patients. Headache and sore throat were more frequent in patients with GI symptoms compared to those without GI symptoms; 83.4% of patients with GI symptoms had non-severe COVID-19 presentations with fewer ICU admissions. There was no difference in FIB-4 scores among patients with and without GI symptoms (Table 7).
| Variable | Respiratory, n = 369 (86.02) | Respiratory and GIT, n = 60 (13.98) | Total | P value | |
| Age | < 18 | 2 (0.5) | 0 (0.0) | 2 (0.5) | 0.12 |
| 18:60 | 271 (73.4) | 51 (85.0) | 322 (75.1) | ||
| > 60 | 96 (26.0) | 9 (15.0) | 105 (24.5) | ||
| Gender | Male | 210 (56.9) | 22 (36.7) | 232 (54.1) | 0.004 |
| Female | 159 (43.1) | 38 (63.3) | 197 (45.9) | ||
| BMI median (IQR) | 25.96 (23.34:31.25) | 29.02 (26.91:33.36) | 27.76 (23.74:32.01) | 0.04 | |
| Cigarette smoking | 15 (18.3) | 6 (18.2) | 21 (18.3) | 1.00 | |
| Diabetes mellitus | 103 (27.9) | 18 (30.0) | 121 (28.2) | 0.74 | |
| Hypertension | 101 (27.4) | 21 (35.0) | 122 (28.4) | 0.22 | |
| Pulmonary diseases | 5 (1.4) | 0 (0.0) | 5 (1.2) | 0.36 | |
| Symptoms | Fever | 255 (69.1) | 44 (73.3) | 299 (69.7) | 0.51 |
| Headache | 124 (33.6) | 34 (56.7) | 158 (36.8) | 0.001 | |
| Dry cough | 252 (68.3) | 35 (58.3) | 287 (66.9) | 0.13 | |
| Dyspnea | 154 (41.7) | 24 (40.0) | 178 (41.5) | 0.80 | |
| Sore throat | 68 (18.4) | 20 (33.3) | 88 (20.5) | 0.008 | |
| Steroids | 101 (27.4) | 24 (40.0) | 125 (29.1) | 0.05 | |
| Lactoferrin | 28 (7.6) | 18 (30.0) | 46 (10.7) | <0.001 | |
| Hydroxy-chloroquine | 101 (27.4) | 31 (51.7) | 132 (30.8) | <0.001 | |
| Chloroquine sulfate | 200 (54.2) | 24 (40.0) | 224 (52.2) | 0.04 | |
| Vitamin C | 201 (54.5) | 50 (83.3) | 251 (58.5) | <0.001 | |
| Azithromycin | 80 (21.7) | 33 (55.0) | 113 (26.3) | <0.001 | |
| Other parenteral antibiotics | 149 (40.4) | 36 (60.0) | 185 (43.1) | 0.004 | |
| Subcutaneous heparin | 186 (50.4) | 22 (36.7) | 208 (48.5) | 0.05 | |
| Oral anticoagulants | 20 (5.4) | 13 (21.7) | 33 (7.7) | <0.001 | |
| COVID-19 disease Classification | Mild | 122 (33.2) | 16 (26.7) | 138 (32.2) | 0.03 |
| Moderate | 137 (37.2) | 34 (56.7) | 171 (40.0) | ||
| Severe | 53 (14.4) | 6 (10.0) | 59 (13.8) | ||
| Critical | 56 (15.2) | 4 (6.7) | 60 (14.0) | ||
| Outcome | Recovery | 320 (87.2) | 56 (93.3) | 376 (88.1) | 0.17 |
| Death | 47(12.8) | 4 (6.7) | 51(11.9) | ||
| CT chest | Normal | 91 (25.0) | 11 (19.3) | 102 (24.2) | 0.35 |
| Abnormal | 273 (75.0) | 46 (80.7) | 319 (75.8) | ||
| Lug consolidation | 61 (17.0) | 4 (7.1) | 65 (15.7) | 0.06 | |
| ICU admission | 111 (30.2) | 10 (16.7) | 121 (28.3) | 0.03 | |
| Fibrosis 4-score | < 1.45 | 202 (64.7) | 34 (64.2) | 236 (64.7) | 0.99 |
| 1.45:3.25 | 81 (26.0) | 14 (26.4) | 95 (26.0) | ||
| > 3.25 | 29 (9.3) | 5 (9.4) | 34 (9.3) | ||
| Creatinine | Normal | 319 (86.4) | 53 (88.3) | 372 (86.7) | 0.89 |
| 1-2 UNL | 41 (11.1) | 7 (11.7) | 48 (11.2) | ||
| 2-3 UNL | 2 (0.5) | 0 (0.0) | 2 (0.5) | ||
| > 3UNL | 7 (1.9) | 0 (0.0) | 7 (1.6) | ||
| Urea | Normal | 226 (75.8) | 46 (85.2) | 272 (77.3) | 0.49 |
| 1-2 UNL | 57 (19.1) | 7 (13.0) | 64 (18.2) | ||
| 2-3 UNL | 7 (2.3) | 1 (1.9) | 8 (2.3) | ||
| > 3UNL | 8 (2.7) | 0 (0.0) | 8 (2.3) | ||
| Estimated-GFR | < 15 | 7 (1.9) | 0 (0.0) | 7 (1.6) | 0.84 |
| 15:29 | 5 (1.4) | 1 (1.7) | 6 (1.4) | ||
| 30:44 | 17 (4.6) | 1 (1.7) | 18 (4.2) | ||
| 45:59 | 42 (11.4) | 8 (13.3) | 50 (11.7) | ||
| 60:89 | 135 (36.6) | 21 (35.0) | 156 (36.4) | ||
| ≥ 90 | 163 (44.2) | 29 (48.3) | 192 (44.8) | ||
| Hemoglobin (gm/L) | 12.80 (12.00:14.00) | 12.05 (11.00:13.35) | 12.60 (12.00:13.90) | 0.02 | |
| White blood cell count (× 109) | 5.30 (4.35:8.40) | 5.00 (3.63:7.08) | 5.00 (4.40:7.60) | 0.12 | |
| Neutrophils/lymphocytes ratio | 3.71 (1.91:6.81) | 4.20 (2.13:6.83) | 3.11 (1.56:6.16) | 0.85 | |
| Platelet count | 213.00 (175.50:282.50) | 225.50 (187.00:278.25) | 208.00 (184.00:272.00) | 0.87 | |
| AST | 29.00 (20.00:44.00) | 26.00 (17.00:45.63) | 28.00 (19.00:43.00) | 0.63 | |
| ALT | 28.00 (19.00:46.00) | 27.00 (18.00:55.50) | 27.00 (18.00:46.00) | 0.96 | |
| Alkaline phosphatase | 86.50 (70.00:112.25) | 99.00 (78.00:117.00) | 89.00 (71.00:114.50) | 0.18 | |
| Total bilirubin | 0.80 (0.55:0.90) | 0.90 (0.65:1.20) | 0.80 (0.60:1.13) | 0.06 | |
| Serum albumin | 4.20 (3.80:4.50) | 4.20 (3.70:4.40) | 4.20 (3.80:4.50) | 0.75 | |
| D-dimer | 0.58 (0.40:1.10) | 0.60 (0.40:1.22) | 0.58 (0.40:1.10) | 0.08 | |
| Ferritin | 317.80 (156.10:615.90) | 350.00 (112.50:723.98) | 300.00 (132.80:590.00) | 0.93 | |
| C-reactive protein | 47.34 (13.81:64.00) | 40.00 (7.00:64.00) | 45.00 (8.25:64.00) | 0.48 | |
This is the first report from Egypt specifically exploring hepatic and GI disturbances in patients with COVID-19. Within our cohort, AST was more frequently elevated (32.10% of cases), compared with ALT (26% of cases). Only 21 (4.91%) and 16 (3.70%) patients, respectively, had a significant liver injury with AST or ALT elevation > 3-fold. The cholestatic pattern of elevated bilirubin > 1.1 mg/dL, elevated ALP >147 U/L, and GGT > 48 U/L was observed in only 8 (1.86%) patients. Our study findings align with those of other studies showing a direct relationship between elevated liver enzymes and COVID-19 disease activity.
Similar to our study, most other studies reveal a predominant pattern of disturbances in liver enzymes in COVID-19 patients, specifically higher AST elevation than ALT elevation[17-19]. A meta-analysis by Wu et al[20] found that low serum albumin and high GGT were the most frequent abnormalities on admission and that ALT elevation occurred most frequently during hospitalization, which they speculate may be due to the inclusion of patients with pre-existing liver disease. In our study, we observed no difference between survivors and non-survivors in serum albumin level. Wu et al[20] and Liu et al[21] found that serum albumin was significantly lower in non-survivors.
Chronic liver disease was reported in 3.29% of our patients. Phipps et al[4] reported that the severity and type of underlying chronic liver disease (e.g., non-alcoholic fatty liver disease and chronic hepatitis B or C) were not significantly associated with hepatic disturbances related to COVID-19, which could be due to the small numbers of those cases with chronic liver diseases in their study.
In our study, AST was significantly higher in non-survivors, but no significant difference was observed in ALT levels between survivors and non-survivors, which is similar to previous meta-analyses[17,18]. Patients with severe COVID-19 and patients admitted to the ICU had higher transaminase levels, as confirmed by previous meta-analyses[17-19,21,22]. Ponziani et al[23] reported that alterations in transaminase levels in patients with COVID-19 were mild to moderate, whereas significant liver injury with transaminases > 3 times the ULN and pure cholestatic injuries occurred in a minority of patients[23]. Phipps et al[4] showed that 6.4% of their patients had a severe liver injury with ALT > 5 times above the ULN. They also found that peak ALT, older age, diabetes mellitus, and intubation were independent predictors of mortality. A meta-analysis by Kulkarni et al[22] found that severe liver injury occurred in 10.7% of 3440 patients with COVID-19: 24.9% among 358 patients with the non-severe disease and 41.5% among 317 patients with severe disease.
Other causes of liver injury in patients with COVID-19 include medication, such as acetaminophen at doses exceeding 7.5–10 g and combined use of antivirals and antibiotics. Systemic effects of SARS-CoV-2 infection can be hepatotoxic. For example, hypoxia due to lung injury and, in severe cases, sepsis, acute respiratory distress syndrome, and multi-organ failure precipitate hypoxia and ischemia of the liver, causing elevated serum ALT, AST, and total bilirubin levels[17].
In this study, baseline FIB-4 was an independent factor affecting mortality, and it was significantly higher in patients admitted to the ICU, those with more severe COVID-19 disease, and non-survivors. FIB-4, which predicts significant hepatic fibrosis in patients with liver disease, comprises four variables: Age, AST, ALT, and platelet count[11]. These factors are deranged in COVID-19 disease due to systemic inflammation, bone marrow suppression, injury of the skeletal muscles, elevated pressure in the right heart system with subsequent liver congestion, and elevated liver stiffness that occurs in the disease[24,25]. Sterling et al[23] showed that gender, diabetes mellitus, and FIB-4 ≥ 2.67 were associated with increased mortality. Another recent study by Li et al[25] found that FIB-4 correlated with mortality and severe COVID-19, noting that FIB-4 normalized in the survivors only. They also found that baseline FIB-4 correlated with SARS-CoV-2 viral load and their studied monocyte/ interferon-I-related cytokines (interleukin-6 and interferon-gamma–induced protein 10)[25]. Rentsch et al[26] and Price-Haywood et al[27] found that FIB-4 scores > 3.25 and ≥ 2.67, respectively, were associated with hospitalization and with ICU admission and mechanical support.
Within our cohort, 60 patients (13.98%) presented with GI symptoms in addition to respiratory symptoms. The prevalence of diarrhoea, nausea, vomiting, and abdominal pain was 9.51%, 2.01%, 3.11%, and 3.84%, respectively. Diarrhoea was the most common GI symptom in our cohort, similar to findings from Wang et al[28].
The prevalence of diarrhoea, nausea/vomiting, and abdominal pain in their systematic review and meta-analysis was 9.1%, 5.2%, and 3.5%, respectively. The predominant GI symptoms differ among countries[28-30]. In China, for example, Luo et al[29] reported anorexia, nausea, and vomiting as the frequent symptoms in two-thirds of their patients, while diarrhoea and abdominal pain presented in 37%and 25% of their patients, respectively. A meta-analysis by Wan et al[30] (included 55 studies from China, 1 from Austria, 1 from the United States, 1 from Spain, and 2 from Singapore) reported the prevalence of GI symptoms as diarrhoea (53 studies, 8604 patients: 11.2%), nausea and vomiting (33 studies, 6165 patients: 10.0%), loss of appetite (15 studies, 2540 patients: 21.3%), and abdominal pain 14 studies, 2203 patients: (4.6%). Another meta-analysis by Parasa et al[31] reported that diarrhoea occurred in 4.3%–12.2%, and nausea, or vomiting occurred in 2.6%–8.0% of their included 4805 patients[31]. A study from the United States showed that GI symptoms were present in 61.3% of their patients (the most common was loss of appetite (34.8%) followed by diarrhoea (33.7%) and nausea (26.4%))[32].
In our study, 83.4% of patients with GI symptoms had non-severe COVID-19. The presence of diarrhoea or other GI symptoms did not impact COVID-19 mortality. Wang et al[28] also found no difference in the prevalence of diarrhoea among severe and non-severe cases, and the presence of GI symptoms did not affect mortality. Nobel et al[33] found that COVID-19 patients with GI symptoms had lower mortality and no difference in ICU admission rates, compared with patients without GI symptoms[33]. In contrast, Zhong et al[8] and Wan et al[30] reported a correlation between diarrhoea and severity of COVID-19, showing that patients with diarrhoea needed more ICU care and ventilator support.
In our cohort, there was no significant difference in transaminases level among patients with and without GI symptoms, which contrasts with a meta-analysis by Wijarnpreecha et al[17] showing higher transaminases in patients with GI symptoms. The results of our study are similar to Zhou et al[34], who found that GI symptoms were more common in females, sore throat was also more common in their patients with GI symptoms, and haemoglobin level was significantly lower in their patients with GI symptoms. In contrast to our study, ALT was higher in their non-medical group of patients with GI symptoms but not in the medical group, and CRP was higher in their patients with GI symptoms. Limitations of our study include inconclusive reports about medications received before hospitalization and the possibility of underlying undiagnosed liver disease, such as non-alcoholic fatty liver disease and unavailability of creatine phosphokinase enzyme results, as it was not tested at the time of the study in the quarantine hospitals affiliated with the Egyptian Ministry of Health.
In conclusion, within our cohort of Egyptian patients with COVID-19, elevated AST, ALT, total bilirubin ,and GGT were present in 32.1%, 26%, 5.8%, and 1.86% of our patients, respectively. Significant liver injury (AST and ALT three times higher than the ULN) affected 4.91% and 3.70% of patients, respectively. Male gender, smoking, hypertension, chronic hepatitis C, and lung involvement were significantly associated with elevated AST and ALT. FIB-4 scores were significantly higher in patients admitted to the ICU, those with more severe COVID-19, and non-survivors. The independent variables affecting outcome were supplementary vitamin C intake, lung consolidation, ICU admission, and FIB-4 score. GI symptoms were significantly more frequent in women, patients with higher body mass index, and those with non-severe COVID-19.
Hepatic and gastrointestinal (GI) disturbances have been reported in patients with coronavirus disease 2019 (COVID-19) with variable prevalence according to disease severity and population characteristics. This could be due to direct severe acute respiratory syndrome coronavirus 2 invasion through the angiotensin-converting enzyme 2 receptors or indirect effects such as an uncontrolled immune response, drug-induced injury, or sepsis.
Comprehensive researches on hepatic and GI derangements in patients with COVID-19 are still lacking, and they are needed for better understanding of the underlying factors, clinical presentations, and disease outcome
We aimed to study the prevalence and severity of liver and GI derangements in Egyptian patients with COVID-19 infection and their relation to disease outcomes.
This multicentre cohort study was conducted on 547 COVID-19 cases from four quarantine hospitals during the period from April 15, 2020 to July 29, 2020. Clinical, laboratory features, fibrosis-4 (FIB-4) index, COVID-19 severity, and outcomes were recorded. Follow-ups were conducted until discharge or death.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were elevated in 26% and 32% of patients while elevations above 3 fold were recorded in 4.91% and 3.73% patients, respectively. Male gender, smoking, hypertension, chronic hepatitis C, and lung involvement were associated with elevated AST or ALT. FIB-4 was significantly higher in patients admitted to the intensive care unit (ICU), those with more severe COVID-19, and non-survivors. The independent variables affecting outcome were supplementary vitamin C intake, lung consolidation, ICU admission, and FIB-4 score > 3.25. GI symptoms were present in 60 (13.98%) patients. They were predominantly females with higher body mass index, and 50 (83.40%) patients had non-severe COVID-19.
Significant liver injury was uncommon among Egyptian patients with COVID-19. The independent variables affecting mortality were supplementary vitamin C intake, lung consolidation, ICU admission, and FIB-4 score.
Variables independently affecting mortality were supplementary vitamin C intake, FIB-4 score > 3.25, lung consolidation, and ICU admission. GI symptoms occurred in patients with non-severe COVID-19.
| 1. | Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 285] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 4. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (3)] |
| 5. | Ghoda A, Ghoda M. Liver Injury in COVID-19 Infection: A Systematic Review. Cureus. 2020;12:e9487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 364] [Article Influence: 60.7] [Reference Citation Analysis (4)] |
| 7. | Suresh Kumar VC, Harne PS, Mukherjee S, Gupta K, Masood U, Sharma AV, Lamichhane J, Dhamoon AS, Sapkota B. Transaminitis is an indicator of mortality in patients with COVID-19: A retrospective cohort study. World J Hepatol. 2020;12:619-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 8. | Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 9. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010-1018. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 385] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 10. | Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 11. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3806] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 12. | Frater JL, Zini G, d'Onofrio G, Rogers HJ. COVID-19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42 Suppl 1:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Ministry of Health and Population, Egypt Management protocol for COVID-19 Patients. [cited 30 May 2020]. Available from: https://www.elwatannews.com/data/iframe/pdf/17175200761591035127.pdf. |
| 14. | Chan YH. Biostatistics 102: quantitative data--parametric & non-parametric tests. Singapore Med J. 2003;44:391-396. [PubMed] |
| 15. | Chan YH. Biostatistics 103: qualitative data - tests of independence. Singapore Med J. 2003;44:498-503. [PubMed] |
| 16. | Chan YH. Biostatistics 202: logistic regression analysis. Singapore Med J. 2004;45:149-153. [PubMed] |
| 17. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Xing QQ, Dong X, Ren YD, Chen WM, Zeng DY, Cai YY, Hong MZ, Pan JS. Liver Chemistries in Patients with COVID-19 Who Discharged alive or Died: A Meta-analysis. Hepatol Commun. 2020;5:12-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Liu C, Yang J, Wang W, Zheng P, Tang Y. Liver injury could be associated with severe disease in COVID-19 patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 23. | Ponziani FR, Del Zompo F, Nesci A, Santopaolo F, Ianiro G, Pompili M, Gasbarrini A; “Gemelli against COVID-19” group. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Sterling RK, Oakes T, Gal TS, Stevens MP, deWit M, Sanyal AJ. The Fibrosis-4 Index Is Associated With Need for Mechanical Ventilation and 30-Day Mortality in Patients Admitted With Coronavirus Disease 2019. J Infect Dis. 2020;222:1794-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Li Y, Regan J, Fajnzylber J, Coxen K, Corry H, Wong C, Rosenthal A, Atyeo C, Fischinger S, Gillespie E, Chishti R, Baden L, Yu XG, Alter G, Kim A, Li JZ. Liver Fibrosis Index FIB-4 Is Associated With Mortality in COVID-19. Hepatol Commun. 2020;5:434-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, Hauser RG, Schultze A, Jarvis CI, Holodniy M, Lo Re V, Akgun KM, Crothers K, Taddei TH, Freiberg MS, Justice AC. Covid-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54-75 Years. 2020 Preprint. Available from: medRxiv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 27. | Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1214] [Cited by in RCA: 1266] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 28. | Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44:653-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 30. | Wan J, Wang X, Su S, Zhang Y, Jin Y, Shi Y, Wu K, Liang J. Digestive symptoms and liver injury in patients with coronavirus disease 2019 (COVID-19): A systematic review with meta-analysis. JGH Open. 2020;4:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 32. | Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020; 159: 765-767. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 33. | Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology 2020; 159: 373-375. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 34. | Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvatore S S-Editor: Liu M L-Editor: Filipodia P-Editor: Li JH