Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6939
Peer-review started: July 20, 2021
First decision: August 19, 2021
Revised: September 1, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: October 28, 2021
Processing time: 98 Days and 21.7 Hours
When Echinococcus multilocularis infects humans as a false intermediate host, alveolar echinococcosis (AE) usually manifests primarily intrahepatically and is initially asymptomatic. If the disease remains undiagnosed and untreated, progressive growth occurs, reminiscent of malignant tumours. The only curative therapy is complete resection, which is limited to localised stages, and palliative drug therapy is used otherwise. Consequently, early diagnosis and reliable detec
To investigate how hepatic AE lesion sonomorphology changes over time in the Echinococcosis Multilocularis Ulm Classification (EMUC)-ultrasound (US) classification.
Based on data from Germany’s national echinococcosis database, we evaluated clinical and US imaging data for 59 patients according to the AE case definition in our preliminary retrospective longitudinal study. There had to be at least two liver sonographies ≥ 6 mo apart, ≥ 1 hepatic AE lesion, and complete documen
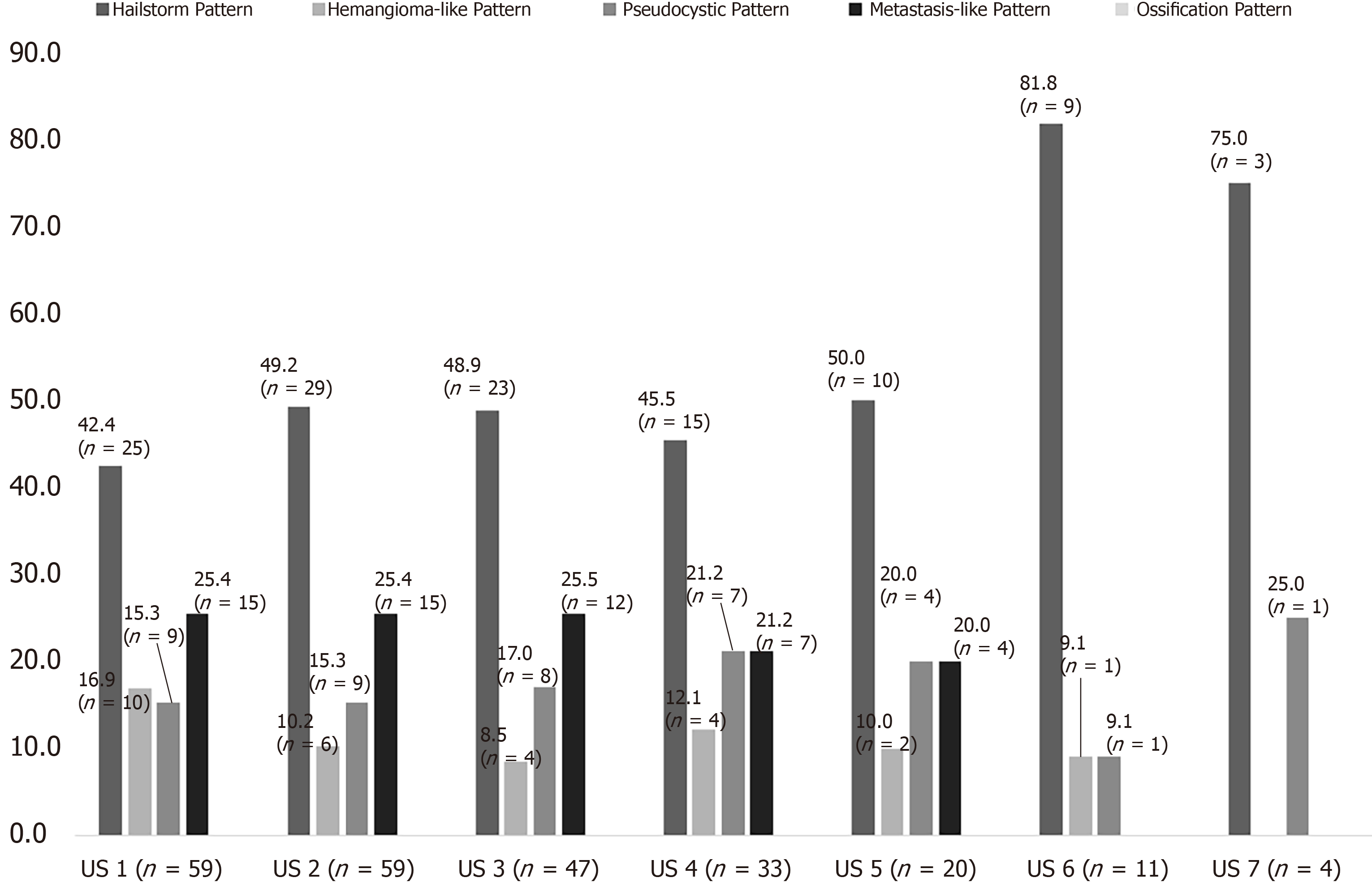
The preliminary study included 59 patients, 38 (64.5%) women and 21 (35.6%) men. The mean age at initial diagnosis was 59.9 ± 16.9 years. At the time of initial ultrasonography, a hailstorm pattern was present in 42.4% (25/59) of cases, a hemangioma-like pattern in 16.9% (10/59), a pseudocystic pattern in 15.3% (9/59), and a metastasis-like pattern in 25.4% (15/59). For the hailstorm pattern, the average lesion size was 67.4 ± 26.3 mm. The average lesion size was 113.7 ± 40.8 mm with the pseudocystic pattern and 83.5 ± 27.3 mm with the hemangioma-like pattern. An average lesion size of 21.7 ± 11.0 mm was determined for the metastasis-like pattern. Although the sonomorphologic pattern remained unchanged in 84.7% (50/59) of AE reference lesions, 15.3% (9/59) showed a change over time. A change in pattern was seen exclusively for AE lesions initially classified as hemangioma-like or pseudocystic. A total of 70% (7/10) of AE lesions initially classified as hemangioma-like showed a relevant change in pattern over time, and 85.7% (6/7) of these were secondarily classified as having a hailstorm pattern, with the remainder (1/7; 14.3%) classified as having a pseudocystic pattern. A total of 22.2% (2/9) of AE lesions initially classified as pseudocystic showed a relevant change in pattern over time and were classified as having a hailstorm pattern. For AE lesions initially classified as having a hailstorm or metastatic pattern, no pattern change was evident. All patients with pattern change were on continuous drug therapy with albendazole.
The sonomorphology of hepatic AE lesions may change over time. The heman
Core Tip: Alveolar echinococcosis is potentially fatal. In approximately 98% of cases, it manifests in the liver, similar to a primary malignant or metastatic tumour. The sonomorphological appearance of the disease is varied and easily confused with other differential diagnoses. Sonography is the most important tool in diagnostics, but how the known patterns change over time is unclear. The evidence that certain sonographic patterns in particular change over time shows a possible evolutionary approach to the disease and may, in the long term, make lifelong drug therapy unnecessary in non-operable patients when non-active stages can be clearly identified.
- Citation: Schuhbaur J, Schweizer M, Philipp J, Schmidberger J, Schlingeloff P, Kratzer W. Long-term follow-up of liver alveolar echinococcosis using echinococcosis multilocularis ultrasound classification. World J Gastroenterol 2021; 27(40): 6939-6950
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6939
Fox tapeworm disease (alveolar echinococcosis, AE) is a zoonotic disease caused by the larval stage of the cestode Echinococcus multilocularis, in which humans act as the major false intermediate host[1,2]. After a host accidentally ingests infectious worm eggs, the eggs penetrate the wall of the small intestine and usually first reach the liver, the most common primary site of AE in humans, Via the enterohepatic circulation[1,3,4].
AE is primarily asymptomatic and progressive in the therapy-naive state[5]. Only as the disease progresses do patients develop nonspecific symptoms, including abdominal tenderness or pain, jaundice, unwanted weight loss, and diffuse deterioration of general condition[6,7]. In Europe, the diagnosis of AE is usually made as an incidental finding in asymptomatic stages, and in China, diagnosis frequently is made in the setting of locally advanced growth with a complicated clinical course. Radical surgical resection of an AE lesion is considered the only potentially curative therapeutic option, followed by anthelmintic therapy for 2 years[8]. In case of an unre
The high rate of advanced AE foci and inoperable disease at diagnosis underscore the need for early, definitive diagnosis. In clinical practice, B-scan ultrasonography is the most common primary imaging modality for evaluating nonspecific abdominal complaints and for the differential diagnostic assessment of hepatic incidentalomas[9].
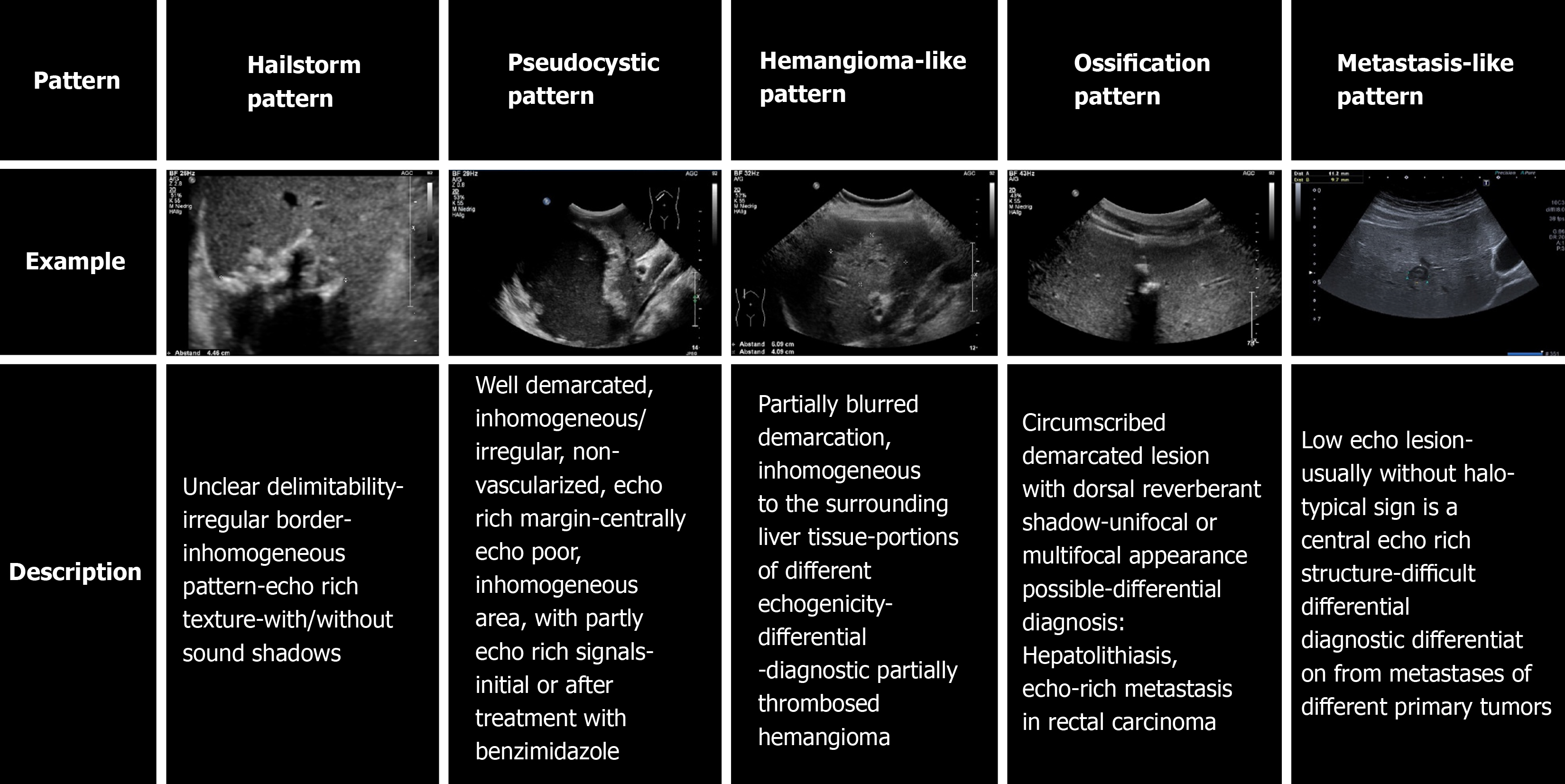
The sonomorphology of hepatic AE foci is heterogeneous. The Echinococcosis Multilocularis Ulm Classification (EMUC)-ultrasound (US) classification, published in 2015, describes five sonomorphologic appearances of hepatic AE lesions and provides important guidance for their recognition and follow-up[10]. Five patterns are distinguished: hailstorm, hemangioma-like, pseudocystic, metastasis-like, and ossification. Conventional cross-sectional imaging [computed tomography (CT), magnetic resonance imaging (MRI)], with the appropriate classification schemes, is particularly useful for assessing spatial spread, potential infiltration of adjacent organs, and extrahepatic manifestations[11]. The gold standard for estimating parasitic activity is 18FDG positron emission tomography (PET)[12,13]. Contrast-enhanced sonography of the liver also plays an increasing role in the evaluation of hepatic AE[14-16]. Available studies and analyses on the sonomorphology of AE have largely been cross-sectional, and we are not aware of any longitudinal studies assessing sonomorphology and a classification scheme for individual AE lesions.
The aim of our preliminary study is to investigate whether a change in pattern and size over time can be identified in patients with hepatic AE during follow-up, using the EMUC-US classification.
The study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki (ref. No. 166/13). Because of its retrospective design and pseudonymised evaluation of imaging, no ethics approval was necessary. All data were analysed anonymously.
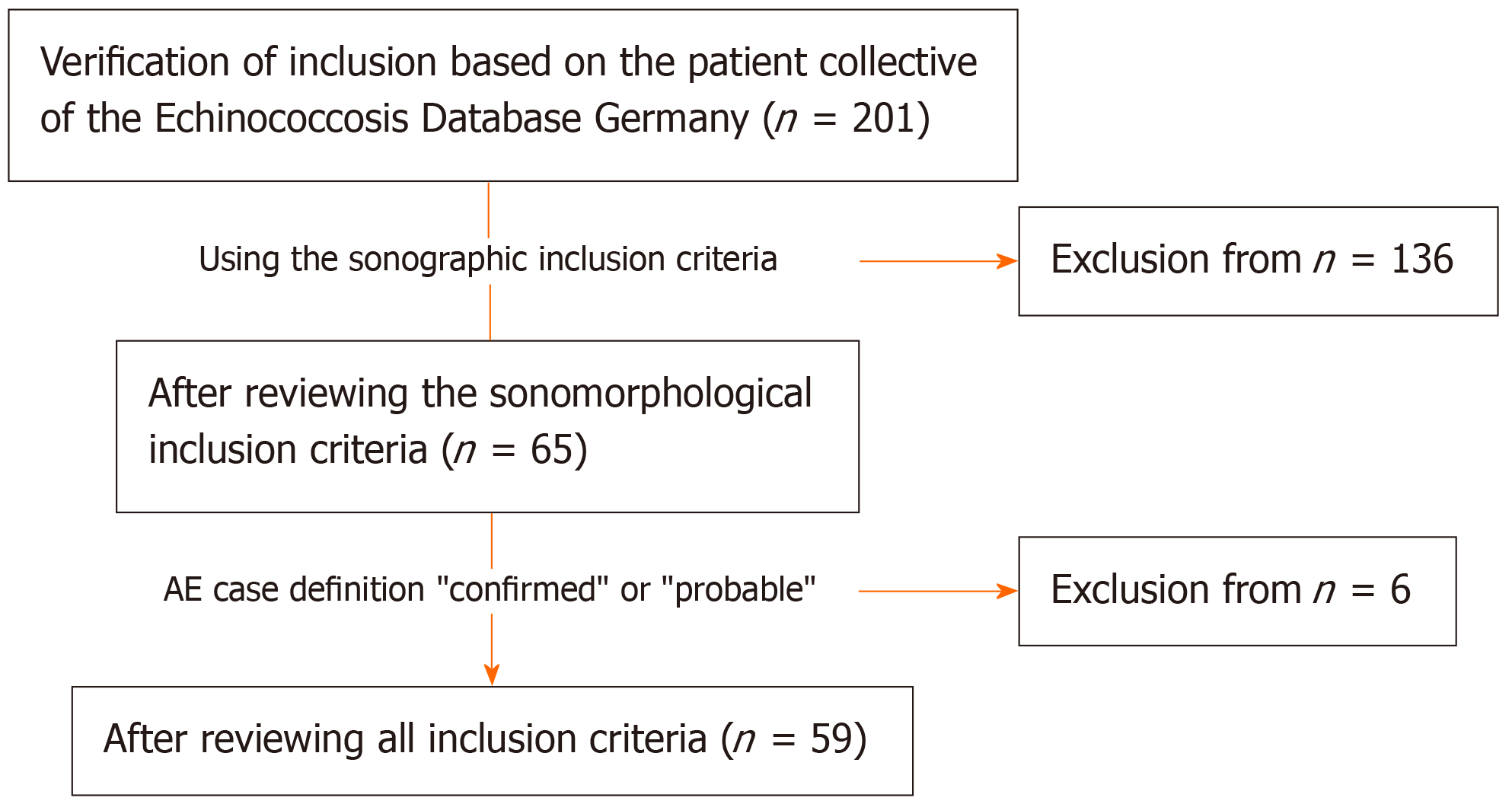
For our preliminary retrospective evaluation, we used the clinical and imaging data of the national Echinococcosis Database in Germany. A main prerequisite for inclusion was confirmed or probable AE disease according to the AE case definition “confirmed” or “probable”[5]. Other inclusion criteria were at least two liver sonographies performed over a period of at least 6 mo, the presence of at least one hepatic AE lesion at the time of US 1, and complete documentation of the AE lesion defined as the reference lesion at all further observation time points. In total, 59 patients with data included in the Echinococcosis Database Germany were included (Figure 1 and Table 1).
| n (%) | mean ± SD | |
| Number of patients | 59 (100.0) | |
| Number of sonographies | 244 (100.0) | |
| Sex | ||
| Female | 38 (64.5) | |
| Male | 21 (35.6) | |
| AE case definition | ||
| Confirmed | 26 (44.1) | |
| Probable | 33 (55.9) | |
| Age at first diagnosis (yr) | 59.9 ± 16.9 | |
| Age at initial ultrasound (yr) | 60.4 ± 16.8 | |
| Localisation of the reference lesion | ||
| Right hepatic | 34 (57.6) | |
| Left hepatic | 19 (32.3) | |
| Bihepatic | 6 (10.2) | |
| Number of AE lesions on US 1 | ||
| 1 | 29 (49.2) | |
| 2-5 | 28 (47.5) | |
| 6-10 | 1 (1.7) | |
| > 10 | 1 (1.7) | |
| Lesion size according to EMUC-US pattern (mm) | 65.6 ± 39.7 | |
| Hailstorm pattern | 67.4 ± 26.3 | |
| Pseudohemangioma-like pattern | 83.5 ± 27.3 | |
| Pseudocystic pattern | 113.7 ± 40.8 | |
| Metastasis-like pattern | 21.7 ± 11.0 | |
| Drug therapy | ||
| Initial albendazole | 58 (98.3) | |
| Initial mebendazole | 0 (0) | |
| Therapy change to mebendazole | 2 (3.4) | |
| Continuous therapy | 50 (86.2) | |
| Discontinuous therapy | 8 (13.8) | |
| No therapy (rejection by patients) | 1 (1.7) |
Initially, a hepatic AE reference lesion was defined per patient; this was measured by the largest diameter (in mm) at the time of the first US examination (US 1). At each time point, the number of all hepatic AE lesions in each patient was documented. The reference lesion was evaluated according to its maximum diameter and its EMUC-US pattern classification[10]. In addition to the AE case definition (“confirmed”, “pro
All US examinations were performed using convex probes (C5-1 MHz, C9-1 MHz) with state-of-the-art US equipment (Aplio 500 Toshiba, Siemens S3000, Philips Epiqu 7, Philips IU22). The sonomorphology of the hepatic AE reference lesion was assigned to a pattern according to EMUC-US in each examination[10]. At each examination time point, three different examiners performed a pattern assignment of the reference lesion. The pattern assignment of examiner 1 was taken from the written documentation as the initial examiner. Investigators 2 and 3 performed follow-up of all US images relevant to the study in all included patients. The physicians who performed the follow-up were blinded to the initial examination and independently performed an EMUC-US assessment (Figure 2).
We performed statistical analyses using SAS Version 9.4 (SAS Institute Inc., Cary, NC, United Stated). Descriptive analysis of the data was performed to obtain absolute and relative frequencies, as well as measures of central tendency and dispersion. Pearson's χ2 and exact fisher tests were used to determine possible relationships and differences in the frequency distribution between dichotomous variables. Wilcoxon rank sum test was performed to determine differences between variables without a normal distribution. All tests were performed two-sided. The level of significance was set at α = 0.05, and a P < 0.05 was considered to be statistically significant with a 5% probability of error.
The statistical methods of this study were reviewed by Dr. Schmidberger J, MPH, Ph.D., from the Department of Internal Medicine I, University Hospital Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany.
The study included 59 patients, including 38 (64.5%) women and 21 (35.6%) men. The mean age at initial diagnosis was 59.9 ± 16.9 years, with a mean age at first sonographic examination of 60.4 ± 16.8 years. A World Health Organization case definition of “confirmed” was present in 26 (44.1%) patients, and a case definition of “probable” was present in 33 (55.9%). The 59 included patients underwent a total of 244 US examinations for a mean of 4.1 sonographic examinations per patient (2-15 ± 2.1 US examinations). A total of 58/59 (98.3%) received initial benzimidazole therapy using albendazole (Tables 1 and 2).
| Number of AE lesions US 1 | n (%) | P value | ||
| Hailstorm pattern (n = 25) | ||||
| 1 | 15 (60) | Reference | 1.0000 | 0.4385 |
| > 1 | 10 (40) | |||
| 2-5 | 9 (36) | - | ||
| 6-10 | 1 (4) | |||
| > 10 | 0 (0) | |||
| Pseudohemangioma-like pattern (n = 10) | ||||
| 1 | 6 (60) | 1.0000 | Reference | 0.6284 |
| > 1 | 4 (40) | |||
| 2-5 | 4 (40) | - | ||
| 6-10 | 0 (0) | |||
| > 10 | 0 (0) | |||
| Pseudocystic pattern (n = 9) | ||||
| 1 | 7 (77.8) | 0.4385 | 0.6284 | Reference |
| > 1 | 2 (22.2) | |||
| 2-5 | 2 (22.2) | - | ||
| 6-10 | 0 (0) | |||
| > 10 | 0 (0) | |||
| Metastasis-like pattern (n = 15) | ||||
| 1 | 1 (6.7) | 0.0009 | 0.0068 | 0.0007 |
| > 1 | 14 (93.4) | |||
| 2-5 | 13 (86.7) | - | ||
| 6-10 | 0 (0) | |||
| > 10 | 1 (6.7) | |||
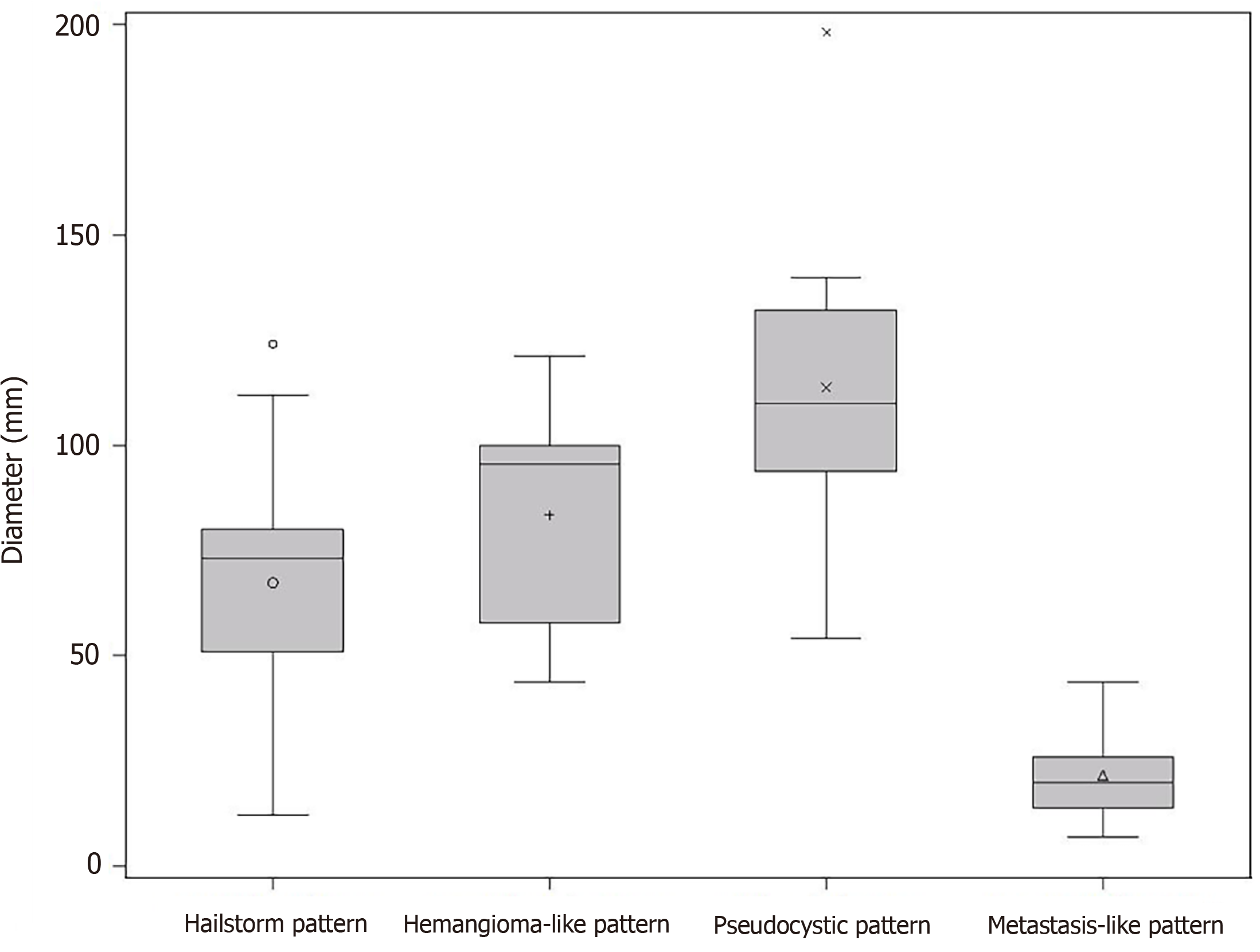
At the time of initial US, 42.4% (25/59) corresponded to the hailstorm pattern, 16.9% (10/59) to the hemangioma-like pattern, 15.3% (9/59) to the pseudocystic pattern, and 25.4% (15/59) to the metastasis-like pattern. None of the reference lesions were initially assigned to the ossification pattern. For those with the hailstorm pattern, the mean lesion size was 67.4 ± 26.3 mm. The average lesion size with the pseudocystic pattern was 113.7 ± 40.8 mm, and that of the hemangioma-like pattern was 83.5 ± 27.3 mm. An average lesion size of 21.7 ± 11.0 mm was determined for the metastasis-like pattern (Figure 3).
In 34/59 (57.6%) of patients, the reference echinococcosis-associated lesion was right hepatic, and in 19/59 (32.3%), it was left hepatic. Bihepatic involvement was identified in 6/59 (10.2%).
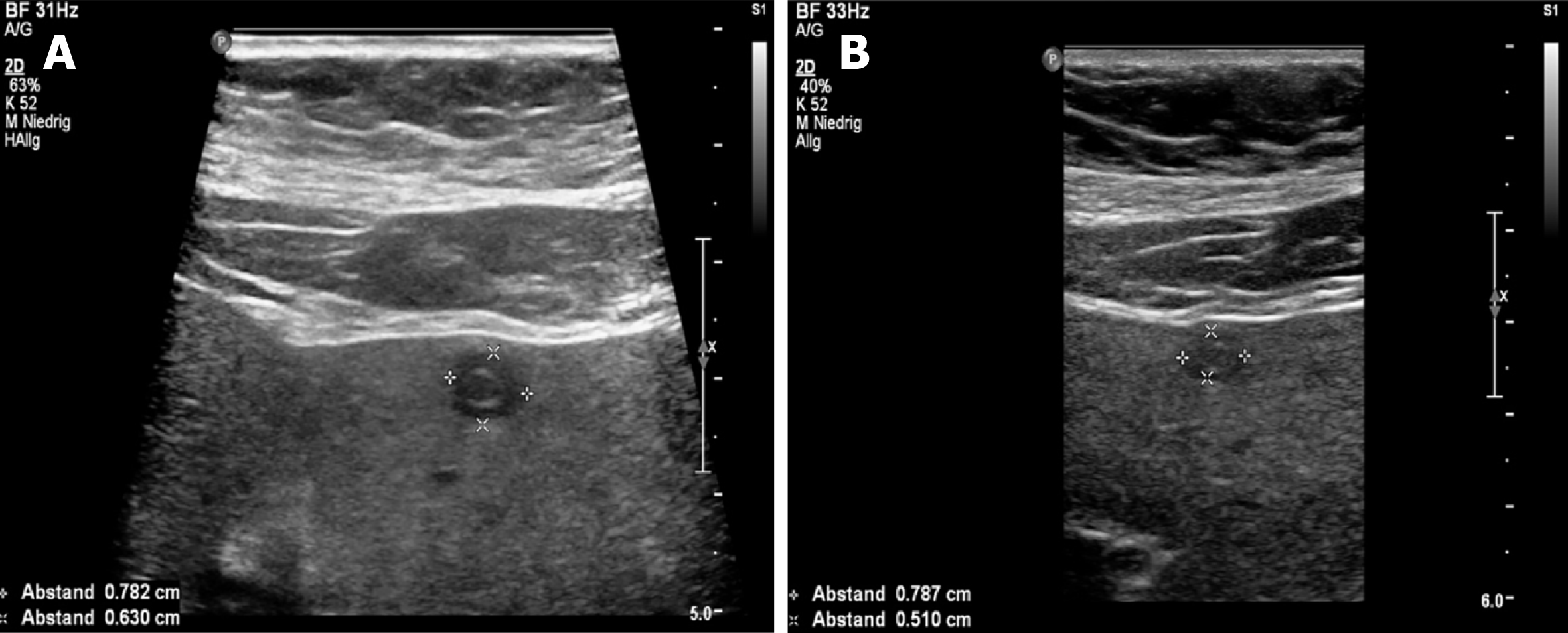
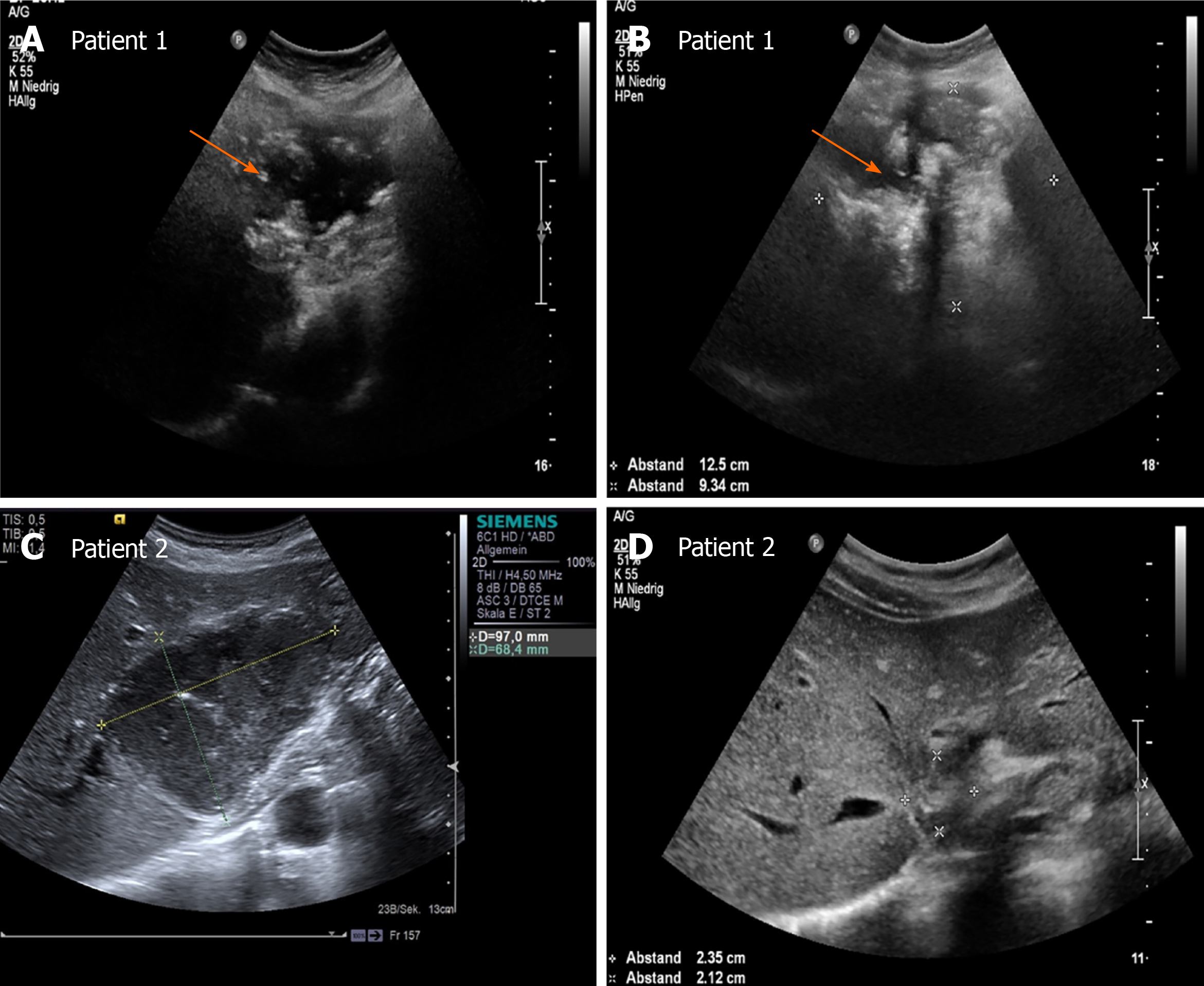
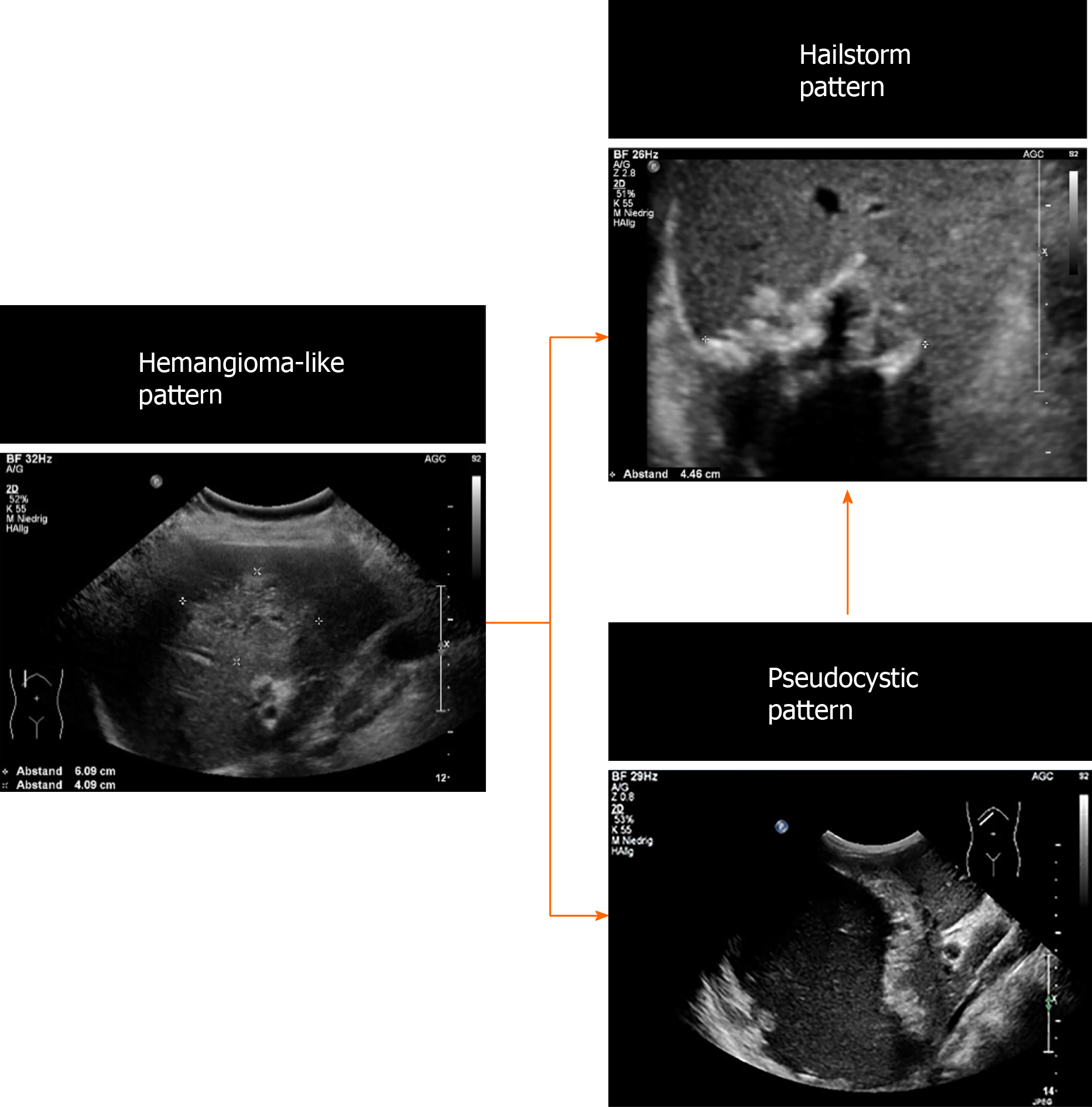
Over the whole observation period, the predominant pattern of hepatic AE reference lesions according to EMUC-US remained unchanged in 50/59 (84.7%). In 9/59 (15.3%) of the findings, however, a change in sonomorphology was observed. Of the 10 reference lesions initially classified as hemangioma-like, 7/10 (70.0%) showed a relevant change in sonomorphologic criteria. Of these, 6/7 (85.7%) were assigned to the hailstorm pattern in subsequent US examinations, and 1/7 (14.3%) showed a change toward the pseudocystic pattern. Lesions initially classified as having a pseudocystic pattern showed a change to a hailstorm pattern over time in 2/9 (22.2%) of cases. In all cases of a change in the pattern of AE reference lesions over time, the patients had taken albendazole continuously through the observation period (Figures 4 and 5). For the metastatic pattern, there were no pattern changes over time, but an increase in the size of the central echo-rich laminar body was observed (Figure 6). The mean time from initial diagnosis of AE to a pattern change was 24.2 ± 35.6 mo.
The study further revealed statistically significant differences in the number of hepatic AE lesions among EMUC-US types (P < 0.05) (Table 2). There were further statistically significant differences in lesion size between the metastatic pattern compared to the hailstorm pattern (21.7 ± 11.0 vs 67.4 ± 26.3; P < 0.0001), the pseudocystic pattern (21.7 ± 11.0 vs 113.7 ± 40.8; P < 0.0001), and the hemangioma-like pattern (21.7 ± 11.0 vs 83.5 ± 27.3; P < 0.0001) (Table 3). In the 9/59 (15.3%) cases with morphologic change in the EMUC-US pattern, there was a nonsignificant size regrowth from the initial examination (88.8 ± 30.6 vs 71.9 ± 29.6; P = 0.1641). For the 7/9 (77.8%) cases initially classified as having a hemangioma-like pattern, the mean average lesion size was 81.4 ± 29.4 mm initially vs 72.4 ± 21.4 mm after the pattern change. For the 2/9 (22.2%) cases initially classified as having a pseudocystic pattern, the mean lesion size at initial examination was 114.5 ± 24.8 mm vs 70.0 ± 65.1 mm after the pattern change.
| mean ± SD, min-max | P value | ||
| EMUC-US | Hailstorm pattern (n = 25) | ||
| Pseudohemangioma-like pattern (n = 10) | 83.5 ± 27.3, 44-121 | 67.4 ± 26.3, 12-124 | 0.1491 |
| Pseudocystic pattern (n = 9) | 113.7 ± 40.8, 54-198 | 67.4 ± 26.3, 12-124 | 0.0017 |
| Metastasis-like pattern (n = 15) | 21.7 ± 11.0, 7-44 | 67.4 ± 26.3, 12-124 | < 0.0001 |
| EMUC-US | Pseudohemangioma-like pattern (n = 10) | ||
| Pseudocystic pattern (n = 9) | 113.7 ± 40.8, 54-198 | 83.5 ± 27.3, 44-121 | 0.1304 |
| Metastasis-like pattern (n = 15) | 21.7 ± 11.0, 7-44 | 83.5 ± 27.3, 44-121 | < 0.0001 |
| EMUC-US | Pseudocystic pattern (n = 9) | ||
| Metastasis-like pattern (n = 15) | 21.7 ± 11.0, 7-44 | 113.7 ± 40.8, 54-198 | < 0.0001 |
Our work is the first preliminary longitudinal study tracking changes in sonomorphology during the follow-up of hepatic AE lesions using a sonomorphologic classification scheme. We found that the sonographic characteristics of individual subtypes of hepatic AE lesions change over time under anthelmintic therapy and exhibit size regrowth, resulting in a pattern classification that diverges from that assigned at the initial examination. A change in sonomorphology leading to pattern reclassification was seen for the hemangioma-like and pseudocystic patterns. A total of 70% of the reference lesions initially classified as hemangioma-like shifted to a different pattern classification. Of these, 85% were assigned to the hailstorm pattern and 15% to the pseudocystic pattern. A total of 22.2% (2/9) of the hepatic AE lesions initially described as pseudocystic were assigned to the hailstorm pattern during follow-up (Figures 5 and 7).
In agreement with our results, a Polish working group using the EMUC-US classification confirmed the hailstorm pattern as the most frequent[14]. However, a correlation between the sonographic EMUC-US patterns and the PNM (P = parasitic mass in the liver, N = involvement of neighbouring organs, and M = metastasis) scheme could not be demonstrated in this retrospective work[16]. In addition to the EMUC-US scheme, classification schemes are currently available for CT and MRI[11]. In a recent 39.8-mo follow-up study of our working group 72 patients on albendazole therapy with hepatic AE using the EMUC-CT classification, only one case had a pattern change reassignment from type IIIa (primarily cystoid) to type V (calcified). This result clearly contrasts with our current findings. To compare the different patterns of hepatic AE between the different imaging modalities, appropriate prospective comparative studies are necessary and as yet are unavailable. However, in a further retrospective study we compared EMUC-US and EMUC-CT, no clear assignment could be found for the sonographic hemangioma-like pattern in the EMUC-CT classification. In that study, the sonographic pseudocystic pattern could be predominantly classified as type I (diffuse-infiltrating) of the EMUC-CT classification. A study comparing MRI and PET-CT demonstrated an association between positron emission activity of hepatic AE lesions and microcysts[17]. A correlation between increased PET activity and calcifications could not be shown in this work[17]. This result contrasts with those of others results of our group who reported increased PET activity depending in particular on microcalcifications. The hailstorm pattern has significantly more calcifications than the hemangioma-like pattern, so these findings offer support for our hypothesis that a shift from hemangioma-like to hailstorm may indicate disease progression. In a recent retrospective study using the EMUC-CT classification in comparison to histology, a possible disease progression could be postulated for EMUC-CT patterns I-III[18]. In further confirmation of our hypothesis that the hemangioma-like pattern may represent an earlier disease stage, Zeng et al[19], using contrast-enhanced sonography with a four-type classification, visualised echo-rich masses corresponding to the EMUC-US hemangioma-like pattern.
The development of different manifestation patterns does not seem to be exclusively an expression of the temporal course of the disease, however, and appears to be significantly related to the immunological and constitutional state of the host/ misintermediate host (patient)[20]. In addition to the intraindividual immune response of the patient, one of the major difficulties in performing longitudinal imaging morphological studies is the apparent slow growth of the parasite with corresponding morphological changes in the liver. The use of international prospective database analysis studies could address this problem in the long term.
The retrospective design of the preliminary cohort study must be seen as a limitation. It must also be taken into account that the number of cases in the individual EMUC-US patterns is very small, so that small effects may not be validly detected. Due to the sample size and study design, no calculation of interobserver variability between examiner 1 and examiner 2 and 3 was performed. Further prospective studies with larger numbers of cases, especially multicentre and international studies, are necessary to confirm the preliminary study results.
Our preliminary study demonstrates not only great heterogeneity in the sonomorphology of hepatic AE lesions at the initial diagnosis but also that sonomorphologic classification can change during disease progression.
Alveolar echinococcosis (AE) is a human parasitosis caused by Echinococcus multilocularis.
Early diagnosis of AE is important to initiate prompt therapy and improve patient prognosis. Abdominal ultrasonography is the most common primary imaging modality and allows for classification of hepatic lesion morphology.
We address the question of whether (and how) the sonomorphology of individual AE lesions can change over time.
In our preliminary retrospective longitudinal study, based on data from the national echinococcosis database in Germany, we evaluated clinical and ultrasound (US) imaging data for 59 patients according to AE case definition of confirmed (n = 26) or probable (n = 33) AE. The AE reference lesion was the largest hepatic AE lesion at the time of the first US examination. We used the Echinococcosis Multilocularis Ulm Classification (EMUC)-US to classify the sonomorphologic pattern.
The study included 59 patients, 38 (64.5%) women and 21 (35.6%) men. The mean median age at initial diagnosis was 59.9 ± 16.9 years. At the time of initial US, 42.4% (25/59) had a hailstorm pattern, 16.9% (10/59) had a pseudohemangioma-like pattern, 15.3% (9/59) had a pseudocystic pattern, and 25.4% (15/59) had a metastasis-like pattern. Although the sonomorphologic pattern remained unchanged in 84.7% (50/59) of the AE reference lesions, 15.3% (9/59) showed a pattern change over time. A change in pattern was seen exclusively for AE lesions initially classified as hemangioma-like or pseudocystic.
The sonomorphology of hepatic AE lesions may change over time, particularly hemangioma-like and pseudocystic patterns.
Further research should clarify whether the patterns evolve and change according to a sonomorphological evolution.
Members of the Echinococcosis Working Group Ulm: Barth TFE, Baumann S, Fischer I, Graeter T, Henne-Bruns D, Hillenbrand A, Kratzer W, Schlingeloff P, Schmidberger J, and Shi R.
| 1. | Pawlowski ZS, Vuitton DA, Ammann RW, Kern P, Craig P; World Health Organization. WHO/OIE Manual on echinococcosis in humans and animals: a public health problem of global concern. Paris, France: World Organization for Animal Health, 2001. |
| 2. | Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1222] [Article Influence: 55.5] [Reference Citation Analysis (1)] |
| 3. | Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. Radiographics. 2012;32:2053-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475-94; quiz 536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 5. | Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1416] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 6. | Vuitton DA, Azizi A, Richou C, Vuitton L, Blagosklonov O, Delabrousse E, Mantion GA, Bresson-Hadni S. Current interventional strategy for the treatment of hepatic alveolar echinococcosis. Expert Rev Anti Infect Ther. 2016;14:1179-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: Advances in the 21st Century. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 704] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 8. | Yang C, He J, Yang X, Wang W. Surgical approaches for definitive treatment of hepatic alveolar echinococcosis: results of a survey in 178 patients. Parasitology. 2019;146:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Qin Y, Vuitton DA, Wen H. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Kratzer W, Gruener B, Kaltenbach TE, Ansari-Bitzenberger S, Kern P, Fuchs M, Mason RA, Barth TF, Haenle MM, Hillenbrand A, Oeztuerk S, Graeter T. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J Gastroenterol. 2015;21:12392-12402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 11. | Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Brumpt E, Blagosklonov O, Calame P, Bresson-Hadni S, Vuitton DA, Delabrousse E. AE hepatic lesions: correlation between calcifications at CT and FDG-PET/CT metabolic activity. Infection. 2019;47:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Yangdan CR, Wang C, Zhang LQ, Ren B, Fan HN, Lu MD. Recent advances in ultrasound in the diagnosis and evaluation of the activity of hepatic alveolar echinococcosis. Parasitol Res. 2021;120:3077-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Cai D, Li Y, Jiang Y, Wang H, Wang X, Song B. The role of contrast-enhanced ultrasound in the diagnosis of hepatic alveolar echinococcosis. Medicine (Baltimore). 2019;98:e14325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Wa ZC, Du T, Li XF, Xu HQ, Suo-Ang QC, Chen LD, Hu HT, Wang W, Lu MD. Differential diagnosis between hepatic alveolar echinococcosis and intrahepatic cholangiocarcinoma with conventional ultrasound and contrast-enhanced ultrasound. BMC Med Imaging. 2020;20:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Sulima M, Nahorski W, Gorycki T, Wołyniec W, Wąż P, Felczak-Korzybska I, Szostakowska B, Sikorska K. Ultrasound images in hepatic alveolar echinococcosis and clinical stage of the disease. Adv Med Sci. 2019;64:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Azizi A, Blagosklonov O, Lounis A, Berthet L, Vuitton DA, Bresson-Hadni S, Delabrousse E. Alveolar echinococcosis: correlation between hepatic MRI findings and FDG-PET/CT metabolic activity. Abdom Imaging. 2015;40:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Grimm J, Beck A, Nell J, Schmidberger J, Hillenbrand A, Beer AJ, Dezsényi B, Shi R, Beer M, Kern P, Henne-Bruns D, Kratzer W, Moller P, Barth TF, Gruener B, Graeter T. Combining Computed Tomography and Histology Leads to an Evolutionary Concept of Hepatic Alveolar Echinococcosis. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Zeng H, Wang J, Xie W, Liu W, Wen H. Assessment of early hepatic echinococcus multilocularis infection in rats with real-time contrast-enhanced ultrasonography. Ultrasound Med Biol. 2012;38:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Deplazes P, Grimm F, Sydler T, Tanner I, Kapel CM. Experimental alveolar echinococcosis in pigs, lesion development and serological follow up. Vet Parasitol. 2005;130:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Okasha HH S-Editor: Gao CC L-Editor: A P-Editor: Yu HG