Published online Oct 21, 2021. doi: 10.3748/wjg.v27.i39.6689
Peer-review started: April 14, 2021
First decision: July 14, 2021
Revised: July 15, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 21, 2021
Processing time: 188 Days and 23.3 Hours
The implementation of a colorectal cancer (CRC) screening programme may increase the awareness of Primary Care Physicians, reduce the diagnostic delay in CRC detected outside the scope of the screening programme and thus improve prognosis.
To determine the effect of implementation of a CRC screening programme on diagnostic delays and prognosis of CRC detected outside the scope of a screening programme.
We performed a retrospective intervention study with a pre-post design. We identified 322 patients with incident and confirmed CRC in the pre-implantation cohort (June 2014 – May 2015) and 285 in the post-implantation cohort (June 2017 - May 2018) in the Cancer Registry detected outside the scope of a CRC screening programme. In each patient we calculated the different healthcare diagnostics delays: global, primary and secondary healthcare, referral and colonoscopy-related delays. In addition, we collected the initial healthcare that evaluated the patient, the home location (urban/rural), and the CRC stage at diagnosis. We determined the two-year survival and we performed a multivariate proportional hazard regression analysis to determine the variables associated with survival.
We did not detect any differences in the patient or CRC baseline-related variables. A total of 20.1% of patients was detected with metastatic disease. There was a significant increase in direct referral to colonoscopy from primary healthcare (25.5%, 35.8%; P = 0.04) in the post-implantation cohort. Diagnostic delay was reduced by 24 d (106.64 ± 148.84 days, 82.84 ± 109.31 d; P = 0.02) due to the reduction in secondary healthcare delay (46.01 ± 111.65 d; 29.20 ± 60.83 d; P = 0.02). However, we did not find any differences in CRC stage at diagnosis or in two-year survival (70.3%; P = 0.9). Variables independently associated with two-year risk of death were age (Hazard Ratio-HR: 1.06, 95%CI: 1.04-1.07), CRC stage (II HR: 2.17, 95%CI: 1.07-4.40; III HR: 3.07, 95%CI: 1.56-6.08; IV HR: 19.22, 95%CI: 9.86-37.44; unknown HR: 9.24, 95%CI: 4.27-19.99), initial healthcare consultation (secondary HR: 2.93, 95%CI: 1.01-8.55; emergency department HR: 2.06, 95%CI: 0.67-6.34), hospitalization during the diagnostic process (HR: 1.67, 95%CI: 1.17-2.38) and urban residence (HR: 1.44, 95%CI: 1.06-1.98).
Although implementation of a CRC screening programme can reduce diagnostic delays for CRC detected in symptomatic patients, this has no effect on CRC stage or survival.
Core Tip: We have designed a retrospective intervention study with a pre-post design to confirm the hypothesis that the implementation of a colorectal cancer (CRC) screening program may increase the awareness of primary care physicians and, thus, reduce the diagnostic delays in CRC detected outside the screening program and improve prognosis. Our results confirm that the implementation of the CRC screening program reduced the diagnostic delays due to an increase in the direct referrals to colonoscopy from primary healthcare. However, this reduction in the delays had no effect on the stage at diagnosis or in the two year survival. These later results were confirmed in a multivariable Cox regression analysis.
- Citation: Cubiella J, Lorenzo M, Baiocchi F, Tejido C, Conde A, Sande-Meijide M, Castro M. Impact of a colorectal cancer screening program implantation on delays and prognosis of non-screening detected colorectal cancer. World J Gastroenterol 2021; 27(39): 6689-6700
- URL: https://www.wjgnet.com/1007-9327/full/v27/i39/6689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i39.6689
Colorectal cancer (CRC) is one of the most important health problems in the Western world. In 2018, almost half a million new cases were diagnosed in Europe and 250,000 patients died due to CRC[1]. In order to reduce the disease burden, population-based CRC screening programmes have been established in the Western world. This strategy has demonstrated its efficacy to reduce CRC mortality and incidence in randomized controlled trials. Furthermore, we have real data showing that implementation of CRC screening programmes has achieved its expected efficiency in reducing both CRC mortality and incidence[2,3].
In spite of the implementation of CRC screening programmes, most CRC are detected among symptomatic patients outside the scope of CRC screening mainly due to the limited participation and the detection in age cohorts that are not candidates for CRC screening[4,5]. However, as in breast cancer screening, the implementation of CRC screening may have an additional positive effect on these patients due to increased awareness and creation of multidisciplinary teams[6]. In this sense, CRC screening may increase the CRC awareness of patients and primary care physicians (PCPs) and promote use of faecal immunochemical test (FIT) as a triage test to refer patients to colonoscopy[7].
The delay to diagnosis in cancer is due to factors related to the patient and health system. The period from initial symptoms until final diagnosis is made can be highly variable. Although the common belief is that a longer delay can lead to an advanced stage at diagnosis and worse prognosis, evidence on CRC is controversial[8]. Patients seeking assistance with more severe symptoms are diagnosed in a shorter period and have more advanced disease[9]. In contrast, there is no evidence that a health system delay lower than six months worsens prognosis in the context of an outpatient diagnosis[10].
Based on the hypothesis that implementation of a mass CRC screening programme could raise awareness of patients and PCPs, we decided to design a retrospective intervention study to determine whether implementation of a CRC screening programme could reduce health system delays and, secondarily, improve CRC staging at diagnosis and long term survival.
We designed a retrospective intervention study with a pre-post design without a control group.
The intervention was the first round of the Galician CRC screening programme that took place between 1 July 2015 and 30 June 2017 in Ourense, Spain. Galician CRC mass screening is based on biennial FIT with a 20 µg haemoglobin/g of faeces threshold. FIT is offered to subjects aged 50 to 69 years. It is coordinated by the Public Health Department of the Galician Regional Health Department. They are in charge of the identification of subjects, invitation to participate, reception of FIT results, citation of patients with a positive result to perform a colonoscopy and final evaluation of the endoscopic and histological results. Primary healthcare clinics are in charge of promoting participation in the screening programme, collecting FIT kits and evaluation of subjects with a positive FIT prior to colonoscopy. The hospitals in each health area are responsible for FIT analysis, colonoscopies, histological analysis and evaluation and treatment of patients with a CRC. Finally, personnel at the Coordination Unit key in data into the screening programme’s information system regarding CRC stage according to the AJCC classification,[11]. the final classification of patients with a positive result[12]. as well as several quality endoscopist indicators according to the Spanish guideline on quality in screening colonoscopy[13]. During the implantation of the CRC screening program no change was performed in the diagnostic pathways for CRC diagnosis in symptomatic patients.
Pre cohort: We included all invasive incident CRC histologically confirmed detected in the natural year before implementation of the CRC screening programme (1 July 2014 – 30 June 2015) in Ourense.
Post cohort: We included all invasive incident CRC histologically confirmed and detected outside the scope of the CRC screening programme in the natural year after the first round: (1 July 2017- 30 June 2018).
We identified the incident using the case identification structure developed and validated by the project for implementation of the Galician Tumour Registry (Project REGAT). REGAT uses the topographic codes ICD-O-3.1 C18-C19-C20 to identify the CRC[14]. Codes C18.1 (appendix), C21 (anus and anal canal) were excluded. REGAT data were crosslinked with the Galician CRC screening information system to exclude those patients with a CRC diagnosed within the screening programme.
We collected information regarding: (1) Demographics (age and sex); and (2) Tumour location in relation to the splenic flexure: proximal (caecum, ascending colon, hepatic flexure and transverse colon) and distal (rectum, sigma, descending colon and splenic flexure).
Cancer stage at diagnosis according to the TNM classification (AJCC 7th edition)[11]. We used the following data to determine the stage at diagnosis: clinical or anatomo-pathological stage for metastatic disease, imaging tests for the local rectal cancer stage (T and N), anatomo-pathological evaluation for the remaining situations (colon cancer T and N).
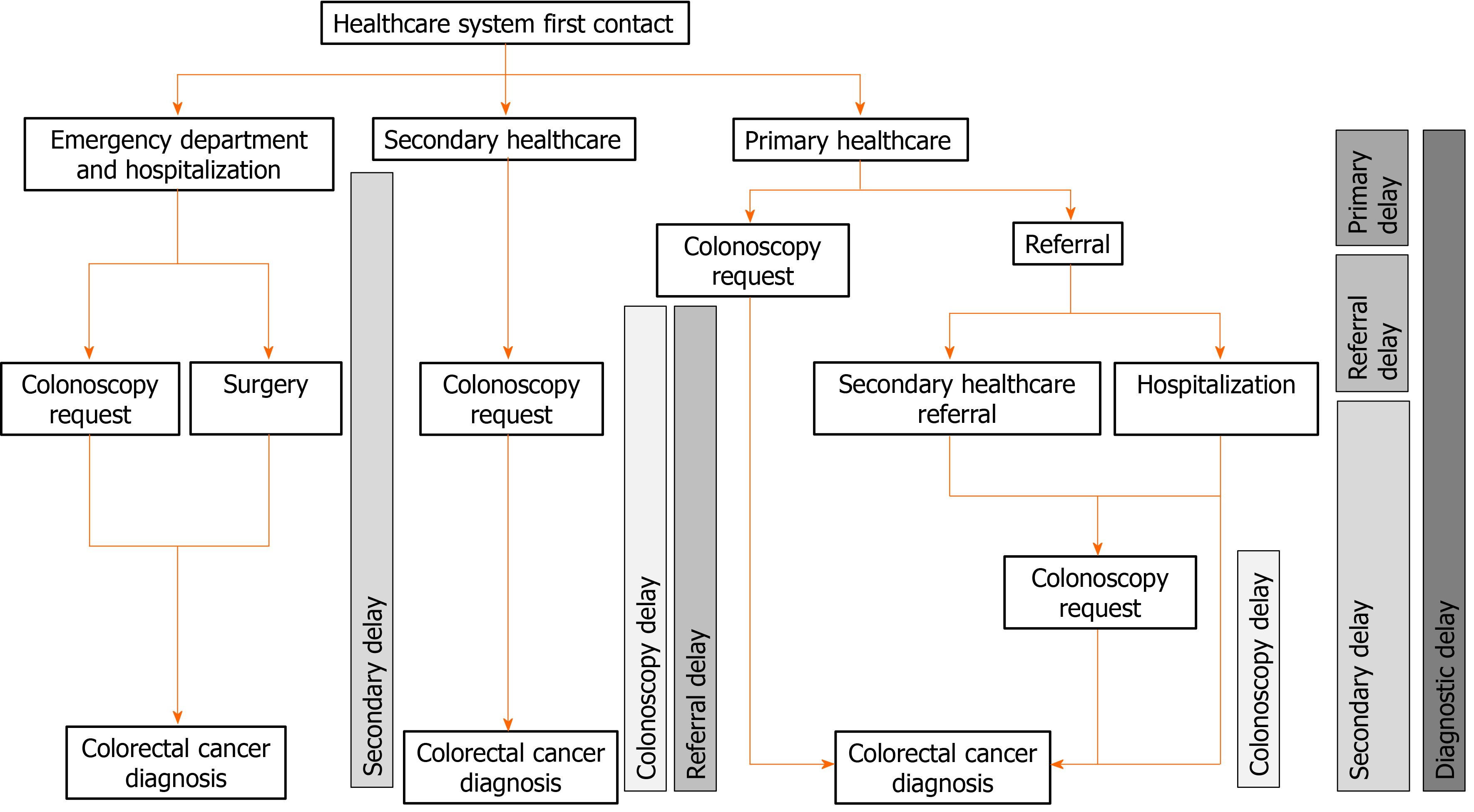
We searched in IANUS, the unified clinical record database of the Galician Health Department, for information regarding the contacts and referrals in the healthcare system. IANUS includes all information regarding attendance in primary and secondary healthcare as well as emergency departments and hospitalization. We determined the first contact in the health system (primary, secondary, emergency), whether the patient required hospitalization during the diagnostic process and the diagnostic delays. We defined five diagnostic delays (Figure 1): (1) Global diagnostic delay: Overall delay from the first consultation to definitive diagnosis; (2) Primary healthcare delay: Delay from the initial evaluation in primary healthcare until the decision to refer to secondary healthcare. In the event of colonoscopy being directly requested from primary healthcare, this date was considered as the referral date; (3) Referral delay: Delay from the primary healthcare referral to the first attendance in secondary healthcare (either clinical consultation or performing of colonoscopy); (4) Secondary healthcare delay: Delay from the first attendance in secondary healthcare to final diagnosis; and (5) Colonoscopy delay: Delay from the colonoscopy request to the performing of colonoscopy.
First, we performed a descriptive analysis of the variables included: number and frequencies in the qualitative variables and mean and standard deviation in the quantitative variables. We determined whether there were differences between both cohorts in the diagnostic pathways (hospitalization, direct referral to colonoscopy from primary healthcare) using the Chi-square test. In order to detect whether there were differences in the referral delays between both cohorts we used the Student t test. We analyzed whether there were differences in two-year survival between both cohorts in the Kaplan-Meier analysis using the log-rank test. Finally, to control confounding variables we performed a Cox multivariate regression analysis and we determined which variables were independently associated with survival after diagnosis. The study was statistically reviewed by a biomedical statistician.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Galicia, Spain (code 2016/274). As long as the study was based on database use, no informed consent was required. The information was accessed according to prevailing European and Spanish legislation.
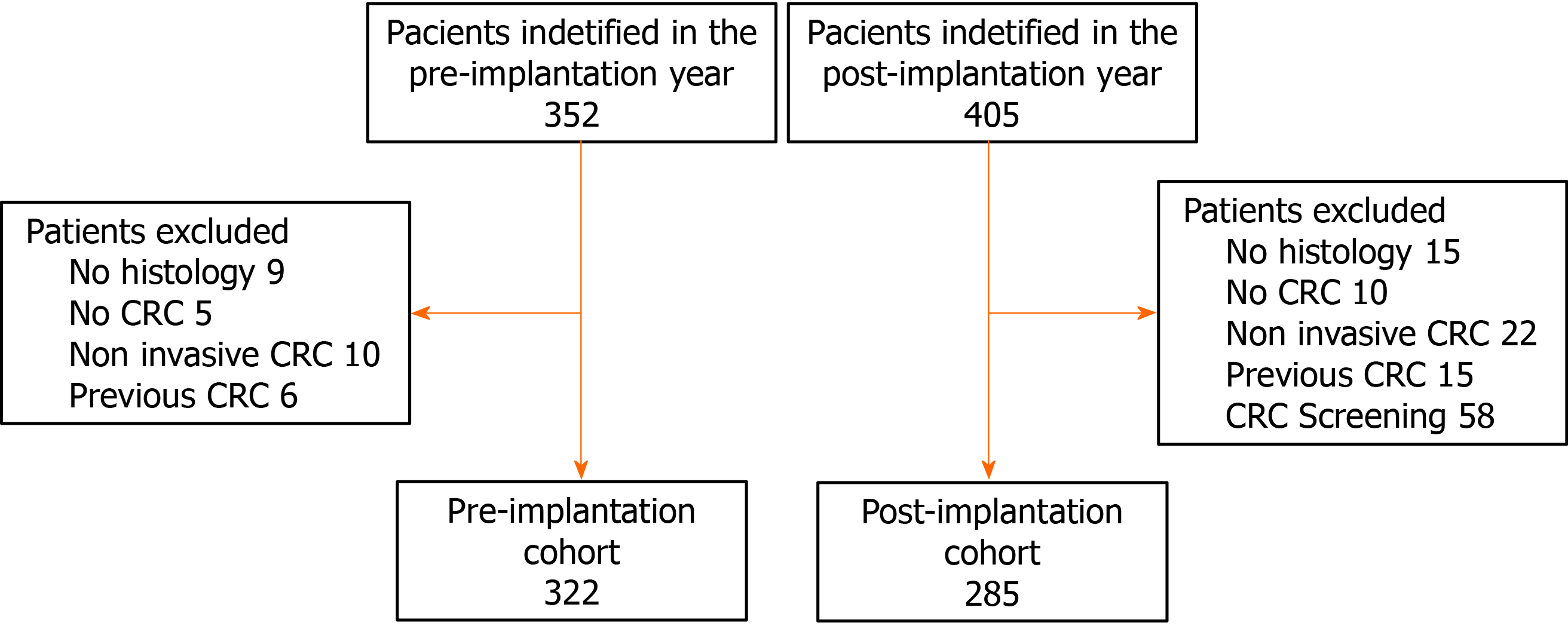
We identified records from 757 patients in the two periods analyzed in the cancer registry. We excluded 92 patients that did not meet the inclusion criteria and 58 patients with CRC detected within the CRC screening programme in the post-implantation cohort. Finally, the pre-implantation and post-implantation cohort consisted of 322 and 285 patients, respectively (Figure 2).
As we show in Table 1, we did not detect baseline differences between both cohorts. CRC was detected more commonly in males (59.6%) with a mean age of 74.5 ± 11.5 years and more than two thirds were distal to the splenic flexure. There were no differences with respect to the place of residence either. Most patients were initially evaluated in primary healthcare but up to 41.0% required hospitalization before reaching final diagnosis. Diagnosis was made through colonoscopy in 89.8% of detected CRC. In this sense, we detected a significant increase in the colonoscopy directly requested from primary healthcare in the post-implantation cohort (P = 0.04). When we limited the analysis to those patients initially seen in primary healthcare (522), the results were similar. In this sense, we only found differences in the rate of colonoscopy directly referred from primary healthcare (29.2%, 42.3%; P = 0.005).
| Pre-implantation cohort (n = 322) | Post-implantation cohort (n = 285) | P value1 | |
| Sex | 0.1 | ||
| Male | 184 (57.1%) | 178 (62.5%) | |
| Female | 138 (42.9%) | 107 (37.5%) | |
| Age (yr) | 74.1 ± 11.8 | 74.8 ± 11.1 | 0.4 |
| Colorectal location | 0.8 | ||
| Distal to splenic | 221 (68.6%) | 197 (69.1%) | |
| Proximal to splenic | 101 (31.4%) | 88 (30.9%) | |
| TNM | |||
| I | 45 (14.0%) | 47 (16.5%) | |
| II | 93 (26.9%) | 71 (24.9%) | |
| III | 101 (31.4%) | 99 (34.7%) | 0.5 |
| IV | 65 (20.2%) | 57 (20.0%) | |
| Unknown | 18 (5.6%) | 11 (3.9%) | |
| Rural/Urban | |||
| Rural | 218 (67.9%) | 195 (68.4%) | 0.8 |
| Urban | 103 (32.1%) | 90 (31.6%) | |
| Initial consultation | |||
| Primary healthcare | 281 (87.3%) | 241 (84.6%) | |
| Secondar y healthcare | 33 (10.2%) | 37 (13.0%) | 0.5 |
| Emergency department | 8 (2.5%) | 7 (2.5%) | |
| Hospitalization | |||
| Yes | 135 (41.9%) | 114 (40.0%) | 0.6 |
| No | 187 (52.2%) | 171 (60.0%) | |
| Colonoscopy request | |||
| Primary healthcare | 82 (25.5%) | 102 (35.8%) | |
| Secondary healthcare | 0.04 | ||
| After referral | 160 (49.7%) | 116 (40.7%) | |
| Direct request | 45 (14.0%) | 40 (14.0%) | |
| No colonoscopy | 35 (10.9%) | 27 (9.5%) |
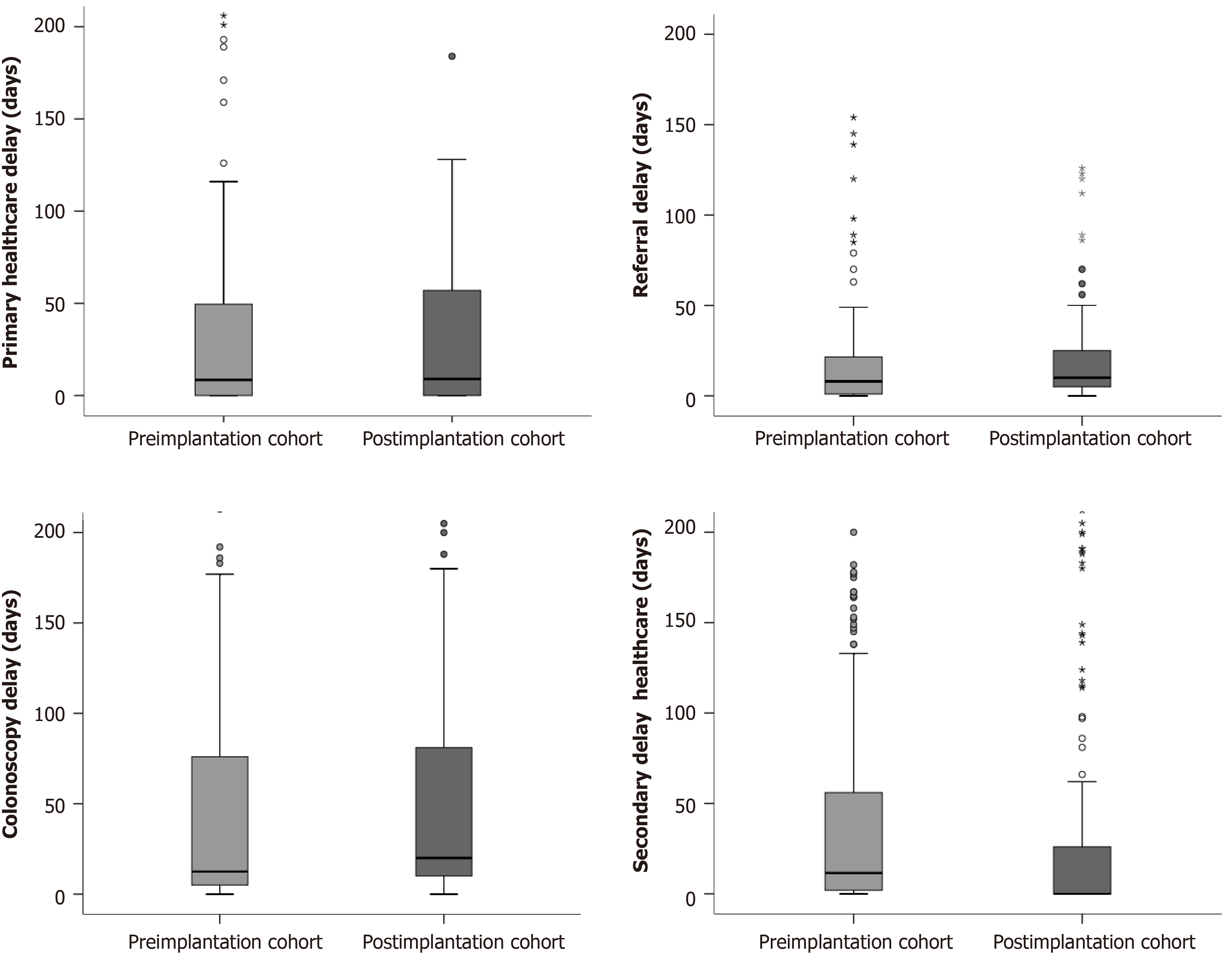
The delay to diagnosis was reduced in 24 d after implantation of the CRC screening programme (P = 0.02). We did not detect any differences in the primary healthcare, referral or colonoscopy delay. The reduction was due to a secondary healthcare delay in relation to an increased rate of direct referral to colonoscopy from primary healthcare, as we show in Table 2 and Figure 3.
| Pre-implantation cohort (n = 322) | Post-implantation cohort (n = 285) | P value1 | |
| Global diagnostic delay (d) | 106.64 ± 148.84 | 82.84 ± 109.31 | 0.02 |
| Primary healthcare delay (d) | 35.88 ± 84.47 | 39.28 ± 98.03 | 0.7 |
| Referral delay (d) | 13.18 ± 25.77 | 16.02 ± 41.63 | 0.4 |
| Secondary healthcare delay (d) | 46.01 ± 111.65 | 29.20 ± 60.83 | 0.02 |
| Colonoscopy delay (d) | 43.71 ± 78.22 | 37.75 ± 53.37 | 0.3 |
The global delay was also reduced by 27 d in patients evaluated initially in primary healthcare (117.66 ± 154.08 days, 90.06 ± 111.31 days; P = 0.02) also due to a reduction in secondary healthcare delay (48.17 ± 116.42 d, 26.89 ± 54.50 d; P = 0.02). There were no differences in primary healthcare, referral or colonoscopy delay.
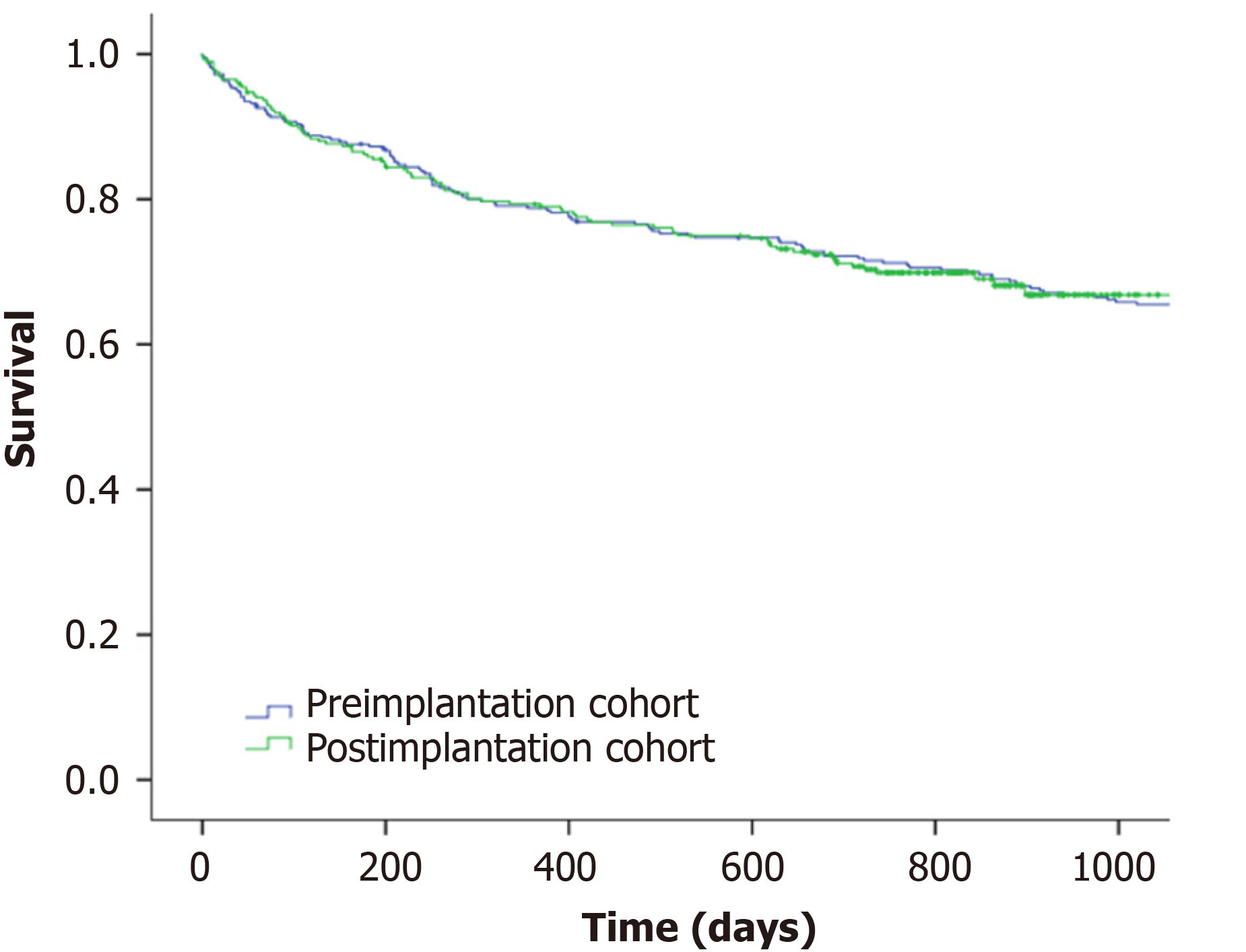
The incidence of metastatic CRC remained stable (20.1%) in both cohorts and overall survival after one and two years was 71.3% and 70.3% without differences in the log-rank test (P = 0.9) as we show in Figure 4. These results were confirmed in the Cox multivariate regression analysis and there were no differences in the survival between both cohorts (post-implantation cohort HR: 1.12, 95%CI: 0.83-1.51). As we show in Table 3, only age, CRC staging according to TNM classification, initial healthcare consultation, hospitalization during the diagnostic process and residence were independently associated with death after CRC diagnosis.
| Hazard ratio1 (95%CI) | |
| Sex | |
| Male | 1 |
| Female | 1.18 (0.89-1.58) |
| Age (yr) | 1.06 (1.04-1.07) |
| Colorectal location | |
| Distal to splenic | 1 |
| Proximal to splenic | 0.84 (0.62-1.13) |
| Cohort | |
| Pre-implantation | 1 |
| Post-implantation | 1.12 (0.83-1.51) |
| TNM | |
| I | 1 |
| II | 2.17 (1.07-4.40) |
| III | 3.07 (1.56-6.08) |
| IV | 19.22 (9.86-37.44) |
| Unknown | 9.24 (4.27-19.99) |
| Initial consultation | |
| Primary healthcare | 1 |
| Secondary healthcare | 2.93 (1.01-8.55) |
| Emergency department | 2.06 (0.67-6.34) |
| Hospitalization | |
| Yes | 1.67 (1.17-2.38) |
| No | 1 |
| Colonoscopy request | |
| Primary healthcare | 1.79 (0.96-3.35) |
| Secondary healthcare | 1.54 (0.92-2.58) |
| After referral from Primary Healthcare | 0.74 (0.25-2.21) |
| Direct request | 1 |
| No colonoscopy | |
| Rural/Urban | |
| Rural | 1 |
| Urban | 1.44 (1.06-1.98) |
| Diagnostic delay (d) | 1.001 (1.00-1.002) |
Our study shows that implementation of the CRC screening programme reduced healthcare referral delays due to the direct request of colonoscopy from primary healthcare. Unfortunately, this reduction in referral delay had no effect on CRC staging at diagnosis nor on the two-year survival. Finally, although we detected several variables associated with overall survival, multivariate logistic analysis confirms that neither implantation of the CRC screening nor diagnostic delay were related to the prognosis of CRC detected outside the scope of CRC screening.
PCPs play an important role in CRC care, from encouraging screening and accurate diagnosis to providing care during and after treatment for cancer and any comorbid complications. The implication of PCPs on CRC screening is variable according to the screening programme. Participation rates are increased when PCPs are involved in the invitation process. However, in the European population-based programmes in Europe PCPs play a rather supportive, informative or facilitating role[15]. In our case, PCPs receive full information on the screening programme organization and they are in charge of promoting participation as well as resolving any doubts. Within the training, PCPs are reminded which symptoms may lead to suspicion of CRC as well as the established referral pathways, including direct referral criteria for colonoscopy evaluation from primary healthcare[8,16].
We designed this analysis under the hypothesis that increased awareness on CRC and training in the diagnosis of CRC and the established protocols could reduce delays attributed to the health system. In this sense, our results confirm that implementation of the screening programme enabled a reduction in the diagnostic delay due to an increase in direct referrals to colonoscopy from primary healthcare. PCPs, as demonstrated in our study, are the main gateway and responsible for a significant part of the delay[17-19]. The role of PCPs in CRC diagnosis is complex since gastro
However, it is relevant that, despite the reduction in delay, we have not detected any changes in the stage at diagnosis or in the prognosis of CRC. These data are in accordance with results previously published by our group[8]. and with the data in the available meta-analysis on the effect of diagnostic delays in CRC prognosis[9]. In this sense, the prognosis of patients with shorter diagnostic delay is worse due to presentation with urgent symptoms that require hospitalization or more serious systemic symptoms[19]. In fact, in our study, hospitalization during admission was associated with a higher risk of mortality after diagnosis. In our research, although initial urgent presentation was rare, up to 40% of patients required hospitalization during the diagnostic process, similar to the information available on literature[22-24]. This lack of relationship between delay and prognosis may be related to different forms of presentation. In this sense, a prospective study on patients that met the National Institute for Health and Care Excellence referral criteria demonstrated that a delay of more than six months was associated with a worse prognosis compared to patients with the same symptoms diagnosed in an interval of less than one month[10].
Our study has two main strengths. We had the opportunity to evaluate the effect of the CRC screening programme on the diagnostic delays of CRC detected in symptomatic patients. This is the first study that evaluates additional impacts of the implementation of CRC screening on CRC diagnosis. No study has evaluated whether a CRC screening programme can increase the awareness of patients and PCPs, reduce delays and improve prognosis. However, we could identify all the CRC through the Galician cancer registry, confirm the diagnosis in IANUS, the centralized clinical record and determine when the patient was evaluated in the health system and thus calculate all the referral delays[25].
There are several limitations. Due to the design of the study, we could not evaluate the effect of CRC screening on the patient delays to seek assistance. Patient delay accounts for a relevant proportion of the delay between the onset of symptoms and the final diagnosis[26]. Moreover, we did not collect the initial symptoms as long as they were not collected uniformly in the clinical records.
To conclude, the implementation of a CRC screening programme enabled reduction of health system diagnostic delay by means of increased patients referred directly by PCPs to colonoscopy. However, this reduction in referral delay did not modify either CRC stage at diagnosis or two-year survival.
In spite of the implementation of colorectal cancer (CRC) screening programmes, most CRC are detected among symptomatic patients outside the scope of CRC screening. However, they may increase the CRC awareness of patients and primary care physicians (PCP).
The implementation of a mass CRC screening programme could raise awareness of patients and PCPs, we decided to design a retrospective intervention study to determine whether implementation of a CRC screening programme could reduce health system delays and, secondarily, improve CRC staging at diagnosis and long term survival.
To determine the effect of implementation of a CRC screening programme on diagnostic delays and prognosis of CRC detected outside the scope of a screening programme.
We designed a retrospective intervention study with a pre-post design without a control group. We compared diagnostic delays, CRC stage and two year survival of a yearly CRC diagnosed before the implementation of a CRC screening programa with a CRC cohort diagnosed the year after the first round.
There was a significant increase in direct referral to colonoscopy from primary healthcare (25.5%, 35.8%; P = 0.04) in the post-implantation cohort. Diagnostic delay was reduced by 24 d (106.64 ± 148.84 d, 82.84 ± 109.31 d; P = 0.02) due to the reduction in secondary healthcare delay (46.01 ± 111.65 d; 29.20 ± 60.83 d; P = 0.02). However, we did not find any differences in CRC stage at diagnosis or in two-year survival (70.3%; P = 0.9).
Although implementation of a CRC screening programme can reduce diagnostic delays for CRC detected in symptomatic patients, this has no effect on CRC stage or survival.
We need more research on the motivations and perspectives of patients seeking help in primary healthcare.
| 1. | World Health Organization. Cancer Today. International Agency for Research on Cancer [Internet]. [cited 23 May 2020]. Available from: https://gco.iarc.fr/today/home. |
| 2. | Zorzi M, Fedeli U, Schievano E, Bovo E, Guzzinati S, Baracco S, Fedato C, Saugo M, Dei Tos AP. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N, Ghai NR, Lee AT, Quesenberry CP, Fireman BH, Doubeni CA. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology. 2018;155:1383-1391.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 407] [Article Influence: 50.9] [Reference Citation Analysis (3)] |
| 4. | Mansouri D, McMillan DC, Crearie C, Morrison DS, Crighton EM, Horgan PG. Temporal trends in mode, site and stage of presentation with the introduction of colorectal cancer screening: a decade of experience from the West of Scotland. Br J Cancer. 2015;113:556-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Gutierrez-Stampa MA, Aguilar V, Sarasqueta C, Cubiella J, Portillo I, Bujanda L. Impact of the faecal immunochemical test on colorectal cancer survival. BMC Cancer. 2020;20:616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Gutierrez-Stampa MA, Aguilar V, Sarasqueta C, Cubiella J, Portillo I, Bujanda L. Colorectal Cancer Survival in 50- to 69-Year-Olds after Introducing the Faecal Immunochemical Test. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Fernández-de Castro JD, Baiocchi Ureta F, Fernández González R, Pin Vieito N, Cubiella Fernández J. The effect of diagnostic delay attributable to the healthcare system on the prognosis of colorectal cancer. Gastroenterol Hepatol. 2019;42:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Tørring ML, Murchie P, Hamilton W, Vedsted P, Esteva M, Lautrup M, Winget M, Rubin G. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017;117:888-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Alonso-Abreu I, Alarcón-Fernández O, Gimeno-García AZ, Romero-García R, Carrillo-Palau M, Nicolás-Pérez D, Jiménez A, Quintero E. Early Colonoscopy Improves the Outcome of Patients With Symptomatic Colorectal Cancer. Dis Colon Rectum. 2017;60:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL. In: Trotti A, editor. AJCC Cancer Staging Manual [Internet]. 7th edition. Springer, 2010. [cited 23 May 2020]. Available from: http://www.springer.com/medicine/surgery/book/978-0-387-88440-0. |
| 12. |
|
| 13. | Jover R, Herráiz M, Alarcón O, Brullet E, Bujanda L, Bustamante M, Campo R, Carreño R, Castells A, Cubiella J, García-Iglesias P, Hervás AJ, Menchén P, Ono A, Panadés A, Parra-Blanco A, Pellisé M, Ponce M, Quintero E, Reñé JM, Sánchez del Río A, Seoane A, Serradesanferm A, Soriano Izquierdo A, Vázquez Sequeiros E; Spanish Society of Gastroenterology; Spanish Society of Gastrointestinal Endoscopy Working Group. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (5)] |
| 14. | World Health Organization. The International Classification of Diseases for Oncology (ICD-O) - 3rd edition, 1st revision [Internet]. 2013. [cited 31 January 2021]. Available from: https://apps.who.int/iris/handle/10665/96612. |
| 15. | Triantafillidis JK, Vagianos C, Gikas A, Korontzi M, Papalois A. Screening for colorectal cancer: the role of the primary care physician. Eur J Gastroenterol Hepatol. 2017;29:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 16. | Vega-Villaamil P, Salve-Bouzo M, Cubiella J, Valentín-Gómez F, Sánchez-Hernández E, Gómez-Fernández I, Fernández-Seara J. Evaluation of the implementation of Galician Health Service indications and priority levels for colonoscopy in symptomatic patients: prospective, cross-sectional study. Rev Esp Enferm Dig. 2013;105:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | van Erp NF, Helsper CW, Olyhoek SM, Janssen RRT, Winsveen A, Peeters PHM, de Wit NJ. Potential for Reducing Time to Referral for Colorectal Cancer Patients in Primary Care. Ann Fam Med. 2019;17:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Brandenbarg D, Groenhof F, Siewers IM, van der Voort A, Walter FM, Berendsen AJ. Possible missed opportunities for diagnosing colorectal cancer in Dutch primary care: a multimethods approach. Br J Gen Pract. 2018;68:e54-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Esteva M, Leiva A, Ramos M, Pita-Fernández S, González-Luján L, Casamitjana M, Sánchez MA, Pértega-Díaz S, Ruiz A, Gonzalez-Santamaría P, Martín-Rabadán M, Costa-Alcaraz AM, Espí A, Macià F, Segura JM, Lafita S, Arnal-Monreal F, Amengual I, Boscá-Watts MM, Hospital A, Manzano H, Magallón R; DECCIRE GROUP. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer. 2013;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Cubiella J, Marzo-Castillejo M, Mascort-Roca JJ, Amador-Romero FJ, Bellas-Beceiro B, Clofent-Vilaplana J, Carballal S, Ferrándiz-Santos J, Gimeno-García AZ, Jover R, Mangas-Sanjuán C, Moreira L, Pellisè M, Quintero E, Rodríguez-Camacho E, Vega-Villaamil P; Sociedad Española de Medicina de Familia y Comunitaria y Asociación Española de Gastroenterología. Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 Update. Gastroenterol Hepatol. 2018;41:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Pin Vieito N, Zarraquiños S, Cubiella J. High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis. World J Gastroenterol. 2019;25:2383-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 22. | Renzi C, Lyratzopoulos G, Card T, Chu TP, Macleod U, Rachet B. Do colorectal cancer patients diagnosed as an emergency differ from non-emergency patients in their consultation patterns and symptoms? Br J Cancer. 2016;115:866-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Esteva M, Ruidíaz M, Sánchez MA, Pértega S, Pita-Fernández S, Macià F, Posso M, González-Luján L, Boscá-Wats MM, Leiva A, Ripoll J; DECCIRE GROUP. Emergency presentation of colorectal patients in Spain. PLoS One. 2018;13:e0203556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Borowski DW, Cawkwell S, Zaidi SM, Toward M, Maguire N, Gill TS. Primary care referral practice, variability and socio-economic deprivation in colorectal cancer. Colorectal Dis. 2016;18:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Leiva A, Esteva M, Llobera J, Macià F, Pita-Fernández S, González-Luján L, Sánchez-Calavera MA, Ramos M. Time to diagnosis and stage of symptomatic colorectal cancer determined by three different sources of information: A population based retrospective study. Cancer Epidemiol. 2017;47:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Weller D, Menon U, Zalounina Falborg A, Jensen H, Barisic A, Knudsen AK, Bergin RJ, Brewster DH, Cairnduff V, Gavin AT, Grunfeld E, Harland E, Lambe M, Law RJ, Lin Y, Malmberg M, Turner D, Neal RD, White V, Harrison S, Reguilon I; ICBP Module 4 Working Group, Vedsted P. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP). BMJ Open. 2018;8:e023870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bordonaro M, Ji J, Rompianesi G S-Editor: Wang LL L-Editor: A P-Editor: Xing YX