Published online Sep 14, 2021. doi: 10.3748/wjg.v27.i34.5764
Peer-review started: March 23, 2021
First decision: April 29, 2021
Revised: May 11, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: September 14, 2021
Processing time: 169 Days and 16.2 Hours
Gastric cancer remains a leading cause of cancer death worldwide. In Taiwan, gastric cancer is the sixth leading cause of cancer mortality in both males and females.
To evaluate secular trends in gastric cancer incidence according to age, sex, and Helicobacter pylori (H. pylori) treatment in Taiwan.
In this population-based study, we used the national Taiwan Cancer Registry database. Annual percent changes in incidence rates were used to describe secular trends in incidence rates and sex ratios of gastric cancer in Taiwan. Pearson’s product-moment correlation coefficients were used to analyze the correlation between annual age-adjusted incidence rates and the annual number of patients treated with antibiotic therapy for H. pylori infection.
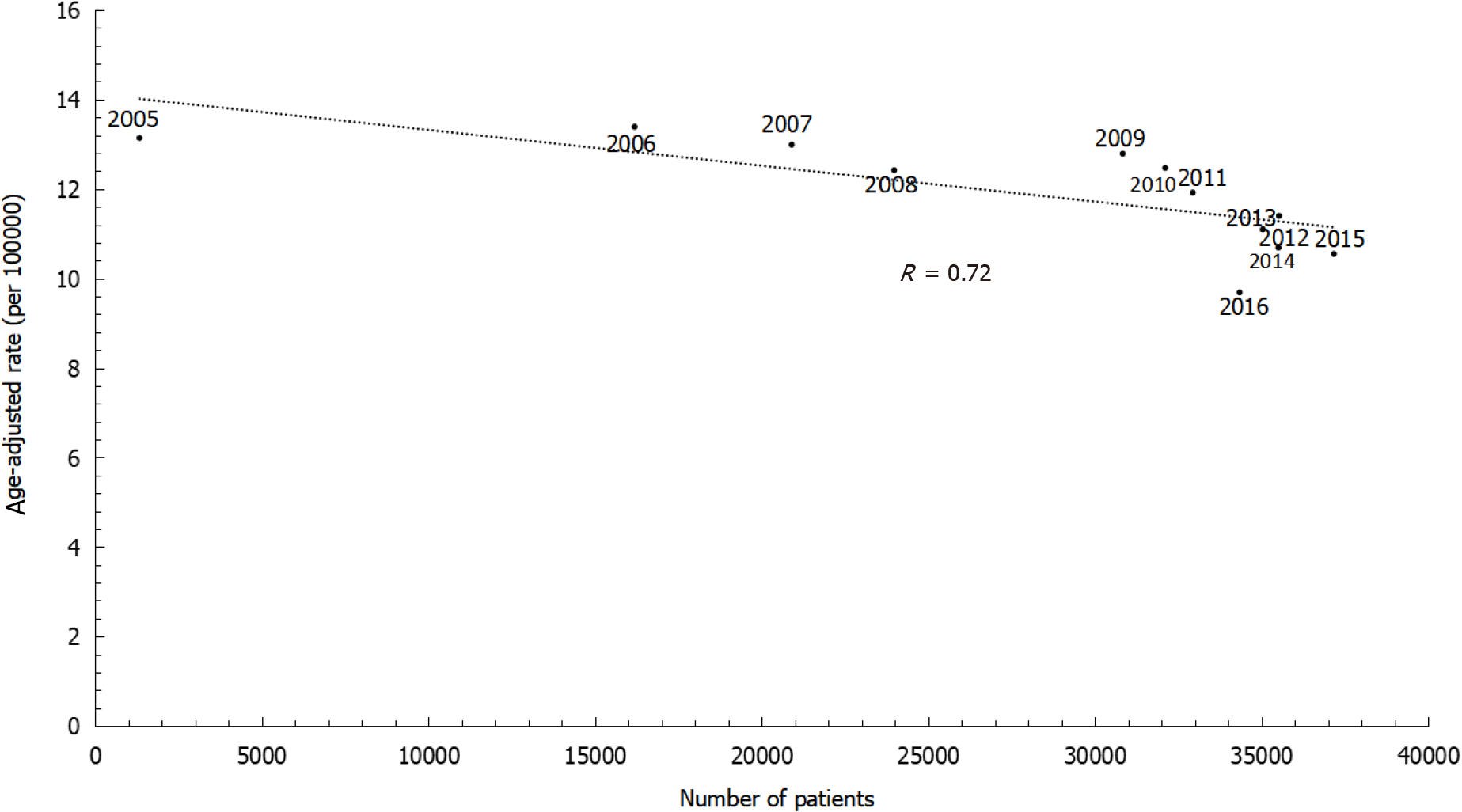
The annual percent changes showed continuously decreasing rates of gastric cancer among both males and females. However, the decreasing trends differed by sex, with an annual percent change of -2.58% in males and -2.14% in females. The age-specific incidence rates increased with age. Within the same age group, more recent time periods showed lower incidence rates than greater time periods. Similarly, the sex ratio was lower in later birth cohorts than in earlier birth cohorts. Age-adjusted incidence rates substantially decreased with increasing numbers of patients being treated with antibiotic therapy for H. pylori infection during 2005 to 2016 (r = 0.72).
We observed steadily decreasing trends with differential sex ratios in the inci
Core Tip: Gastric cancer remains a leading cause of cancer death worldwide. In this population-based study, we used the national Taiwan Cancer Registry database to evaluate secular trends in gastric cancer incidence in Taiwan. Annual percent changes showed continuously decreasing rates of gastric cancer among both males and females, with an annual percent change of -2.58% in males and -2.14% in females. Age-specific incidence rates increased with age. Within the same age group, the sex ratio was lower in later birth cohorts than in earlier birth cohorts. Age-adjusted incidence rates substantially decreased with the increase in patients newly treated with anti-Helicobacter pylori treatment.
- Citation: Lin YT, Chiang CJ, Yang YW, Huang SP, You SL. Secular decreasing trends in gastric cancer incidence in Taiwan: A population-based cancer registry study. World J Gastroenterol 2021; 27(34): 5764-5774
- URL: https://www.wjgnet.com/1007-9327/full/v27/i34/5764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i34.5764
Gastric cancer is one of the leading causes of cancer death worldwide. Data from GLOBOCAN 2020 shows an age-standardized incidence rate of gastric cancer of 15.8 per 100000 persons for males and 7.0 per 100000 persons for females, with a mortality rate of 11.0 per 100000 persons in males and 4.9 per 100000 persons in females[1]. In Taiwan, gastric cancer was the sixth leading cause of cancer death for both males and females in 2018[2].
Gastric cancer is a multifactorial disease; its risk factors include alcohol drinking, tobacco smoking, gastric ulcer, and atrophic gastritis, whereas the intake of fresh fruits and vegetables is probably protective[3]. A previous study demonstrated that anti-Helicobacter pylori (H. pylori) treatment has the potential to decrease the risk of gastric cancer[4]. Moreover, a study by Chiang et al[5] showed that the effectiveness of H. pylori eradication in reducing gastric cancer incidence and mortality was 53% [95% confidence interval (CI): 30% to 69%, P < 0.001] and 25% (95%CI: -14% to 51%, P = 0.18), respectively. This study aimed to investigate secular trends in the incidence of gastric cancer and its association with H. pylori eradication programs in Taiwan.
We obtained data on gastric cancer incidence from 1979 to 2016 from the national population-based Taiwan Cancer Registry (TCR) database. The TCR database has been collecting data on newly diagnosed cancer cases since 1979. The TCR’s accuracy was such that the percentage of death certificate only cases was 0.91%, and the proportion of morphologically verified cases reached 92.23%[6]. In addition, the completeness of the TCR database was reported to be 98.44% in 2016[6]. All cases of primary gastric cancer in this analysis were classified based on the International Classification of Diseases for Oncology, third edition (codes C16.0–C16.9)[7]. We identified subjects who received anti-H. pylori treatment from 2005 to 2016, based on Anatomical The
Age-specific and age-adjusted incidence rates per 100000 person-years were calculated according to the 2000 World Health Organization standard population[9]. The in
To describe linear trends by time period, we computed the annual percent change (APC) in incidence rate and tested whether the corresponding changes in rates were significant. We also used the Pearson’s product-moment correlation coefficient to analyze the correlation between the annual age-adjusted incidence rate and the annual number of patients treated with antibiotic therapy for H. pylori infection. The research protocol was approved by the Institutional Review Board of Fu-Jen Catholic Uni
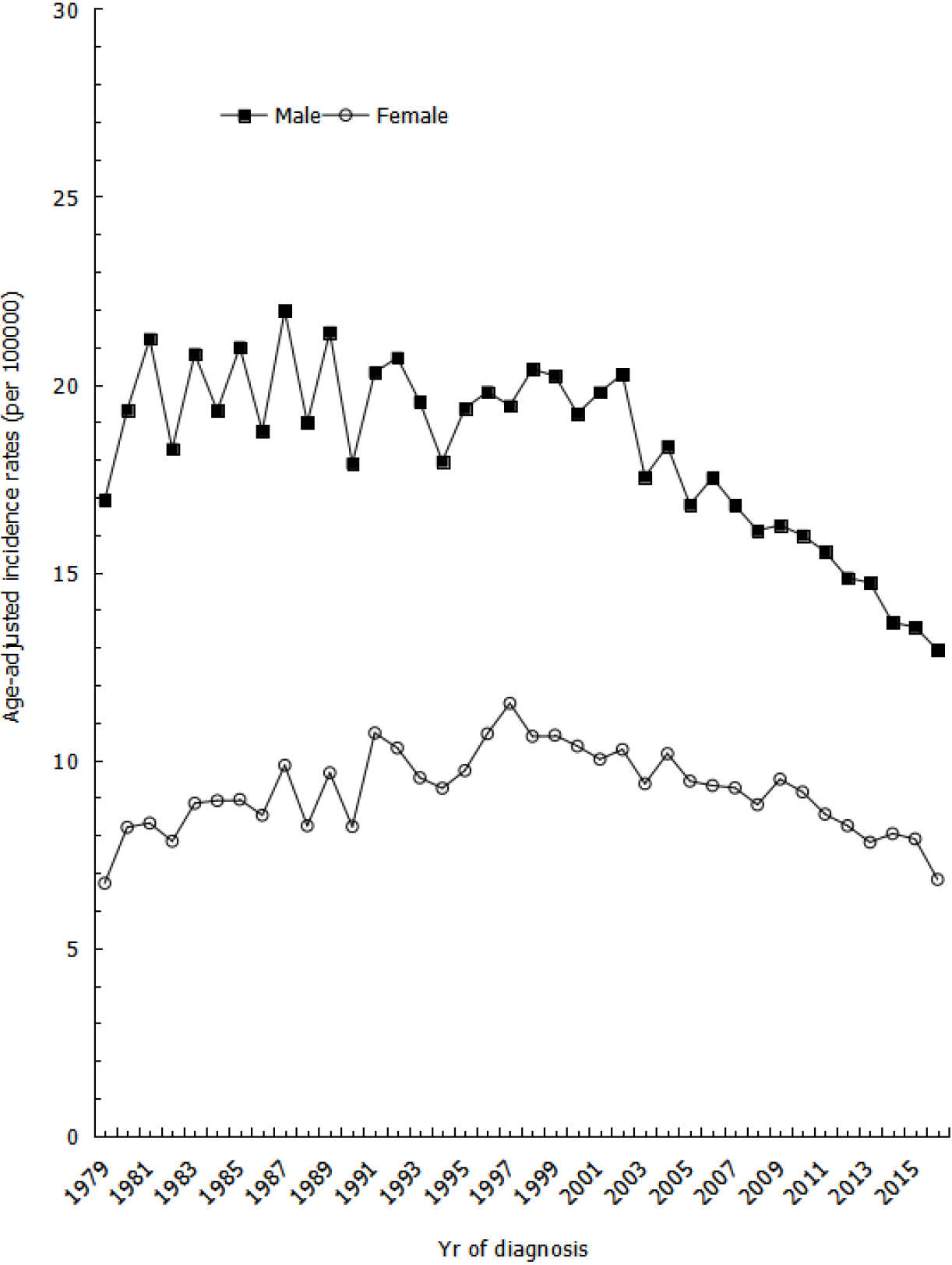
The age-adjusted incidence rates of gastric cancer show that rates for males were volatile between the years of 1981 to 2002 but steadily decreased from 2002 to 2016, with an average APC (AAPC) of -2.58% (Figure 1). However, the incidence rates for females slightly increased between the years of 1979 to 1997 and then gradually decreased from 1997 to 2016, with an AAPC of -2.14% (Figure 1).
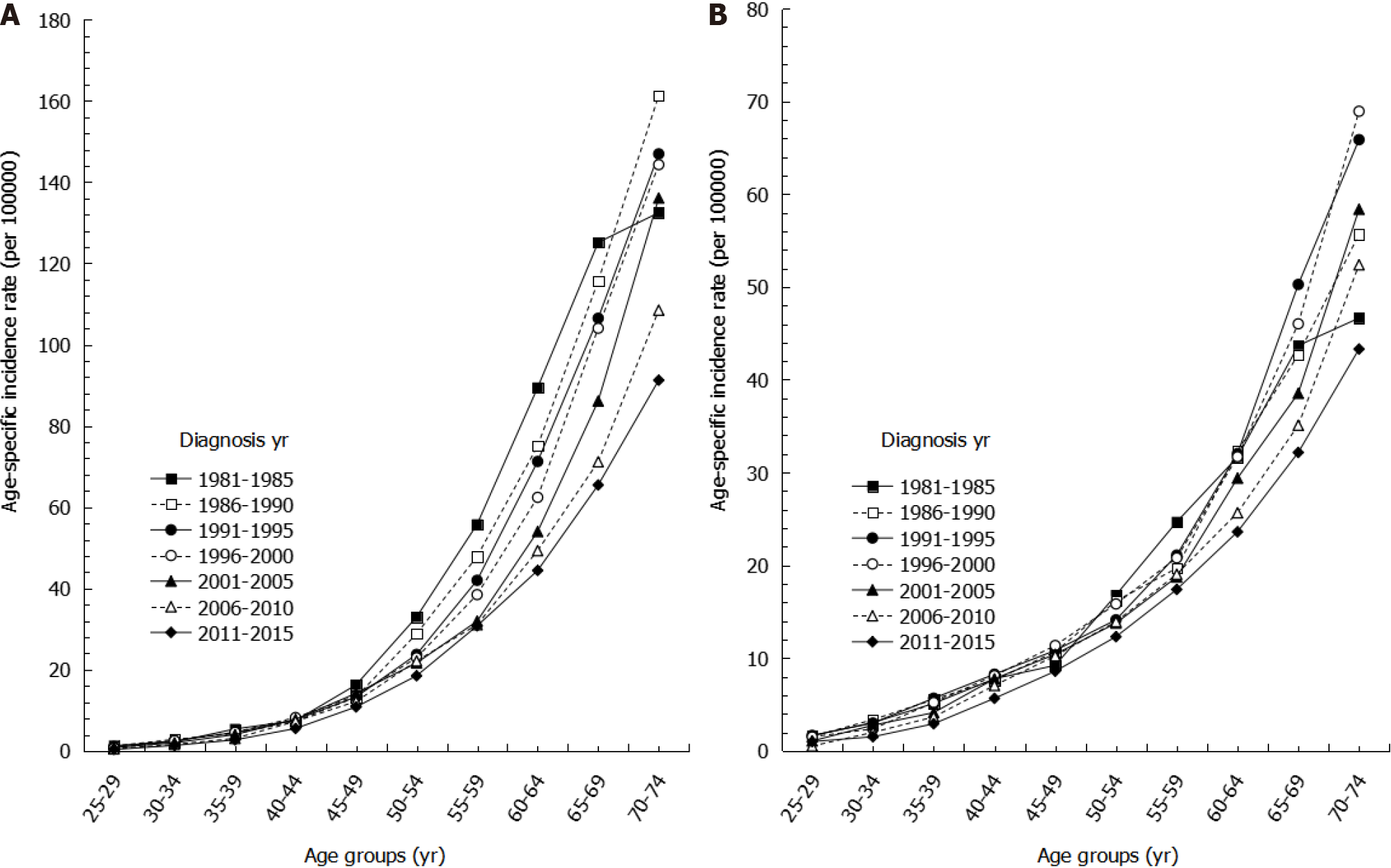
Looking at age-specific incidence rates by sex for the years of 1981 to 2015, we found that age-specific incidence rates of gastric cancer increased with age, with the highest incidence rate occurring among the oldest age group across all time periods (Figure 2). Among the same age group, more recent time periods showed lower incidence rates than earlier time periods. For example, among males aged 60-64 years, the incidence rate per 100000 persons during 2011-2015 was 44.42, which was lower than the incidence rate for 1996-2000, which had an incidence rate of 62.41. Moreover, among the same age group, the decreasing trend in age-specific incidence was more evident in males than in females (Figure 2).
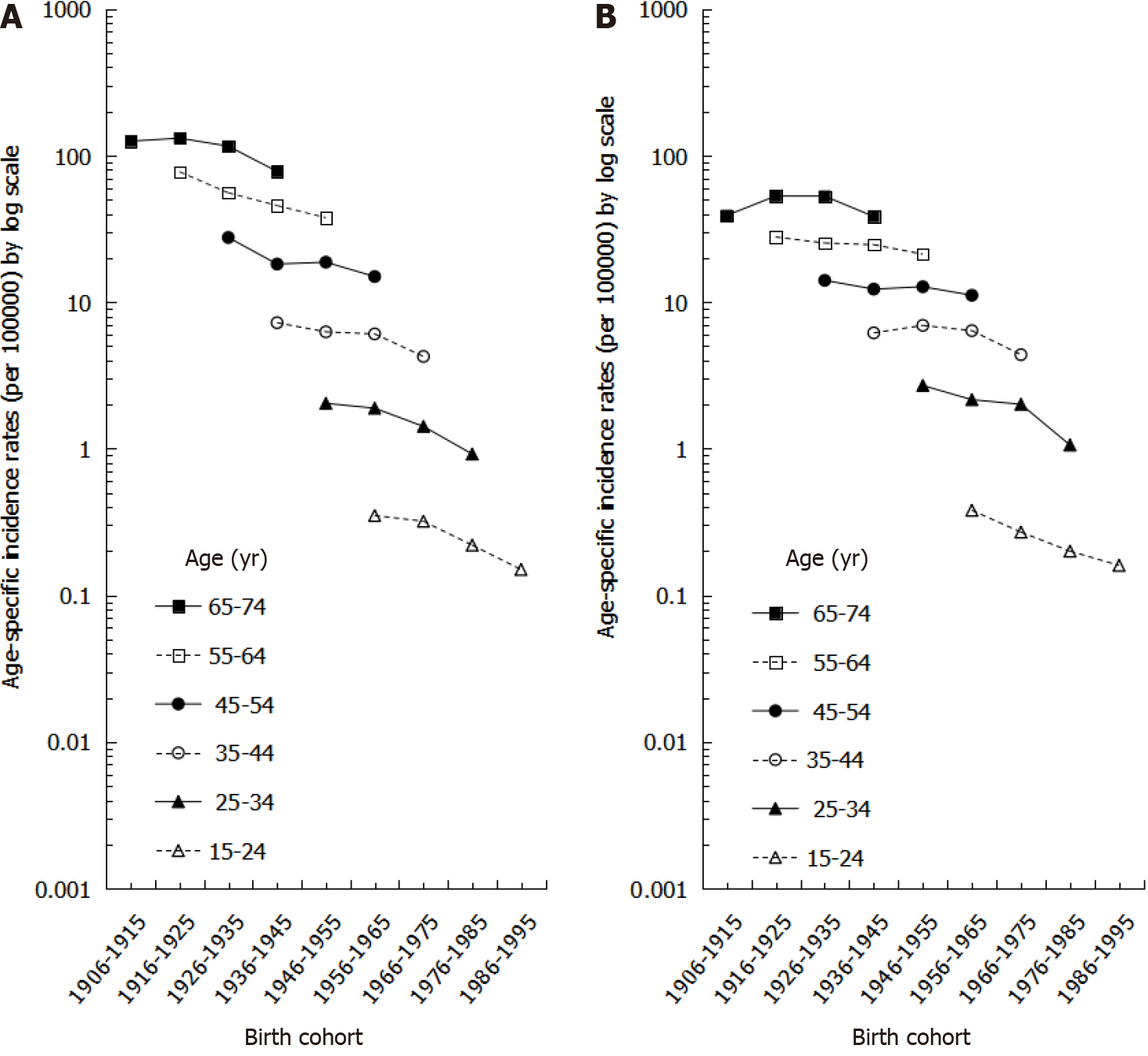
The age-specific incidence rates by birth cohort for both males and females are shown in Figure 3. In both males and females, older birth cohorts had higher incidence rates than younger birth cohorts within the same age group. Moreover, among the same birth cohort, incidence rates increased more significantly by age in males than in females (Figure 3).
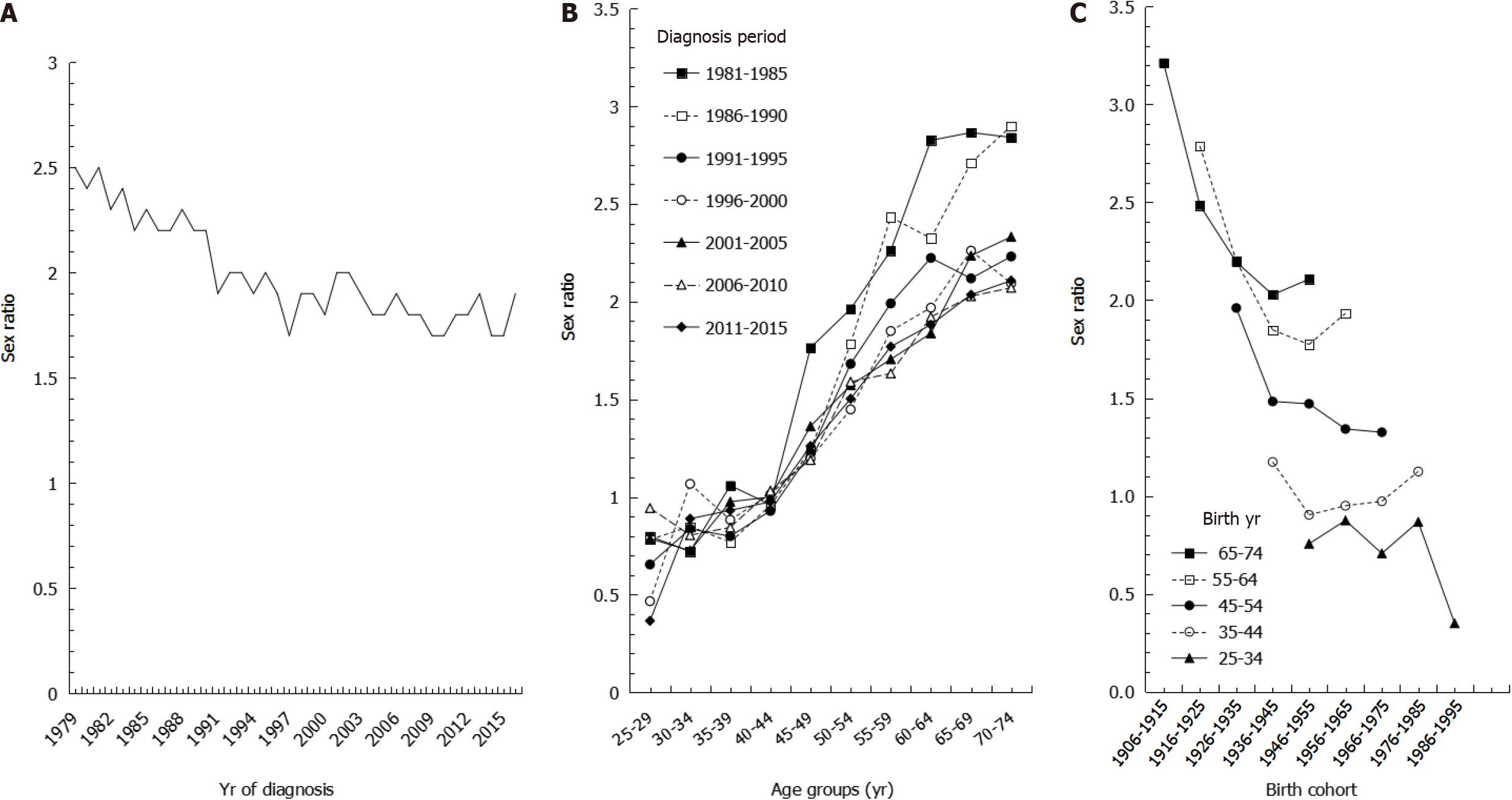
We also found that the sex ratios of age-adjusted incidence rates decreased from 1981 to 2016 (Figure 4A). Males had higher age-adjusted incidence rates than females, with sex ratios ranging from 2.5 in 1979 to 1.9 in 2016, with an AAPC of -0.67% during the study period. During time periods prior to 1990, age-specific sex ratios were higher in older age groups, whereas the sex ratios were more consistent during time periods after 1996 (Figure 4B). Sex ratios of age-specific incidence rates by birth cohort showed that among the same age group, sex ratios were higher in earlier birth cohorts than in later birth cohorts. Whereas the sex ratios were close to 1 in the 35-44 age group, the sex ratios were all less than 1 in the 25-34 age group (Figure 4C).
Furthermore, we also investigated the association between gastric cancer incidence and H. pylori treatment (Figure 5). During 2005 to 2016, the age-adjusted incidence rates decreased substantially along with increasing numbers of patients newly treated with antibiotic therapy for H. pylori infection (r = 0.72).
This population-based study found an overall secular trend of declining gastric cancer incidence in Taiwan from 1979 to 2016. Age-adjusted incidence rates decreased after 2002, with a statistically significant trend in both males and females; although, our results were slightly lower than estimates from other East Asian countries in terms of proportion changes. For example, one study from Japan found a decreasing trend with an AAPC of -1.4%, while another study from China found an AAPC of -3.7%[10]. Meanwhile, Taiwan showed an AAPC in incidence rate of -1.62% from 1996 to 2013[11]. The downward trend in incidence was greater in males during earlier years, but this difference has since diminished in recent years. Furthermore, the downward trend observed from 2002 to 2016 was higher in males than in females, with an AAPC of -2.59% in males and -2.40% in females. Our proportion changes were slightly lower than estimates from other Asian countries, as shown in a study by Bertuccio et al[12]. One possible reason for the discrepancy may be that our study period began in the 1980s, after the incidence of gastric cancer had already been steadily declining for several decades.
As the development of gastric cancer is a multistage process arising from different risk factors, the decline in incidence rates in Taiwan may be attributable to the following reasons. First, the Taiwanese government launched an H. pylori eradication program in 1997[13]. Although anti-H. pylori treatments started in 1997, the number of patients treated with antibiotic therapy was unstable during the first decade. There
Several clinical studies have demonstrated the prophylactic role of H. pylori-eradication in the prevention of gastric cancer. For instance, in a Swedish study[14] of 39154 patients undergoing hip replacement, prophylactic antibiotic treatment conferred a reduction in gastric cancer risk after 5 years (odds ratio: 0.6; 95%CI: 0.3 to 1.2). In addition, another Japanese study reported that H. pylori eradication inhibited the development of gastric cancer after endoscopic resection during a follow-up period of 3 years[15]. Ever since the government approved H. pylori eradication, there has been a decreasing trend in peptic ulcers in Taiwan[13,16]. The mass eradication of H. pylori infection was launched in 2004 among a high-risk Taiwanese population with prevalent H. pylori infection that were living on the Matsu Islands, and continued until 2018. As a result of this effort, the prevalence rates of H. pylori decreased from 64.2% to 15.0%, with reinfection rates of less than 1% per person-year[5]. In one Taiwanese study of 80255 peptic ulcer patients, early H. pylori eradication conferred a reduction in early gastric cancer risk (hazard ratio: 0.77)[17]. In addition, this study also showed that early H. pylori eradication reduced the standardized incidence ratio of gastric cancer from 1.60 to 1.05 (95%CI: 0.96 to 1.14). Together with results from these studies, our findings provide supportive evidence that the eradication of H. pylori infection may be associated with a substantial reduction in gastric cancer incidence.
Second, dietary and lifestyle habits in Taiwan have improved in the past few decades. Earlier studies showed that the consumption of foods containing nitrosamine[18] and cigarette smoking[19] were risk factors for gastric cancer. However, the increased use of refrigerators as a method of food preservation led to a decreased consumption of preserved foods containing nitrosamine and provided the constant availability of fresh food[20]. In Taiwan, a national public survey claimed that the popularization rate of refrigerators was approximately 99.14% in 1991 compared to a popularization rate of only 74.19% in 1976, with 3.6% of families even owning two refrigerators[21]. In regard to cigarette smoking, the Tobacco Hazards Prevention Act was implemented in January 2009, and the prevalence of smoking in adults over 40 years of age dropped from 12.25% in 2001 to 8.18% in 2013[22].
Our age-adjusted incidence rates were slightly lower than estimates from other East Asian countries in terms of proportion change. The reason may be that there has been no evident decline in alcohol consumption, another known risk factor for gastric cancer[23]. Surveys in Taiwan showed that the prevalence of alcohol drinking among males was 38.2% in 2001 and 24.2% in 2013, while the prevalence among females was 9.2% in 2001 and 9.3% in 2013[24]. Furthermore, obesity is also associated with an increased risk of gastric cancer[25-27]. The body mass index of the Taiwanese po
We also showed a steady decrease in sex ratios. In our results examining sex ratios by birth cohort, younger age groups had lower sex ratios, especially in the 25-34 age group, which had a ratio lower than 1, indicating that males accounted for a lower incidence of gastric cancer. The trends in sex ratios by birth cohort are similar to the intestinal type of gastric cancer based on histopathology and non-cardia site based on anatomic site[29]. We speculate that the decreasing trend in gastric cancer may be mainly attributed to declines in the intestinal type histology because the intestinal type of gastric cancer affects males and older age groups more frequently[30], leading to a greater decrease in incidence among males than among females. Furthermore, the dominant risk factors for gastric cancer, especially for non-cardia cancer, are diet, cigarette smoking, and H. pylori infection, whereas the risk factors for cardia cancer are obesity, gastroesophageal reflux, and Barrett’s esophagus[31]. This may explain why the corresponding decrease in Taiwan is mainly in non-cardia gastric cancer, as the prevalence of risk factors such as poor diet, smoking, and H. Pylori infection are decreasing in Taiwan.
Regarding the sex ratios in age-specific incidence, we found a large curve in sex ratios around 45-59 years of age. Lindblad et al[32] indicated that estrogen may prevent gastric cancer, and our results are in accordance with females in this age group being exposed to some protective factors against gastric cancer, such as estrogen and reproductive factors. The growth of gastric cancer cells may be stimulated during low concentrations of estrogen, such as in young females and postmenopausal women, while the growth of cancer cells may be inhibited in females with high concentrations of estrogen[33,34].
In this study, we summarize the descriptive epidemiology of gastric cancer in
In conclusion, we show continuously decreasing rates of gastric cancer in both sexes, increasing age-specific incidence rates with age, lower sex ratios in later birth cohorts, and decreasing age-adjusted incidence rates with increasing anti-H. pylori treatment. The results support the need to further address the issue of mass anti-H. pylori treatment in areas with a high prevalence rate of H. pylori infection in order to elimi
Gastric cancer is a multifactorial cancer and is a leading cause of cancer death world
We desired to discover the association between gastric cancer incidence and potential factors, including anti-Helicobacter pylori (H. pylori) treatment.
The objective of this study was to observe secular trends in gastric cancer incidence according to age, sex, and the implementation of a nationwide H. pylori treatment program in Taiwan.
We used the national Taiwan Cancer Registry database in this population-based study. Annual percent changes in incidence rates were used to describe secular trends in incidence rates and sex ratios of gastric cancer in Taiwan and to study the relationship between its incidence and potential risk factors, including anti-H. pylori treatment. Pearson’s product-moment correlation coefficients were used to analyze the corre
The annual percent changes showed steadily decreasing rates of gastric cancer in both sexes. However, the decreasing trends differed by sex, with an annual percent change of -2.58% in males and -2.14% in females. The age-specific incidence rates increased with age. Among the same age group, more recent time periods showed lower in
We observed steadily decreasing trends with differential sex ratios in the incidence of gastric cancer in Taiwan. The results also support the association between trends in gastric cancer incidence and the implementation of H. pylori eradication programs in Taiwan.
Further long-term cohort studies are needed to investigate the relationship between H. pylori treatment and gastric cancer histologic type and anatomic site and should include adjusting for other confounders, such as dietary and lifestyle habits and genetic susceptibility.
| 1. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, Znoar A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020. |
| 2. | Ministry of Health and Welfare. Cancer Registry Annual Report. Department of Health, editor. Taiwan, Republic of China, 2021. |
| 3. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 4. | IARC Helicobacter pylori Working Group. Helicobater pylori Eradication as a Strategy for Gastric Cancer Prevention. In: IARC Working Group Reports, No 8. International Agency for Research on Cancer, editor. Lyon, France, 2014. |
| 5. | Chiang TH, Chang WJ, Chen SL, Yen AM, Fann JC, Chiu SY, Chen YR, Chuang SL, Shieh CF, Liu CY, Chiu HM, Chiang H, Shun CT, Lin MW, Wu MS, Lin JT, Chan CC, Graham DY, Chen HH, Lee YC. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Ministry of Health and Welfare. Cancer Registry Annual Report, 2016. In: Health Promotion Administration. Taiwan, Republic of China, 2016. |
| 7. | Clarke CA, Undurraga DM, Harasty PJ, Glaser SL, Morton LM, Holly EA. Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer Epidemiol Biomarkers Prev. 2006;15:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Liou JM, Chang CY, Chen MJ, Chen CC, Fang YJ, Lee JY, Wu JY, Luo JC, Liou TC, Chang WH, Tseng CH, Wu CY, Yang TH, Chang CC, Wang HP, Sheu BS, Lin JT, Bair MJ, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. The Primary Resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors-A Nationwide Study. PLoS One. 2015;10:e0124199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Ahmad OB, Boschi-Pinto C, Lopez AD. Age standardization of rates: A new WHO standard. 2001. [cited 10 March 2021]. Available from: https://www.who.int/healthinfo/paper31.pdf. |
| 10. | Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer. 2017;141:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Chang JS, Kuo SH, Chu PY, Shan YS, Tsai CR, Tsai HJ, Chen LT. The Epidemiology of Gastric Cancers in the Era of Helicobacter pylori Eradication: A Nationwide Cancer Registry-Based Study in Taiwan. Cancer Epidemiol Biomarkers Prev. 2019;28:1694-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 486] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 13. | Wu CY, Wu CH, Wu MS, Wang CB, Cheng JS, Kuo KN, Lin JT. A nationwide population-based cohort study shows reduced hospitalization for peptic ulcer disease associated with H pylori eradication and proton pump inhibitor use. Clin Gastroenterol Hepatol. 2009;7:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 14. | Akre K, Signorello LB, Engstrand L, Bergström R, Larsson S, Eriksson BI, Nyrén O. Risk for gastric cancer after antibiotic prophylaxis in patients undergoing hip replacement. Cancer Res. 2000;60:6376-6380. [PubMed] |
| 15. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] |
| 16. | Lee YC, Lin JT. Screening and treating Helicobacter pylori infection for gastric cancer prevention on the population level. J Gastroenterol Hepatol. 2017;32:1160-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 17. | Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 18. | Hsiung HY, Fann JC, Yen AM, Chen SL, Chiu SY, Ku TH, Liu TY, Chen HH, Lin MW. Stage-specific Dietary Factors Associated with the Correa Multistep and Multifactorial Process of Human Gastric Carcinogenesis. Nutr Cancer. 2016;68:598-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kneller RW, You WC, Chang YS, Liu WD, Zhang L, Zhao L, Xu GW, Fraumeni JF Jr, Blot WJ. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst. 1992;84:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 20. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (2)] |
| 21. | Directorate-General of Budget. Report on the survey of personal income & distribution in Taiwan area of the republic of China. [cited 4 August 2021]. In: Taiwan, Republic of China: Executive Yuan, 1994 [Internet]. Available from: https://win.dgbas.gov.tw/fies/doc/result/83.pdf. |
| 22. | Shih YH, Chang HY, Lu MI, Hurng BS. Time trend of prevalence of self-reported cataract and its association with prolonged sitting in Taiwan from 2001 and 2013. BMC Ophthalmol. 2014;14:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Scherübl H. Alcohol Use and Gastrointestinal Cancer Risk. Visc Med. 2020;36:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 24. | Lai YJ, Hu HY, Lee YL, Ko MC, Ku PW, Yen YF, Chu D. Frequency of alcohol consumption and risk of type 2 diabetes mellitus: A nationwide cohort study. Clin Nutr. 2019;38:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Wang FW, Tu MS, Mar GY, Chuang HY, Yu HC, Cheng LC, Hsu PI. Prevalence and risk factors of asymptomatic peptic ulcer disease in Taiwan. World J Gastroenterol. 2011;17:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 26. | Lee HH, Wu HY, Chuang YC, Chang AS, Chao HH, Chen KY, Chen HK, Lai GM, Huang HH, Chen CJ. Epidemiologic characteristics and multiple risk factors of stomach cancer in Taiwan. Anticancer Res. 1990;10:875-881. [PubMed] |
| 27. | Song M, Choi JY, Yang JJ, Sung H, Lee Y, Lee HW, Kong SH, Lee HJ, Kim HH, Kim SG, Yang HK, Kang D. Obesity at adolescence and gastric cancer risk. Cancer Causes Control. 2015;26:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Lai YJ, Hu HY, Lee YL, Ku PW, Yen YF, Chu D. Association between obesity and risk of chronic kidney disease: A nationwide Cohort study in Taiwan. Nutr Metab Cardiovasc Dis. 2017;27:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 29. | Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (3)] |
| 30. | Vauhkonen M, Vauhkonen H, Sipponen P. Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:651-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 31. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1262] [Article Influence: 63.1] [Reference Citation Analysis (20)] |
| 32. | Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13:2203-2207. [PubMed] |
| 33. | Ur Rahman MS, Cao J. Estrogen receptors in gastric cancer: Advances and perspectives. World J Gastroenterol. 2016;22:2475-2482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 34. | Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Doorakkers E, Lagergren J, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut. 2018;67:2092-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai ZL, Dong QJ, Keikha M S-Editor: Liu M L-Editor: Wang TQ P-Editor: Yuan YY