Published online Sep 14, 2021. doi: 10.3748/wjg.v27.i34.5753
Peer-review started: January 6, 2021
First decision: May 5, 2021
Revised: May 15, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: September 14, 2021
Processing time: 245 Days and 17 Hours
Non-invasive fibrosis scores are not yet validated in the newly defined metabolic associated fatty liver disease (MAFLD).
To evaluate the diagnostic performance of four non-invasive scores including aspartate aminotransferase to platelet ratio index (APRI), fibrosis-4 index (FIB-4), body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes score (BARD), and nonalcoholic fatty liver disease fibrosis score (NFS) in patients with MAFLD.
Consecutive patients with histologically confirmed MAFLD were included. The discrimination ability of different non-invasive scores was compared.
A total of 417 patients were included; 156 (37.4%) of them had advanced fibrosis (Metavir ≥ F3). The area under receiver operating characteristic curve of FIB-4, NFS, APRI, and BARD for predicting advanced fibrosis was 0.736, 0.724, 0.671, and 0.609, respectively. The area under receiver operating characteristic curve of FIB-4 and NFS was similar (P = 0.523), while the difference between FIB-4 and APRI (P = 0.001) and FIB-4 and BARD (P < 0.001) was statistically significant. The best thresholds of FIB-4, NFS, APRI, and BARD for diagnosis of advanced fibrosis in MAFLD were 1.05, -2.1, 0.42, and 2. A subgroup analysis showed that FIB-4, APRI, and NFS performed worse in the pure MAFLD group than in the hepatitis B virus-MAFLD group.
APRI and BARD scores do not perform well in MAFLD. The FIB-4 and NFS could be more useful, but a new threshold is needed. Novel non-invasive scoring systems for fibrosis are required for MAFLD.
Core Tip: Metabolic associated fatty liver disease (MAFLD) is a new concept proposed in 2020 to redefine fatty liver disease. The utility of non-invasive fibrosis scores as well as their optimal thresholds for MAFLD remains unknown. We validated the conventional non-invasive scores including aspartate aminotransferase to platelet ratio index, fibrosis-4 index, body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes score, and nonalcoholic fatty liver disease fibrosis score in patients with MAFLD. The results indicate that the conventional scores may lead to a high rate of misdiagnosis in MAFLD. A novel scoring system for fibrosis is urgently needed.
- Citation: Wu YL, Kumar R, Wang MF, Singh M, Huang JF, Zhu YY, Lin S. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol 2021; 27(34): 5753-5763
- URL: https://www.wjgnet.com/1007-9327/full/v27/i34/5753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i34.5753
Nonalcoholic fatty liver disease (NAFLD), defined as excessive fat accumulation in liver cells in the absence of other liver diseases, has become a new epidemic due to its growing prevalence[1,2]. To date, NAFLD is believed to affect more than a quarter of the global population[2,3]. The natural history of NAFLD is highly variable; however, it is believed to progress through various fibrosis stages to end up in liver cirrhosis in a significant number of patients. The development and grade of liver fibrosis are strongly related with the adverse outcomes of NAFLD[4-6]. Thus, it is critical to identify patients with advanced fibrosis to optimize the management of NAFLD.
Liver biopsy is currently regarded as the “gold standard” for the diagnosis of liver fibrosis. However, due to the high prevalence of NAFLD, it is impossible to perform a biopsy for each patient. Moreover, the inherent issues including safety, sampling errors, and the inter- and intraobserver variation limit its application[7,8]. These limitations warrant the need for non-invasive scores for assessing liver fibrosis.
Numerous non-invasive assessment tools have been developed for diagnosis of advanced fibrosis[9]. The most widely used non-invasive scores include aspartate aminotransferase (AST) to platelet ratio index (APRI), fibrosis-4 index (FIB-4), body mass index (BMI), AST/alanine aminotransferase (ALT) ratio, diabetes score (BARD), and NAFLD fibrosis score (NFS). Most of them have been tested in subjects with NAFLD, showing great diagnostic accuracy in predicting fibrosis[10-13].
Metabolic associated fatty liver disease (MAFLD) is a recently proposed concept to replace NAFLD[14]. Significantly different from NAFLD, the MAFLD definition does not require the exclusion of any chronic liver diseases; however, the presence of metabolic associated disease or dysfunction is required[15,16]. It is known that metabolic profiles are associated with a risk for severe fibrosis in patients with NAFLD[17]. The MAFLD population has been found to have higher non-fibrosis scores than NAFLD individuals[18]. Thus, in the light of the new concept of MAFLD, which incorporates metabolic disorder, the performance of these non-invasive models requires re-evaluation and further validation. This study aimed to evaluate the utility of conventional non-invasive scoring systems in MAFLD.
The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University and was in accordance with the Declaration of Helsinki. Written consent forms were obtained from all patients. The data was anonymized prior to the analysis.
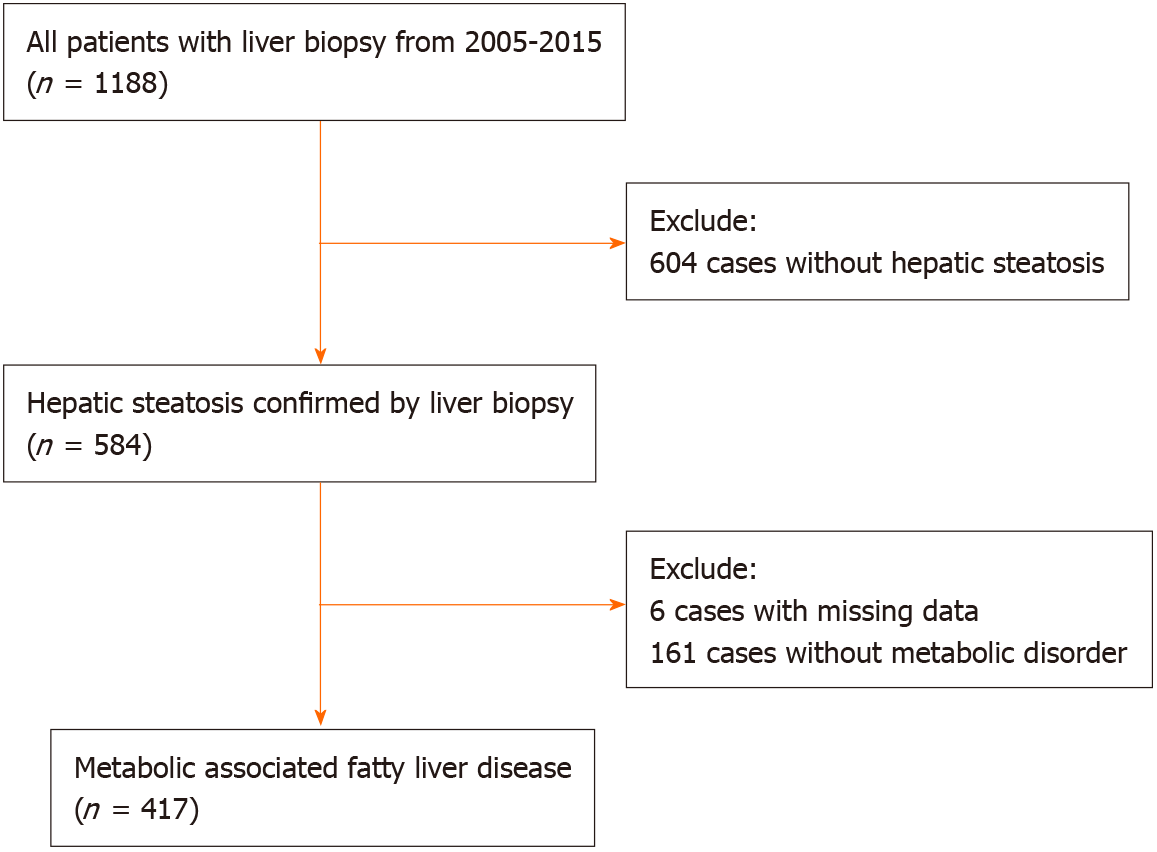
Consecutive patients with histologically confirmed MAFLD admitted to the First Affiliated Hospital of Fujian Medical University from 2005 to 2015 were retro
All patients enrolled in this study underwent percutaneous liver biopsy under ultrasonic guidance. The liver specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin and Masson’s trichrome. The minimum biopsy length was 15 mm, and at least six portal areas were required[19]. Histopathological slides were reviewed independently by two pathologists experienced in reading liver histopathology slides and were blinded to the patient’s clinical data.
Fatty liver was defined as the presence of steatosis in at least 5% of hepatocytes. The liver fibrosis was graded as 0 to 4 points according to the Metavir fibrosis stage[20], where 0 = absence of fibrosis; 1 = perisinusoidal or periportal; 2 = perisinusoidal and portal/periportal; 3 = bridging fibrosis; 4 = cirrhosis. Advanced fibrosis was defined as stage 3 or 4 fibrosis (bridging fibrosis or cirrhosis).
The diagnosis of MAFLD was based on the following criteria[15]: A histological evidence of hepatic steatosis and the presence of one of the following three conditions: BMI ≥ 23 kg/m2, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation. The metabolic dysregulation was defined by the presence of at least two of the following conditions: (1) Waist circumference ≥ 90 cm in men and 80 cm in women; (2) Blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) Plasma triglycerides ≥ 1.70 mmol/L or specific drug treatment; (4) Plasma high-density lipoprotein cholesterol < 1.0 mmol/L for men and < 1.3 mmol/L for women or specific drug treatment; (5) Prediabetes (i.e. fasting glucose levels 5.6-6.9 mmol/L or 2 h post-load glucose levels 7.8-11.0 mmol/L or glycated hemoglobin 5.7%-6.4%; (6) Homeostasis model assessment-insulin resistance score ≥ 2.5; and (7) Plasma high-sensitivity C-reactive protein level > 2 mg/L.
According to the result of hepatitis B surface antigen (HBsAg) seropositivity, patients were divided into pure MAFLD group (HBsAg negative) and hepatitis B virus (HBV)-MAFLD group (HBsAg positive for > 6 mo) for the purpose of subgroup analysis.
The following data were collected at the time of biopsy from all patients: age, gender, BMI, waist circumference, history of diabetes, and hypertension. Laboratory parameters were as follows: Blood cell count, AST, ALT, γ-glutamyl transpeptidase, albumin, globulin, bilirubin, fasting plasma glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, urea, creatinine, uric acid, fasting insulin, glycated hemoglobin, high-sensitivity C-reactive protein, and HBsAg.
All biochemical assessments were performed by standard laboratory methods. Non-invasive liver fibrosis assessment scores included APRI, FIB-4, BARD, and NFS, which were calculated based on previously published formulas as shown in Table 1[10,12,21,22].
| Formula | Equation | Lower cutoff | Higher cutoff |
| FIB-4 | [Age (yr) × AST (IU/L)]/{platelet count (109/L) × [ALT(IU/L)]1/2} | 1.3 | 2.67[11] |
| 1.45 | 3.25[22] | ||
| APRI | [(AST/ULN)/platelet count (109/L)] × 100 | 0.5 | 1.5[21] |
| NFS | -1.675 + 0.037 × age (yr) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT-0.013 × platelet count (× 109/L) - 0.66 × albumin (g/dL) | -0.676 | 1.455[10] |
| BARD | Scale 0-4; BMI ≥ 28 kg/m2 = 1 point; AST/ALT ≥ 0.8 = 2 points; Diabetes = 1 point | 2[12] | |
Continuous variables are expressed as mean ± SD or median (interquartile range) and were compared using Student’s t test in the case of normally distributed data or Mann-Whitney test in the remaining cases. Categorical variables are expressed as counts (percentages) and evaluated by χ2 test or the Fisher’s exact test. The diagnostic accuracy of conventional non-invasive scoring systems was evaluated by the receiver operating characteristic (ROC) curve. The best cut-off values to determine the presence of advanced fibrosis were chosen based on Youden’s index. The discrimination ability of the different models was compared using area under ROC curve (AUROC), positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity, and accuracy. Statistical analyses were performed using the SPSS software, version 18.0 (SPSS, Chicago, IL, United States) and MedCalc software version 15.2.2 (MedCalc Software, Mariakerke, Belgium). A P value < 0.05 was considered statistically significant.
A total of 417 patients with biopsy-proven MAFLD were included in this study (Figure 1). All patients were treatment-naïve and did not receive nucleosides analogue treatment before biopsy. The mean age was 40.54 ± 10.95 years, and the BMI was 25.48 ± 2.66 kg/m2. Of them, 354 (84.9%) were male, 82 (19.7%) had type 2 diabetes mellitus, 42 (10.1%) had hypertension, and 156 (37.4%) had advanced fibrosis (Table 2). Liver histological examination showed that 272 (76.0%) HBV-MAFLD patients had significant inflammation (grade ≥ 2). Antiviral therapy was initiated when chronic hepatitis B was diagnosed with the presence of significant inflammation (grade ≥ 2) or significant liver fibrosis (stage ≥ 2). Patients with advanced fibrosis were significantly older and had higher AST and lower albumin, triglyceride, and platelet counts. In addition, the FIB-4 (P < 0.001), NFS (P < 0.001), APRI (P = 0.003) and BARD (P < 0.001) scores were all significantly higher in patients with advanced fibrosis compared with patients with no/mild fibrosis.
| Overall, n = 417 | No/mild fibrosis, n = 261 | Advanced fibrosis, n = 156 | P value | |
| Age (yr) | 40.54 ± 10.95 | 39.42 ± 11.39 | 42.41 ± 9.94 | 0.007 |
| Male, n (%) | 354 (84.9) | 219 (83.9) | 135 (86.5) | 0.468 |
| BMI (kg/m2) | 25.48 ± 2.66 | 25.51 ± 2.56 | 25.42 ± 2.83 | 0.757 |
| Diabetes mellitus, n (%) | 82 (19.7) | 46 (17.6) | 36 (23.1) | 0.175 |
| Hypertension, n (%) | 42 (10.1) | 30 (11.5) | 12 (7.7) | 0.212 |
| ALB (g/L) | 42.53 ± 4.96 | 43.65 ± 5.14 | 40.65 ± 3.98 | < 0.001 |
| GLO (g/L) | 29.88 ± 14.11 | 29.11 ± 6.00 | 31.13 ± 21.53 | 0.160 |
| ALT (U/L) | 133.07 ± 199.05 | 126.93 ± 151.37 | 143.33 ± 260.27 | 0.416 |
| AST (U/L) | 70.69 ± 85.14 | 63.24 ± 62.19 | 83.17 ± 112.77 | 0.021 |
| GGT (U/L) | 89.64 ± 109.58 | 88.75 ± 117.46 | 91.11 ± 95.28 | 0.832 |
| FPG (mmol/L) | 5.34 ± 1.42 | 5.28 ± 1.20 | 5.45 ± 1.73 | 0.251 |
| Cr (μmol/L) | 73.41 ± 13.71 | 74.00 ± 13.50 | 72.41 ± 14.07 | 0.273 |
| UA (μmol/L) | 368.49 ± 82.08 | 377.02 ± 83.87 | 353.77 ± 76.98 | 0.007 |
| TG (mmol/L) | 1.67 ± 1.12 | 1.78 ± 1.45 | 1.48 ± 0.71 | 0.016 |
| HDL-C (mmol/L) | 1.12 ± 0.30 | 1.12 ± 0.29 | 1.12 ± 0.33 | 0.959 |
| PLT (× 109/L) | 204.00 ± 64.22 | 221.00 ± 62.76 | 175.52 ± 56.22 | < 0.001 |
| FIB-4 | 1.48 ± 1.64 | 1.19 ± 1.65 | 1.97 ± 1.49 | < 0.001 |
| APRI | 1.03 ± 1.72 | 0.84 ± 1.33 | 1.34 ± 2.19 | 0.003 |
| NFS | -2.16 ± 1.38 | -2.56 ± 1.24 | -1.50 ± 1.33 | < 0.001 |
| BARD (%) | < 0.001 | |||
| 0 | 209 (50.1) | 145 (55.6) | 64 (41.0) | |
| 1 | 86 (20.6) | 59 (22.6) | 27 (17.3) | |
| 2 | 92 (22.1) | 48 (18.4) | 44 (28.2) | |
| 3 | 25 (6.0) | 8 (3.1) | 17 (10.9 ) | |
| 4 | 5 (1.2) | 1 (0.4) | 4 (2.6) |
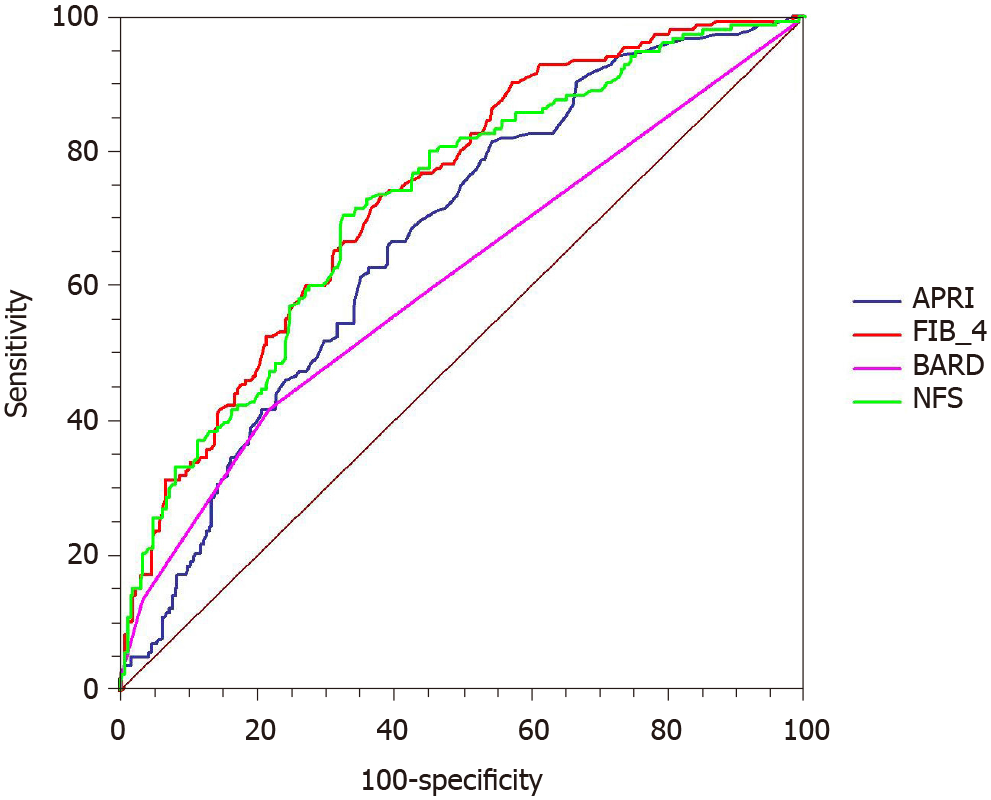
The ROC curves were used to evaluate the utility of non-invasive scoring systems to identify advanced fibrosis (Figure 2). The AUROC was greatest for FIB-4 [0.736; 95% confidence interval (CI): 0.691-0.778] followed by NFS (0.724; 95%CI: 0.679-0.767), APRI (0.671; 95%CI: 0.623-0.715), and BARD (0.609; 95%CI: 0.560-0.656). The comparison between AUROCs showed that the discrimination abilities of FIB-4 and NFS were similar (P = 0.523). Similar results were also noted in NFS and APRI (P = 0.080). The differences between FIB-4 and APRI (P = 0.001), FIB-4 and BARD (P < 0.001), as well as NFS and BARD (P < 0.001) were statistically significant.
Table 3 summarizes the best cutoff values developed for prediction of advanced fibrosis by the four non-invasive models and the validation of previously reported cutoffs (for NAFLD) in this MAFLD cohort[10-12,21,22]. The best cutoff scores of FIB-4, APRI, NFS, and BARD for the diagnosis of advanced fibrosis in MAFLD were 1.05, 0.42, -2.1, and 2, respectively. Most cutoff scores were lower than the prior reported thresholds for each model. Using the newly developed thresholds, the sensitivity, specificity, PPV, and NPV of the above four models ranged from 41.7%-81.4%, 44.4%-78.2%, 46.7%-55.8%, and 69.2%-80.0%, respectively (Table 3).
| Cutoffs | AUROC | Accuracy, % | Advanced fibrosis being missed, %1 | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Youden index | |
| FIB-4 | 1.05 | 0.736 | 66.2 | 20.3 | 73.7 | 61.7 | 53.5 | 79.7 | 0.354 |
| 1.30 | 68.1 | 25.4 | 57.7 | 74.3 | 57.3 | 74.6 | 0.320 | ||
| 1.45 | 68.8 | 26.5 | 52.6 | 78.5 | 59.4 | 73.5 | 0.311 | ||
| 2.67 | 66.2 | 34.1 | 17.3 | 95.4 | 69.2 | 65.9 | 0.127 | ||
| 3.25 | 66.7 | 34.4 | 14.1 | 98.1 | 81.5 | 65.6 | 0.122 | ||
| NFS | -2.100 | 0.724 | 68.1 | 20.9 | 70.5 | 66.7 | 55.8 | 79.1 | 0.372 |
| -1.455 | 66.2 | 29.5 | 44.9 | 78.9 | 56.0 | 70.6 | 0.238 | ||
| 0.676 | 63.8 | 36.5 | 4.5 | 99.2 | 77.8 | 63.5 | 0.037 | ||
| APRI | 0.42 | 0.671 | 58.3 | 20.0 | 81.4 | 44.4 | 46.7 | 80.0 | 0.258 |
| 0.50 | 59.2 | 24.7 | 71.2 | 52.5 | 47.2 | 75.3 | 0.237 | ||
| 1.50 | 63.3 | 34.6 | 22.4 | 87.7 | 52.2 | 65.4 | 0.101 | ||
| BARD | 2 | 0.609 | 64.5 | 30.8 | 41.7 | 78.2 | 53.3 | 69.2 | 0.199 |
With the previously reported cutoff value of 1.30 (lower) and 2.67 (higher) of FIB-4 for advanced fibrosis, the PPV was only 57.3% and 69.2%, and the NPV was 74.6% and 65.9%, respectively. Similar results were found for the NFS score; the PPV and NPV of the well-accepted threshold (-1.455) were only 56.0% and 70.6% (Table 3).
The accuracy of the FIB-4 and NFS scores, irrespective of the numerical value of cutoff used, was only 63.8%-68.8%. The accuracy was even lower for APRI and BARD (58.3%-64.5%). On the other hand, 20%-35% patients with biopsy-proven advanced fibrosis were misdiagnosed as no advanced fibrosis by these four scores (Table 3).
According to the result of HBsAg, patients were divided into HBV-MAFLD (359, 86.1%) and pure MAFLD (58, 13.9%) subgroups. The difference in the baseline characteristics between HBV-MAFLD and pure MAFLD had been presented in our previous work[23] and is shown in Supplementary Table 1. Briefly, the HBV-MAFLD group had higher ALT (135.82 ± 210.31 U/L vs 119.76 ± 118.19 U/L) and AST (71.70 ± 88.63 U/L vs 65.55 ± 63.28 U/L) levels and lower platelet (198.59 ± 60.33) × 109/L vs (237.47 ± 76.97) × 109/L levels than the pure MAFLD group. The AUROCs of FIB-4, NFS, and APRI in the HBV-MAFLD group was 0.738, 0.725, and 0.671, respectively, which were all higher than in the pure MAFLD group (FIB-4 0.658, NFS 0.692, and APRI 0.633). The AUROC of BARD was lower in the HBV-MAFLD group than in the pure MAFLD group (0.609 vs 0.644). Using different thresholds mentioned above, the overall performance of the FIB-4, APRI, and NFS, including sensitivity, specificity, PPV and NPV, was better in the HBV-MAFLD group. The BARD score performed better in the pure MAFLD population (Table 4).
| Cutoffs | AUROC | Accuracy, % | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |||||||
| A | B | A | B | A | B | A | B | A | B | A | B | ||
| FIB-4 | 1.05 | 0.738 | 0.658 | 67.7 | 56.9 | 74.8 | 55.6 | 62.7 | 57.1 | 58.2 | 19.2 | 78.2 | 87.5 |
| 1.30 | 68.2 | 67.2 | 58.5 | 44.4 | 75.0 | 71.4 | 61.9 | 22.2 | 72.3 | 87.5 | |||
| 1.45 | 68.5 | 70.7 | 54.4 | 22.2 | 78.3 | 79.6 | 63.5 | 16.7 | 71.2 | 84.8 | |||
| 2.67 | 63.2 | 84.5 | 17.0 | 22.2 | 95.3 | 95.9 | 71.4 | 50.0 | 62.3 | 87.0 | |||
| 3.25 | 63.5 | 86.2 | 13.6 | 22.2 | 98.1 | 98.0 | 83.3 | 66.7 | 62.1 | 87.3 | |||
| NFS | -2.100 | 0.725 | 0.692 | 68.2 | 67.2 | 70.1 | 77.8 | 67.0 | 65.3 | 59.5 | 29.2 | 76.3 | 94.1 |
| -1.455 | 64.9 | 74.1 | 44.2 | 55.6 | 79.2 | 77.6 | 59.6 | 31.3 | 67.2 | 90.5 | |||
| 0.676 | 60.4 | 84.5 | 4.1 | 11.1 | 99.5 | 98.0 | 85.7 | 50.0 | 59.9 | 85.7 | |||
| APRI | 0.42 | 0.671 | 0.633 | 59.3 | 51.7 | 81.6 | 77.8 | 43.9 | 46.9 | 50.2 | 21.2 | 77.5 | 92.0 |
| 0.50 | 60.2 | 55.2 | 70.7 | 77.8 | 52.8 | 51.0 | 51.0 | 22.6 | 72.3 | 92.6 | |||
| 1.50 | 61.0 | 77.6 | 23.1 | 11.1 | 87.3 | 89.8 | 55.7 | 16.7 | 62.1 | 84.6 | |||
| BARD | 2 | 0.609 | 0.644 | 63.0 | 74.1 | 42.2 | 33.3 | 77.4 | 81.6 | 56.4 | 25.0 | 65.9 | 87.0 |
Non-invasive scoring systems are widely used to identify or exclude advanced fibrosis in patients with chronic liver disease. The main finding of our study is that FIB-4 and NFS performed better than APRI and BARD in MAFLD patients. These scores are more useful in HBV-MAFLD than in pure MAFLD population. However, the performance of all above models was not as good as previously reported in NAFLD.
The non-invasive fibrosis scoring systems are derived from widely available clinical, laboratory, and anthropometric parameters. Although the APRI and BARD scores are more user-friendly, their discrimination ability in prediction of advanced fibrosis is not satisfactory in this group of MAFLD patients. The AUROC of BARD was 0.609, and the accuracy was less than 65%. It is not a surprising result as the two variables for calculating BARD score (BMI and diabetes) were also variables for the diagnosis of MAFLD. This led to lower sensitivity and higher false positivity for detecting advanced fibrosis in MAFLD patients, thus the BARD score should not be recommended for MAFLD patients in clinical practice. Although APRI score was easy to calculate, it did not perform well in MAFLD as well. The NPV did not exceed 80%, and the PPV was only around 50% at any cutoff value tested. Thus, APRI should not be used for assessment of advanced fibrosis in the MAFLD population either.
The calculations of FIB-4 and NFS require the use of more complex formulas and may not be easy to use. Even though the results of our study indicate that both FIB-4 and NFS significantly outperform APRI and BARD for the prediction of advanced fibrosis in MAFLD, the NPV and PPV of these two models did not reach the similar values reported by previous studies using the cohort of NAFLD patients[10,11,24]. According to the previously reported studies on patients with NAFLD[10,11,24], when the cutoff of the FIB-4 and NFS was set at 1.3 and -1.455, respectively, the NPV increased from 90% to 93%. When the cutoff of the FIB-4 was set at 2.67, the PPV could reach 80%. However, in our cohort of MAFLD patients, by using the aforementioned cutoffs, or the new threshold found in the present study, the NPV did not exceed 75%, and PPV did not exceed 70%. This finding is of utmost clinical importance and indicates that in the light of the new concept of MAFLD, newer non-invasive fibrosis scores need to be developed and validated to assess the presence of advanced fibrosis in patients with MAFLD. Another worrying yet important finding of our study is that the use of these non-invasive fibrosis scores, even with the new cutoffs, led to a misdiagnosis of no advanced fibrosis in 20%-35% of the patients in this cohort (Table 3) when the histology actually showed the presence of advanced fibrosis.
The occurrence of HBV infection and fatty liver disease is frequently seen in Asian countries[25]. As our cohort consisted of 359 (86.1%) patients with chronic HBV infection, we performed a subgroup analysis to test the performance of the non-invasive scores in a pure MAFLD group, which is very close to previous NAFLD and HBV-MAFLD group. Three of the four non-invasive models (FIB-4, NFS, and APRI) performed even worse in the pure MAFLD group than in the HBV-MAFLD group. We speculate that the higher levels of ALT and AST and lower platelets in HBV-MAFLD might result in higher scores of the non-invasive models and make them easier to discriminate the advanced fibrosis. As MAFLD is a new entity, this result further reinforces the need to develop and validate novel scoring systems for fibrosis in the MAFLD population.
The strength of our study is that, to our knowledge, this is the first validation of conventional non-invasive fibrosis scoring systems in a large sample of histology-proven MAFLD. However, this study has some limitations. Firstly, a large proportion of included patients (86%) had concomitant chronic HBV infection, which could be a potential limitation as the western MAFLD population differs substantially from Asia’s population in the HBsAg seropositivity rates, the results may only be applicable to a subset of the entire MAFLD pool namely HBV-MAFLD, which is the most important subtype of MAFLD in clinical practice in Asia. Secondly, as our study is a single-center study with only an Asian population, the findings will need further validation in other Asian and western cohorts.
APRI and BARD scores do not perform well and are not suitable for the diagnosis of advanced fibrosis in MAFLD. The FIB-4 and NFS could be more useful, and we propose a new threshold of 1.05 and -2.1, respectively, which had the best diagnostic performance for advanced fibrosis. There is an urgent need of a novel non-invasive scoring system for predicting advanced fibrosis in patients with MAFLD including its subtypes.
Metabolic associated fatty liver disease (MAFLD) is a new concept proposed in 2020. The clinical features of MAFLD would be different from nonalcoholic fatty liver disease.
Non-invasive fibrosis scores have been tested in subjects with nonalcoholic fatty liver disease showing great diagnostic accuracy in predicting fibrosis. But the utility of non-invasive fibrosis scores, as well as their optimal thresholds, needs re-evaluation in MAFLD.
This study aimed to evaluate the diagnostic performance of four non-invasive scores including aspartate aminotransferase to platelet ratio index (APRI), fibrosis-4 index (FIB-4), body mass index, aspartate aminotransferase/alanine aminotransferase ratio, and diabetes score (BARD), and nonalcoholic fatty liver disease fibrosis score (NFS) in patients with MAFLD.
Consecutive patients with histologically-confirmed MAFLD admitted to a single medical center were included. The discrimination ability of different non-invasive scores was compared.
A total of 417 patients were included; 156 (37.4%) of them had advanced fibrosis. The area under receiver operating characteristic curve of FIB-4, NFS, APRI, and BARD for predicting advanced fibrosis were 0.736, 0.724, 0.671, and 0.609, respectively. The area under receiver operating characteristic curve of FIB-4 and NFS was similar (P = 0.523), while the difference between FIB-4 and APRI (P = 0.001) and FIB-4 and BARD (P < 0.001) was statistically significant. The best thresholds of FIB-4, NFS, APRI, and BARD for diagnosis of advanced fibrosis in MAFLD were 1.05, -2.1, 0.42, and 2, respectively. A subgroup analysis showed that FIB-4, APRI, and NFS performed worse in the pure MAFLD group than the HBV-MAFLD group.
APRI and BARD scores do not perform well in MAFLD. The FIB-4 and NFS could be more useful, but a new threshold is needed.
MAFLD is a new entity. The results of this study indicate the conventional scores may lead to misdiagnosis, and the development of novel scoring systems to assess fibrosis in the MAFLD population is urgently needed.
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5209] [Article Influence: 651.1] [Reference Citation Analysis (9)] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4012] [Article Influence: 501.5] [Reference Citation Analysis (2)] |
| 3. | Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 398] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 4. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 5. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 822] [Article Influence: 91.3] [Reference Citation Analysis (7)] |
| 6. | Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443-457.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 7. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1590] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 8. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 9. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1119] [Article Influence: 159.9] [Reference Citation Analysis (1)] |
| 10. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2369] [Article Influence: 124.7] [Reference Citation Analysis (2)] |
| 11. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1230] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 12. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 649] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 13. | Kolhe KM, Amarapurkar A, Parikh P, Chaubal A, Chauhan S, Khairnar H, Walke S, Ingle M, Pandey V, Shukla A. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol. 2019;6:e000288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2408] [Article Influence: 401.3] [Reference Citation Analysis (1)] |
| 15. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3158] [Article Influence: 526.3] [Reference Citation Analysis (2)] |
| 16. | Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What's in a name? Liver Int. 2020;40:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 17. | Petta S, Eslam M, Valenti L, Bugianesi E, Barbara M, Cammà C, Porzio M, Rosso C, Fargion S, George J, Craxì A. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver Int. 2017;37:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 19. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 20. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3128] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 21. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3339] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 22. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3806] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 23. | Wang MF, Wan B, Wu YL, Huang JF, Zhu YY, Li YB. Clinic-pathological features of metabolic associated fatty liver disease with hepatitis B virus infection. World J Gastroenterol. 2021;27:336-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 25. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1262] [Article Influence: 157.8] [Reference Citation Analysis (6)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sawada K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH