Published online Aug 21, 2021. doi: 10.3748/wjg.v27.i31.5288
Peer-review started: March 22, 2021
First decision: June 3, 2021
Revised: June 8, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: August 21, 2021
Processing time: 148 Days and 17 Hours
Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a rare mesenchymal tumor characterized by multiple nodular plexiform growth patterns and an immunophenotype with myofibroblasts. The pathological characteristics, immunohistochemistry, diagnostic criteria, differential diagnosis, and gene-level changes of PAMT have been reported in many studies. At present, the main treatment for PAMT in the reported cases is surgery; only eight cases were treated via endoscopy (excluding 1 thoracoscopic resection), and the lesions were all smaller than 5 cm. There are no reports on the prognosis and follow-up of young patients with lesion sizes reaching 5 cm who undergo endoscopic submucosal dissection (ESD). Herein, we present the first case of a young patient with a lesion size reaching 5 cm who was diagnosed with PAMT via endoscopic submucosal dissection.
A 15-year-old young man with upper abdominal pain for 2 years presented to the Gastroenterology Department of our hospital. Painless gastroscopy showed a semicircular bulge approximately 5 cm in size in the lesser curvature near the cardia of the fundus; the surface was eroded, and shallow ulcers had formed. The pathological manifestations of the biopsy were spindle cell proliferative lesions with interstitial mucinous changes, and the surface mucosa showed chronic inflammatory changes with active lesions; immunohistochemistry showed smooth muscle actin (SMA) (+), CD117 (-), CD34 (-), DOG-1 (-), S-100 (-), and Ki67 (LI: < 1%). We performed ESD on the patient. The lesion that we removed was 5 cm × 4 cm × 2 cm in size. Pathologically, the resected tissue displayed typical manifestations, such as fat spindle-shaped fibroblasts and myofibroblast-like cells showing irregular nodular hyperplasia. Immunohistochemistry staining of the tumor cells revealed the following: CD34 (partially +), SMA (weakly +), CD117 (-), DOG-1 (-), S-100 (-), SDHB (+), PCK (-), and Ki67 (labelling index: 2%). There was no recurrence or metastasis during the 3-mo follow-up after the operation, and the treatment effect was good. We also performed a review of the literature on the clinical manifestations, pathological features, immunohistochemistry, and differential diagnosis of PAMT.
At present, the diagnostic criteria for PAMT are relatively clear, but the pathogenesis and genetic changes require further study. PAMT is benign in nature, and these patients are less likely to experience local or metastatic recurrence. The main treatment is still surgery if the lesion is in the stomach. Partial gastrectomy and distal gastrectomy are the most frequently performed surgical treatments for PAMT, followed by local resection, subtotal gastrectomy, and wedge resection. But for comprehensive evaluation of the disease, ESD can be considered a suitable method to avoid excessive treatment.
Core Tip: Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a rare mesenchymal tumor. The main treatment for PAMT in the reported cases is surgery. We present a rare case of PAMT in a young patient who underwent endoscopic submucosal dissection (ESD). The biopsied and resected tissue showed interstitial mucinous changes and myofibroblast-like cells showing irregular nodular hyperplasia, and immunohistochemistry revealed that the tissue was SMA (+). There was no recurrence during the 3-mo follow-up after ESD, and the treatment effect was good. For comprehensive evaluation of the disease, ESD can be considered a suitable method to avoid excessive treatment.
- Citation: Wu JD, Chen YX, Luo C, Xu FH, Zhang L, Hou XH, Song J. Plexiform angiomyxoid myofibroblastic tumor treated by endoscopic submucosal dissection: A case report and review of the literature. World J Gastroenterol 2021; 27(31): 5288-5296
- URL: https://www.wjgnet.com/1007-9327/full/v27/i31/5288.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i31.5288
Plexiform angiomyxoid myofibroblastic tumor (PAMT), also termed plexiform fibromyxoma (PF), is a rare benign tumor of the gastrointestinal tract that was first proposed by Takahashi et al[1] in 2007. This tumor occurs mainly in the antrum of the stomach and shows unique histological features, including myxoid extracellular matrix and ovoid or spindle cells as a plexiform intramural growth pattern. Tumor cells show differentiation into myofibroblasts with SMA expression and have limited cytological atypia[2]. Although some reports showed extragastric expansion and vascular infiltration, no local metastasis or distant recurrence after resection was reported. The main treatment for PAMT in the reported cases is surgery. Herein, we present the first case of a young patient with a lesion size reaching 5 cm who was diagnosed with PAMT via endoscopic submucosal dissection.
A 15-year-old patient arrived at the Gastroenterology Department of our hospital complaining of abdominal pain for 2 years.
The patient had intermittent abdominal pain over the past 2 years without special treatment.
The patient had no previous medical history.
The patient never smoked or drank. He had no history of food or drug allergy and no family history of gastrointestinal tumors.
The temperature of the patient was 36.5 °C, heart rate was 73 beats per minute, breathing rate was 16 beats per minute, and blood pressure was 130/79 mmHg. Examination of the chest, lung, and abdomen showed no abnormalities.
The patient’s alkaline phosphatase level was 197 U/L, glutamyl transfer peptidase was 6 U/L, uric acid was 431.6 μmol/L, and phosphorus was 1.41 mmol/L. Other routine blood tests, routine urine tests, routine stool tests, and biochemical and erythrocyte sedimentation rates were all normal.
Computed tomography (CT) of the lungs and abdomen revealed no obvious abnormalities in both the lungs and mediastinum, but a space-occupying lesion in the gastric fundus area. Our clinical diagnostic consideration was gastric stromal tumor.
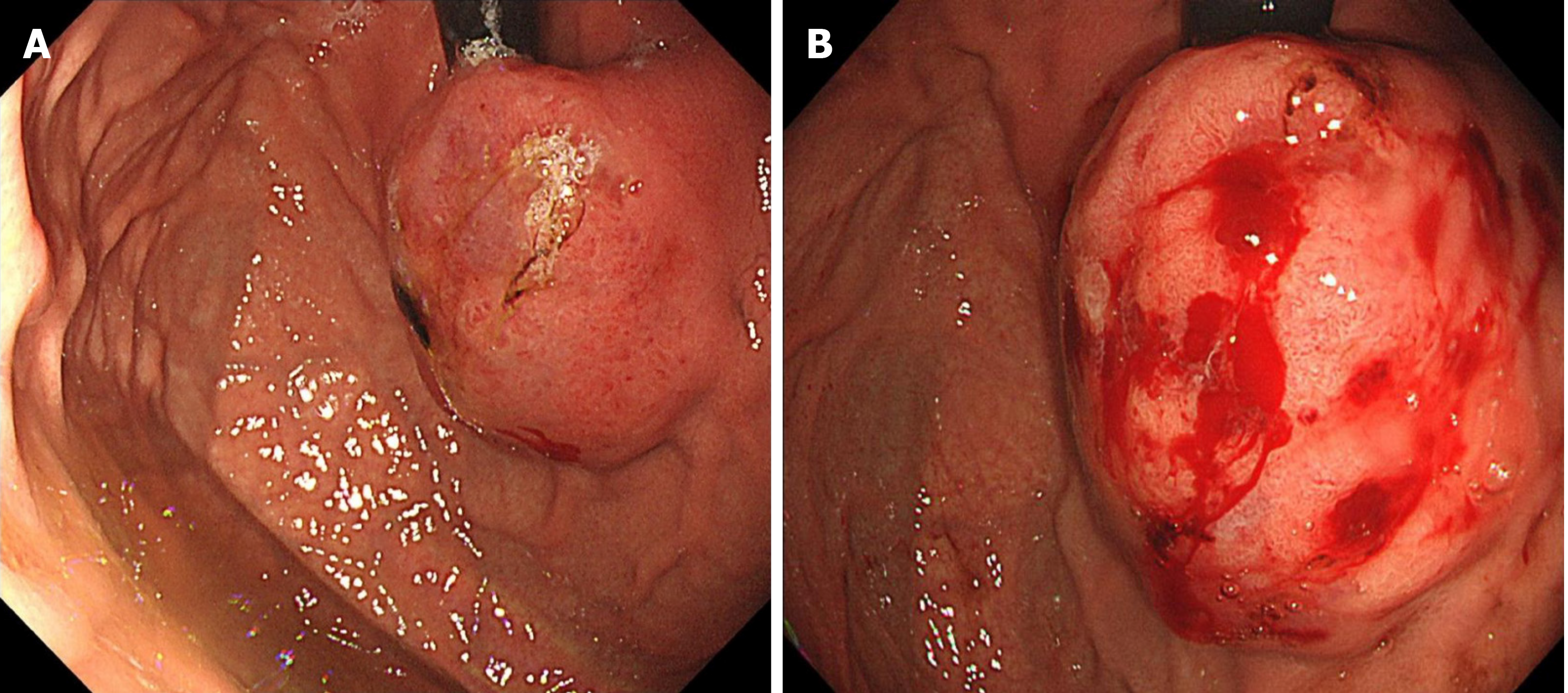
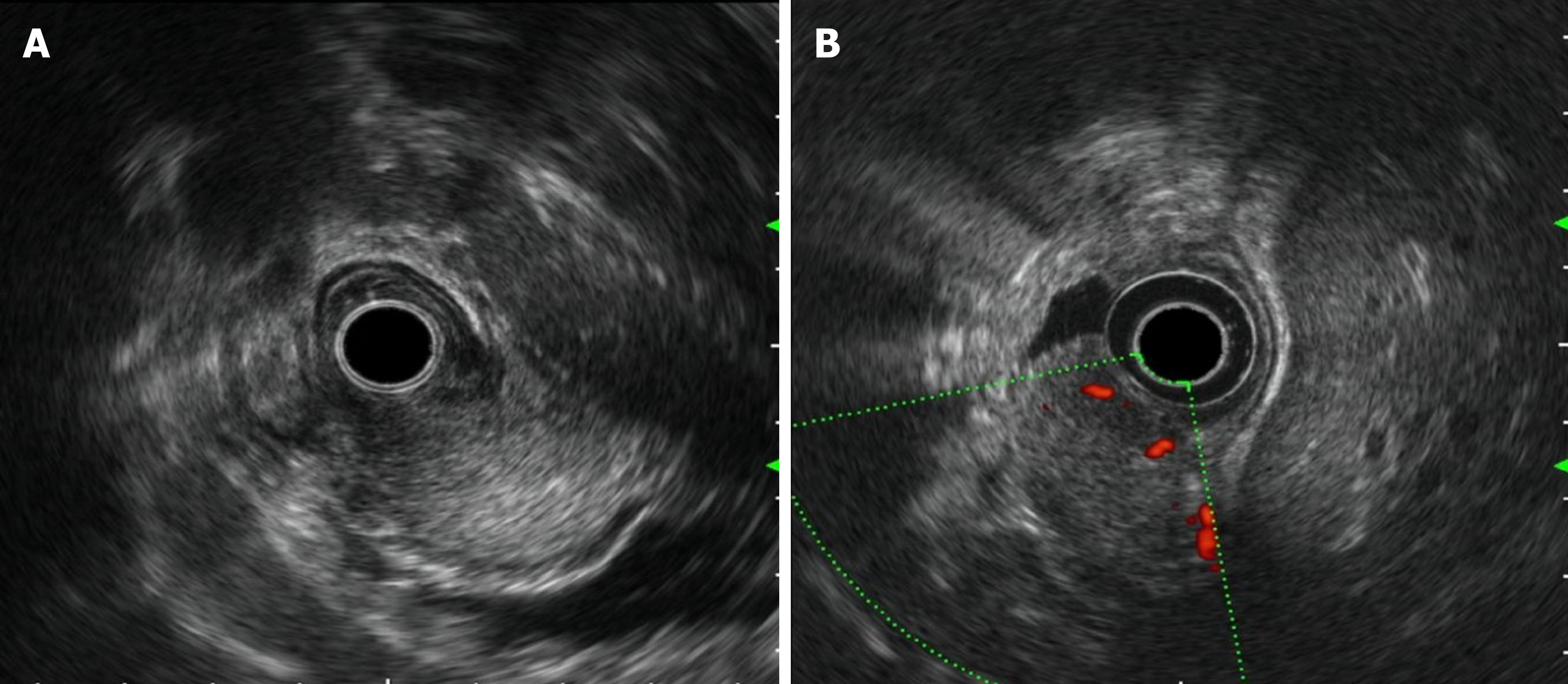
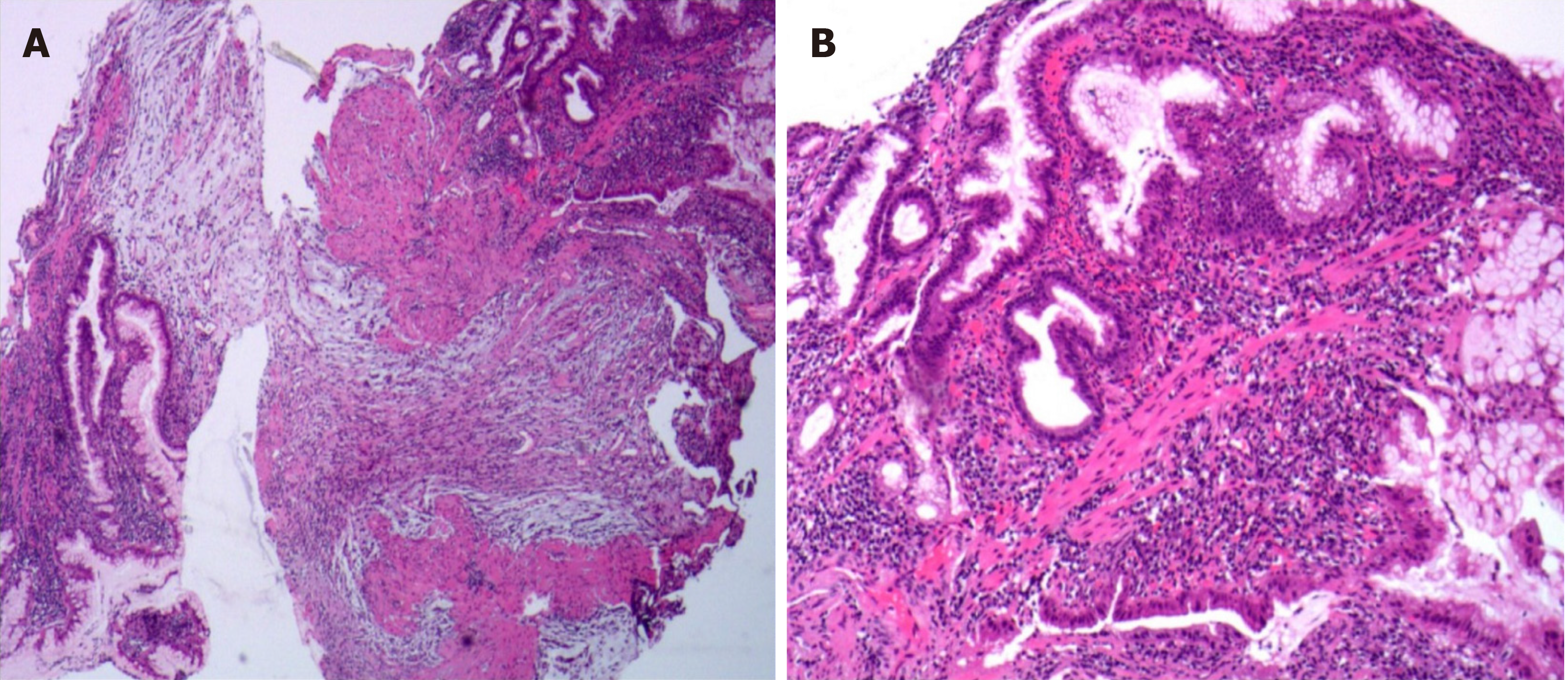
Painless gastroscopy showed a semicircular bulge approximately 5 cm in size in the lesser curvature near the cardia of the fundus; the surface was eroded, and shallow ulcers had formed. A biopsy sample was taken from the surface of the lesion (Figure 1). Endoscopic ultrasonography revealed that the lesion originated from the submucosal layer, which was heterogeneous and hyperechoic, and the posterior muscularis propria and serosal surface were present. The blood supply to the lesion was abundant by Doppler ultrasound (Figure 2). Pathologically, the biopsy sample revealed spindle cell proliferative lesions with interstitial mucinous changes, and the surface mucosa showed chronic inflammatory changes with active lesions (Figure 3). Immunohistochemistry showed smooth muscle actin (SMA) (+), CD117 (-), CD34 (-), DOG-1 (-), S-100 (-), and Ki67 (labelling index: < 1%).
Based on the pathological results and clinical results, the final diagnosis was PAMT of the stomach.
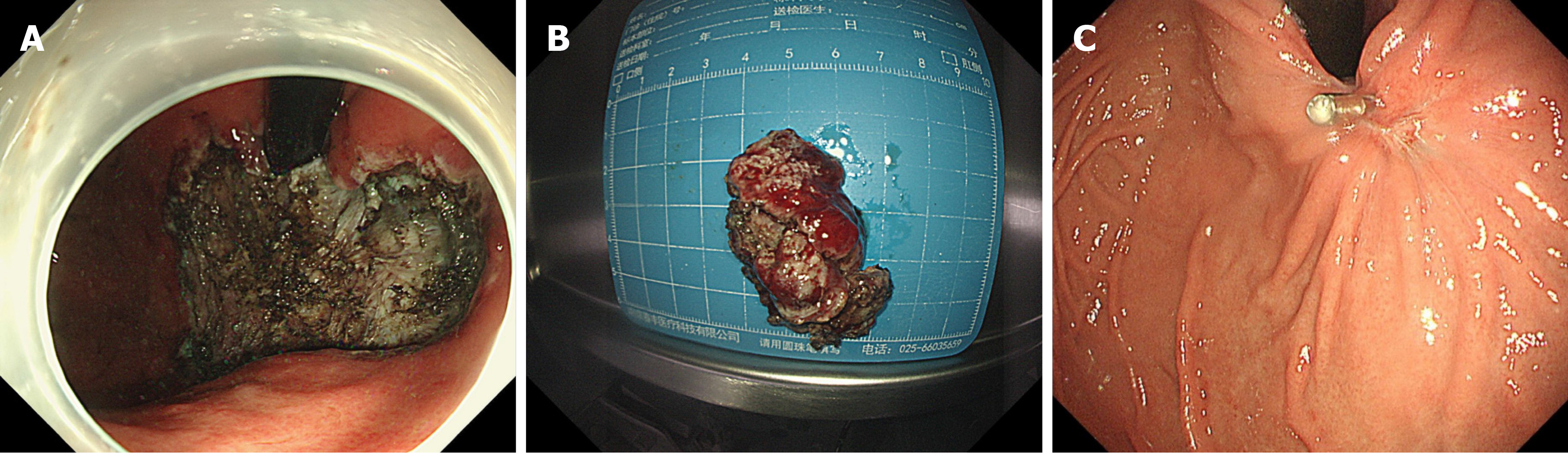
Because the lesion was near the cardiac area and the patient was young, we decided to remove the lesion by endoscopy. We performed ESD of the lesion, and as expected, the operation procedure was very difficult. The method of ESD was described everywhere. Simply, we marked the extent of the lesion first. Then, we performed submucosal injections and incised the mucosa at the outer edge of the lesion with a Dual knife and IT knife. After separating the submucosa, the lesion was exposed and dissected with the aid of the snare traction. After nearly 6 h of effort, we resected the lesion successfully (Figure 4A). The blood vessels of the lesion were treated with hemostatic agents during and after the operation. There was no active bleeding or perforation after washing. The cardia was preserved, and the lesion that we removed was 5 cm × 4 cm × 2 cm in size (Figure 4B).
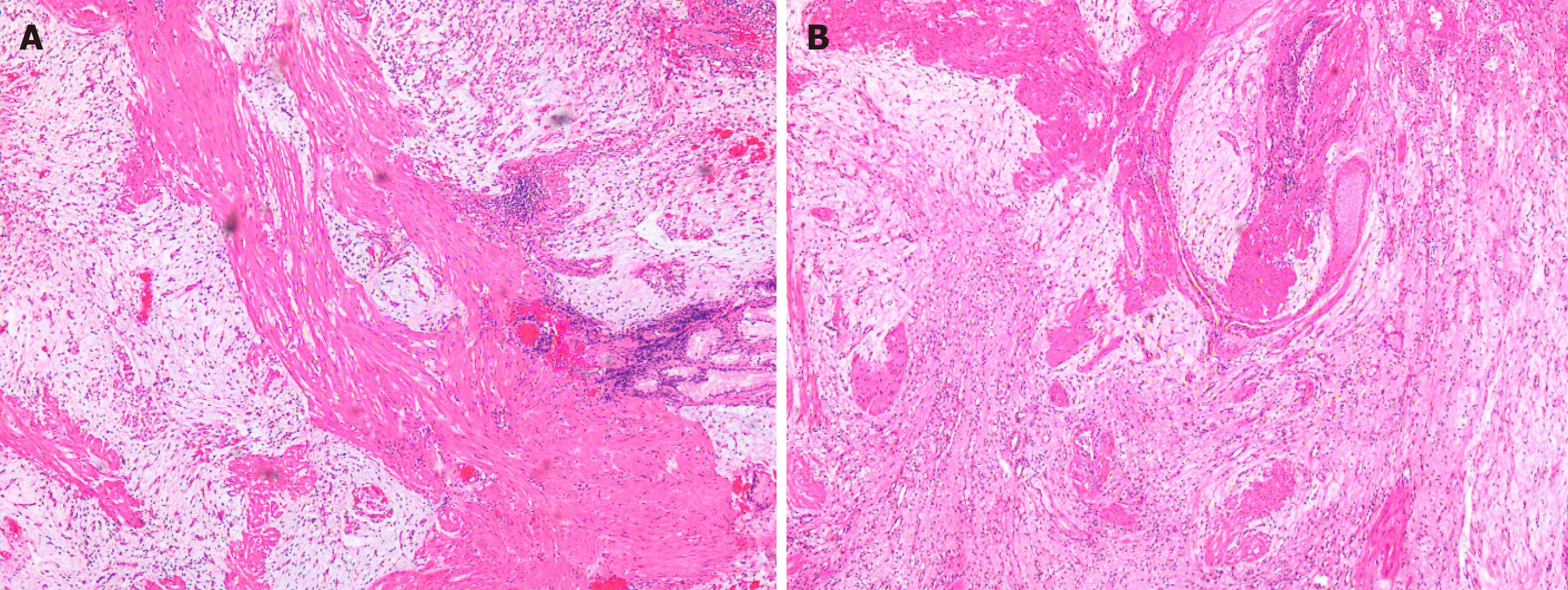
The patient had sudden abdominal pain on the second day after the operation, and the upper abdomen showed signs of abdominal muscle tension, tenderness, and rebound pain. The results of emergency CT examination showed perforation of the gastrointestinal tract and abdominal effusion. The patient was treated by gastrointestinal decompression, anti-inflammatory therapy, acid suppression, fluid rehydration, and pain relief. On the third day after the operation, the patient was accompanied by fever, and the abdominal symptoms and peritoneal irritation did not improve. Therefore, the patient underwent laparoscopic gastric perforation repair, intestinal adhesion release, and abdominal drainage operation. During the operation, there was a 1-cm perforation in the lower anterior wall of the gastric cardia, and the surrounding tissues were congested and edema. A large abscess cavity formed with the spleen and the left lobe of the liver under the diaphragm. More purulent fluid can be seen in the liver, right paracolic sulcus, intestines, and pelvis. We cleaned the abdominal cavity, sutured the perforation, and strengthened the seromuscular layer. The patient fully recovered without any symptoms and was discharged after 14 d. There was no recurrence during the 3-mo follow-up after ESD (Figure 4C). The pathology of the resected tissue showed typical manifestations, such as abundant slender blood vessels, rich mucus-like mesenchyme, and spindle-shaped, fat spindle-shaped fibroblasts and myofibroblast-like cells showing irregular nodular hyperplasia (Figure 5). Immunohistochemistry staining of tumor cells revealed the following: CD34 (partially +), SMA (weakly +), CD117 (-), DOG-1 (-), S-100 (-), SDHB (+), PCK (-), and Ki67 (labelling index: 2%).
In 2007, Takahashi et al[1] first reported a rare gastric mesenchymal tissue tumor and named it PAMT[1,4]. Miettinen et al[3] first coined the term PF for this type of lesion in 2009, and subsequently, the term was adopted in WHO 2010 classification of digestive system tumors in the chapter “Mesenchymal Tumours of the Stomach,” which was wrote in collaboration with Miettinen[23]. The term PF was used due to its cellular architecture and fibromyxoid nature, allowing nonprofessionals to recognize that it is a benign disease when encountering this spectrum of lesions. Despite that the WHO classification established the nomenclature, many authors still believe that the term PAMT better describes the histogenesis and histology of such tumors[16,26,27].
Since then, until 2019, only 121 additional cases have been published in the literature[5], and two more cases have been added in 2020. Miettinen et al[3] reported that the incidence of gastric PAMT is very low, less than 1/150 of gastrointestinal stromal tumors (GISTs). The range in patient ages is broad, from 5 to 81 years old (average age, 43.17 ± 18.00 years; median age, 46 years). The patients were mostly middle-aged and elderly people, and the peak was usually between 30 and 60 years old[4]. Lesions are frequently located in the gastric antrum but occasionally located in the small intestine, other parts of the stomach, large intestine, mediastinum, and esophagus[24]. According to a recent review report, the maximum diameter of the tumor ranged from 0.8-17 cm, with a mean size of 4.81 ± 3.30 cm and median size of 4.0 cm[4].
Although PAMT is benign, it shows extensive vascular proliferation; therefore, clinical manifestations can be accidental discovery, general gastrointestinal symptoms, and gastrointestinal bleeding. Typical PAMT manifests as general gastrointestinal symptoms, including abdominal pain, abdominal distension, indigestion, loss of appetite, heartburn, diarrhea, and epigastric discomfort. Manifestations of gastrointestinal bleeding are also common and can lead to gastrointestinal bleeding-related manifestations such as anemia, melena, and hematemesis[6-9]. Some patients experience dull upper abdominal pain, along with the presence of a lump in the right hypochondrium[24].
The currently reported cases of PAMT have unique characteristics: (1) The tumor can be polypoid, nodular, or cystic. PAMT forms a leaf-like intramural/submucosal mass, which is light pink, brown, or red in color, usually shiny and mucinous, and very hard. The margins usually have a good outline, such as polypoid, nodular, and cystic lesions, but they are not completely different[1,3,6,13-16,21,25]. The mass is usually visible endoscopically[5,16,21,25] and radiologically[14,21,25]. The tumor originates from the submucosa or muscularis propria and can infiltrate into other tissue layers, protruding from the mucosa or serous membrane. The surface can be accompanied by ulcers and erosions; (2) histologically, tumor tissue is mainly composed of bland ovoid to spindle-shaped fibroblast-like cells, abundant thin-walled blood vessels, and fibrous mucous matrix. Tumor cells are arranged in an irregular plexiform or multinodular pattern, and are separated by the abundant mucus-like or mucous-fibrous matrix around the small blood vessels. These features are helpful to differentiate PF from other gastrointestinal mesenchymal tumors, such as myxoid GISTs, but further differentiation and confirmation of the diagnosis can be identified by immunostaining[3]. The myxoid matrix is always positive for Alcian blue. The tumor cells show monomorphous oval nuclei containing fuzzy nucleoli and fine chromatin, surrounded by mild eosinophilic cytoplasm with indistinct boundary. Nucleolus and chromatin are delicate and fuzzy[4]. Some cases showed vascular and lymphatic infiltration[3,28], but metastasis or recurrence did not occur after surgery; and (3) in immunohistochemistry and special staining of PAMT, vimentin, SMA, muscle-specific actin, and Alcian blue are generally positive[10], indicating the fibroblastic, myofibroblastic, and smooth-muscle-cell characteristics of PAMT. Current research reports show that vimentin, SMA, and MSA have a high sensitivity and specificity for the diagnosis of PAMT, mainly because they are important markers of smooth muscle tumors and mesenchymal tissues. The immunochemical staining indicators in some specimens such as H-caldesmon, calponin, and desmin were partially positive[1,3,11-17], suggesting possible myofibroblastic differentiation, while these stains have been negative in other specimens[1,3,18-21]. For the muscular lineage toward terminal muscle cell differentiation, desmin and caldesmon are more specific markers. These markers show limited and focal reactive results in PAMT, which is consistent with the myofibroblastic spectrum of PF cell development[4], but they are also expressed in desmoid-type fibromatosis. Equivocal results were found for estrogen receptor (ER) and progesterone receptor (PR) staining. Some case reports have shown that specimens are focally or diffusely positive for PR but negative for ER. Thus, hormone therapy is also a direction to consider, but it may be of little significance[24]. For c-kit, pancytokeratin, S-100 protein, B-catenin, neurofilament, cytokeratin, DOG-1, CD34, epithelial membrane antigen, and ALK-1, all reported cases have negative results[10]. Ki-67 staining commonly illustrates very low proliferation rates, mostly < 2%. CD34 is not usually expressed in leiomyomas or desmoid-type fibromatoses; however, it is normally expressed in endothelial cells, embryonic cells of the hematopoietic system, and solitary fibrous tumors. S100 protein can be expressed in spindle cell neoplasms of the gastrointestinal (GI) tract, a characteristic of schwannomas. Combination of these markers can help make a differential diagnosis of spindle cell tumors in the GI tract[25]. B-catenin and ALK-1 are always negative in PAMT. CD117 or DOG1 is usually negative in PAMT but positive in GISTs, which do not show a unique plexiform wall growth pattern; thus, it can be separated from PAMT.
The diagnosis of PAMT depends on pathological and immunochemical examinations, and typical microscopic manifestations under high magnification should be observed, including bland ovoid to spindle-shaped fibroblast-like cells, abundant thin-walled blood vessels, and fibrous mucous matrix. Clinicians generally speculate that PAMT is benign based on the limited atypicality of tumor cells and low mitotic rate[22]. Vimentin, SMA, and muscle-specific actin were at least partially positive; Alcian blue was positive; and pancytokeratin, c-kit, DOG-1, CD34, S-100 protein, B-catenin, and ALK-1 were negative[4]. In our case, the tumor was positive for SMA, partly positive for CD34, and negative for DOG-1, S-100, PCK, and Ki67 (labelling index: 2%), which immunohistochemically confirmed PAMT.
For genes, mutations in c-kit and PDGFRA genes are important and characteristic in GISTs[29], but no studies have reported mutations in these two genes in PAMT. Therefore, PAMT can be further distinguished from GISTs in terms of gene mutations. Some cases have been described as having a repeated translocation, t(11;12)(q11;q13), involving the long noncoding gene metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and the gene glioma-associated oncogene homologue 1 (GLI1). Through repeated MALAT1-GLI1 translocation or GLI1 upregulation, GLI1 overexpression depicts a different pathogenic subgroup of plexiform fibromyxomas with activation of the Sonic Hedgehog signaling pathway. Inappropriate reactivation of the Hedgehog signaling pathway is responsible for the formation and progression of several cancers[30]. In addition to the canonical pathway of Hedgehog signaling, some canonical pathways that may be important for the development of gastrointestinal tumors has been recently described, including a noncanonical, Patched-dependent, and Smoothened-independent pathway[37]. Base on the molecular pathway of PF development, neoplasms with GLI1 oncogenesis may be sensitive to Hedgehog pathway inhibitors, targeting either Patched, Smoothened, GLI1, or other Hedgehog pathway components[31].
For the differential diagnosis of myxoid mesenchymal tumors in the gastrointestinal tract, the main considerations include GIST, leiomyomata, solitary fibrous tumor, leiomyosarcoma (LMS), desmoid tumor, inflammatory fibroid polyp, neurilemmoma, inflammatory myofibroblastic tumor, low-grade fibromyxoid sarcoma, Plexiform nerve fibroma, myxoma and, in women, myxoid low-grade endometrial stromal sarcoma[1,3,11-13,32]. PF is positive for SMA, DES, and vimentin and negative for c-kit, CD34, S-100, DOG1, and CD117. However, GIST may have CD117, CD34, and DOG-1 immunoreactivity and c-kit or PDGFRA mutation. Leiomyoma can be differentiated by the bundle like and braided arrangement of tumor tissue and the eosinophilic cytoplasm of fusiform tumor cells. SMA, MSA, desmin, and caldesmon were positive in leiomyoma, but desmin was negative in PAMT. Myxoid and leiomyoma have positive reaction for SMA, desmin, and caldesmon, and Plexiform neurofibroma have positive reaction for S-100 protein. Solitary fibrous tumors are positive for CD34, and consist of altered hypercellular and hypocellular areas, deposits of dense keloid-type collagen, and hemangiopericytoma-like areas[25].
The main treatment for PAMT is surgery. Current literature reviews up to 2019 reporting on 121 patients demonstrated no local or metastatic reoccurrence among the 84 patients who were followed[4,5]. Some reports also presented cases of PAMT treated via endoscopy, but the lesions were smaller than 5 cm[20,33-36]. Our case is the ninth case of endoscopic resection, and it is the largest mass that has been removed endoscopically so far. The lesion reached 5 cm × 4 cm × 2 cm in size and was near the cardia. The diseased tissue was completely removed, and the cardia was preserved. Our patient had no recurrence or metastasis during the 3-mo follow-up after the operation, and the treatment effect was good.
At present, the diagnostic criteria for PAMT are relatively clear, but the pathogenesis and genetic changes require further study. PAMT is benign in nature, and these patients are less likely to experience local or metastatic recurrence. The main treatment is still surgery, and when the lesion is in the stomach, partial gastrectomy and distal gastrectomy are the most commonly performed surgical treatments for PAMT, followed by local gastrectomy, subtotal gastrectomy, and wedge-shaped gastrectomy. But for comprehensive evaluation of the disease, ESD can be considered a suitable method to avoid excessive treatment.
The authors thank the patient.
| 1. | Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2007;31:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Lu B, Ye W, Liu H. A Rare Gastric Tumor in a Young Woman. Gastric Plexiform Angiomyxoid Myofibroblastic Tumor. Gastroenterol. 2015;149:294-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009;33:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 4. | Su HA, Yen HH, Chen CJ. An Update on Clinicopathological and Molecular Features of Plexiform Fibromyxoma. Can J Gastroenterol Hepatol. 2019;2019:3960920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Perry L, McCann C, Schwartz J, Gott M, Senatore P, Slotman G. Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach. Am Surg. 2020;3134820951487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol. 2010;16:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Lee PW, Yau DT, Lau PP, Chan JK. Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor) of stomach: an unusual presentation as a fistulating abscess. Int J Surg Pathol. 2014;22:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Xu J, Jiang G, Ma Y, Qi J, Li W, Zhang D. Gastrointestinal stromal tumor with a PDGFRA mutation masquerading as gastric plexiform fibromyxoma: A comparative clinicopathological study of two cases. Oncol Lett. 2017;13:887-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zhang WG, Xu LB, Xiang YN, Duan CH. Plexiform fibromyxoma of the small bowel: A case report. World J Clin Cases. 2018;6:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Duckworth LV, Gonzalez RS, Martelli M, Liu C, Coffin CM, Reith JD. Plexiform fibromyxoma: report of two pediatric cases and review of the literature. Pediatr Dev Pathol. 2014;17:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Rau TT, Hartmann A, Dietmaier W, Schmitz J, Hohenberger W, Hofstaedter F, Katenkamp K. Plexiform angiomyxoid myofibroblastic tumour: differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin Pathol. 2008;61:1136-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol. 2008;32:1910-1912; author reply 1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Pailoor J, Mun KS, Chen CT, Pillay B. Plexiform angiomyxoid myofibroblastic tumour of the stomach. Pathology. 2009;41:698-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sing Y, Subrayan S, Mqadi B, Ramdial PK, Reddy J, Moodley MS, Bux S. Gastric plexiform angiomyxoid myofibroblastic tumor. Pathol Int. 2010;60:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Tan CY, Santos LD, Biankin A. Plexiform angiomyxoid myofibroblastic tumour of the stomach: a case report. Pathology. 2010;42:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kim A, Bae YK, Shin HC, Choi JH. Plexiform angiomyxoid myofibroblastic tumor of the stomach: a case report. J Korean Med Sci. 2011;26:1508-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Bi R, Yin W, Liu XL, Wei HM, Sheng WQ, Wang J. [Plexiform angiomyxoid myofibroblastic tumor of stomach]. Zhonghua Bing Li Xue Za Zhi. 2012;41:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Galant C, Rousseau E, Ho Minh Duc DK, Pauwels P. Re: Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2008;32:1910; author reply 1912-1910; author reply 1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Schulz T, Drgac J, Chmelar C, Höhler T, Agaimy A, Vieth M. [Plexiform angiomyxoid myofibroblastic tumour of the stomach]. Pathologe. 2012;33:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kang Y, Jung W, Do IG, Lee EJ, Lee MH, Kim KM, Choi J. Plexiform angiomyxoid myofibroblastic tumor of the stomach: report of two cases and review of the literature. Korean J Pathol. 2012;46:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Li P, Zhang Q, Jia X, Li Q, Li Z, Wang Z. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Open J Pathol. 2012;2:147-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Li B, Zhang QF, Han YN, Ouyang L. Plexiform myxoid gastrointestinal stromal tumor: a potential diagnostic pitfall in pathological findings. Int J Clin Exp Pathol. 2015;8:13613-13618. [PubMed] |
| 23. | Miettinen M, Fletcher CD, Kindblom LG, Tsui WM. ‘‘Mesenchymal tumours of the stomach.’’ In: Bosman FT, Carneiro F, Hruban R, Teise ND, eds. WHO Classification of Tumours of the Digestive System. Lyon: IARC, 2010: 74-79. |
| 24. | Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kim SM, An JY, Choi MG, Lee JH, Sohn TS, Kim KM, Kim S, Bae JM. Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: a Rare Case. J Gastric Cancer. 2017;17:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Dixit JD, Sharief SA, Goyal MK, Khan S, Kauser L. Plexiform Angiomyxoid Myofibroblastic Tumor (PAMT) of Stomach with Synchronous Bilateral Cystic Ovarian Neoplasms, a Rare Case Presentation. Indian J Surg Oncol. 2016;7:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Akai H, Kiryu S, Shinozaki M, Ohta Y, Nakano Y, Yasaka K, Ohtomo K. Computed tomography and magnetic resonance imaging of a plexiform angiomyxoid myofibroblastic tumor: a case report. BMC Med Imaging. 2017;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kawara F, Tanaka S, Yamasaki T, Morita Y, Ohara Y, Okabe Y, Hoshi N, Toyonaga T, Umegaki E, Yokozaki H, Hirose T, Azuma T. Gastric plexiform fibromyxoma resected by endoscopic submucosal dissection after observation of chronological changes: A case report. World J Gastrointest Oncol. 2017;9:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (2)] |
| 30. | Spans L, Fletcher CD, Antonescu CR, Rouquette A, Coindre JM, Sciot R, Debiec-Rychter M. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol. 2016;239:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC. A Distinct Malignant Epithelioid Neoplasm with GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am J Surg Pathol. 2018;42:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 32. | Oliva E, Young RH, Clement PB, Scully RE. Myxoid and fibrous endometrial stromal tumors of the uterus: a report of 10 cases. Int J Gynecol Pathol. 1999;18:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Wang WY, Li JN, Li GD. Plexiform angiomyxoid myofibroblastic tumour of the gastric fundus: successful diagnosis and treatment by endoscopy. J Clin Pathol. 2010;63:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Li X, Li S, Xiong S, Wang Z, Zhang H. A rare case of plexiform angiomyxoid myofibroblastic tumor in the stomach which was diagnosed at the earliest stage in the literature. Gastroenterol Rep (Oxf). 2018;6:313-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Hu G, Chen H, Liu Q, Wei J, Feng Y, Fu W, Zhang M, Wu H, Gu B, Ren J. Plexiform fibromyxoma of the stomach: a clinicopathological study of 10 cases. Int J Clin Exp Pathol. 2017;10:10926-10933. [PubMed] |
| 36. |
Qi G, Zheng J, Yang Z, Ru G and He X, “Clinicopathological characteristic analysis of gastric plexiform fibromyxoma” J Pract Oncol 2017; 5: 464-466 [DOI: 10.
Qi G, Zheng J, Yang Z, Ru G and He X, “Clinicopathological characteristic analysis of gastric plexiform fibromyxoma” |
| 37. | Faria AVS, Akyala AI, Parikh K, Brüggemann LW, Spek CA, Cao W, Bruno MJ, Bijlsma MF, Fuhler GM, Peppelenbosch MP. Smoothened-dependent and -independent pathways in mammalian noncanonical Hedgehog signaling. J Biol Chem. 2019;294:9787-9798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sikiric P, Wang Z S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Xing YX