Published online Aug 21, 2021. doi: 10.3748/wjg.v27.i31.5272
Peer-review started: February 12, 2021
First decision: April 18, 2021
Revised: May 3, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 21, 2021
Processing time: 186 Days and 14.8 Hours
The rate of positive tests using fecal immunochemical test (FIT) does not decrease with subsequent campaigns, but the positive predictive value of advanced neoplasia significantly decreases in subsequent campaign after a first negative test. A relationship between the fecal hemoglobin concentration (Fhb) and the opportunity to detect a colorectal cancer in subsequent campaign has been shown.
To predict the severity of colorectal lesions based on Fhb measured during previous colorectal cancer screening campaign.
This etiological study included 293750 patients aged 50-74, living in Auvergne-Rhône-Alpes (France). These patients completed at least two FIT [test(-1) and test(0)] between June 2015 and December 2019. Delay between test(-1) and test(0) was > 1 year and test(-1) result was negative (< 150 ngHb/mL). The severity of colorectal lesions diagnosed at test(0) was described according to Fhb measured at test(-1) [Fhb(-1)]. The relationship between the severity classified in seven ordinal categories and the predictive factors was analyzed in an ordered multivariate polytomous regression model.
The test(0) positive rate was 4.0%, and the colonoscopy completion rate was 97.1% in 11594 patients who showed a positive test(0). The colonoscopy detection rate was 77.7% in those 11254 patients who underwent a colonoscopy. A total of 8748 colorectal lesions were detected (including 2182 low-risk-polyps, 2400 high-risk-polyp, and 502 colorectal cancer). The colonoscopy detection rate varied significantly with Fhb(-1) [0 ngHb/mL: 75.6%, (0-50 ngHb/mL): 77.3%, (50-100 ngHb/mL): 88.7%, (100-150 ngHb/mL): 90.3%; P = 0.001]. People with a Fhb(-1) within (100-150 ngHb/mL) (P = 0.001) were 2.6 (2.2; 3.0) times more likely to have a high severity level compared to those having a Fhb(-1) value of zero. This risk was reduced by 20% in patients aged 55-59 compared to those aged < 55 [adjusted odds ratio: 0.8 (0.6; 1.0)].
The study showed that higher Fhb(-1) is correlated to an increased risk of severity of colorectal lesions. This risk of severity increased among first-time participants (age < 55) and the elderly (≥ 70). To avoid the loss of chance in these age groups, the FIT positivity threshold should be reduced to 100 ngHb/mL. The other alternative would be to reduce the time between the two tests in these age groups from the current 2 years to 1 year.
Core Tip: The study showed that the severity of the colonic lesions increases with a high concentration of fecal hemoglobin measured in previous test. The elderly (≥ 70 years) had a high proportion of positive colonoscopy when the fecal hemoglobin concentration measured in previous campaign was between 100 and 150 ngHb/mL. Younger patients (age < 54) were likely to have a high-severity neoplasia. Given these results, the recommendation to reduce the FIT positivity threshold to 100 ngHb/mL for first-time participants and the elderly (aged ≥ 70) should attract the attention of the decision-making authority.
- Citation: Balamou C, Koïvogui A, Rodrigue CM, Clerc A, Piccotti C, Deloraine A, Exbrayat C. Prediction of the severity of colorectal lesion by fecal hemoglobin concentration observed during previous test in the French screening program. World J Gastroenterol 2021; 27(31): 5272-5287
- URL: https://www.wjgnet.com/1007-9327/full/v27/i31/5272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i31.5272
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer-related death[1]. To address these major public health issues, several countries have launched nationwide CRC screening program (CRCSP) in order to reduce the incidence and mortality of CRC.
In 2015, in France, the fecal immunochemical test (FIT) OC-Sensor® was introduced as part of the CRCSP based on its performance to detect advanced adenoma and CRC[2-5]. The positivity threshold has been set at 150 ngHb/mL (30 µgHb/g) of stool. Anyone who tested negative was re-invited for testing 2 years later. Anyone who tested positive was advised to complete a colonoscopy. Several studies showed a reduction in the CRC incidence several years after FIT introduction in the CRCSP[6,7].
Some colonic polyps such as adenomatous and serrated polyps exhibit a malignant potential, while others do not (hyperplastic, post-inflammatory, hamartomatous)[8]. Surveillance of polyp characteristic over time should lead to a significant increase in the clinical performance of the detection of polyps with high malignant potential[9]. The variability in growth behavior supports the hypothesis that some polyps continue to grow, other are remaining stable, whereas some polyps regress over time. Some authors have reported that advanced adenoma may have the potential to grow faster than non-advanced adenoma, while most small polyps remain stable or are regress over time[10,11].
It was shown that the rate of positive test results with FIT does not decrease during the subsequent campaigns, but the positive predictive value for advanced neoplasia, especially for CRC, significantly decreased in participants during the subsequent campaign after a first negative test[12,13]. A relationship between the fecal hemoglobin concentration (Fhb) and the risk to detect a CRC or an advanced adenoma has also been shown[14-21], but the severity risk of the colorectal lesions according to the earlier Fhb was poorly described.
Looking for less invasive methods for monitoring patients at moderate risk for CRC, this study aims to predict the severity of colorectal lesions based on the Fhb measured during a previous colorectal cancer screening test.
This etiological study included 293750 patients living in one of the six French departments (Ain, Ardèche, Drôme, Isère, Savoie, & Haute-Savoie) of the Auvergne-Rhône-Alpes region. These patients were aged between 50 and 74 and had completed at least two FIT screening tests between June 1, 2015 and December 31, 2019. The delay between the last test [test(0)] and the penultimate test [test(-1)] was > 1 year, with a negative test(-1) result (< 150 ngHb/mL). The diagnosis of a colorectal lesion as well as its severity were described according to the Fhb measured at test(-1) [Fhb(-1)].
All study data were extracted on the same date (November 30, 2020) from departmental databases. These databases were regularly enriched by socio-demographic data, diagnosis (colonoscopy, histopathology), and follow-up data provided by partners (Health Insurance Plans, Medical Information Services, Gastroenterologists, Surgeons & GPs).
In each selected department, the CRCSP campaigns were organized according to the CRCSP national specifications[22,23]. As a reminder, this nationwide screening program started in 2009, and the CRCSP target was every 2 years in an eligible population, i.e. asymptomatic patients aged 50 to 74, with no risk factors other than their age (according to the CRCSP national specification). This program was based on the guaiac fecal occult blood test (gFOBT) or hemoccult© II, which has been replaced by the FIT in 2015. The CRC screening test was a two-step method: The first step consisted in the completion of a FIT, the second-step consisted in the completion of a colonoscopy in case of positive FIT. The positivity threshold of the test was set at 150 ngHb/mL of stool. In case of normal colonoscopy, the patient received an invitation to the CRCSP after 5 years. In case of positive colonoscopy (high risk polyps or cancer) the patient was excluded from the nationwide program.
As a study result criterion, test(0) was positive at the threshold of 150 ngHb/mL. The value of the Fhb measured during this test(0) [Fhb(0)] was treated as a discrete variable (0 ngHb/mL, 0-50 ngHb/mL, 50–100 ngHb/mL, 100-150 ngHb/mL, 150-300 ngHb/mL, > 300 ngHb/mL). The colonoscopy completion rate was defined by the proportion of patients who had a colonoscopy (complete or incomplete) among those with a positive test(0) result. Colonoscopy was considered positive when a colorectal neoplasia was diagnosed by the gastroenterologist or by a cytopathological examination of the specimens. In the CRCSP, no specific training other than specialized training as an endoscopic physician was required for the practice of colonoscopy by gastroenterologists. The colonoscopy detection rate was defined by the proportion of positive colonoscopies among those performed (complete or incomplete) after a positive test. In the event of a positive result, the diagnostic course was analyzed in terms of types of diagnosed lesions including: Low risk polyps (LRP), high risk polyps (HRP), unspecified-polyp (UP), and CRC. HRP included: Adenomas ≥ 10 mm (except hyperplastic polyps), serrated adenomas, adenomas with high grade dysplasia, and villous or tubulo-villous adenomas. For these colorectal tumors detected, five localizations were described during colonoscopy: Cecum, right colon (ascending colon and right angle), transverse colon, left colon (left angle and descending colon), and rectosigmoid (rectum and sigmoid colon). The diagnoses associated with CRC and polyps or adenoma were those related to C18–C20 and D12 of the 10th version of the World Health Organization International Classification of Diseases[24]. CRC lesions were described by stage of severity, using the tumor, node, and metastasis (TNM) classification based on tumor size, lymph node involvement, and the possible presence of metastases[25]: CRC Stage-0 (pTisN0M0), Stage-I (pT1-2N0M0), Stage-II (pT3N0M0 or pT4N0M0), Stage-III (pT1-T2N1M0 or pT1N2M0 or pT3-T4N1M0 or pT2-T3N2M0 or pT4N2M0), and Stage-IV (any T, any N, M1). The size of the polyp, its dysplasia, its cytopathological aspect, and the TNM classification were used to define a scale of severity of colorectal lesions in 7 ordinal categories: Severity level-0 (LRP), level -1 (HRP), level-2 (Stage-0), level-3 (Stage-I), level-4 (Stage-II), level-5 (Stage-III), and level-6 (Stage-IV). The main studied factors were the value of Fhb(-1) and the variation of the Fhb between test(-1) and test(0). The value of Fhb(-1) expressed in ngHb/mL (0, 0-50, 50-100, 100-150) and its variation in ngHb/mL (≤ 0, 0-50, 50-100, 100-150, 150-300, ≥ 300) were treated as discrete variables. The cofactors studied were: (1) the participation in at least one gFOBT campaign (new participant: -the person under 50-years-old at the time the gFOBT was used in the program-, No-gFOBT: -the person has never completed a gFOBT despite being often invited to the gFOBT campaigns-, Yes-gFOBT: -the person completed at least one gFOBT campaign-); (2) the age at the time of performing test(-1) (50-54, 55-59, 60-64, 65-69, ≥ 70-years-old); (3) the gender (female vs male); (4) the delay (mo) between test(-1) and test(0) (≤ 24, 25-30, > 30) and; (5) the number of tests completed before the test(-1) (0, 1, 2, 3, ≥ 4).
The main characteristics were described in frequencies for qualitative variables and in mean ± SD for quantitative variables. Proportions were compared using Pearson Chi-2 test or Fisher’s exact test when appropriate.
The relationship between the diagnosis of neoplasia (positive colonoscopy vs negative colonoscopy) and the predictive factors [Fhb(-1) , participation in at least one gFOBT campaign, age at test(-1), gender, delay between test(-1) and test(0), number of tests completed before test(-1)] was analyzed in a multivariate logistic regression model, with the estimation of the adjusted odds ratio (OR) and its 95% confidence interval (CI). For the construction of the multivariate model, all the adjustment covariates regardless of the strength of association in univariate analysis were used. In addition, a strong correlation existed between several covariates [age, delay, number of tests completed before test(-1), participation in at least one gFOBT campaign], the model was extended to the terms of interaction between these covariates. Only the significant interaction terms (P < 0.05 in univariate analysis) were retained in the final model evaluated by the likelihood ratio test. Positive tests without colonoscopy at the time of the study (n = 340) were excluded from this logistic regression analysis.
The relationship between the severity of the lesions (ordinal variable; 0 to 6) and the predictive factors [Fhb(-1), participation in at least one gFOBT campaign, age at test(-1), gender, delay between test(-1) and test(0), number of tests completed before test(-1)] was analyzed in an ordered, multivariate polytomous regression model, with the estimation of the risk and its 95%CI. All the adjustment covariates regardless of the strength of association in univariate analysis were used in the multivariate model. In addition, the model was extended to terms of interaction between covariates with a strong correlation (cited above). Only the significant interaction terms (P < 0.05 during a univariate analysis) were retained in the final model evaluated by the likelihood ratio test. In this analysis, the unspecified polyps (n = 3664) were classified as the polyps not at risk. Similarly, cancers without any precision (unknown/unspecified, n = 101) on the TNM classification were classified as tumors in situ. As a reminder, negative colonoscopies (n = 2846) were excluded from this ordered, univariate, and multivariate polytomous regression. The differences were significant at the 5% level with version 13 of the STATA software (College Station, TX, United States).
Before analysis, all data were anonymized. The screening database had a favorable opinion from the institution that oversees the ethics of data collection (“Commission nationale de l'Informatique et des libertés”)[26]. According to the current French legislation, a study that does not change the care of patients did not require the opinion of the Clinical Research Centers Ethics Committee. This article does not contain any studies with human participants performed by any of the authors. This study does not involve human participants, and an informed consent was therefore not required. This article does not contain any studies with animals performed by any of the authors.
A total of 575587 patients completed at less one FIT between 2015 and 2019. Among them, 281837 (49.0%) people were excluded from this etiological analysis because they had completed only one FIT during the study duration. Finally, our study focused on the 293750 people who completed at least two FIT such as test(-1) and test(0). With a mean age of 61.1 ± 6.7 when completing the test(-1), the positivity rate of the test(0) was 4.0% in these 293750 people. This positivity rate was significantly higher (P < 0.001) among patients who have a Fhb(-1) between 100 and 150 ngHb/mL (Table 1).
| Characteristics | Result of the last test [test(0)] | |||||||||||||||
| Positive test and colonoscopy completion rate | Colonoscopy detection rate | Number of the colorectal lesions | ||||||||||||||
| Nb. (%T+) | P value1 | Number T+ (% Colo in T+) | Number Colo (% Positive Colo) | P value1 | All lesions | Polyps | CRC | |||||||||
| LRP | HRP | UP | S-0 | S-I | S-II | S-III | S-IV | USC | ||||||||
| Overall | 293750 (4.0) | 11594 (97.1) | 11254 (77.7) | 8748 | 2182 | 2400 | 3664 | 133 | 130 | 53 | 64 | 21 | 101 | |||
| Fhb(-1) (ngHb/mL) | < 10-3 | < 10-3 | ||||||||||||||
| 0 | 280012 (3.5) | 9655 (97.0) | 9368 (75.6) | 7085 | 1890 | 1769 | 3070 | 85 | 99 | 31 | 47 | 15 | 79 | |||
| 0-50 | 1680 (10.4) | 174 (98.9) | 172 (77.3) | 133 | 27 | 41 | 56 | 3 | 4 | 1 | 0 | 0 | 1 | |||
| 50–100 | 8776 (13.0) | 1144 (96.9) | 1108 (88.7) | 983 | 177 | 360 | 370 | 25 | 16 | 12 | 9 | 2 | 12 | |||
| 100-150 | 3282 (18.9) | 621 (97.6) | 606 (90.3) | 547 | 88 | 230 | 168 | 20 | 11 | 9 | 8 | 4 | 9 | |||
| gFOBT campaign participation | < 10-3 | 0.03 | ||||||||||||||
| Yes-gFOBT | 213476 (4.2) | 8874 (97.0) | 8605 (77.8) | 6695 | 1760 | 1906 | 2603 | 112 | 109 | 44 | 59 | 17 | 85 | |||
| New entrant | 40199 (2.9) | 1156 (97.5) | 1127 (75.1) | 846 | 168 | 203 | 456 | 8 | 6 | 0 | 0 | 1 | 4 | |||
| No-gFOBT | 40075 (3.9) | 1564 (97.3) | 1522 (79.3) | 1207 | 254 | 291 | 605 | 13 | 15 | 9 | 5 | 3 | 12 | |||
| Age (yr) at test(-1) | < 10-3 | < 10-3 | ||||||||||||||
| 50-54 | 57625 (3.0) | 1727 (97.9) | 1690 (73.7) | 1245 | 276 | 348 | 582 | 10 | 14 | 1 | 6 | 1 | 7 | |||
| 55-59 | 62489 (3.5) | 2166 (97.3) | 2107 (75.6) | 1592 | 389 | 445 | 687 | 21 | 19 | 4 | 7 | 3 | 17 | |||
| 60-64 | 64141 (3.9) | 2514 (97.4) | 2449 (78.4) | 1920 | 509 | 503 | 807 | 28 | 19 | 17 | 15 | 7 | 15 | |||
| 65-69 | 63915 (4.7) | 2997 (97.0) | 2907 (78.7) | 2287 | 592 | 647 | 886 | 38 | 54 | 14 | 17 | 5 | 34 | |||
| ≥ 70 | 45580 (4.8) | 2190 (95.9) | 2101 (81.1) | 1704 | 416 | 457 | 702 | 36 | 24 | 17 | 19 | 5 | 28 | |||
| Gender | < 10-3 | < 10-3 | ||||||||||||||
| Female | 160181 (3.3) | 5343 (97.1) | 5188 (72.8) | 3778 | 962 | 937 | 1663 | 59 | 51 | 15 | 33 | 11 | 47 | |||
| Male | 133569 (4.7) | 6251 (97.0) | 6066 (81.9) | 4970 | 1220 | 1,463 | 2001 | 74 | 79 | 38 | 31 | 10 | 54 | |||
| Delay (mo) between test(-1) and test(0) | < 10-3 | < 10-3 | ||||||||||||||
| ≤ 24 | 40422 (5.2) | 2111 (94.3) | 1991 (72.2) | 1437 | 435 | 435 | 482 | 21 | 23 | 12 | 12 | 0 | 17 | |||
| 24-30 | 163470 (4.0) | 6538 (97.7) | 6390 (76.9) | 4916 | 1322 | 1440 | 1833 | 99 | 83 | 29 | 39 | 14 | 57 | |||
| > 30 | 89858 (3.3) | 2945 (97.6) | 2873 (83.4) | 2395 | 425 | 525 | 1349 | 13 | 24 | 12 | 13 | 7 | 27 | |||
| Nb. of tests completed before test(-1) | < 10-3 | 0.01 | ||||||||||||||
| 0 | 68839 (3.5) | 2421 (97.1) | 2351 (76.1) | 1790 | 396 | 465 | 855 | 19 | 21 | 9 | 5 | 4 | 16 | |||
| 1 | 54790 (3.7) | 2044 (97.7) | 1996 (77.6) | 1548 | 364 | 432 | 667 | 20 | 26 | 6 | 13 | 6 | 14 | |||
| 2 | 55794 (4.0) | 2245 (97.1) | 2180 (76.6) | 1670 | 434 | 472 | 678 | 25 | 17 | 11 | 12 | 2 | 19 | |||
| 3 | 56716 (4.4) | 2514 (97.3) | 2445 (77.9) | 1905 | 553 | 562 | 649 | 34 | 37 | 13 | 24 | 5 | 28 | |||
| 4 | 57611 (4.1) | 2370 (96.3) | 2282 (80.4) | 1835 | 435 | 469 | 815 | 35 | 29 | 14 | 10 | 4 | 24 | |||
A total of 282156 people (i.e. 96.2% of the sample) had a negative test(0) [Fhb(0) < 150 ngHb/mL]. The colonoscopy completion rate was globally estimated at 97.1% in 1594 people who had a positive test(0) [Fhb(0) ≥ 150 ngHb/mL]. The colonoscopy detection rate was 77.7% among those 11254 people who had a colonoscopy, with a total of 8745 colonic lesions detected (including 2182 LRP, 2400 HRP, 502 CRC). This colonoscopy detection rate varied significantly (P < 10-3) with the Fhb(-1) [0: 75.6%, (0-50): 77.3%, (50-100): 88.7%, (100-150): 90.3%; P < 0.001) (Table 1).
In total, 27.4% of the lesions detected (i.e. 2395 cases) were detected in people who completed a test(0) more than 30 mo after the test(-1). However, these 2395 lesions were mostly polyps compared to the lesions detected in people who completed a test(0) within a reasonable delay between test(0) and test(-1) (delay ≤ 24 m -LRP: 63.8%, HRP: 30.3%, CRC: 5.9%-; delay in 24-30 m -LRP: 64.2%, HRP: 29.3%, CRC: 6.5%-; delay > 30 m -LRP: 74.1%, HRP: 21.9%, CRC: 4.0%-; P = 0.0001).
For the same value of the previous Fhb [Fhb(-1)], the colonoscopy detection rate was not significantly different between the positive values of Fhb(0) [i.e. Fhb(-1) = 0 & Fhb(0) = (150-300): rate = 75.5% vs Fhb(-1) = 0 & Fhb(0) ≥ 300: rate = 75.7%; P = 0.8). However, the colonoscopy detection rate was significantly higher when the Fhb(0) was higher, from 75.6% when Fhb(0) was 0 ngHb/mL to 90.3% at (100-150) ngHb/mL (Table 2).
| Fhb(0) by Fhb(-1) | Colonoscopy detection rate | Number of colorectal lesions by Severity | ||||||||||||
| Fhb(-1) (ngHb/mL) | Fhb(0) (ngHb/mL) | Number (% in subtotal) | Number Colo (%Positive Colo) | P value1 | Nb. of colorectal lesions | Polyps | CRC | |||||||
| LRP | HRP | UP | S-0 | S-I | S-II | S-III | S-IV | USC | ||||||
| 0 | 0.8 | |||||||||||||
| 0 | 257589 (92.0) | |||||||||||||
| 0-50 | 1805 (0.6) | |||||||||||||
| 50-100 | 8275 (3.0) | |||||||||||||
| 100-149 | 2688 (1.0) | |||||||||||||
| 150-300 | 4903 (1.7) | 4778 (75.5) | 3609 | 980 | 918 | 1601 | 35 | 38 | 5 | 6 | 2 | 24 | ||
| ≥ 300 | 4752 (1.7) | 4590 (75.7) | 3476 | 910 | 851 | 1469 | 50 | 61 | 26 | 41 | 13 | 55 | ||
| Subtotal | 280012 (100.0) | 9368 (75.6) | 7085 | 1890 | 1769 | 3070 | 85 | 99 | 31 | 47 | 15 | 79 | ||
| 0–50 | 0.3 | |||||||||||||
| 0 | 1328 (79.1) | |||||||||||||
| 0-50 | 30 (1.8) | |||||||||||||
| 50-100 | 112 (6.7) | |||||||||||||
| 100-149 | 36 (2.1) | |||||||||||||
| 150-300 | 86 (5.1) | 85 (74.1) | 63 | 17 | 18 | 24 | 1 | 2 | 1 | 0 | 0 | 0 | ||
| ≥ 300 | 88 (5.2) | 87 (80.5) | 70 | 10 | 23 | 32 | 2 | 2 | 0 | 0 | 0 | 1 | ||
| Subtotal | 1680 (100.0) | 172 (77.3) | 133 | 27 | 41 | 56 | 3 | 4 | 1 | 0 | 0 | 1 | ||
| 50–100 | 0.7 | |||||||||||||
| 0 | 6589 (75.1) | |||||||||||||
| 0-50 | 106 (1.2) | |||||||||||||
| 50-100 | 700 (7.9) | |||||||||||||
| 100-149 | 237 (2.7) | |||||||||||||
| 150-300 | 540 (6.2) | 525 (88.4) | 464 | 97 | 174 | 175 | 7 | 4 | 2 | 2 | 0 | 3 | ||
| ≥ 300 | 604 (6.9) | 583 (89.0) | 519 | 80 | 186 | 195 | 18 | 12 | 10 | 7 | 2 | 9 | ||
| Subtotal | 8776 (100.0) | 1108 (88.7) | 983 | 177 | 360 | 370 | 25 | 16 | 12 | 9 | 2 | 12 | ||
| 100–149 | 0.3 | |||||||||||||
| 0 | 2244 (68.4) | |||||||||||||
| 0-50 | 49 (1.5) | |||||||||||||
| 50-100 | 259 (7.9) | |||||||||||||
| 100-149 | 109 (3.3) | |||||||||||||
| 150-300 | 293 (8.9) | 286 (88.8) | 254 | 52 | 103 | 87 | 4 | 2 | 3 | 1 | 0 | 2 | ||
| ≥ 300 | 328 (10.0) | 320 (91.6) | 293 | 36 | 127 | 81 | 16 | 9 | 6 | 7 | 4 | 7 | ||
| Subtotal | 3282 (100.0) | 606 (90.3) | 547 | 88 | 230 | 168 | 20 | 11 | 9 | 8 | 4 | 9 | ||
Regardless of the age group, the colonoscopy detection rate was significantly different between the values of Fhb(-1) [i.e. age ≥ 70 years: Fhb(-1) = 0: rate = 79.1%, Fhb(-1) = (0-50): Rate = 75.9%, Fhb(-1) = (50-100): Rate = 88.2%, Fhb(-1) = (100-150): Rate = 93.8%; P = 0.001). In the age groups of 65-69 years and ≥ 70 years, the proportion of CRC among colorectal lesions was significantly higher when the Fhb(-1) was between 100 and 150 [i.e. age in 65-69 years: Fhb(-1) = 0: %CRC = 6.2, Fhb(-1) = (0-50): %CRC = 8.2, Fhb(-1) = (50-100): %CRC = 8.1, Fhb(-1) = (100-150): %CRC = 15.8; P = 0.001) (Table 3).
| Fhb(-1) by age at test(-1) | Colonoscopy detection rate and CRC proportion | ||||
| Number Colo (%Positive colo) | P value1 | Number of lesions (%CRC) | P value2 | ||
| Age (yr) at test(-1) | Fhb(-1) (ngHb/mL) | ||||
| Overall | 11254 (77.7) | 8748 (5.7) | |||
| 50-54 | <10-3 | 0.7 | |||
| 0 | 1471 (71.6) | 1053 (3.1) | |||
| 0-50 | 21 (71.4) | 15 (0.0) | |||
| 50-100 | 130 (90.8) | 118 (2.5) | |||
| 100-150 | 68 (86.8) | 59 (5.1) | |||
| Subtotal | 1690 (73.7) | 1245 (3.1) | |||
| 55-59 | < 10-3 | 0.1 | |||
| 0 | 1797 (73.7) | 1324 (3.9) | |||
| 0-50 | 31 (67.7) | 21 (4.8) | |||
| 50-100 | 178 (87.1) | 155 (7.1) | |||
| 100-150 | 101 (91.1) | 92 (7.6) | |||
| Subtotal | 2107 (75.6) | 1592 (4.5) | |||
| 60-64 | < 10-3 | 0.05 | |||
| 0 | 2044 (76.4) | 1562 (4.6) | |||
| 0-50 | 37 (70.3) | 26 (7.7) | |||
| 50-100 | 247 (90.3) | 223 (8.1) | |||
| 100-150 | 121 (90.1) | 109 (8.3) | |||
| Subtotal | 2449 (78.4) | 1920 (5.3) | |||
| 65-69 | < 10-3 | 0.001 | |||
| 0 | 2373 (76.5) | 1815 (6.2) | |||
| 0-50 | 54 (90.7) | 49 (8.2) | |||
| 50-100 | 308 (88) | 271 (8.1) | |||
| 100-150 | 172 (88.4) | 152 (15.8) | |||
| Subtotal | 2907 (78.7) | 2287 (7.1) | |||
| ≥70 | < 10-3 | 0.01 | |||
| 0 | 1683 (79.1) | 1331 (6.5) | |||
| 0-50 | 29 (75.9) | 22 (9.1) | |||
| 50-100 | 245 (88.2) | 216 (10.2) | |||
| 100-150 | 144 (93.8) | 135 (13.3) | |||
| Subtotal | 2101 (81.1) | 1704 (7.6) | |||
Overall, the proportion of recto-sigmoid lesions was 57.2% among the 5100 lesions for which the location was provided. The proportion of CRC among these colorectal lesions was significantly higher (P = 0.01) in cecal localization compared to other colonic locations. However, this increase in the proportion of CRC among the cecal lesions was not statistically significant regardless of the stratum combining Fhb(-1) and Fhb(0) (Table 4).
| Fhb(0) by Fhb(-1) | Colorectal | Number of colorectal lesions by localization and proportion of CRC | |||||||
| Specified localization: number (% CRC) | Cecum: number (% CRC) | Right colon: number (% CRC) | Transverse colon: number (%CRC) | Left colon: number (%CRC) | Rectosi | P value1 | |||
| Fhb(-1) (ngHb/mL) | Fhb(0) (ngHb/mL) | ||||||||
| Overall | 150-300 | 4390 (57.0) | 2500 (5.4) | 195 (6.7) | 490 (5.3) | 221 (3.2) | 252 (3.2) | 1342 (6.1) | 0.2 |
| > 300 | 4358 (59.7) | 2600 (13.4) | 179 (16.8) | 432 (11.8) | 176 (11.4) | 235 (11.1) | 1578 (14.1) | 0.3 | |
| Total | 8748 (58.3) | 5100 (9.5) | 374 (11.5) | 922 (8.4) | 397 (6.8) | 487 (7.0) | 2920 (10.4) | 0.01 | |
| 0 | 150-300 | 3609 (55.7) | 2011 (5.3) | 154 (7.8) | 391 (5.1) | 176 (2.8) | 200 (3.0) | 1090 (5.8) | 0.2 |
| > 300 | 3476 (58.2) | 2022 (12.0) | 145 (14.5) | 355 (10.1) | 139 (8.6) | 185 (10.3) | 1198 (12.9) | 0.3 | |
| Subtotal | 7085 (56.9) | 4033 (8.6) | 299 (11.0) | 746 (7.5) | 315 (5.4) | 385 (6.5) | 2288 (9.5) | 0.02 | |
| 0-50 | 150-300 | 63 (61.9) | 39 (10.3) | 2 (0.0) | 6 (16.7) | 4 (0.0) | 8 (12.5) | 19 (10.5) | 1.0 |
| > 300 | 70 (55.7) | 39 (12.8) | 2 (0.0) | 4 (25.0) | 2 (0.0) | 3 (0.0) | 28 (14.3) | 0.8 | |
| Subtotal | 133 (58.7) | 78 (11.5) | 4 (0.0) | 10 (20) | 6 (0.0) | 11 (9.1) | 47 (12.8) | 0.9 | |
| 50-100 | 150-300 | 464 (62.1) | 288 (5.6) | 24 (0.0) | 66 (4.6) | 23 (4.4) | 23 (0.0) | 152 (7.9) | 0.5 |
| > 300 | 519 (63.0) | 327 (17.1) | 22 (27.3) | 46 (17.4) | 21 (23.8) | 29 (10.3) | 209 (16.3) | 0.5 | |
| Subtotal | 983 (62.6) | 615 (11.7) | 46 (13.0) | 112 (9.8) | 44 (13.6) | 52 (5.8) | 361 (12.7) | 0.6 | |
| 100-149 | 150-300 | 254 (63.8) | 162 (6.2) | 15 (6.7) | 27 (7.4) | 18 (5.6) | 21 (4.8) | 81 (6.2) | 1.0 |
| > 300 | 293 (72.4) | 212 (21.7) | 10 (30.0) | 27 (22.2) | 14 (21.4) | 18 (22.2) | 143 (21.0) | 1.0 | |
| Subtotal | 547 (68.4) | 374 (15) | 25 (16.0) | 54 (14.8) | 32 (12.5) | 39 (12.8) | 224 (15.6) | 1.0 | |
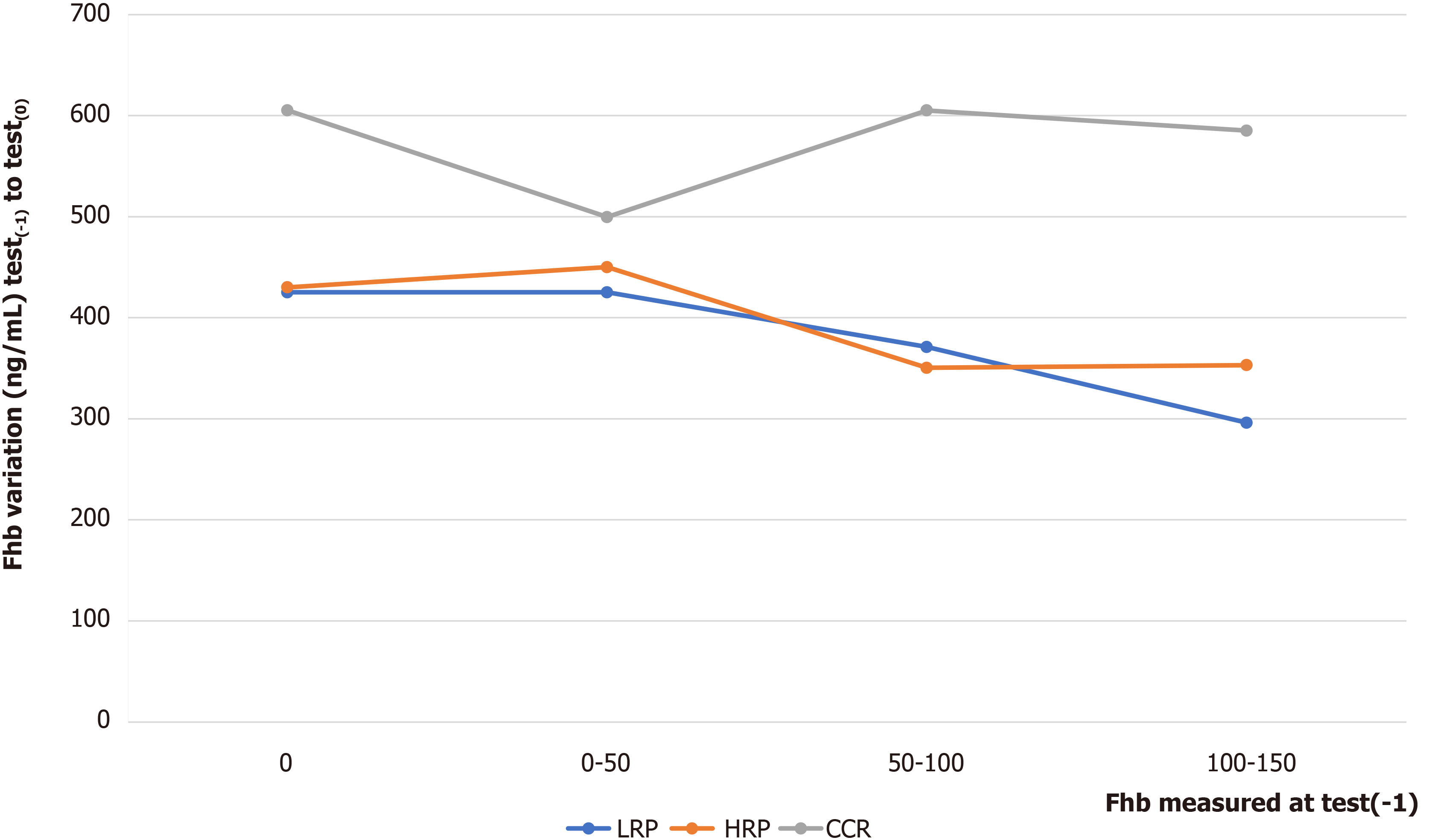
Regardless of the Fhb(-1) modalities [except for modality (0-50)], the variation in Fhb between test(0) and test(-1) was significantly greater when the lesion was CRC colorectal cancer. For this modality (0-50), the number of lesions was relatively lower (CRC = 9, LRP = 27, UP = 56, HRP = 41) (Figure 1).
The multivariate logistic regression included 11254 people who completed a colonoscopy. This analysis shows that people with a Fhb(-1) in (100-150) were 2.9 times more likely to be diagnosed with a colorectal lesion in test(0) compared to those having a Fhb(-1) value of zero (adjusted OR = 2.9; 95%CI: 2.2-3.8). This risk of detecting a neoplastic lesion increased significantly with the age, the gender, the delay between test(-1) and test(0), and the number of tests performed before test(-1) (Table 5).
| Predictive factors | Risk analysis of colorectal lesions in a logistic regression model | Risk analysis of the severity of colorectal lesions in an ordered polytomous regression model | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Unadjusted OR (95%CI) | P value1 | Adjusted OR (95%CI) | P value1 | Unadjusted OR (95%CI) | P value2 | Adjusted OR (95%CI) | P value2 | |
| Fhb-1 (ngHb/mL) (Ref.: 0) | ||||||||
| 0-50 | 1.1 (0.8; 1.6) | 0.6 | 1.1 (0.8; 1.6) | 0.5 | 1.4 (1.0; 2.0) | 0.1 | 1.3 (0.9; 1.8) | 0.1 |
| 50-100 | 2.5 (2.1; 3.1) | < 10-3 | 2.4 (2.0; 3.0) | < 10-3 | 1.8 (1.6; 2.1) | < 10-3 | 1.8 (1.6; 2.0) | < 10-3 |
| 100-150 | 3.0 (2.3; 3.9) | < 10-3 | 2.9 (2.2; 3.8) | < 10-3 | 2.6 (2.2; 3.1) | < 10-3 | 2.6 (2.2; 3.0) | < 10-3 |
| gFOBT campaign participation (Ref.: Yes-gFOBT) | ||||||||
| New entrant | 0.9 (0.7; 1.0) | 0.04 | 3.3 (2.2; 5.0) | < 10-3 | 0.6 (0.6; 0.8) | < 10-3 | 0.1 (0.1; 0.2) | < 10-3 |
| No-gFOBT | 1.1 (1.0; 1.2) | 0.2 | 2.8 (1.9; 4.1) | < 10-3 | 0.8 (0.7; 0.9) | < 10-3 | 0.2 (0.1; 0.3) | < 10-3 |
| Age (yr) at test(-1) (Ref.: 50-54) | ||||||||
| 55-59 | 1.1 (1.0; 1.3) | 0.2 | 1.3 (1.0; 1.6) | 0.04 | 1.1 (0.9; 1.3) | 0.4 | 0.8 (0.6; 1.0) | 0.02 |
| 60-64 | 1.3 (1.1; 1.5) | < 10-3 | 1.4 (1.2; 1.8) | 0.001 | 1.0 (0.9; 1.2) | 0.5 | 0.7 (0.6; 0.9) | 0.004 |
| 65-69 | 1.3 (1.1; 1.5) | < 10-3 | 1.4 (1.1; 1.8) | 0.002 | 1.3 (1.1; 1.5) | 0.002 | 0.9 (0.7; 1.1) | 0.2 |
| ≥ 70 | 1.5 (1.3; 1.8) | < 10-3 | 1.7 (1.3; 2.1) | < 10-3 | 1.2 (1.0; 1.4) | 0.01 | 0.8 (0.6; 1.0) | 0.07 |
| Gender (Ref.: Female) | ||||||||
| Male | 1.7 (1.5; 1.9) | < 10-3 | 1.7 (1.5; 1.8) | < 10-3 | 1.2 (1.1; 1.3) | 0.002 | 1.2 (1.1; 1.3) | < 10-3 |
| Delay (mo) between test(-1) and test0 (Ref.: < 24) | ||||||||
| 24-30 | 1.3 (1.1; 1.4) | < 10-3 | 1.3 (1.1; 1.5) | < 10-3 | 1.0 (0.9; 1.1) | 0.9 | 1.0 (0.9; 1.1) | 0.9 |
| > 30 | 1.9 (1.7; 2.2) | < 10-3 | 2.1 (1.7; 2.6) | < 10-3 | 0.6 (0.5; 0.7) | < 10-3 | 0.6 (0.5; 0.7) | < 10-3 |
| Number of tests completed before test(-1) (Ref.: 0) | ||||||||
| 1 | 1.1 (0.9; 1.2) | 0.3 | 2.7 (1.9; 3.9) | < 10-3 | 1.2 (1.0; 1.4) | 0.03 | 0.3 (0.2; 0.4) | < 10-3 |
| 2 | 1.0 (0.9; 1.2) | 0.7 | 2.7 (1.8; 4.1) | < 10-3 | 1.2 (1.0; 1.3) | 0.03 | 0.2 (0.1; 0.3) | < 10-3 |
| 3 | 1.1 (1.0; 1.3) | 0.1 | 2.9 (1.9; 4.4) | < 10-3 | 1.4 (1.2; 1.6) | < 10-3 | 0.3 (0.2; 0.4) | < 10-3 |
| 4 | 1.3 (1.1; 1.5) | < 10-3 | 3.5 (2.3; 5.2) | < 10-3 | 1.1 (1.0; 1.3) | 0.1 | 0.2 (0.1; 0.3) | < 10-3 |
The ordered polytomous regression included 8748 people having exhaustive information on the colorectal lesion. The analysis showed that people with a Fhb(-1) in (100-150) (P = 0.001) were 2.6 (2.2; 3.0) times more likely to have a high severity level, compared to those having a Fhb(-1) value of zero. The risk to have a high severity level was higher in male compared to female (P = 0.001). This risk was reduced by 20% in age group 55-59 years compared to age group < 55 years [adjusted OR: 0.8 (0.6; 1.0), P = 0.02] (Table 5).
This study showed that the risk of detecting a colorectal lesion during a campaign was proportional to the Fhb observed in the previous test completed. Above all, it highlighted that an increase in the probability of detecting a colorectal neoplasia with a high level of severity was proportional to the Fhb observed in the previous test completed. These probabilities varied with the socio-demographic characteristics, especially with age.
The proportion of positive tests and the colonoscopy detection rate increased proportionally with the Fhb(-1). Furthermore, the detection of colorectal lesions according to the variation in the Fhb between test(-1) and test(0) allows to support an association between the detection of cancerous lesions and the strong Fhb variations between two consecutive tests. These results agree with previous data already described in the literature. Furthermore, the increased risk of detecting a neoplasia during a new screening 2 years after a negative screening test result has already been reported in Ireland[14] and recently in the Ile-de-France region[27]. These authors observed variable proportions of pathologies (advanced adenomas or CRC) during a subsequent campaign and hypothesized that these lesions could have been diagnosed during the previous campaign if the FIT positivity threshold was not relatively high. However, they admitted that lowering the positivity threshold would both allow the diagnosis of lesions that could be serious only 2 years later and create an unsustainable endoscopy referral burden[14].
The current positivity threshold in the French screening program induces a considerable loss of chance. Indeed, in most countries, the threshold of FIT positivity is chosen in part to adapt to the offer of colonoscopies. The need to readjust this strategy in patients with a Fhb between 100 and 150 ngHb/mL should be assessed[27]. This study highlights the need for a strategy taking into account the age of people participating in the screening campaign. The elderly (≥ 70 years) had a high proportion of positive colonoscopy when the Fhb measured in the previous campaign was between 100 and 150 ngHb/mL. Younger people (< 54 years) had a likelihood of having high-severity neoplasia. Given these results, the recommendation to reduce the FIT positivity threshold to 100 ngHb/mL for first-time arrivals and the elderly (≥ 70 years) should attract the attention of the French health authority.
Another alternative would be to reduce the delay between two tests for these first-time participants and the elderly (age ≥ 70 years) from the current 2 years to 1 year. This alternative could have its main justification in the enthusiasm of the elderly towards screening campaigns in France, described in a previous study[28]. The reduction in the time between two tests has the advantage of allowing the recovery of false negative results that a decrease in the positivity threshold cannot recover. Indeed, after finding that the Fhb was less than 4 µgHb/g in 94.0% of false negative individuals, Ibañez-Sanz et al[29] concluded that the decrease in the positivity threshold of the FIT does not increase the detection rate of advanced neoplasia but may increase costs and potential adverse effects. Whatever strategy chosen, it should also include the 65-69-age group, which accounts for more than 33.3% of the CRCs detected and presents a significantly increased proportion of CRC when Fhb(-1) was between 100 and 150.
In terms of the location of colorectal lesions, it has been argued that FITs are possibly less effective at detecting lesions located in the proximal colon than distally[30]. Digby et al[19] showed that 77.8% of adenomas and 69.2% of cancers were located in the distal colon. The results of this study are consistent with this proximal location. However, in the Ibañez-Sanz study[29], about 60% of the lesions were localized in the proximal colon, while the expected percentage was 30%.
The optimal interval for CRC screening using FIT remains unclear[5]. In terms of the impact of the delay between the tests on the risk of detection of a colorectal lesion, the results of this study are not consistent with Van Roon's analysis, certainly because of the lower test positivity threshold (≥ 50 ng/mL) in their study carried out in a small sample (7501 people)[31]. In addition, this study found an unexpected reverse direction in the analysis of the lesion severity risk according to the delay between test(0) and test(-1). This paradoxical reduction in the risk of severity could be explained by the high proportion of polyps among the lesions detected in patients with an abnormally long delay between test(0) and test(-1). We can also hypothesize that some patients with a time between the two tests greater than 2 years may be symptomatic and therefore will not appear in the screening program as mentioned by Liao et al[21].
The main limitation of this study is the amount of missing data, especially on the stages of the lesions and their colonic locations. However, this is a consequence inherent to retrospective studies that cannot question the results of this study. In addition, the absence of a cancer registry in the "Auvergne Rhône-Alpes Region” does not allow interval cancers to be included in this study.
An increased risk of severity of the colorectal lesion was observed in proportion to the increase in Fhb(-1). This risk of severity varied with the socio-demographic characteristics of the patients, especially among first-time participants. An increased colonoscopy detection rate was observed in the elderly in correlation with the increase in Fhb(-1). According to these results, the FIT positivity threshold should be reduced to 100 ngHb/mL for first-time participants and patients aged ≥ 70. The other alternative should be to reduce the delay between the two tests for these first-time participants and the elderly (age ≥ 70) from the current 2 years to 1 year.
The rate of positive tests using fecal immunochemical test (FIT) does not decrease with subsequent campaigns, but the positive predictive value of advanced neoplasia significantly decreases in subsequent campaign after a first negative test. A relationship between the fecal hemoglobin concentration (Fhb) and the opportunity to detect a colorectal cancer in subsequent campaign has been shown.
In this period of implementation of the optimization strategies of the French program, our motivation was to alert the health authority on the severity of the lesions not diagnosed because of the high positivity threshold of the current screening FIT.
Our objective was to predict the severity of colorectal lesions based on Fhb measured during previous colorectal cancer screening campaign.
The etiological study included 293750 patients aged 50-74, living in Auvergne-Rhône-Alpes (France). These patients completed at least two FIT [test(-1) and test(0)] between June 2015 and December 2019. Delay between test(-1) and test(0) was > 1 year, and test(-1) result was negative (< 150 ngHb/mL). The severity of colorectal lesions diagnosed at test(0) was described according to Fhb measured at test(-1) [Fhb(-1)]. The relationship between the severity classified in seven ordinal categories and the predictive factors was analyzed in an ordered multivariate polytomous regression model.
The test(0) positive rate was 4.0% and the colonoscopy completion rate was 97.1% in 11594 patients who showed a positive test(0). The colonoscopy detection rate was 77.7% in those 11254 patients who underwent a colonoscopy. In total, 8748 colorectal lesions were detected (including 2182 low-risk-polyps, 2400 high-risk-polyp and 502 colorectal cancer). The colonoscopy detection rate varied significantly with Fhb(-1) [0 ngHb/mL: 75.6%, (0-50): 77.3%, (50-100): 88.7%, (100-150): 90.3%; P = 0.001). People with a Fhb(-1) within (100-150 ngHb/mL) (P = 0.001) were 2.6 (2.2; 3.0) times more likely to have a high severity level compared to those having a Fhb(-1) value of zero. This severity risk was reduced by 20% in patients aged 55-59 compared to those aged < 55 [adjusted odds ratio: 0.8 (0.6; 1.0)].
The study showed that higher Fhb(-1) is correlated to an increased risk of severity of colorectal lesions. This risk of severity increased among first-time participants (age < 55) and the elderly (≥ 70). To avoid the loss of chance in these age groups, the FIT positivity threshold should be reduced to 100 ngHb/mL. The other alternative would be to reduce the time between the two tests in these age groups from the current 2 years to 1 year.
At the end of this study, we aim to conduct an experiment with a screening program considering the age of patients and the previous values of the fecal hemoglobin concentration.
The authors are grateful to their colleagues from the colorectal cancer screening program management structures for the good collaboration and the quality of the data used in this study. The authors are grateful to Charlène Vandenbroucke for the thorough editing of the final version of the manuscript.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56651] [Article Influence: 7081.4] [Reference Citation Analysis (134)] |
| 2. | Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 512] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 4. | Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C, Jover R, Montalvo I, Arenas J, Laredo E, Hernández V, Iglesias F, Cid E, Zubizarreta R, Sala T, Ponce M, Andrés M, Teruel G, Peris A, Roncales MP, Polo-Tomás M, Bessa X, Ferrer-Armengou O, Grau J, Serradesanferm A, Ono A, Cruzado J, Pérez-Riquelme F, Alonso-Abreu I, de la Vega-Prieto M, Reyes-Melian JM, Cacho G, Díaz-Tasende J, Herreros-de-Tejada A, Poves C, Santander C, González-Navarro A; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 674] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 5. | Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Rex DK. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Ventura L, Mantellini P, Grazzini G, Castiglione G, Buzzoni C, Rubeca T, Sacchettini C, Paci E, Zappa M. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis. 2014;46:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Vicentini M, Zorzi M, Bovo E, Mancuso P, Zappa M, Manneschi G, Mangone L, Giorgi Rossi P; Colorectal Cancer Screening IMPATTO study working group. Impact of screening programme using the faecal immunochemical test on stage of colorectal cancer: Results from the IMPATTO study. Int J Cancer. 2019;145:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol. 2016;22:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 9. | Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The Natural History of Colorectal Polyps: Overview of Predictive Static and Dynamic Features. Gastroenterol Clin North Am. 2018;47:515-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol. 2009;193:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Pickhardt PJ, Kim DH, Pooler BD, Hinshaw JL, Barlow D, Jensen D, Reichelderfer M, Cash BD. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol. 2013;14:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E. Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology. 2012;142:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Koïvogui A, Mab GL, Benamouzig R. Detection of Colorectal Neoplasia in a Cohort Before and After the Change of Fecal Occult Blood Test in a French Colorectal Cancer Screening Program. Am J Gastroenterol. 2018;113:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Gibson DJ, Mooney T, Mooney J, Mulcahy HE, O'Donoghue D. Impact of a higher fecal immunochemistry test cut-off on pathology detected in subsequent rounds of a colorectal screening program. Gastrointest Endosc. 2019;89:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Grobbee EJ, Schreuders EH, Hansen BE, Bruno MJ, Lansdorp-Vogelaar I, Spaander MCW, Kuipers EJ. Association Between Concentrations of Hemoglobin Determined by Fecal Immunochemical Tests and Long-term Development of Advanced Colorectal Neoplasia. Gastroenterology. 2017;153:1251-1259.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Hernandez V, Cubiella J, Gonzalez-Mao MC, Iglesias F, Rivera C, Iglesias MB, Cid L, Castro I, de Castro L, Vega P, Hermo JA, Macenlle R, Martínez-Turnes A, Martínez-Ares D, Estevez P, Cid E, Vidal MC, López-Martínez A, Hijona E, Herreros-Villanueva M, Bujanda L, Rodriguez-Prada JI; COLONPREV Study Investigators. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J Gastroenterol. 2014;20:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Pin Vieito N, Zarraquiños S, Cubiella J. High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis. World J Gastroenterol. 2019;25:2383-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 18. | Ribbing Wilén H, Blom J, Höijer J, Hultcrantz R. Fecal immunochemical test in colorectal cancer screening: Colonoscopy findings by different cut-off levels. J Gastroenterol Hepatol. 2019;34:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Digby J, Fraser CG, Carey FA, McDonald PJ, Strachan JA, Diament RH, Balsitis M, Steele RJ. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol. 2013;66:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Kawamura T, Inoue T, Shinomiya R, Sakai H, Amamiya K, Sakiyama N, Shirakawa A, Okada Y, Sanada K, Nakase K, Mandai K, Suzuki A, Kamaguchi M, Morita A, Nishioji K, Tanaka K, Uno K, Yokota I, Kobayashi M, Yasuda K. Significance of fecal hemoglobin concentration for predicting risk of colorectal cancer after colonoscopy. JGH Open. 2020;4:898-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Liao CS, Lin YM, Chang HC, Chen YH, Chong LW, Chen CH, Lin YS, Yang KC, Shih CH. Application of quantitative estimates of fecal hemoglobin concentration for risk prediction of colorectal neoplasia. World J Gastroenterol. 2013;19:8366-8372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Goulard H, Boussac-Zarebska M, Bloch J. Epidemiological assessment of the pilot programme for organized colorectal cancer screening, France, 2007. BEH. 2009;2-3:22-25. |
| 23. | Leuraud K, Jezewski-Serra D, Viguier J, Salines E. Colorectal cancer screening by guaiac faecal occult blood test in France: Evaluation of the programme two years after launching. Cancer Epidemiol. 2013;37:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | World Health Organization. 10th International Classification of Diseases, Version: 2008. Geneve: World Health Organization, 1990. Available from: https://icd.who.int/browse10/2008/fr. |
| 25. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017. |
| 26. | Journal Officiel de la République Française. Délibération n 2017-215 du 13 juillet 2017 portant adoption d'une norme destinée à simplifier l'obligation de déclaration des traitements de données à caractère personnel ayant pour finalité le dépistage organisé du cancer du sein, du cancer colorectal et du cancer du col de l'utérus mis en œuvre par les structures de gestion conventionnées, et abrogeant la délibération n°2015-175 du 11 juin 2015 (décision d'autorisation unique n AU-043) (NS-059). Journal Officiel de la République Française (JORF) 2017; NOR: CNIL1724568X. Available from: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000035484848&categorieLien=id. |
| 27. | Kaufmanis A, Vincelet C, Koivogui A, Delattre-Massy H, Deyra J, Ait Hadad H, Brixi Z, Bercier S, Le Trung T. Devenir en deuxième campagne de dépistage organisé du cancer colorectal d’un test négatif en première campagne selon le dosage d’hémoglobine fécale. Journées Francophones d’Hépato-Gastroentérolgie et d’Oncologie Digestive (JFHOD) de la Société Nationale Française de Gastro-Entérologie (SNFGE); 2019 March 21-24; Paris, France.Paris: SNFG, 2019: Poster P.395. Available from: https://www.snfge.org/content/devenir-en-deuxieme-campagne-de-depistage-organise-du-cancer-colorectal-dun-test-negatif-en. |
| 28. | Koïvogui A, Balamou C, Rymzhanova R, Letrung T, Hadad HA, Brixi Z, Cornelis S, Delattre-Massy H, Aparicio T, Benamouzig R. Colorectal cancer fecal screening test completion after age 74, sources and outcomes in French program. World J Gastrointest Oncol. 2019;11:729-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Ibañez-Sanz G, Garcia M, Milà N, Rodríguez-Moranta F, Binefa G, Gómez-Matas J, Benito L, Padrol I, Barenys M, Moreno V. False-negative rate cannot be reduced by lowering the haemoglobin concentration cut-off in colorectal cancer screening using faecal immunochemical test. Eur J Cancer Prev. 2017;26:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Haug U, Kuntz KM, Knudsen AB, Hundt S, Brenner H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer. 2011;104:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | van Roon AH, Goede SL, van Ballegooijen M, van Vuuren AJ, Looman CW, Biermann K, Reijerink JC, Mannetje H', van der Togt AC, Habbema JD, van Leerdam ME, Kuipers EJ. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut. 2013;62:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yao K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX