Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4603
Peer-review started: February 27, 2021
First decision: March 27, 2021
Revised: April 11, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: July 28, 2021
Processing time: 148 Days and 21.3 Hours
In this review the current overall knowledge on hepatitis A, B, C, D, and E will be discussed. These diseases are all characterized by liver inflammation but have significant differences in distribution, transmission routes, and outcomes. Hepatitis B virus and hepatitis C virus are transmitted by exposure to infected blood, and in addition to acute infection, they can cause chronic hepatitis, which in turn can evolve into cirrhosis. It is estimated that more than 300 million people suffer from chronic hepatitis B or C worldwide. Hepatitis D virus, which is also transmitted by blood, only affects hepatitis B virus infected people, and this dual infection results in worse liver-related outcomes. Hepatitis A and E spread via the fecal–oral route, which corresponds mainly to the ingestion of food or water contaminated with infected stools. However, in developed countries hepatitis E is predominantly a zoonosis. Although hepatitis A virus and hepatitis E virus are usually responsible for a self-limiting hepatitis, a serious, rarely fatal illness is also possible, and in immunosuppressed patients, such as organ transplant recipients, hepatitis E virus infection can become chronic. The description of goals achieved, unresolved issues, and the latest research on this topic may make it possible to speculate on future scenarios in the world of viral hepatitis.
Core Tip: Viral hepatitis still endangers the health of millions of people around the world. In this review the path that led to the current knowledge in the field of viral hepatitis will be reported.
- Citation: Torre P, Aglitti A, Masarone M, Persico M. Viral hepatitis: Milestones, unresolved issues, and future goals. World J Gastroenterol 2021; 27(28): 4603-4638
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4603.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4603
Hepatitis viruses are a leading cause of morbidity and mortality as the consequences of their acute or chronic infections. The World Health Organization (WHO) estimated 1.4 million deaths per year due to viral hepatitis worldwide, most of which are a consequence of hepatitis B or C. Significant progress has been made in the prevention and treatment of hepatitis B, C, and D viruses (HBV, HCV, and HDV) infections, but chronic hepatitis by these viruses still represents the most common etiology of cirrhosis and hepatocellular carcinoma (HCC). Hepatitis A virus (HAV), HBV, HDV, and hepatitis E virus (HEV) are a frequent cause of clinical hepatitis and acute liver failure. Although it is rare, the latter is a life-threatening condition. In 2016, the WHO Global Health Sector Strategy established the goal of eliminating viral hepatitis “as a major public health threat” by 2030. However, great efforts are still needed to bridge the gap between the current situation and this ambitious goal.
HCV is a single strand positive sense RNA virus of the Flaviviridae family (Hepacivirus genus) discovered in 1989 by Michael Houghton and colleagues[1,2]. This discovery, which was awarded the 2020 Nobel Prize in Physiology or Medicine, shed light on blood-transmitted chronic non-A, non-B hepatitis cases, and through the subsequent advent of specific therapies is estimated to have saved millions of lives. HCV is blood-borne transmitted[3], and currently eight viral genotypes are recognized[4,5]. In 15% of cases HCV infection is responsible for acute hepatitis[6], while approximately 80% of infected patients progress to chronic infection; 20% of chronically infected patients develop cirrhosis within 25 years, and among patients with cirrhosis 25% develop HCC and/or decompensated liver disease[7].
HCV is widespread all over the world, with different prevalence of viral genotypes within different geographical areas. Genotype 1 is the most common, especially in North America, Europe, and Asia. Genotype 2 is endemic in parts of West Africa and Latin America. Genotype 3 is present in Southeast Asia and Eastern Europe. Genotype 4 is endemic in North Africa and Sub-Saharan Africa. Genotype 5 is particularly common in Southern Africa[8]. This virus, whose only host is humans, is transmitted by blood. In high-income countries this infection occurs particularly in “at risk” populations, such as people who inject drugs who may share infected needles, men who have sex with other men, and prisoners. In low-middle-income countries, healthcare associated transmission (e.g., during invasive procedures or the execution of blood transfusions with infected blood) is still an important problem[9,10]. Perinatal transmission from infected mothers occurs in 3%-10% of cases[11].
Acute HCV infection is usually asymptomatic and characterized by increased transaminases usually occurring between 6 wk and 8 wk after exposure[12]. Symptoms such as nausea, asthenia, and weight loss appear in less than 30% of patients, and in most cases they do not interfere with normal daily activities[13]. The presence of jaundice is also infrequent, occurring in less than one third of patients[14]. Acute liver failure due to HCV infection is unusual[12].
About 30% of people who contract the infection spontaneously eliminate the HCV within 6 mo. On the contrary, at least 60% to 70% of infected people develop chronic infection, defined as the presence of anti-HCV immunoglobulin in the blood and the persistence of HCV-RNA for at least 6 mo[12,15]. In these cases, transaminases are usually moderately increased but can also be normal[16]. Most patients with chronic hepatitis C are asymptomatic, but some may experience asthenia, anorexia, and weight loss. Jaundice occurs only in the case of advanced liver cirrhosis. Physical examination can reveal hepatomegaly and splenomegaly (this latter in case of cirrhosis or blood malignancies)[12]. Chronic HCV infection could induce liver fibrosis, which can progress to the development of cirrhosis, decompensation, and HCC[17]. In HCV patients, HCC usually appears in patients with liver cirrhosis, but it can also occur in the absence of such condition[12]. The proportion of patients who develop liver cirrhosis after 20 years of infection is quite varied, ranging between 2%-3%[18,19] and 51%[20], depending on the study population and various risk factors, such as male gender, alcohol consumption, older age at the time of infection, coinfection with other viruses [human immunodeficiency virus (HIV) and HBV], and others[7].
The HCV primarily affects the liver, but a high percentage of HCV patients (up to 74%) was observed to have at least one extrahepatic manifestation[21]. For some extrahepatic manifestations the link with HCV was strongly demonstrated by epidemiological and pathogenetic data (mixed cryoglobulinemia/cryoglobulinemic vasculitis and B-cell non-Hodgkin’s lymphoma), while others have a less close connection with this virus[22,23]. Mixed cryoglobulinemia is a small vessel vasculitis that predominantly affects the skin, joints, peripheral nerves, and kidneys, and in 80% of cases is caused by HCV. Clinical manifestations are variable, ranging from mild manifestations (purpura or arthralgia) to more serious conditions such as glomerulonephritis or generalized vasculitis[24]. B-cell non-Hodgkin’s lymphoma is particularly common in HCV infected patients[25,26], and over the last years the association between these two diseases has been confirmed in some meta-analyses[27,28]. The association between HCV infection and lymphoma seems to be greater in Mediterranean countries, Japan, and Brazil[29], while it seems less strong in the United Kingdom, Northern Europe, and Canada[30]. In the United States, two extensive studies showed a modest increased risk of developing B-cell non-Hodgkin’s lymphoma in infected HCV patients compared to negative controls[31,32].
Other pathological conditions that have been associated with chronic HCV infection include autoimmune disorders (such as arthritis, Sjogren’s syndrome, hemolytic anemia, autoimmune thrombocytopenia)[33], chronic kidney failure secondary to the onset of glomerulonephritis[24], cardiovascular diseases[34], endocrine disorders (such as thyroiditis), and type 2 diabetes mellitus[35].
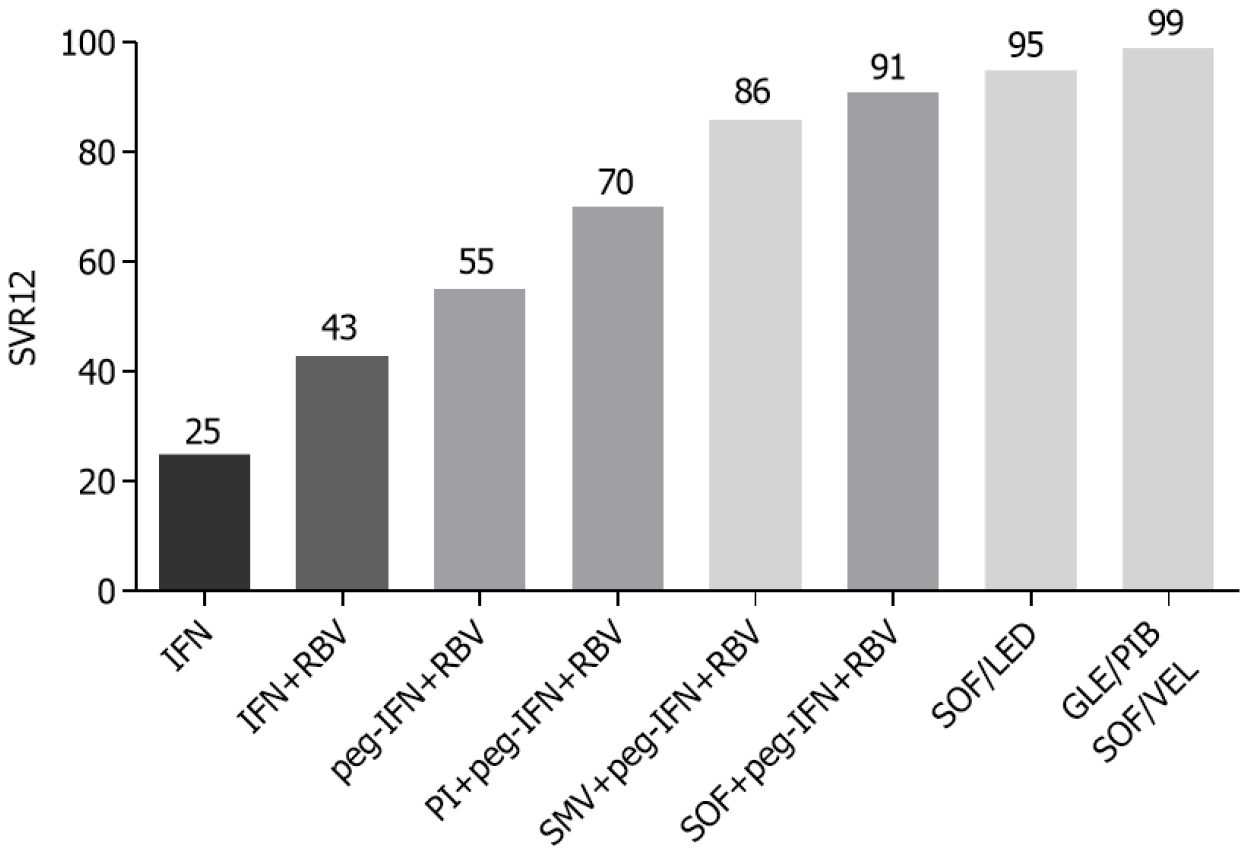
The discovery of second-generation direct-acting antiviral agents (DAAs) was a real revolution in the treatment of hepatitis C (Figure 1). In the early 1990s, the use of alpha interferon (IFN) (3 million units three times a week for 6 mo) gave rather unsatisfactory results, with a sustained virological response rate (SVR) of about 25%[36]. The addition of ribavirin (RBV) combined with IFN 2α for 48 wk provided a good increase in the SVR rate, showing an efficacy of 43% compared to 19% of SVR in patients treated with IFN in monotherapy for 48 wk. However, patients receiving combination therapy showed a higher rate of adverse events (19% vs 13%) than patients in monotherapy, particularly anemia and leukopenia[37]. In the early 2000s, the use of pegylated IFN (peg-IFN) with the addition of RBV further improved viral eradication rates, with an SVR greater than 50%[38,39].
In 2011 the addition of protease inhibitors, such as boceprevir and telaprevir, to the standard therapy with peg-IFN + RBV allowed the achievement of eradication rates higher than 60% in the treatment of genotype 1 chronic HCV infection[40-43]. This triple therapy was, however, burdened by a greater number of adverse events than the standard therapy, particularly anemia, dysgeusia, and skin rash for boceprevir[41,44]. The use of telaprevir was more associated with the onset of skin rash, itching, anemia, nausea, and diarrhea[40,42]. In 2013 a new protease inhibitor, simeprevir, was used in combination with peg-IFN and RBV, which allowed a further increase in the success rate of antiviral therapy (86%) and a reduction of adverse events associated with previous regimens[45,46].
The big breakthrough came with the commercial introduction of sofosbuvir, a polymerase nucleotide inhibitor. Initially, it was used in combination with peg-IFN and RBV, obtaining a SVR rate of 90% and allowing a reduction of the treatment period to 12 wk[47-49]. It subsequently became the fundamental drug for the first IFN-free regimens[50], exceeding 95% of SVR in combination with ledipasvir in the treatment of genotype 1 HCV chronic infection[51]. Finally, a further step in the treatment of chronic HCV infection was the advent of pangenotypic regimens, such as sofosbuvir/velpatasvir and glecaprevir/pibrentasvir that guarantee an SVR rate close to 100% in all HCV genotypes[52-55]. The glecaprevir/pibrentasvir combination allowed a further reduction in the treatment period by showing a 99% of SVR in non-cirrhotic patients treated for 8 wk[56,57] (Table 1).
| DAAs | Clinical indications |
| Glecaprevir/Pibrentasvir | Pangenotypic regimen |
| 8 wk treatment in naïve/experienced (except GT3) non-cirrhotic pts and naïve CPT A5 cirrhotic pts | |
| 12 wk treatment in experienced (except GT3) CPT A5 cirrhotic pts | |
| 16 wk treatment in experienced GT3 non-cirrhotic or CPT A5 cirrhotic pts | |
| ESRD and/or hemodialysis (same treatment duration of pts without CKD) | |
| Contraindicated in decompensated cirrhosis | |
| Grazoprevir/Elbasvir | GT1b, GT4 |
| 12 wk treatment in GT1b or GT4 with HCV-RNA < 800000 IU/mL, naïve/experienced, CPT A5 cirrhotic or non-cirrhotic pts | |
| ESRD and/or hemodialysis (same treatment duration of pts without CKD) | |
| Contraindicated in decompensated cirrhosis | |
| Sofosbuvir/Velpatasvir | Pangenotypic regimen |
| 12 wk treatment in naïve/experienced, cirrhotic or non-cirrhotic pts | |
| Indicated in decompensated cirrhosis | |
| Add RBV (if tolerated) in GT3 cirrhotic pts or in decompensated cirrhosis | |
| Optimal profile for drug interactions | |
| Contraindicated in pts with eGFR < 30 mL/min | |
| Sofosbuvir/Velpatasvir/Voxilaprevir1 | Pangenotypic regimen |
| 8 wk treatment in naïve/experienced non cirrhotic pts or GT3 naïve/experienced CPT A5 cirrhotic pts | |
| 12 wk treatment in GT1, 2, 4, 5, 6 naïve/experienced CPT A5 cirrhotic pts | |
| First line therapy for retreatment after DAAs failure | |
| Contraindicated in pts with eGFR < 30 mL/min | |
| Contraindicated in decompensated cirrhosis |
The second generation DAAs, thanks to their effectiveness and high safety profile, have allowed the treatment of patients once considered difficult to treat. In patients with compensated cirrhosis DAAs showed a 95% eradication rate, slightly lower (86.1% and 88.3%) in patients with Child Pugh score B[58,59]. Even in cirrhotic patients the treatment period has been further reduced. In a recent trial, the administration of glecaprevir/pibrentasvir for 8 wk in treatment-naïve patients with compensated cirrhosis showed efficacy and safety comparable to the 12 wk regimen[60]. A recent study of 667 patients with advanced/decompensated cirrhosis treated with DAAs showed an improvement of at least 3 points in the MELD score in 29% of patients at a long-term follow-up. This improvement was more frequent in patients with MELD score ≥ 16 at baseline (45%)[61]. In addition, DAA treatments reduced the hospitalization rate of patients with HCV-related liver cirrhosis[62] and allowed a reduction of liver transplantation for related HCV diseases in Europe from 22.8% in the IFN/RBV era to 10.6% in the first half of 2017[63]. Therefore, treating patients with compensated or decompensated cirrhosis with DAAs not only allows viral eradication, but also an improvement in the natural history of this disease.
IFN-free therapies have shown efficacy and safety even in patients with HCC. In a recent meta-analysis by He et al[64], 3126 patients with a prior history of HCC or with present HCC were compared with 49138 cirrhotic patients without HCC showing a percentage of SVR of 88.2% and 92.4%, respectively (P > 0.001). After initial controversy over the possibility that the DAAs could increase the occurrence or recurrence of HCC[65], in recent years several studies have denied this possibility. In a large cohort study of about 60000 patients, Ioannou et al[66] compared the incidence of HCC in patients treated with IFN-only, DAA + IFN, and DAA-only. Authors showed that SVR was associated with a significantly decreased risk of HCC in multivariable models irrespective of whether the antiviral treatment was DAA-only, DAA + IFN, or IFN-only. Particularly, DAA-induced SVR is associated with a 71% reduction in HCC risk.
In a study involving 18076 patients treated with DAAs, with a follow-up of about 3 years, Kanwal et al[67] showed a cumulative incidence of HCC occurrence at 1, 2, and 3 years of 1.1%, 1.9%, and 2.8%, respectively. In patients with cirrhosis the cumulative incidence was 2.2%, 3.8%, and 5.6% at 1, 2, and 3 years, respectively. Two recent prospective Italian studies showed an incidence of HCC in patients treated with DAAs less than 3%, and in both studies the lack of SVR was a risk factor for the development of HCC[68,69].
Careful screening for hepatic nodules is essential before starting treatment with DAAs as the presence of undefined/benign hepatic nodules is significantly associated with HCC occurrence during or after treatment with DAA[70,71]. The recurrence rate of HCC in patients treated with DAAs is quite varied in literature, ranging from 2.2% in a cohort of liver transplant recipients for HCC and then treated with DAAs to 45.1% in cirrhotic patients[72]. In a broad, retrospective cohort study, patients treated with DAAs were compared with HCV-infected, cirrhotic patients that had not received any antiviral treatment, showing a lower early recurrence rate of HCC in the group of patients treated with DAAs (42.1% vs 58.9%)[73]. Finally, treatment with DAAs showed an improvement in survival in cirrhotic patients with HCC compared to patients with HCC and chronic HCV infection not treated with DAAs, thus resulting in effective, safe and beneficial treatment for patients[74-76].
DAA regimen has been shown to be highly effective in treating patients with chronic HCV infection and the concomitant presence of extrahepatic manifestations, such as mixed cryoglobulinemia and/or lymphoma. The SVR rate is similar compared to patients with no extrahepatic symptoms, exceeding 90%[77]. In a study by Passerini et al[78], 93 patients with chronic HCV infection and mixed cryoglobulinemia treated with DAAs were enrolled and compared with a control group of 89 HCV infected without mixed cryoglobulinemia also treated with an IFN-free regimen. The SVR rate was superimposable between the two groups, and the percentage of adverse events was similar. The authors demonstrated the high efficacy and safety of DAA treatment in patients with mixed cryoglobulinemia.
Conflicting data have been collected on the effectiveness of DAA regimen in the treatment of mixed cryoglobulinemia. In a study involving 148 patients with HCV infection and secondary mixed cryoglobulinemia vasculitis treated with DAAs with an average follow-up of about 15 mo, it was observed that 72.6% of patients obtained a complete response (defined as improvement of all organs involved at baseline without subsequent relapse), 22% a partial response, while 5% had no clinical response. Cryoglobulins were eliminated in the blood in about 50% of patients, while in over 90% of patients vasculitic and renal manifestations were eliminated; in 86% of patients an improvement of arthralgia was observed. Factors associated with a partial or absent response were a severe form of vasculitis and peripheral neuropathy[79]. In a recent work by Colantuono et al[80], 70 patients with HCV and mixed cryoglobulinemia vasculitis treated with DAAs were compared with 39 patients with the same clinical condition treated with IFN. The percentage of patients achieving at any time point of follow-up (more than 2 years) a complete or partial response was similar in the two groups, but patients treated with an IFN-free regimen had less effectively eliminated cryoglobulins and B-cell clones in the blood. Most importantly, they had a significantly higher percentage of vasculitis relapse (18% vs 3%, P = 0.028) and a greater need for treatment with rituximab.
In 2017, our group demonstrated that DAA therapy was safe and effective in patients with chronic HCV infection and with diffuse large B cell non-Hodgkin lymphoma (DLBCL) in current chemotherapy. In this small cohort of patients the SVR rate was 100%, and it emerged that patients treated with DAAs had a greater disease-free survival than HCV+ patients with DLBCL not treated with antiviral therapy (historical cohort). In multivariate analysis, DAAs have proven effective (P = 0.012) in improving remission of aggressive lymphoma in HCV+ patients[81]. Our data were subsequently confirmed in a study by Pellicelli et al[82], in which they demonstrated how HCV+ patients with DLBCL treated with DAAs or with peg-IFN + RBV who had not obtained SVR showed a greater relapse of DLBCL after chemotherapy than patients who reached a SVR. Furthermore, an Italian-French cohort of 47 HCV+ patients with DLBCL showed an SVR rate of 96% and a progression of lymphoproliferative disease in only three patients who had obtained SVR[83].
In a recent paper by Frigeni et al[84], 100 HCV+ patients with indolent non-Hodgkin lymphoma treated with an IFN-free regimen were enrolled. They showed an SVR rate of 99%, with an overall hematological response rate of 66%, with 23% of complete response and 43% of partial response; a higher overall hematological response rate was observed in marginal zone lymphoma than in non-marginal zone lymphoma (73% vs 48%, P = 0.021). Moreover, in this study a comparison between patients treated with DAAs alone as first-line therapy for indolent non-Hodgkin lymphoma (excluding patients previously treated with IFN) and a historical cohort of HCV+ patients with indolent non-Hodgkin lymphoma treated with IFN was performed. Although the SVR rate was higher in patients treated with DAAs, IFN therapy had a higher percentage of complete response and a higher median duration of response than DAAs, thus demonstrating a greater anti-lymphoma action than IFN-free regimens, secondary to the well-known antiproliferative activity of IFN. The hematological outcomes in terms of overall survival and progression-free survival (PFS) were overlapping[84]. Finally, a recent meta-analysis of our group comprising 317 HCV+ patients with non-Hodgkin lymphoma treated with DAAs and/or an IFN-based regimen and 250 controls not treated with antiviral therapy showed how the achievement of an SVR, regardless of the antiviral treatment performed, has a strong positive effect on the non-Hodgkin lymphoma outcome in terms of progression-free survival[85].
DAAs are effective and safe in patients with chronic kidney disease (CKD) and/or hemodialysis. Antiviral treatments containing sofosbuvir are currently not recommended as first-line therapy in patients with CKD and or end stage renal disease due to its renal metabolism and possible adverse events on kidney function[86]. The first combination to be used was ombitasvir/paritaprevir/ritonavir + dasabuvir in the treatment of patients with genotype 1 and 4[87]. Subsequently, the combination grazoprevir/elbasvir showed efficacy and safety in patients with CKD and HCV chronic infection genotypes 1 and 4[88,89]. The pangenotypic regimen glecaprevir/ pibrentasvir showed high SVR rates and satisfactory safety profile in the treatment of moderate/advanced CKD and/or hemodialysis patients with HCV chronic infection genotype 1-6 for 12 wk of treatment[90]. In a recent trial, the administration of glecaprevir/pibrentasvir for 8 wk in non-cirrhotic patients with moderate/advanced CKD and/or in hemodialysis with chronic HCV infection (all genotypes) showed SVR rates comparable to the 12 wk, thus allowing a significant reduction in the treatment period[91]. The effectiveness and safety of glecaprevir/pibrentasvir in patients with CKD or end stage renal disease has also been tested in real life settings[92,93], showing data superimposable with controlled trials. The efficacy and safety of sofosbuvir/ velpatasvir has recently been tested in patients with CKD and/or hemodialysis. Although the two cohorts are limited (51 and 59 patients, respectively), the SVR rate was high (96% and 95%) with an acceptable safety profile[94,95]. Studies with larger populations will be needed to confirm these data.
In 2016, the WHO set the challenge of eliminating HCV infection and blocking its spread worldwide by 2030[96]. In order to achieve this objective, the European Association for the Study of the Liver has proposed to strengthen the measures of case finding and treatment in populations at greater risk of infection, such as people who inject drugs, prisoners, the homeless, and men who have sex with other men, so as to obtain a microelimination in these populations and subsequently the macroelimination of HCV infection[97]. In a recent study of our group in which 160 people who inject drugs with HCV infection, afferent to drug addiction centers were treated, the SVR rate was 98.5% without significant side effects[98]. Furthermore, the concomitant opioid substitution therapy does not affect the efficacy and safety of DAAs[99]. Even in a complicated context such as prison, therapy with DAAs has proved highly effective, with a 100% SVR in 46 convicts in southern Italy. This study highlights how difficult it is to reach these patients not to treat them[100].
The conditions for achieving microelimination in 2030 were quite satisfactory, but the recent global pandemic caused by severe acute respiratory syndrome coronavirus 2 infection has suddenly interrupted this process. Indeed, the national health systems have had to concentrate all their resources (human, structural, and economic) on the fight against this infection, temporarily putting aside the activities of screening and treatment of chronic diseases, such as chronic HCV infection. In a study conducted at the Boston Medical Center, it was shown that between March and June 2019, there was a 49% reduction in the performance of HCV tests and a 42% reduction in new HCV+ patient identification hospital wide. These numbers are even higher in ambulatory clinics connected to this center for screening programs for HCV infection with a reduction of 72% and 63% in the number of HCV tests performed and in the new HCV+ patient identification, respectively[101].
In Italy, a recent survey proposed by the Italian Association for the Study of the Liver showed that outpatient activities concerning viral hepatitis were reduced in 99% of hospitals involved in the study, and the start of antiviral treatment has been postponed in 23% of centers, even for patients considered at risk of disease evolution[102]. In Germany between March and May 2020 there was also a sharp reduction in outpatient hepatological activities and in the prescription of anti-HCV treatments[103]. The consequences of this reduction in the screening and treatment of hepatitis C could be serious in the near future. In a study by Blach et al[104], it was estimated that in the decade 2020-2030 there could be 900000 missed new diagnoses of HCV infection and a reduction of about 750000 treatments due to the global pandemic. This could result in an increase in end-stage disease outcomes, with 44800 excess HCC cases and 72300 excess liver-related deaths predicted over 2020-2030.
HBV, the pathogen responsible for hepatitis B, is a major public health problem, as both its acute and chronic infections are still responsible for numerous cases of morbidity and mortality. International guidelines provide specific indications (with few differences from one another) on antiviral therapy against HBV, but this field still represents an open challenge because despite the increasing effectiveness of anti-HBV drugs approved over the years, a definitive cure for hepatitis B does not yet exist.
In 2016, a worldwide prevalence of hepatitis B surface antigen (HBsAg) positivity of about 292 million people was estimated and that in the same year only 10% of these were identified[105]. Moreover, it was calculated that this virus caused 887000 deaths in 2015, mainly from cirrhosis or HCC. Africa and the Western Pacific are the areas with the higher prevalence of infection, and the WHO African Region was estimated to have the lowest vaccine birth dose coverage[105,106]. Despite the open challenges, important results have already been achieved, such as the reduction in chronic hepatitis B (CHB)[106-108], HCC[109], extrahepatic manifestations, and decreased mortality for fulminant hepatitis[110] due to vaccination against this virus.
HBV can spread in different ways. In high prevalence areas, transmission during the first years of life is the most common. In this age group, they can be distinguished by perinatal transmission (from infected mother to newborn), that is the most widespread worldwide and occurs at the time of birth, shortly after, or rarely in the intrauterine life and horizontal transmission during childhood that is prevalent in certain countries and can occur via small lesions through contaminated environmental surfaces[106,108]. Horizontal infections in adulthood occur mainly through the parenteral route in both health care settings (due to the use of contaminated blood derivatives or contaminated instruments) or in the context of intravenous drug use, such as opioids. Getting tattooed and performing piercings are other procedures that can allow HBV spread[106]; sexual intercourse is a consistent risk factor for certain categories of people, such as male homosexuals or people who have multiple partners[106,108]. In addition to blood, HBV is found in saliva, seminal, vaginal, or menstrual fluids[106,111].
In 1965, Blumberg et al[112] described the formation of precipitins (precipitating antibodies) after contact between serum from two hemophilic patients and that of an Australian aborigine. Since this antigen (called “Australia antigen”) was subsequently found also in the serum of patients suffering from acute hepatitis, it was hypothesized that it could be linked to a hepatitis virus[113]. Following observations through electron microscopy, in 1970 David Dane made it known for the first time that the agent responsible for the “Australian antigen-associated hepatitis” was a virus of 42 nm in diameter[114], therefore called “Dane particles.” The term “hepatitis B virus,” however, appeared several years before identification of this virus (in 1947) and was proposed to distinguish the two different types of hepatitis that were known at the time[115,116].
HBV belongs to a family of hepatotropic, DNA viruses named Hepadnaviridae. This family includes five genera: Avihepadnavirus, Herpetohepadnavirus, Metahepadnavirus, Orthohepadnavirus, and Parahepadnavirus. The genus Orthohepadnavirus comprises 12 species (which can be hosted by a wide range of mammals) among which there is the HBV species that infects humans and apes[117,118]. HBV is divided into four serotypes (adw, ayw, ayr, and adr) based on HBsAg and depending on nucleotide sequences in ten different genotypes (A, B, C, D, E, F, G, H, I, J)[118,119]. There are some subtypes of the different genotypes. The various genotypes have a different geographical distribution, are associated with different responses to therapies (genotypes A and B have a better response to IFN than C and D), and outcomes (genotype C is associated with an increased risk of advanced fibrosis and HCC)[119].
The HBV genome consists of a 3.2 kb long, partial double stranded, relaxed circular DNA molecule, formed by a complete coding negative strand, linked to the viral polymerase, and an incomplete noncoding positive strand. The minus strand contains four open reading frames (ORF) that partly overlap: preC/C, P, preS/S, and X[117,119,120].
PreC region (pre-core) encodes the E antigen (HBeAg) which unlike HBc (that remains in the cell) is secreted into the circulation; this protein has immunomodulatory/tolerogenic functions, and it is believed to give the virus an evolutionary advantage (in fact it is found in all Orthohepadnaviruses). It is still unclear if it could also have other roles[121,122]. ORF C encodes the highly antigenic and self-assembling core protein HBc, which constitutes the structural protein (HBcAg) that covers the HBV DNA forming the nucleocapsid. It also participates in other steps of the HBV life cycle, including viral replication[123]. ORF P encodes the viral polymerase (P protein), a multifunctional protein responsible for viral replication; this latter is divided into four (TP, spacer region, RT, and RNase H) domains that allow the DNA or RNA reverse transcription and the ribonuclease activity[124]. Pre-S (1 and 2)/S ORF encode the HBsAg that form the viral envelope; they are distinguished in small, medium, and large surface proteins. Small surface proteins are the most abundant envelope protein and includes the “a” determinant, which is the main target of the neutralizing anti-HBs response. HBV results in an icosahedral shaped, enveloped particle of about 42 nm in diameter[125]. ORF X encodes the HBx protein, whose functions have long been unclear; it can transactivate several viral and cellular gene promoters and cause epigenetic modifications of covalently closed circular DNA (cccDNA)[126].
The life cycle of HBV has been described in detail. Briefly, the virus initially attaches to the hepatocyte surface through a low affinity binding of S-HBsAg with heparan sulfate proteoglycans. Glypican-5 was recently found to be involved in the early stages of HBV attack on the host cell. Hence, a highly specific bond between L-HBsAg and sodium-taurocholate cotransporting polypeptide takes part, and this is followed by entry via endocytosis[120,127,128]. Once inside the cell, the fusion of the envelope with the endosome membrane causes the release of the nucleocapsid into the cytoplasm, and subsequently the HBV relaxed circular DNA is transported to the nucleus. After completion of the positive strand, the HBV DNA is converted into a cccDNA molecule that remains stable in the nucleus in the form of a chromatin-like minichromosome[120,129]. Here it can undergo epigenetic modifications that modify its expression. The cccDNA probably persists for the entire life of the hepatocyte and explains the persistence of this virus and the viral reactivations that occur in certain patients, such as immunosuppressed or those who stop antiviral therapy[128]. The cccDNA serves as a template for the transcription of viral mRNAs by the host’s RNA polymerase II. The envelope and the HBx proteins originate from the subgenomic RNAs, while the core protein and viral polymerase derive from pregenomic RNA (pgRNA). This latter is also encapsulated together with HBV polymerase by core proteins to form immature particles. After encapsulation, the pgRNA will serve as a template for the reverse transcription of HBV DNA that is first synthesized in its minus strand[120,128,129]. The latter acts as a template for the synthesis of the positive HBV strand, which will result as partially incomplete. These nucleocapsids are supplied with envelope proteins in the endoplasmic reticulum (ER), and further modifications occur in the Golgi complex[120]. Finally, complete particles and sub-viral particles (including “S” antigen) are released from the host cell. Other nucleocapsids, on the other hand, recirculate in the nucleus to supply it with viral genome. HBV is not cytopathic, and liver damage is mediated by the immune system[107].
In the acute HBV infection, the appearance of HBV DNA is followed by HBsAg and HBeAg (the latter, as well as HBV DNA, is a marker of viral replication). HBsAg appears and increases approximately by 1-10 wk after contact with HBV, and anti-HBc IgM and then IgG are found in the serum about 2 wk after the appearance of HBsAg[130,131]. HBV DNA and HBsAg persist for the time of duration of clinical manifestations, while HBe seroconversion usually coincides with the peak of clinical illness[131]. Following the disappearance of HBsAg, anti-HBc IgM may be the only detectable marker. In those who develop them, anti-HBs appear during or after recovery from the disease; they are a marker of immunity against HBV and are found together with anti-HBc IgG in those who had a past infection or alone in vaccinated people[131].
An occult HBV infection (OBI) is defined by the combination of HBsAg absent/HBV DNA present in the liver (e.g., cccDNA) and HBV DNA absent or present in the serum. This phase is distinguished in seropositive OBI [(anti-HBc and/or anti-HBs positive) that may occur both after overcoming an acute infection or following a chronic infection/hepatitis] and seronegative OBI [(anti-HBc and anti-HBs negative) that may result from the progressive loss of antibodies against HBV or because they never developed][132]. It is of crucial importance to recognize an OBI because of the high risk of viral reactivation that exists for such patients in certain circumstances (e.g., anti-CD20 chemotherapy regimens for hematological malignancies)[133].
HBsAg persistence for at least 6 mo is defined as chronic hepatitis B[108,131]. In this case, the HBeAg and HBV DNA persist for a long time. Levels of HBsAg vary in the different phases of the disease and as well as the values of HBV DNA correlate with the risk of developing cirrhosis and HCC[134,135]. As discussed below, HBsAg loss is associated with a reduction (but not abolition) in the occurrence of HBV related complications, but it is a rare event. Furthermore, it is not yet known whether the appearance of anti-HBs confer greater advantages in terms of clinical outcomes than the disappearance of HBsAg alone[135]. In recent years, new biomarkers have been studied to overcome the limitations offered by those currently in use.
HBcrAg is made up of three related viral proteins (HBcAg, HBeAg, and p22cr; the latter is a 22-kDa pre-core protein) that share a 149 amino acids long identical sequence. HBcrAg measurement is not redundant to that of HBsAg, and this marker can also be detected in HBsAg negative patients. It showed an association with intrahepatic cccDNA, intrahepatic pgRNA, HBV translational activity, and serum HBV DNA[136]. From a practical point of view, higher levels of HBcrAg were found to be associated with an increased risk of HCC onset [in nucleos(t)ide analogues (NAs) treated or untreated patients] and recurrence. Moreover, this marker was linked to the risk of viral reactivation, response to treatment, and relapse after therapy withdrawal[136-138]. The latest guidelines for the management of HBV infection of the Japan Society of Hepatology recommend HBcrAg dosage to predict the risk of relapse after cessation of therapy, while in Europe and the United States it has not yet entered into daily practice. Other tools that have been explored in the context of HBV infection include circulating HBV RNA (prediction of response to treatment and relapse after the end of treatment), anti-HBc titers, and HBeAg levels[138].
This virus causes acute and chronic infections. After contact with HBV, the incubation period is approximately 75 d, but it can be very variable[106]. Acute hepatitis B can be asymptomatic or symptomatic, with varying severity. Newborns and children usually have an asymptomatic and anicteric course, while adults can develop a symptomatic hepatitis in 30%-50% of cases. Fulminant hepatitis occurs in about 0.1%-0.5% of infected patients[131,139]; it is probably due to an abnormal response of the immune system and has a very high rate of mortality. In symptomatic patients, the disease can run through the typical course of acute hepatitis (incubation, prodromal, icteric, and resolution periods). A few weeks after the appearance of viral markers in the serum, abnormalities of liver biochemistry can be found[131]; transaminases can exceed 1000 IU/L [with alanine aminotransferase (ALT) greater than aspartate aminotransferase], bilirubin increase can be of varying degrees depending on the severity of the disease, whilst an increase in international normalized ratio (> 1.5) is typical of severe forms[107]. Clinical and laboratory resolution occur in highly variable timescales, depending on the severity of the disease. In adults, the disease spontaneously resolves itself in more than 95% of cases, and this is followed by the appearance of anti-HBs.
Progression to chronic hepatitis mainly depends on the age at which the infection is acquired. It occurs in about 90% of cases if the infection is acquired in the perinatal or infantile period, 30%-50% if acquired by children up to 6-years-old, and less than 5% if acquired in adulthood[106,139]. Chronic hepatitis B proceeds through various phases characterized by different laboratory findings, and chronically HBV infected and untreated people have a 5-year cumulative incidence of cirrhosis of 8%-20%. Therefore, the disease can further progress to the onset of complications and HCC. The latter was found to arise with an incidence rate of 2%-5% per year in HBV cirrhotic patients[106,107]. Viral, host, or environmental related factors (genotype C, older age, male gender, coinfection with HDV, HCV, and HIV, alcohol, diabetes, and others) can increase the probability of worse outcomes, including HCC[107,139]. Several extrahepatic manifestations can be observed in the context of HBV infection and are thought to be due to the formation of immune complexes with HBsAg or HBeAg. Among these, there are systemic syndromes (serum sickness-like or flu-like), vasculitis (e.g., polyarteritis nodosa), glomerulonephritis, skin manifestations (e.g., purpura and lichen planus), polyarthritis, neurological disorders (e.g., Guillain-Barré syndrome), cryoglobulinemia, and increased inflammatory indices[107,140]. It has also been found that HBV infected people have a higher risk of developing B-cell lymphoma[141].
HBV chronic infection is a dynamic condition, and its course depends on the virus-host relationship. To characterize the disease and choose whether to start the treatment, viral (HBsAg, HBeAg, anti-HBe, HBV DNA) and liver damage (ALT, fibrosis, or necroinflammation through non-invasive tests such as liver elastography or score results or with liver biopsy) markers must be evaluated. Based on the results of these tests, various phases (Table 2) of CHB can be identified[107,142].
| HBsAg+ ≥ 6 mo | |
| HBeAg-positive chronic infection (1) | |
| HBe: HBeAg+; HBV DNA: very high; ALT: persistently normal; Liver disease: minimal or absent | Ex “immune-tolerant;” Typical of children and young adults infected at birth; HBV DNA: > 1 million IU/mL; highly contagious people; Low rate of spontaneous HBeAg loss. |
| HBeAg-positive chronic hepatitis (2) | |
| HBe: HBeAg+; HBV DNA: high; ALT: elevated; Liver disease: moderate or severe | Ex “immune reactive HBeAg-positive;” People infected in adulthood are likely to enter this phase; HBV DNA > 20000 IU/mL; rapid progression to fibrosis; Frequent HBe seroconversion. |
| HBeAg-negative chronic infection (3) | |
| HBe: HBeAg-, anti-HBe+; HBV DNA: low or undetectable; ALT: normal; Liver disease: low | Ex “inactive HBV carrier;” HBV DNA: < 2000 IU/mL; it can also be > 2000 IU/mL but usually < 20000 IU/mL; Low risk of progression to cirrhosis or HCC; HBsAg loss in 1%–3%/yr. |
| HBeAg-negative chronic hepatitis (4) | |
| HBe: HBeAg-, anti-HBe+; HBV DNA: moderate or high; ALT: elevated (variable); Liver disease: moderate or severe | HBV DNA: 2000-20000 IU/mL; Older people, frequent mutant precore HBV; Fluctuating levels of HBV DNA and ALT; Rare spontaneous remission and rapid progression to cirrhosis. |
The last phase, described above, is the occult phase (also named HBsAg loss phase, resolved CHB, or phase 5)[109,143,144]. Gish et al[144] also proposed two theoretical phases: “clearance of cccDNA” and “clearance of cells that have integrated HBV.” Progression from one phase to another is not necessarily sequential (e.g., the phase 2 can be followed by phase 3 or directly by phase 4), and the return to a previous phase is also possible. Moreover, in daily practice, several cases of CHB may not belong to any of these phases (for example during transition between phases or in the presence of concomitant liver diseases)[107,143].
HCC has variable incidence rates worldwide, depending on the prevalence of the various risk factors around different geographical areas. Most cases, however, are documented in the Asian regions[145]. In 2015, there were about 854000 incident cases of HCC, and this cancer caused 810000 deaths worldwide; these numbers are expected to increase in the coming years[146]. In most cases, HCC develops in the context of cirrhosis, but it is well known that many HCCs arise in the absence of such disease. Although the prevailing causes of HCC vary by region, HBV is the overall leading cause. Despite an observed increase in the overall number of cases in the 1990–2015 time frame, a deflection of incidence rates of liver cancer due to HBV was also reported during the same period[146]. This virus can cause HCC in the absence of cirrhosis and at a younger age compared to other etiologies[147]. In addition to viral, host, and environmental risk factors mentioned above, several conditions have been associated with the onset of HBV-related HCC[148]: host genetic predisposition (e.g., single nucleotide polymorphisms). Immune response to HBV, cell death, hepatic inflammation, and liver fibrosis create an optimal substrate for the onset of HCC. Mutations in the HBV preS1/S2 domain cause an accumulation of altered large surface proteins in the ER, resulting in the development of oxidative stress. Integration of HBV-DNA into the host genome causes genomic instability or direct involvement (insertional mutagenesis) of cancer-associated genes (e.g., TERT, MLL4, CCNE1).
Viral proteins (HBx, preS and HBc) interfere with critical steps of cell life cycle.
The HBx protein can have a procarcinogenic role as it causes reactive oxygen species production in mitochondria and ER stress. It can interfere in cancer-related pathways (e.g., Jak/STAT, RAS/RAF/MAPK, or Wnt/β-catenin; moreover HBx is able to inactivate p53) and can cause epigenetic modifications of the host DNA. It alters the expression of microRNAs and long noncoding RNAs, and it interferes with processes involved in DNA repair. It was proposed to promote the “stemness” of HCC cells[147-149]. Much knowledge on HBV-related HCC has been accumulated, but there is still a gap compared to what is clinically useful.
The aim of antiviral therapies against HBV is to increase the survival of people suffering from CHB by reducing cases of liver failure, cirrhosis, and HCC and prevent the transmission of the infection. Specific terms are used to indicate the goals of antiviral therapy. With the currently available drugs a “definitive,” “sterilizing,” or “true” cure for hepatitis B (defined by HBsAg/serum HBV DNA/hepatic cccDNA/integrated HBV DNA not detected) is not possible, given the persistence of HBV DNA in the nucleus[107,150]. The “functional cure” corresponds to HBsAg loss, with or without seroconversion, and undetectable HBV DNA following a course of antiviral therapy. This event, related to both therapy and disease factors, is associated with reduced risk of HCC, liver failure, and other liver-related adverse outcomes[151]. This “ideal endpoint” is uncommon with currently available drugs, but after HBsAg disappearance (both after therapy with NAs or peg-IFN or spontaneous) this result is generally stable and allows patients to stop the therapy[107,135,143]. Experts believe that near-future therapies will have to achieve this goal rather than others (that however may appear more radical), and it is believed that HBsAg loss should be the primary endpoint of phase III trials[150]. In a recent meta-analysis that included 42588 CHB adult patients, it was observed that HBsAg loss occurred in 3194 of them, with an annual pooled seroclearance rate of 1.02%. This event was more likely in patients who were HBeAg negative and those who had lower HBsAg and HBV DNA levels at baseline. On the other hand, the treatment did not have a major effect in achieving this goal[151]. Other studies also obtained similar results regarding the rate of spontaneous or post therapy HBsAg loss. In any case, even after HBsAg loss, the risk of HCC is not eliminated[107,143].
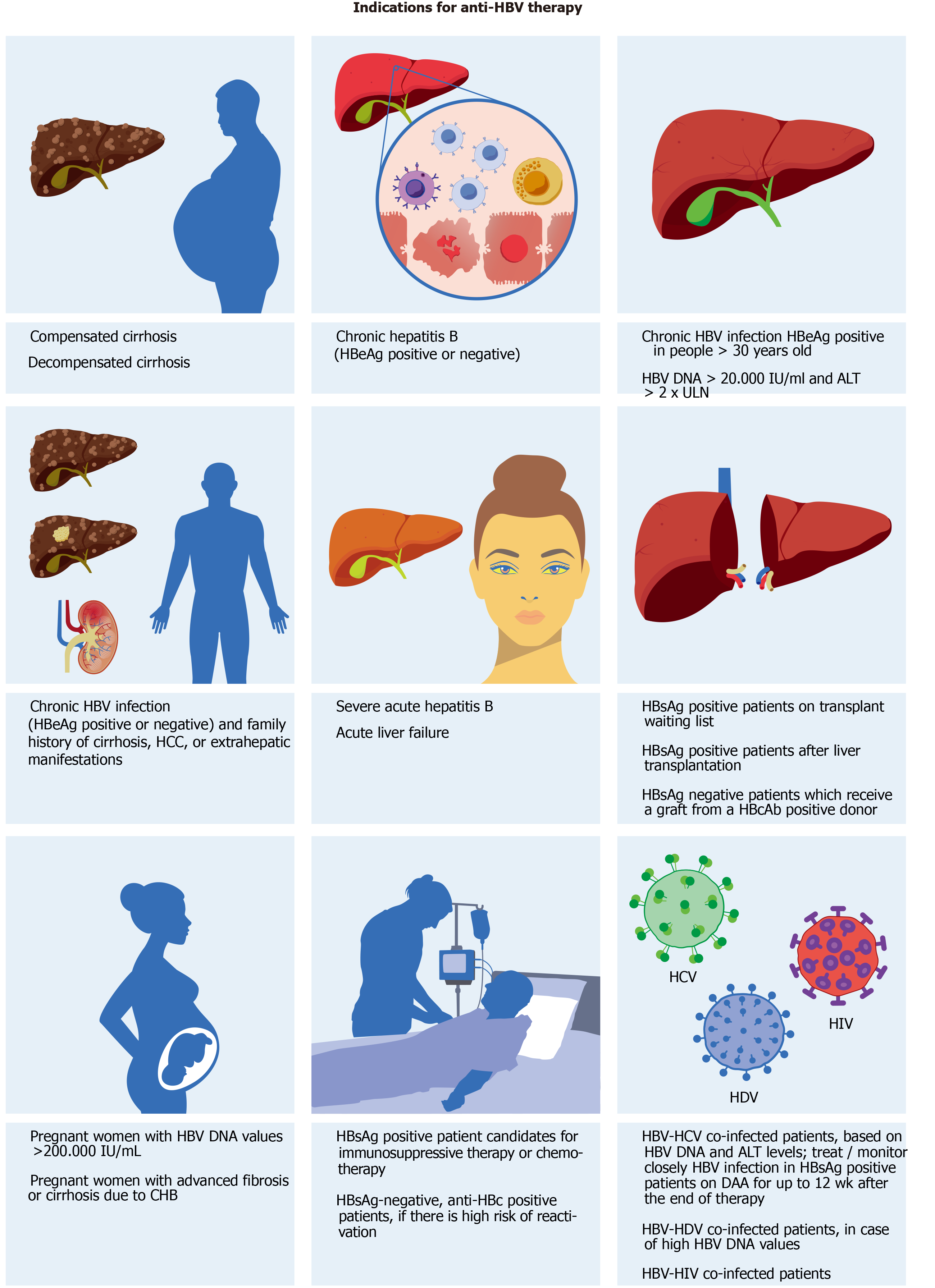
Therefore, the main and “realistic” endpoint that current therapies can offer correspond to suppression of HBV DNA or virological response, defined in different ways depending on type and timing of therapy. Normalization of ALT (biochemical response), loss of HBeAg ± seroconversion, or reduction in necroinflammatory activity (histological response) are other surrogate but important endpoints. The indications for treatment (Figure 2) derive mainly from ALT, HBV DNA values, and from the severity of liver damage defined by liver biopsy or non-invasive tests such as transient elastography or scores such as FIB-4 and FibroTest[107,142]. A recent meta-analysis that included 162 studies and 145789 people with chronic HBV infection from all WHO regions estimated that 19% of infected people were eligible for treatment according to current indications[152].
Antiviral therapy is usually not indicated in those who have HBeAg-positive (ex-immunotolerant phase, IT) or negative (ex-inactive carrier phase) chronic infection[107,150]. IT patients who remain in this phase showed a minimal or no progression of liver disease[153]. Some studies seemed to provide evidence against such indications, showing, for example, a greater risk of worse outcomes in untreated IT patients than those in immune-active phase on NAs[154]. However, these and conflicting results from other studies were partly attributed to patient inclusion criteria[155]. Anyhow, it is not excluded that despite current statements IT patients may benefit in the future from more effective therapies to reduce the viral replication (and the risk of HBV transmission) and potential complications[150].
The drugs currently used for the treatment of HBV infection are: NAs: nucleoside analogues [lamivudine, telbivudine, entecavir (ETV)]; nucleotide analogues [adefovir dipivoxil, tenofovir disoproxil fumarate (TDF), tenofovir alafenamide fumarate; besifovir dipivoxil maleate was recently approved in Korea]; and peg-IFNα.
Between these options, the most used are NAs[107,119]. This class of drugs inhibits reverse transcription activity of HBV DNA polymerase; NAs compete with natural substrates, and their integration in the nascent viral DNA chain interrupts its formation[156]. Lamivudine, adefovir dipivoxil (the first two approved NAs for the treatment of hepatitis B), and telbivudine have a low barrier to resistance and therefore are characterized by a high percentage of cases of resistance during therapy (70% for lamivudine and 29% for adefovir dipivoxil after 5 years of therapy)[107,157]. Despite this, these drugs are still used in some countries mainly for their low cost[157].
On the other hand, ETV, TDF, and tenofovir alafenamide fumarate, the most powerful NAs available today, have a high barrier to resistance. They are associated with higher HBV DNA suppression rates and are the recommended first-line drugs for treatment of CHB. These NAs have a high tolerability and can be used regardless of the severity of the liver disease (even in decompensated cirrhosis) and comorbidities[107]. The occurrence rate of resistance to ETV after 5 years of therapy was shown to be approximately 1% in nucleoside naïve CHB patients, while no cases of resistance were recorded in a 7 year-long study in which 437 CHB patients received TDF monotherapy[158] and in other long-standing studies[107]. Accordingly, the discovery of tenofovir resistant strains would be of considerable impact.
Two cases of viral breakdown addressed to a quadruple mutation (CYEI) were recently reported in CHB patients and linked to the ongoing therapy, which included TDF. It was therefore emphasized that in these cases there would be no consolidated possibilities of therapy[159]. In a response study, however, it was pointed out that CYEI mutation was already documented before the availability of TDF, suggesting that it could (rarely) arise in therapy naïve people. Other evidence behind the reduced susceptibility/resistance to tenofovir has recently been claimed, but currently the clinical impact of this phenomenon is limited[160,161].
Although NAs have been shown to effectively suppress HBV DNA and reduce the progression of liver disease, the disappearance of HBsAg, as already mentioned, rarely occurs, and they have no direct effect on cccDNA. Nevertheless, a study on patients on long-term therapy with NAs showed that they can achieve a reduction in intrahepatic HBV DNA and cccDNA and that the latter can also become not detectable[162]. It was recently observed, however, that among patients that were part of the same study who had undetectable cccDNA, all those who discontinued antiviral therapy (n = 13) experienced an HBV DNA rebound, unlike those who continued treatment. These results suggested that even small, not detectable amounts of cccDNA may allow the virus to reactivate[163]. NAs require frequent monitoring, and usually they must be assumed for an indefinite period. Moreover, the risk of HCC is not eliminated, and long-term therapy is associated with some adverse events. To reduce renal or bone toxicity, ETV or tenofovir alafenamide fumarate should be preferred over TDF[107,142].
Conversely, peg-IFNα is administered for a finite period, generally 48 wk, but longer durations are also recommended in certain cases[107]. The choice between peg-IFNα and the NAs depends on the patient and his preferences, previous treatments, virological factors, and the liver disease. However, it requires subcutaneous injection, and it is associated with well-known side effects. Moreover, due to its non-optimal safety profile, it is contraindicated in patients with decompensated cirrhosis, in cases of other co-morbidities (e.g., autoimmune diseases, psychiatric disorders, cytopenias), during pregnancy, and in severe acute hepatitis B or liver failure, and it is not recommended in some patients with compensated cirrhosis (e.g., those with portal hypertension)[107,142]. Combination therapy (NA-IFN) increased response rates but is currently not recommended as further confirmations are needed[107,119]. The response to anti-HBV therapy and its effect on liver-related outcomes, such as the onset of HCC, were found to vary also by ethnicity (e.g., Asian patients are less likely to experience HBsAg loss following NA therapy than Caucasian ones)[107,119,150].
Many molecules are being studied as a therapy for hepatitis B to improve the results obtainable with the drugs currently available. Among the various approaches, some aim to strengthen the immune response against HBV, while others interfere with specific steps in its life cycle. Direct elimination of the HBV genome is not yet close to clinical practice[119,164].
HBV entry inhibitors: Bulevirtide binds and inhibits the sodium-taurocholate cotransporting polypeptide, preventing the intrahepatic entry of HBV. The same mechanism of action is shared by Cyclosporin A and its analogues, but the block of bile acids transport should be considered. Other strategies to block cell entry include attack inhibition and antibodies against HBV antigens.
cccDNA inhibitors: TALENs and CRISPR/Cas9 can lead to genome rearrangement by acting in specific sites and are under investigation to evaluate their efficiency in targeting cccDNA. In these cases, the potential risk of making changes to non-target genes has to be considered.
Core protein assembly/allosteric modulators: these molecules interfere with the formation of viral capsid. The class I core protein assembly/allosteric modulators lead to the appearance of abnormal, misassembled, capsid structures, while the class II core protein assembly/allosteric modulators accelerate the formation of morphologically intact but empty capsids (without pgRNA).
RNAi-based agents: in this case HBV transcripts are recognized and degraded by small RNA molecules, resulting in post-transcriptional silencing.
HBsAg release inhibitors: REP 2139 inhibits the release of HBsAg by targeting sub-viral particles, with the aim of counteracting its tolerogenic effect.
RNaseH inhibitors: they inhibit the RNase activity of DNA polymerase, an important step for viral replication. Other options under study include new NAs, innate response stimulating drugs (TLR-7, TLR8, or STING agonists, RIG-1 activator), and therapeutic vaccines.
Hepatitis D, or delta, is the liver disease caused by HDV that was discovered by Rizzetto et al[165] in 1977. A recent review and meta-analysis estimated a 4.5% prevalence of anti-HDV among HBsAg-positive people, corresponding to about 12 million HDV infections worldwide. Other recent estimates, however, provided much larger numbers[166,167]. Its real spread remains elusive probably due to the non-systematic and universal screening in HBsAg positive patients[166,167]. Over recent years a drop in the prevalence and incidence of HDV infection in developed countries, such as Italy, was recorded as presumably the result of HBV vaccination and the increased sensitivity and precautionary measures to counteract risk factors for parenteral and sexually transmitted infections[167-169]. A current issue is delta hepatitis cases due to immigration from high prevalence countries. Nowadays, this infection is highly prevalent in several Asian countries (e.g., Mongolia), West and central Africa, Moldova, and the Amazon region[167-169].
HDV is a defective, satellite virus, 36-43 nm in diameter, which belongs to the Deltaviridae family that in turn includes a single genus (Deltavirus). The latter contains a single species, the hepatitis delta virus. Eight distinct genotypes, with different geographical distribution, have been identified[167]. Its genome consists of a single-stranded negative circular RNA molecule ~1.7 kb long, and encodes only the hepatitis delta antigen (HDAg, distinct in HDAg-S, small, and HDAg-L, large), which together with viral RNA form the HDV ribonucleoprotein[170,171]. HDV exploits the host RNA polymerase II for the replication of its genome. HDV envelope is formed by the S, M, and L proteins of HBV, and the latter is necessary for HDV to enter the host cell as well as in the assembly and exit phases[171]. However, replication within the hepatocyte can occur in the absence of HBV components, and it is thought that in addition to the extracellular propagation pathway, HDV can also spread by a cell-division mediated pathway[172]. This virus has the same modes of transmission as HBV (parenteral, inapparent parenteral, sexual, and rarely vertical)[168,171].
Only HBV infected people can contract delta hepatitis. Coinfection that occurs when HBV and HDV are acquired simultaneously usually manifests itself as an acute hepatitis B, with varying severity up to fulminant hepatitis and has a chronicization rate of 2%-5% in adults. Superinfection refers to an HDV infection in a chronically HBV infected person. In this case, there is a higher risk of severe acute hepatitis and the probability of chronicity is about 80%-90%[168,171-173]. Chronic hepatitis D evolves rapidly towards cirrhosis and its complications[173]. The diagnosis consists in the detection of anti-HDV IgM or IgG and HDV RNA in the serum[168,171]. Current guidelines recommend looking for this infection systematically in HBV infected patients (European Association for the Study of the Liver, Asian-Pacific Association for the Study of the Liver) or preferentially in certain categories of people (American Association for the Study of Liver Diseases).
Until recently, there were no specific approved therapies for hepatitis D. The latest international guidelines recommend using peg-IFNα to treat this infection (in association with NAs if patients have high HBV DNA levels). This drug demonstrated HDV RNA suppression in 19%-57% of treated patients at the end of the treatment but is characterized by high rates of post-therapy relapse[169]. In addition to the well-known side effects, it should not be used in patients with decompensated disease. Recently, 24 wk of peg-IFN-lambda showed similar antiviral effect but better tolerability compared to peg-IFNα[174].
Bulevirtide acetate (Hepcludex®, previously known as Myrcludex B, MyrB) was approved in 2020 in the European Union for the treatment of chronic HDV infection in adults with compensated liver disease[175]. The recommended dosage is 2 mg s.c. once daily, but the optimal duration of therapy is currently not specified. This drug blocks the entry of HBV and HDV into cells by binding sodium-taurocholate cotransporting polypeptide[175]. The results of a phase 2b clinical trial with primary endpoint HDV RNA reduction by 2 log or HDV negativity showed that 24 wk of treatment with MyrB in combination with TDF allowed patients to reach the endpoint in a significantly higher percentage of cases compared to those on TDF monotherapy (46.4% in the MyrB at 2 mg/die plus TDF arm vs 3.3% in the TDF in mono one) and that HDV RNA was suppressed in a dose-dependent manner. Twelve weeks after the end of therapy, however, the relapse was a frequent event[176]. Currently, some studies on bulevirtide are evaluating mono or combination therapies, different dosages, and durations, and the effect on patients with liver disease of varied severity.
Prenylation inhibitors block the prenylation of HDAg-L mediated by farnesyl transferase, preventing the bond of HDAg-L to HBsAg. Oral lonafarnib (LNF) inhibits the farnesyl transferase and makes it impossible for HDV particles to form. It reduced HDV RNA significantly more than the placebo in a study where it was used for 28 d; this therapy was associated with the appearance of some side effects, including gastrointestinal that were the most frequent[177]. Subsequently, LNF was tested in combination with ritonavir (ritonavir inhibits the CYP3A4 that metabolizes LNF, allowing the LNF dosage to be lowered), peg-IFNα, or lambda. The combination peg-IFN-lambda/LNF/ritonavir taken for 24 wk recently showed that it lowered HDV RNA > 2 log in most (25/26) treated chronic HDV patients and led to undetectable or “below the lower limit of quantification” HDV RNA in more than 50% of them at the end of treatment[178].
Among HBsAg secretion inhibitors, there is REP 2139, which in combination with peg-IFNα 2α (REP 301 study) was safe and was associated with HBsAg suppression in 5/12 patients and HDV RNA control in 7/12 patients after 1 year from the end of treatment[179]. This protocol showed persistence of the results in terms of safety and virological control of HBV and HDV over an extended follow-up period of 3.5 years[180].
Liver transplant is a therapeutic option for HDV patients who have not benefited or cannot use medical therapy[169].
HAV is a single-strand RNA virus belonging to the Hepatovirus genus of the Picornaviridae family that was discovered by Feinstone et al[181] in 1973. HAV is able to withstand moderate temperatures and to survive in the environment. Therefore, it is transmitted through the orofecal route[182]. The clinical manifestations of HAV infection depend on the age at which the infection is contracted. In children, it occurs in less than 30% of cases with symptomatic hepatitis, while 80% of infected adults have a severe hepatitis with jaundice and a significant increase in transaminases[183].
HAV infection is widespread worldwide, with a higher prevalence in low- and middle-income countries[184] where the hygienic-sanitary conditions are scarce. Most of the children (90%) contract the infection before the age of 10, often without clinical manifestations. Epidemics rarely occur as older children and adults are generally immune. Intermediate endemicity is observed in North Africa, Eastern Europe, the Middle East, Latin America, and middle-income regions in Asia[185]. High-income countries such as the United States and West European countries have lower rates of HAV infection, but most of their adult population is not immune to this virus, resulting in a higher probability for this category of developing clinical manifestations due to HAV infection than in the low-income countries[186]. In areas with high endemic and poor hygienic and sanitary conditions, HAV infection is transmitted mainly by contaminated water. In intermediate endemic areas HAV is mainly transmitted through contaminated food and water[187], while in non-endemic countries, risk factors are travel to endemic areas and oroanal sex[188,189].
Clinical manifestations of HAV infection range from the total absence of signs and symptoms to acute hepatitis. Acute liver failure (ALF) is also possible, but it is a rare event. This infection usually resolves itself spontaneously and is not responsible for chronic hepatitis. After a period of 2-7 wk from the time of infection, the patient may develop typical symptoms of acute hepatitis, such as jaundice, asthenia, vomiting, dark urine, clay-colored stools, fever, or general malaise[190]. Blood tests show a severe increase in transaminases (also 100 times the upper limit of normal), usually with ALT > aspartate aminotransferase, increased total bilirubin, and increased alkaline phosphatase. It is important to monitor PT and the onset of portosystemic encephalopathy to assess the presence of ALF[191], although this is a very rare occurrence (less than 1%)[192]. Atypical manifestations may also be present, including relapse hepatitis, autoimmune hepatitis, prolonged cholestasis, and acute kidney failure[193]. Considering that the clinical presentation of hepatitis by HAV is similar to any other hepatitis, the diagnosis of HAV infection requires the dosage of IgM anti-HAV. IgM anti-HAV becomes detectable in the blood about 5 d after the onset of the disease and remains positive for a short period after healing. The recent execution of the HAV vaccine can determine a positive test result for IgM anti-HAV. The presence of IgG anti-HAV indicates a previous HAV infection[194].
There is no specific cure for HAV acute hepatitis. Therefore, supportive therapy is indicated, trying to alleviate the symptoms of the infection (fever, nausea, vomiting) and intervening on any other complication (dehydration, electrolytic abnormalities, etc.). IFN has been evaluated in the past for the treatment of hepatitis A. This drug has been shown to reduce viral replication in cell cultures[195] and has been administered in sporadic cases of patients with severe or fulminant hepatitis secondary to HAV infection[196]. Sofosbuvir showed the ability to inhibit replication of HAV in vitro[197], but there are no reported cases in literature where this drug was administered in vivo. Viral hepatitis are responsible for 12% of acute liver failure, and of these only in 0.35% of cases the causative agent is HAV[198]. HAV infection is the main cause of ALF in the pediatric population in some countries[199-201]. Liver transplantation is necessary in 30% of ALF due to HAV infection, therefore it is a rare indication[198]. In some case reports, the effectiveness of plasma exchange in ALF therapy due to HAV infection is reported[202,203], while the use of corticosteroids in a small cohort of children was associated with reduced hepatocyte destruction and with higher survival rates compared to controls[204]. N-acetyl cysteine, although very effective in ALF secondary to paracetamol abuse, does not appear to provide any benefit in the treatment of HAV related ALF[205]. In the case of worsening liver function, liver transplantation is required[206]. Liver transplantation in ALF secondary to HAV infection is burdened with a high mortality[207] and is rarely complicated even by a post-transplant HAV reinfection[208].
The improvement of sanitary conditions and vaccination play a fundamental role in the prevention of HAV infection[209,210]. Available vaccine formulations for hepatitis A (inactivated and attenuated) have proven effective and safe in immunizing in large trials involving nearly 750000 patients worldwide[211]. The vaccine can be admin
Hepatitis E, the liver disease caused by HEV, is a health problem that is receiving increasing attention over the years, although many aspects concerning this infection are still lacking. It has a self-limiting and benign course in most people, but some categories are particularly at risk of serious outcomes, such as pregnant women who have a mortality risk of about 25%, immunocompromised patients who can develop a chronic hepatitis (and its consequences), and patients with previous liver disease who are at risk of acute-on-chronic liver failure.
The strong negative impact of HEV on human health can be deduced from data estimating that in 2005 there were about 20.1 million cases of hepatitis E (genotypes 1 and 2) infections and 70000 deaths worldwide[219]. A recent study[220] estimated that more than 900 million people have had contact with this virus; moreover, in many regions of the world HEV is the leading cause of acute viral hepatitis[221]. While in some countries HEV infections appear to have remained stable, in others, including European ones, there has been an increase over time (data probably explained also by an increase in identifications)[221,222]. However, the real burden of hepatitis E on human health is not yet fully known[220].
The transmission mode and diffusion of HEV infection vary greatly depending on the countries, and in the same country there are some epidemiological differences depending on the areas (e.g., southwest compared to other regions in France)[221,223]. The most frequent transmission mode in areas of the world with poor hygiene standards is the oral-fecal route, mediated by water (genotypes 1 and 2), while in developed countries is zoonosis (genotypes 3 and 4)[223].
The existence of an enterically transmitted non-A, non-B hepatitis virus was first hypothesized in 1978 by Khuroo[224], while identification under microscope occurred in 1983. The name “hepatitis E virus” was proposed for the first time in 1988[225].
HEV is a non- or quasi-enveloped icosahedral virus, 27-34 nm in size that contains a single strand, positive-sense RNA 7.2 kb long. The quasi-enveloped variant that exists in circulation is due to the lipid membrane originating from infected cells. The HEV RNA genome contains three ORF: ORF1 encodes a polyprotein that includes an RNA-dependent RNA polymerase; ORF2 encodes the capsid protein; and ORF3 encodes a protein involved in the viral egress[221,225]. ORF 4, recently described for HEV-1, has a supporting role for viral polymerase[226]. Despite the advances in knowledge of this virus, several aspects of the HEV life cycle are not yet known, including the exact mechanism by which the virus enters the host’s cells (e.g., entry receptors), some aspects of the relationship between host components and virus, and many processes concerning assembly and cell exit[227].
It is likely that, as well as other major hepatotropic viruses, HEV causes liver damage mainly through an immune-mediated mechanism[228]. Briefly, the host response starts with an initial phase in which the innate immune system detects the virus through pathogen recognition receptors such as TLR3, TLR7, RIG-I, and MDA5[229,230]. Subsequently, the production of IFN and other cytokines or chemokines that amplify the response against HEV take part[229,230]. Liver migration and activation of innate cells such as natural killer and neutrophils and then recruitment of T lymphocytes determine the antiviral response and contextually the immune-mediated liver injury[228,231]. The immune response (including antibodies) against specific regions of the ORF2 protein is critical in the fight against the virus. Although the HEV can hinder some steps of both innate and adaptive responses[228,229,232], the combination of these events usually leads to the clearance of the virus. Anyhow, the role of anti-HEV antibodies in the resolution of the infection[232] as well as the specific role of other elements of the immune system in the host-HEV relationship is not yet fully understood.
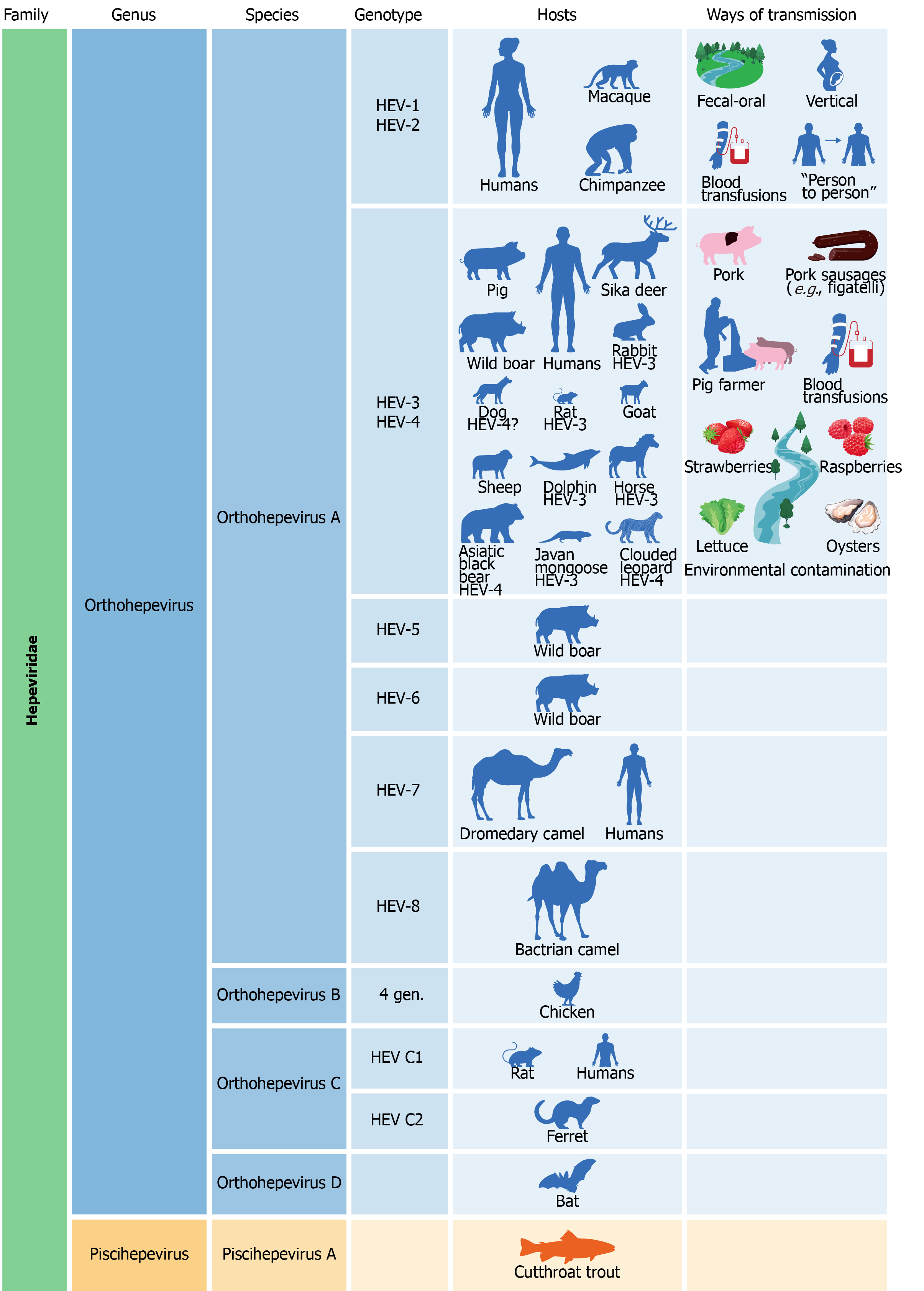
HEV is part of the Herpesviridae family that is divided into two genera, Orthohepevirus and Piscihepevirus. The genus Orthohepevirus includes four species (A-D), while the genus Piscihepevirus has only the Piscihepevirus A species. Eight distinct genotypes belong to the Orthohepevirus A species, and some viral subtypes of these genotypes have been recognized[225,233].
Orthohepevirus A genotypes 1, 2, 3, and 4 (HEV-1, HEV-2, HEV-3, and HEV-4) infect humans differently from the other genotypes of this species[225,226]. However, a case of a middle-aged liver transplanted Somalian man who regularly consumed camelid meat and milk infected with dromedary HEV (HEV-7) was also described[234].
HEV-1 and HEV-2 are restricted to higher primates (humans and several species of monkeys), but the finding of HEV-1 in the serum of Egyptian horses suggested that in rare circumstances transmission of these genotypes to other animals is also possible[233].
HEV-1 and HEV-2 spread among the human population mainly by the oral-fecal route that consists in ingestion of water contaminated with human feces[221,223,225,235]. This phenomenon typically occurs in regions of the developing world with poor hygiene standards, such as those in which untreated water, for example of a river, is drunk or used for cooking, bathing, and waste disposal. In such contexts, climatic changes (e.g., heavy rainfall or monsoons) can increase the risk of HEV infection spreading[235,236]. These genotypes are responsible for the occurrence of epidemics and outbreaks. The first one to be retrospectively documented broke out in Delhi (India) in 1955[225]. Today HEV infection is considered hyperendemic and associated with the development of outbreaks in various parts of north, east, and west Africa, south and central Asia, the Middle East, and Latin America[223,236]. In such areas, a high percentage of inhabitants were found to be anti-HEV IgG positive[220]. The typical route of transmission of these genotypes makes it conceivable that an improvement in hygiene conditions could result in a containment of the spread of infection[221,225]. In addition to the orofecal route, vertical via blood transfusion and person-to-person transmissions were documented[225,236]. In developed countries, where a better level of sanitation is guaranteed, HEV infection is sporadic and it is acquired as zoonoses, by travelling to endemic areas, or by blood transfusion[221,225].
HEV-3 and HEV-4 infect animals (including pigs that are the main reservoir and source of contagion for humans, wild boars, and deer) and humans and are responsible for the zoonotic form of the disease. They can be transmitted to humans in several ways: consumption of undercooked or raw meat (a recurring example in literature of a source of infection are the Corsican sausages “figatelli” that contain pork liver) or milk from infected animals; consumption of fruits, vegetables (strawberries, raspberries, lettuce, etc.), or seafood contaminated with feces of infected animals, and direct contact with infected animals (farmers, veterinarians, slaughterhouse workers)[221,223,225,236]. Today it is known that in addition to the most often mentioned animals (pig, wild boar, and deer), many others can serve as reservoirs for HEV-3 and HEV-4 (e.g., rabbits, cattle, rats, goats, sheep, dogs, horses, Java mongoose, dolphins, clouded leopard, Asiatic black bear, and others)[233]. An aspect not yet well known is which animals are “true hosts” for HEV and which others host it transiently. Blood transmission is also possible for HEV-3 and HEV-4[221].
The other Orthohepevirus A genotypes were identified in animals: HEV-5 and HEV-6 in wild boar, HEV-7 and HEV-8 in camelidae (dromedary and bactrian camel, respectively)[233].
Orthohepevirus B, of which four genotypes are known, has been isolated mainly in chickens but also in turkeys, ducks, and other birds. However, the possibility that some of its strains can infect mammals cannot be ruled out[233]. In the Orthohepevirus C species there are two different genotypes (HEV-C1, HEV-C2), whose presence has been documented in different types of rodents (rats, mice, shrews, ferrets, minks, and others). Some cases of human infections from rat HEV have been recently discovered in patients from Hong Kong and in a man suffering from acute hepatitis, who probably contracted the infection in central Africa[233,237,238]. Orthohepevirus D species was isolated in the bat. The Piscihepevirus genus includes only the Piscihepevirus A species that infects the cutthroat trout and other fish[233]. An illustration of the link between HEV genotypes, hosts, and routes of transmission is provided in Figure 3.
Anti-HEV IgM appear about 4 wk after infection, approximately with the onset of symptoms, and their persistence in the serum is variable (they usually last 3 or 4 mo, but cases in which they were detectable after a longer time were also described). Given their kinetics, they reveal a recent infection and can be considered a marker of acute hepatitis E[221,239,240]. In addition to anti-HEV IgM, anti-HEV IgA antibodies are considered markers of acute hepatitis E, but it is not a widely used test[221].
Conversely, anti-HEV IgG appear after anti-HEV IgM and last several years. They are used to diagnose a past infection and are indispensable for seroprevalence studies of such disease. Because anti-HEV IgG appear quite early in the course of the disease they can be detected together with IgM in the acute phase; in this way they help to reduce the misdiagnosis rate that occurs if IgM are considered alone[221,240]. The antibodies against HEV are determined by enzyme immunoassays[239,240]. For diagnostic purposes, due to the non-optimal reliability of antibody assays, guidelines of the European Association for the Study of the Liver recommend using the anti-HEV IgM (or anti-HEV IgG) in combination with the HEV RNA, or antibodies in combination with each other, or HEV RNA (in blood or stool) alone to reveal a current infection[221].
HEV RNA is detectable soon after infection before symptoms appear and generally lasts a maximum of 6 wk; it confirms the diagnosis of infection. To improve comparability of nucleic acid amplification test results and ensure a correct genotype identification, the WHO defined international standard and an international reference panel for HEV genotypes 1-4. Another direct test consists of the search for the HEV capsid antigen, but its role in the diagnosis is still unclear[221,240]. Currently it is difficult to know how widespread screening is for HEV, but the raising awareness of the spread of hepatitis E even in high-income countries over the years will likely result in a reduction of undiagnosed cases in the near future.
After HEV contact, the incubation period is about 2-6 wk, but it can be very variable. HEV infection is generally asymptomatic or with very few symptoms and self-limiting. This aspect also contributes to the non-definitive knowledge on the real diffusion of the disease. In about 5%-30% such infection is symptomatic and has a similar course to that of other acute hepatitis. In these cases, the infection is responsible for nonspecific symptoms, such as asthenia, nausea, vomiting, fever, muscle, and joint pains and then jaundice, itching, dark urine, and light-colored stools. Contextually, blood tests can show alterations in liver biochemistry such as high transaminases (ALT often exceeds 1000 IU/L), bilirubin, gamma glutamyl transferase, and alkaline phosphatase. It was observed that HEV-3 and HEV-4 tend to be associated with clinically more severe hepatitis in older males, but the underlying reasons are still unclear. Rarely can HEV infection result in ALF[219,223,225,239,240].
While healthy, immunocompetent people usually have a benign course, there are some categories for whom HEV is more dangerous. Patients with pre-existing chronic liver disease can develop acute-on-chronic liver failure due to hepatitis E virus infection[223,226]. Nowadays it is estimated that many cases of liver damage of undetermined etiology or suspected drug origin are caused by HEV. For this reason, it is recommended to test patients with liver biochemistry compatible with hepatitis or diagnosed with ALF or acute-on-chronic liver failure of still undetermined etiology for HEV infection in addition to other causes of liver injury.
Immunosuppressed patients, including organ or stem-cell transplant recipients, HIV infected patients, patients receiving chemotherapy for hematological diseases, or long-term therapy for autoimmune disorders are another category at risk of worse outcomes[221,226,240-242]. In these patients the risk of developing chronic hepatitis E is consistent, given the inability of their immune system to eliminate the virus. Studies on organ transplant patients infected with HEV showed a chronicization rate of more than 60%[243]. In this group, it is debated whether to consider “chronic” an infection from HEV when the RNA has been identifiable for at least 6 mo, or if it is detectable for more than 3 mo after infection[221,244]. Chronic HEV infection has been described mainly for genotype 3, but chronic infections from HEV-4 were also reported, and one case of chronic infection by HEV-7 (as reported above) was described.
Some cases of chronic hepatitis E by HEV-1 have recently been claimed[245,246]. Chronic hepatitis E generally has a slightly symptomatic or asymptomatic course, with only mild alterations of liver biochemistry[240,241,243]. Moreover, anti-HEV antibodies can often give false negative results, and therefore the search for HEV RNA is particularly useful in these people[240]. This form can progress through the development of increasingly severe fibrosis up to decompensated cirrhosis. Liver injury, as has already been observed for other viral hepatitis, can regress after the elimination of the virus[221,240].
HEV-1 and HEV-2 are responsible for a high percentage of liver failure and mortality (25%-30%) in pregnant women during the third trimester, and HEV infection during pregnancy was also associated with increased neonatal morbidity (e.g., preterm birth) and mortality[223,247]. Several factors have been hypothesized to explain the high severity of HEV infection in pregnancy, including host (hormonal and immune changes) and virus-related factors, but the exact causes are not known[247]. Another noteworthy aspect is that there are considerable geographical differences regarding the severity of the course of HEV infection in pregnancy[223].
The hepatitis E virus is responsible for several extrahepatic manifestations. The pathophysiology of these events is not yet fully understood, but direct damage by the virus is not excluded[221,226,240]. It has been observed that HEV can replicate in many other locations besides the liver, and these findings raised the hypothesis that the entry receptors of HEV are not limited to hepatocytes. Among the extrahepatic manifestations there are neurological disorders (Guillain–Barré syndrome, neuralgic amyotrophy, Bell’s palsy, meningoencephalitis, peripheral neuropathy, mononeuritis multiplex), kidney injury (membranoproliferative and membranous glomerulonephritis, IgA nephropathy), hematologic disturbances (thrombocytopenia, hemolysis, aplastic anemia, monoclonal immunoglobulin, cryoglobulinemia), acute pancreatitis, and some reports of thyroiditis, arthritis, and myocarditis[221,240].
Antiviral therapy is usually not necessary for acute hepatitis E, and in any case, there are no specific or extensively tested treatments. In cases of severe forms of hepatitis E and liver failure, RBV was successfully used and resulted in improvement of liver biochemistry and clearance of HEV RNA; corticosteroids have also been shown to be effective in some patients[221,240].
For patients diagnosed with a chronic form of hepatitis E in the context of transplant recipients, it is recommended to reduce immunosuppressive therapy as a first step. This measure alone proved to cause HEV clearance in a consistent percentage of patients (nearly one-third)[221,243]. Currently, RBV represents the therapy of choice for chronic hepatitis E, but its mechanism of action against this virus remains unknown. In a retrospective study on 59 organ transplant patients suffering from chronic hepatitis E treated with RBV, the SVR rate was 78% after a first course, and 85% if re-treatments are considered[248]. In another study of 255 organ transplant patients with chronic HEV infection, 81.2% of them reached SVR after the first course[249]. In these studies, RBV was used with a median dose of 600 mg/d for a median duration of 3 mo, and this is the most used protocol. As known, RBV treatment can be associated with the onset of side effects including hemolytic anemia, skin reactions, or a dry cough. Furthermore, due to its teratogenic potential, the use of RBV is not recommended in pregnancy, and for this category careful monitoring and supportive care must be provided; therefore, prevention of infection is a key point[221,247].
Peg-IFNα is another drug used for hepatitis E in liver transplanted patients and people undergoing hemodialysis, but it is contraindicated in kidney, heart, lung, and pancreas transplant recipients due to the risk of rejection[221,240]. In cases of non-response or intolerance to RBV and the patient is not eligible for IFN, there are no options to continue therapy[221,249,250]. A recent study in which sofosbuvir in monotherapy was used in nine chronic hepatitis E patients showed the ineffectiveness of this drug in eliminating HEV[250]. For these reasons, there is a need for new effective drugs with specific anti-HEV activity[250,251].
An HEV vaccine (Hecolin®) has been available in China since 2011; this vaccine was designed based on the ORF2 region of HEV-1 and showed very high efficacy in preventing symptomatic hepatitis E in a randomized placebo-controlled trial[252]. It proved to be effective for several years, but the overall duration of protection is yet to be known. The vaccine looks safe in pregnant women, but further concrete data is needed. The other open questions are efficacy and safety in specific categories of patients (such as those suffering from chronic liver diseases) and effectiveness against other genotypes[221].
In conclusion, although viral hepatitis still represents a major public health problem and a leading cause of mortality, important milestones have been achieved in this field. Nowadays, hepatitis C is curable in almost all patients by simple, safe, and short-course therapies. To eradicate it, it is crucial to focus on case-finding and linkage to care initiatives. The scenario of hepatitis B and D is changing, with a reduction in their prevalence largely due to the anti-HBV vaccination. Currently available anti-HBV therapies are effective in suppressing viral replication, but they do not eradicate HBV. However, several new therapeutic approaches are being studied for HBV and HDV infections, and in 2020 an entry inhibitor for the treatment of chronic hepatitis D was approved in the European Union. Improvements in hygiene conditions and vaccination can prevent hepatitis caused by HAV and HEV, but the vaccine against the latter virus is not available in all countries. Hepatitis E still represents a challenge as its pathophysiology and real spread are not fully known. Moreover, currently available treatment options are not feasible in all patients.
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4669] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 2. | Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2495] [Cited by in RCA: 2345] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 3. | Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 551] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 991] [Article Influence: 82.6] [Reference Citation Analysis (2)] |
| 5. | Borgia SM, Hedskog C, Parhy B, Hyland RH, Stamm LM, Brainard DM, Subramanian MG, McHutchison JG, Mo H, Svarovskaia E, Shafran SD. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J Infect Dis. 2018;218:1722-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 425] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 8. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1513] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 9. | Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Falla AM, Hofstraat SHI, Duffell E, Hahné SJM, Tavoschi L, Veldhuijzen IK. Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at-risk groups. BMC Infect Dis. 2018;18:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, Chuang CK. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J Hepatol. 2014;6:643-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Dickson RC. Clinical manifestations of hepatitis C. Clin Liver Dis. 1997;1:569-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Alter MJ. Community acquired viral hepatitis B and C in the United States. Gut. 1993;34:S17-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1114] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 15. | World Health Organization. Hepatitis C. [cited 11 April 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 16. | Alberti A, Morsica G, Chemello L, Cavalletto D, Noventa F, Pontisso P, Ruol A. Hepatitis C viraemia and liver disease in symptom-free individuals with anti-HCV. Lancet. 1992;340:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 256] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 623] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 18. | Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in germany: a 20-year multicenter study. Hepatology. 2000;32:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Wiese M, Grüngreiff K, Güthoff W, Lafrenz M, Oesen U, Porst H; East German Hepatitis C Study Group. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany--a 25-year multicenter study. J Hepatol. 2005;43:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 819] [Article Influence: 26.4] [Reference Citation Analysis (6)] |
| 21. | Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204-2212. [PubMed] [DOI] [Full Text] |
| 22. | Zignego AL, Bréchot C. Extrahepatic manifestations of HCV infection: facts and controversies. J Hepatol. 1999;31:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB; Italian Association of the Study of Liver Commission on Extrahepatic Manifestations of HCV infection. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46 Suppl 5:S165-S173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Ferri C, La Civita L, Caracciolo F, Zignego AL. Non-Hodgkin's lymphoma: possible role of hepatitis C virus. JAMA. 1994;272:355-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ferri C, Caracciolo F, La Civita L, Monti M, Longombardo G, Greco F, Zignego AL. Hepatitis C virus infection and B-cell lymphomas. Eur J Cancer. 1994;30A:1591-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Negri E, Little D, Boiocchi M, La Vecchia C, Franceschi S. B-cell non-Hodgkin's lymphoma and hepatitis C virus infection: a systematic review. Int J Cancer. 2004;111:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol. 2007;60:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Engels EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, Whitby D, Colt JS, Hartge P. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer. 2004;111:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Calvaruso V, Craxì A. Immunological alterations in hepatitis C virus infection. World J Gastroenterol. 2013;19:8916-8923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Domont F, Cacoub P. Chronic hepatitis C virus infection, a new cardiovascular risk factor? Liver Int. 2016;36:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Sherlock S. Antiviral therapy for chronic hepatitis C viral infection. J Hepatol. 1995;23 Suppl 2:3-7. [PubMed] |
| 37. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1640] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 38. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4753] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 39. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4559] [Article Influence: 182.4] [Reference Citation Analysis (4)] |
| 40. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L, McNair L, George S, Kieffer T, Kwong A, Kauffman RS, Alam J, Pawlotsky JM, Zeuzem S; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 795] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 41. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1984] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 42. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1865] [Article Influence: 124.3] [Reference Citation Analysis (4)] |
| 43. | Pawlotsky JM. The results of Phase III clinical trials with telaprevir and boceprevir presented at the Liver Meeting 2010: a new standard of care for hepatitis C virus genotype 1 infection, but with issues still pending. Gastroenterology. 2011;140:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1311] [Article Influence: 87.4] [Reference Citation Analysis (2)] |
| 45. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F, Sherman M, Zeuzem S, Scott J, Gilles L, Lenz O, Peeters M, Sekar V, De Smedt G, Beumont-Mauviel M. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 46. | Fujii H, Nishimura T, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Sumida Y, Mitsuyoshi H, Yokomizo C, Tanaka S, Ishikawa H, Nishioji K, Kimura H, Takami S, Nagao Y, Takeuchi T, Shima T, Sawa Y, Minami M, Yasui K, Itoh Y. Comparison of peg-interferon, ribavirin plus telaprevir vs simeprevir by propensity score matching. World J Hepatol. 2015;7:2841-2848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1330] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 48. | Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, Dejesus E, Freilich B, Nelson DR, Dieterich DT, Jacobson IM, Jensen D, Abrams GA, Darling JM, Rodriguez-Torres M, Reddy KR, Sulkowski MS, Bzowej NH, Hyland RH, Mo H, Lin M, Mader M, Hindes R, Albanis E, Symonds WT, Berrey MM, Muir A. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, Afdhal N, Vierling JM, Gordon SC, Anderson JK, Hyland RH, Dvory-Sobol H, An D, Hindes RG, Albanis E, Symonds WT, Berrey MM, Nelson DR, Jacobson IM. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 50. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, Schiff E, Al-Assi MT, Subramanian GM, An D, Lin M, McNally J, Brainard D, Symonds WT, McHutchison JG, Patel K, Feld J, Pianko S, Nelson DR; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 51. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 52. | Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S; ASTRAL-1 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 889] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 53. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 658] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 54. | Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, Felizarta F, Hassanein T, Hinrichsen H, Rincon D, Morillas R, Zeuzem S, Horsmans Y, Nelson DR, Yu Y, Krishnan P, Lin CW, Kort JJ, Mensa FJ. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 55. | Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, Felizarta F, Sulkowski MS, Gane E, Maliakkal B, Overcash JS, Gordon SC, Muir AJ, Aguilar H, Agarwal K, Dore GJ, Lin CW, Liu R, Lovell SS, Ng TI, Kort J, Mensa FJ. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017;67:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 56. | Persico M, Aglitti A, Milella M, Coppola C, Messina V, Claar E, Gentile I, Sogari F, Pierri P, Surace LA, Morisco F, Tundo P, Brancaccio G, Serviddio G, Gatti P, Termite AP, Di Costanzo GG, Caroleo B, Cozzolongo R, Coppola N, Longo A, Fontanella L, Federico A, Rosato V, Terrenato I, Masarone M. Real-life glecaprevir/pibrentasvir in a large cohort of patients with hepatitis C virus infection: The MISTRAL study. Liver Int. 2019;39:1852-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | D'Ambrosio R, Pasulo L, Puoti M, Vinci M, Schiavini M, Lazzaroni S, Soria A, Gatti F, Menzaghi B, Aghemo A, Capelli F, Rumi MG, Morini L, Giorgini A, Pigozzi MG, Rossini A, Maggiolo F, Pan A, Memoli M, Spinelli O, Del Poggio P, Saladino V, Spinetti A, De Bona A, Capretti A, Uberti-Foppa C, Bonfanti P, Terreni N, Menozzi F, Colombo AE, Giglio O, Centenaro R, Borghi M, Baiguera C, Picciotto V, Landonio S, Gori A, Magnani C, Noventa F, Paolucci S, Lampertico P, Fagiuoli S; NAVIGATORE-Lombardia Study Group. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. 2019;70:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 58. | Persico M, Aglitti A, Aghemo A, Rendina M, Lleo A, Ciancio A, Di Marco V, Lampertico P, Brunetto MR, Zuin M, Andreone P, Villa E, Troshina G, Calvaruso V, Degasperi E, Coco B, Giorgini A, Conti F, Di Leo A, Marzi L, Boccaccio V, Bollani S, Maisonneuve P, Bruno S. High efficacy of direct-acting anti-viral agents in hepatitis C virus-infected cirrhotic patients with successfully treated hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;47:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V; Rete Sicilia Selezione Terapia–HCV (RESIST-HCV). Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411-421.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 60. | Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horváth G, Zuckerman E, Carrion BR, Rodriguez-Perez F, Urbánek P, Abergel A, Cohen E, Lovell SS, Schnell G, Lin CW, Zha J, Wang S, Trinh R, Mensa FJ, Burroughs M, Felizarta F. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol. 2020;72:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 61. | Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, Zeuzem S, Landis CS, Kwo P, Hassan M, Manns MP, Vainorius M, Akushevich L, Nelson DR, Fried MW, Reddy KR. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 62. | Rodríguez-Tajes S, Pocurull A, Castillo J, Casanova G, Vega L, Lens S, Mariño Z, Londoño MC, Forner A, Torres F, Forns X. Hepatitis C-related cirrhosis will be a marginal cause of hospital admissions by 2025. J Hepatol. 2020;73:1360-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, Karam V, Salizzoni M, Andujar RL, Fondevila C, De Simone P, Morelli C, Fabregat-Prous J, Samuel D, Agarwaal K, Moreno Gonzales E, Charco R, Zieniewicz K, De Carlis L, Duvoux C; all the contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 64. | He S, Lockart I, Alavi M, Danta M, Hajarizadeh B, Dore GJ. Systematic review with meta-analysis: effectiveness of direct-acting antiviral treatment for hepatitis C in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:34-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 66. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 67. | Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. 2020;71:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 68. | Lleo A, Aglitti A, Aghemo A, Maisonneuve P, Bruno S, Persico M; collaborators. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig Liver Dis. 2019;51:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Romano A, Angeli P, Piovesan S, Noventa F, Anastassopoulos G, Chemello L, Cavalletto L, Gambato M, Russo FP, Burra P, Vincenzi V, Scotton PG, Panese S, Tempesta D, Bertin T, Carrara M, Carlotto A, Capra F, Carolo G, Scroccaro G, Alberti A. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J Hepatol. 2018;69:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 70. | Sangiovanni A, Alimenti E, Gattai R, Filomia R, Parente E, Valenti L, Marzi L, Pellegatta G, Borgia G, Gambato M, Terreni N, Serio I, Belli L, Oliveri F, Maimone S, Brunacci M, D'Ambrosio R, Forzenigo LV, Russo FP, Rumi M, Barone M, Fracanzani AL, Raimondo G, Giannini EG, Brunetto MR, Villa E, Biganzoli E, Colombo M, Lampertico P. Undefined/non-malignant hepatic nodules are associated with early occurrence of HCC in DAA-treated patients with HCV-related cirrhosis. J Hepatol. 2020;73:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Muzica CM, Stanciu C, Huiban L, Singeap AM, Sfarti C, Zenovia S, Cojocariu C, Trifan A. Hepatocellular carcinoma after direct-acting antiviral hepatitis C virus therapy: A debate near the end. World J Gastroenterol. 2020;26:6770-6781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 74. | Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, Madonia S, Rossi M, Magro B, Rini F, Distefano M, Larocca L, Prestileo T, Malizia G, Bertino G, Benanti F, Licata A, Scalisi I, Mazzola G, Di Rosolini MA, Alaimo G, Averna A, Cartabellotta F, Alessi N, Guastella S, Russello M, Scifo G, Squadrito G, Raimondo G, Trevisani F, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia – HCV (RESIST-HCV) and Italian Liver Cancer (ITA. LI.CA.) Group. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 75. | Dang H, Yeo YH, Yasuda S, Huang CF, Iio E, Landis C, Jun DW, Enomoto M, Ogawa E, Tsai PC, Le A, Liu M, Maeda M, Nguyen B, Ramrakhiani N, Henry L, Cheung R, Tamori A, Kumada T, Tanaka Y, Yu ML, Toyoda H, Nguyen MH. Cure With Interferon-Free Direct-Acting Antiviral Is Associated With Increased Survival in Patients With Hepatitis C Virus-Related Hepatocellular Carcinoma From Both East and West. Hepatology. 2020;71:1910-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 76. | Singal AG, Rich NE, Mehta N, Branch AD, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Ioannou GN, Huang A, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Kagan S, Gopal P, Wong R, Parikh ND, Murphy CC. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology. 2019;157:1253-1263.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 77. | Comarmond C, Cacoub P, Saadoun D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Therap Adv Gastroenterol. 2020;13:1756284820942617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Passerini M, Schiavini M, Magni CF, Landonio S, Niero F, Passerini S, Croci AL, Bolis M, Scalzi V, Gubertini G, Ricci ED, Galli M, Rizzardini G. Are direct-acting antivirals safe and effective in hepatitis C virus-cryoglobulinemia? Eur J Gastroenterol Hepatol. 2018;30:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Cacoub P, Si Ahmed SN, Ferfar Y, Pol S, Thabut D, Hezode C, Alric L, Comarmond C, Ragab G, Quartuccio L, Hegazy M, Poynard T, Resche Rigon M, Saadoun D. Long-term Efficacy of Interferon-Free Antiviral Treatment Regimens in Patients With Hepatitis C Virus-Associated Cryoglobulinemia Vasculitis. Clin Gastroenterol Hepatol. 2019;17:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 80. | Colantuono S, Marrapodi R, Del Padre M, Collalti G, Garzi G, De Santis A, Fiorilli M, Basili S, Visentini M, Casato M. Clinico-immunological outcomes of HCV-cured cryoglobulinemia: Lower relapse rate with interferon-based than interferon-free therapy. Liver Int. 2021;41:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Persico M, Aglitti A, Caruso R, De Renzo A, Selleri C, Califano C, Abenavoli L, Federico A, Masarone M. Efficacy and safety of new direct antiviral agents in hepatitis C virus-infected patients with diffuse large B-cell non-Hodgkin's lymphoma. Hepatology. 2018;67:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 82. | Pellicelli A, Giannelli V, Zoli V, Pellicelli V, Zignego AL. Antiviral therapy in hepatitis C-infected patients prevents relapse of diffuse large B cell lymphoma. Clin Exp Hepatol. 2018;4:197-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Merli M, Frigeni M, Alric L, Visco C, Besson C, Mannelli L, Di Rocco A, Ferrari A, Farina L, Pirisi M, Piazza F, Loustaud-Ratti V, Arcari A, Marino D, Sica A, Goldaniga M, Rusconi C, Gentile M, Cencini E, Benanti F, Rumi MG, Ferretti VV, Grossi P, Gotti M, Sciarra R, Tisi MC, Cano I, Zuccaro V, Passamonti F, Arcaini L. Direct-Acting Antivirals in Hepatitis C Virus-Associated Diffuse Large B-cell Lymphomas. Oncologist. 2019;24:e720-e729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Frigeni M, Besson C, Visco C, Fontaine H, Goldaniga M, Visentini M, Pulsoni A, Torres HA, Peveling-Oberhag J, Rossotti R, Zaja F, Rigacci L, Merli M, Dorival C, Alric C, Piazza F, Gentile M, Ferrari A, Pirisi M, Nassi L, Rattotti S, Frustaci A, Milella M, Cencini E, Defrancesco I, Ferretti VV, Bruno R, Hermine O, Arcaini L. Interferon-free compared to interferon-based antiviral regimens as first-line therapy for B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Leukemia. 2020;34:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Masarone M, Persico M. Hepatitis C virus infection and non-hepatocellular malignancies in the DAA era: A systematic review and meta-analysis. Liver Int. 2019;39:1292-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, Nelson DR, Fried MW, Terrault NA; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 87. | Sanai FM, Alghamdi AS, Afghani AA, Alswat K, AlZanbagi A, Alghamdi MN, AlMousa A, Aseeri M, Assiri AM, Babatin MA. High Efficacy of ombitasvir/paritaprevir/ritonavir plus dasabuvir in hepatitis C genotypes 4 and 1-infected patients with severe chronic kidney disease. Liver Int. 2018;38:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 534] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 89. | Choi DT, Puenpatom A, Yu X, Erickson KF, Kanwal F, El-Serag HB, Kramer JR. Effectiveness of Elbasvir/Grazoprevir in patients with hepatitis C virus genotype 1 infection and chronic kidney disease in the United States veterans population. Antiviral Res. 2020;174:104698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 91. | Lawitz E, Flisiak R, Abunimeh M, Sise ME, Park JY, Kaskas M, Bruchfeld A, Wörns MA, Aglitti A, Zamor PJ, Xue Z, Schnell G, Jalundhwala YJ, Porcalla A, Mensa FJ, Persico M. Efficacy and safety of glecaprevir/pibrentasvir in renally impaired patients with chronic HCV infection. Liver Int. 2020;40:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Atsukawa M, Tsubota A, Toyoda H, Takaguchi K, Nakamuta M, Watanabe T, Michitaka K, Ikegami T, Nozaki A, Uojima H, Fukunishi S, Genda T, Abe H, Hotta N, Tsuji K, Ogawa C, Tachi Y, Shima T, Shimada N, Kondo C, Akahane T, Aizawa Y, Tanaka Y, Kumada T, Iwakiri K. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment: a prospective, multicenter study. Aliment Pharmacol Ther. 2019;49:1230-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Persico M, Aglitti A, Caruso R, Calvanese G, Di Siervi P, Masarone M. Real-life efficacy and safety of glecaprevir/pibrentasvir in HCV-infected patients with chronic kidney disease. GastroHep. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 94. | Taneja S, Duseja A, Mehta M, De A, Verma N, Premkumar M, Dhiman RK, Singh V, Singh MP, Ratho RK, Ramachandran R, Kumar V, Kohli HS. Sofosbuvir and Velpatasvir combination is safe and effective in treating chronic hepatitis C in end-stage renal disease on maintenance haemodialysis. Liver Int. 2021;41:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Borgia SM, Dearden J, Yoshida EM, Shafran SD, Brown A, Ben-Ari Z, Cramp ME, Cooper C, Foxton M, Rodriguez CF, Esteban R, Hyland R, Lu S, Kirby BJ, Meng A, Markova S, Dvory-Sobol H, Osinusi AO, Bruck R, Ampuero J, Ryder SD, Agarwal K, Fox R, Shaw D, Haider S, Willems B, Lurie Y, Calleja JL, Gane EJ. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 96. | World Health Organization. Global health sector strategy on viral hepatitis, 2016–2021: towards ending viral hepatitis. [cited 11 April 2021]. Available from: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. |
| 97. | Lazarus JV, Wiktor S, Colombo M, Thursz M; EASL International Liver Foundation. Micro-elimination - A path to global elimination of hepatitis C. J Hepatol. 2017;67:665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 98. | Persico M, Masarone M, Aglitti A, Armenante C, Giordano A, Guardiola A, Raimondi G, Contaldi C, Nigro C, Marena G, De Luna A. HCV point-of-care screening programme and treatment options for people who use drugs in a metropolitan area of Southern Italy. Liver Int. 2019;39:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Grebely J, Feld JJ, Wyles D, Sulkowski M, Ni L, Llewellyn J, Mir HM, Sajed N, Stamm LM, Hyland RH, McNally J, Brainard DM, Jacobson I, Zeuzem S, Bourlière M, Foster G, Afdhal N, Dore GJ. Sofosbuvir-Based Direct-Acting Antiviral Therapies for HCV in People Receiving Opioid Substitution Therapy: An Analysis of Phase 3 Studies. Open Forum Infect Dis. 2018;5:ofy001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | Masarone M, Caruso R, Aglitti A, Izzo C, De Matteis G, Attianese MR, Pagano AM, Persico M. Hepatitis C virus infection in jail: Difficult-to-reach, not to-treat. Results of a point-of-care screening and treatment program. Dig Liver Dis. 2020;52:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Sperring H, Ruiz-Mercado G, Schechter-Perkins EM. Impact of the 2020 COVID-19 Pandemic on Ambulatory Hepatitis C Testing. J Prim Care Community Health. 2020;11:2150132720969554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Aghemo A, Masarone M, Montagnese S, Petta S, Ponziani FR, Russo FP; Associazione Italiana Studio Fegato (AISF). Assessing the impact of COVID-19 on the management of patients with liver diseases: A national survey by the Italian association for the study of the Liver. Dig Liver Dis. 2020;52:937-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 103. | Hüppe D, Niederau C, Serfert Y, Hartmann H, Wedemeyer H; für das DHC-R. [Problems in treating patients with chronic HCV infection due to the COVID-19 pandemic and during the lockdown phase in Germany]. Z Gastroenterol. 2020;58:1182-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 105. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1263] [Article Influence: 157.9] [Reference Citation Analysis (6)] |
| 106. | World Health Organization. Hepatitis B. [cited 11 April 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 107. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4009] [Article Influence: 445.4] [Reference Citation Analysis (1)] |
| 108. | Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis. 2016;20:607-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 109. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472-480.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 110. | Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 111. | Petersen NJ, Barrett DH, Bond WW, Berquist KR, Favero MS, Bender TR, Maynard JE. Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages. Appl Environ Microbiol. 1976;32:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Blumberg BS, ALTER HJ, VISNICH S. A "NEW" ANTIGEN IN LEUKEMIA SERA. JAMA. 1965;191:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 736] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 113. | Blumberg BS, Gerstley BJ, Hungerford DA, London WT, Sutnick AI. A serum antigen (Australia antigen) in Down's syndrome, leukemia, and hepatitis. Ann Intern Med. 1967;66:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 352] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 114. | Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 562] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 115. | Serum Hepatitis. The Lancet. 1947;250:691-692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 116. | Block TM, Alter HJ, London WT, Bray M. A historical perspective on the discovery and elucidation of the hepatitis B virus. Antiviral Res. 2016;131:109-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | ICTV. Hepadnaviridae. [cited 11 April 2021]. Available from: https://ictv.global/report/hepadnaviridae. |
| 118. | Magnius L, Mason WS, Taylor J, Kann M, Glebe D, Dény P, Sureau C, Norder H; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepadnaviridae. J Gen Virol. 2020;101:571-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 119. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 120. | Schädler S, Hildt E. HBV life cycle: entry and morphogenesis. Viruses. 2009;1:185-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Milich DR. Is the function of the HBeAg really unknown? Hum Vaccin Immunother. 2019;15:2187-2191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 122. | Kramvis A, Kostaki EG, Hatzakis A, Paraskevis D. Immunomodulatory Function of HBeAg Related to Short-Sighted Evolution, Transmissibility, and Clinical Manifestation of Hepatitis B Virus. Front Microbiol. 2018;9:2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Diab A, Foca A, Zoulim F, Durantel D, Andrisani O. The diverse functions of the hepatitis B core/capsid protein (HBc) in the viral life cycle: Implications for the development of HBc-targeting antivirals. Antiviral Res. 2018;149:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 124. | Clark DN, Hu J. Unveiling the roles of HBV polymerase for new antiviral strategies. Future Virol. 2015;10:283-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Venkatakrishnan B, Zlotnick A. The Structural Biology of Hepatitis B Virus: Form and Function. Annu Rev Virol. 2016;3:429-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 126. | Slagle BL, Bouchard MJ. Hepatitis B Virus X and Regulation of Viral Gene Expression. Cold Spring Harb Perspect Med. 2016;6:a021402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 127. | Verrier ER, Colpitts CC, Bach C, Heydmann L, Weiss A, Renaud M, Durand SC, Habersetzer F, Durantel D, Abou-Jaoudé G, López Ledesma MM, Felmlee DJ, Soumillon M, Croonenborghs T, Pochet N, Nassal M, Schuster C, Brino L, Sureau C, Zeisel MB, Baumert TF. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology. 2016;63:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 128. | Rybicka M, Bielawski KP. Recent Advances in Understanding, Diagnosing, and Treating Hepatitis B Virus Infection. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Song JE, Kim DY. Diagnosis of hepatitis B. Ann Transl Med. 2016;4:338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 131. | Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 747] [Cited by in RCA: 667] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 132. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 133. | Masarone M, De Renzo A, La Mura V, Sasso FC, Romano M, Signoriello G, Rosato V, Perna F, Pane F, Persico M. Management of the HBV reactivation in isolated HBcAb positive patients affected with Non Hodgkin Lymphoma. BMC Gastroenterol. 2014;14:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Wong VW, Janssen HL. Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J Hepatol. 2015;63:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 135. | Loglio A, Lampertico P. How Durable Is Functional Cure (Hepatitis B Surface Antigen Loss) in Patients With Chronic Hepatitis B Treated With Current Antivirals? Hepatol Commun. 2020;4:5-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Baudi I, Inoue T, Tanaka Y. Novel Biomarkers of Hepatitis B and Hepatocellular Carcinoma: Clinical Significance of HBcrAg and M2BPGi. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Mak LY, Seto WK, Fung J, Yuen MF. New Biomarkers of Chronic Hepatitis B. Gut Liver. 2019;13:589-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 138. | Coffin CS, Zhou K, Terrault NA. New and Old Biomarkers for Diagnosis and Management of Chronic Hepatitis B Virus Infection. Gastroenterology. 2019;156:355-368.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 139. | Pan CQ, Zhang JX. Natural History and Clinical Consequences of Hepatitis B Virus Infection. Int J Med Sci. 2005;2:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Kappus MR, Sterling RK. Extrahepatic manifestations of acute hepatitis B virus infection. Gastroenterol Hepatol (N Y). 2013;9:123-126. [PubMed] |
| 141. | Li M, Gan Y, Fan C, Yuan H, Zhang X, Shen Y, Wang Q, Meng Z, Xu D, Tu H. Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat. 2018;25:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 142. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3090] [Article Influence: 386.3] [Reference Citation Analysis (1)] |
| 143. | Suk-Fong Lok A. Hepatitis B Treatment: What We Know Now and What Remains to Be Researched. Hepatol Commun. 2019;3:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 144. | Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, Schluep T. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 145. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 851] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 146. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1573] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 147. | Jia L, Gao Y, He Y, Hooper JD, Yang P. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol Res. 2020;159:104992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 148. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 149. | Matsuda Y, Ichida T. Impact of hepatitis B virus X protein on the DNA damage response during hepatocarcinogenesis. Med Mol Morphol. 2009;42:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 151. | Yeo YH, Ho HJ, Yang HI, Tseng TC, Hosaka T, Trinh HN, Kwak MS, Park YM, Fung JYY, Buti M, Rodríguez M, Treeprasertsuk S, Preda CM, Ungtrakul T, Charatcharoenwitthaya P, Li X, Li J, Zhang J, Le MH, Wei B, Zou B, Le A, Jeong D, Chien N, Kam L, Lee CC, Riveiro-Barciela M, Istratescu D, Sriprayoon T, Chong Y, Tanwandee T, Kobayashi M, Suzuki F, Yuen MF, Lee HS, Kao JH, Lok AS, Wu CY, Nguyen MH. Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:635-646.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 152. | Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, Ford N, Han Q, Lu Y, Bulterys M, Hutin Y. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:106-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 153. | Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK; Hong Kong Liver Fibrosis Study Group. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 154. | Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, Lee HC, Lee YS. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 155. | Attar BM. CON: All Patients With Immune-Tolerated Hepatitis B Virus Do Not Need to Be Treated. Clin Liver Dis (Hoboken). 2020;15:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 156. | Menéndez-Arias L, Álvarez M, Pacheco B. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol. 2014;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 157. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2053] [Article Influence: 205.3] [Reference Citation Analysis (12)] |
| 158. | Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P, Kitrinos KM, Subramanian GM, Gane E, Marcellin P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 159. | Park ES, Lee AR, Kim DH, Lee JH, Yoo JJ, Ahn SH, Sim H, Park S, Kang HS, Won J, Ha YN, Shin GC, Kwon SY, Park YK, Choi BS, Lee YB, Jeong N, An Y, Ju YS, Yu SJ, Chae HB, Yu KS, Kim YJ, Yoon JH, Zoulim F, Kim KH. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol. 2019;70:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 160. | Blackard JT, Kwara A, Sherman KE. In response to identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol. 2019;71:1259-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | Mokaya J, McNaughton AL, Bester PA, Goedhals D, Barnes E, Marsden BD, Matthews PC. Hepatitis B virus resistance to tenofovir: fact or fiction? Wellcome Open Res. 2020;5:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 162. | Lai CL, Wong D, Ip P, Kopaniszen M, Seto WK, Fung J, Huang FY, Lee B, Cullaro G, Chong CK, Wu R, Cheng C, Yuen J, Ngai V, Yuen MF. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 163. | Lai CL, Wong DK, Wong GT, Seto WK, Fung J, Yuen MF. Rebound of HBV DNA after cessation of nucleos/tide analogues in chronic hepatitis B patients with undetectable covalently closed. JHEP Rep. 2020;2:100112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 164. | Tu T, Urban S. Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol. 2018;30:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 165. | Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 654] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 166. | Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C, Hutin Y, Geretti AM. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 167. | Rizzetto M, Hamid S, Negro F. The changing context of hepatitis D. J Hepatol. 2021;74:1200-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 168. | World Health Organization. Hepatitis D. [cited 11 April 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-d. |
| 169. | Hercun J, Koh C, Heller T. Hepatitis Delta: Prevalence, Natural History, and Treatment Options. Gastroenterol Clin North Am. 2020;49:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 170. | Magnius L, Taylor J, Mason WS, Sureau C, Dény P, Norder H; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Deltavirus. J Gen Virol. 2018;99:1565-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Botelho-Souza LF, Vasconcelos MPA, Dos Santos AO, Salcedo JMV, Vieira DS. Hepatitis delta: virological and clinical aspects. Virol J. 2017;14:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 172. | Zhang Z, Urban S. New insights into HDV persistence: The role of interferon response and implications for upcoming novel therapies. J Hepatol. 2021;74:686-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 173. | Negro F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med. 2014;4:a021550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 174. | Etzion O, Hamid SS, Lurie Y, Gane E, Bader N, Yardeni D, Nevo-Shor a, channa s, Mawani M, Parkash O, Yang K, Longo D, Gish RG, Glenn J, Apelian D. PS-052-End of study results from LIMT HDV study: 36% durable virologic response at 24 wk post-treatment with pegylated interferon lambda monotherapy in patients with chronic hepatitis delta virus infection. J Hepatol. 2019;70:e32. [DOI] [Full Text] |
| 175. | Kang C, Syed YY. Bulevirtide: First Approval. Drugs. 2020;80:1601-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 176. | Wedemeyer H, Bogomolov P, Blank A, Allweiss L, Dandri-Petersen M, Bremer B, Voronkova N, Schöneweis K, Pathil A, Burhenne J, Haag M, Schwab M, Haefeli WE, Wiesch JSZ, Alexandrov A, Urban S. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol. 2018;68:S3. [DOI] [Full Text] |
| 177. | Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, Subramanya G, Cooper SL, Pinto P, Wolff EF, Bishop R, Ai Thanda Han M, Cotler SJ, Kleiner DE, Keskin O, Idilman R, Yurdaydin C, Glenn JS, Heller T. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (3)] |
| 178. | Journal of Hepatology: The Home of Liver Research. [cited 11 Apr 2021]. Available from: https://ilc-congress.eu/wp-content/uploads/2020/08/digital-ilc-2020-abstract-book-20-august.pdf. |
| 179. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 180. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Anderson M, Gersch J, Holzmayer V, Elsner C, Krawczyk A, Kuhns MC, Cloherty G, Dittmer U, Vaillant A. Persistent Control of Hepatitis B Virus and Hepatitis Delta Virus Infection Following REP 2139-Ca and Pegylated Interferon Therapy in Chronic Hepatitis B Virus/Hepatitis Delta Virus Coinfection. Hepatol Commun. 2020;5:189-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 181. | Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973;182:1026-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 420] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 182. | Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43:S164-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 183. | Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171 Suppl 1:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 184. | Jacobsen KH. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 185. | Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653-6657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (3)] |
| 186. | Abutaleb A, Kottilil S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol Clin North Am. 2020;49:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 187. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 188. | Tanaka S, Kishi T, Ishihara A, Watanabe D, Uehira T, Ishida H, Shirasaka T, Mita E. Outbreak of hepatitis A linked to European outbreaks among men who have sex with men in Osaka, Japan, from March to July 2018. Hepatol Res. 2019;49:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 189. | Aasheim ET, Seymour M, Balogun K, Ngui SL, Williams CJ, Shankar AG. Acute hepatitis A in an elderly patient after care worker travel to high endemicity country. Hum Vaccin Immunother. 2013;9:2480-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 190. | Shin EC, Jeong SH. Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 191. | European Association for the Study of the Liver; Clinical practice guidelines panel; Wendon, J; Panel members; Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative; Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 192. | Ajmera V, Xia G, Vaughan G, Forbi JC, Ganova-Raeva LM, Khudyakov Y, Opio CK, Taylor R, Restrepo R, Munoz S, Fontana RJ, Lee WM; Acute Liver Failure Study Group. What factors determine the severity of hepatitis A-related acute liver failure? J Viral Hepat. 2011;18:e167-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 193. | Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology. 2010;53:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 194. | Nainan OV, Xia G, Vaughan G, Margolis HS. Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev. 2006;19:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 195. | Crance JM, Lévêque F, Chousterman S, Jouan A, Trépo C, Deloince R. Antiviral activity of recombinant interferon-alpha on hepatitis A virus replication in human liver cells. Antiviral Res. 1995;28:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 196. | Yoshiba M, Inoue K, Sekiyama K. Interferon for hepatitis A. Lancet. 1994;343:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 197. | Jiang W, Muhammad F, Ma P, Liu X, Long G. Sofosbuvir inhibits hepatitis A virus replication in vitro assessed by a cell-based fluorescent reporter system. Antiviral Res. 2018;154:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 198. | Navarro MED, Yao CC, Whiteley A, Movahedi B, Devuni D, Barry C, Zacharias I, Theodoropoulos NM, Bozorgzadeh A, Martins PN. Liver transplant evaluation for fulminant liver failure due to acute hepatitis A infection: Case series and literature review. Transpl Infect Dis. 2021;23:e13476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 199. | Moreira-Silva SF, Frauches DO, Almeida AL, Mendonça HF, Pereira FE. Acute liver failure in children: observations in Vitória, Espírito Santo State, Brazil. Rev Soc Bras Med Trop. 2002;35:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 200. | Sood V, Lal BB, Gupta E, Khanna R, Siloliya MK, Alam S. Hepatitis A Virus-related Pediatric Liver Disease Burden and its Significance in the Indian Subcontinent. Indian Pediatr. 2019;56:741-744. [PubMed] |
| 201. | Ciocca M, Moreira-Silva SF, Alegría S, Galoppo MC, Ruttiman R, Porta G, Da Silvera TR, Rubio P, Macias M, Cervantes Y, Avila-Aguero ML, Clemens SA, Clemens R, Weil J. Hepatitis A as an etiologic agent of acute liver failure in Latin America. Pediatr Infect Dis J. 2007;26:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 202. | Bajpai M, Kakkar B, Patale D. Role of high-volume plasma exchange in a case of a G6PD deficient patient presenting with HAV related acute liver failure and concomitant acute renal failure. Transfus Apher Sci. 2019;58:102677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 203. | Caballol B, Reverter E, Cid J, Hernández-Tejero M, Triolo M, Lozano M, Fernández J. Fulminant hepatitis A complicated by Takotsubo syndrome successfully treated with standard volume plasma exchange. JHEP Rep. 2019;1:445-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 204. | Zakaria HM, Salem TA, El-Araby HA, Salama RM, Elbadry DY, Sira AM, Ali MA, Salem ME, Abd-Alaaty BM, Goda SS, Eltaras SM, Khalil FO, Abou-Zeinah SS, Sira MM. Steroid therapy in children with fulminant hepatitis A. J Viral Hepat. 2018;25:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 205. | Gunduz H, Karabay O, Tamer A, Ozaras R, Mert A, Tabak OF. N-acetyl cysteine therapy in acute viral hepatitis. World J Gastroenterol. 2003;9:2698-2700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 206. | Kim JD, Cho EJ, Ahn C, Park SK, Choi JY, Lee HC, Kim DY, Choi MS, Wang HJ, Kim IH, Yeon JE, Seo YS, Tak WY, Kim MY, Lee HJ, Kim YS, Jun DW, Sohn JH, Kwon SY, Park SH, Heo J, Jeong SH, Lee JH, Nakayama N, Mochida S, Ido A, Tsubouchi H, Takikawa H, Shalimar, Acharya SK, Bernal W, O'Grady J, Kim YJ. A Model to Predict 1-Month Risk of Transplant or Death in Hepatitis A-Related Acute Liver Failure. Hepatology. 2019;70:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 207. | Mallick S, Nair K, Thillai M, Manikandan K, Sethi P, Madhusrinivasan D, Johns SM, Binoj ST, Mohammed Z, Ramachandran NM, Balakrishnan D, Unnikrishnan G, Dhar P, Sudheer OV, Sudhindran S. Liver Transplant in Acute Liver Failure - Looking Back Over 10 Years. J Clin Exp Hepatol. 2020;10:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 208. | Eisenbach C, Longerich T, Fickenscher H, Schalasta G, Stremmel W, Encke J. Recurrence of clinically significant hepatitis A following liver transplantation for fulminant hepatitis A. J Clin Virol. 2006;35:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 209. | Yan BY, Lv JJ, Liu JY, Feng Y, Wu WL, Xu AQ, Zhang L. Changes in seroprevalence of hepatitis A after the implementation of universal childhood vaccination in Shandong Province, China: A comparison between 2006 and 2014. Int J Infect Dis. 2019;82:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 210. | Sharp A, Coles S, Pegorie M, Harwood C, Ngui SL, Welfare W, Mandal S, Balogun K, Gent M. Vaccination strategies for control of community outbreaks of hepatitis A: A comparison of two outbreaks in England. Vaccine. 2019;37:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 211. | Irving GJ, Holden J, Yang R, Pope D. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev. 2012;CD009051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 212. | Bell BP. Hepatitis A vaccine. Semin Pediatr Infect Dis. 2002;13:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Lin KY, Chen GJ, Lee YL, Huang YC, Cheng A, Sun HY, Chang SY, Liu CE, Hung CC. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: A review. World J Gastroenterol. 2017;23:3589-3606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 214. | Link-Gelles R, Hofmeister MG, Nelson NP. Use of hepatitis A vaccine for post-exposure prophylaxis in individuals over 40 years of age: A systematic review of published studies and recommendations for vaccine use. Vaccine. 2018;36:2745-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 215. | Moorman AC, Xing J, Nelson NP; CHeCS Investigators. Need for Increasing Hepatitis A Virus Vaccination Among Patients Infected With Hepatitis B Virus and Hepatitis C Virus. Gastroenterology. 2018;154:2015-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 216. | Pedersini R, Marano C, De Moerlooze L, Chen L, Vietri J. HAV & HBV vaccination among travellers participating in the National Health and Wellness Survey in five European countries. Travel Med Infect Dis. 2016;14:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 217. | Beauté J, Westrell T, Schmid D, Müller L, Epstein J, Kontio M, Couturier E, Faber M, Mellou K, Borg ML, Friesema I, Vold L, Severi E. Travel-associated hepatitis A in Europe, 2009 to 2015. Euro Surveill. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 218. | Victor JC, Monto AS, Surdina TY, Suleimenova SZ, Vaughan G, Nainan OV, Favorov MO, Margolis HS, Bell BP. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 219. | Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 527] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 220. | Li P, Liu J, Li Y, Su J, Ma Z, Bramer WM, Cao W, de Man RA, Peppelenbosch MP, Pan Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020;40:1516-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 221. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 481] [Article Influence: 60.1] [Reference Citation Analysis (3)] |
| 222. | Lapa D, Capobianchi MR, Garbuglia AR. Epidemiology of Hepatitis E Virus in European Countries. Int J Mol Sci. 2015;16:25711-25743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 223. | Seth A, Sherman KE. Hepatitis E: What We Think We Know. Clin Liver Dis (Hoboken). 2020;15:S37-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 224. | Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 428] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 225. | Pallerla SR, Harms D, Johne R, Todt D, Steinmann E, Schemmerer M, Wenzel JJ, Hofmann J, Shih JWK, Wedemeyer H, Bock CT, Velavan TP. Hepatitis E Virus Infection: Circulation, Molecular Epidemiology, and Impact on Global Health. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 226. | Thakur V, Ratho RK, Kumar S, Saxena SK, Bora I, Thakur P. Viral Hepatitis E and Chronicity: A Growing Public Health Concern. Front Microbiol. 2020;11:577339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 227. | Wißing MH, Brüggemann Y, Steinmann E, Todt D. Virus-Host Cell Interplay during Hepatitis E Virus Infection. Trends Microbiol. 2021;29:309-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 228. | Wedemeyer H, Rybczynska J, Pischke S, Krawczynski K. Immunopathogenesis of hepatitis E virus infection. Semin Liver Dis. 2013;33:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 229. | Li Y, Qu C, Yu P, Ou X, Pan Q, Wang W. The Interplay between Host Innate Immunity and Hepatitis E Virus. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 230. | Hakim MS, Ikram A, Zhou J, Wang W, Peppelenbosch MP, Pan Q. Immunity against hepatitis E virus infection: Implications for therapy and vaccine development. Rev Med Virol. 2018;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 231. | Peron JM, Danjoux M, Kamar N, Missoury R, Poirson H, Vinel JP, Mansuy JM, Bureau C, Izopet J, Brousset P, Selves J. Liver histology in patients with sporadic acute hepatitis E: a study of 11 patients from South-West France. Virchows Arch. 2007;450:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 232. | Walker CM. Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 233. | Kenney SP. The Current Host Range of Hepatitis E Viruses. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 234. | Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355-357.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 235. | Corwin AL, Tien NT, Bounlu K, Winarno J, Putri MP, Laras K, Larasati RP, Sukri N, Endy T, Sulaiman HA, Hyams KC. The unique riverine ecology of hepatitis E virus transmission in South-East Asia. Trans R Soc Trop Med Hyg. 1999;93:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 236. | Khuroo MS, Khuroo MS, Khuroo NS. Transmission of Hepatitis E Virus in Developing Countries. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 237. | Sridhar S, Yip CC, Wu S, Chew NF, Leung KH, Chan JF, Zhao PS, Chan WM, Poon RW, Tsoi HW, Cai JP, Chan HS, Leung AW, Tse CW, Zee JS, Tsang OT, Cheng VC, Lau SK, Woo PC, Tsang DN, Yuen KY. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology. 2021;73:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 238. | Andonov A, Robbins M, Borlang J, Cao J, Hatchette T, Stueck A, Deschambault Y, Murnaghan K, Varga J, Johnston L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J Infect Dis. 2019;220:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 239. | Lhomme S, Marion O, Abravanel F, Chapuy-Regaud S, Kamar N, Izopet J. Hepatitis E Pathogenesis. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 240. | Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26:5543-5560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (4)] |
| 241. | Zhou X, de Man RA, de Knegt RJ, Metselaar HJ, Peppelenbosch MP, Pan Q. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev Med Virol. 2013;23:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 242. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1016] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 243. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 244. | Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 245. | Robins AEM, Bowden DJ, Gelson WTH. Chronic genotype 1 hepatitis E infection from immunosuppression for ileo-colonic Crohn's disease. Oxf Med Case Reports. 2018;2018:omy059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 246. | Rathi S, Duseja A, Thakur V, Ratho RK, Singh MP, Taneja S, Das A, Aggarwal R, Dhiman RK, Chawla YK. Chronic Hepatitis E With Genotype 1-Masquerading as Allograft Rejection After LiverTransplantation. J Clin Exp Hepatol. 2021;11:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 247. | Wu C, Wu X, Xia J. Hepatitis E virus infection during pregnancy. Virol J. 2020;17:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 248. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 249. | Kamar N, Abravanel F, Behrendt P, Hofmann J, Pageaux GP, Barbet C, Moal V, Couzi L, Horvatits T, De Man RA, Cassuto E, Elsharkawy AM, Riezebos-Brilman A, Scemla A, Hillaire S, Donnelly MC, Radenne S, Sayegh J, Garrouste C, Dumortier J, Glowaki F, Matignon M, Coilly A, Figueres L, Mousson C, Minello A, Dharancy S, Rerolle JP, Lebray P, Etienne I, Perrin P, Choi M, Marion O, Izopet J; Hepatitis E Virus Ribavirin Study Group. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin Infect Dis. 2020;71:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 250. | Cornberg M, Pischke S, Müller T, Behrendt P, Piecha F, Benckert J, Todt D, Steinmann E, Papkalla A, von Karpowitz M, Koch A, Lohse A, Hardtke S, Manns MP, Wedemeyer H. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E - The HepNet SofE pilot study. J Hepatol. 2020;73:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 251. | Kinast V, Burkard TL, Todt D, Steinmann E. Hepatitis E Virus Drug Development. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 252. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (1)] |
| 253. | AISF. Documento di indirizzo dell’Associazione Italiana per lo Studio del Fegato per l’uso razionale dei farmaci anti-HCV disponibili in Italia. [cited 11 Apr 2021]. Available from: https://www.webaisf.org/wp-content/uploads/2020/12/DOCUMENTO-HCV-14_12_20.pdf. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yin GQ S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL