Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3682
Peer-review started: January 24, 2021
First decision: February 23, 2021
Revised: March 8, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: June 28, 2021
Processing time: 151 Days and 14.6 Hours
With increasing rates of liver transplantation and a stagnant donor pool, the annual wait list removals have remained high. Living donor liver transplantation (LDLT) is an established modality in expanding the donor pool and is the primary method of liver donation in large parts of the world. Marginal living donors, including those with hepatic steatosis, have been used to expand the donor pool. However, due to negative effects of steatosis on graft and recipient outcomes, current practice excludes overweight or obese donors with more than 10% macro vesicular steatosis. This has limited a potentially important source to help expand the donor pool. Weight loss is known to improve or resolve steatosis and rapid weight loss with short-term interventions have been used to convert marginal donors to low-risk donors in a small series of studies. There is, however, a lack of a consensus driven standardized approach to such interventions.
To assess the available data on using weight loss interventions in potential living liver donors with steatotic livers and investigated the feasibility, efficacy, and safety of using such donors on the donor, graft and recipient outcomes. The principal objective was to assess if using such treated donor livers, could help expand the donor pool.
We performed a comprehensive literature review and meta-analysis on studies examining the role of short-term weight loss interventions in potential living liver donors with hepatic steatosis with the aim of increasing liver donation rates and improving donor, graft, and recipient outcomes.
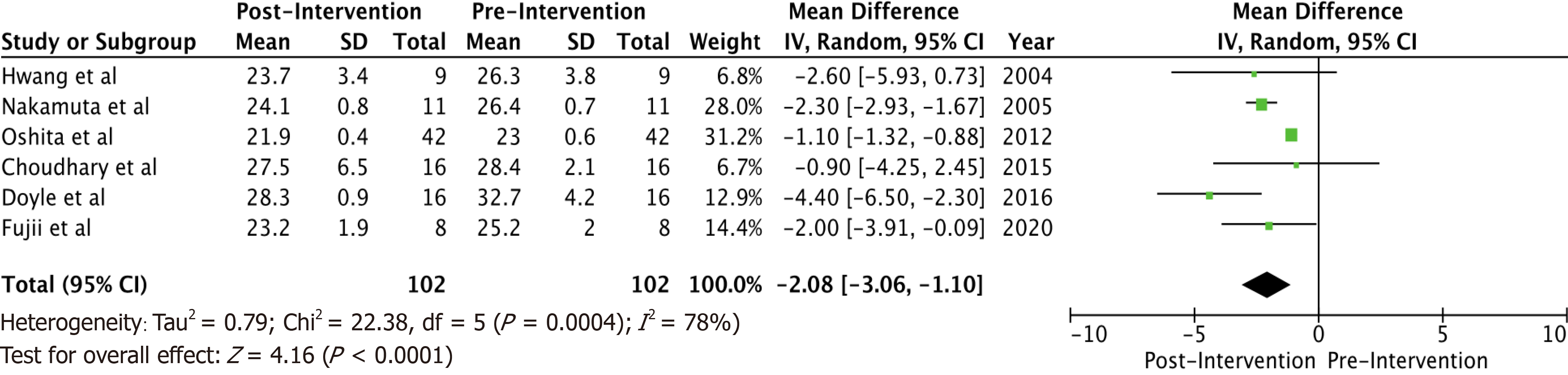
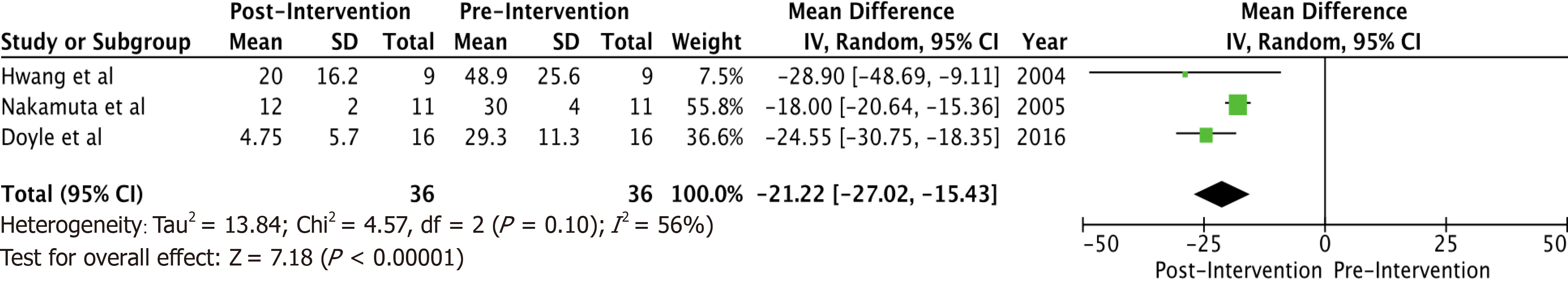
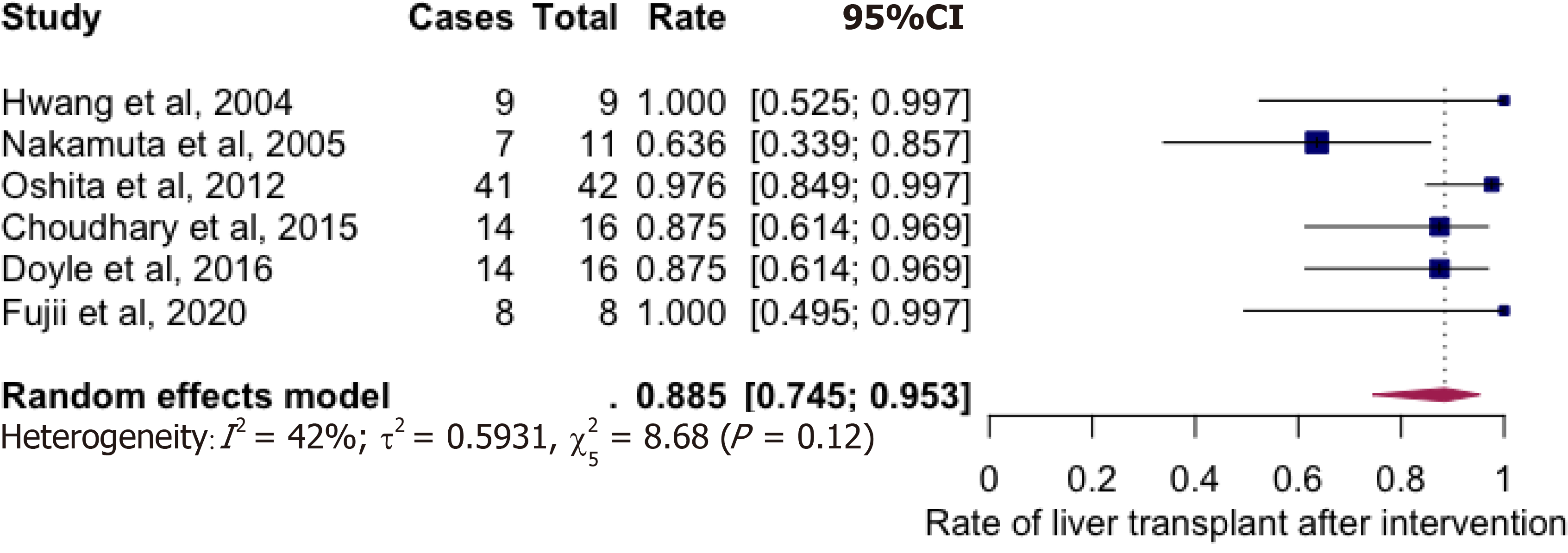
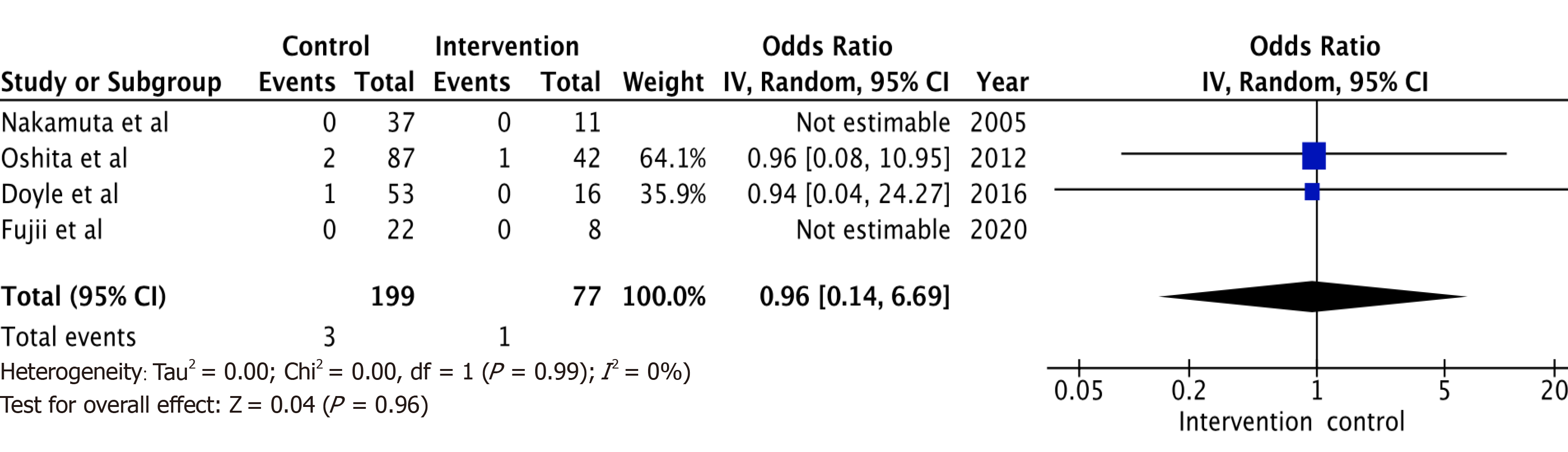
A total of 6 studies with 102 potential donors were included. Most subjects were males (71). All studies showed a significant reduction in body mass index post-intervention with a mean difference of -2.08 (-3.06, 1.10, I2 = 78%). A significant reduction or resolution of hepatic steatosis was seen in 93 of the 102 (91.2%). Comparison of pre- and post-intervention liver biopsies showed a significant reduction in steatosis with a mean difference of -21.22 (-27.02, -15.43, I2 = 56%). The liver donation rates post-intervention was 88.5 (74.5, 95.3, I2 = 42%). All donors who did not undergo LDLT had either recipient reasons or had fibrosis/steatohepatitis on post intervention biopsies. Post-operative biliary complications in the intervention group were not significantly different compared to controls with an odds ratio of 0.96 [(0.14, 6.69), I2 = 0]. The overall post-operative donor, graft, and recipient outcomes in treated donors were not significantly different compared to donors with no steatosis.
Use of appropriate short term weight loss interventions in living liver donors is an effective tool in turning marginal donors to low-risk donors and therefore in expanding the donor pool. It is feasible and safe, with comparable donor, graft, and recipient outcomes, to non-obese donors. Larger future prospective studies are needed.
Core Tip: Living donor liver transplantation is an established modality in expanding the donor pool but is limited by donor safety concerns and recipient and graft outcomes due to high prevalence of hepatic steatosis in obese or overweight donors. Weight loss is known to improve or resolve steatosis and help convert marginal donors to low-risk donors in a small series of studies. Our meta-analysis demonstrates that short term weight loss intervention, is feasible and safe in significantly reducing hepatic steatosis in living liver donors undergoing donor evaluation and has the potential to safely expand the donor pool.
- Citation: Trakroo S, Bhardwaj N, Garg R, Modaresi Esfeh J. Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: A systematic review and meta-analysis. World J Gastroenterol 2021; 27(24): 3682-3692
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3682.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3682
With the steady increase in liver transplantation (LT) over the last 2 decades, and the donor pool remaining largely stagnant, the shortage of organs for transplantation has become even more pressing. This has led to an increase in median time on the wait list for transplantation, especially in patients with a model for end-stage liver disease (MELD) greater than 15[1]. Consequently, as per United Network for Organ Sharing (UNOS) data, every year more than 1200 patients are being removed from the liver transplant wait list[1].
Living donor LT (LDLT) has the potential of increasing the donor pool and lowering the wait list mortality. LDLT offers recipients the advantage of a high-quality graft and the possibility of avoiding delisting, deconditioning over time, or death due to a change in clinical status. In addition, LDLT has the benefit of scheduling the transplantation as an elective surgery[2]. In parts of the world including South Korea, Japan, India, and Taiwan, LDLT is the primary modality of offering organs to patients in need for LT[3].
Although the number of LDLTs has steadily increased in the United States in the last few years, the total number of LDLTs has lagged in comparison to high volume centers in Asia. The number of deceased donor liver transplants in the United States in 2019 was 8372. The number of LDLTs during the same year was 524, a mere 6% of total liver transplants performed[4].
Most patients listed for liver transplant struggle to find a suitable living donor[5,6]. One way to address the shortage of donors is to use marginal living donors, including those with hepatic steatosis. The negative effects of such steatotic grafts on liver donation and transplantation are well known, including higher incidence of severe ischemic damage resulting in primary dysfunction or primary non function of graft, biliary strictures, and decreased one-year graft survival[7-9]. In a study by Gabrielli et al[10], recipients who received non-heart beating liver grafts with macrovesicular steatosis had significantly lower 3-year overall survival.
Compounding the problem of organ shortage is the dramatically rising rate of obesity around the world[11]. In the United States in 2012, 69% of the population was overweight [body mass index (BMI) > 25] and 35% was obese (BMI > 30)[12]. Obesity is a strong risk factor for hepatic steatosis and steatosis is seen on liver biopsy in 76% of potential living liver donors with a BMI of more than 28[13].
The aim of our study was to summarize the current evidence on the role of short-term interventions for weight loss, such as diet and medications, in obese or overweight potential living liver donors with steatotic livers. The objectives were to assess the effectiveness of these interventions in reducing donor BMI and liver steatosis, turning marginal donors to low-risk donors, and examining the impact of steatosis reduction or resolution on short-term donor and recipient morbidity, mortality, and graft outcomes.
We used PubMed as our primary electronic search database. Keywords used for search criteria included LDLT, living liver donors, diet therapy, fatty liver, steatosis, and short-term weight loss interventions. Studies were analyzed using the Population, Intervention, Comparison, and Outcomes methodology and all studies that met our eligibility criteria were included (Table 1).
| Study number | Ref. | n | Type of intervention | Treatment duration | BMI reduction | Steatosis reduction | Liver donation | Donor, graft, recipient outcomes |
| 1 | Fujii et al[15], 2020 | 8 | < 1600 Kcal/d + exercise 20 min x 3/wk ± statins | Median of 58 d | Yes (P = 0.0009) | Yes (P = 0.0006) | 8 | No significant difference from controls |
| 2 | Doyle et al[16], 2016 | 16 | Optifast VLCD: 1000 kcal/ d | Median of 7.3 wk | Yes (P < 0.001) | Yes (P < 0.001) | 14 (1 inadequate volume, 1 fibrosis) | No significant difference from controls |
| 3 | Choudhary et al[17], 2015 | 16 | 1200 kcal/d + 200 to 400 kcal/d exercise ± statins | Mean 28 ± 10 d | Yes (P = 0.006) | Yes (P = 0.008) | 14 (2 had NASH/fibrosis) | No reported complication in perioperative period |
| 4 | Oshita et al[18], 2012 | 42 | 800 to 1400 kcal/d diet + 100 to 400 kcal/d exercise | Median 2.9 mo | Yes (P < 0.0001) | Yes, to < 20 % | 41 (1 had stage 2 fibrosis) | No different from control group |
| 5 | Nakamuta et al[19], 2005 | 11 | 1000 kcal/d diet + exercise (600 kcal/d) + Bezafibrate | Mean 37.8 ± 4.6 d | Yes(P = 0.0033) | Yes(P= 0.0028) | 7 (2 recipient deaths, 1 inadequate GRWR) | No different from control group |
| 6 | Hwang et al[20], 2004 | 9 | Diet (25-30 calories x ideal body weight) + exercise | Median of 3 mo | Yes (P = 0.0001) | Yes (P = 0.006) | 9 | No different from control group |
Studies that investigated weight loss strategies for potential living liver donors were reviewed. Our eligibility criteria included adult (age > 18 years), overweight or obese potential living liver donors with biopsy proven and or radiologically assessed hepatic steatosis who underwent weight loss interventions and who had post intervention assessment of liver steatosis, with liver biopsy with or without radiologic modalities and post intervention assessment of weight loss. Studies should have also analyzed donor, recipient, and graft outcomes, including perioperative complications as graded by Clavien-Dindo classification[14], and donor and recipient morbidity and mortality. Six studies that met our criteria were finally included in our study.
Variables that were examined in each study included exclusion criteria, treatment modality, diagnostic modalities to assess for pre- and post-intervention hepatic steatosis (such as liver biopsies, computed tomography, or magnetic resonance imaging), pre- and post-intervention BMIs, total bilirubin, liver transaminases [aspartate transaminase, alanine amiotransferase (ALT)]. In addition, liver donation rates, donor and recipient perioperative complications (graded according to Clavien’s scale), and donor, graft and recipient outcomes were examined with each study.
We used meta-analysis techniques to calculate the pooled estimates following the methods suggested by DerSimonian and Laird using the random-effects model. Mean difference and odds ratio were calculated using random-effects model for continuous and binary variables, respectively. When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis. Heterogeneity was assessed between study-specific estimates by using Cochran Q statistical test for heterogeneity, and the I2statistics. I2values of < 30%, 30%-60%, 61%-75%, and > 75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively. A P value of ≥ 0.05 was used ‘a-priori’ to define statistical significance. The analysis was done using RStudio and RevMan software.
Six studies were included following our literature search. Data and results from these studies are outlined in Table 1. The largest study had a sample size of 42 patients and the smallest study, had a sample size of 8.
Some inferences can be made based on the data analyzed. Most subjects (71) were males. There were no reported dropouts from the treated group due to adverse events. All studies showed a significant reduction in BMI post-interventions (Figure 1) with a mean difference of -2.08 (-3.06, 1.10, I2 = 78%). All six studies showed a significant reduction (P < 0.05) in steatosis (Table 1). A significant reduction or resolution of hepatic steatosis was seen in 93 of the 102 in the intervention group (91.2%). Three of the 6 included studies had both pre- and post-intervention liver biopsies, and these studies were included in the Forest plot (Figure 2) to allow for a standardized comparison of intervention outcomes; they showed a significant reduction in steatosis with a mean difference of -21.22 (-27.02, -15.43, I2 = 56%).
Majority of donors who underwent weight loss interventions successfully underwent living liver donation (Figure 3) with the rate being at 88.5% (74.5-95.3%, I2 = 42%). Prospective donors, who did not undergo donor hepatectomy, were either waiting to donate at study conclusion or had either steatohepatitis (2 in Choudhary group, 1 in Doyle group), inadequate Graft weight/recipient weight ratios (GRWR) (1 in Nakamuta group, 1 in Doyle group) or recipient causes for not donating (2 recipient deaths in the Nakamuta group).
Post-operative biliary complications (Figure 4) in the intervention group were not significantly different compared to control (non-intervention) donors with odds ratio of 0.96 [(0.14, 6.69), I2 = 0]. The overall post-operative donor, graft, and recipient outcomes in the diet treated donors were also not significantly different when compared to non-diet treated donors. All studies were limited by their small sample size, and some by their retrospective study design.
With increasing rates of LT and a stagnant donor pool, the annual wait list removals have remained high. The use of extended criteria donors including those with steatotic livers to expand the donor pool is a viable option in expanding the donor pool. The data on donor, graft and recipient outcomes in grafts used for potential donors with steatotic livers who have undergone weight loss interventions, is however sparse. Compounding this issue is the increasing rates of obesity has made donor safety and successful recipient outcomes, an even greater challenge.
We, therefore, analyzed current literature on the role of short-term dietary interventions in preparing potential donors with hepatic steatosis for LDLT with the aim of safely and effectively expanding the donor pool and improving donor and recipient outcomes. Studies included, have been summarized in Table 1.
In study number 1 by Fujii et al[15], 8 potential donors were examined from October 2009 to August 2015. Exclusion criteria were age greater than 65 and steatohepatitis. Steatosis was diagnosed based on a liver to spleen (L/S) ratio of < 1.1 and/or hepatic attenuation of < 55 Hounsfield units (HU) on non-enhanced CT. Donors without fatty liver (n = 21) during the study period, were selected as a control group. Treatment efficacy was serially evaluated and when L/S was ≥ 1.1, and hepatic attenuation was ≥ 55 HU, a liver biopsy was performed. When macrovesicular steatosis of < 10% was confirmed, donors were taken up for partial hepatectomy. A significant reduction in mean BMI (25 ± 2.0 to 23.2 ± 1.9, P = 0.0009) and L/S ratio [0.95 (0.62-1.06) to 1.2 (1.12-1.46), P = 0.003] were seen. All 8 in the study group showed < 10% steatosis on intra-operative biopsy and underwent partial donor hepatectomy. No major complications (Clavien grade IIIa or greater) were seen. No significant difference in graft function were observed between the 2 groups with 100% Graft and patient survival at 3 mo. They concluded that preoperative treatment for fatty liver was effective and treated potential donors can undergo LDLT without jeopardizing donor safety.
Study number 2 from University of Toronto by Doyle et al[16], retrospectively analyzed 16 potential donors from September 2011 to December 2014. Subjects were followed until September 2015. Potential donors with nonalcoholic steatohepatitis (NASH) were excluded and those with steatosis of > 10% who underwent treatment with Optifast, were included. Baseline pre-treatment liver biopsies were performed in the first 8 but after observing promising preliminary results, the authors proceeded directly to dietary intervention in the remaining 8, based on imaging. All underwent liver biopsies at treatment completion. A targeted BMI reduction of 10%, guided treatment duration. The control group (n = 53) included all non-Optifast donors had intraoperative liver biopsy showing < 10% macrovesicular steatosis, as part of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) consortium. The pre-intervention mean BMI of Optifast donors was 32.7 kg/m2 [95% confidence interval (CI): 30.5-34.9 kg/m2] and was higher than non-Optifast donors at 26.4 kg/m2 (95%CI: 25.4-27.4 kg/m2; P < 0.001).
Optifast was well tolerated and all 16 completed intervention. The mean BMI decreased to 28.3 kg/m2 (95%CI: 26.3-30.2 kg/m2; P < 0.001). All post-intervention biopsies demonstrated ≤ 10% macrovesicular steatosis with mean steatosis reduction from 29.3% to 4.75% (P < 0.001). Fourteen underwent partial hepatectomy with no reported donor mortality and no significant difference in surgical complications (P = 0.11), Clavien scores (P = 0.28), or mean length of stay (P = 0.82) between recipients of both groups. The authors concluded that Optifast can potentially eliminate or significantly reduce steatosis in donors being evaluated for LDLT, with donor and recipient outcomes equivalent to outcomes in non-steatotic donors.
Study number 3 by Choudhary et al[17], from July 2010 to January 2015, prospec
The mean weight loss was 7 ± 4.3 kg with a significant post-intervention BMI reduction (P = 0.006) and improvement in LAI (P = 0.008). A median decrease in steatosis from 15% to 5% was seen in fifteen, including normalization in 7. Two donors had steatohepatitis, steatosis > 20% with borderline liver remnant and did not undergo liver donation. Fourteen underwent liver donation with all donors and their recipients having an uneventful post-operative course. The authors concluded that, in motivated younger liver donors with no comorbidities, steatosis is reversible in a short duration by aggressive lifestyle modifications.
Study number 4 by Oshita et al[18] compared outcomes of diet treated (n = 42) to non-diet treated donors (n = 87), from April 2003 to March 2010. Steatosis was assessed by pre-intervention L/S ratio and post-intervention biopsy. Pre-intervention exclusion criteria were diabetes mellitus and L/S ratio of ≥ 1.2. Post-intervention exclusion criteria were macrovesicular steatosis of > 20%.
BMI was reduced from 23.3 ± 0.6 to 21.9 ± 0.4 kg/m2 (P < 0.0001). ALT, γ-GTP, and total cholesterol showed significant improvements (P = 0.0128, 0.0016, and 0.0004, respectively). Forty in the intervention group had stage 0/1 fibrosis with ≤ 20% steatosis and one had stage 2 fibrosis. One had inflammation and did not undergo liver donation. Forty-one treated donors underwent LDLT with no significant differences in perioperative lab data and complications (Clavien grading), including recipient biliary complications compared to controls. Overall, 1-, 3-, and 5-year recipient survival were not significantly different between the study and control groups (P = 0.455). The authors concluded that with appropriate selection criteria, use of diet-treated donors is feasible and safe with respect to donor and recipient outcomes.
Study number 5 by Nakamuta et al[19], tested short-term weight loss interventions on 11 potential donors with ≤ 30% combined microvesicular and macrovesicular steatosis. All had pre- and post-intervention liver biopsies. The control group included 37 donors without hepatic steatosis. The study was conducted from May 2003 to July 2004.
A significant reduction in steatosis (30% ± 4% to 12% ± 2%, P = 0.0028) and BMI (26.4 ± 0.7 kg/m2 to 24.1 ± 0.8 kg/m2, P = 0.0033) was seen. All had post-intervention normalization of liver enzymes, total cholesterol, and triglycerides. Seven underwent LDLT and one at study conclusion was waiting for donation. No adverse postoperative events were observed in study group donors or recipients with no difference in graft function. The authors concluded that short-term interventions are effective in reducing steatosis and can contribute to a safer LDLT.
In study number 6 by Hwang et al[20], from January 2001 to December 2002, 9 potential liver donors were examined. Exclusion criteria were a combined macro- and microvesicular steatosis of > 30% and or alcohol intake > 40 gm/wk. All underwent pre- and post-intervention liver biopsies and CT assessment of steatosis. In addition, all in the study group had intra-operative liver biopsies. All except one potential donor had pre-intervention elevation in LFTs. All nine in the intervention group showed a significant reduction in BMI (25.3 ± 3.8 to 23.7 ± 3.4, P = 0.0001) and in steatosis (48.9% ± 25.6% to 20.0% ± 16.2%, P = 0.006). All nine underwent donor hepatectomy with an uneventful post-operative course recovered and all recipients survived at 15 mo post-transplantation (study completion). They concluded that short-term weight loss in donors reduces steatosis and can contribute to expanding the donor pool.
Prior research has shown that hepatic steatosis adversely affects donor and recipient outcomes in LT and increases the likelihood of graft damage[21,22]. Marsman et al[23] reported that transplantation of livers with up to 30% steatosis resulted in a decreased 4-mo graft survival and 2-year patient survival rate. These findings, along with several other studies showing adverse outcomes with steatotic grafts[7-9], has led to the current practice of excluding potential overweight or obese donors with more than 10% macro vesicular steatosis[24]. In an analysis of the A2ALL database, only 15% of all living donors had a BMI of 30 or more[25]. As per UNOS database, in 2019, of the 874 donor livers discarded, 650 (74%) were in donor BMIs of 25 or more[1].
A few studies have used overweight or obese donors. Knaak et al[26], showed that donors with BMI of > 30 but < 35, had equivalent outcomes to non-obese donors. However, all potential donors with > 10% hepatic steatosis were excluded from their study. Also, certain donor characteristics separated them from other LDLT programs, including the use of Graft with higher GRWR in the obese donor group (mean of 1.42 ± 0.44%), a number much higher than the standard practice of using a GRWR cutoff of ≥ 0.8% and the greater use of male donors who tend to have larger liver volumes.
To avoid graft size mismatch, preoperative donor liver volumetry is done using the standardized GRWR. The donor graft weight is derived from CT volumetric assessment of the proposed graft to be harvested and the recipient's required standard liver volume (SLV) is calculated from the recipient's body weight[27]. GRWR is then expressed as the ratio of graft volume (expressed in kg) to the recipient's SLV calculated from the recipient's weight. Calculating GRWR is important in preventing overestimation of the donor’s standard liver volume (that can result in excessive hepatic resection and consequent liver failure) and in preventing underestimation of the recipient's standard liver volume that could lead to small-for-size syndrome. The generally accepted GRWR threshold is 0.8%. Some authors have proposed the lowering of threshold to between 0.6 to 0.8% under specific circumstances including donor age < 45 years, MELD score < 20, no graft steatosis and specific anatomic graft requirements. In such highly select cases, using a lower GRWR threshold in combination with grafts with no steatosis could lead to safe expansion of the donor pool with additional decrease in donor morbidity by preferentially selecting left lobe over right lobe grafts.
Calorie restriction, weight loss, and exercise are still recommended as the initial treatment for fatty liver. In a recent randomized control trial using paired biopsies of 261 patients with NASH who underwent dietary and lifestyle changes for a duration of 52 wk, 72 (25%) achieved resolution of steatohepatitis, 138 (47%) had reductions in NAFLD activity score (NAS) and 56 (19%) had regression of fibrosis[28]. The degree of weight loss correlated independently with all NASH histology. In those who achieved 10% or more weight loss, 90% had resolution of NASH and 45% had regression of fibrosis[28].
As our analysis has shown, there is promising data regarding short term interventions in decreasing or eliminating macro-vesicular steatosis, turning marginal steatotic donors to low-risk donors, and in positively impacting donor and recipient outcomes. All six studies included in this review (Table 1), however, are limited by their small sample size. All studies except one[15], used liver biopsy to quantify steatosis pre-donor hepatectomy and used non-invasive modalities for fat estimation, as an adjunct. Only one of the included studies is in Western population[16] making it difficult to extrapolate findings to our patient population. In addition, study inclusion and exclusion criteria were varied and so were the interventions. Despite these variabilities, most in the study groups tolerated the interventions well, and showed no increase in donor, graft or recipient morbidity or mortality as compared to non-diet treated donors.
Overall, the combination of short-term dietary intervention with low calorie diet (most studies had < 1200 kcal/d) for a duration ranging from 4 to 12 wk with exercise, and/or pharmacotherapy, was safe, well tolerated, and showed good treatment adherence. These interventions were effective in significantly reducing donor BMI with a pooled weighted difference of -1.6 (-4.4 to -1.1, CI of 0.95) and significantly reduced liver steatosis, leading to successful liver donation (88.5%) in the diet treated group. With respect to complications, diet-treated donors did extremely well, with only one donor in the Oshita group having Clavien grade III biliary stenosis. Outcomes of recipients who received grafts from diet-treated donor were not significantly different from recipients of grafts from non-diet treated donor. Grafts from diet treated donors functioned similarly to grafts from donors without obesity. The use of diet-treated donors is feasible with respect to safety of the donor and the outcome of the recipient in LDLT when strict selection criteria are used.
Short term dietary interventions, in conjunction with exercise and pharmacotherapy, is feasible and safe with good donor adherence. Our study has shown that such interventions significantly reduce and, in some help resolve hepatic steatosis in potential donors undergoing evaluation for LDLT. We conclude that, carefully selected steatotic diet treated living liver donors have equivalent donor, graft and recipient outcomes compared to those receiving grafts from non steatotic donors. It therefore has the potential to safely expand the donor pool and consequently, decrease the number of wait list removals.
The rates of liver transplantation having increasing but the donor pool has largely remained stagnant leading to high removals from liver transplant waitlists. Living donor liver transplantation (LDLT) using fatty liver could potentially be used to expand the donor pool. However, due to negative effects of steatosis on Graft and recipient outcomes, current practice is to exclude overweight or obese donors with steatosis livers. Data on feasibility, efficacy, and safety of using weight loss interventions marginal donors to low-risk donors is lacking. The aim of the study was to evaluate the feasibility safety and efficacy of short-term weight loss interventions in converting marginal living liver donors to low-risk donors.
Data on safety, efficacy and donor, graft and recipient outcomes when using short term weight loss interventions to convert marginal steatotic liver grafts in LDLT, to low-risk grafts, is lacking. With continuing shortage of organs for transplantation, we looked into the safety and efficacy of using treated steatotic donors, for LDLT.
We did a meta-analysis on the feasibility, safety, and efficacy of weight loss interventions in converting marginal living liver donors to low-risk donors and analyzed the perioperative donor, graft and recipient outcomes.
We performed a systematic review and meta-analysis on studies examining the role of short-term weight loss interventions in potential living liver donors with hepatic steatosis with the aim of increasing liver donation rates and improving donor, graft, and recipient outcomes.
A total of 6 studies with 102 potential donors were included. Most subjects were males (n = 71). All studies showed a significant reduction in body mass index post-intervention with a mean difference of -2.08 (-3.06, 1.10, I2 = 78%). A significant reduction or resolution of hepatic steatosis was seen in 93 of the 102 (91.2%). Comparison of pre- and post-intervention liver biopsies showed a significant reduction in steatosis with a mean difference of -21.22 (-27.02, -15.43, I2 = 56%). The liver donation rates post-intervention was 88.5 (74.5, 95.3, I2 = 42%). All donors who did not undergo LDLT had either recipient reasons or had fibrosis/steatohepatitis on post intervention biopsies. Post-operative biliary complications in the intervention group were not significantly different compared to controls with an odds ratio of 0.96 [(0.14, 6.69), I2 = 0]. The overall post-operative donor, graft, and recipient outcomes in treated donors were not significantly different compared to donors with no steatosis.
Our study has shown that using liver grafts from potential living liver donors with hepatic steatosis undergoing short term weight loss interventions, have comparable donor, graft, and recipient outcomes, to donors with no hepatic steatosis.
Use of appropriate short term weight loss interventions in living liver donors is a feasible, safe, and effective tool in turning marginal donors with liver steatosis to low-risk donors and therefore can help in expanding the donor pool.
| 1. | U S. Department of Health and Human Services. Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data; 2020. Database: Organ Procurement and Transplantation Network [Internet]. [cited 3 February 2020]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. |
| 2. | Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, Ahn CS, Moon DB, Hwang GS, Kim KM, Ha TY, Kim DS, Jung JP, Song GW. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | International Registry in Organ Donation and Transplantation. Donation Activity Charts; 2019. Database: IRODaT – DTI Foundation [Internet]. [cited 3 February 2020]. Available from: https://www.irodat.org/?p=database. |
| 4. | Moriarty S. The Most Active Living Donor Liver Transplant Programs. UNOS. [cited 3 February 2020]. Available from: https://unos.org/news/improvement/most-active-living-donor-liver-transplant-programs/. |
| 5. | Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 329] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Brown RS Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor Hepatic Steatosis and Outcome After Liver Transplantation: a Systematic Review. J Gastrointest Surg. 2015;19:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Hisatake G, Yang L. Liver-specific deceased donor risk indices. Hepatol Res. 2014;44:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gabrielli M, Moisan F, Vidal M, Duarte I, Jiménez M, Izquierdo G, Domínguez P, Méndez J, Soza A, Benitez C, Pérez R, Arrese M, Guerra J, Jarufe N, Martínez J. Steatotic livers. Can we use them in OLTX? Ann Hepatol. 2012;11:891-898. [PubMed] |
| 11. | Robertson C, Archibald D, Avenell A, Douglas F, Hoddinott P, van Teijlingen E, Boyers D, Stewart F, Boachie C, Fioratou E, Wilkins D, Street T, Carroll P, Fowler C. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess. 2014;18:v-vi, xxiii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6227] [Cited by in RCA: 5962] [Article Influence: 496.8] [Reference Citation Analysis (1)] |
| 13. | Rinella ME, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M, Superina R, Flamm SL, Blei AT. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl. 2001;7:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9238] [Article Influence: 543.4] [Reference Citation Analysis (1)] |
| 15. | Fujii Y, Kawamura N, Zaitsu M, Watanabe M, Goto R, Kamiyama T, Taketomi A, Shimamura T. Outcome of Living-Donor Liver Transplantation Using Grafts from Donors Treated for Fatty Liver. Ann Transplant. 2020;25:e920677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Doyle A, Adeyi O, Khalili K, Fischer S, Dib M, Goldaracena N, Dillon J, Grant D, Cattral M, McGilvray I, Greig P, Ghanekar A, Lilly L, Renner E, Levy G, Selzner N. Treatment with Optifast reduces hepatic steatosis and increases candidacy rates for living donor liver transplantation. Liver Transpl. 2016;22:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Choudhary NS, Saraf N, Saigal S, Gautam D, Lipi L, Rastogi A, Goja S, Menon PB, Bhangui P, Ramchandra SK, Soin AS. Rapid Reversal of Liver Steatosis With Life Style Modification in Highly Motivated Liver Donors. J Clin Exp Hepatol. 2015;5:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Oshita A, Tashiro H, Amano H, Kobayashi T, Onoe T, Ide K, Takaki S, Takahashi S, Arihiro K, Chayama K, Ohdan H. Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation. Transplantation. 2012;93:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, Yoshimitsu K, Enjoji M, Kotoh K, Taketomi A, Uchiyama H, Shimada M, Nawata H, Maehara Y. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Hwang S, Lee SG, Jang SJ, Cho SH, Kim KH, Ahn CS, Moon DB, Ha TY. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. 2004;10:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Seifalian AM, Chidambaram V, Rolles K, Davidson BR. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg. 1998;4:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, Gores GJ, Krom RA. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 240] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Levy GA, Selzner N, Grant DR. Fostering liver living donor liver transplantation. Curr Opin Organ Transplant. 2016;21:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Trotter JF, Wisniewski KA, Terrault NA, Everhart JE, Kinkhabwala M, Weinrieb RM, Fair JH, Fisher RA, Koffron AJ, Saab S, Merion RM; A2ALL Study Group. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. 2007;46:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Knaak M, Goldaracena N, Doyle A, Cattral MS, Greig PD, Lilly L, McGilvray ID, Levy GA, Ghanekar A, Renner EL, Grant DR, Selzner M, Selzner N. Donor BMI >30 Is Not a Contraindication for Live Liver Donation. Am J Transplant. 2017;17:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 735] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 28. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015; 149: 367-378. quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1786] [Article Influence: 162.4] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Gendy HA, Joven J, Xu ZL S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX