Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3630
Peer-review started: February 5, 2021
First decision: March 6, 2021
Revised: March 20, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: June 28, 2021
Processing time: 139 Days and 21.6 Hours
Liver transplantation (LT) presents a curative treatment option in patients with early stage hepatocellular carcinoma (HCC) who are not eligible for resection or ablation therapy. Due to a risk of up 30% for waitlist drop-out upon tumor progression, bridging therapies are used to halt tumor growth. Transarterial chemoembolization (TACE) and less commonly stereotactic body radiation therapy (SBRT) or a combination of TACE and SBRT, are used as bridging therapies in LT. However, it remains unclear if one of those treatment options is superior. The analysis of explant livers after transplantation provides the unique opportunity to investigate treatment response by histopathology.
To analyze histopathological response to a combination of TACE and SBRT in HCC in comparison to TACE or SBRT alone.
In this multicenter retrospective study, 27 patients who received liver transplantation for HCC were analyzed. Patients received either TACE or SBRT alone, or a combination of TACE and SBRT as bridging therapy to liver transplantation. Liver explants of all patients who received at least one TACE and/or SBRT were analyzed for the presence of residual vital tumor tissue by histopathology to assess differences in treatment response to bridging therapies. Statistical analysis was performed using Fisher-Freeman-Halton exact test, Kruskal-Wallis and Mann-Whitney-U tests.
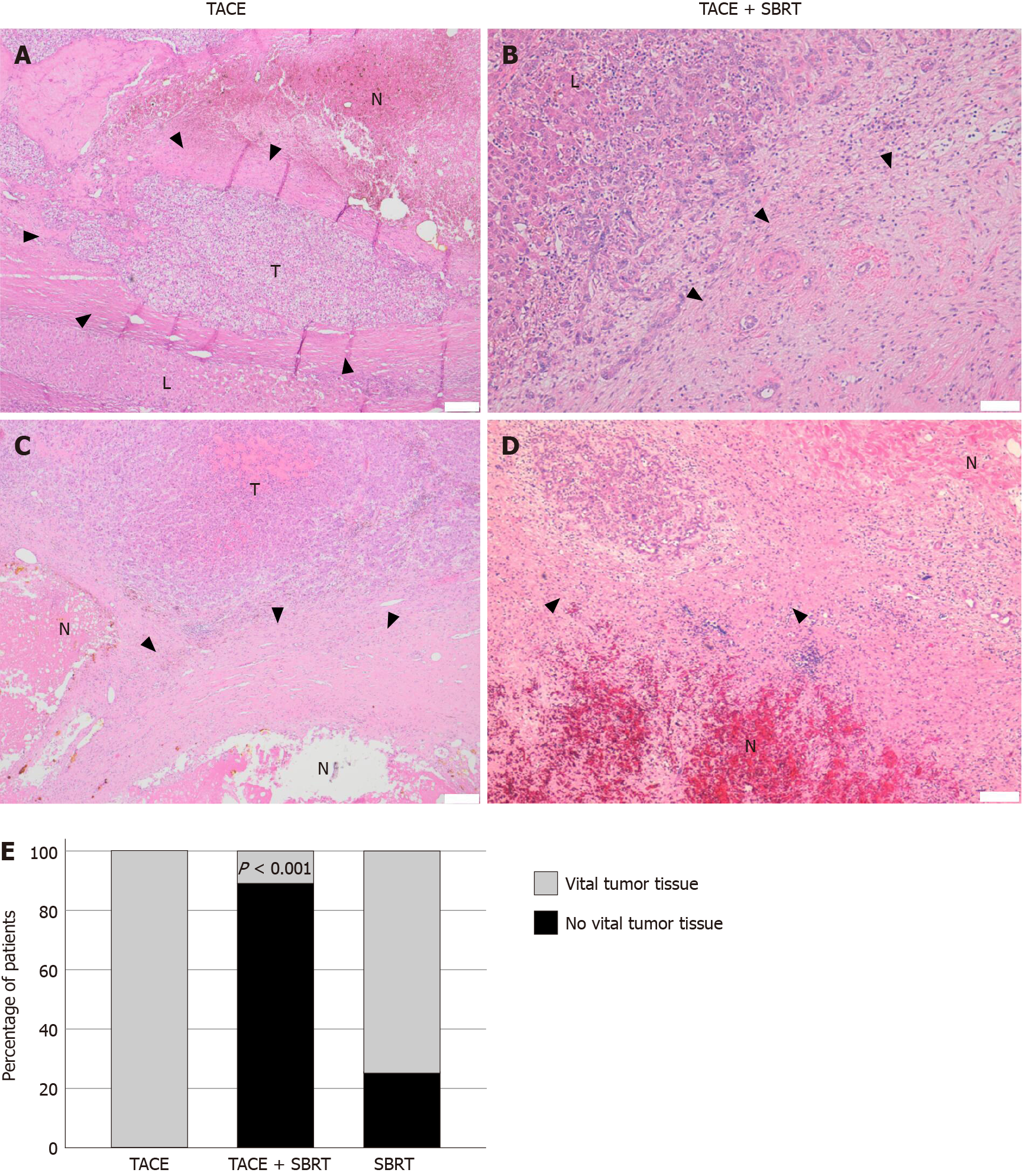
Fourteen patients received TACE only, four patients SBRT only, and nine patients a combination therapy of TACE and SBRT. There were no significant differences between groups regarding age, sex, etiology of underlying liver disease or number and size of tumor lesions. Strikingly, analysis of liver explants revealed that almost all patients in the TACE and SBRT combination group (8/9, 89%) showed no residual vital tumor tissue by histopathology, whereas TACE or SBRT alone resulted in significantly lower rates of complete histopathological response (0/14, 0% and 1/4, 25%, respectively, P value < 0.001).
Our data suggests that a combination of TACE and SBRT increases the rate of complete histopathological response compared to TACE or SBRT alone in bridging to liver transplantation.
Core Tip: In patients with early-stage hepatocellular carcinoma (HCC) who are not eligible for resection or ablation, liver transplantation presents a curative treatment option. To halt tumor growth during waiting time, bridging therapies such as transarterial chemoembolization (TACE), ablation, and stereotactic body radiation therapy (SBRT) are used prior to liver transplantation. In a multicenter retrospective trial with 27 HCC patients who received either TACE or SBRT alone, or a combination of TACE and SBRT, explant histopathology was analyzed to assess treatment response. Strikingly, almost all patients in the combination group exhibited no residual vital tumor by histopathology, whereas TACE or SBRT alone resulted in significantly lower rates of complete histopathological response.
- Citation: Bauer U, Gerum S, Roeder F, Münch S, Combs SE, Philipp AB, De Toni EN, Kirstein MM, Vogel A, Mogler C, Haller B, Neumann J, Braren RF, Makowski MR, Paprottka P, Guba M, Geisler F, Schmid RM, Umgelter A, Ehmer U. High rate of complete histopathological response in hepatocellular carcinoma patients after combined transarterial chemoembolization and stereotactic body radiation therapy. World J Gastroenterol 2021; 27(24): 3630-3642
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3630
Hepatocellular carcinoma (HCC) ranks among the leading causes of cancer-associated deaths worldwide. In very early [1 tumor < 2 cm, Barcelona clinic liver cancer (BCLC) 0, United Network for Organ Sharing (UNOS) T1] and early (1 tumor 2-5 cm or 2-3 tumors ≤ 3 cm, BCLC A, UNOS T2) stage HCC, surgical resection or local ablation is the treatment of choice. However, accompanying cirrhosis and tumor location often preclude these curative treatment approaches. Additionally, recurrence rates after resection of early HCC (BCLC A) are high with up to 70% after 2 years[1]. In contrast, liver transplantation (LT) is a curative treatment option not only for the tumor but also for the underlying precancerous condition (i.e. liver cirrhosis, chronic HBV infection, non-alcoholic fatty liver disease) with excellent 5-year survival (65%-78%) and low recurrence rates (11%-18%) if Milan criteria (MC, 1 tumor ≤ 5 cm or 3 tumors ≤ 3 cm, without vascular invasion) are fulfilled[2,3]. Acceptable outcomes after LT are even achieved in patients outside MC, though 5-year survival rates are noticeably lower (46%-60%), depending on size and number of tumor lesions, alpha-fetoprotein (AFP), and treatment response[2,4,5]. Therefore, MC are widely accepted to identify HCC patients who will benefit from LT.
Currently about 30%-35% of patients on the waiting list for LT in Europe suffer from HCC[3]. In the US, the percentage is lower, but has been steadily rising over the recent years[6]. Due to organ shortage, waiting periods are long with a high risk for tumor progression and therefore drop-out from the waiting list. Without any bridging therapy, tumor progression beyond MC has been reported in up to 30% of cases[7]. To avoid tumor progression, locoregional therapies are used as bridging to LT and several countries have now implemented response to locoregional therapies into their transplant allocation systems[8,9].
The most commonly used locoregional bridging therapies are transarterial chemoembolization (TACE) and thermal ablation, such as radio frequency ablation (RFA) and microwave ablation (MWA). These therapies are also recommended in current guidelines for the treatment of BCLC A or B stage HCC[3,9]. However, in patients with tumors not suitable for these standard treatment modalities, individual treatment approached such as 90Y radioembolization or stereotactic body radiation therapy (SBRT) have been used to control tumor growth[10-12].
RFA or MWA are established in the treatment of early and very early stage HCC (BCLC A and 0, respectively) as a curative approach or as bridging with excellent long term outcomes after LT[13-16]. However, thermal ablation is not technically feasible in all patients. Tumor location can preclude safe and successful treatment, for example in subcapsular HCC close to the diaphragm or in lesions close to the liver hilum[17]. If these lesions are not amenable for resection, TACE and less commonly alternative treatment options such as SBRT or radioembolization are used to achieve tumor control in a pre-transplant setting[12].
TACE, which also presents the standard of care in patients with intermediate stage HCC, is a widely used bridging therapy that can efficiently halt tumor growth in the pre-transplant setting[2,3,9,18-21]. Even in patients initially outside MC, who achieve down-staging to fulfill MC after TACE, overall survival rates are comparable to patients that were never outside MC [5,22]. On the other hand, insufficient response to TACE is a predictor of post-transplant HCC recurrence[20]. With longer waiting times due to organ shortage, the risk of tumor progression after treatment with TACE remains a substantial concern.
SBRT is a local ablative treatment option for patients not suitable for resection or thermal ablation. In particular, SBRT can also be applied to tumors close to large blood vessels or wherever tumor location precludes RFA or MWA[17]. Even with excellent local control of tumor lesions and a good safety profile, current guidelines do not regard SBRT as primary treatment option due to a lack of large randomized trials[23-26]. SBRT is therefore mainly performed as an individualized treatment approach in selected cases. In a pre-transplant setting, complete histopathological response after SBRT in 3 of 11 tumor lesions (27%) in a small cohort of 10 patients has been reported[27]. In a larger retrospective analysis of 30 patients treated with SBRT prior to transplantation, drop-out rate (16.7%) and 5-year survival (61%) were not different from patients treated with TACE or RFA[10].
A combination of TACE and SBRT is an alternative local treatment option with therapeutic benefits and a good safety profile, though data from large randomized controlled trials is still missing[28,29]. A recent retrospective analysis of SBRT and TACE (n = 49) compared to TACE alone (n = 98) showed significantly better disease control, progression free survival, and 3-year overall survival for the TACE and SBRT combination group[30]. In a larger study of 199 patients with tumors ≤ 5 cm, combination therapy lead to improved local control rates, but did not have any effect on overall survival[31]. To date, TACE and SBRT combination therapy has been mainly used as a palliative treatment approach and only rarely as bridging to LT. Therefore, data on histopathologic response is limited with only one study reporting on two tumors which showed near complete tumor response after treatment[12]. Three ongoing prospective trials are currently recruiting patients to evaluate TACE and SBRT combination therapy in comparison to TACE (NCT01918683; NCT02513199; NCT03895359).
Given the promising results achieved with TACE and SBRT combination therapy, we aimed to analyze treatment response prior to liver transplantation in patients within MC who could not be treated with resection or ablation and were treated with TACE and SBRT.
This multi-center retrospective trial was conducted to specify treatment options that may improve prognosis in patients with HCC and possible LT. Three German transplant centers, University Hospital rechts der Isar, Munich, University Hospital of Munich, and Hannover Medical School participated in the study. Protocols for patient analysis were reviewed and approved by the local ethics committee of each participating center. Decisions for tumor treatment were discussed in a multidisciplinary tumor board. Patients received treatment as standard of care and data were collected retrospectively.
For this study, medical records of all patients with liver cirrhosis and HCC within MC who underwent LT between 2007 and 2019 were retrospectively reviewed. From Hannover Medical School, only patients who received TACE and SBRT or SBRT alone prior to LT were screened. Patients who received at least one TACE with or without SBRT (TACE only: 8 patients at University Hospital rechts der Isar, Munich; 6 patients at University Hospital of Munich; TACE + SBRT: 4 patients at University Hospital rechts der Isar, Munich; 5 patients at University Hospital of Munich), or at least one SBRT alone (2 patients at University Hospital of Munich; 2 patients at Hannover Medical School), were included in our study. Patients who received additional tumor therapies as bridging such as resection of individual lesions, RFA, or MWA were not included into our study since these therapies are established as a curative treatment option. Additionally, data showing excellent response by radiology and histo
Observation period started with initial diagnosis through December 2019. To compare the different dose and fractionation regimens used for SBRT, the biological equivalent dose (BED) of the surrounding isodose was calculated according to the formula BED = nd (1+ d/alphabeta) (with n: Number of fractions, d: Single dose and alpha/beta set to 10).
Number and size of HCCs were documented by magnetic resonance imaging or computed tomography scan at the time of diagnosis. Number of treatment cycles, time of treatment, and radiation dose were assessed when applicable. Additionally, age, sex, cause of liver cirrhosis (alcohol, chronic viral hepatitis, other) and serum AFP levels were analyzed. After transplantation, the presence of vital tumor tissue in explant livers was analyzed. Specifically, size and number of any remaining tumor nodules were determined macroscopically and by histopathology in order to identify differences in tumor response. The absence of vital tumor tissue was considered as complete response.
The study was designed as a retrospective multicenter longitudinal survey. All data were analyzed using Microsoft Excel (version 16) and SPSS (version 25). Statistics were performed using Fisher-Freeman-Halton test. Due to the limited sample size, no multivariate analysis was performed. Kruskal-Wallis tests as well as Mann-Whitney-U tests were used for comparisons of variables between groups, when appropriate. All statistical tests were performed two-sided using a significance level of α = 5%.
The study cohort comprised 27 subjects with HCC of whom 14 received TACE only, four SBRT only, and nine a combination of TACE and SBRT. Within the study cohort, 20 (74%) patients were male, 7 (26%) female. Mean patient age was 60 (SD ± 6) years ranging from 48 to 71 years. All patients suffered from cirrhosis, mostly due to alcohol (11/27; 40%) or hepatitis C (10/27; 37%).
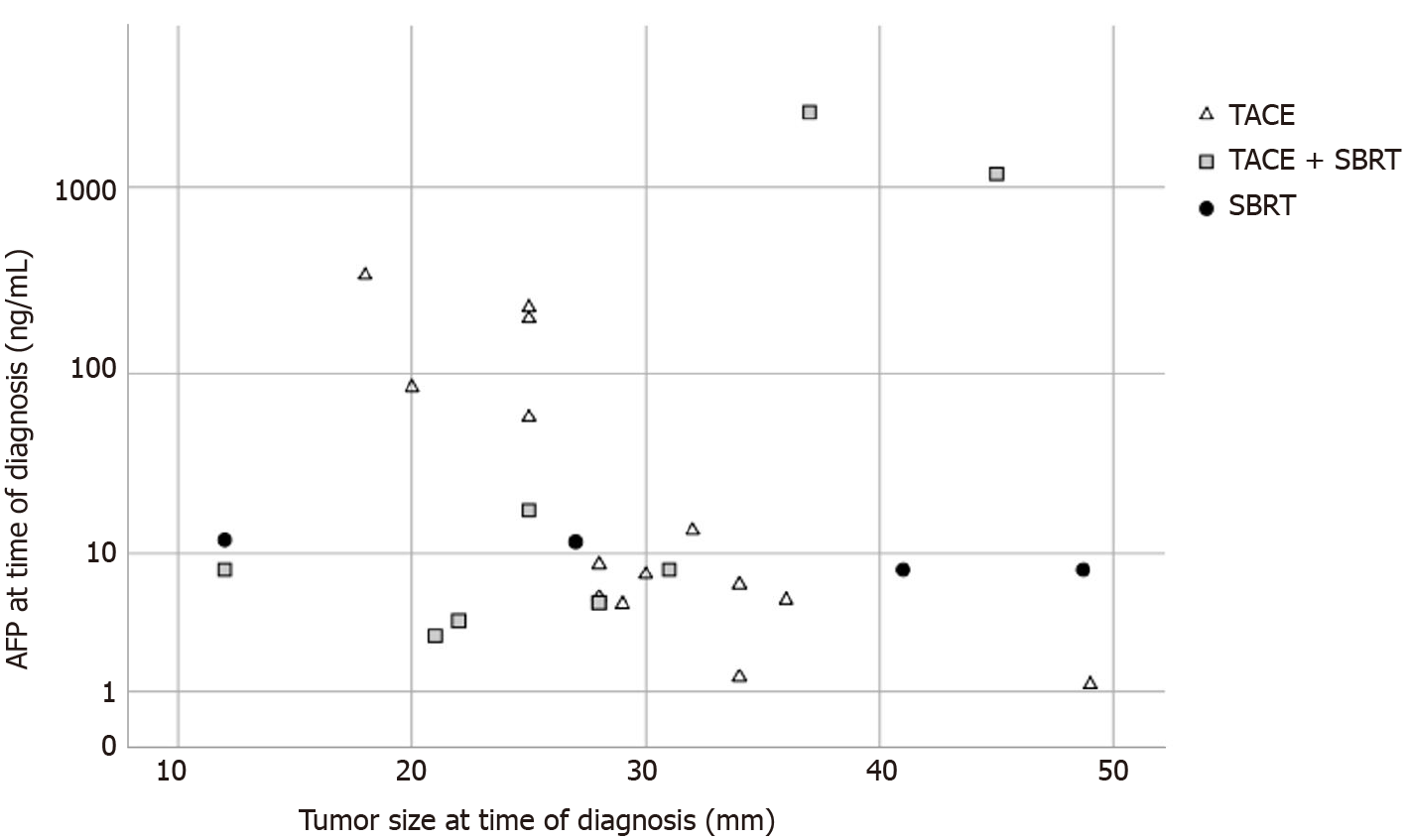
Most patients had a single tumor lesion (20/27; 74%). Of the seven patients with two lesions, one patient received a combination of TACE and SBRT and one patient SBRT only, the others were in the TACE only group. At the time of diagnosis, mean tumor size was 29.3 mm (SD ± 9.5 mm). Median AFP was 8.0 ng/mL, with 1st quartile 5.0 ng/mL and 3rd quartile 58.0 ng/mL (range 1.2 to 2515 ng/mL).
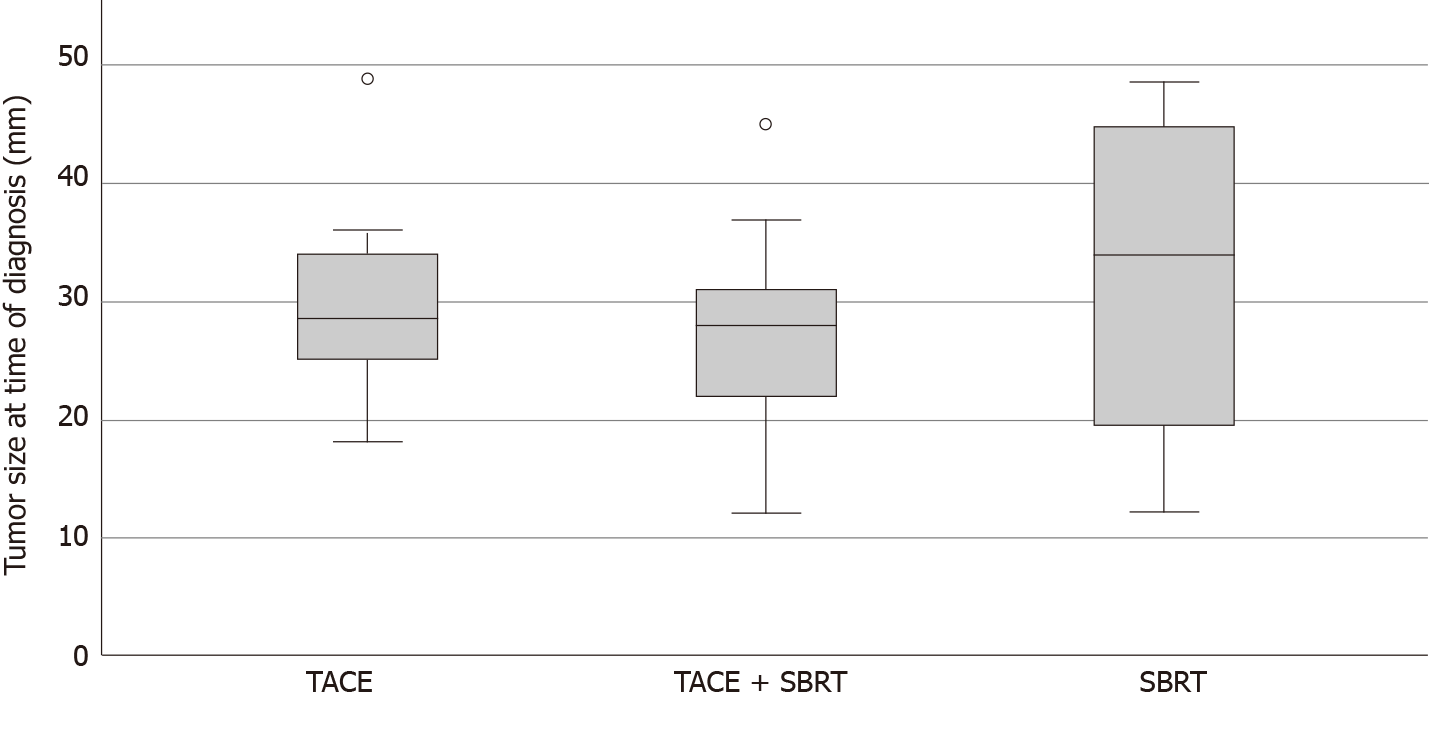
Treatment plans were tailored to each individual patient and varied in number of TACE cycles (median = 2, range 1 to 5) and SBRT radiation dose (range 18.9 to 54 Gy, in 3 to 9 fractions, prescribed to the surrounding isodose) (Table 1). The most common schemes were 3 × 12.5 Gy prescribed to the 65% -isodose and 3 × 15 Gy prescribed to the 60%-isodose delivered every other day. There were no statistically significant differences in age, gender, origin of cirrhosis, tumor size or number of tumor lesions between groups (Table 1, Figure 1). LT was performed after a median interval of 114 d (range 1 to 786 d) from SBRT treatment (Supplementary Figure 1).
| Characteristics | Total number of patients (n1 = 27) | TACE only (n = 14) | Combination of TACE and SBRT (n = 9) | SBRT only (n = 4) | P value |
| Male/female | 20 (74)/7 (26) | 12 (86)/2 (14) | 5 (56)/4 (44) | 3 (75)/1 (25) | |
| age < 60/≥ 60 yr | 13 (48)/14 (52) | 6 (43)/8 (57) | 5 (56)/4 (44) | 2 (50)/2 (50) | |
| mean age yr ± SD | 60 ± 6 | 59.5 ± 8 | 61 ± 4 | 60 ± 2 | 0.963 |
| Genesis of cirrhosis | |||||
| 1 alcohol | 11 (41) | 6 (44) | 3 (33) | 2 (50) | |
| 2 viral2 | 12 (44) | 8 (57) | 3 (33) | 1 (25) | |
| 3 others3 | 4 (15) | 0 (0) | 3 (33) | 1 (25) | |
| Numbers of TACE treatment cycle4 | |||||
| 1 | 12 (44) | 5 (36) | 7 (78) | NA | |
| 2 | 6 (22) | 4 (29) | 2 (22) | ||
| 3 or more | 5 (19) | 5 (36) | 0 (0) | ||
| Mean radiation dose in Gy | NA | 40.00 ± 3.75 | 36.80 ± 17.56 | 0.586 | |
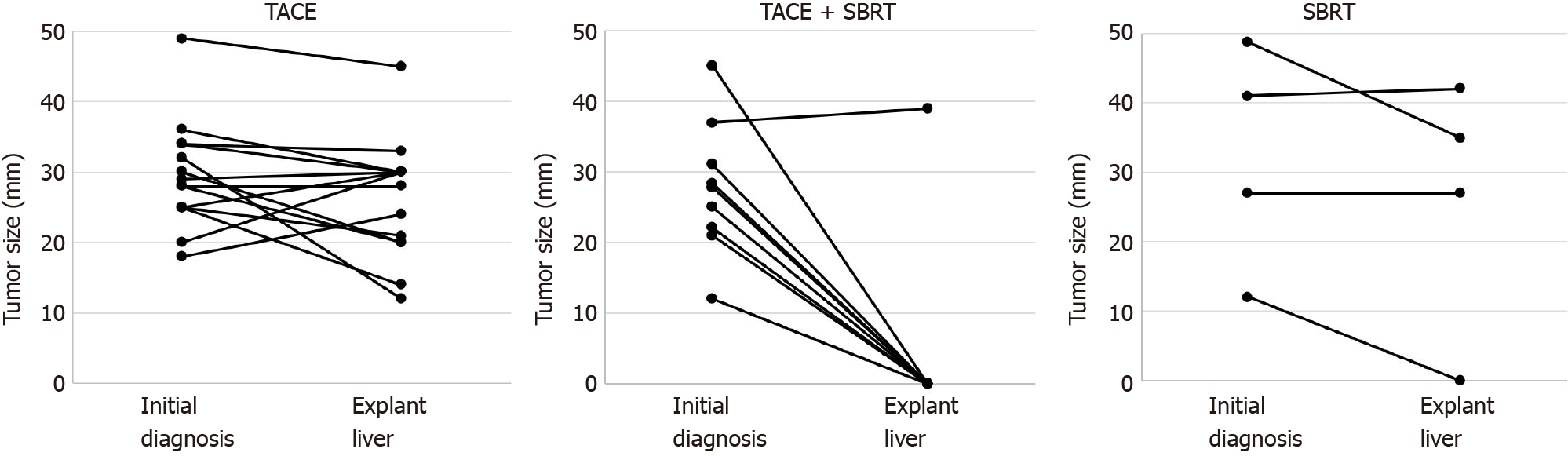
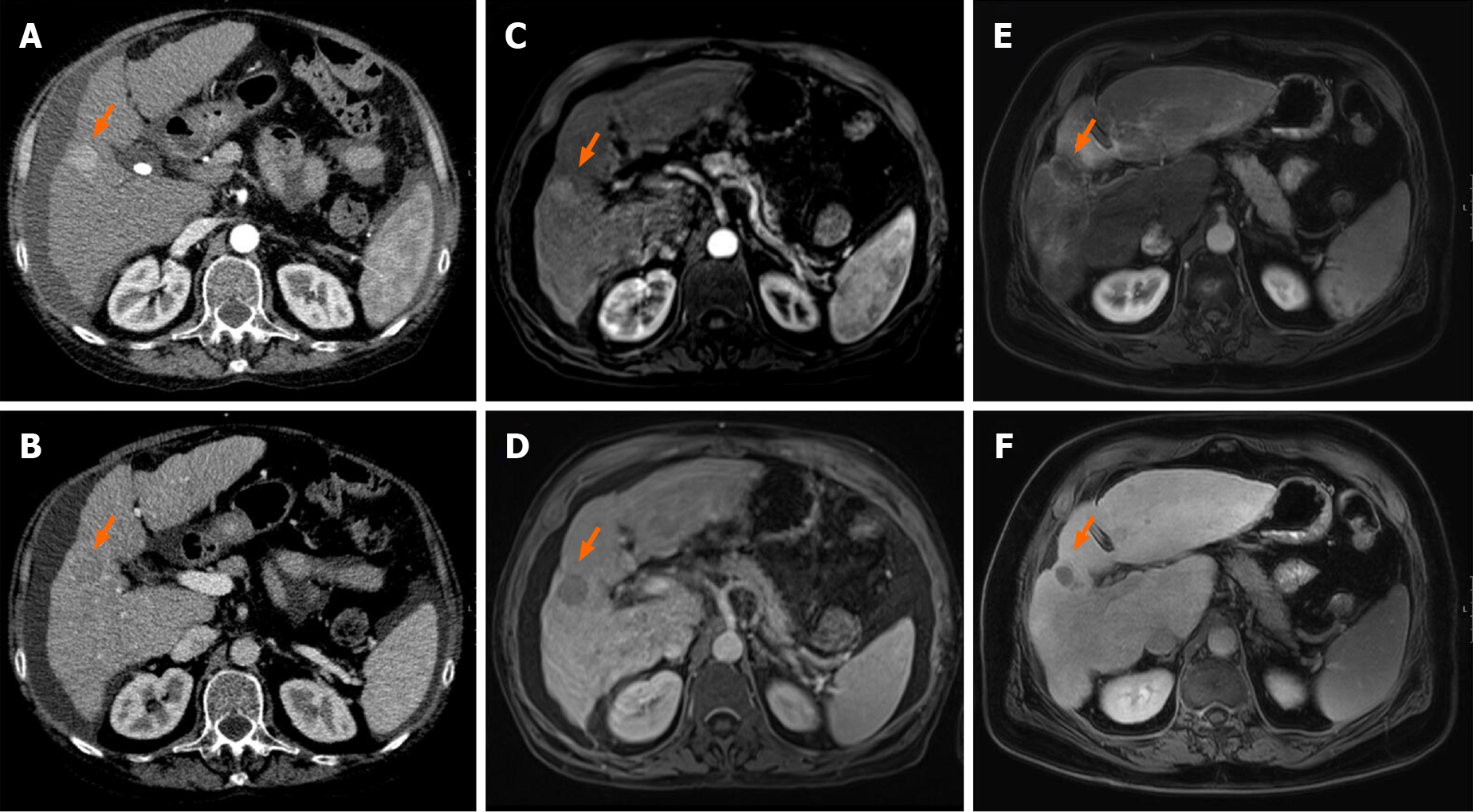
Analysis of explant livers by histopathology showed different treatment responses. In 9/27 patients (33%), no vital tumor was detected microscopically, which was considered as complete response (Figure 2A-D, Supplementary Figure 2). Strikingly, for the majority of patients in the TACE and SBRT combination therapy group a complete response was observed (8/9, 89%), compared to none in the TACE only group (0/14, 0%) and only one in the SBRT only (1/4, 25%) group (P < 0.001) (Table 2, Figure 2E). When tumor size at the time of initial diagnosis was compared to tumor size in liver explants, treatment with TACE alone led to a stabilization or a decrease in tumor size in the majority of patients, but could not stop tumor growth in all cases. In the combination group, the only sample with vital tumor showed disease stabilization (increase in size < 20%) with most lesions being completely necrotic by histopathology as described above. In the SBRT group, one completely necrotic tumor was observed, but no conclusions on treatment response could be made due to the small sample size (Figure 3).
| Total number of patients (n1 = 27) | TACE only (n = 14) | Combination of TACE and SBRT (n = 9) | SBRT only (n = 4) | P value | |
| Complete response | 9 (33.3) | 0 (0) | 8 (88.89) | 1 (25) | < 0.001 |
| Number of tumor lesions | 0.517 | ||||
| 1 | 20 (74) | 9 (64) | 8 (89) | 3 (75) | |
| 2 | 7 (26) | 5 (36) | 1 (11) | 1 (25) | |
| Mean tumor size2 | 29.3 ± 9.46 | 29.50 ± 7.63 | 27.67 ± 9.54 | 26.67 ± 14.50 | 0.389 |
| BCLC4 | |||||
| 0 | 1 (4) | 0 (0) | 0 (0) | 1 (25) | |
| A | 26 (96) | 14 (100) | 9 (100) | 3 (75) | |
| Median AFP3,5 | 8.0, 5.0/58.0 | 8.05, 5.2/84.2 | 8.0, 5.0/17.7 | 9.85, 8.0/11.85 | |
Of note, the only patient with vital tumor in the TACE and SBRT group had by far the highest AFP level (2515 ng/mL, Figure 4) and the shortest time interval between SBRT and LT (29 d). The only patient with a complete response in the SBRT only group had the smallest tumor in this group (12 mm, BCLC 0), the longest time between SBRT and LT (256 d) and was transplanted due to deterioration of liver function. While there was a weak correlation between tumor size and treatment response in the overall patient cohort, the difference was not statistically significant (Supplementary Figure 3).
On follow-up, two patients suffered from extrahepatic recurrence after LT, of whom one was in the TACE only group (with vital tumor tissue by explant histology) and one patient was in the TACE and SBRT group (no vital tumor detected in explanted liver).
In this study, patients with HCC who received a combination therapy of TACE and SBRT before LT had a significantly higher rate of complete histopathological response than patients who received TACE or SBRT alone.
Current bridging strategies to LT aim to stabilize the disease but are not sufficient to delay tumor growth in all patients[7]. Thermal ablation and TACE are the most commonly used bridging therapies before LT. However, RFA or MWA are not technically feasible in all patients mostly due to tumor location, and a complete pathologic response to TACE alone is found in less than 35% of patients receiving LT for HCC[21,33]. Disease progression poses a risk in these patients – especially in countries with long waiting times such as Germany, with an average of two to three patients per center removed due to tumor progression each year, revoking any curative treatment option. Based on the outcomes of previous studies indicating improved response after TACE and SBRT combination vs TACE alone in HCC[34,35], we used TACE and SBRT combination therapy as an individualized treatment approach in patients at risk for tumor progression beyond MC to achieve long term disease stabilization.
While a better outcome of the combination of TACE and SBRT was expected[28,30], the rate of complete tumor response by histopathology was surprisingly high in our patient cohort. In almost all patients who received combined TACE and SBRT, no residual vital tumor was detected in explant livers (TACE and SBRT 89% vs TACE alone 0%; P < 0.001). The only patient in the TACE and SBRT group with vital tumor tissue by histopathology had a very high AFP (2515 ng/mL) and was transplanted less than one month after SBRT treatment. On the other hand, one patient with complete response in the SBRT-only group had a lesion < 2 cm, an interval of more than 6 mo between SBRT and LT, and was transplanted for deterioration of liver function.
In the small group of patients treated with SBRT alone (four patients in which chemoembolization was not feasible for anatomical reasons or where treatment decision was made at an external hospital), only one of four patients had no vital tumor by histomorphology. Importantly, we had no indications for differences regarding SBRT schemes between groups in our study cohort (Table 1, Supplementary Figure 4 and 5). However, from a sample size this small and above all a very short time interval between SBRT and LT in three out of four patients in the SBRT only group (Supplementary Figure 1) it cannot be ruled out that SBRT alone might be equally efficient to TACE and SBRT combination therapy. However, recently published data from a cohort of 14 patients showing complete response by histopathology in 23.1% of tumor nodules in liver explants is in line with our data[36] – indicating that complete tumor necrosis is not commonly achieved after SBRT alone.
Importantly, none of our patients showed any higher grade treatment-related toxicities, which is in line with previous analyses[37,38]. While treatment side effects were not prospectively evaluated, there were no reports of deterioration of liver function in the TACE and SBRT group, or other higher-grade side effects observed at our centers. However, our study comprises a relatively small group of patients and almost all patients of our cohort had well-preserved liver function. Therefore, outcomes might have been different in patients with impaired liver function[39,40]. Clearly, long-term hepatic toxicity, which is mostly negligible in a pre-transplant setting, might be limiting in palliative treatment strategies[38].
Together, data from our study strongly indicate that TACE and SBRT combination therapy might lead to higher rates of complete histopathological tumor response than TACE alone. Nevertheless, this study has some limitations. A sample bias due to the multicenter, retrospective design, large duration of study recruitment and little opportunity to adjust for possible confounders due to small sample size cannot be ruled out. Furthermore, most of the patients with two tumor lesions were in the TACE group, which might have biased the results towards a lower percentage of complete response in this cohort. Additionally, the limited number of patients in this study does not allow to draw any conclusions on tumor recurrence or even overall survival. Our cohort accounts for less than 25% of all patients that were transplanted with HCC in Munich transplant centers as most HCC patients received additional bridging therapies such as thermal ablation or even resection whenever feasible. Therefore, whether these histopathological findings will translate into a survival benefit remains to be investigated prospectively in a larger patient cohort. For example, one patient who received TACE and SBRT combination therapy and showed a complete response in the explant liver developed metachronous metastatic disease less than 6 mo after liver transplantation. In this patient, metastases first occurred in the skull that was not routinely screened by standard tumor staging procedures while the patient was on the waiting list. If bone metastases to the skull were already present before completing SBRT bridging therapy before LT cannot be determined retrospectively but is certainly a possibility. The development of extrahepatic metastases therefore remains an eminent risk even with excellent local tumor control, yet it occurs very rarely at this stage. On the other hand, a patient with high AFP (2515 ng/mL) indicating limited prognosis was successfully transplanted after TACE and SBRT combination therapy. Despite only partial response with 20% of vital tumor tissue by histopathology, he shows no signs of tumor recurrence more than 4 years after LT.
More recently, down-staging to MC has been implemented in organ allocation criteria in several countries. In our cohort, a complete response with decrease of tumor size and loss of arterial hyperperfusion was routinely observed in the combination cohort (Figure 5).
Even though our study did not include any patients beyond MC, TACE and SBRT combination therapy might be efficient for down-staging patients to MC to reach requirements for LT. As a sample bias cannot be excluded due to the limited number of patients, retrospective design, and long recruitment time, further studies in a larger patient cohort are needed to confirm high treatment response to TACE and SBRT combination therapy and to clarify if these findings translate into a decreased number of waitlist removals due to tumor progression or into reduced rates of tumor recurrence after liver transplantation.
In summary, data from our study shows that patients not eligible for ablation or resection who received TACE and SBRT combination therapy were significantly more likely to have complete histopathological tumor response in explanted livers compared to patients treated with TACE or SBRT only. Whether TACE and SBRT combination therapy results in decreased number of waiting list removals and/or a reduced rate of tumor recurrence after LT needs to be evaluated prospectively in a larger patient cohort. Additionally, future studies will need to show if patients within MC who are not eligible for LT because of old age or relevant co-morbidities could benefit from TACE and SBRT combination therapy if curative resection or ablation is not possible due to tumor location.
In patients with hepatocellular carcinoma (HCC) who are not eligible for resection or ablation therapy, liver transplantation presents a curative treatment option. Due to organ shortage there are long waiting times with the risk of tumor progression. Therefore, efficient bridging therapies are needed.
This study evaluated the treatment response to a combination therapy of transarterial chemoembolization (TACE) and stereotactic body radiation therapy (SBRT) as bridging to liver transplantation.
This study aimed to establish a pathologic response in explant livers after TACE and SBRT.
Retrospective multicenter analysis of 27 patients that underwent liver transplantation and received either TACE or SBRT alone or a combination therapy of TACE and SBRT as bridging to liver transplantation.
About 89% of the patients in the TACE and SBRT combination group had no residual tumor tissue by histopathology, whereas 0% in the TACE only and 25% in the SBRT only group had a complete histopathological response.
A combination of TACE and SBRT shows superior pathologic response in comparison to TACE or SBRT alone for bridging to liver transplantation in patients with HCC.
If complete histopathological response in the TACE and SBRT combination group translates into a better progression free and overall survival needs to be evaluated in larger studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boteon YL, Huang CY, Jeruc J, Yu JI S-Editor: Wang JL L-Editor: A P-Editor: Liu JH
| 1. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 449] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6396] [Article Influence: 799.5] [Reference Citation Analysis (9)] |
| 4. | Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8:1982-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 793] [Article Influence: 56.6] [Reference Citation Analysis (2)] |
| 6. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 7. | Bhoori S, Sposito C, Germini A, Coppa J, Mazzaferro V. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int. 2010;23:712-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Cillo U, Burra P, Mazzaferro V, Belli L, Pinna AD, Spada M, Nanni Costa A, Toniutto P; I-BELT (Italian Board of Experts in the Field of Liver Transplantation). A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a "Blended Principle Model". Am J Transplant. 2015;15:2552-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3426] [Article Influence: 428.3] [Reference Citation Analysis (3)] |
| 10. | Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Bauschke A, Altendorf-Hofmann A, Ardelt M, Kissler H, Tautenhahn HM, Settmacher U. Impact of successful local ablative bridging therapy prior to liver transplantation on long-term survival in patients with hepatocellular carcinoma in cirrhosis. J Cancer Res Clin Oncol. 2020;146:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Rubinstein MM, Kaubisch A, Kinkhabwala M, Reinus J, Liu Q, Chuy JW. Bridging therapy effectiveness in the treatment of hepatocellular carcinoma prior to orthotopic liver transplantation. J Gastrointest Oncol. 2017;8:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Lee MW, Raman SS, Asvadi NH, Siripongsakun S, Hicks RM, Chen J, Worakitsitisatorn A, McWilliams J, Tong MJ, Finn RS, Agopian VG, Busuttil RW, Lu DSK. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: A 10-year intention-to-treat analysis. Hepatology. 2017;65:1979-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and Safety of Radiofrequency Ablation Combined with Transcatheter Arterial Chemoembolization for Hepatocellular Carcinomas Compared with Radiofrequency Ablation Alone: A Time-to-Event Meta-Analysis. Korean J Radiol. 2016;17:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Wells SA, Hinshaw JL, Lubner MG, Ziemlewicz TJ, Brace CL, Lee FT Jr. Liver Ablation: Best Practice. Radiol Clin North Am. 2015;53:933-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | De Giorgio M, Vezzoli S, Cohen E, Armellini E, Lucà MG, Verga G, Pinelli D, Nani R, Valsecchi MG, Antolini L, Colledan M, Fagiuoli S, Strazzabosco M. Prediction of progression-free survival in patients presenting with hepatocellular carcinoma within the Milan criteria. Liver Transpl. 2010;16:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg. 2017;402:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Tsochatzis E, Garcovich M, Marelli L, Papastergiou V, Fatourou E, Rodriguez-Peralvarez ML, Germani G, Davies N, Yu D, Luong TV, Dhillon AP, Thorburn D, Patch D, O'Beirne J, Meyer T, Burroughs AK. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int. 2013;33:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 23. | Liu E, Stenmark MH, Schipper MJ, Balter JM, Kessler ML, Caoili EM, Lee OE, Ben-Josef E, Lawrence TS, Feng M. Stereotactic body radiation therapy for primary and metastatic liver tumors. Transl Oncol. 2013;6:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, Levendag PC. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 356] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, Kwo P, Cárdenes H. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e443-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Nabavizadeh N, Mitin T, Dawson LA, Hong TS, Thomas CR Jr. Stereotactic body radiotherapy for patients with hepatocellular carcinoma and intermediate grade cirrhosis. Lancet Oncol. 2017;18:e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Huo YR, Eslick GD. Transcatheter Arterial Chemoembolization Plus Radiotherapy Compared With Chemoembolization Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2015;1:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 29. | Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, Imajo K, Aoki Y, Saito H, Kunieda E. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Wong TC, Chiang CL, Lee AS, Lee VH, Yeung CS, Ho CH, Cheung TT, Ng KK, Chok SH, Chan AC, Dai WC, Wong FC, Luk MY, Leung TW, Lo CM. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg Oncol. 2019;28:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Jun BG, Kim SG, Kim YD, Cheon GJ, Han KH, Yoo JJ, Kim YS, Jeong SW, Jang JY, Lee SH, Park S, Kim HS. Combined therapy of transarterial chemoembolization and stereotactic body radiation therapy vs transarterial chemoembolization for ≤5cm hepatocellular carcinoma: Propensity score matching analysis. PLoS One. 2018;13:e0206381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Bale R, Schullian P, Eberle G, Putzer D, Zoller H, Schneeberger S, Manzl C, Moser P, Oberhuber G. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: a Histopathological Study in Explanted Livers. Hepatology. 2019;70:840-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Zhang W, Xu AH, Wang W, Wu YH, Sun QL, Shu C. Radiological appearance of hepatocellular carcinoma predicts the response to trans-arterial chemoembolization in patients undergoing liver transplantation. BMC Cancer. 2019;19:1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, Nakahara T, Naeshiro N, Ono A, Miyaki D, Nagaoki Y, Kawaoka T, Takaki S, Hiramatsu A, Ishikawa M, Kakizawa H, Kenjo M, Takahashi S, Awai K, Nagata Y, Chayama K. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Jacob R, Turley F, Redden DT, Saddekni S, Aal AK, Keene K, Yang E, Zarzour J, Bolus D, Smith JK, Gray S, White J, Eckhoff DE, DuBay DA. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥ 3 cm. HPB (Oxford). 2015;17:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Wang YF, Dai YH, Lin CS, Chang HC, Shen PC, Yang JF, Hsiang CW, Lo CH, Huang WY. Clinical outcome and pathologic correlation of stereotactic body radiation therapy as a bridge to transplantation for advanced hepatocellular carcinoma: a case series. Radiat Oncol. 2021;16:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Honda Y, Kimura T, Aikata H, Nakahara T, Naeshiro N, Tanaka M, Miyaki D, Nagaoki Y, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Ishikawa M, Kakizawa H, Kenjo M, Awai K, Nagata Y, Chayama K. Pilot study of stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatogastroenterology. 2014;61:31-36. [PubMed] |
| 38. | Jang WI, Bae SH, Kim MS, Han CJ, Park SC, Kim SB, Cho EH, Choi CW, Kim KS, Hwang S, Kim JH, Chang AR, Park Y, Kim ES, Kim WC, Jo S, Park HJ. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: Safety and efficacy. Cancer. 2020;126:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Gerum S, Heinz C, Belka C, Walter F, Paprottka P, De Toni EN, Roeder F. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol. 2018;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |