Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3556
Peer-review started: January 29, 2021
First decision: February 25, 2021
Revised: March 11, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: June 28, 2021
Processing time: 147 Days and 7 Hours
Chronic infections due to hepatitis B and hepatitis C viruses are responsible for most cases of hepatocellular carcinoma (HCC) worldwide, and this association is likely to remain during the next decade. Moreover, viral hepatitis-related HCC imposes an important burden on public health in terms of disability-adjusted life years. In order to reduce such a burden, some major challenges must be faced. Universal vaccination against hepatitis B virus, especially in the neonatal period, is probably the most relevant primary preventive measure against the develo
Core Tip: Hepatitis B and C are associated with most cases of hepatocellular carcinoma, and it is estimated that this scenario will remain for the next decade. This review highlights the impact of viral hepatitis on the development of liver cancer, the characteristics of viral hepatitis-related hepatocellular carcinoma, and the challenges that must be faced in order to reduce their burden.
- Citation: de Mattos ÂZ, Debes JD, Boonstra A, Yang JD, Balderramo DC, Sartori GDP, de Mattos AA. Current impact of viral hepatitis on liver cancer development: The challenge remains. World J Gastroenterol 2021; 27(24): 3556-3567
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3556.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3556
Viral infections are the leading cause of hepatitis, with hepatitis B virus (HBV) and hepatitis C virus (HCV) being the most important causes of chronic viral hepatitis worldwide. It was estimated that nearly 292 million individuals had chronic HBV infection in 2016 (prevalence of 3.9%) and 71 million people had chronic HCV infection in 2015 (prevalence of 1%)[1-3]. Hepatitis D virus (HDV) is a defective virus, requiring HBV coexistence in order to replicate. It was estimated that HDV caused chronic infection in approximately 12 million individuals in 2016[4]. Most HBV, HCV and HDV infections are concentrated in low- and lower middle-income countries[3].
The incidence of liver cancer is the sixth highest of all malignant neoplasms (905677 new cases in 2020, an incidence of 11.6/100000 inhabitants). Moreover, it is the second cause of cancer-related death in the world (830180 deaths in 2020, mortality of 10.7/100000 inhabitants)[5]. Hepatocellular carcinoma (HCC) accounts for approximately 75%-85% of primary liver cancers[6,7]. HCC frequently develops in patients with cirrhosis, with an annual incidence of 1%-4%[8]. HBV and HCV are currently the two most important risk factors for HCC development worldwide. This is reflected by the geographical distribution of liver cancer, which coincides with those of HBV and HCV, predominating in transitioning countries, particularly in Asia and Africa[6,7,9]. In a systematic review of 260 studies involving 119006 patients with HCC, the seroprevalence of HBV and/or HCV was over 60% in most of the 50 countries which contributed with data[10]. Furthermore, it is noteworthy that, according to the Global Burden of Disease Study, HBV-related liver cancer was associated with 5.80 million disability-adjusted life years, and HCV-related liver cancer, with 2.88 million disability-adjusted life years in 2019[11]. The impact of HBV may be further increased by the fact that HCC usually develops at an earlier age in patients infected with this virus[12].
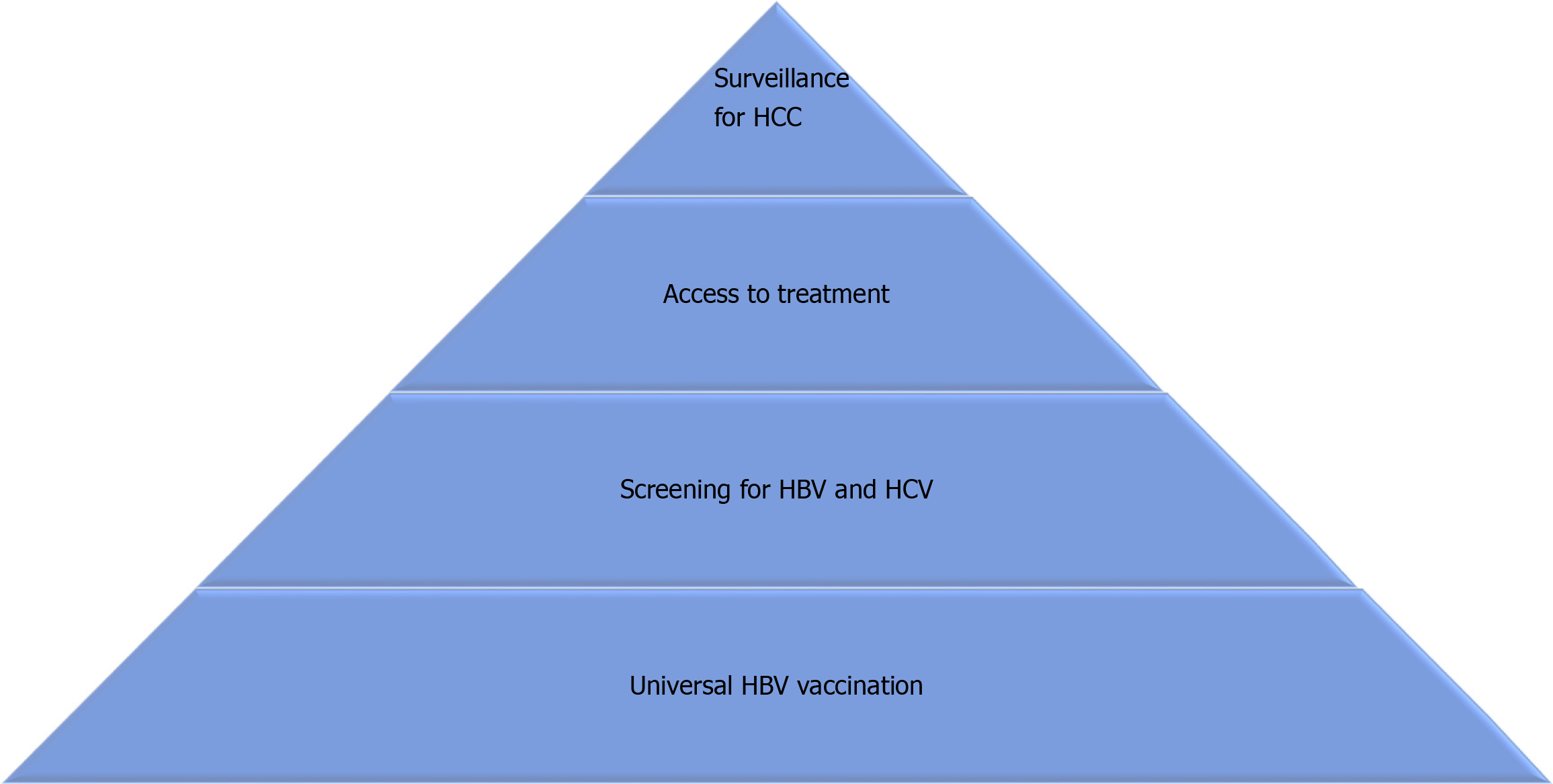
Despite vaccination against HBV and effective treatments for HBV and HCV, it is estimated that their impact on liver health will remain for the next decade at least. Using data from 195 countries or territories between 1990 and 2017, a recent study proposed Bayesian models to project primary liver cancer incidence through 2030. The authors estimated that the age-standardized incidence rate of liver cancer will increase globally from 11.80 in 2017 to 14.08 per 100000 inhabitants in 2030. According to the etiology of liver disease, HBV was responsible for 46.5% of cases of liver cancer in 1990 and 42.4% in 2017, and it is estimated that it will be associated with 40.7% of cases in 2030. With regard to HCV, it was associated with 25.2% of cases of primary liver cancer in 1990 and 27.0% in 2017, and it is projected that it will be responsible for 26.8% of cases in 2030[13]. The aim of this article is to review the current impact of viral hepatitis on the development of liver cancer, the characteristics of viral hepatitis-related HCC and the challenges to reduce their burden (Figure 1).
In chronic viral hepatitis, the development of fibrosis, cirrhosis and HCC is linked to the activity of the immune response in the infected liver. Both HBV and HCV uniquely infect hepatocytes and replicate in a non-cytopathic manner. The ensuing liver damage that is observed during the acute and the chronic phase of infection is the consequence of virus-specific as well as non-specific immune activity within the inflamed liver. Histologically, active phases of chronic HBV and HCV infections are characterized by extensive infiltration around the portal tract areas consisting predominantly of CD4+ and CD8+ T cells, but these cells are incapable of clearing these viruses. Multiple mechanisms have been described that explain the weak activity of CD4+ and CD8+ T cells, and that are the main reasons for persistence of HBV and HCV in the liver, and the slowly progressing liver disease[14-16]. The mechanisms include impairment of dendritic cells and natural killer (NK) cells, increased production of immunosuppressive cytokines and an increase in the numbers of regulatory T cells. In addition, the continuous exposure of immune cells to high levels of viral antigens for many years further contributes and maintains the persistent infection due to exhaustion of HBV and HCV-specific T cells, which renders these cells functionally impaired mediated via triggering of exhaustion markers, such as programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and others[17].
In the liver, the balance between immune tolerance and immune activation is normally tightly controlled to ensure efficient elimination of pathogen products and transformed cells. Dysregulation of this balance due to viral infection has severe consequences for the process of immune surveillance that is highly efficient in the detection and elimination of transformed cells, and contributes to HCC development[18]. Also, alterations in lipid metabolism and the release of reactive oxygen species contribute to tumor initiation[19].
Interestingly, the status of tumor-infiltrating immune cells in HCC has a high degree of resemblance to that observed in an HBV or HCV inflamed liver: an increased T cell infiltrate is observed in HCC, but the CD8+ T cells are dysfunctional with low production of the cytolytic enzymes granzyme and perforin, low overall cytokine production, and relatively high expression of the exhaustion markers PD-1 and CTLA-4[20,21]. In addition, increased numbers of regulatory T cells and reduced numbers and functionality of NK cells have been reported by many groups[18,22,23]. These immune dysfunctions are generally more pronounced in the advanced HCC stages.
The importance of the immune system in controlling the development and progression to advanced stages of HCC is highlighted by observations that increased T cell infiltrates are associated with improved overall survival in HCC and lower tumor recurrence following resection[24-26]. Furthermore, the enrichment of exhausted T cells in HCC is associated with poorer survival, while higher numbers of infiltrating NK cells are associated with better survival. Using cytometry by time of flight and RNA sequencing, a recent study defined different immune subsets and showed that the tumor microenvironment in HBV-related HCC consisted of a more immunosuppressive and exhausted phenotype with an enrichment of regulatory T cells and resident memory T cells that differs from the non-viral-related HCC environment. The study further demonstrated that regulatory T cells are associated with poor prognosis, and memory T cells, with good prognosis[27].
The crucial importance of the patient’s immune activity against the tumor has initiated the development of numerous immunotherapeutic approaches in recent years, all aimed at boosting anti-tumor immunity[28]. The approaches are diverse and include strategies using peptide vaccines and cell-based therapies. Moreover, therapies aimed at neutralizing exhaustion markers using immune checkpoint inhibitors have shown promising results and PD-1 blockade has been approved as therapy for HCC. However, many studies are ongoing to improve therapy success by examining the therapeutic outcomes using different combinations of checkpoint inhibitors.
Advances in next-generation sequencing technology and integrative studies combining multiomic approaches, encompassing genomic and epigenomic characterization and transcriptome, proteomic, and metabolomic analysis of HCCs have shown that HCC is heterogeneous at the histomolecular level, with variable molecular features and clinical outcomes[29-31]. While ongoing hepatic inflammation, increased cell turnover, repeated cycles of cell death and regeneration, DNA instability leading to an alteration in DNA and dysregulation of CpG island methylation are common pathways leading to HCC regardless of the underlying etiology of liver disease[32], there are HBV specific pathogenesis pathways in HCC development. HBV promotes HCC through viral DNA integration into host genes that can provide a growth advantage to the host cell. HBV has been found to be integrated into or near cancer-related genes[33,34]. For example, promoter mutation of telomerase reverse transcriptase, which is often integrated by HBV DNA, is the most frequent genetic event in HBV-associated HCC. The translated protein products of the viral HBV genome have also been implicated in promoting carcinogenesis. For instance, HBx protein, a regulatory protein required for viral replication, has been shown to regulate hepatocyte proliferation, regeneration and apoptosis by modulating the PI3K/Akt-mTOR, STAT3/Nanog, and Wnt/β-catenin signaling pathways to promote the progression of HCC[35-37].
Occult HBV infection is a condition where HBV DNA replication occurs in the liver in patients with negative serum HBsAg[38]. While detection of anti-HBc (hepatitis B core antibody) in the blood indicates prior HBV infection, 20% of patients with occult HBV infection are seronegative for anti-HBc. HBV DNA analysis should be further pursued to confirm the diagnosis[39]. Occult HBV infection increases the risk of HCC development in patients with or without chronic liver disease, which is thought to be mediated by HBV DNA integration into the host genome, the production of pro-oncogenic proteins such as HBx protein, and persistent low-grade necroinflammation leading to fibrosis progression[39]. Currently, HCC surveillance is not routinely recommended in patients with occult HBV infection in the absence of advanced fibrosis/cirrhosis.
HCC continues to be one of the leading causes of cancer-related death throughout the world and it frequently develops within the context of cirrhosis[40]. This has also led to the development of guidelines by all the major liver societies for HCC surveillance in patients with cirrhosis given its potential for early-stage cancer detection, which likely links to curative treatment[41,42]. Given its high oncogenic potential and its tendency to develop HCC in the absence of cirrhosis, screening for HCC is indicated in a broader population of HBV patients. All HBV patients with HBV cirrhosis (except for patients with severe liver dysfunction and not eligible for liver transplantation) are recommended to undergo HCC surveillance. Surveillance is also recommended in a selected high-risk population of non-cirrhotic HBV patients, as demonstrated in Table 1[43].
| Indications for surveillance |
| Asian males > 40 yr |
| Asian females > 50 yr |
| African ancestry |
| First degree relative with HCC |
| HDV co-infection |
HBV vaccination is an effective primary prevention method for HCC. The effectiveness of HBV vaccination in reducing HCC incidence was reported in a nationwide population-based study from Taiwan. The study used data from 2 Taiwanese HCC registry systems and showed that HCC incidence was four-fold higher in the HBV unvaccinated cohort than in the vaccinated at birth cohorts. Among the 1509 patients who were 6-26 years old diagnosed with HCC from 1983 through 2011, 1343 were born before, and 166 were born after the HBV vaccination program began. Moreover, the relative risks for HCC in patients vaccinated at 6-9 years old, 10-14 years old, 15-19 years old, and 20-26 years old in comparison to those who were not vaccinated were 0.26 [95% confidence interval (CI): 0.17-0.40], 0.34 (95%CI: 0.25-0.48), 0.37 (95%CI: 0.25-0.51), and 0.42 (95%CI: 0.32-0.56), respectively[44]. Although neonatal HBV vaccination is recommended in most countries, vaccine coverage is still less than 70% in most African countries where the incidence rates of HCC remain high[45], providing a clear window of opportunity for improvement in primary prevention of HCC[40].
Aside from identifying infected patients, offering effective treatment against HBV and surveying those at high risk for HCC, the major challenge in order to reduce HBV-related HCC burden is widening the vaccination coverage against the virus. Despite the increase in vaccination coverage between 2010 and 2018 in 97% of the 194 member states of the World Health Organization (WHO), with an increment from 73% to 84% in the completion of the three-dose vaccination schedule and with an increase from 28% to 42% in the neonatal vaccination coverage in the 108 countries in which newborns are vaccinated, improvements are still necessary[46]. For instance, vaccination coverage requires improvement in countries such as Nigeria, India and Indonesia, which together are responsible for 50% of HBV infections in children under 5 years of age[3].
HCV is one of the most important risk factors for HCC. As previously mentioned, HCV is a non-cytopathic virus, which leads to liver damage through immune-mediated mechanisms. However, the virus itself also plays a role in the development of HCC, which becomes clear when considering, for instance, that HCV genotype 3 is associated with a higher risk of HCC than other genotypes[47]. The mechanism through which HCV genotype 3 is involved in hepatocarcinogenesis is not yet completely understood, but the down-regulation of the phosphatase and tensin homolog (PTEN) gene, which has a tumor suppressor role, might be implicated[48].
Direct-acting antiviral (DAA) therapy in HCV-infected patients has been shown to reduce all-cause mortality (including liver-related and unrelated causes), as well as the incidence of HCC in patients with cirrhosis[49]. However, in the subgroup of patients with advanced fibrosis or cirrhosis, the risk of developing HCC after achieving sustained virologic response (SVR) remains high, ranging from 0.3% to 1.8% per year[50]. For this reason, different guidelines recommend lifelong surveillance for patients with advanced fibrosis or cirrhosis even after SVR[51]. Interestingly, recent studies have attempted to differentiate subgroups of patients with cirrhosis at the highest risk for HCC after SVR. In an Italian study, the combination of clinical predictors (male sex and diabetes), albumin, and genetics identified patients at high risk for HCC[52]. Furthermore, it is suggested that changes in liver stiffness measured by transient elastography at 1 and 3 years after the end of HCV treatment could identify patients at low risk for HCC[53].
In 2017, the WHO Global Hepatitis Report stated that 71 million people worldwide were infected by HCV[54]. About 3.5 million to 5.0 million of these individuals are children and adolescents, and 2.3 million are co-infected with the human immunodeficiency virus (HIV)[51,54]. HCV infection causes approximately 400000 deaths annually, mainly from cirrhosis-related complications and HCC[54]. Despite the current availability of DAA therapies with high rates of SVR, the prevalence of HCV infection has not changed. One of the reasons that explain this situation is that only 20% of individuals with HCV infection worldwide are aware of their diagnosis. Another reason is that access to DAA treatments continues to be limited mainly by costs in several countries[54].
The WHO Global Health Sector Strategy on Viral Hepatitis recommended that an effort should be made in order to increase the HCV diagnosis rate to 30% by 2020 and to 90% by 2030[55]. Overall, HCV screening consists in a universal, one-time, and opt-out screening strategy in adults 18 years of age and older with an upper age limit of 79 years. However, periodic screening tests should be offered to individuals with behaviors, conditions or circumstances associated with an increased risk of exposure to HCV[51].
Vertical transmission could be present in about 5% of deliveries from mothers with HCV and accounts for the majority of HCV infections in the pediatric population[56]. Universal prenatal screening for HCV facilitates better identification of at-risk infants who require HCV testing[57]. This evaluation results in better detection of HCV infection in the pediatric population and enables early therapeutic interventions.
DAA regimens have a high cure rate for HCV infection[51]. Furthermore, new formulations have simplified the duration and administration of the treatment. However, in 2015, only 7% of people diagnosed with HCV had started antiviral treatment with DAA[54]. According to the strategy suggested by the WHO, access to DAA should reach 80% of eligible people by 2030[55]. After this declaration, access to treatment has improved in different countries. An example is the strategy in Australia, where more than 80% of the HCV-infected population was diagnosed during the last two decades and where there is currently an unrestricted DAA access program that permits prescriptions by any registered medical practitioner. This allowed for the initiation of DAA treatment in Australia for approximately 70% of the total population with HCV-related cirrhosis between 2014 and 2017[58].
Simultaneously with screening and therapy access, different preventive measures must be carried out in people capable of transmitting HCV (e.g., people who inject drugs) in order to avoid new infections or reinfections in those who have been previously treated successfully. Global coverage of harm reduction programs for people who inject drugs, including needle and syringe programs, is currently less than 10%[54].
Currently, the major challenge in order to reduce HCV-related HCC burden is identifying infected patients. The silent course of the early stages of this disease makes this challenge even harder to overcome. Therefore, efforts should be made in order to improve awareness of the population and health care workers regarding the underdiagnosis of HCV[54]. It is also of great importance to shorten the pathway leading from the diagnosis to the extremely effective treatments against HCV, which could even allow for subgroups of patients to be treated in primary care[59].
Yet another challenge relates to the coronavirus disease 2019 pandemic. It is estimated that the impact of the pandemic on the global efforts towards HCV eradication could lead to an excess of 44800 cases of HCC[60].
As previously mentioned, HBV and HCV infections are the most common risk factors for HCC worldwide[61]. Individuals living with HIV are a high-risk population for developing HCC, mostly as a consequence of HCV and HBV co-infection[62,63]. Indeed, HCC has become a major clinical problem in HIV. Studies from Europe suggest that the incidence of HCC has risen in HIV-infected patients over the last 20 years, and in Spain alone HCC is the second cause of death in HIV-HCV co-infected patients with cirrhosis[64]. Studies from both the United States and Europe suggest that HIV may hasten the evolution of HBV-related HCC, resulting in an earlier age of HCC presentation in HIV co-infected patients[65,66].
Although the implementation of new treatments for HCV with DAA agents is expected to decrease HCC incidence, new cases will continue to emerge in the near-medium term[67,68]. Moreover, a recent study that reported an HCC risk increase of 1% every year in HIV-HCV co-infected individuals in the HEPAVIR cohort in Europe included in their cohort 61% of individuals with cured HCV, indicating that this population remains at risk for HCC[63].
In HIV-HBV co-infection, a recent study from the EuroSIDA cohort showed a stable incidence of HCC in non-cirrhotic individuals under HBV therapy (tenofovir specifically), while increasing rates of HCC were seen over time in those not on tenofovir. This suggests a potential threshold to not survey the former group for HCC. However, the same study did show a continued increase in HCC risk among those that had cirrhosis, indicating the importance of surveillance in this population[69].
Most of the data reported on HIV and HCC originate in resource-rich settings. These dynamics related to poor outcomes are likely to be increased in resource-limited settings, where most of HIV infections occur. A small African study of 60 patients found that those infected with HIV developed HCC at a younger age (32 years) compared to those without HIV (49 years)[70], and a more recent study from South Africa showed the age of HCC development to be lower in those co-infected with HBV and HIV (mean age of 36 years vs 46 years in HIV-uninfected). Interestingly, this study showed that females were more impacted by HCC when co-infected with HIV, and the age of HCC presentation was much lower in females with HIV (mean age of 36 years vs 50 years in HIV-uninfected)[71]. In South America and Asia overall, data are scarce on the interrelation between HCC and HIV infection. The specific mechanisms underlying younger HCC occurrence in co-infected individuals with viral hepatitis and HIV is unclear. However, a large Swiss study found a direct association between CD4+ T cells and the risk of developing HCC, suggesting that impairment of the immune system could be implicated[72].
Surveillance for HCC represents a major challenge in co-infected populations not only due to the complex nature of dual disease, but also because most guidelines are tailored towards mono-infected individuals at risk for HCC. In addition, recent studies show that ultrasound surveillance has a low performance for HCC in HBV or HCV individuals co-infected with HIV, with a suboptimal 43% rate of early-stage diagnosis, compared to 63%-71% found in studies on HIV-uninfected cohorts[73,74].
Triple co-infection of HIV, HBV and HDV is rather uncommon, ranging from 1% to 20% depending on the geographical area[75]. However, studies performed in small cohorts have shown that the main impact of HDV on HBV-HIV disease is an accelerated pace to cirrhosis and hepatic decompensation[76]. In this context, a Swiss HIV cohort reported a 9-fold increase in HCC in those triple co-infected with HIV-HBV-HDV compared to HDV-negative individuals[77].
Despite the advances in prevention and treatment of viral hepatitis, it is clear that many challenges remain in order to reduce the burden of viral hepatitis-related HCC. Universal neonatal vaccination against HBV, as well as vaccination of at-risk adult populations, screening for HBV and HCV, and access to highly-effective treatments against both viruses are instrumental measures that must be pursued with the objective of diminishing their impact on global health and particularly on the development of HCC. Finally, effective surveillance for HCC must be offered to patients who already have advanced fibrosis or cirrhosis and to high-risk HBV-infected individuals, so that HCC is detected at earlier stages, allowing for curative treatments and longer survival.
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1513] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 2. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1263] [Article Influence: 157.9] [Reference Citation Analysis (6)] |
| 3. | Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C, Hutin Y, Geretti AM. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Global Cancer Observatory - GLOBOCAN 2020. [cited 17 January 2021]. In: World Health Organization [Internet]. Available from: https://gco.iarc.fr/. |
| 6. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1507] [Article Influence: 301.4] [Reference Citation Analysis (2)] |
| 7. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020; 159: 335-349. e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1442] [Article Influence: 240.3] [Reference Citation Analysis (16)] |
| 8. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 794] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 9. | Debes JD, Chan AJ, Balderramo D, Kikuchi L, Gonzalez Ballerga E, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Diaz Ferrer J, Yang JD, Carrera E, Garcia JA, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 394] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 11. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13226] [Cited by in RCA: 12082] [Article Influence: 2013.7] [Reference Citation Analysis (42)] |
| 12. | Chan AJ, Balderramo D, Kikuchi L, Ballerga EG, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Ferrer JD, Yang JD, Carrera E, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR, Debes JD. Early Age Hepatocellular Carcinoma Associated With Hepatitis B Infection in South America. Clin Gastroenterol Hepatol. 2017;15:1631-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Xu K, Jiang Y, Cai N, Fan J, Mao X, Suo C, Jin L, Zhang T, Chen X. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. 2021;50:128-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 16. | Boeijen LL, Hoogeveen RC, Boonstra A, Lauer GM. Hepatitis B virus infection and the immune response: The big questions. Best Pract Res Clin Gastroenterol. 2017;31:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Wieland D, Hofmann M, Thimme R. Overcoming CD8+ T-Cell Exhaustion in Viral Hepatitis: Lessons from the Mouse Model and Clinical Perspectives. Dig Dis. 2017;35:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 792] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 19. | Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 2010;28:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M, Dong H, Thielemans K, Pan Q, IJzermans JNM, Bruno MJ, Kwekkeboom J. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017; 153: 1107-1119. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 335] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 22. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 716] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 23. | Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, Blum HE, Neumann-Haefelin C, Thimme R. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 26. | Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, Lim TKH, Yeong J, Toh HC, Lee SY, Chan CY, Goh BK, Chung A, Heikenwälder M, Ng IO, Chow P, Albani S, Chew V. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68:916-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 28. | Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, Mukherjee SK, Thursz M, Wong CN, Sharma R, Rimassa L. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620-3637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 29. | Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017; 169: 1327-1341. e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1823] [Article Influence: 202.6] [Reference Citation Analysis (1)] |
| 30. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1410] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 31. | Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-7392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 992] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 32. | Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, Mulawadi FH, Wong KF, Liu AM, Poon RT, Fan ST, Chan KL, Gong Z, Hu Y, Lin Z, Wang G, Zhang Q, Barber TD, Chou WC, Aggarwal A, Hao K, Zhou W, Zhang C, Hardwick J, Buser C, Xu J, Kan Z, Dai H, Mao M, Reinhard C, Wang J, Luk JM. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 747] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 34. | Zhao LH, Liu X, Yan HX, Li WY, Zeng X, Yang Y, Zhao J, Liu SP, Zhuang XH, Lin C, Qin CJ, Zhao Y, Pan ZY, Huang G, Liu H, Zhang J, Wang RY, Wen W, Lv GS, Zhang HL, Wu H, Huang S, Wang MD, Tang L, Cao HZ, Wang L, Lee TL, Jiang H, Tan YX, Yuan SX, Hou GJ, Tao QF, Xu QG, Zhang XQ, Wu MC, Xu X, Wang J, Yang HM, Zhou WP, Wang HY. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. 2016;7:12992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 35. | Wang P, Guo QS, Wang ZW, Qian HX. HBx induces HepG-2 cells autophagy through PI3K/Akt-mTOR pathway. Mol Cell Biochem. 2013;372:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Ching RHH, Sze KMF, Lau EYT, Chiu YT, Lee JMF, Ng IOL, Lee TKW. C-terminal truncated hepatitis B virus X protein regulates tumorigenicity, self-renewal and drug resistance via STAT3/Nanog signaling pathway. Oncotarget. 2017;8:23507-23516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Chen Z, Tang J, Cai X, Huang Y, Gao Q, Liang L, Tian L, Yang Y, Zheng Y, Hu Y, Tang N. HBx mutations promote hepatoma cell migration through the Wnt/β-catenin signaling pathway. Cancer Sci. 2016;107:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 39. | Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 40. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 3120] [Article Influence: 445.7] [Reference Citation Analysis (17)] |
| 41. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3439] [Article Influence: 429.9] [Reference Citation Analysis (3)] |
| 42. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6419] [Article Influence: 802.4] [Reference Citation Analysis (9)] |
| 43. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin Liver Dis (Hoboken). 2018;12:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 44. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016; 151: 472-480. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 45. | World Health Organization. WHO-UNICEF estimates of HepB3 coverage. [cited 17 January 2021]. In: World Health Organization [Internet]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragehepb3.html. |
| 46. | Peck M, Gacic-Dobo M, Diallo MS, Nedelec Y, Sodha SV, Wallace AS. Global Routine Vaccination Coverage, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:937-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 47. | Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 48. | Goossens N, Negro F. Is genotype 3 of the hepatitis C virus the new villain? Hepatology. 2014;59:2403-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 483] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 50. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017; 153: 996-1005. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 719] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 51. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 52. | Degasperi E, Galmozzi E, Pelusi S, D'Ambrosio R, Soffredini R, Borghi M, Perbellini R, Facchetti F, Iavarone M, Sangiovanni A, Valenti L, Lampertico P. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology. 2020;72:1912-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Alonso López S, Manzano ML, Gea F, Gutiérrez ML, Ahumada AM, Devesa MJ, Olveira A, Polo BA, Márquez L, Fernández I, Cobo JCR, Rayón L, Riado D, Izquierdo S, Usón C, Real Y, Rincón D, Fernández-Rodríguez CM, Bañares R. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology. 2020;72:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 54. | Centre for Disease Analysis. Web Annex B. WHO estimates of the prevalence and incidence of hepatitis C virus infection by World Health Organization region, 2015. Geneva: World Health Organization, 2018: 1-68. |
| 55. | World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis. [cited 17 January 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1. |
| 56. | Indolfi G, Azzari C, Resti M. Perinatal transmission of hepatitis C virus. J Pediatr 2013; 163: 1549-1552. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Epstein RL, Sabharwal V, Wachman EM, Saia KA, Vellozzi C, Hariri S, Linas BP. Perinatal Transmission of Hepatitis C Virus: Defining the Cascade of Care. J Pediatr 2018; 203: 34-40. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Dore GJ, Hajarizadeh B. Elimination of Hepatitis C Virus in Australia: Laying the Foundation. Infect Dis Clin North Am. 2018;32:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Pawlotsky JM, Ramers CB, Dillon JF, Feld JJ, Lazarus JV. Simplification of Care for Chronic Hepatitis C Virus Infection. Semin Liver Dis. 2020;40:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 61. | Debes JD, de Knegt RJ, Boonstra A. The Path to Cancer and Back: Immune Modulation During Hepatitis C Virus Infection, Progression to Fibrosis and Cancer, and Unexpected Roles of New Antivirals. Transplantation. 2017;101:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Merchante N, Merino E, Rodríguez-Arrondo F, Tural C, Muñoz J, Delgado-Fernández M, Jover F, Galindo MJ, Rivero A, López-Aldeguer J, Aguirrebengoa K, Romero-Palacios A, Martínez E, Pineda JA. HIV/hepatitis C virus-coinfected patients who achieved sustained virological response are still at risk of developing hepatocellular carcinoma. AIDS. 2014;28:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Merchante N, Rodríguez-Arrondo F, Revollo B, Merino E, Ibarra S, Galindo MJ, Montero M, García-Deltoro M, Rivero-Juárez A, Téllez F, Delgado-Fernández M, Ríos-Villegas MJ, García MA, Vera-Méndez FJ, Ojeda-Burgos G, López-Ruz MA, Metola L, Omar M, Alemán-Valls MR, Aguirrebengoa K, Portu J, Raffo M, Macías J, Pineda JA; GEHEP-002 Study Group. Hepatocellular carcinoma after sustained virological response with interferon-free regimens in HIV/hepatitis C virus-coinfected patients. AIDS. 2018;32:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Merchante N, Merino E, López-Aldeguer J, Jover F, Delgado-Fernández M, Galindo MJ, Ortega E, Rivero A, Mínguez C, Romero-Palacios A, Padilla S, Márquez-Solero M, Amador C, Ríos-Villegas MJ, Téllez F, Portilla J, Pineda JA. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis. 2013;56:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Sahasrabuddhe VV, Shiels MS, McGlynn KA, Engels EA. The risk of hepatocellular carcinoma among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2012;118:6226-6233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Bräu N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, Trikha A, Sherman M, Sulkowski MS, Dieterich DT, Rigsby MO, Wright TL, Hernandez MD, Jain MK, Khatri GK, Sterling RK, Bonacini M, Martyn CA, Aytaman A, Llovet JM, Brown ST, Bini EJ; North American Liver Cancer in HIV Study Group. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, Sheen IS, Chang WY, Lee CM, Liaw YF. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985-994. [PubMed] |
| 68. | Merchante N, Rodríguez-Fernández M, Pineda JA. Screening for Hepatocellular Carcinoma in HIV-Infected Patients: Current Evidence and Controversies. Curr HIV/AIDS Rep. 2020;17:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Wandeler G, Mauron E, Atkinson A, Dufour JF, Kraus D, Reiss P, Peters L, Dabis F, Fehr J, Bernasconi E, van der Valk M, Smit C, Gjærde LK, Rockstroh J, Neau D, Bonnet F, Rauch A; Swiss HIV Cohort Study; Athena Observational Cohort Study, EuroSIDA, ANRS CO3 Aquitaine Cohort. Incidence of hepatocellular carcinoma in HIV/HBV-coinfected patients on tenofovir therapy: Relevance for screening strategies. J Hepatol. 2019;71:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Tanon A, Jaquet A, Ekouevi DK, Akakpo J, Adoubi I, Diomande I, Houngbe F, Zannou MD, Sasco AJ, Eholie SP, Dabis F, Bissagnene E; IeDEA West Africa Collaboration. The spectrum of cancers in West Africa: associations with human immunodeficiency virus. PLoS One. 2012;7:e48108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Maponga TG, Glashoff RH, Vermeulen H, Robertson B, Burmeister S, Bernon M, Omoshoro-Jones J, Ruff P, Neugut AI, Jacobson JS, Preiser W, Andersson MI. Hepatitis B virus-associated hepatocellular carcinoma in South Africa in the era of HIV. BMC Gastroenterol. 2020;20:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, Rauch A, Probst-Hensch NM, Bouchardy C, Levi F, Franceschi S; Swiss HIV Cohort. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 74. | Rodríguez de Lope C, Reig M, Matilla A, Ferrer MT, Dueñas E, Mínguez B, F Castroagudín J, Ortiz I, Pascual S, Lledó JL, Gallego A, Arenas JI, Aracil C, Forne M, Muñoz C, Pons F, Sala M, Iñarrairaegui M, Martin-Llahi M, Andreu V, Garre C, Rendón P, Fuentes J, Crespo J, Rodríguez M, Bruix J, Varela M; en representación del Grupo de Estudio de Cáncer Hepático (GECH). Clinical characteristics of hepatocellular carcinoma in Spain. Comparison with the 2008-2009 period and analysis of the causes of diagnosis out of screening programs. Analysis of 686 cases in 73 centers. Med Clin (Barc). 2017;149:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Ferrante ND, Lo Re V 3rd. Epidemiology, Natural History, and Treatment of Hepatitis Delta Virus Infection in HIV/Hepatitis B Virus Coinfection. Curr HIV/AIDS Rep. 2020;17:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Soriano V, Grint D, d'Arminio Monforte A, Horban A, Leen C, Poveda E, Antunes F, de Wit S, Lundgren J, Rockstroh J, Peters L. Hepatitis delta in HIV-infected individuals in Europe. AIDS. 2011;25:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Béguelin C, Moradpour D, Sahli R, Suter-Riniker F, Lüthi A, Cavassini M, Günthard HF, Battegay M, Bernasconi E, Schmid P, Calmy A, Braun DL, Furrer H, Rauch A, Wandeler G; Swiss HIV Cohort Study. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Zheng SJ S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL