Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3516
Peer-review started: February 28, 2021
First decision: March 27, 2021
Revised: April 16, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: June 28, 2021
Processing time: 116 Days and 12.8 Hours
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has a tremendous impact on the health of millions of people worldwide. Unfortunately, those suffering from previous pathological conditions are more vulnerable and tend to develop more severe disease upon infection with the new SARS-CoV-2. This coronavirus interacts with the angiotensin-converting enzyme 2 receptor to invade the cells. Recently, another receptor, neuropilin-1 (NRP-1), has been reported to amplify the viral infection. Interestingly, NRP-1 is expressed in nonparenchymal liver cells and is related to and upregulated in a wide variety of liver-related pathologies. It has been observed that SARS-CoV-2 infection promotes liver injury through several pathways that may be influenced by the previous pathological status of the patient and liver expression of NRP-1. Moreover, coronavirus disease 2019 causes an inflammatory cascade called cytokine storm in patients with severe disease. This cytokine storm may influence liver sinusoidal-cell phenotype, facilitating viral invasion. In this review, the shreds of evidence linking NRP-1 with liver pathologies such as hepatocellular carcinoma, liver fibrosis, nonalcoholic fatty liver disease and inflammatory disorders are discussed in the context of SARS-CoV-2 infection. In addition, the involvement of the infection-related cytokine storm in NRP-1 overexpression and the subsequent increased risk of SARS-CoV-2 infection are also analyzed. This review aims to shed some light on the involvement of liver NRP-1 during SARS-CoV-2 infection and emphasizes the possible involvement this receptor with the observed liver damage.
Core Tip: Severe acute respiratory syndrome coronavirus 2 uses angiotensin-converting enzyme 2 and neuropilin-1 (NRP-1) receptors to infect cells. NRP-1 expression is upregulated in several liver pathologies, which may facilitate viral infection. Moreover, the cytokine storm might increase liver permeability and NRP-1 expression, giving rise to an increased severity of infection and a worse prognosis.
- Citation: Benedicto A, García-Kamiruaga I, Arteta B. Neuropilin-1: A feasible link between liver pathologies and COVID-19. World J Gastroenterol 2021; 27(24): 3516-3529
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3516.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3516
The identification of a new coronavirus from patients suffering from an outbreak of pneumonia of unknown origin in the city of Wuhan, China, in December 2019[1], alarmed the scientific and medical community. This coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), spread all over the globe, becoming a public health emergency and a pandemic that has paralyzed the world for a year. Moreover, the high incidence of newly infected individuals has pushed health systems worldwide to the limit, with a continuous influx of patients requiring hospitalization because of complications related to this new coronavirus. After the chaotic initial months of the pandemic, an increasing number of studies have shown that the severity of SARS-CoV-2 is directly related to the health status of the infected individuals[2] and also with gender[3]. Severe complications and an increased risk of mortality have been linked with various pathologies[2,4] including obesity, diabetes, lung disease, and hypertension[2,4-7]. SARS-CoV-2 invades the mucosal cells of the host, mainly through the nose and mouth. Once in the body, SARS-CoV-2 infects the epithelial cells of the nasal cavity using a specific receptor present in those cells[8]. In detail, angiotensin-converting enzyme 2 (ACE2) protein expressed in the membrane of the epithelial cell surface serves as the entry for the spike protein of the coronavirus, facilitating the infection[2,8]. Once in the cell, SARS-CoV-2 kidnaps the genetic machinery of the host cell to increase its copy number, leading to virus amplification.
Interestingly, there is recent evidence of another SARS-CoV-2 receptor expressed on the surface of host cells during infection, neuropilin-1 (NRP-1)[9,10]. NRP-1 is a non-tyrosine kinase receptor isoform of the NRP protein family, which also includes NRP2. These transmembrane glycoproteins consist of a common short cytoplasmic domain and a large extracellular domain of 840 amino acid residues[11]. NRPs are present in all vertebrates and initially found in neurons, where they function as adhesion molecules[12]. NRPs lack catalytic activity, but they closely interact with the cytosolic adaptor protein synectin or GIPC1[13] to participate in signaling events. NRPs play a critical role during vasculogenesis[14,15] because of their ability to interact with vascular endothelial growth factor (VEGF)[16]. NRP-1 also interacts with transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF)[17] to regulate a broad spectrum of processes under both normal physiological conditions and pathological responses. The findings led to an increasing number of studies of NRPs in health and disease that have shown their involvement in diverse diseases, including pathologic angiogenesis, fibrosis, cirrhosis, and cancer[18-22]. The expression of NRPs is upregulated in those diseases, which suggests that they are potential therapeutic targets.
NRP-1 was initially found in the nervous system, but is expressed in many cell types in tissues of the heart, lung, pancreas, skeletal muscle, and liver. This wides
The pathologies associated with NRP-1 are common to several organs, and many involve the liver, including fibrosis, cirrhosis, malignancies (hepatocellular carcinoma, liver metastasis, cholangiocarcinoma, and others), and angiogenesis. NRP-1 is expressed in liver-resident cells, especially nonparenchymal cells, such as liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSC). Some NRP-1 ligands are involved in liver pathologies. For example, VEGF mediates the angiogenic response of LSECs[23] and is associated with metastatic growth[24]. Another NRP-1 ligand, TGF-β, mediates the activation of HSCs during fibrogenesis[25,26], leading to liver fibrosis and extracellular matrix (ECM) remodeling during liver metastasis and the creation of a premetastatic niche[27]. Platelet-derived growth factor (PDGF) is also involved in HSC activation, a required step in the pathogenesis of liver fibrosis, cholangiocarcinoma, liver metastasis, and HCC[28-31]. It is tempting to hypothesize that the increased expression of NRP-1 in the liver under both physiological conditions and in patients with liver diseases modulates coronavirus disease 2019 (COVID-19) infection. This review summarizes the potential implications of liver expression of NRP-1 during SARS-CoV-2 infection and its possible role in COVID-19 disease progres
The liver is a functionally complex organ that not only maintains metabolic homeostasis but also has immune functions, such as the elimination of pathogens. Recently, NRP-1 has been identified as a facilitating receptor for SARS-CoV-2 infection. As it is expressed in some types of liver cells, it may affect the status of liver disease in COVID-19 patients[10]. Liver functions are carried out by a number of different cell populations, including hepatocytes, which make up about 92.5% of the liver volume[32], and nonparenchymal cells that include LSECs, Kupffer cells (KCs)[33], and HSCs[34]. Small, but important percentages of leukocytes, such as natural killer (NK) cells, natural killer T (NKT) cells, myeloid-derived suppressor cells, and T cells[35,36].
NRP-1 expression has been detected in LSECs[37] and HSCs[38] in the adult liver. Although the expression of NRP-1 is weak in HSCs, it increases following activation associated with diseases with various etiologies. NRP-1 expression in HSCs will be discussed in later sections. Bergé et al[39] reported that NRP-1 was not expressed in the hepatocytes of healthy adult livers[39]. Aung et al[40] reported weak expression in the cytoplasm of adult hepatocytes but no expression in KCs[40]. There is no doubt of the NRP-1 expression in fetal hepatic monocytes observed by Rantakari et al[41] and the absence of NRP-1 in adult hepatic macrophages.
Hepatic sinusoidal endothelium is characterized by the presence of fenestrae and the absence of a true basement membrane. This characteristic of LSECs allows direct contact between blood components and other hepatic cell types[42]. In the fetal liver, NRP-1 is expressed in LSECs in close association with plasmalemma vesicle associated protein (also known as PV-1 and MECA32) during biogenesis of the fenestra, an association that is lost in the adult liver[43]. In fetal LSECs, plasmalemma vesicle associated protein forms additional associations with components of the VEGF signaling pathway. Despite the unknown functional implication of this association, mice deficient in this protein present with significant leukocyte infiltration and an evident steatosis[44].
NRP-1 is expressed in HSCs, but its expression is largely confined to LSECs and co-distributed with that of VEGFR in normal liver tissue. NRP-1 regulates the expression of VEGFR2 at both the transcriptional and post-translational levels by the activation of focal adhesion kinase. The activation of NRP-1 in LSECs thus initiates multiple intracellular signal transduction pathways that regulate cell proliferation, survival, and migration, which are essential for angiogenesis[20].
During physiological aging, the expression of NRP-1 increases in LSECs and is associated with factors present in the lumens of the sinusoids. NRP-1 interacts with hypoxic inducing factor (HIF)-2α to suppress anti-thrombotic and anti-inflammatory pathways that are correlated with profibrotic aggregation of macrophages and platelets. The inhibition of NRP-1 or its association with HIF-2α normalizes the profibrotic niche, with restores the regenerative ability of the liver[45]. It is interesting to note that during aging, LSECs undergo a pseudo capillarization associated with a decrease in endocytic capacity and an increase in leukocyte adhesion, with reduced liver perfusion[46]. However, whether there is a direct relationship between increased expression of NRP-1 in LSECs or pseudo capillarization with a decrease in endocytic capacity is unknown at this time.
The presence of multiple ligands for NRP-1 underscores the importance of this receptor in the liver environment[47]. During adulthood, liver vascularization is stimulated by low blood flow associated with an increase in VEGF and the consequent proliferation of LSECs[48]. Under physiological conditions, hepatic angiogenesis that takes place during regeneration contributes to the formation of new functional sinusoids. As a VEGF coreceptor NRP-1 is the main mediator of angiogenesis[48], although the role of this coreceptor in intrahepatic angiogenesis is currently unclear. NRP-1 may regulate the action of VEGF on vascular permeability, the proliferation of endothelial cells, and leukocyte adhesion. Some studies suggest that NRP-1 acts independently of VEGFR2 or modulates its activity by stimulating cell migration and adhesion, which are essential for the development of an angiogenic response. In addition to its direct action on VEGFR phosphorylation, NRP-1 indirectly stimulates the VEGFR-dependent angiogenic pathway by preventing the binding of VEGF and its decoy receptor, placental growth factor[16,49].
The interaction of NRP-1 with VEGFR and VEGF is dependent on heparin, which can alter VEGF signaling in endothelial cells to either stimulate or inhibit angiogenesis[50]. Heparin is an anticoagulant that is synthesized in the body, eliminated by receptor-mediated endocytosis in LSECs, and accumulates in the liver. The formation of complexes consisting of heparin and other proteins has important clinical implications[51]. Heparin increases the binding of VEGF with the VEGFR/NRP-1[52] complex and the binding of NRP-1 to placental growth factor-2[53,54] by increasing the number of binding sites without affecting the affinity itself.
As mentioned previously, hepatic macrophages in the adult liver do not express NRP-1. However, they do produce a wide range of angiogenic factors that interact with NRP-1, including HIF-2α and VEGF in addition to TGFβ[55], which requires extracellular activation by NRP-1 to increase its affinity for its receptors. The effects of TGFβ on angiogenesis depend on the receptor with which it interacts. Both receptors are expressed in LSECs, suggesting a balancing function in the angiogenic process. The signaling pathways initiated after the interaction of TGFβ with its receptor are highly dependent on the specific microenvironment in the liver at the time. Indeed, signaling via TFGβ receptor binding of anaplastic lymphoma kinase promotes angiogenesis; while the interaction with activin receptor-like kinase 5 inhibits angiogenesis. Although this factor has long been considered profibrogenic in cooperation with other NRP-1 ligands such as PDGF-B, its inhibition causes undesired effects. Expression of both NRP-1 and TGF-β is low in quiescent HSCs in the normal liver and both increase immediately upon liver damage[56]. That suggests a complex scenario for NRP-1 as a coreceptor with angiogenic and profibrogenic receptors.
We must not forget the functions of the liver as an immune organ, in which NRP-1 participates. In addition to the liver lymphocyte population, which is selectively enriched with NK and NKT cells, circulating lymphocytes interact closely with LSECs, KCs, and dendritic cells present in liver sinusoids[57]. NRP-1 has been associated with inhibition of immune responses[58], which may also occur in the liver, an organ that is part of the innate immune system.
COVID-19 affects mainly the upper airways. In some cases, involvement of the lower airways, gives rise to pneumonia[59]. Along with the ability to infect other tissues in addition to the airways, the impact of SARS-CoV-2 infection is detectable in various organs even without local viral invasion[59]. Liver injury occurs in a large proportion of COVID-19 patients and is characterized by elevated levels of gamma-glutamyl transferase, alanine aminotransferase, and/or aspartate aminotransferase enzymes; and occasionally, moderate hyperbilirubinemia[60-63]. The persistence and degree of liver damage caused by COVID-19 seem to vary. In most cases, liver function recovers soon after viral infection, but patients with severe disease may develop irreversible hepatic injury[64-66]. In line with that observation, increased severity of SARS-CoV-2 infection is related to reduced hepatic function[62,67]. A recent postmortem analysis found microvesicular steatosis along with lobular and portal activity in a CODIV-19 patient[68].
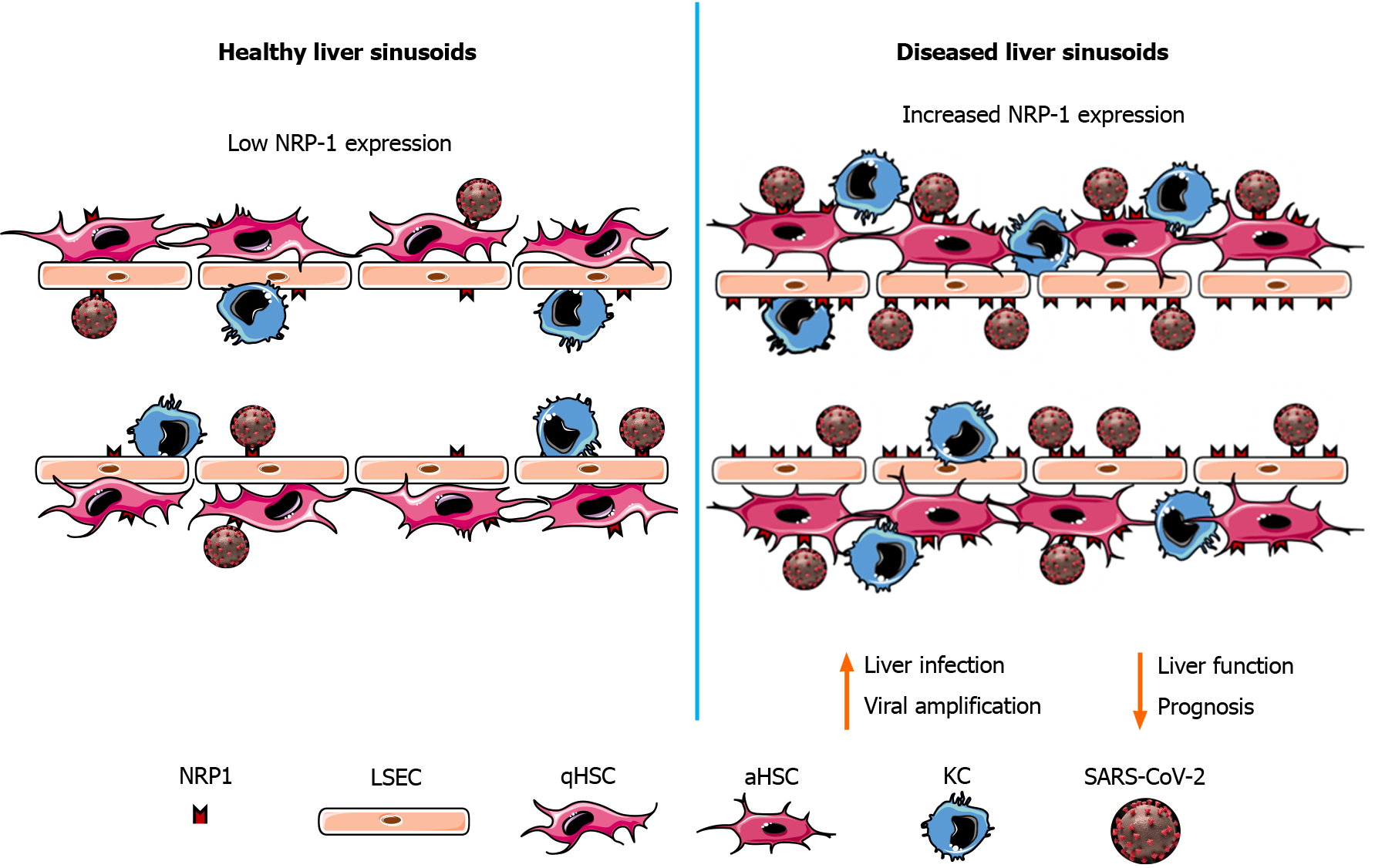
To date, apart from immune-related inflammation and hypoxia generated by airway malfunction, the mechanisms proposed to explain the reported liver damage were drug toxicity, inflammation, and hypoxia resulting from lung malfunction[60,69]. The expression of ACE2 in cholangiocytes has also been proposed as a possible mechanism of liver injury. NRP-1 expression in LSECs and HSCs may thus act to amplify the liver damage as a consequence of SARS-CoV-2 infection (Figure 1).
Hepatic fibrosis is a scarring response involving various types of cells affected by liver diseases of different etiologies[70], including metabolic diseases, exacerbated immune responses, and viral infections. Hepatitis C virus (HCV) infection has some similarities to infections of the SARS-CoV-2 virus family[71]. In SARS, the defects observed in the liver are more the result of viral infection than other factors, such as drug toxicity or systemic inflammatory responses[71]. However, the cause of the liver disease observed in a high percentage of COVID-19 patients following SARS-CoV-2 infection is not clear, but steatosis and fibrosis are observed in liver biopsies.
Wang et al[72] pointed to direct infection of the liver by SARS-CoV-2 as the cause of the steatosis, lobular inflammation, endothelitis, and fibrosis in patients with COVID-19. Even though the ACE2 receptor has been identified as the main mediator of virus entry[73], the probability that SARS-CoV-2 infects the liver by that route is low given its low or null expression in hepatocytes, HSCs, and LSECs[74]. The development of the fibrotic response in COVID-19 patients may then depend on other routes. It is interesting to note that high levels of other molecules involved in the infectivity of SARS-CoV-2 such as furin, TMPRSS11a, and NRP-1 were detected in infected cells[75] with and without ACE2 receptors. Apart from its involvement in the entry of SARS-CoV-2, the role of NRP-1 as a signaling platform has been established. Cao et al[38] found that NRP-1 was a signaling element in HSCs during fibrotic processes caused by viral infections. NRP-1 could thus be the link between infection with SARS-CoV-2 and the development of hepatic steatosis with the presence of a fibrotic response.
HSCs play a central role during liver fibrosis. Following activation, HSCs undergo a change from a quiescent phenotype to a myofibroblastic phenotype characterized by increased proliferation, motility, and accumulation of extracellular matrix. They also contribute to angiogenesis and vascular remodeling processes[70]. HSC activation is initiated by the binding of PDGF-B to its receptor along with a temporal increase in NRP-1. NRP-1 also promotes signaling via other key growth factors involved the development of liver fibrosis, such as TGF-β and VEGF[38]. The overexpression of NRP-1 in patients with cirrhosis caused by HCV infection contributes to the progression of liver fibrosis either by influencing the angiogenic response or effects on the PDGF and TGF-β signaling pathways. NRP-1 regulates not only the motility of HSCs but also collagen deposition, and the severity of fibrosis associated with steatohepatitis and HCV infection are related to the expression of NRP-1. To date, no studies have linked NRP-1 expression with liver involvement in COVID-19 patients. However, it is tempting to hypothesize that the fibrotic response is related to the expression of NRP-1 as an extracellular coreceptor. In other scenarios, such as acute lung SARS or Middle East respiratory syndrome (MERS) infection, cell entry is mediated by TGF-β[76], a mechanism possibly relevant to SARS-CoV-2 and its coreceptor, NRP-1. In the acute phase of COVID-19, the levels of TGF-β are directly related to the development of pulmonary fibrosis[77] and liver fibrosis in pathologies derived from different etiologies[78]. Currently, no studies have found a similar relationship of TGF-β to the fibrotic processes observed in the livers in patients with COVID-19.
Additionally, the mechanical ventilation required by many COVID-19 patients alters hepatic hemodynamics, with reduced portal flow that can result in acute liver damage and activation of HSCs[79]. Although it has not been possible to relate the presence of metabolic fatty liver diseases with the increased risk of hospitalization, nor to the outcomes of hospitalized patients with both diseases, Campos-Murguía et al[80] observed an increased risk of the need for mechanical ventilation with the development of liver fibrosis, among other symptoms. However, other studies observed an increase in the severity of the disease in the presence of a fibrotic development. Even today, it is not clear whether SARS-CoV-2 is solely responsible for the development of liver damage or if the damage is a consequence of systemic inflammation caused by the virus or its treatment[81]. Regardless of the cause of liver damage, conditions such as hypoxia, inflammation, and fibrotic responses that develop as a result of the viral infection are related to elevated levels of NRP-1 in both HSCs and LSECs. Indeed, in hypoxic states, such as those observed during COVID-19 progression, HSCs respond through an increase in VEGF. As a result, there is a concomitant increase in the expression of NRP-1 in LSECs, HSC motility, and TGF-β-dependent collagen production[47]. In the presence of TGF-β the glycome of activated HSCs favors the binding of galectins to NRP-1, which promotes migration and further activation[19,82]. It is interesting to note that the levels of both galectin-1 and -3 are increased in COVID-19 patients and have been significantly associated with the severity of the disease[83].
In addition to all the above, the activation of HSCs, which drives liver fibrosis, is induced by profibrotic and proinflammatory cytokines. The resulting inflammatory environment during the development and progression of COVID-19 may be one of the causes of the reported liver damage and a cause of HSC activation, with the consequent induction of the fibrotic response. In fact, during the development of liver fibrosis, infiltration of a subgroup of macrophages enriched in genes associated with tissue repair has been observed[84,85]. The genes encode coreceptors of NRP-1 and the inflammatory cytokines that regulate its expression[86]. One of those cytokines is IL-6, which results from activation of the immune system by SARS-CoV-2 in COVID-19 patients and is associated with altered levels of liver enzymes[87]. In various viral infections, IL-6 is associated with the development of liver fibrosis[88] and with an increase in the expression of NRP-1[89]. In addition, the level of galectin-3, which binds to NRP-1 and has a structure similar to a part of the SARS-CoV-2 spike protein[90], leads to dysregulated expression of proinflammatory cytokines[83,91]. Its expression is increased secondary to diverse types of injury mediated by viral diseases including hepatitis B, hepatitis C, and, SARS-CoV-2[92]. The findings suggest a nexus between systemic inflammation and subsequent liver fibrosis in patients with COVID-19 through NRP-1.
Viral infection of the liver is the leading cause of hepatocellular carcinoma (HCC) worldwide[93]. Changes in signaling pathways during viral infection promote inflammation that contributes to the development and progression of chronic liver disease beginning with hepatic cirrhosis and finally, HCC[94]. Interestingly, HCV elimination improves the overall survival of HCC patients, indicating that viral infections complicate the outcome[95]. Similarly, SARS-CoV-2 viral infection may facilitate HCC or complicate the outcome of the disease. In the context of the SARS-CoV-2 pandemic, several reports have linked the presence of cancer with an increase of a worse outcome in COVID-19 patients[96,97]. Furthermore, a prospective nationwide cohort study carried out in China revealed that 1% of COVID-19 patients had a history of cancer. Those patients had an increased risk of complications, ICU admission, and a fatal outcome compared with patients without cancer. Interestingly, cancer survivors had more likely to suffer disease complications than healthy people but less likely than cancer patients[98]. Recent studies describe a possible link between SARS-CoV-2 receptor ACE2 in cancer tissues and increased risk of infection[99]. In liver cancer, Dai et al[100] found that upregulated expression of ACE2, the primary SARS-CoV-2 ligand in infected cells, was related to improved survival in HCC patients. However, an increase in ACE2 expression might make such patients more vulnerable to SARS-CoV-2 infection. The link between NRP-1 and liver cancer was discovered some years ago, with increased NRP-1 expression in hepatocellular carcinoma[39]. NRP-1 expression was higher in LSECs from HCC biopsies than from healthy liver biopsies. Hepatocyte expression of NRP-1 correlated with primary HCC and increased with tumor progression. Blocking NRP-1 protein led to impaired tumor growth and vascular remodeling[39]. Interestingly, NRP-1 expression stimulated the activation of HSCs[101], liver-resident myofibroblast-like cells that contribute to the malignant growth of liver metastasis[23,24,102]. The NRP-1-dependent activation boosted tumor proliferation, cell migration, and invasiveness[101]. Therefore, it is tempting to hypothesize that HCC patients with increased NRP-1 expression in LSECs, tumor cells, and HSCs may have an increased risk of SARS-CoV-2 infection.
Cholangiocarcinoma (CCA) is a relatively uncommon liver malignancy, accounting for about 10% of liver cancers[103]. The risk of CCA appears to be increased by both HCV and hepatitis B virus (HBV) infection[104], which may also account for increased SARS-CoV-2 risk. Little is known about the involvement of NRP-1 in CCA. There is evidence that NRP-1 expression is elevated in intrahepatic CCA tissue compared with normal biliary tissue. Moreover, the association of NRP-1 and CCA development has been confirmed by NRP-1 knockdown leading to impaired cancer cell proliferation, blocked cell cycle, reduced cell migration, and reduced focal adhesion kinase expression[105]. In line with that finding, NRP-1 overexpression in intrahepatic CCA cells that was associated with miR-320a downregulation boosted cell proliferation and epithelial to mesenchymal transition and stimulated tumor angiogenesis[106]. Recently, high NRP-1 expression was correlated with poor prognosis and reduced overall survival of intrahepatic CCA patients[107]. Intrahepatic CCA may favor development of an inflammatory milieu driving to vascular permeabilization and increased expression of NRP-1 upon activation of liver-resident cells. The inflammation may be mediated by IL-13, which significantly increased in CCA patients and is known to promote the expression of adhesion molecules in endothelial cells[108,109]. Therefore, CCA could indirectly promote NRP-1 expression in both LSECs and HSCs and facilitate liver damage by immune infiltration. Based on the data, SARS-CoV-2 infection may be facilitated by CCA through NRP-1 overexpression. HCV, HBV, or SARS-CoV-2 infection could drive to liver inflammation and LSEC and HSC activation. The process would lead to increased recruitment of inflammatory cells, NRP-1 overexpression, and assisted viral infection accompanied by liver injury.
Cytokine storm is a potentially fatal consequence of SARS-CoV-2 infection. The release of inflammatory cytokines into the blood of infected individuals is a major turning point in the prognosis and survival of patients with severe disease[110]. The liver filters and detoxifies the blood supply coming from the portal vein and hepatic artery. During COVID-19, both amplifying viruses and cytokines released during the cytokine storm enter the liver and flow through the liver sinusoids and small diameter capillaries in contact with nonparenchymal liver cells, LSECs, KCs, and HSCs[51]. These small vessels have specific properties adapted for their function, such as fenestrations in the endothelial layer, tightly controlled immune tolerance, close contact with nonparenchymal-cell subsets and hepatocytes, and a wide array of cell adhesion molecules and receptors[111,112].
As mentioned previously, the cytokine storm is induced by the release of inflammatory mediators such as IL-6, IL-1β, IL-10, TNF-α, interferon-γ, macrophage inflammatory protein (MIP) 1α and 1β, and VEGF[67,113] and complicates the course of the disease, driving to multiorgan failure and coagulation. The role of these inflammatory mediators in the recruitment of immune and nonimmune cells into the infectious foci, mainly the lungs, is widely recognized and well characterized. However, these soluble proteins have the intrinsic ability to switch on a wide spectrum of cellular responses in both immune cells and nonimmune cells in epithelial, endothelial, and other tissues[114]. Some of the soluble mediators released during the cytokine storm may increase liver permeability. The effects of TNF-α, IL-6, and IL-1β increase the expression of intercellular adhesion molecule 1 (ICAM-1) in endothelial cells[115-117], which may take place during SARS-CoV-2 infection. LSEC ICAM-1 mediates the adhesion and infiltration of immune cells in the liver[118], which may further increase the number of inflammatory cells and increase the extent of liver damage.
VEGF mediates the recruitment of several immune populations[119] and is considered the main stimulus for the proliferation and migration of endothelial cells[120]. It is also a ligand of NRP-1[53]. Interestingly, VEGF acts on endothelial cells not only as a proliferative and promigratory signal, but it can stimulate the expression of NRP-1[121,122], creating a feedback loop. Consequently, VEGF may facilitate SARS-CoV-2 infection in the liver through the upregulation of the local expression of NRP-1, the recently discovered SARS-CoV-2 receptor. It is tempting to hypothesize that this process may take place in other tissues such as the lung epithelium and that the involvement of IL-6 in promoting increased NRP-1 expression might be underestimated. Previous reports have described NRP-1 upregulation in pancreatic cancer[89,123], which was stimulated by IL-6 and mediated by STAT3 transcription activity[89]. Interestingly, the IL-6/STAT3 pathway plays a significant role during HSC activation[124] and might boost the expression of NRP-1 in these liver-specific cells.
The severity of SARS-CoV-2 infection is increased in patients with previous pathologies. A high proportion of COVID-19 patients experience liver damage. NRP-1 is a recently discovered coreceptor for SARS-CoV-2 virus, and is overexpressed in the injured and pathologic liver. Patients with SARS-CoV-2 infection and liver diseases should be followed to monitor the liver response and overall health status. The increased expression in NRP-1 in the pathologic liver of patients suffering from COVID-19 may represent an amplifying pathway to further complicate the infection and worsen the prognosis and severity of the disease. There is evidence of a link between liver NRP-1 and SARS-CoV-2 infection, but further study is needed to determine whether previous liver disease and NRP-1 influence COVID-19 progression, severity and mortality. Even though the presence of liver disease can promote the increased severity of COVID-19 disease, direct infection and liver injury by SARS-COV-2 virus cannot be ruled out. Liver diseases, such as fibrosis and different types of liver cancers, share active mediators that are directly linked to NRP-1, indicating a feasible and direct relationship of NRP-1, liver disease with COVID-19.
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17906] [Article Influence: 2984.3] [Reference Citation Analysis (2)] |
| 2. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18404] [Article Influence: 3067.3] [Reference Citation Analysis (13)] |
| 3. | Belice T, Demir I. The gender differences as a risk factor in diabetic patients with COVID-19. Iran J Microbiol. 2020;12:625-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Abe T, Egbuche O, Igwe J, Jegede O, Wagle B, Olanipekun T, Onwuanyi A. Cardiovascular complications in COVID-19 patients with or without diabetes mellitus. Endocrinol Diabetes Metab. 2020;e00218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Al Heialy S, Hachim MY, Hachim IY, Bin Naeem K, Hannawi H, Lakshmanan J, Al Salmi I, Hannawi S. Combination of obesity and co-morbidities leads to unfavorable outcomes in COVID-19 patients. Saudi J Biol Sci. 2021;28:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Signes-Costa J, Núñez-Gil IJ, Soriano JB, Arroyo-Espliguero R, Eid CM, Romero R, Uribarri A, Fernández-Rozas I, Aguado MG, Becerra-Muñoz VM, Huang J, Pepe M, Cerrato E, Raposeiras S, Gonzalez A, Franco-Leon F, Wang L, Alfonso E, Ugo F, García-Prieto JF, Feltes G, Abumayyaleh M, Espejo-Paeres C, Jativa J, Masjuan AL, Macaya C, Carbonell Asíns JA, Estrada V; HOPE COVID-19 investigators. Prevalence and 30-Day Mortality in Hospitalized Patients With Covid-19 and Prior Lung Diseases. Arch Bronconeumol. 2021;57 Suppl 2:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 667] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 8. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14589] [Article Influence: 2431.5] [Reference Citation Analysis (3)] |
| 9. | Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Antón-Plágaro C, Shoemark DK, Simón-Gracia L, Bauer M, Hollandi R, Greber UF, Horvath P, Sessions RB, Helenius A, Hiscox JA, Teesalu T, Matthews DA, Davidson AD, Collins BM, Cullen PJ, Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 969] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 10. | Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1438] [Article Influence: 239.7] [Reference Citation Analysis (24)] |
| 11. | Schwarz Q, Ruhrberg C. Neuropilin, you gotta let me know: should I stay or should I go? Cell Adh Migr. 2010;4:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Parker MW, Guo HF, Li X, Linkugel AD, Vander Kooi CW. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51:9437-9446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 13. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 13501] [Article Influence: 964.4] [Reference Citation Analysis (0)] |
| 14. | Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895-4902. [PubMed] |
| 15. | Yuan L, Moyon D, Pardanaud L, Bréant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797-4806. [PubMed] |
| 16. | Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 1882] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 17. | Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol. 2016;23:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Mohseni N, Roshan R, Naderi S, Behdani M, Kazemi-Lomedasht F. In vitro combination therapy of pathologic angiogenesis using anti-vascular endothelial growth factor and anti-neuropilin-1 nanobodies. Iran J Basic Med Sci. 2020;23:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Wu MH, Chen YL, Lee KH, Chang CC, Cheng TM, Wu SY, Tu CC, Tsui WL. Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells. Sci Rep. 2017;7:11006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Wang L, Feng Y, Xie X, Wu H, Su XN, Qi J, Xin W, Gao L, Zhang Y, Shah VH, Zhu Q. Neuropilin-1 aggravates liver cirrhosis by promoting angiogenesis via VEGFR2-dependent PI3K/Akt pathway in hepatic sinusoidal endothelial cells. EBioMedicine. 2019;43:525-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | De Vlaeminck Y, Bonelli S, Awad RM, Dewilde M, Rizzolio S, Lecocq Q, Bolli E, Santos AR, Laoui D, Schoonooghe S, Tamagnone L, Goyvaerts C, Mazzone M, Breckpot K, Van Ginderachter JA. Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Cao H, Li Y, Huang L, Bai B, Xu Z. Clinicopathological Significance of Neuropilin 1 Expression in Gastric Cancer: A Meta-Analysis. Dis Markers. 2020;2020:4763492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Benedicto A, Herrero A, Romayor I, Marquez J, Smedsrød B, Olaso E, Arteta B. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci Rep. 2019;9:13111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Herrero A, Benedicto A, Romayor I, Olaso E, Arteta B. Inhibition of COX-2 Impairs Colon Cancer Liver Metastasis through Reduced Stromal Cell Reaction. Biomol Ther (Seoul). 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Okuno M, Sato T, Kitamoto T, Imai S, Kawada N, Suzuki Y, Yoshimura H, Moriwaki H, Onuki K, Masushige S, Muto Y, Friedman SL, Kato S, Kojima S. Increased 9,13-di-cis-retinoic acid in rat hepatic fibrosis: implication for a potential link between retinoid loss and TGF-beta mediated fibrogenesis in vivo. J Hepatol. 1999;30:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hong J, Kim S, Lin PC. Interleukin-33 and ST2 Signaling in Tumor Microenvironment. J Interferon Cytokine Res. 2019;39:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1554] [Cited by in RCA: 2162] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 28. | Kocabayoglu P, Lade A, Lee YA, Dragomir AC, Sun X, Fiel MI, Thung S, Aloman C, Soriano P, Hoshida Y, Friedman SL. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 29. | Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, Fingas C, Cristina Malerba M, Nardo G, Dall'Olmo L, Milani E, Mariotti V, Stecca T, Massani M, Spirli C, Fiorotto R, Indraccolo S, Strazzabosco M, Fabris L. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 30. | Yuge R, Kitadai Y, Shinagawa K, Onoyama M, Tanaka S, Yasui W, Chayama K. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am J Pathol. 2015;185:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Patel S, Nanavati P, Sharma J, Chavda V, Savjani J. Functional Role of Novel Indomethacin Derivatives for the Treatment of Hepatocellular Carcinoma Through Inhibition of Platelet-Derived Growth Factor. Arch Med Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 645] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 33. | Smedsrød B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, Geerts A. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Friedman SL. Hepatic stellate cells. Prog Liver Dis. 1996;14:101-130. [PubMed] |
| 35. | Freitas-Lopes MA, Mafra K, David BA, Carvalho-Gontijo R, Menezes GB. Differential Location and Distribution of Hepatic Immune Cells. Cells. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | McNamara HA, Cockburn IA. The three Rs: Recruitment, Retention and Residence of leukocytes in the liver. Clin Transl Immunology. 2016;5:e123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, Spira G. Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol. 2004;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, Charlton M, Watts RJ, Mukhopadhyay D, Shah VH. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Bergé M, Allanic D, Bonnin P, de Montrion C, Richard J, Suc M, Boivin JF, Contrerès JO, Lockhart BP, Pocard M, Lévy BI, Tucker GC, Tobelem G, Merkulova-Rainon T. Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Aung NY, Ohe R, Meng H, Kabasawa T, Yang S, Kato T, Yamakawa M. Specific Neuropilins Expression in Alveolar Macrophages among Tissue-Specific Macrophages. PLoS One. 2016;11:e0147358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Rantakari P, Patten DA, Valtonen J, Karikoski M, Gerke H, Dawes H, Laurila J, Ohlmeier S, Elima K, Hübscher SG, Weston CJ, Jalkanen S, Adams DH, Salmi M, Shetty S. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci USA. 2016;113:9298-9303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver Sinusoidal Endothelial Cells. Compr Physiol. 2015;5:1751-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Herrnberger L, Hennig R, Kremer W, Hellerbrand C, Goepferich A, Kalbitzer HR, Tamm ER. Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage. PLoS One. 2014;9:e115005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Auvinen K, Lokka E, Mokkala E, Jäppinen N, Tyystjärvi S, Saine H, Peurla M, Shetty S, Elima K, Rantakari P, Salmi M. Fenestral diaphragms and PLVAP associations in liver sinusoidal endothelial cells are developmentally regulated. Sci Rep. 2019;9:15698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Chen Y, Pu Q, Ma Y, Zhang H, Ye T, Zhao C, Huang X, Ren Y, Qiao L, Liu HM, Esmon CT, Ding BS, Cao Z. Aging Reprograms the Hematopoietic-Vascular Niche to Impede Regeneration and Promote Fibrosis. Cell Metab 2021; 33: 395-410. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 46. | LE Couteur DG, Cogger VC, McCuskey RS, DE Cabo R, Smedsrød B, Sorensen KK, Warren A, Fraser R. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Elpek GÖ. Neuropilins and liver. World J Gastroenterol. 2015;21:7065-7073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Fleischer JR, Jodszuweit CA, Ghadimi M, De Oliveira T, Conradi LC. Vascular Heterogeneity With a Special Focus on the Hepatic Microenvironment. Front Physiol. 2020;11:591901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 558] [Article Influence: 24.3] [Reference Citation Analysis (6)] |
| 50. | Teran M, Nugent MA. Synergistic Binding of Vascular Endothelial Growth Factor-A and Its Receptors to Heparin Selectively Modulates Complex Affinity. J Biol Chem. 2015;290:16451-16462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Oie CI, Olsen R, Smedsrød B, Hansen JB. Liver sinusoidal endothelial cells are the principal site for elimination of unfractionated heparin from the circulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G520-G528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci USA. 2007;104:6152-6157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Klagsbrun M, Takashima S, Mamluk R. The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol. 2002;515:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818-24825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Roth S, Gong W, Gressner AM. Expression of different isoforms of TGF-beta and the latent TGF-beta binding protein (LTBP) by rat Kupffer cells. J Hepatol. 1998;29:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 605] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 57. | Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 995] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 58. | Prud'homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 59. | Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC, Chang L, Gendelman HE, Kevadiya BD. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol. 2020;15:359-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 359] [Article Influence: 59.8] [Reference Citation Analysis (16)] |
| 60. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 577] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 61. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11616] [Article Influence: 1936.0] [Reference Citation Analysis (2)] |
| 62. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19026] [Article Influence: 3171.0] [Reference Citation Analysis (9)] |
| 63. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6712] [Article Influence: 1118.7] [Reference Citation Analysis (1)] |
| 64. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13064] [Article Influence: 2177.3] [Reference Citation Analysis (4)] |
| 65. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1309] [Article Influence: 218.2] [Reference Citation Analysis (8)] |
| 66. | Sun LJ, Yu JW, Shi YG, Zhang XY, Shu MN, Chen MY. Hepatitis C virus core protein induces dysfunction of liver sinusoidal endothelial cell by down-regulation of silent information regulator 1. J Med Virol. 2018;90:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30483] [Article Influence: 5080.5] [Reference Citation Analysis (13)] |
| 68. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5833] [Article Influence: 972.2] [Reference Citation Analysis (3)] |
| 69. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 446] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 70. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 71. | Humar A, McGilvray I, Phillips MJ, Levy GA. Severe acute respiratory syndrome and the liver. Hepatology. 2004;39:291-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 73. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4644] [Article Influence: 201.9] [Reference Citation Analysis (0)] |
| 74. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 75. | Cantuti-Castelvetri L, Ojha R, Pedro L, Djannatian M, Franz J, Kuivanen S, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier F, Butcher S, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. 2020 Preprint. Available from: bioRxiv: 2020.06.07. 137802;. [DOI] [Full Text] |
| 76. | Evans RM, Lippman SM. Shining Light on the COVID-19 Pandemic: A Vitamin D Receptor Checkpoint in Defense of Unregulated Wound Healing. Cell Metab. 2020;32:704-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Zhao X, Nicholls JM, Chen YG. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem. 2008;283:3272-3280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 79. | Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver disease and COVID-19: from Pathogenesis to Clinical Care. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 80. | Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, González-Regueiro JA, Solís-Ortega AA, Kúsulas-Delint D, Cruz-Contreras M, Cruz-Yedra N, Cubero FJ, Nevzorova YA, Martínez-Cabrera CF, Moreno-Guillén P, Lozano-Cruz OA, Chapa-Ibargüengoitia M, Gulías-Herrero A, Aguilar-Salinas CA, Ruiz-Margáin A, Macías-Rodríguez RU. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 82. | Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060-5065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 83. | Kazancioglu S, Yilmaz FM, Bastug A, Ozbay BO, Aydos O, Yücel Ç, Bodur H, Yilmaz G. Assessment of Galectin-1, Galectin-3, and PGE2 Levels in Patients with COVID-19. Jpn J Infect Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1866] [Article Influence: 311.0] [Reference Citation Analysis (0)] |
| 85. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 850] [Article Influence: 70.8] [Reference Citation Analysis (4)] |
| 86. | Levi M, Nieuwdorp M, van der Poll T, Stroes E. Metabolic modulation of inflammation-induced activation of coagulation. Semin Thromb Hemost. 2008;34:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Da BL, Kushner T, El Halabi M, Paka P, Khalid M, Uberoi A, Lee BT, Perumalswami PV, Rutledge SM, Schiano TD, Friedman S, Saberi B. Liver Injury in Hospitalized Patients with COVID-19 Correlates with Hyper Inflammatory Response and Elevated IL-6. Hepatol Commun. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 88. | Fuster D, Tsui JI, Cheng DM, Quinn EK, Armah KA, Nunes D, Freiberg MS, Samet JH. Interleukin-6 is associated with noninvasive markers of liver fibrosis in HIV-infected patients with alcohol problems. AIDS Res Hum Retroviruses. 2013;29:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Yang Y, Yang L, Li Y. Neuropilin-1 (NRP-1) upregulated by IL-6/STAT3 signaling contributes to invasion in pancreatic neuroendocrine neoplasms. Hum Pathol. 2018;81:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Caniglia JL, Guda MR, Asuthkar S, Tsung AJ, Velpula KK. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ. 2020;8:e9392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 91. | Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology. 1998;94:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2035] [Cited by in RCA: 2243] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 93. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264-1273. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2564] [Article Influence: 183.1] [Reference Citation Analysis (3)] |
| 94. | Wu JW, Kao JH, Tseng TC. Three Heads are Better than Two: HBcrAg as a New Predictor of HBV-related HCC. Clin Mol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 95. | Nahon P, Layese R, Cagnot C, Asselah T, Guyader D, Pol S, Pageaux GP, De Lédinghen V, Ouzan D, Zoulim F, Audureau E; ANRS CO12 CirVir group. HCV Eradication in Primary or Secondary Prevention Optimizes Hepatocellular Carcinoma Curative Management. Cancer Prev Res (Phila). 2021;14:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care. 2020;24:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 97. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4321] [Article Influence: 720.2] [Reference Citation Analysis (1)] |
| 98. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3332] [Cited by in RCA: 3154] [Article Influence: 525.7] [Reference Citation Analysis (0)] |
| 99. | Hoang T, Nguyen TQ, Tran TTA. Genetic Susceptibility of ACE2 and TMPRSS2 in Six Common Cancers and Possible Impacts on COVID-19. Cancer Res Treat. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Dai YJ, Hu F, Li H, Huang HY, Wang DW, Liang Y. A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann Transl Med. 2020;8:481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 101. | Xu ZC, Shen HX, Chen C, Ma L, Li WZ, Wang L, Geng ZM. Neuropilin-1 promotes primary liver cancer progression by potentiating the activity of hepatic stellate cells. Oncol Lett. 2018;15:2245-2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Romayor I, Badiola I, Benedicto A, Márquez J, Herrero A, Arteta B, Olaso E. Silencing of sinusoidal DDR1 reduces murine liver metastasis by colon carcinoma. Sci Rep. 2020;10:18398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Cardinale V, Bragazzi MC, Carpino G, Torrice A, Fraveto A, Gentile R, Pasqualino V, Melandro F, Aliberti C, Bastianelli C, Brunelli R, Berloco PB, Gaudio E, Alvaro D. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 104. | Tan JH, Zhou WY, Zhou L, Cao RC, Zhang GW. Viral hepatitis B and C infections increase the risks of intrahepatic and extrahepatic cholangiocarcinoma: Evidence from a systematic review and meta-analysis. Turk J Gastroenterol. 2020;31:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Zhu H, Jiang X, Zhou X, Dong X, Xie K, Yang C, Jiang H, Sun X, Lu J. Neuropilin-1 regulated by miR-320 contributes to the growth and metastasis of cholangiocarcinoma cells. Liver Int. 2018;38:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Zhu H, Zhai B, He C, Li Z, Gao H, Niu Z, Jiang X, Lu J, Sun X. LncRNA TTN-AS1 promotes the progression of cholangiocarcinoma via the miR-320a/neuropilin-1 axis. Cell Death Dis. 2020;11:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Wu YN, He LH, Bai ZT, Li X. NRP1 is a Prognostic Factor and Promotes the Growth and Migration of Cells in Intrahepatic Cholangiocarcinoma. Cancer Manag Res. 2020;12:7021-7032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G, Destro A, Sozio F, Di Tommaso L, Roncalli M, Banales JM, Coulouarn C, Bujanda L, Torzilli G, Invernizzi P. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 109. | Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799-803. [PubMed] |
| 110. | Kovalchuk A, Wang B, Li D, Rodriguez-Juarez R, Ilnytskyy S, Kovalchuk I, Kovalchuk O. Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? Aging (Albany NY). 2021;13:1571-1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol. 2018;15:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 112. | Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 447] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 113. | Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 114. | Schumacher N, Rose-John S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Eaton KV, Yang HL, Giachelli CM, Scatena M. Engineering macrophages to control the inflammatory response and angiogenesis. Exp Cell Res. 2015;339:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | McHale JF, Harari OA, Marshall D, Haskard DO. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha. J Immunol. 1999;162:1648-1655. [PubMed] |
| 118. | Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 567] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 119. | Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 120. | Djordjevic S, Driscoll PC. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov Today. 2013;18:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Oh H, Takagi H, Otani A, Koyama S, Kemmochi S, Uemura A, Honda Y. Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc Natl Acad Sci USA. 2002;99:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 122. | Mehta V, Fields L, Evans IM, Yamaji M, Pellet-Many C, Jones T, Mahmoud M, Zachary I. VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling. Arterioscler Thromb Vasc Biol. 2018;38:1845-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 123. | Feurino LW, Zhang Y, Bharadwaj U, Zhang R, Li F, Fisher WE, Brunicardi FC, Chen C, Yao Q, Min L. IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer Biol Ther. 2007;6:1096-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Kagan P, Sultan M, Tachlytski I, Safran M, Ben-Ari Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS One. 2017;12:e0176173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan X S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Ma YJ