Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3466
Peer-review started: January 26, 2021
First decision: February 24, 2021
Revised: March 13, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: June 28, 2021
Processing time: 149 Days and 14.3 Hours
Primary liver cancers carry significant morbidity and mortality. Hepatocellular carcinoma (HCC) develops within the hepatic parenchyma and is the most common malignancy originating from the liver. Although 80% of HCCs develop within background cirrhosis, 20% may arise in a non-cirrhotic milieu and are referred to non-cirrhotic-HCC (NCHCC). NCHCC is often diagnosed late due to lack of surveillance. In addition, the rising prevalence of non-alcoholic fatty liver disease and diabetes mellitus have increased the risk of developing HCC on non-cirrhotic patients. Viral infections such as chronic Hepatitis B and less often chronic hepatitis C with advance fibrosis are associated with NCHCC. NCHCC individuals may have Hepatitis B core antibodies and occult HBV infection, signifying the role of Hepatitis B infection in NCHCC. Given the effectiveness of current antiviral therapies, surgical techniques and locoregional treatment options, nowadays such patients have more options and potential for cure. However, these lesions need early identification with diagnostic models and multiple surveillance strategies to improve overall outcomes. Better under
Core Tip: Non-cirrhotic hepatocellular carcinoma (HCC) accounts for 20% of reported HCCs. Such tumors are typically diagnosed late, compromising the outcome. The discovery of direct antivirals, loco-regional treatments and systemic novel immune-chemotherapies, along with advancements of complex hepatobiliary surgery, and the genesis of transplant oncology have revolutionized the management of these aggressive primary liver tumors. Coordinated care at tertiary high-volume HCC, preferably liver transplant centers, remains critical. It is time the stakeholders pursued a consensus approach in developing universal HCC surveillance and treatment strategies on non-cirrhotic patients at risk, such as patients with non-alcoholic steatohepatitis and/or patients with advanced fibrosis.
- Citation: Perisetti A, Goyal H, Yendala R, Thandassery RB, Giorgakis E. Non-cirrhotic hepatocellular carcinoma in chronic viral hepatitis: Current insights and advancements. World J Gastroenterol 2021; 27(24): 3466-3482
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3466
Primary liver cancer originates from the liver parenchyma, the bile ducts or both. Worldwide, as per 2018 statistics, primary liver cancer is the second most lethal cancer (only next to pancreatic cancer), fourth leading cause of cancer-related mortality and the sixth most frequently diagnosed with an incidence of 841000 cases per year[1]. Hepatocellular carcinoma (HCC) is the most common primary malignant tumor (90%) originating from the liver[2]. HCC commonly develops within a background of chronic liver disease, characterized by progressive hepatic fibrosis, loss of architecture and formation of regenerative nodules (cirrhosis). This is referred to as cirrhotic-HCC and is present in the majority of the cases (80%). However, 20% of HCC cases develop on a non-cirrhotic background, and therefore referred to as non-cirrhotic-HCC (NCHCC)[3]. Fibrolamellar HCC, angiosarcoma, lymphoma, embryonic sarcoma are other non-cirrhotic liver malignancies, but are rare in occurrence. Due to the lack of surveillance strategies, NCHCC is often diagnosed late, leading to poor prognosis[4,5].
NCHCC risk factors include alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), hepatitis B [hepatitis B virus, (HBV)], hepatitis C [hepatitis C virus (HCV)], hepatitis D virus, tobacco abuse, diabetes mellitus, genetic factors and environmental toxins. Among these, NAFLD and viral hepatitis (HBV and HCV) have been the most common. Given the obesity pandemic, NAFLD burden on the health systems has been expanding. Chronic HBV can be associated with high viral loads, hepatitis D coinfection, prolonged replication phase, concomitant tobacco use and alcohol intake, which can predispose to NCHCC pathogenesis. Similarly, chronic HCV can be associated with carcinogenesis due to multiple gene products development during viremia, altered cell cycle resulting in NCHCC. Further, this risk can continue even after eradication of HCV. It is critical to understand the underlying mechanisms associated with chronic viral hepatitis leading to NCHCC. A comprehensive strategy is needed for surveillance, diagnosis, and management of these tumors[6].
In this review, we discuss NCHCC epidemiology, risk factors, and pathogenesis, and elaborate on NCHCC diagnosis and treatment strategies.
An online search was performed using databases PubMed/Medline, EMBASE, Cochrane, Web of Science and CINAHL from January 1, 2000 to January 1, 2021 to identify published reports on HCC in chronic viral hepatitis without cirrhosis. We used following search terms- “carcinoma, hepatocellular” or “cancer, hepatocellular” and ”viral hepatitis” and “HBV” and “HCV” excluding “liver cirrhosis.” This resulted in 705 published reports. With use of filters (human species and English language), 648 published reports were obtained. After removing articles not relevant/duplicates/ non-English language including a manual search, 677 published articles were reviewed.
HCC is the most common primary liver cancer and a leading cause of cancer-related deaths[5]. HCC-related deaths have been increasing globally. In the United States, in 2001-2006, average HCC incidence increased from 2.7 (2001) per 100,000 to 3.2 per 100,000 (2006)[7]. During this time, HCC was considered ninth leading cause of cancer death. From 2011-2014, mortality from HCC increased by 2.7%[8]. Geographically, HCC is 72% in Asia[9]. World’s highest incidence is noted in Mongolia with an incidence of 93.7 per 100,000[1]. Racial and ethnic variations are present in HCC with Asian/Pacific Islands, Blacks, Native Americans having a higher prevalence compared to whites[7,10].
Up to 20% of HCC can grow in a non-cirrhotic liver[11]. While HCC shows a unimodal distribution with peak at the seventh decade of life, NCHCC shows bimodal age distribution, with peaks at second and seventh decade of life[11,12]. This could be related to HBV infection at birth and during adult life[13]. Fibrolamellar HCC is commonly seen in younger adults (second and third decades of life). Male to female ratio is 3:2 in HCC vs 1.3-2:1 for NCHCC[14]. Fibrolamellar HCC does not show any sex predilection. Contrary to HCC which is seen frequently in Asians, prevalence of fibrolamellar HCC is higher in Europe and North America[15]. Shim et al[16] reported that in Korea, prevalence of HBV infection among NCHCC can range up to 77%. NCHCC individuals, even without active HBV infection, can have antibodies against HBV core (indicative of prior HBV infection) and occult HBV infection [HBV DNA in the liver/blood without hepatitis B surface antigen (HBsAg)], signifying the role of HBV infection in NCHCC[16]. Despite the disappearance of HBV antigen (with treatment or spontaneous regression), some patients continue to be at risk of developing HCC[17].
Obesity, overweight, and diabetes mellitus are considered NCHCC risk factors. Fatty liver disease can lead to inflammation and hepatocellular carcinogenesis (Table 1)[18]. Tumor suppressor gene dysregulation plays an important role in steatosis development, hepatocyte injury, and NCHCC tumorigenesis[4,19]. Increased tumor necrosis factor-alfa, interleukin-6, leptin, resistin and decreased adiponectin contribute to carcinogenesis in non-cirrhotic livers[20]. Recently, Sydor et al[21] reported an association with gut microbiota and primary conjugated bile acid composition in CHCC and NCHCC carcinogenesis among NASH patients[21]. Microbiota-associated changes in bile acid homeostasis and farnesoid X receptor signaling via fibroblast growth factor-19 might contribute to the tumorigenesis in these patients[21]. Given the promising results obtained from direct-acting antiviral (DAA) agents in the treatment of HCV, metabolic syndrome and NAFLD will likely predominate and may become the leading risk factor for the pathogenesis of HCC and NCHCC.
| HCC | NCHCC | |
| Epidemiology | Eighty percent of HCC develops with a cirrhotic background. A unimodal age distribution (peak in 7th decade) noted. Male:female ratio - 3:2 | Twenty percent of tumors develop in non-cirrhotic liver. A bimodal age distribution (peak in 2nd and 7th decade) noted. Male:female ratio-2:1 |
| Risk factors | Development of cirrhosis from any etiology can progress to HCC. Hepatotropic viruses, environmental and life-style factors (alcohol, tobacco), metabolic conditions (nonalcoholic fatty liver disease, diabetes mellitus, obesity) play a predominant role | NCHCC develops without a background of underlying cirrhosis. Viral (HBV, HCV infection) and non-viral risk factors (obesity, diabetes mellitus, toxin exposure, germline mutations and genetic disorders) noted |
| Clinical features | Symptoms could be related to underlying cirrhosis (from portal hypertension) or HCC (early satiety, upper abdominal pain) itself. Paraneoplastic signs such as hypercalcemia, hypoglycemia have been reported | Generalized fatigue, abdominal pain and weight loss are common symptoms. Can present at late stage with large tumor burden, extrahepatic metastasis |
| Diagnosis | High quality cross-sectional imaging (CT/MRI) are used with typical arterial phase hyper-enhancement and portal venous washout. LI-RADS classification is used in classification of radiological findings in HCC | Although CT and MRI are increasingly utilized for diagnosis, liver biopsy are utilized in patients when cross-sectional imaging is equivocal. LI-RADS classification cannot be utilized for NCHCC and instead tumor characteristics (size, imaging features) are utilized for staging |
| Treatment | Given the underlying cirrhosis, liver transplant candidacy need to be evaluated for HCC patients. Resectability of the lesion, amount of liver reserve, vascular invasion, performance status determines the treatment outcomes | Antiviral treatment recommended when etiology of NCHCC is HBV/HCV. Surgery remains the main treatment modality. Systemic and local therapy options are increasingly being utilized for NCHCC |
Other risk factors include toxin exposure (alcohol, aflatoxin B1, industrial agents, genotoxins, anabolic steroids, iron excess)[22-25], genetic conditions, such as Wilson’s disease, glycogen storage disease, Alpha-1 antitrypsin deficiency, hereditary hemochromatosis, acute hepatic porphyria’s, hypercitrullinemia, Budd-Chiari syndrome, nodular regenerative hyperplasia[26-30], and germline mutations (telomerase reverse transcriptase gene mutation is the most commonly described mutation in NCHCC)[31]. Excess alcohol intake may play a role in NCHCC carcinogenesis[32]. Studies reported that prevalence of alcohol abuse among patients with NCHCC range up to 12%-21%[4,11,33,34]. If alcohol leads to significant inflammation with or without fibrosis in these NCHCC is unclear. Further, a synergism with other risk factors, such as viral hepatitis and metabolic syndrome could likely play a role in hepatic carcinogenesis.
Hepatotropic viral infections such as HBV and less often HCV are major contributors to NCHCC. Hepatitis D can cause coinfection with HBV and lead to high risk of HCC compared to HBV alone [odds ratio (OD) 1.28, 95% confidence interval (CI) 1.05-1.57][35]. Few studies report hepatitis E may affect chronic HBV infection as they already have compromised liver function[36,37]. If similar risk exists for NCHCC is unclear. Additionally, NCHCC can exhibit more genomic variants, suggestive of a separate tumor biology in these patients[38,39]. Further, HBV-human immunodeficiency virus (HIV) coinfection has been reported to cause higher risk of HCC (OD 7.1, 95%CI: 2.8-17.9)[40].
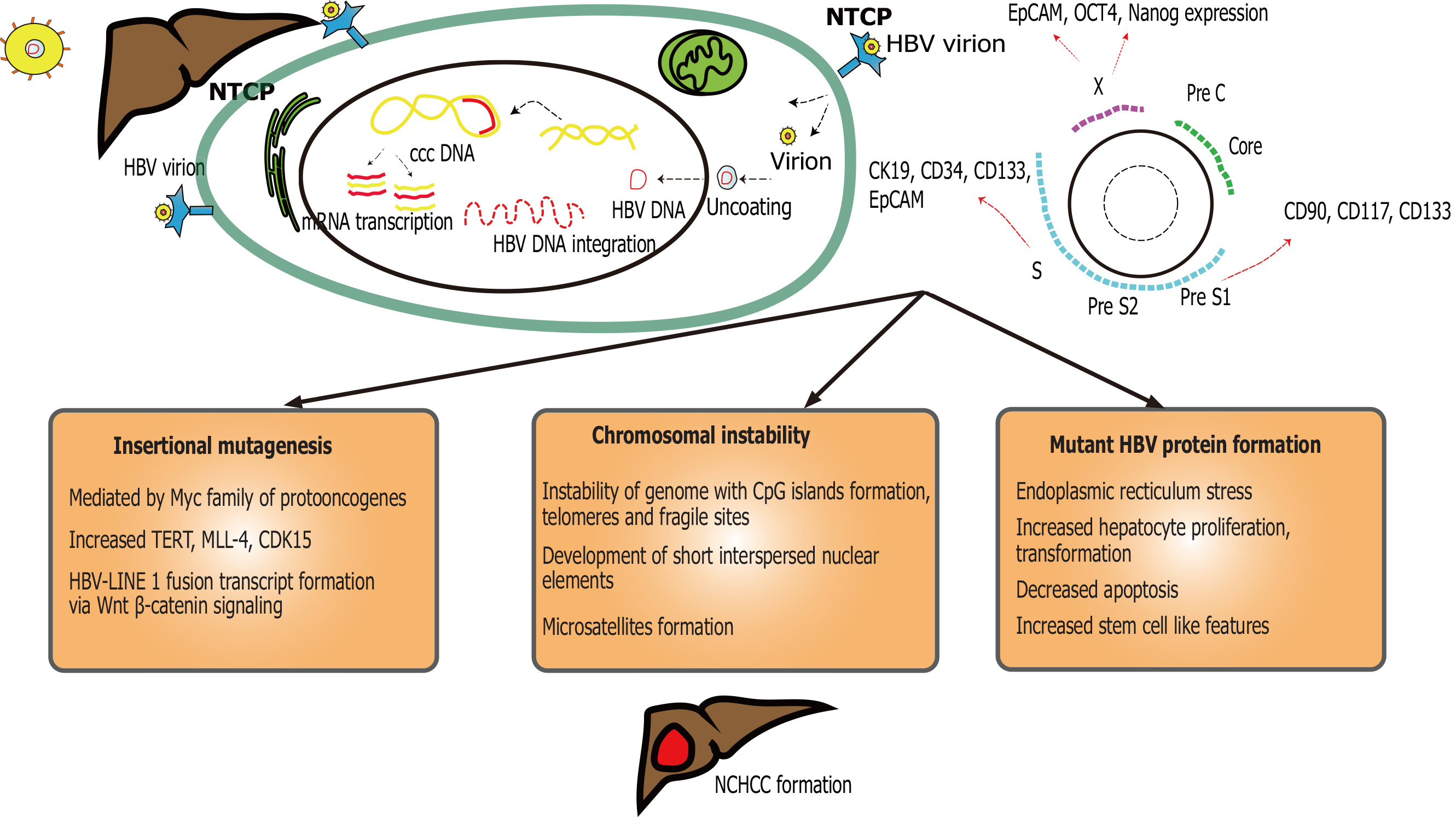
HBV virus is a DNA virus (Figure 1), a member of the Hepadnaviridae family. It is composed of viral core [nucleocapsid, HBV core antigen (HBcAg) and DNA polymerase] and surface [formed by surface antigen (HBsAg)]. The genes coding for HBcAg code for HBV e antigen (HBeAg). Worldwide, there are 292 million people living with chronic HBV, with 2.2 million reported in the United States[41,42]. HBV (+/- HDV) associated HCC is more common in low and middle socio-economic population. Chronic HBV coinfection can been seen with HDV and/ or HCV.
Ten genotype variants (A to J) are known for HBV of which genotype B and C are highly relevant to HCC development[43]. In general, patients with genotype B have lower risk of progression towards cirrhosis (due to less active disease and earlier HBeAg seroconversion)[44], compared to genotype C. Few studies from Japan demonstrated that genotype B is more common in younger NCHCC[45]. Some studies suggest that individuals infected with either B or C HBV genotype with high T1762/A1764 basal core promotor mutation have a higher risk of HCC development, especially among non-cirrhotic younger adults (< 50 years)[46,47]. Notably, most of these studies were of Asian origin, known to have higher HBV prevalence compared to the West. Mutations are frequently noticed during HBV replication, due to its lack of proofreading activity during reverse transcription. Girones et al[48] reported that hepadnavirus mutation rate is 100 times higher than other DNA viruses[48]. Thirty percent of these HCC patients have no underlying cirrhosis (NCHCC)[49]. Pooled HCC surveillance adherence is much lower in non-cirrhotics (23%) compared to their cirrhotic counterparts, despite recommendations[50]. This can lead to tumors, which are advanced at the time of diagnosis, limiting treatment options and outcomes.
Transforming hepatocytes without significant fibrosis or inflammation remains a hallmark of HBV-induced NCHCC[51]. The underlying mechanism remains unclear, even though related to cellular transformation with epigenetic alteration, telomere shortening and instability of the genome system, insertional proto-oncogenes/tumor suppressor mutagenesis, and expression of mutant HBV proteins following integration[52,53]. A variety of factors can predispose HBV positive individuals to develop HCC through either cirrhosis or non-cirrhotic pathways. HBV genome, due to its ability to integrate into the host, can cause genomic instability, disrupting normal regulatory mechanisms. Further, and most interestingly, these mechanisms continue to exist even after seroconversion, due to HBV genome integration. This has been related to HBV highly stable minichromosome and/ or covalently closed circular DNA (HBV cccDNA) which resides inside the hepatocyte nucleus[54]. Tu et al[53] compared HBV integrated sites between tumor and matched non-tumor tissues and found that HCC tumors have higher integration event frequency in coding/promoter regions. In the non-cirrhotic setting, recurrent enhancer II/core HBV promoter integration near telomerase reverse transcriptase, myeloid/lymphoid or mixed leukemia 4 genes cause upregulation of oncogenes during early and late tumorigenesis[53,55]. This is critical for HBV non-cirrhotic carcinogenesis, even after HBV treatment or spontaneous resolution. High viral loads of HBV are independently associated with NCHCC, indicative of genetic transformation of the hepatocytes induced by the HBV[47].
Many cellular processes modulate the HBV cccDNA such as histone acetylation, epigenetic modification and activation of signal transduction[56]. These processes could be a target for pharmaceutical agents, however remaining challenges are cccDNA longevity and stability in the host and viremia recurrence once the antiviral therapy is stopped[57]. The host immune response is unable to eliminate infected cells, resulting instead in immune-mediated damage. This can lead to repeat bouts of hepatitis and inflammation-necrosis-proliferation cycles, resulting in production of reaction oxygen species, genetic mutations and carcinogenesis[58].
NCHCC individuals are more likely African American (OR 6.8, 95%CI: 2.1-22.4), Asian (OR 11.6, 95%CI 2.63-50.8) or have a family history of HCC (OR 32.9, 95%CI: 3.76-288)[51]. In addition, some of the NCHCC from chronic HBV can have multiple risk factors, such as HCV coinfection, alcohol abuse and cryptogenic etiologies[16]. It has been widely contested that viral infection alone might not be sufficient for carcinogenesis; an interplay of host factors, environment and time are needed for full blown cancer development. This probably explains the variation in HCC prevalence among different population groups, and the protracted timeline for HBV-related tumorigenesis, which may span at least one to two decades of life.
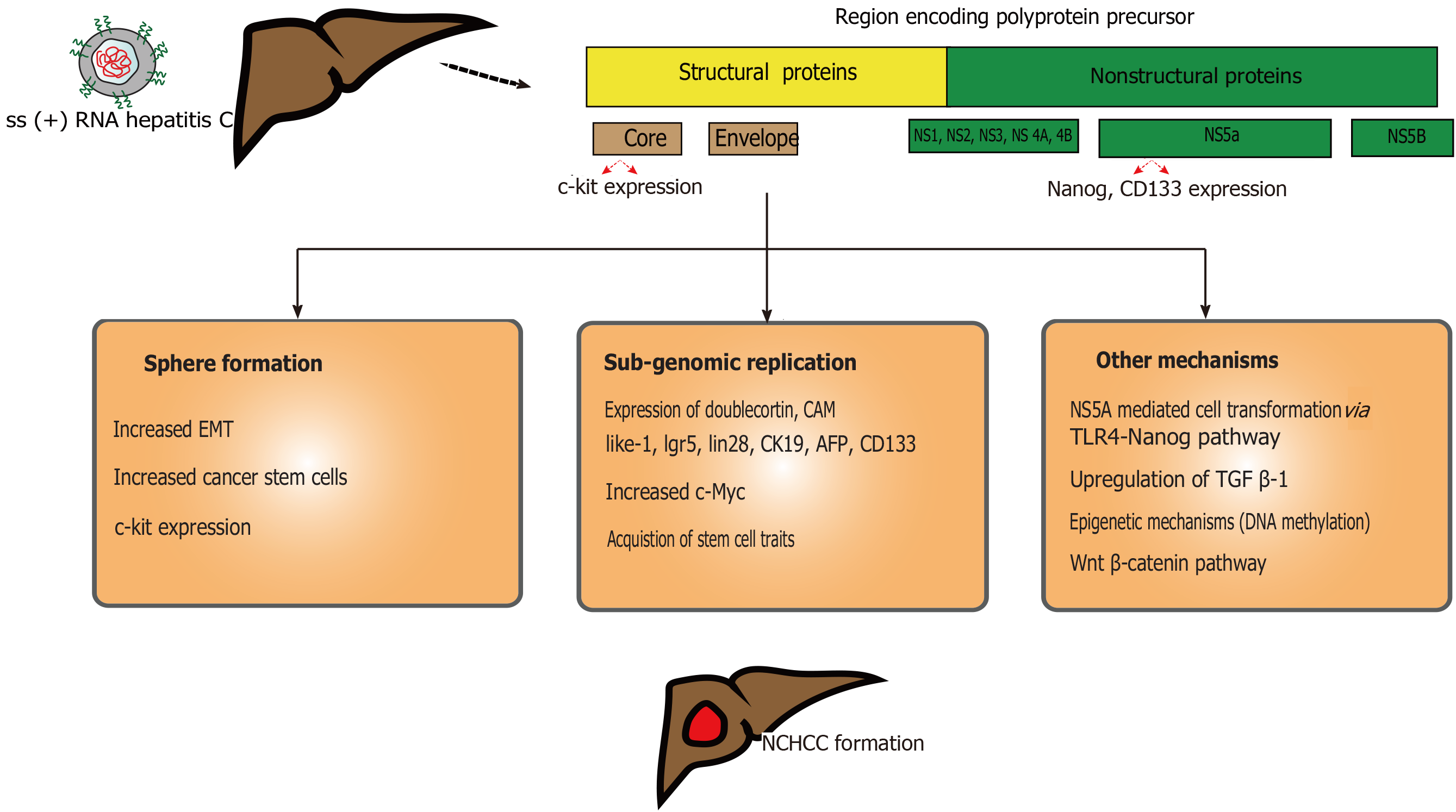
HCV is a part of Flaviviridae family. It is an single-stranded enveloped RNA virus which primarily infects hepatocytes (Figure 2) and targets liver-specific cellular host factors[59]. Precise HCV NCHCC prevalence is unclear. Few reports suggested a possible incidence of up to 10.6%[60]. This risk decreases to 4.2% after achieved sustained viral response (SVR)[61]. Few risk factors, such as male sex, advance age (> 60 years), F3 fibrosis, steatosis, elevated ALT at the end of treatment and history of alcohol abuse contribute to this risk. Furthermore, HCV genotype 3 with steatosis may correlate with HCC[62].
Similar to HBV, successful HCV treatment would not completely eliminate HCC risk, even though risk would be much lower compared to HBV[63]. Attributed risk factors are HCV-genotype 1b[64], viral co-infection infections (HBV, HIV), alcohol or tobacco abuse, and metabolic syndrome. HCV core protein can alter the telomerase activity and immortalize the hepatocytes (along with loss of tumor suppressor function)[65]. Upon hepatocyte entry, the viral RNA undergoes replication and translation in the rough endoplasmic reticulum[66]. This translated product is cleaved by host and viral proteases to form different proteins (core, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, NS5B). Among these, NS3, NS4B, NS5A can induce carcinogenesis coupled with host cellular proteins[65]. Release of reactive oxygen species remains a critical part for HCV-induced HCC. Chronic HCV infection leads to milder liver inflammation, facilitating release of pro-inflammatory cytokines and growth factors, and the generation of inflammation-necrosis-proliferation cycles[67,68]. This is precipitated by increased reactive oxygen species production, mediated by calcium release and high expression of HCV core protein, and cytochrome p450 2E1 oxidase[69]. Decades of this cycles lead to fibrosis, cirrhosis and, eventually HCC.
Due to the ongoing risk of carcinogenesis despite achieving SVR, active evaluation for non-viral risk factors (co-infection, alcohol, tobacco, fatty liver, and metabolic syndrome) should be performed. Recommendations on the surveillance of non-cirrhotic patients with stage 2 or 3 fibrosis and history of treatment-naïve or SVR HCV infection have been unclear. The American Association for the Study of Liver Diseases do not recommend active surveillance. The European Association of the Study of Liver recommended that surveillance would be useful[70].
Co-infection of hepatotropic viruses can occur among each other and rarely with other virus infection such as the HIV. Due to identical modes of transmission (contaminated needles, blood and sexual routes), co-infection with viral hepatitis and HIV have been observed in certain populations (sub-Saharan Africa)[71]. Although the rates of infection differ based on region and risk-based groups, 5%-20% of HIV patients could be co-infected with HBV. Further, the rates of morbidity and mortality are significantly higher among HIV and HBV co-infection even after adequate viral suppression[72]. HCV and HIV co-infection, although lower (1%-7%) compared to HBV, can lead to evolution of fibrosis (especially with low CD4 counts) and early onset cirrhosis[73]. Chronic viral hepatitis D, being a defective virus, depends on HBV for propagation and occurs in patients concomitantly infected with HDV and HBV. HDV could infect up to 5%-15% of all HBV carriers[74]. This co-infection can cause severe form of chronic viral hepatitis, with 2-fold risk of mortality and 3-fold risk of cirrhosis[75]. The risk of HCC increased 1.2 fold with this co-infection and even higher (7.1%) with HIV-infection. HDV-associated HCC can be due to oncogenesis induced by impact of viral replication/epigenetic events and abnormal cell methylation processes[76]. Despite these findings, future studies are needed to evaluate the carcinogenesis induced by co-infection and long-term effects in the patients.
NCHCC usually presents at late stage, with a large tumor size, extrahepatic metastases, and heavy tumor burden. Symptoms may include malaise, fatigue, weight loss, abdominal pain, gastrointestinal bleeding and distension, even though often patients are asymptomatic[77-79].
Diagnosis of HCC is mostly performed with non-invasive techniques and rarely histological diagnosis is needed (Table 2). Imaging has replaced histology and conventional angiography[80]. Most of the radiological features of CHCC and NCHCC are similar, except, of course, for the background of cirrhosis among the CHCC patients. Multiple imaging modalities are available, including ultrasound (US), contrast enhanced US, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography scan[81], CT-based radiomics nomogram [82,83], and angiography-assisted CT hepatic angiography[84,85]. Classical CT/MRI findings are arterial phase hyper-enhancement (due to hepatic arterial supply of the tumor), followed by portal venous washout.
| CHCC | NCHCC | |
| Imaging modality | Background of advance fibrosis (cirrhosis) | No background of advance fibrosis (cirrhosis) |
| CT | Homogenous with irregular but well defined margin | Initially hypoattenuating mass which can be come heterogenous (areas of necrosis/hemorrhage within the tumor) when tumor attains bigger size |
| Multiple masses | ||
| Large solitary mass (/dominant mass) with satellite nodules | ||
| Extrahepatic extension less common | ||
| Extrahepatic extension (with direct adjacent organ) is more often seen | ||
| Metastasis frequently seen, vascular invasion less common (15%) | ||
| Vascular invasion (encasement) more common (85%) | ||
| Lymphadenopathy seen in 20% of cases. | ||
| MR | T1: Variable but mostly hypointense. T2: Hyperintense/isointense compared to surrounding liver | Unenhanced T1 image - Hypointense lesion (presence of hemorrhage/fat can increase the signal). T2 - Hyperintense (low grade/well differentiated can be iso/hypointense) |
| DWI-high ADC when lesion is well differentiated | DWI - Used for small lesions. Shows low ADC |
Few studies evaluated clinico-radiological characteristics in NCHCC and found that they differ compared to CHCC[86]. Time of contrast washout correlates with histopathological grade in HCC[86]. US is rarely used for diagnosis of the NCHCC due to its non-specific findings, especially for tumors which are less than 2 cm in size, obesity and background of cirrhosis[87]. In rare conditions, such as undifferentiated embryonal sarcoma, ultrasound shows solid component of the tumor (and can exclude purely cystic lesions which are benign)[88]. The sensitivity of US can be significantly limited (up to 21%) due to abdominal fat and obesity; CT/MR is frequently used in these situations[89].
CT is one of the most commonly used modality of imaging for diagnosis of HCC and can be critical in identifying salient features of HCC. Patients with NCHCC can present with large tumor size lesions, solitary mass (with or without satellite lesions)[90]. They can have well-defined margins with mosaic pattern of enhancement, complete capsule and delayed washout on the CT[86]. This in comparison to CHCC which showed smaller lesions, ill-defined margins, heterogeneous enhancement, no capsule and portal venous phase washout (Table 2). Extrahepatic lesions are more commonly noted in NCHCC (20.5%) compared to CHCC (6.5%). Calcifications are rare in HCC, but in fibrolamellar subtype, they can be found up to 70% and can be associated with central scar.
MRI imaging is superior and often diagnostic. Although several imaging protocols and contrast media are available, hepatocyte specific contrast media are beneficial for accurate diagnosis in NCHCC. MRI Imaging characteristics of NCHCC can differ from classical CHCC in few important aspects (Table 2). Some of independent predictors that were utilized in prior studies include T1-hypointensity, T2-hypo/hyperintensity, lack of central tumor-enhancement, and satellite lesions. Use of these features improved the sensitivity and specificity of identifying lesions as high as 91% and 98% respectively[91]. Although superior in nature, MRI features can differ. T1 images are mostly hypointense. Degree of tumor differentiation, iron or glycogen, lipid, copper could make the lesion appear hyperintense. Further lipid could be distributed diffusely, which can manifest as signal drop outs on the MRI[92]. The mosaic pattern often seen in the NCHCC may be better seen on MRI compared to CT, due to better soft tissue enhancement. HCCs are mildly hyperintense or isointense compared to the surrounding liver on T2- weighted images.
Histological diagnosis for CHCC is rarely needed due to availability of high quality imaging such as CT and MRI. However, NCHCC often requires biopsy confirmation as the standard LIRADS classification can only be applied in the presence of cirrhosis. If lesions are smaller (< 1 cm) especially in NCHCC with atypical features, it is reasonable to repeat imaging in 6 months or obtain histological diagnosis with a liver biopsy based upon the pretest probably and HCC risk factors. Multiple routes of liver biopsy can tried; percutaneous or endoscopic ultrasound guided[93]. Risk of bleeding, pain, tumor seeding of the needle track should be weighed against obtaining adequate tissue for histological diagnosis[94]. Multidisciplinary team HCC diagnosis and management has now become the standard of care. Multidisciplinary approach can improve the overall care in these patients[95].
Treatment options for NCHCC depend on the etiology, tumor size, extent, vascular invasion, performance status of the individual, and their transplant candidacy (Table 3). Surgery remains the mainstay, if the lesion is resectable. With the introduction of antiviral agents for HCV and HBV, there has been significant improvement on HCC occurrence. Advanced surgical techniques have improved survivability in these patients. In recent years, the implementation of locoregional and novel systemic therapies have been added to the armamentarium of the treatment options in these patients.
| Treatment | Comments |
| Antiviral therapy | If HBV or HCV are identified as potential causes of NCHCC, aggressive treatment should be pursued. Entecavir, tenofovir have been used for HBV and DAA agents are used for HCV infection |
| Surgery | Mainstay for the treatment of NCHCC. BCLC staging cannot be used for NCHCC patients. Tumor size, elevated bilirubin level, low platelet count, vascular invasion can predict prognosis in NCHCC individuals |
| Locoregional therapy | Limited data available in NCHCC patients. Isolated cases and case series showed improved prognosis with these treatment options |
| Systemic therapy | Multikinase inhibitors (sorafenib, regorafenib), immunotherapy (nivolumab), chemotherapeutic agents (epirubicin, cisplatin, 5-fluororuacil, capecitabine, docetaxel, GEMOX) have been used in NCHCC with various success |
All patients with NCHCC should be evaluated for underlying cause. If HBV or HCV infection is detected, the patient should be offered the option of antiviral treatment.
Entecavir and tenofovir are used as first-line therapies for CHB infection. Multiple risk prediction models have been developed for the study of HCC prevalence in HBV patients[96]. Some of these models include individual prediction model, Chinese University-HCC, Guide with Age, Gender, HBV DNA, Core Promoter Mutation and Cirrhosis-HCC, Risk Estimation for Hepatocellular Carcinoma in Chronic HBV and Nomogram-HCC[97]. Arends et al[98] noted that HCC incidence is lower in patients who have received antiviral therapy, even though it did not eliminate the risk: The reported 5-year cumulative incidence rate of NCHCC was 2.1%. Although both therapeutic agents reduce the risk of HCC, few studies compared their effectiveness. A systematic review reported that tenofovir was associated with lower risk of HCC development [adjusted hazard ratio (HR) 0.81; 95%CI: 0.62-0.85], however the beneficial effect did not reach statistical significance for non-cirrhotic patients (adjusted HR 0.83; 95%CI: 0.51-1.35)[99].
DAA agents have revolutionized HCV care. This in turn, has decreased the risk of HCC on both cirrhotics and non-cirrhotics. Tanaka et al[100] analyzed 5814 patients (5646 SVR and, 168 non-SVR) from Asia and noted that HCC incidence was higher in the non-SVR group (5.26 vs 1.94, P < 0.001). Among the SVR group, in non-cirrhotic SVR patients, baseline alfa fetoprotein of ≥ 10 ng/mL was significant (adjusted HR: 4.26, P = 0.005)[100]. SVR in HCV patients depended on the type of treatment regimen. Few studies showed that these regimens might achieve SVR (especially in genotype-2) at higher rate in non-cirrhotics compared to cirrhotics (98.2% vs 89.4%)[101]
Surgical resection remains the mainstay of NCHCC treatment. Survival of these patients is excellent if the lesions are at an early stage[5]. Unfortunately, NCHCC lesions are usually large due to their late diagnosis[102]. Long-term overall survival (OS) after hepatic resection for NCHCC showed an OS rate of 62%-100% (1 year), 46%-78% (3 year) and 30%-64% (5 year)[103,104]. Multiple synchronous lesions, large tumor size, non-clear resection margin, poor tumor staging and lymphatic invasion were indicators of poor prognosis[104]. Subject to the presence of adequate future liver remnant, these patients may undergo repeat resections. Advanced surgical resection modalities, such as non-anatomical (parenchymal sparing liver resections), ex-situ resections along with the application of future liver remnant growth techniques, such as portal venous embolization, two-stage hepatectomies, associating liver partition with portal vein ligation, hepatic venous deprivation with/without locoregional treatments, have allowed for extended repeat resections on these patients, extending survival.
Tumor size (> 5 cm), elevated bilirubin levels (> 5.6 mg/L), low platelet count, portal vein thrombus development can predict vascular invasion and prognosis in the patients[105,106]. Few studies in the past debated if tumor size along predicts survival in these patients. Pommergaard et al[39] studied 22787 HCC patients from European Liver Transplant Registry transplanted between 1990 and 2016 and noted that HCC in non-cirrhotic livers had similar overall mortality (adjusted HR 1.11, 95%CI: 0.99-1.25), but higher HCC-specific mortality (adjusted HR 1.62, 95%CI: 1.31-2); perhaps due to a more aggressive biology of the tumors in the non-cirrhotic livers. NCHCC patients were younger, had lower MELD scores and higher risk of microvascular invasion and received more locoregional treatment. Irrespective of the background liver quality, vascular invasion remains critical, underlining the need for early diagnosis and management strategies[107].
In the absence of extrahepatic disease or vascular invasion, liver transplantation (LT) has been the gold standard of HCC treatment among cirrhotics based on the Milan criteria, with the number and size of lesions the being the main determinants[108]. If outside Milan criteria, the patients’ lesions may be down-staged under specific guidelines (University of California San Francisco criteria), and then be reassessed for transplantability. On patients beyond the established criteria, novel surgical approaches can be pursued, such as living donor transplantation, split transplantation or the use of marginal grafts, such as donation after circulatory death allografts[109]. On non-cirrhotics, macrovascular invasion and extrahepatic spread are the only recommended exclusion criteria for LT[39]. As per Mazzaferro et al[108] if trans
Locoregional therapies such as radiofrequency ablation or transarterial embolization modalities, have been highly successful in the management of HCC on cirrhotics. However, little has been reported in the non-cirrhotic setting. Wagle et al[79] retrospectively noted ten NCHCC patients with outcomes with LRT and surgery. One patient underwent trans-arterial embolization and three underwent sequential TACE-portal vein embolization. No mortality was noted in these patients and risk-free survival in all these ten patients was 100% at year 1, 62% at year 3 and OS was 100% at year 1, 72% at year 3 and 72% at year 5. These findings indicate the developing role for LRTs for NCHCC and overall improved prognosis.
Systemic therapy with multikinase inhibitors (Sorafenib, regorafenib) and programmed cell death protein 1 inhibitor (nivolumab) have been tried for HCC and extended their use in NCHCC[113]. Sorafenib is effective in advanced HCC and can prolong survival in these patients. However, owing to its adverse events (nausea, excessive fatigue, diarrhea and skin reactions), its widespread use is limited[114]. Use of chemotherapeutic agents such as epirubicin (E), cisplatin (C), 5-fluororuacil (F), capecitabine, docetaxel, GEMOX haven tried in NCHCC and fibrolamellar HCC. Variable responses have noted with these regiments. For instance, epirubicin, cisplatin, capecitabine showed 52% disease control and epirubicin, cisplatin and 5-flurouracil. GEMOX showed complete response in fibrolamellar HCC. Combination therapy such as use of Sorafenib and mammalian target of rapamycin inhibitors[115] has been attempted with one year survival of 82% and five year survival of 33%[116]. New immunotherapy protocols such as atezolizumab-bevacizumab combination has not been studied in NCHCC, however future trials can be expected with these agents in NCHCC[117]. Although these modalities seem promising, further studies are needed to evaluate their role in survivability in NCHCC patients.
Improved cross-sectional imaging characteristics are expected to identify NCHCC at an earlier stage and provide increasing treatment options in the future. Survival and recurrence rates in NCHCC have been improving and expected to reach HCC patients with efficacy of antiviral treatment options, living donor liver transplantation, parenchymal sparing liver resection and two stage liver resections. Use of artificial intelligence, deep learning models (convolutional neural network) are being utilized for identification of NCHCC[118]. Messenger RNA (mRNA) is a family of RNA molecules which are involved in coding proteins and convey genetic information. On the contrary, microRNAs (miRNAs) are non-coding molecules (22 nucleotide length) that regulate gene expression especially in post-transcriptional state[119]. Dysregulated miRNA can lead to DNA damage with alter gene expression playing a role in NCHCC tumor pathogenesis. Use of micro RNA and messenger RNA (miRNA-mRNA) networks with bioinformatic analysis and experimental validation are being development for therapeutics for NHCCC[119]. Use of miRNA as a potential serum biomarker for diagnosis, prognostication, survival after liver resection and systemic therapy have been studied[120,121]. Despite these advances, further research on molecular mechanism of mRNA and miRNA regulation in NCHCC, and validation of genes involved in NCHCC are urgently needed.
Following the introduction of direct antiviral treatments and the increasing prevalence of NAFLD, NCHCC has been on the rise. Even though less common than the hepatocellular carcinoma encountered on cirrhotics, NCHCC still accounts for 20% of reported hepatomas. Such tumors are typically diagnosed late, thus compromising the outcome. The discovery and generalization of use of direct antivirals, loco-regional treatments and systemic novel immune-chemotherapies, the advancements of complex hepatobiliary surgery and the genesis of transplant oncology have added to the treatment armamentarium of these aggressive primary liver tumors. Multidisciplinary approach and coordinated care at tertiary high-volume HCC, preferably liver transplant centers, remain critical. It is time the stakeholders pursued a consensus approach in developing universal HCC surveillance and treatment strategies on non-cirrhotic patients at risk, such as NAFLD and/or patients with advanced fibrosis.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56698] [Article Influence: 7087.3] [Reference Citation Analysis (135)] |
| 2. | Davis GL, Dempster J, Meler JD, Orr DW, Walberg MW, Brown B, Berger BD, O'Connor JK, Goldstein RM. Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent). 2008;21:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 4. | Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Sigurdsson B, Sigurdardottir R, Arnardottir MB, Lund SH, Jonasson JG, Björnsson ES. A nationwide study on hepatocellular carcinoma. Cancer Epidemiol. 2020;69:101835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Papatheodoridis GV, Sypsa V, Dalekos GN, Yurdaydin C, Van Boemmel F, Buti M, Calleja JL, Chi H, Goulis J, Manolakopoulos S, Loglio A, Voulgaris T, Gatselis N, Keskin O, Veelken R, Lopez-Gomez M, Hansen BE, Savvidou S, Kourikou A, Vlachogiannakos J, Galanis K, Idilman R, Esteban R, Janssen HLA, Berg T, Lampertico P. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J Hepatol. 2020;72:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 7. | Centers for Disease Control and Prevention (CDC). Hepatocellular carcinoma - United States, 2001-2006. Morb Mortal Wkly Rep. 2010;59:517-520. [PubMed] |
| 8. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13305] [Article Influence: 1663.1] [Reference Citation Analysis (4)] |
| 9. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 853] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 10. | Kao JH. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2015;29:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Trevisani F, D'Intino PE, Caraceni P, Pizzo M, Stefanini GF, Mazziotti A, Grazi GL, Gozzetti G, Gasbarrini G, Bernardi M. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer. 1995;75:2220-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Smalley SR, Moertel CG, Hilton JF, Weiland LH, Weiand HS, Adson MA, Melton LJ 3rd, Batts K. Hepatoma in the noncirrhotic liver. Cancer. 1988;62:1414-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Schmit N, Nayagam S, Thursz MR, Hallett TB. The global burden of chronic hepatitis B virus infection: comparison of country-level prevalence estimates from four research groups. Int J Epidemiol. 2021;50:560-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 14. | Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MM, Dubinsky TJ, Kolokythas O, Lalwani N. Hepatocellular carcinoma in the noncirrhotic liver. AJR Am J Roentgenol. 2014;203:W34-W47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transpl Surg. 1999;5:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Shim CW, Park JW, Kim SH, Kim JS, Kim BH, Hong EK. Noncirrhotic hepatocellular carcinoma: etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol. 2017;10:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Tamori A, Nishiguchi S, Kubo S, Narimatsu T, Habu D, Takeda T, Hirohashi K, Shiomi S. HBV DNA integration and HBV-transcript expression in non-B, non-C hepatocellular carcinoma in Japan. J Med Virol. 2003;71:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Billeter AT, Müller PC, Albrecht T, Roessler S, Löffler M, Lemekhova A, Mehrabi A, Müller-Stich BP, Hoffmann K. Impact of Type 2 Diabetes on Oncologic Outcomes of Hepatocellular Carcinomas in Non-Cirrhotic, Non-alcoholic Steatohepatitis: a Matched-Pair Analysis. J Gastrointest Surg. 2021;25:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 20. | Perumpail RB, Liu A, Wong RJ, Ahmed A, Harrison SA. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J Hepatol. 2015;7:2384-2388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Sydor S, Best J, Messerschmidt I, Manka P, Vilchez-Vargas R, Brodesser S, Lucas C, Wegehaupt A, Wenning C, Aßmuth S, Hohenester S, Link A, Faber KN, Moshage H, Cubero FJ, Friedman SL, Gerken G, Trauner M, Canbay A, Bechmann LP. Altered Microbiota Diversity and Bile Acid Signaling in Cirrhotic and Noncirrhotic NASH-HCC. Clin Transl Gastroenterol. 2020;11:e00131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 22. | Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (14)] |
| 23. | Hardt A, Stippel D, Odenthal M, Hölscher AH, Dienes HP, Drebber U. Development of hepatocellular carcinoma associated with anabolic androgenic steroid abuse in a young bodybuilder: a case report. Case Rep Pathol. 2012;2012:195607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Perisetti A, Raghavapuram S, Sheikh AB, Yendala R, Rahman R, Shanshal M, Thein KZ, Farooq A. Mushroom Poisoning Mimicking Painless Progressive Jaundice: A Case Report with Review of the Literature. Cureus. 2018;10:e2436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, Lee MH, Jen CL, You SL, Santella RM, Chen CJ. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Britto MR, Thomas LA, Balaratnam N, Griffiths AP, Duane PD. Hepatocellular carcinoma arising in non-cirrhotic liver in genetic haemochromatosis. Scand J Gastroenterol. 2000;35:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Evert M, Dombrowski F. [Hepatocellular carcinoma in the non-cirrhotic liver]. Pathologe. 2008;29:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Topic A, Ljujic M, Radojkovic D. Alpha-1-antitrypsin in pathogenesis of hepatocellular carcinoma. Hepat Mon. 2012;12:e7042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Deybach JC, Puy H. Hepatocellular carcinoma without cirrhosis: think acute hepatic porphyrias and vice versa. J Intern Med. 2011;269:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Nakayama M, Okamoto Y, Morita T, Matsumoto M, Fukui H, Nakano H, Tsujii T. Promoting effect of citrulline in hepatocarcinogenesis: possible mechanism in hypercitrullinemia. Hepatology. 1990;11:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Müller M, Bird TG, Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. 2020;72:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 32. | Tobari M, Hashimoto E, Taniai M, Kodama K, Kogiso T, Tokushige K, Yamamoto M, Takayoshi N, Satoshi K, Tatsuo A. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (3)] |
| 33. | Wörns MA, Bosslet T, Victor A, Koch S, Hoppe-Lotichius M, Heise M, Hansen T, Pitton MB, Niederle IM, Schuchmann M, Weinmann A, Düber C, Galle PR, Otto G. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol. 2012;47:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Giannini EG, Marenco S, Bruzzone L, Savarino V, Farinati F, Del Poggio P, Rapaccini GL, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F. Hepatocellular carcinoma in patients without cirrhosis in Italy. Dig Liver Dis. 2013;45:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Alfaiate D, Clément S, Gomes D, Goossens N, Negro F. Chronic hepatitis D and hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. J Hepatol. 2020;73:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 36. | Tseng TC, Liu CJ, Chang CT, Su TH, Yang WT, Tsai CH, Chen CL, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Perisetti A, Laoveeravat P, Inamdar S, Tharian B, Thandassery R, Goyal H. Hepatitis E virus infection in liver transplant recipients: a descriptive literature review. Eur J Gastroenterol Hepatol. 2020;32:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Wong N, Lai P, Lee SW, Fan S, Pang E, Liew CT, Sheng Z, Lau JW, Johnson PJ. Assessment of genetic changes in hepatocellular carcinoma by comparative genomic hybridization analysis: relationship to disease stage, tumor size, and cirrhosis. Am J Pathol. 1999;154:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Pommergaard HC, Rostved AA, Adam R, Rasmussen A, Salizzoni M, Bravo MAG, Cherqui D, De Simone P, Houssel-Debry P, Mazzaferro V, Soubrane O, García-Valdecasas JC, Prous JF, Pinna AD, O'Grady J, Karam V, Duvoux C, Thygesen LC. Mortality after Transplantation for Hepatocellular Carcinoma: A Study from the European Liver Transplant Registry. Liver Cancer. 2020;9:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Béguelin C, Moradpour D, Sahli R, Suter-Riniker F, Lüthi A, Cavassini M, Günthard HF, Battegay M, Bernasconi E, Schmid P, Calmy A, Braun DL, Furrer H, Rauch A, Wandeler G; Swiss HIV Cohort Study. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1261] [Article Influence: 157.6] [Reference Citation Analysis (6)] |
| 42. | Lim JK, Nguyen MH, Kim WR, Gish R, Perumalswami P, Jacobson IM. Prevalence of Chronic Hepatitis B Virus Infection in the United States. Am J Gastroenterol. 2020;115:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 43. | Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol. 2000;81:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 621] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 44. | Chan HL, Wong GL, Tse CH, Chim AM, Yiu KK, Chan HY, Sung JJ, Wong VW. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin Gastroenterol Hepatol. 2009;7:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Chen CH, Eng HL, Lee CM, Kuo FY, Lu SN, Huang CM, Tung HD, Chen CL, Changchien CS. Correlations between hepatitis B virus genotype and cirrhotic or non-cirrhotic hepatoma. Hepatogastroenterology. 2004;51:552-555. [PubMed] |
| 46. | Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 47. | Do AL, Wong CR, Nguyen LH, Nguyen VG, Trinh H, Nguyen MH. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol. 2014;48:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Girones R, Miller RH. Mutation rate of the hepadnavirus genome. Virology. 1989;170:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 50. | Wang C, Chen V, Vu V, Le A, Nguyen L, Zhao C, Wong CR, Nguyen N, Li J, Zhang J, Trinh H, Nguyen MH. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine (Baltimore). 2016;95:e4744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, Kanwal F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 52. | Mani SKK, Andrisani O. Hepatitis B Virus-Associated Hepatocellular Carcinoma and Hepatic Cancer Stem Cells. Genes (Basel). 2018;9:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Tu T, Budzinska MA, Shackel NA, Urban S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses. 2017;9:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (2)] |
| 54. | Turton KL, Meier-Stephenson V, Badmalia MD, Coffin CS, Patel TR. Host Transcription Factors in Hepatitis B Virus RNA Synthesis. Viruses. 2020;12:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (2)] |
| 55. | Dong H, Zhang L, Qian Z, Zhu X, Zhu G, Chen Y, Xie X, Ye Q, Zang J, Ren Z, Ji Q. Identification of HBV-MLL4 Integration and Its Molecular Basis in Chinese Hepatocellular Carcinoma. PLoS One. 2015;10:e0123175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 495] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 57. | Iwamoto M, Saso W, Sugiyama R, Ishii K, Ohki M, Nagamori S, Suzuki R, Aizaki H, Ryo A, Yun JH, Park SY, Ohtani N, Muramatsu M, Iwami S, Tanaka Y, Sureau C, Wakita T, Watashi K. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc Natl Acad Sci USA. 2019;116:8487-8492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 58. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48816] [Article Influence: 3254.4] [Reference Citation Analysis (12)] |
| 59. | Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 606] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 60. | Alotaibi AS, Alghamdi W, Marotta P, Qumosani K. A266 Hepatocellular carcinoma prevalence in non-cirrhotic hepatitis c patients. J Can Assoc Gastroenterol. 2018;1 suppl 2:383-384. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, Watanabe H. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 63. | Mattos AA, Marcon Pdos S, Araújo FS, Coral GP, Tovo CV. Hepatocellular carcinoma in a non-cirrhotic patient with sustained virological response after hepatitis c treatment. Rev Inst Med Trop Sao Paulo. 2015;57:519-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, L'Italien G, Chen CJ, Yuan Y; REVEAL-HCV Study Group. Hepatitis C virus genotype 1b increases cumulative lifetime risk of hepatocellular carcinoma. Int J Cancer. 2014;135:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | D'souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26:5759-5783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (8)] |
| 67. | Valgimigli M, Valgimigli L, Trerè D, Gaiani S, Pedulli GF, Gramantieri L, Bolondi L. Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and cell proliferation. Free Radic Res. 2002;36:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Kim CW, Chang KM. Hepatitis C virus: virology and life cycle. Clin Mol Hepatol. 2013;19:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 69. | Smirnova OA, Ivanova ON, Bartosch B, Valuev-Elliston VT, Mukhtarov F, Kochetkov SN, Ivanov AV. Hepatitis C Virus NS5A Protein Triggers Oxidative Stress by Inducing NADPH Oxidases 1 and 4 and Cytochrome P450 2E1. Oxid Med Cell Longev. 2016;2016:8341937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4563] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 71. | Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol. 2014;61:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 72. | Rajbhandari R, Jun T, Khalili H, Chung RT, Ananthakrishnan AN. HBV/HIV coinfection is associated with poorer outcomes in hospitalized patients with HBV or HIV. J Viral Hepat. 2016;23:820-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Akinyemi JO, Ogunbosi BO, Fayemiwo AS, Adesina OA, Obaro M, Kuti MA, Awolude OA, Olaleye DO, Adewole IF. Demographic and epidemiological characteristics of HIV opportunistic infections among older adults in Nigeria. Afr Health Sci. 2017;17:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Miao Z, Zhang S, Ou X, Li S, Ma Z, Wang W, Peppelenbosch MP, Liu J, Pan Q. Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection. J Infect Dis. 2020;221:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 75. | Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol. 2016;64:S102-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 76. | Diaz G, Engle RE, Tice A, Melis M, Montenegro S, Rodriguez-Canales J, Hanson J, Emmert-Buck MR, Bock KW, Moore IN, Zamboni F, Govindarajan S, Kleiner DE, Farci P. Molecular Signature and Mechanisms of Hepatitis D Virus-Associated Hepatocellular Carcinoma. Mol Cancer Res. 2018;16:1406-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Augustine T, Perisetti A, Tharian B. Unusual Cause of Upper Gastrointestinal Bleeding in Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:e60-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Polavarapu AD, Ahmed M, Samaha G, Weerasinghe CK, Deeb L, Sokoloff A. Spontaneous Rupture of Fibrolamellar Variant Hepatocellular Carcinoma. Gastroenterology Res. 2019;12:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Wagle P, Narkhede R, Desai G, Pande P, Kulkarni DR, Varty P. Surgical management of large hepatocellular carcinoma: the first single-center study from western India. Arq Bras Cir Dig. 2020;33:e1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Huo TI, Ho SY, Liu PH. Changing faces of hepatocellular carcinoma: East vs West. Liver Int. 2021;41:1430-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Chalaye J, Costentin CE, Luciani A, Amaddeo G, Ganne-Carrié N, Baranes L, Allaire M, Calderaro J, Azoulay D, Nahon P, Seror O, Mallat A, Soussan M, Duvoux C, Itti E, Nault JC. Positron emission tomography/computed tomography with 18F-fluorocholine improve tumor staging and treatment allocation in patients with hepatocellular carcinoma. J Hepatol. 2018;69:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 82. | Nie P, Wang N, Pang J, Yang G, Duan S, Chen J, Xu W. CT-Based Radiomics Nomogram: A Potential Tool for Differentiating Hepatocellular Adenoma From Hepatocellular Carcinoma in the Noncirrhotic Liver. Acad Radiol. 2021;28:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Nie P, Yang G, Guo J, Chen J, Li X, Ji Q, Wu J, Cui J, Xu W. A CT-based radiomics nomogram for differentiation of focal nodular hyperplasia from hepatocellular carcinoma in the non-cirrhotic liver. Cancer Imaging. 2020;20:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 84. | Guniganti P, Kierans AS. PET/MRI of the hepatobiliary system: Review of techniques and applications. Clin Imaging. 2021;71:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 85. | Liang Y, Xu F, Guo Y, Lai L, Jiang X, Wei X, Wu H, Wang J. Diagnostic performance of LI-RADS for MRI and CT detection of HCC: A systematic review and diagnostic meta-analysis. Eur J Radiol. 2021;134:109404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 86. | Jamwal R, Krishnan V, Kushwaha DS, Khurana R. Hepatocellular carcinoma in non-cirrhotic versus cirrhotic liver: a clinico-radiological comparative analysis. Abdom Radiol (NY). 2020;45:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3443] [Article Influence: 430.4] [Reference Citation Analysis (3)] |
| 88. | Psatha EA, Semelka RC, Fordham L, Firat Z, Woosley JT. Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging. 2004;22:897-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Esfeh JM, Hajifathalian K, Ansari-Gilani K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin Mol Hepatol. 2020;26:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 90. | Di Martino M, Saba L, Bosco S, Rossi M, Miles KA, Di Miscio R, Lombardo CV, Tamponi E, Piga M, Catalano C. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: clinical, radiological and pathological findings. Eur Radiol. 2014;24:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Fischer MA, Raptis DA, Donati OF, Hunziker R, Schade E, Sotiropoulos GC, McCall J, Bartlett A, Bachellier P, Frilling A, Breitenstein S, Clavien PA, Alkadhi H, Patak MA. MR imaging features for improved diagnosis of hepatocellular carcinoma in the non-cirrhotic liver: Multi-center evaluation. Eur J Radiol. 2015;84:1879-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Mitchell DG, Palazzo J, Hann HW, Rifkin MD, Burk DL Jr, Rubin R. Hepatocellular tumors with high signal on T1-weighted MR images: chemical shift MR imaging and histologic correlation. J Comput Assist Tomogr. 1991;15:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Johnson KD, Laoveeravat P, Yee EU, Perisetti A, Thandassery RB, Tharian B. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J Gastrointest Endosc. 2020;12:83-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (3)] |
| 94. | Palmer QD, Perisetti A, Broadfoot B, Johnson KD, Garcia-Saenz-de-Sicilia M. Recurrent Hepatocellular Carcinoma From Needle Tract Seeding 13 Years Post-Transplant: 2218. Am J Gastroenterol. 2019;114:S1242-S1243. |
| 95. | Lhewa D, Green EW, Naugler WE. Multidisciplinary Team Management of Hepatocellular Carcinoma Is Standard of Care. Clin Liver Dis. 2020;24:771-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Dasari BV, Kamarajah SK, Hodson J, Pawlik TM, Vauthey JN, Ma YT, Punia P, Coldham C, Abradelo M, Roberts KJ, Marudanayagam R, Sutcliffe RP, Muiesan P, Mirza DF, Isaac J. Development and validation of a risk score to predict the overall survival following surgical resection of hepatocellular carcinoma in non-cirrhotic liver. HPB (Oxford). 2020;22:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Chon HY, Lee JS, Lee HW, Chun HS, Kim BK, Park JY, Kim DY, Ahn SH, Kim SU. Impact of antiviral therapy on risk prediction model for hepatocellular carcinoma development in patients with chronic hepatitis B. Hepatol Res. 2021;51:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bömmel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL; VIRGIL Surveillance Study Group. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2015;64:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 99. | Cheung KS, Mak LY, Liu SH, Cheng HM, Seto WK, Yuen MF, Lai CL. Entecavir vs Tenofovir in Hepatocellular Carcinoma Prevention in Chronic Hepatitis B Infection: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2020;11:e00236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Tanaka Y, Ogawa E, Huang CF, Toyoda H, Jun DW, Tseng CH, Hsu YC, Enomoto M, Takahashi H, Furusyo N, Yeh ML, Iio E, Yasuda S, Lam CP, Lee DH, Haga H, Yoon EL, Ahn SB, Wong G, Nakamuta M, Nomura H, Tsai PC, Jung JH, Song DS, Dang H, Maeda M, Henry L, Cheung R, Yuen MF, Ueno Y, Eguchi Y, Tamori A, Yu ML, Hayashi J, Nguyen MH; REAL-C Investigators. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int. 2020;14:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Cheng TS, Liang PC, Huang CF, Yeh ML, Huang CI, Lin ZY, Chen SC, Huang JF, Dai CY, Hsieh PH, Chuang WL, Yu ML. Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C patients with genotype-2 infection after completed treatment. Kaohsiung J Med Sci. 2021;37:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, Scuderi V, Barkhatov L, Edwin B, Troisi RI, Dagher I, Reggiani P, Belli G, Aldrighetti L, Abu Hilal M. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc. 2018;32:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 103. | Xie QS, Chen ZX, Zhao YJ, Gu H, Geng XP, Liu FB. Systematic review of outcomes and meta-analysis of risk factors for prognosis after liver resection for hepatocellular carcinoma without cirrhosis. Asian J Surg. 2021;44:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Martínez-Mier G, Esquivel-Torres S, Casanova-Sánchez IE, Escobar-Ríos AY, Troche-Gutiérrez JM, Yoldi-Aguirre CA. Hepatocellular carcinoma in the noncirrhotic liver: Clinical features and outcomes in Veracruz, Mexico. Rev Gastroenterol Mex (Engl Ed). 2021;86:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Lim C, Compagnon P, Sebagh M, Salloum C, Calderaro J, Luciani A, Pascal G, Laurent A, Levesque E, Maggi U, Feray C, Cherqui D, Castaing D, Azoulay D. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford). 2015;17:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Abdalla EK, Denys A, Hasegawa K, Leung TW, Makuuchi M, Murthy R, Ribero D, Zorzi D, Vauthey JN, Torzilli G. Treatment of large and advanced hepatocellular carcinoma. Ann Surg Oncol. 2008;15:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Panwar R, Pal S, Dash NR, Shalimar, Sahni P, Acharya SK, Pande GK, Chattopadhyay TK, Nundy S. Hepatic resection for predominantly large size hepatocellular carcinoma: Early and long-term results from a tertiary care center in India. Indian J Gastroenterol. 2016;35:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5397] [Article Influence: 179.9] [Reference Citation Analysis (7)] |
| 109. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 110. | McPeake JR, O'Grady JG, Zaman S, Portmann B, Wight DG, Tan KC, Calne RY, Williams R. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Halazun KJ, Sapisochin G, von Ahrens D, Agopian VG, Tabrizian P. Predictors of outcome after liver transplantation for hepatocellular carcinoma (HCC) beyond Milan criteria. Int J Surg. 2020;82S:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, Seo S, Machimoto T, Uemoto S. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Nivolumab Approved for Liver Cancer. Cancer Discov. 2017;7:OF3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Hussaarts KGAM, van Doorn L, Bins S, Sprengers D, de Bruijn P, van Leeuwen RWF, Koolen SLW, van Gelder T, Mathijssen RHJ. Combining Sorafenib and Immunosuppression in Liver Transplant Recipients with Hepatocellular Carcinoma. Pharmaceuticals (Basel). 2021;14:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Li T, Zhang G, Wang L, Li S, Xu X, Gao Y. Defects in mTORC1 Network and mTORC1-STAT3 Pathway Crosstalk Contributes to Non-inflammatory Hepatocellular Carcinoma. Front Cell Dev Biol. 2020;8:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, Antonelli B, Airoldi A, Manini MA, Sangiovanni A, Rossi G, Donato MF, Saverio Belli L, Lampertico P. Experience With Early Sorafenib Treatment With mTOR Inhibitors in Hepatocellular Carcinoma Recurring After Liver Transplantation. Transplantation. 2020;104:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 117. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5332] [Article Influence: 888.7] [Reference Citation Analysis (29)] |
| 118. | Oestmann PM, Wang CJ, Savic LJ, Hamm CA, Stark S, Schobert I, Gebauer B, Schlachter T, Lin M, Weinreb JC, Batra R, Mulligan D, Zhang X, Duncan JS, Chapiro J. Deep learning-assisted differentiation of pathologically proven atypical and typical hepatocellular carcinoma (HCC) versus non-HCC on contrast-enhanced MRI of the liver. Eur Radiol. 2021;epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 119. | Ding B, Lou W, Liu J, Li R, Chen J, Fan W. In silico analysis excavates potential biomarkers by constructing miRNA-mRNA networks between non-cirrhotic HCC and cirrhotic HCC. Cancer Cell Int. 2019;19:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Zhang Y, Li T, Qiu Y, Zhang T, Guo P, Ma X, Wei Q, Han L. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e5642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Nishida N, Arizumi T, Hagiwara S, Ida H, Sakurai T, Kudo M. MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma. Liver Cancer. 2017;6:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: FRCS (Glasg); ESOT; ASTS; ILTS; ACS (Associate Fellow; FEBS- Transplant (Fellow); TTS.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balaban YH, Lun YZ S-Editor: Liu M L-Editor: A P-Editor: Wang LL